Abstract
Social comparisons are an important means by which we gain information about the self, but little is known about the neural mechanisms underlying comparative social judgment, as most prior functional magnetic resonance imaging research on this topic has investigated judgments of self or others in isolation. Ventromedial prefrontal cortex (vmPFC) has routinely been implicated in social cognitive tasks that rely on such absolute judgments about the self or others, but it is unclear whether activity in this region is modulated by personal relevance of social stimuli or self-similarity of judgment targets. Using functional magnetic resonance imaging, we demonstrate that these forces interact to determine vmPFC response during social comparisons, as well as neural activity in the bilateral anterior insulae. Comparisons between the self and similar others exhibit a unique response in this region when compared with other judgment contexts, suggesting that the special psychological status afforded to these social comparisons is indexed by activity in the vmPFC and insula.
Keywords: self, vmPFC, insula, social comparison, pACC
INTRODUCTION
Choosing a seat at a high-school reunion dinner party may require intricate feats of coordination at the neural level in order to apprehend and traverse space, but the challenge in deciding where to sit does not lie solely in locating and walking to an available chair. Successfully navigating the social environment demands, among many things, both self-relevant and other-focused, person-specific knowledge. Few would choose to sit next to the creepy former classmate with whom one finds little common ground, and most would rather sit next to the easygoing peer with whom one quickly (re)connects. Such situations also seem to naturally lend themselves to comparing one’s own attributes and preferences with those of the other partygoers, in ways that inform one’s own sense of self. Although people can draw on a rich personal history of social interaction, a lack of absolute standards for concepts like how ‘attractive’, ‘shy’ or ‘rebellious’ someone is means that, in order to satisfy the long-hypothesized drive to evaluate our own opinions and abilities, humans must often rely on social comparison processes to shape and refine our conceptualizations of self (Festinger, 1954).
Effective mapping of the social world further requires that we make dispositional inferences about others, which are heavily biased by degree of similarity to or difference from the self (Mussweiler, 2003). Ventromedial prefrontal cortex (vmPFC) is widely regarded as an important neural substrate for this sort of social cognition, and meta-analyses have specifically linked activity in this region to making judgments about the enduring characteristics of others (van Overwalle, 2009), to explicit self-reflection (van der Meer et al., 2010) and to processing social stimuli with a high degree of self-relevance (Northoff et al., 2010). The vmPFC also exhibits heightened responses during non-comparative judgments about self-similar (as opposed to dissimilar), unfamiliar social targets (Mitchell et al., 2006). More recently, the vmPFC has been linked to degree of personal closeness, responding preferentially to close others (as opposed to unknown persons) due to their heightened social relevance, regardless of the degree of similarity between the self and judgment target (Krienen et al., 2010).
When explicitly comparing the self to others, people seem to rely on the self as a relative calibration point or anchor, and then make incremental, serial adjustments to better approximate a true estimate of the judgment target. It is faster to make an inference that requires only slight adjustment, as demonstrated by a positive linear relationship between self-other distance and reaction time (RT) (Tamir and Mitchell, 2012), and neuroimaging research on this effect has demonstrated that the self-other difference in response latency also scales in a linear fashion with social cognition associated with blood oxygen level-dependent (BOLD) signal in the vmPFC (Tamir and Mitchell, 2010).
Extrapolation of this line of research to the study of social comparison processes is somewhat complicated by the fact that the majority of neuroimaging investigations of social inference to date have relied upon judgment targets with whom participants were highly unlikely to ever engage in a true social interaction (e.g. George Bush and Harry Potter), meaning that spontaneous social comparisons to such targets are probably quite rare. Another common feature of prior studies is that judgment targets are almost invariably evaluated in isolation, which is somewhat artificial and may affect self-referential social cognition in a manner that is difficult to assess (Goffin and Olson, 2011). Because people appear to spontaneously evoke the self when evaluating other people in the absence of a more appropriate comparison target (Mussweiler, 2003), we cannot rule out that the BOLD signal elicited in vmPFC by self-similar targets reflects unintentional self-specific cognition as well.
One notable exception reported that comparative social judgments (e.g. ‘Which person is taller?’) have been shown to elicit stronger vmPFC responses than non-comparative judgments about social targets (e.g. ‘Is this person a rock star?’) (Lindner et al., 2008). It should be pointed out, however, that the social targets comprised famous individuals not personally known to subjects, and there was no attempt made to control for spontaneous self-comparisons. Additionally, a recent study made the important demonstration that comparative social judgments between evaluations of stimuli valence made by oneself and a close other (e.g. who thinks ‘spy’ is more unpleasant?) engage lateral, rather than medial, prefrontal cortex when contrasted directly against judgments of the self in isolation (Raposo et al., 2011). Finally, two recent experiments endeavored to disentangle explicit social evaluations from spontaneous judgments by alternating between directly instructing subjects to make a trait inference (based on a verbal description of the target) or else simply presenting the behavioral description, prompting spontaneous social judgment; the results of these studies were interpreted as suggesting that spontaneous judgments elicit a stronger signal in the mPFC (Ma et al., 2011; Kestemont et al., 2012).
In conceptually related work, social comparison tasks involving discrepant financial payoffs have also demonstrated reward-related activity in the ventral striatum and vmPFC for relative social gains compared with absolute gains (Fliessbach et al., 2007), and reward-related signal during social gambling tasks is known to predict both subsequent risky decision-making and vmPFC activity for future social comparison trials (Bault et al., 2011). Finally, large-scale meta-analysis of the PFC points to a broader role for the vmPFC in the generation of affective meaning, synthesizing social knowledge with emotions to create contextually appropriate models of the self (Roy et al., 2012). Taken together, these findings suggest that social comparison processes involve value computation at both cognitive and affective levels, providing emotional coloring to our relationships with others as a fuzzy means of quantifying our similarities and differences to our peers.
No experiment to date has simultaneously compared the neural correlates of comparative social judgment for actual peers who vary in perceived self-similarity, across a variety of psychological traits. The use of judgment targets with whom participants have a personal history allows us to test the way that inclusion in a comparison and similarity of comparators interact in a task that draws upon the sort of social cognitive faculties people regularly employ in their everyday lives. If personal relevance of comparison targets were the sole driving force behind vmPFC activity, then we would expect only a main effect of self-similarity and no interaction between: (i) direct personal involvement as a comparison target and (ii) self-similarity of comparators. We hypothesize, however, that ‘both’ explicit involvement of the self ‘and’ perceived self-similarity of (non-self) targets modulate the neural activity underlying comparative social judgment in vmPFC.
Specifically, we expect that comparisons between the self and a similar peer will exhibit a distinct neural signature from other social comparison conditions. Consistent with this notion, repetition-related suppression has been demonstrated in vmPFC for judgments about the self-following judgments about similar, but not dissimilar others (Jenkins et al., 2008). Half a century of research has explored when, why and with whom people engage in social comparisons (Mussweiler, 2003), but no study to date has investigated the central importance Festinger (1954) placed on comparisons between the self and similar others by looking to the brain. A unique response in vmPFC to comparisons between the self and similar peers could identify neural substrates underlying their hypothetically vital psychological status. If comparisons between others depend on anchoring to the self and then adjusting to the targets, then we would also expect differences in response latency across conditions. Trials in which the self is explicitly involved should be more quickly resolved than those involving only others, because comparisons between peers would require more steps of serial adjustment from the self. However, comparing two similar peers requires more finely tuned adjustments, and thus should be a more lengthy process than comparing a similar peer to a different one.
METHODS
Participants
Participants were 19 right-handed individuals (10 women; age, mean = 25.95, s.d. = 2.32 years), recruited via flyers. Prior to scanning, all participants provided the names of three similar and three dissimilar peers, known for at least 1 year and with whom they interact regularly (excluding ‘best’ friends, as this relationship lacks a parallel dissimilar category for most people).
Functional magnetic resonance imaging paradigm
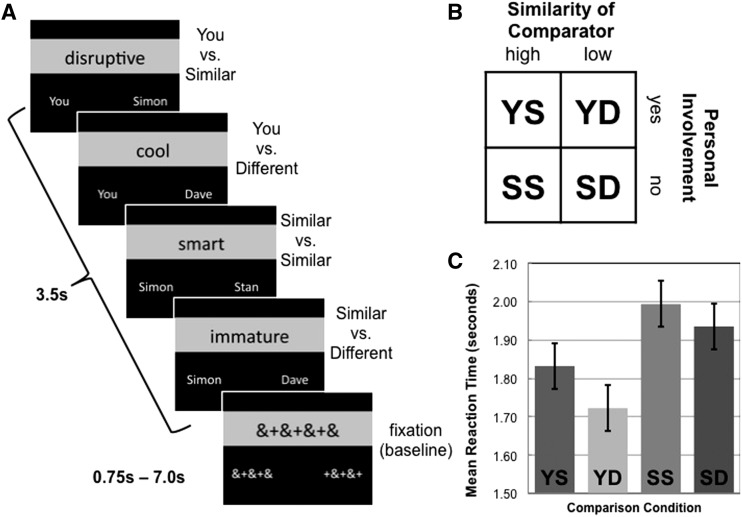
In the task at hand participants were shown a series of trait adjectives, each accompanied by the names of two individuals and presented for 3.5 s and asked to decide via button press ‘Who is more _____?’ A blank screen was presented for 0.5 s between trials, and fixation trials ranging from 0.75 to 7.0 s were inserted into the design sequence at optimal junctures, both to introduce jitter and serve as a baseline condition with comparable visual complexity to stimuli of interest. An optimized presentation sequence was determined through use of a genetic algorithm designed to obtain maximal contrast detection between the conditions of interest (Kao et al., 2009). Each of 40 trait adjectives was presented once per condition (160 total trials; see Figure 1). Participants completed two functional runs, each of which lasted 6 min 42 s.
Fig. 1.
Diagram illustrating (A) experimental paradigm, (B) experimental design and (C) mean reaction time for each comparison condition.
Functional magnetic resonance imaging acquisition and analysis
All data were acquired on a 3T Siemens Allegra MRI scanner at the Robert and Beverly Lewis Center for Neuroimaging at the University of Oregon, including pre-subtracted phase and magnitude maps, both T1-weighted (MP-RAGE) and T2-weighted (coplanar high resolution) anatomical images and two functional runs of BOLD, echo-planar images (BOLD-EPI) with the following scanning parameters: TR = 2000 ms, TE = 30 ms, field of view = 200 mm, matrix size = 64 × 64, 32 oblique slices, slice thickness = 4 mm, 200 images. The BOLD-EPI sequence used also prospectively corrected for motion during acquisition (Thesen et al., 2000). DICOM images were converted to NIfTI format via MRIConvert (http://lcni.uoregon.edu/∼jolinda/MRIConvert/) and non-brain tissue was removed using FSL’s Brain Extraction Tool (Smith, 2002). Voxel displacement maps were generated to correct for field inhomogeneities using the FieldMap toolkit and then used to unwarp and realign functional images in SPM8. Anatomical images were co-registered to the mean functional image and then segmented into six tissue types using the unified segmentation approach (Ashburner and Friston, 2005). DARTEL was used to create a group anatomical template, transformations from which were applied to warp functional data to the ICBM-152 template supplied with SPM8 (Ashburner, 2007).
For each participant, condition effects were estimated according to the general linear model in using a canonical hemodynamic response function, high-pass filtering (90 s), correction for serial autocorrelation (AR1) and an optimally thresholded explicit mask (Ridgway et al., 2009). RT was entered into the model as a parametric modulator in order to control for potential discrepancies in BOLD signal due to differences in RT across conditions. In order to assess population level effects, voxelwise statistical parametric maps summarizing differences between conditions were calculated for each participant and then entered into a random effects (group level) repeated measures analysis of variance (ANOVA) with personal involvement and similarity of comparators as factors (thresholded at P < 0.05 FWE, corrected for multiple comparisons across the whole brain; see Table 1 for results). This correction was determined via a Monte Carlo Simulation implemented in NeuroElf (http://www.neuroelf.net) and based on AlphaSim with a height threshold of P < 0.005 and an extent threshold of 24 voxels. Whole-brain contrast images from the repeated measures ANOVA for (YS-YD), (YS-SS) and (YS-SD) were combined and tested against the conjunction null (Nichols et al., 2005) at the FWE-corrected height and extent thresholds. All reported coordinates were converted into the Talairach coordinate system via icbm2tal. Mean parameter estimates from significant clusters were extracted in NeuroElf, converted to percent signal change and subjected to further statistical assessment in MATLAB 2011a (http://www.mathworks.com).
Table 1.
BOLD activity associated with interaction between personal relevance and self-similarity
| Neural region (Talairach coordinates) | x | y | z | No. of voxels | Peak F |
|---|---|---|---|---|---|
| Left anterior insula | −28 | 28 | 8 | 127 | 29.453 |
| Right anterior insula | 31 | 31 | 6 | 81 | 18.778 |
| vmPFC (pACC) | 4 | 33 | 6 | 78 | 14.968 |
Correction for multiple comparisons (FWE P < 0.05) applied via height threshold of P < 0.005 and extent threshold of k = 24. vmPFC, ventromedial prefrontal cortex; pACC, perigenual anterior cingulate cortex.
RESULTS
Behavioral results
Mean RT per condition for each participant was entered into a 2 × 2 repeated measures ANOVA with two factors: (i) direct personal involvement as a comparison target (ipseity) and (ii) self-similarity of comparators as within-subjects factors, revealing significant main effects of both ipseity [F(1,18) = 50.97, P < 0.001, d = 3.37] and similarity [F(1,18) = 7.29, P < 0.05, d = 1.27], but no significant interaction [F(1,18) = 2.05, P = 0.17, d = 0.67; Figure 1C]. Pairwise comparisons demonstrated that participants responded more quickly to YD judgments compared with YS [F(1,18) = 9.80, P < 0.01, d = 1.48], but more slowly to both SS [F(1,18) = 19.62, P < 0.001, d = 2.09] and SD [F(1,18) = 5.31, P < 0.05, d = 1.09] judgments than YS judgments. SS and SD did not differ from each other [F(1,18) = 2.52, P = 0.13, d = 0.75]. In other words, participants were fastest when comparing themselves to someone different and slowest when comparing only others, with judgments between the self and a similar other falling in between.
Functional magnetic resonance imaging results
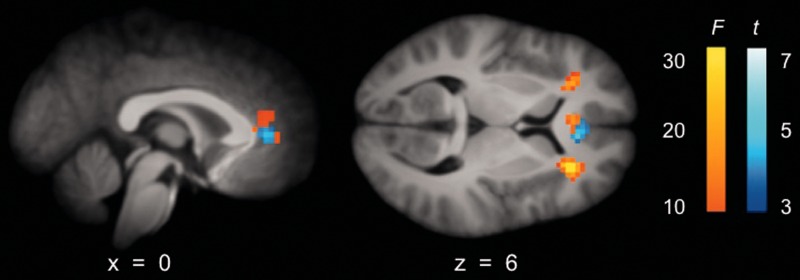
Linear contrast images from each condition (controlling for RT) for each participant were entered into a 2 × 2, repeated measures ANOVA with personal relevance and similarity as factors. No main effects of either personal relevance or similarity were detected at our statistical thresholds. As predicted, the group-level repeated measures ANOVA revealed a significant interaction in the vmPFC, specifically, the perigenual anterior cingulate cortex (pACC; k = 78, P < 0.05, FWE corrected; see ‘hot’ colors in Figure 2 and Table 1). This interaction effect demonstrates that neither degree of personal involvement nor self-similarity of comparators alone can explain the role of vmPFC in comparative social judgment.
Fig. 2.
BOLD activity associated with comparative social judgment in the ventromedial prefrontal cortex (vmPFC; specifically, perigenual anterior cingulate cortex) and anterior insula, displayed on composite image of participants’ T1-weighted anatomical scans. Warm: interaction between personal involvement and similarity of comparator (Talairach coordinates, [4 33 6], k = 78, P < 0.05, FWE), F(1,72) = 14.97. Cool: Conjunction of (YS > YD), (YS > SS) and (YS > SD) demonstrating activity unique to comparisons between the self and a similar peer (Talairach coordinates, [−3 36 3], k = 40, t(18) = 4.05).
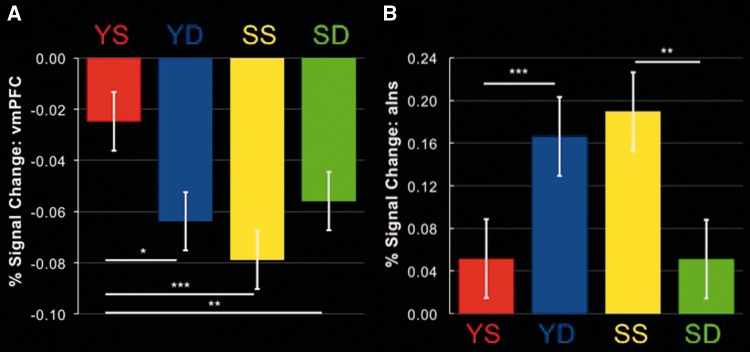
Mean parameter estimates for each condition were extracted from the vmPFC cluster, converted to percent-signal change and interrogated using pair-wise t-tests in order to decompose the interaction (see Figure 3A). Note that, consistent with our data, when neurotypical adults make trait judgments involving the self, there is typically a mild reduction in the high rate of vmPFC baseline metabolic activity, but thinking about others elicits considerably stronger ‘deactivations’ (Kelley et al., 2002; Heatherton et al., 2006). Results indicated that similarity had a significant effect when making comparisons involving the self [YS vs YD: t(18) = 2.99, P < 0.01, d = 0.68], but not during comparisons between others [SS vs SD: t(18) = 1.77, P = 0.09, d = 0.77]. Likewise, personal relevance had a significant effect for comparisons with similar others [YS vs SS: t(18) = 6.26, P < 0.00001, d = 1.44], but not when targets included dissimilar others [YD vs SD: t(18) = 0.95, P = 0.36, d = 0.22]. Meanwhile, a reciprocal pattern was observed bilaterally in the anterior insula (see Figures 2 and 3B; Table 1), such that comparisons between two similar peers or between oneself and a different peer elicited more activity than comparisons between similar and dissimilar others or between oneself and a similar other.
Fig. 3.
Parameter estimates extracted from ventromedial prefrontal cortex (vmPFC; A) and anterior insula (aIns; B) from clusters in Figure 2 for each comparison condition. Parameter estimates for (B) are from the right anterior insula cluster, but an identical pattern was reflected by parameter estimates extracted from left anterior insula as well. *P < 0.01, **P < 0.005, ***P < 0.00001. YS = You vs Similar, YD = You vs Different, SS = Similar vs Similar, SD = Similar vs Different.
Finally, to substantiate the uniqueness of the vmPFC response for comparisons between the self and a similar other, whole-brain contrast images for (YS > YD), (YS > SS) and (YS > SD) were combined and tested against the conjunction null, revealing a solitary cluster in vmPFC (specifically, pACC, Talairach coordinates, [−3 36 3], k = 40, t(18) = 4.05) situated almost entirely within the ventral aspect of the interaction cluster(see ‘cool’ colors in Figure 2). Whole-brain contrast images for pairwise comparisons revealed no additional clusters beyond those described by the repeated measures or conjunction analyses, and significant patterns of activity observed in the whole-brain pairwise comparison analyses were consistent with the aforementioned clusters.
DISCUSSION
In this study, social comparisons between the self and similar peers elicited relatively more activity in vmPFC than social comparisons between the self and dissimilar peers, between two similar peers, or between one similar peer and one dissimilar peer. Meanwhile, bilateral anterior insula was more engaged when making social comparisons between two similar peers, or between the self and dissimilar peers, than during social comparisons between the self and similar peers, or between one similar peer and one dissimilar peer. These results represent the only significant clusters of activity that distinguished between these highly similar and relevant comparative social judgments, which notably included peers known to participants, selected on the basis of their general similarity or dissimilarity to the self.
The sort of person-specific cognition underlying social comparison processes has been repeatedly linked to activity in the vmPFC (Northoff et al., 2006; van Overwalle, 2009; van der Meer et al., 2010), and neural responses to tasks involving self-relevant stimuli appear much like those associated with judgments of similar others (Mitchell et al., 2006; Ames et al., 2008). Comparative judgments between personally unknown others are likewise known to elicit activity in the vmPFC, especially for psychological rather than physical traits (Lindner et al., 2008), although comparative judgments between evaluations of stimuli valence made by the self and a close other elicit responses in lateral PFC (Raposo et al., 2011). The neural mechanisms for engaging in comparisons between the self and persons with whom participants have active and meaningful relationships have received considerably less attention, although one recent exception suggests that pACC is implicated in positivity bias exhibited during self-perception, which may extend to romantic partners as compared with non-close others (Beer, 2012).
One limitation of the current experiment is that the decision to regard peers as similar or different was entirely determined by participants in a generalized fashion. Closeness was controlled by requesting that participants chose peers with whom they had roughly equivalent degrees of personal history and excluding ‘best friends’, but as a friend who is generally regarded as different may be quite similar with respect to a particular trait adjective, the category into which such comparative judgments were placed may have been less than ideally defined. Future studies could account for this by obtaining ratings for all trait adjectives employed for the participants and all selected peers, as well as obtaining more detailed information about relationship histories that may contribute additional variance. However, such a limitation only served to make our test of the neural mechanisms supporting social comparison processes more conservative.
Despite this potential limitation, our findings demonstrate that vmPFC concurrently tracks both personal relevance and self-similarity during social comparisons. As evidenced by the interaction and conjunction analyses, vmPFC activity is modulated by social judgment context. Moreover, the unique BOLD response to relative judgments between the self and a close, similar other points to the inclusion of those who are ‘like me’ in self-representation at the neural level (Mussweiler, 2003). Rather than simply distinguishing between friends and strangers or evaluating the degree of similarity between the self and others, we suggest that the pACC aspect of vmPFC encodes the dynamic interplay between such judgments, providing emotive clues about aspects of person perception that are difficult to quantify explicitly. The vmPFC is central to supplying this sort of ‘affective meaning’ (Roy et al., 2012), a property that emerges from the integration of conceptual information from long-term memory with the sort of emotion-laden, subjective evaluations known to be tightly linked with stimuli involving the self (Northoff et al., 2006). Higher levels of activity in this region when considering the self in relation to similar peers speak to the special significance long ascribed to this particular social comparison process (Festinger, 1954), and an enhanced vmPFC signal during such judgments aligns well with the notion that the sort of information derived from such comparisons is critically involved in updating representations of the self in a given decision-making context.
It is somewhat surprising that comparisons between one similar peer and one different peer (SD) elicit profiles of activity in vmPFC that appear more closely related to comparisons between the self and similar peers (YS), than to judgments associated with the other social comparison contexts assessed. One possibility is that comparisons between similar and dissimilar peers are most conducive to simulation-oriented types of person perception (Benoit et al., 2010), in which the self is readily projected into the role of similar other. The patterns of activity in bilateral anterior insula also exhibit a tight reciprocity with those observed in vmPFC, such that comparisons between the self and a similar peer (YS) as well as those between one similar and one different peer (SD) elicit less-severe shifts away from baseline activity than do comparisons between the self and a different peer (YD) or between two similar peers (SS). Although the anterior insula is frequently implicated in a host of social, affective and cognitive functions (Chang et al., 2013), prohibiting a clear inference about its precise contribution in this task, we believe it is relevant that anterior insula is engaged during the perception of non-self-relevant (as compared with personally relevant) stimuli (Enzi et al., 2009), as well as in the inhibition of one’s own perspective, a necessary component of simulation (van der Meer et al., 2011). As such, the apparent parallel between responses during YS and SD comparisons in both the anterior insula and vmPFC may suggest that comparisons that readily lend themselves to simulation, such as those between similar and dissimilar peers, are also particularly useful for updating both perceptions of the self and social judgment contexts.
Future behavioral and neuroimaging research should follow up on this unique pairing elucidated at the neural level, to ascertain in what ways social comparisons between the self and a similar peer are more similar to social comparisons between one similar and one different peer, vs other comparative social judgments. It may also be useful to interrogate the utility of this kind of social comparison, relative to those between the self and a similar peer—perhaps by investigating the frequency with which these naturally occur, or the ability of certain groups who may have difficulty with simulation processes (like individuals with autism spectrum disorders; Oberman and Ramachandran, 2007) to engage in these and other kinds of social comparisons.
In conclusion, this study provides further empirical support for the hypothesized functions ascribed to vmPFC with respect to person perception, specifically in the paralimbic pACC region. More importantly, it emphasizes and elucidates in greater detail the contextual specificity of the vmPFC response for different sorts of comparative social judgment. It also suggests a potential neural marker for the special significance long attributed to comparisons between the self and similar others (Festinger, 1954; Mussweiler, 2003), highlighting their informative nature both for self-perception and social cognition, while also drawing attention to the potential importance of comparisons between similar and dissimilar peers. Coupled with these results is evidence suggesting that the anterior insula plays a role in determining appropriate contextual responses for social judgments, pointing to a simulation-oriented account of person perception that involves both enhancement of another person’s perspective (vmPFC) and inhibition of one’s own point of view (insula). Taken together, the current findings highlight the importance of vmPFC and anterior insula in determining the affective meaning ascribed to different kinds of social comparisons, highlighting their supporting role in contrasting the self against personally known others. The mutual activity of these brain regions may very well produce the barely perceptible gut feeling of squeamishness about sitting next to a creepy former classmate, placating implicit concerns about social identity by distinguishing ourselves from them and instead identifying with the easygoing peer, a person readily regarded as more similar to the self.
FUNDING
This work was supported by grants from the National Institutes of Health [L40HD059442 and L40MH087356 to J.H.P.]. Magnetic resonance imaging was performed at the Robert and Beverly Lewis Center for Neuroimaging at the University of Oregon. The authors wish to thank Jolinda Smith for her guidance in sequence selection, Scott Watrous and Chuck Theobold for their assistance in image acquisition, Elliot Berkman for providing feedback throughout our data analysis, and the rest of the Developmental Social Neuroscience Laboratory.
REFERENCES
- Ames DL, Jenkins AC, Banaji MR, Mitchell JP. Taking another person’s perspective increases self-referential neural processing. Psychological Science. 2008;19(7):642. doi: 10.1111/j.1467-9280.2008.02135.x. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bault N, Joffily M, Rustichini A, Coricelli G. Medial prefrontal cortex and striatum mediate the influence of social comparison on the decision process. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(38):16044–9. doi: 10.1073/pnas.1100892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer JS. Orbitofrontal cortex and anterior cingulate cortex are modulated by motivated social cognition. Cerebral Cortex (New York, N.Y.) 2012;22(6):1372–81. doi: 10.1093/cercor/bhr213. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Volle E, Burgess PW. When I think about me and simulate you: Medial rostral prefrontal cortex and self-referential processes. Neuroimage. 2010;50(3):1340–9. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: Functional parcellation and large-scale reverse inference. Cerebral Cortex (New York, N.Y.) 2013;23(3):739–49. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzi BAG, Prösch MA, Tempelmann UA, Northoff CA, Georg Is our self nothing but reward? Neuronal overlap and distinction between reward and personal relevance and its relation to human personality. PLoS ONE. 2009;4(12):e8429. doi: 10.1371/journal.pone.0008429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festinger L. A Theory of Social Comparison Processes. Human Relations. 1954;7(2):117–40. [Google Scholar]
- Fliessbach K, Weber B, Trautner P, et al. Social comparison affects reward-related brain activity in the human ventral striatum. Science (New York, N.Y.) 2007;318(5854):1305–8. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- Goffin RD, Olson JM. Is it all relative? Perspectives on Psychological Science. 2011;6(1):48–60. doi: 10.1177/1745691610393521. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1(1):18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AC, Macrae CN, Mitchell JP. Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(11):4507–12. doi: 10.1073/pnas.0708785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao MH, Mandal A, Lazar N, Stufken J. Multi-objective optimal experimental designs for event-related fmri studies. Neuroimage. 2009;44(3):849–56. doi: 10.1016/j.neuroimage.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fmri study. Journal of Cognitive Neuroscience. 2002;14(5):785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kestemont J, Vandekerckhove M, Ma N, van Hoeck N, van Overwalle F. Situation and person attributions under spontaneous and intentional instructions: an fMRI study. Social Cognitive and Affective Neuroscience. 2012;7:1–13. doi: 10.1093/scan/nss022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Tu P-C, Buckner RL. Clan mentality: evidence that the medial prefrontal cortex responds to close others. The Journal of Neuroscience. 2010;30(41):13906–15. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner M, Hundhammer T, Ciaramidaro A, Linden DE, Mussweiler T. The neural substrates of person comparison—an fMRI study. NeuroImage. 2008;40(2):963–71. doi: 10.1016/j.neuroimage.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Ma N, Vandekerckhove M, van Overwalle F, Seurinck R, Fias W. Spontaneous and intentional trait inferences recruit a common mentalizing network to a different degree: spontaneous inferences activate only its core areas. Social Neuroscience. 2011;6(2):123–38. doi: 10.1080/17470919.2010.485884. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mussweiler T. Comparison processes in social judgment: mechanisms and consequences. Psychological Review. 2003;110(3):472. doi: 10.1037/0033-295x.110.3.472. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25(3):653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P, Feinberg TE. Brain imaging of the self- conceptual, anatomical and methodological issues. Consciousness and Cognition. 2011;20(1):52–63. doi: 10.1016/j.concog.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Pineda JA, Ramachandran VS. The human mirror neuron system: A link between action observation and social skills. Social Cognitive and Affective Neuroscience. 2007;2(1):62. doi: 10.1093/scan/nsl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo A, Vicens L, Clithero JA, Dobbins IG, Huettel SA. Contributions of frontopolar cortex to judgments about self, others and relations. Social Cognitive and Affective Neuroscience. 2011;6(3):260–9. doi: 10.1093/scan/nsq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway GR, Omar R, Ourselin S, Hill DLG, Warren JD, Fox NC. Issues with threshold masking in voxel-based morphometry of atrophied brains. Neuroimage. 2009;44(1):99–111. doi: 10.1016/j.neuroimage.2008.08.045. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16(3):147–56. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. Neural correlates of anchoring-and-adjustment during mentalizing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(24):10827–32. doi: 10.1073/pnas.1003242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. Disclosing information about the self is intrinsically rewarding. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(21):8038–43. doi: 10.1073/pnas.1202129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine. 2000;44(3):457–65. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews. 2010;34(6):935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Groenewold NA, Nolen WA, Pijnenborg M, Aleman A. Inhibit yourself and understand the other: Neural basis of distinct processes underlying theory of mind. Neuroimage. 2011;56(4):2364–74. doi: 10.1016/j.neuroimage.2011.03.053. [DOI] [PubMed] [Google Scholar]
- van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30(3):829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]