Abstract
Functions of the orbitofrontal cortex include diverse social, cognitive and affective processes, many of which are abnormal in autism spectrum disorders (ASDs). Recently, altered orbitofrontal sulcogyral patterns have been revealed in several psychiatric conditions, such as schizophrenia, indicating a possibility that altered orbitofrontal sulcogyral morphology reflects abnormal neurodevelopment. However, the presence of sulcal alterations in ASD remains unexplored. Using structural magnetic resonance imaging, subtypes of the ‘H-shaped’ sulcus (Type I, II and III, in order of frequency), posterior orbital sulcus (POS) and intermediate orbital sulcus were identified in each hemisphere of adult males with ASD (n = 51) and matched normal controls (n = 55) based on the study by Chiavaras and Petrides. ASD showed a significantly altered distribution of H-shaped sulcal subtypes in both hemispheres, with a significant increase of Type III. A significant alteration in the distribution of sulcal subtypes was also identified in the right hemisphere POS of ASD. Categorical regression analysis revealed that Type I and II expressions predicted a reduced total Autism-Spectrum Quotient score. Furthermore, Type I expression was associated with a reduced ‘attention to detail’ subscale score. The results demonstrate that altered sulcogyral morphology can be a marker for abnormal neurodevelopment leading to the increased risk of developing autism.
Keywords: autism spectrum disorder, magnetic resonance imaging, orbitofrontal cortex, brain sulcus, autistic trait
INTRODUCTION
Clinical symptoms of autism spectrum disorders (ASDs) can be characterized by marked impairments in socialization and communication along with restricted and repetitive behaviors (RRBs). Recent advances in clinical neuroscience have highlighted that deficits in a set of brain regions can be responsible for the impairments in social communicative functions and inflexible cognitive styles found in ASD. Among several regions, the importance of the orbitofrontal cortex (OFC) has been widely recognized (Baron-Cohen, 2002), particularly because of its key role in various facets of social cognition and behaviors (Schoenbaum and Roesch, 2005) as well as its dense connections to the amygdala and the anterior cingulate cortex, both of which are crucially involved in the pathophysiology of ASD (Barbas and Pandya, 1989; Ongur et al., 2003). Despite its theoretical significance, empirical evidence for dysfunction of the OFC has not been consistently demonstrated by neuroimaging studies (Girgis et al., 2007; Jiao et al., 2010; Via et al., 2011; Philip et al., 2012). While several reasons for this lack of evidence can be considered, it is of note that there are technical difficulties specific to the OFC and its neighboring regions that hamper conventional magnetic resonance imaging (MRI) approaches; for example, large magnetic field inhomogeneity during functional MRI (Stenger, 2006).
For structural MRI studies, substantial interindividual variability in the sulcogyral pattern in the OFC can hamper interpretation of the volumetric measures derived by standard morphometric approaches, because definition of the region-of-interest (ROI) often relies on sulci in the manual drawing method, and variation of sulcogyral pattern could be a confounding factor at the spatial normalization step in the voxel-based morphometry (VBM). The development of the sulcogyral convolution is thought to reflect critical neurodevelopmental events, such as neuronal migration and local neuronal connection (Rakic, 1988; Armstrong et al., 1995). Therefore, sulcogyral patterns may be significantly altered in neurodevelopmental disorders, which may further result in a systematic bias for structural measurements using VBM and manual ROI tracing methods.
Interindividual variability of the human orbitofrontal sulci has been systematically described in a seminal work using MR images of 100 hemispheres of 50 neurotypical individuals (Chiavaras and Petrides, 2000). Apart from the olfactory sulcus in the medial OFC, which is clearly visible and easily identifiable in every subject, the morphology of the other orbitofrontal sulci is highly complex and variable, and some sulci may not be consistently present between individuals. For instance, an ‘H-shaped’ sulcus refers to a configuration of multiple components of the medial orbital sulcus (MOS), lateral orbital sulcus (LOS) and transverse orbital sulcus (TOS). In a previous study by Chiavaras and Petrides, the H-shaped sulcus has been classified into three major subtypes (Type I, II and III, with Type I being the most common) based on the continuity of the MOS and LOS. A subsequent study reported an additional minor subtype (Type IV) that was found in 3–6% of the neurotypical population (Chakirova et al., 2010). Pattern variations of the other orbitofrontal sulci have also been described, including the posterior orbital sulcus (POS) and the intermediate orbital sulcus (IOS), located in the posterior and anterior orbitofrontal gyri, respectively (Chiavaras and Petrides, 2000).
Several recent MRI studies have examined orbitofrontal sulcogyral variations in psychiatric populations. The sulcogyral formation is strongly regulated by genetic factors that mobilize sequences of early cortical folding developmental processes (Bartley et al., 1997; Lohmann et al., 1999), and after formation, sulcogyral morphology is thought to be stable throughout life (Armstrong et al., 1995; Nakamura et al., 2007; Chakirova et al., 2010). Although there is evidence that environmental and experiential factors later in life can modulate quantitative variables related to the sulcogyral folding, such as local gyrification index (Luders et al., 2012), previous studies have identified series of critical events in early neural development that are thought to play central roles for determining cortical folding patterns [e.g. cortical growth (Toro and Burnod, 2005) and tensions from white matter fibers (Van Essen, 1997)], which led some researchers to propose that the sulcogyral pattern can be a morphological trait marker for psychiatric disorders with developmental origins (Nakamura et al., 2007). Nakamura et al. (2007) were the first to report that the frequency distribution of H-shaped sulcus subtypes was significantly altered in schizophrenia, with an increased occurrence of Type III together with a reduced occurrence of Type I in the right hemisphere. This pattern of alteration was also observed in first-episode schizophrenic patients (Chakirova et al., 2010; Takayanagi et al., 2010) and individuals at high risk of developing schizophrenia (Chakirova et al., 2010). Furthermore, Type III expression was associated with more severe psychotic symptoms and impulsivity in patients (Nakamura et al., 2007) and higher ratings for psychosis in people at risk of developing schizophrenia (Chakirova et al., 2010). These studies illustrate the possibility that the altered orbitofrontal sulcogyral pattern can indeed be a morphological trait marker for a psychiatric condition with neurodevelopmental aberration, predicting the development of particular clinical symptoms in later life.
This study examined the orbitofrontal sulcogyral morphology in adult males with high-functioning ASD. Given that ASD is a neurodevelopmental disorder with a strong genetic involvement (Muhle et al., 2004; Sebat et al., 2007), and that various aspects of social cognition and behavioral regulation functions of the OFC are impaired in ASD (Kringelbach and Rolls, 2004), we hypothesized that the frequency distribution of sulcal pattern variations would be significantly altered in ASD when compared with neurotypical individuals. Prediction of the specific pattern of alteration in the H-shaped sulcus is not straightforward from the results of previous studies of schizophrenia. However, if the increased expression of Type III in schizophrenia reflects neurodevelopmental abnormalities that commonly occur in other developmental disorders, we predict that Type III expression would be elevated in the ASD as well, perhaps in a more enhanced manner than schizophrenia. Furthermore, given the ample evidence of pathophysiological amygdala abnormalities in ASD (Lauvin et al., 2012), it is possible that the POS pattern is also altered because the posterior OFC is considered part of the paralimbic region that has heavy anatomical connectivity with the amygdala. In addition, considering the morphological alterations in several neocortical association areas for social functions in ASD (e.g. inferior frontal gyrus), one might predict alteration of the IOS in the anterior OFC, which is also a part of neocortical association areas in the social brain network (Kringelbach and Rolls, 2004). Rather than focusing on a particular orbitofrontal sulcus, we systematically examined pattern variations in the H-shaped sulcus, POS and IOS separately, each of which has been shown to demonstrate substantial interindividual variability (Chiavaras and Petrides, 2000). Clinical correlation analyses of the OFC sulcogyral patterns were further performed to explore any relationship between orbitofrontal morphological traits and clinical features in ASD.
MATERIALS AND METHODS
Participants
Fifty-one adult males with ASD were recruited from outpatients at the Showa University Hospital. All patients were diagnosed with ASD based on the Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV) criteria and a medical chart review. A team of three experienced psychiatrists and a clinical psychologist performed the assessment. The assessment consisted of participant interviews about developmental history, present illness, life history and family history assessed independently by a psychiatrist and a clinical psychologist in the team. Participants were also asked to bring suitable informants who knew the participant in early childhood and about current mental or psychological symptoms, behavioral symptoms or motor skill deficits. At the end of the clinical interview, participants were formally diagnosed with pervasive developmental disorder by the psychiatrist if there was a consensus between the psychiatrists and the clinical psychologist. The diagnostic process required ∼3 h. Patients with secondary autism related to a specific etiology such as tuberous sclerosis were excluded.
A group of 55 normal male controls (NC) were recruited by advertisements and acquaintances and screened using the Japanese version of the Mini-International Neuropsychiatric Interview (MINI) (Otsubo and Kamijima, 2000) by the same trained interviewers. No control subjects had an Axis-I psychiatric disorder or a first-degree relative with an Axis-I psychiatric disorder.
The intellectual ability was assessed using the Japanese version of the National Adult Reading Test (JART) (Nelson and Willison, 1991; Matsuoka and Kim, 2006). All subjects in both groups had an intelligent quotient (IQ) score > 80. Handedness was assessed using the Edinburgh Inventory of Handedness (Oldfield, 1971). To assess the presence and severity of autistic traits in each participant, the Autism-Spectrum Quotient (AQ) was administered (Baron-Cohen et al., 2001). We used the Japanese version of the AQ, which has been assessed for its internal consistency reliability, test–retest reliability and discriminant validity (Kurita et al., 2005; Wakabayashi et al., 2006). To characterize autistic traits of each participant more specifically, we calculated the subscale scores of ‘social interaction’ and ‘attention to detail’ using the method proposed by Hoekstra et al. (2008).
Table 1 shows the demographic and clinical data of participants in the ASD and NC groups. Age, education and IQ were matched between the two groups (all P > 0.15). Handedness was significantly different (P = 0.005), consistent with several studies reporting an increased prevalence of left handedness in children with autism (Gillberg, 1983; Hauck and Dewey, 2001; Dane and Balci, 2007). At the time of the MRI scan, 11 of the 51 ASD participants were using the following medications: antidepressants (9 patients), anti-anxiety drugs (minor tranquilizer: 7 patients), hypnotic drugs (8 patients), antipsychotic drugs (4 patients) and antiepileptic drugs (1 patient). Most patients began taking medication only after reaching adolescence or adulthood (mean ± s.d.: 26.2 ± 9.2 years), which makes it unlikely that the medication can be a determinant factor of the sulcogyral patterns. Consistent with this view, Nakamura et al. (2007) reported that the OFC sulcogyral pattern was not associated with the antipsychotic dosage in patients with schizophrenia. All subjects participated in this study after providing written informed consent. This study was approved by the Ethics Committee of the Faculty of Medicine of Showa University.
Table 1.
The demographic and clinical data for the participants
| ASD (n = 51) |
NC (n = 55) |
df | t-Value | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Range | Mean | s.d. | Range | ||||
| Age (years) | 30.9 | 8.2 | 19–51 | 32.0 | 7.1 | 19–49 | 104 | 0.676 | 0.501 |
| Education (years) | 15.3 | 2.1 | 9–21 | 14.6 | 2.0 | 12–18 | 85 | 1.41 | 0.161 |
| Handedness | 0.53 | 0.74 | −1.00–1.00 | 0.90 | 0.32 | −0.67–1.00 | 77 | 2.904 | 0.005a |
| ICV × 106 (mm3) | 1.6096 | 0.14931 | 1.1631–1.9910 | 1.6193 | 0.15150 | 1.1809–2.0910 | 104 | 0.33 | 0.743 |
| Estimated IQ | 109.8 | 9.5 | 89.5–119.8 | 107.1 | 9.0 | 87.5–119.8 | 94 | 1.44 | 0.152 |
| AQ | 35.5 | 5.3 | 24–47 | 14.3 | 5.8 | 3–30 | 95 | 18.84 | <0.001a |
| Hoekstra’s subscale | |||||||||
| Social interaction | 121.8 | 11.7 | 96–144 | 82.0 | 12.1 | 52–106 | 95 | 16.44 | <0.001a |
| Attention to detail | 26.2 | 4.5 | 17–35 | 21.6 | 4.0 | 12–37 | 95 | 5.24 | <0.001a |
aSignificant difference thresholded at P = 0.01.
MRI data acquisition
MR images were acquired on a 1.5-T General Electric Signa System (GE Medical Systems, Milwaukee, WI, USA) at the Karasuyama Hospital in Tokyo. In total, 128 contiguous sagittal images were collected from each individual using an eight-channel phased-array whole-head coil and a spoiled gradient-echo (SPGR) sequence with the following parameters: 25 ms repetition time; 5.2 ms echo time; flip angle = 20°; 24 × 24 cm field of view; 256 × 256 matrix and 1.4 mm slice thickness. After acquisition, each image was resampled to 0.9375 × 0.9375 × 0.7 mm, and then realigned along the mid-sagittal anterior–posterior commissure line to correct any head tilt for consistent assessment of sulcogyral patterns.
Sulcogyral pattern identification
We classified sulcogyral pattern variations in the H-shaped sulcus (consisting of the TOS, MOS and LOS), POS and IOS into multiple subtypes in each hemisphere based on the previous description by Chiavaras and Petrides (2000) and a refined description for the H-shaped sulcus by Chakirova et al. (2010). In addition to the TOS, MOS, LOS, POS and IOS, we identified the neighboring olfactory sulcus and sulcus fragmentosus in each hemisphere to assist the precision and consistency of the sulcogyral classifications. We used three-dimensional slicer software (http://www.slicer.org/) to browse the SPGR images for visual identification of each orbitofrontal sulcus. Freesurfer software (http://surfer.nmr.mgh.harvard.edu/) was also used to calculate the intracranial volume (ICV) of individual brains and to generate the pial and white matter surfaces that were used to assist the classification of sulcogyral subtypes.
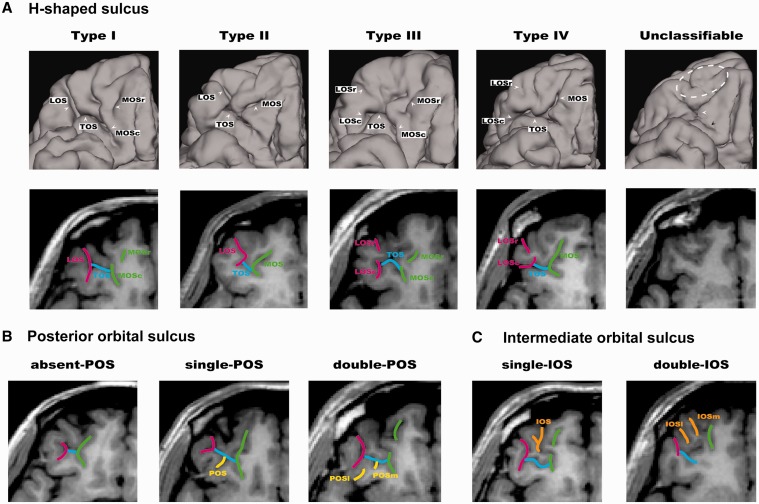
The subtypes of the H-shaped sulci were identified on the basis of the continuity of the LOS and MOS in the rostrocaudal direction (Chiavaras and Petrides, 2000), as demonstrated in Figure 1A. In Type I, the rostral and caudal portions of the MOS were clearly interrupted, whereas the LOS was continuous throughout the entire length. In Type II, rostral and caudal portions of the MOS and LOS were continuous without interruption and were connected by the TOS. In Type III, rostral and caudal portions of both MOS and LOS were interrupted. In Type IV, the rostral and caudal portions of the LOS were interrupted in the presence of a continuous MOS, thus representing the opposite of Type I. The original work by Chiavaras and Petrides (2000) described only the first three subtypes, but Type IV was later identified in a small population of neurotypical individuals (Chakirova et al., 2010). We included Type IV in our classification system to exhaust all four logical possibilities derived by continuity in both the LOS and MOS.
Fig. 1.
Examples of the orbitofrontal sulcogyral patterns. (A) Variations of the H-shaped sulcus. Patterns were classified into four subtypes (Type I–IV) according to the continuity of the LOS and MOS in the rostrocaudal direction (r: rostral, c: caudal) (Type I: continuous LOS and discontinuous MOS, Type II: continuous LOS and MOS, Type III: discontinuous LOS and MOS, Type IV: discontinuous LOS and continuous MOS). The rightmost figure represents an ASD case that cannot be classified to either type using the classification systems of Chiavaras and Petrides (2000) and Chakirova et al. (2010). White dotted area shows clearly small and under-developed anterior orbital gyrus without any visible IOS. The TOS is not clearly identified (white arrow), and the caudal MOS and LOS are also unclear (black arrow). (B) Variants of the POS. The patterns were classified into three subtypes depending on the number of identified POS. (C) Variants of the IOS (m: medial, l: lateral). The patterns were classified into two subtypes depending on the number of identified IOS.
According to the previous study by Chiavaras and Petrides (2000), the POS can be either absent or present in the posterior orbital convexity in a hemisphere. If present, either one or two POS can be identified between the caudal MOS and LOS, which lies posterior to the TOS. In this study, we labeled these three variants as absent-POS, single-POS and double-POS subtypes (Figure 1B). The IOS was identified anterior to the TOS between the rostral MOS and LOS in both hemispheres in all individuals (Chiavaras and Petrides, 2000). Here, the IOS was classified into single-IOS and double-IOS subtypes depending on the number of identified sulci (Figure 1C) (see Supplementary material 1 for the detail).
The sulcogyral pattern classification in each individual was done by H.W., blinded to the diagnostic status of the individual. Inter-rater reliability was assessed by two clinicians (H.W. and T.O.), blinded to the diagnosis, who evaluated the sulcogyral patterns in 25 randomly selected participants. The intraclass correlation coefficients (Cronbach’s α) were 0.958, 0.897 and 0.846 for the H-shaped sulcus, POS and IOS, respectively.
Statistical analysis
We used SPSS software (version 18.0, SPSS, Chicago, IL, USA) for statistical analysis. Initially, we assessed differences between the ASD and NC groups in the distribution of the sulcogyral subtypes of the H-shaped sulcus, POS and IOS separately. For each sulcus, a separate chi-square test was applied to the frequency data in each hemisphere and to the combined data of the two hemispheres. The frequency distribution observed in the NC group was used as the expected number for each subtype. After examining group differences in the frequency distributions between the ASD and NC groups of our data, we also performed the same statistical tests using the frequency data of our NC group and that described by Chiavaras and Petrides (2000) to check the validity of our data. Only Type I, II and III data were used to analyze the H-shaped sulcus because the Type IV classification only occurred in 6 of 212 hemispheres (2.8%; Table 1), providing insufficient data to perform any reliable group comparison.
We then performed categorical regression analysis to determine which variations of the OFC sulci predict autistic traits. Categorical regression analysis was selected, because this model allows us to examine the effects of multiple factors (i.e. sulcogyral subtypes) in a single integrated model without using multiple independent statistical tests (Nakamura et al., 2007, 2008; Roppongi et al., 2010; Uehara-Aoyama et al., 2011). Each individual participant was classified according to whether he possessed the H-shaped sulcus (Type I, II and III) or POS (absent, single and double), subtypes in either hemisphere. In this analysis, the IOS subtypes were not included because the frequency distribution was comparable between the ASD and NC groups (see ‘Results’ section), and were therefore unlikely to predict autistic traits. The classification resulted in six nominal variables (Type I, Type II, Type III, absent POS, single POS and double POS), each of which was entered as an independent variable in a single model of categorical analysis regression. In our categorical regression, each categorical predictor represents whether a particular participant has the sulcal subtype (represented by that predictor) in the left or right hemisphere. This means that, in the Type I predictor, for example, we put a categorical value for participants who had Type I either in the left hemisphere only, or in the right hemisphere only, or in both hemispheres. In contrast, we put another value for participants who did not have Type I in either hemisphere. The single model of categorical regression was adopted rather than multiple univariate comparisons for the six independent variables to reduce the risk of false positives (Nakamura et al., 2007). Separate models were built to predict dependent variables of the total AQ score (Baron-Cohen et al., 2001), ‘social interaction’ and ‘attention to detail’ subscale scores individually (Hoekstra et al., 2008). Three other models consisting of the same six nominal variables were also built for handedness, estimated IQ scores and ICVs. The odds ratio was calculated by cross-tabulation using SPSS to estimate the risk of developing ASD from expression of particular sulcogyral subtypes. The cross-tabulation consisted of three variables of the left H-shaped sulcal types, the right H-shaped sulcal types and the right POS types, all of which turned out to show significant frequency difference between NC and ASD (see ‘Results’ section).
RESULTS
Sulcogyral pattern distribution
Table 2 shows the frequency of the H-shaped sulcus, POS and IOS subtypes in each group. One ASD patient displayed a highly unusual sulcogyral pattern in which rostral components of the H-shaped sulcus were absent or immature in both hemispheres (Figure 1A). In this case, the rostral side of the OFC was extremely small in both hemispheres; therefore, IOS could not be identified. Figure 2 shows the distribution of the H-shaped sulcal subtypes excluding this case and cases showing Type IV (see ‘Materials and methods’ section). The distributions of the POS and IOS subtypes are shown in Figures 3 and 4, respectively.
Table 2.
Distributions of orbitofrontal sulcal patterns of the ASD and NC in this study and NC in Chakirova and Petrides (2000)
| H-shaped sulcus N (%) |
IOS N (%) |
POS N (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | Single | Double | Absent | Single | Double | |
| ASD | |||||||||
| Left | 26 (52) | 12 (24) | 12 (24) | 0 (0) | 42 (84) | 8 (16) | 11 (22) | 33 (66) | 6 (12) |
| Right | 23 (46) | 15 (30) | 10 (20) | 2 (4) | 33 (66) | 17 (34) | 21 (42) | 26 (52) | 3 (6) |
| Total (left + right) | 49 (49) | 27 (27) | 22 (22) | 2 (2) | 75 (75) | 25 (25) | 32 (32) | 59 (59) | 9 (9) |
| NC | |||||||||
| Left | 27 (49) | 19 (35) | 6 (11) | 3 (5) | 44 (80) | 11 (20) | 10 (18) | 36 (66) | 9 (16) |
| Right | 35 (63) | 13 (24) | 6 (11) | 1 (2) | 41 (75) | 14 (25) | 14 (26) | 37 (67) | 4 (7) |
| Total (left + right) | 62 (56) | 32 (29) | 12 (11) | 4 (4) | 85 (77) | 25 (23) | 24 (22) | 73 (66) | 13 (12) |
| NC in Chiavaras and Petrides (2000) | |||||||||
| Left | 24 (48) | 17 (34) | 9 (18) | 0 (0) | 41 (82) | 9 (18) | 12 (24) | 28 (56) | 10 (20) |
| Right | 32 (64) | 13 (26) | 5 (10) | 0 (0) | 40 (80) | 10 (20) | 11 (22) | 34 (68) | 5 (10) |
| Total (left + right) | 56 (56) | 30 (30) | 14 (14) | 0 (0) | 81 (81) | 19 (19) | 23 (23) | 62 (62) | 15 (15) |
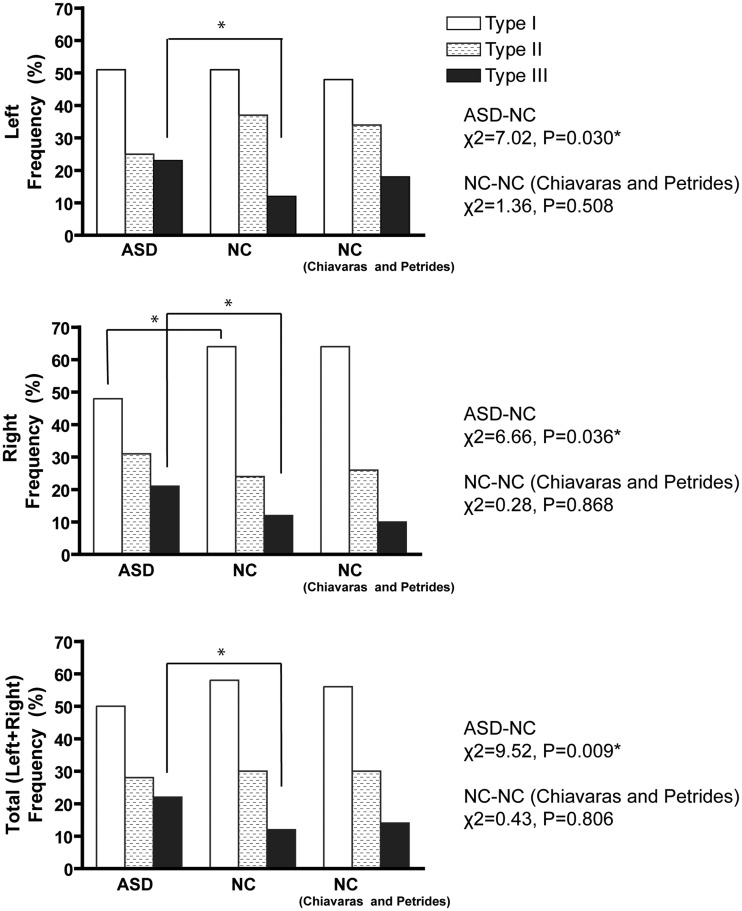
Fig. 2.
Sulcal pattern distribution of the ‘H-shaped’ sulcus in the OFC. Right column shows results from the previous study on neurotypical individuals by Chiavaras and Petrides (2000). According to post hoc test, Type III expression was increased in both hemispheres in the ASD group compared with the NC group (left: χ2 = 5.75, P = 0.016, right: χ2 = 3.80, P = 0.051, bilateral: χ2 = 9.45, P = 0.002), whereas Type I expression was deceased in the right hemisphere (χ2 = 5.93, P = 0.015). *Significant difference thresholded at P = 0.05.
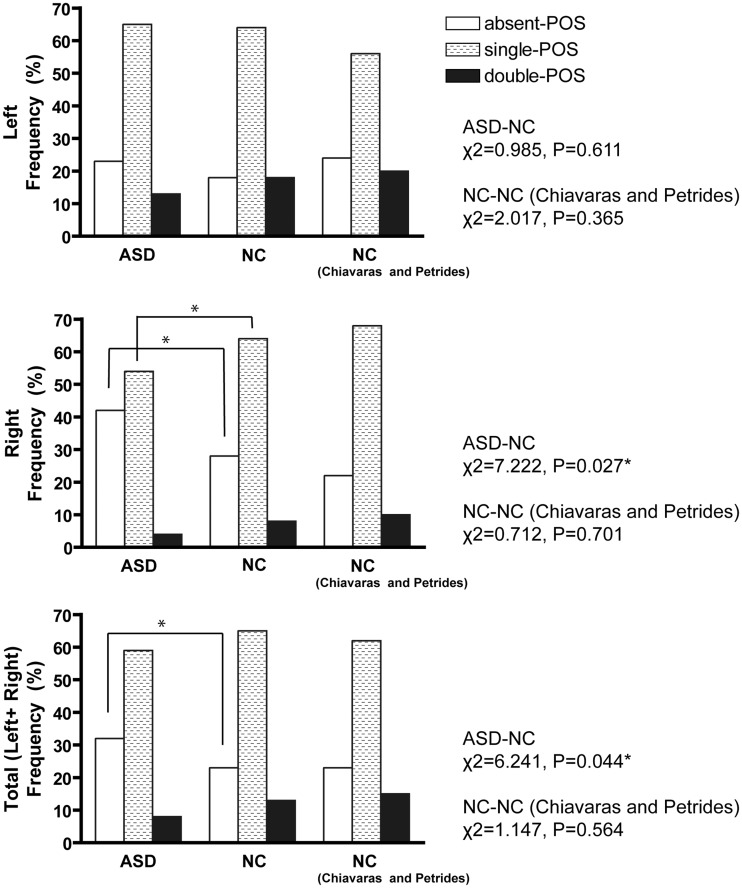
Fig. 3.
Sulcal pattern distribution of the POS in the OFC. Right column shows results from Chiavaras and Petrides (2000). Post hoc tests showed significant increase of the absent POS in the right hemisphere (χ2 = 7.213, P = 0.007) and bilateral hemispheres (χ2 = 6.078, P = 0.014), and decrease of the ‘single POS’ in the right hemisphere (χ2 = 5.297, P = 0.021). *Significant difference thresholded at P = 0.05.
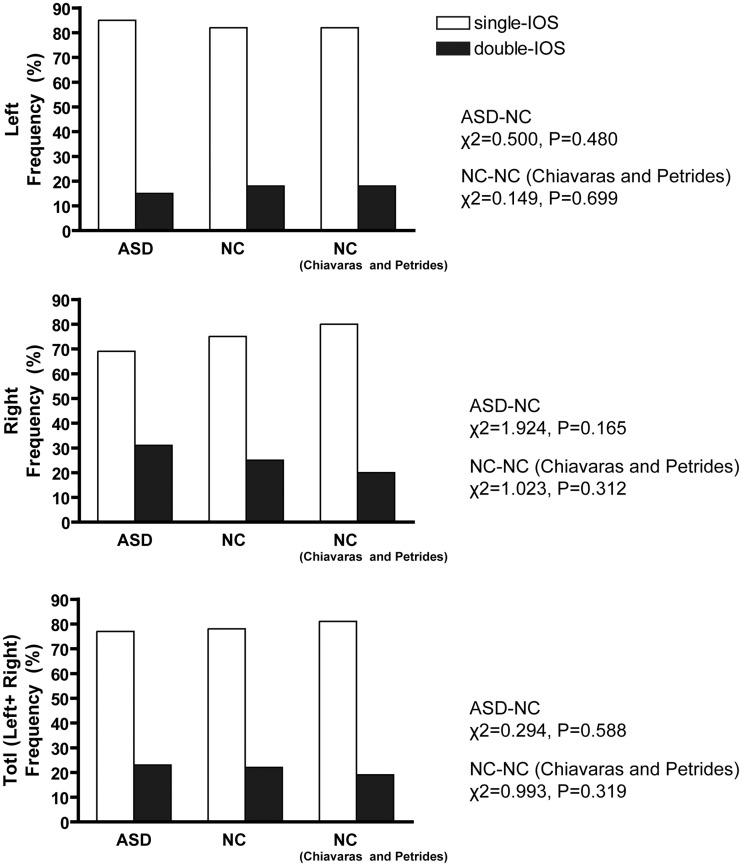
Fig. 4.
Sulcal pattern distribution of the IOS in the OFC. Right column shows results from Chiavaras and Petrides (2000). No significant distribution difference was found between groups.
We found a significant group difference in the distribution of subtypes of the H-shaped sulcus in both hemispheres (Figure 2). Post hoc tests revealed that occurrence of Type III was greater in both hemispheres in the ASD group compared with the NC group, whereas expression of Type I was deceased in the right hemisphere. We confirmed that the distribution of the H-shaped sulcal subtypes in the NC group was consistent with that of the previous study (Chiavaras and Petrides, 2000).
The distribution of the POS subtypes in the ASD group was significantly different from that in the NC group in the right hemisphere and in the combined data of the two hemispheres (Figure 3). Post hoc tests showed a significant increase in the occurrence of the absent-POS subtype and a decrease in the occurrence of the single-POS subtype in the right hemisphere in the ASD group. The distributions of the IOS subtypes were not significantly different between the two groups (Figure 4). The distributions of the POS and IOS subtypes in the NC group were comparable with those reported by Chiavaras (Figures 3 and 4).
The odds ratio calculated by the cross-tabulation showed that, compared to subjects without Type III occurrence, participants with Type III expression in any hemisphere showed a 2.67-fold increase in morbid risk for ASD, and that, compared to those with either single POS or double POS in the right hemisphere, participants with the absent-POS subtype in the right hemisphere showed a 1.40-fold increase in morbid risk. Moreover, compared to participants without Type III expression in both hemispheres and absent POS in the right hemisphere, participants with Type III occurrence in any hemisphere combined with absent-POS subtype in the right hemisphere showed a 4.89-fold increase in morbid risk for ASD.
Categorical regression analysis of OFC sulcogyral patterns
Categorical regression analysis revealed that, within the ASD group, expressions of Type I and II were significantly associated with reduced total AQ scores (Table 3). Moreover, Type I expression was also associated with reduced scores on the ‘attention to detail’ subscale. Type III expression or any of the POS subtypes was not significantly associated with either the total AQ score or the two subscales (Table 3).
Table 3.
Categorical regression analysis
| Clinical/cognitive measures (dependent variables) | ASD group |
NC group |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA | Independent variables | β | F | P | ANOVA | Independent variables | β | F | P | |
| AQ | ||||||||||
| Total score | F(6,47) = 2.689, | Type I | −0.479 | 8.139 | 0.007 | F(6,42) = 0.491, | Type I | 0.043 | 0.055 | 0.816 |
| P = 0.027 | Type II | −0.334 | 4.228 | 0.046 | P = 0.811 | Type II | 0.052 | 0.072 | 0.790 | |
| Type III | 0.084 | 0.245 | 0.623 | Type III | 0.105 | 0.361 | 0.552 | |||
| Absent POS | −0.163 | 1.017 | 0.319 | Absent POS | −0.163 | 0.759 | 0.389 | |||
| Single POS | 0.276 | 3.344 | 0.075 | Single POS | 0.097 | 0.220 | 0.642 | |||
| Double POS | 0.131 | 0.799 | 0.377 | Double POS | 0.021 | 0.011 | 0.918 | |||
| Social interaction | F(6,47) = 1.680, | Type I | 0.173 | 0.945 | 0.337 | F(6,42) = 1.907, | Type I | 0.306 | 3.379 | 0.074 |
| P = 0.150 | Type II | −0.396 | 5.335 | 0.026 | P = 0.106 | Type II | −0.004 | 0.001 | 0.982 | |
| Type III | −0.159 | 0.780 | 0.382 | Type III | 0.242 | 2.321 | 0.136 | |||
| Absent POS | −0.321 | 3.501 | 0.068 | Absent POS | −0.315 | .3471 | 0.071 | |||
| Single POS | −0.153 | 0.916 | 0.344 | Single POS | 0.185 | 0.972 | 0.331 | |||
| Double POS | 0.276 | 3.191 | 0.081 | Double POS | 0.156 | 0.699 | 0.409 | |||
| Attention to detail | F(6,47) = 2.909, | Type I | −0.420 | 6.399 | 0.015 | F(6,42) = 1.735, | Type I | −0.182 | 1.168 | 0.287 |
| P = 0.019 | Type II | −0.101 | 0.399 | 0.531 | P = 0.141 | Type II | 0.048 | 0.071 | 0.792 | |
| Type III | 0.023 | 0.019 | 0.890 | Type III | −0.085 | 0.278 | 0.601 | |||
| Absent POS | 0.097 | 0.367 | 0.548 | Absent POS | 0.320 | 3.507 | 0.069 | |||
| Single POS | −0.217 | 2.103 | 0.155 | Single POS | −0.190 | 1.008 | 0.322 | |||
| Double POS | 0.112 | 0.602 | 0.442 | Double POS | −0.108 | 0.326 | 0.571 | |||
Bold fonts represent statistically significant effects.
Within the NC group, no significant association between a particular sulcal subtype and AQ score was found (Table 3). Categorical regression analysis showed no significant association of sulcogyral subtypes either with handedness [ASD: F(6,41) = 1.315, P = 0.276; NC: F(6,36) = 1.306, P = 0.285], ICV [ASD: F(6,47) = 1.992, P = 0.089; NC: F(6,50) = 0.669, P = 0.675] or estimated IQ [ASD: F(6,42) = 0.539, P = 0.775; NC: F(6,45) = 1.225, P = 0.315].
DISCUSSION
To our knowledge, this study is the first to reveal altered distributions of the orbitofrontal sulcogyral patterns in individuals with high-functioning ASD. Whereas previous psychiatric studies targeted only one of the three orbitofrontal sulci (Nakamura et al., 2007; Chakirova et al., 2010; Roppongi et al., 2010; Takayanagi et al., 2010; Uehara-Aoyama et al., 2011), we performed an extended study on all three sulci that have been shown to display major interindividual variability within the OFC (Chiavaras and Petrides, 2000). For the H-shaped sulcus, the frequency distribution of sulcal subtypes in ASD was significantly altered from that in the NC group in both hemispheres: Type III expression was increased in both hemispheres together with reduced occurrence of Type I in the right hemisphere. Furthermore, in ASD, the absent-POS subtype occurred significantly more frequently, combined with reduced frequency of the single-POS subtype in the right hemisphere. No significant group difference was found for the IOS. Subsequent categorical regression analysis for predicting autistic traits indicated that expressions of Type I and II in the H-shaped sulcus were associated with reduced total AQ score. More specifically, the occurrence of Type I was associated with a decreased subscale score for ‘attention to detail’. These findings have demonstrated altered expressions of the orbitofrontal sulcogyral morphology in individuals with ASD that may predict the severity of general and specific autistic traits.
Variations of the H-shaped sulcal pattern
Altered distribution of sulcal subtypes in ASD was most clearly demonstrated for the H-shaped sulcus in both hemispheres. More specifically, increased expression of Type III was observed in both hemispheres. Compared with other subtypes, the morphology of Type III may be characterized as less mature development of the sulcogyral formation. According to previous research on the development of the human infant brain (Chi and Dooling, 1976; Dubois et al., 2008; Habas et al., 2012), the formation of the orbitofrontal sulcogyral patterns can be identified between 16 and 44 weeks of gestation, starting from the posteromedial region and then extending along the anterior and lateral directions (Kringelbach and Rolls, 2004). Future neurodevelopmental studies of infant brains together with our findings of altered sulcogyral morphology may provide new insight into critical gestation periods during which abnormal developmental processes lead to the altered sulcogyral formation in ASD (Bachevalier and Loveland, 2006).
We also found an interesting ASD case in which the rostral parts of the OFC were extremely small in both hemispheres and therefore could not be classified into any of the known variations of the orbitofrontal sulcogyral pattern. It is of note that this individual is highly intelligent with markedly successful academic achievements. Given the observation that expression of Type III, the least common pattern in a neurotypical population, was increased in the ASD group, this case could be interpreted as an extreme case of underdevelopment of the orbitofrontal sulcogyral formation. Therefore, this ASD case also suggests the possible link between abnormal orbitofrontal sulcogyral formation and the etiology of ASD.
Commonality and differences between ASD and schizophrenia
Increased Type III and decreased Type I expressions have been previously demonstrated in the right hemisphere of schizophrenic individuals, replicated by at least three different institutes (Nakamura et al., 2007; Chakirova et al., 2010; Takayanagi et al., 2010). Converging findings from first-episode schizophrenia and individuals with high risk of developing schizophrenia indicated that the presence of Type III in the right hemisphere may be a trait marker for developing psychotic symptoms in schizophrenia (Nakamura et al., 2007; Chakirova et al., 2010). The same pattern of increased Type III coupled with decreased Type I in the right hemisphere was also identified in our study of ASD. Our finding of a shared morphological feature between ASD and schizophrenia is consistent with that of previous studies emphasizing overlaps between the two diseases at multiple levels, including clinical symptoms (Urfer-Parnas et al., 2010; Parnas, 2011), social cognition (Solomon et al., 2011), brain activity (Sugranyes et al., 2011), brain morphology (Cheung et al., 2010) and gene mutations (Guilmatre et al., 2009; Rapoport et al., 2009). However, an important difference exists such that a significant alteration was found in both hemispheres in ASD, whereas the sulcal pattern was only altered in the right hemisphere in schizophrenia. Previous neuropsychological studies have shown that a bilateral lesion of the OFC causes more severe deficits in social and emotional functioning than does a unilateral lesion (Bramham et al., 2009). Therefore, the manifestation of clear social and behavioral abnormalities characteristic to ASD may become more likely when neurodevelopmental abnormalities affect the OFC in both hemispheres.
Restricted and repetitive behaviors in ASD
Using categorical regression analysis for predicting specific ASD traits, we found that the presence of Type I is associated with a reduced score on the ‘attention to detail’ subscale. This result could be consistent with that of a previous study reporting that Type I expression was associated with milder clinical symptoms in male schizophrenia (Nakamura et al., 2007). The factor of ‘attention to detail’ in the AQ test belongs to ‘high-level’ RRBs (Turner, 1999) characterized by an adherence to some rule or mental set leading to rigidity and inflexibility, and is also observed in individuals with obsessive–compulsive disorder (OCD) (Lewis and Kim, 2009). While previous neuroimaging studies of OCD have accumulated evidence for structural and functional abnormalities of the OFC (see reviews by Maia et al., 2008 and Rotge et al., 2009), involvement of the OFC for RRB has not been clearly demonstrated in ASD, although the implication of other structures has been suggested (e.g. striatum) (Sears et al., 1999; Hollander et al., 2005; Rojas et al., 2006). Given our finding of altered distribution of orbitofrontal sulcogyral subtypes in ASD, it would be interesting in future studies to measure the gray matter volume and/or density in the OFC separately for each subtype and examine possible associations with behavioral measures as previously done for schizophrenia (Nakamura et al., 2008).
Variations in POS and IOS patterns
We found evidence of increased cases of absent POS in the right hemisphere together with decreased cases of single POS in ASD, whereas no significant group difference was observed in the IOS pattern. The POS and IOS are located in the posterior and anterior OFC gyri, respectively. According to a model of OFC function, the posterior OFC houses multimodal representations for visual, auditory, somatosensory, gustatory and olfactory sensations that are then linked to particular reward value representations (Kringelbach and Rolls, 2004). Recently, abnormalities in sensory processing have been demonstrated in all five classic sensory modalities in ASD (Marco et al., 2011), and altered reactivity to sensory inputs is now included as one of the new diagnostic criteria for ASD in DSM-V (Mandy et al., 2012). It is possible that an underdeveloped sulcogyral pattern in the posterior orbitofrontal gyrus may be related to sensory abnormalities and unusual affections associated with sensations.
Limitations
We note several limitations in this study. First, except for the association between the autistic factor of ‘attention to detail’ and Type III expression in the right hemisphere, this study alone does not clearly demonstrate which functions are particularly affected by altered orbitofrontal sulcogyral patterns. Because the entire OFC is engaged in diverse functions, many of which are altered in ASD, future studies may need an extended neuropsychological test battery to examine the relationship between sulcogyral variations and altered functions more thoroughly. Second, we used the AQ for assessing the severity of autistic traits. Although the AQ is a validated self-report questionnaire (Baron-Cohen et al., 2001; Wakabayashi et al., 2006), it would be desirable to have other standard assessment procedures such as the Autism Diagnostic Interview Revised to support the evaluation of autistic traits in each individual. Third, our sample was limited to the male population. While the present approach is advantageous in terms of sample homogeneity, our analyses need to be applied to female patients as well given a recent finding of a significant dimorphic distribution of the H-shaped sulcus in schizophrenia (Uehara-Aoyama et al., 2011). Finally, it remains unclear whether and how expressions of particular sulcogyral subtypes are associated with other structural measures, such as regional tissue density or volume in the OFC. Although our preliminary VBM analysis suggested increased white matter density in the OFC among ASD individuals with the double-POS type, small number of subjects of this subtype did not allow us to draw firm conclusions (see Supplementary material 2).
In conclusion, this study examined morphological variations of the multiple orbitofrontal sulci in ASD and revealed significant alterations in distributions of sulcal subtypes in the H-shaped sulcus in both hemispheres and the right POS. Furthermore, expressions of specific subtypes of the H-shaped sulcus were associated with the degree of general and specific autistic traits. These findings indicate that the altered orbitofrontal sulcogyral pattern may be used as a morphological trait marker that reflects abnormal neurodevelopmental processes leading to an elevated risk of developing autistic symptoms.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank Ms Matsushita and Saito for help with the collection of clinical and demographic data.
This work was supported by a Core Research for Evolutional Science and Technology (CREST) grant from Japan Science and Technology Agency to N.K. and by Grant-in-Aid for Scientific Research on Innovative Areas (23118003; Adolescent Mind and Self-Regulation) from the Ministry of Education, Culture, Sports, Science and Technology of Japan to R.H.
REFERENCES
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cerebral Cortex. 1995;5(1):56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neuroscience and Biobehavioral Reviews. 2006;30(1):97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1989;286(3):353–75. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. Trends in Cognitive Sciences. 2002;6(6):248–54. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120(Pt 2):257–69. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- Bramham J, Morris RG, Hornak RG, Bullock P, Polkey CE. Social and emotional functioning following bilateral and unilateral neurosurgical prefrontal cortex lesions. Journal of Neuropsychology. 2009;3(Pt 1):125–43. doi: 10.1348/174866408X293994. [DOI] [PubMed] [Google Scholar]
- Chakirova G, Welch KA, Moorehead TW, et al. Orbitofrontal morphology in people at high risk of developing schizophrenia. European Psychiatry. 2010;25(6):366–72. doi: 10.1016/j.eurpsy.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Cheung C, Yu K, Fung G, et al. Autistic disorders and schizophrenia: related or remote? An anatomical likelihood estimation. PLoS ONE. 2010;5(8):e12233. doi: 10.1371/journal.pone.0012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi JG, Dooling EC. Gyral development of the human brain. Transactions of the American Neurological Association. 1976;101:89–90. [PubMed] [Google Scholar]
- Chiavaras MM, Petrides M. Orbitofrontal sulci of the human and macaque monkey brain. Journal of Comparative Neurology. 2000;422(1):35–54. [PubMed] [Google Scholar]
- Dane S, Balci N. Handedness, eyedness and nasal cycle in children with autism. International Journal of Developmental Neuroscience. 2007;25(4):223–6. doi: 10.1016/j.ijdevneu.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Cachia A, et al. Mapping the early cortical folding process in the preterm newborn brain. Cerebral Cortex. 2008;18(6):1444–54. doi: 10.1093/cercor/bhm180. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Autistic children's hand preferences: results from an epidemiological study of infantile autism. Psychiatry Research. 1983;10(1):21–30. doi: 10.1016/0165-1781(83)90025-2. [DOI] [PubMed] [Google Scholar]
- Girgis RR, Minshew NJ, Melhem NM, et al. Volumetric alterations of the orbitofrontal cortex in autism. Progress in Neuropsychopharmacology and Biological Psychiatry. 2007;31(1):41–5. doi: 10.1016/j.pnpbp.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmatre A, Dubourg C, Mosca AL, et al. Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Archives of General Psychiatry. 2009;66(9):947–56. doi: 10.1001/archgenpsychiatry.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas PA, Scott JA, Roosta A, et al. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cerebral Cortex. 2012;22(1):13–25. doi: 10.1093/cercor/bhr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck JA, Dewey D. Hand preference and motor functioning in children with autism. Journal of Autism and Developmental Disorders. 2001;31(3):265–77. doi: 10.1023/a:1010791118978. [DOI] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, Cath DC, Boomsma DI, et al. Factor structure, reliability and criterion validity of the Autism-Spectrum Quotient (AQ): a study in Dutch population and patient groups. Journal of Autism and Developmental Disorders. 2008;38(8):1555–66. doi: 10.1007/s10803-008-0538-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Winterrowd E, et al. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biological Psychiatry. 2005;58(3):226–32. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Chen R, Ke X, et al. Predictive models of autism spectrum disorder based on brain regional cortical thickness. Neuroimage. 2010;50(2):589–99. doi: 10.1016/j.neuroimage.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72(5):341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kurita H, Koyama T, Osada H, et al. Autism-Spectrum Quotient-Japanese version and its short forms for screening normally intelligent persons with pervasive developmental disorders. Psychiatry and Clinical Neuroscience. 2005;59(4):490–6. doi: 10.1111/j.1440-1819.2005.01403.x. [DOI] [PubMed] [Google Scholar]
- Lauvin MA, Martineau J, Destrieux C, et al. Functional morphological imaging of autism spectrum disorders: current position and theories proposed. Diagnostic and Interventional Imaging. 2012;93(3):139–47. doi: 10.1016/j.diii.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Lewis M, Kim SJ. The pathophysiology of restricted repetitive behavior. Journal of Neurodevelopmental Disorders. 2009;1(2):114–32. doi: 10.1007/s11689-009-9019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G, von Cramon DY, Steinmetz H, et al. Sulcal variability of twins. Cerebral Cortex. 1999;9(7):754–63. doi: 10.1093/cercor/9.7.754. [DOI] [PubMed] [Google Scholar]
- Luders E, Kurth F, Mayer EA, et al. The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Frontiers in Human Neuroscience. 2012;6:34. doi: 10.3389/fnhum.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia TV, Cooney RE, Peterson BS, et al. The neural bases of obsessive-compulsive disorder in children and adults. Development and Psychopathology. 2008;20(4):1251–83. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandy WP, Charman T, Skuse DH, et al. Testing the construct validity of proposed criteria for DSM-5 autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(1):41–50. doi: 10.1016/j.jaac.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatric Research. 2011;69(5 Pt 2):48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Kim Y. Japanese Adult Reading Test (JART) Tokyo: Shinkou-igaku Publication; 2006. [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):e472–86. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, McCarley RW, et al. Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain. 2007;130(Pt 3):693–707. doi: 10.1093/brain/awm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, Levitt JJ, et al. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131(Pt 1):180–95. doi: 10.1093/brain/awm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE, Willison JR. National Adult Reading Test (NART) 2nd edn. Windsor, UK: NFER-Nelson; 1991. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460(3):425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Otsubo T, Kamijima K. The Japanese Version of The Mini-International NEuropsychiatric Interview (M.I.N.I): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. Tokyo: Seiwa Bookstore; 2000. [Google Scholar]
- Parnas J. A disappearing heritage: the clinical core of schizophrenia. Schizophrenia Bulletin. 2011;37(6):1121–30. doi: 10.1093/schbul/sbr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip RC, Dauvermann MR, Whalley HC, et al. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neuroscience and Biobehavioral Reviews. 2012;36(2):901–42. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241(4862):170–6. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(1):10–8. doi: 10.1097/CHI.0b013e31818b1c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Peterson E, Winterrowd E, et al. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6:56. doi: 10.1186/1471-244X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppongi T, Nakamura M, Asami T, et al. Posterior orbitofrontal sulcogyral pattern associated with orbitofrontal cortex volume reduction and anxiety trait in panic disorder. Psychiatry and Clinical Neurosciences. 2010;64(3):318–26. doi: 10.1111/j.1440-1819.2010.02085.x. [DOI] [PubMed] [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, et al. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biological Psychiatry. 2009;65(1):75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47(5):633–6. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears LL, Vest C, Mohamed S, et al. An MRI study of the basal ganglia in autism. Progress in Neuropsychopharmacology and Biological Psychiatry. 1999;23(4):613–24. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–9. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Olsen E, Niendam T, et al. From lumping to splitting and back again: atypical social and language development in individuals with clinical-high-risk for psychosis, first episode schizophrenia, and autism spectrum disorders. Schizophrenia Research. 2011;131(1–3):146–51. doi: 10.1016/j.schres.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger VA. Technical considerations for BOLD fMRI of the orbitofrontal cortex. In: Zald David H, Rauch Scott L, editors. The Orbitofrontal Cortex. Oxford: Oxford University Press; 2006. pp. 423–46. [Google Scholar]
- Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS ONE. 2011;6(10):e25322. doi: 10.1371/journal.pone.0025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Takahashi T, Orikabe L, et al. Volume reduction and altered sulco-gyral pattern of the orbitofrontal cortex in first-episode schizophrenia. Schizophrenia Research. 2010;121(1–3):55–65. doi: 10.1016/j.schres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Toro R, Burnod Y. A morphogenetic model for the development of cortical convolutions. Cerebral Cortex. 2005;15(12):1900–13. doi: 10.1093/cercor/bhi068. [DOI] [PubMed] [Google Scholar]
- Turner M. Annotation: repetitive behaviour in autism: a review of psychological research. Journal of Child Psychology and Psychiatry. 1999;40(6):839–49. [PubMed] [Google Scholar]
- Uehara-Aoyama K, Nakamura M, Asami T, et al. Sexually dimorphic distribution of orbitofrontal sulcogyral pattern in schizophrenia. Psychiatry and Clinical Neurosciences. 2011;65(5):483–9. doi: 10.1111/j.1440-1819.2011.02229.x. [DOI] [PubMed] [Google Scholar]
- Urfer-Parnas A, Mortensen EL, Parnas J, et al. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300–11. doi: 10.1159/000318814. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385(6614):313–8. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Via E, Radua J, Happe F, Mataix-Cols D. Meta-analysis of gray matter abnormalities in autism spectrum disorder: should Asperger disorder be subsumed under a broader umbrella of autistic spectrum disorder? Archives of General Psychiatry. 2011;68(4):409–18. doi: 10.1001/archgenpsychiatry.2011.27. [DOI] [PubMed] [Google Scholar]
- Wakabayashi A, Baron-Cohen S, Wheelwright S, Tojo Y. The Autism-Spectrum Quotient (AQ) in Japan: a cross-cultural comparison. Journal of Autism and Developmental Disorders. 2006;36(2):263–70. doi: 10.1007/s10803-005-0061-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.