Abstract
Epidemiologic studies suggest that dietary vitamin E is a candidate intervention for atopic disease. We used in vitro and ex vivo exposures to test the hypothesis that the most common dietary isoform of vitamin E, γ-tocopherol (γT), could suppress FcεRI-mediated basophil activation.
Rat Basophilic Leukemia (RBL)-SX38 cells that express human FcεRI were treated with or without γT, followed by stimulation with α-IgE. In the ex vivo study, 20 Der f 1-allergic volunteers consumed a γT-enriched supplement for 7 days. Their basophils were challenged ex vivo with α-IgE and graded doses of Der f 1 before and after the supplementation period.
γT treatment of RBL-SX38 cells significantly reduced basophil degranulation and de novo TH2 cytokine production. Daily consumption of a γT - rich supplement by dust mite-allergic volunteers reduced basophil activation after ex vivo dust mite challenge.
Vitamin E supplements rich in γT may be useful adjuncts in decreasing atopic disease.
Keywords: Basophil, Complementary and Alternative Medicine, Dust Mite, Tocopherol, Vitamin E
Brief Communication:
Complementary and Alternative Medicine therapies have become increasingly popular in the United States and Europe for the treatment of allergies and asthma 1,2. Vitamin E is a nutritional antioxidant which has been studied for its impact in asthma. Decreased levels of IgE and reduced occurrence of childhood asthma have been associated with intake of dietary vitamin E (predominantly gamma tocopherol, γT) early in life 3 or in children of women with high vitamin E intakes during pregnancy 4. In a rodent model of allergic rhinitis and asthma, we found that daily administration of γT prior to Ova challenge depressed eosinophil infiltration into the airways, as well as production of nasal TH2 cytokines and pulmonary eicosanoids 5.
Previous work showed that vitamin E interfered with phosphoinositide-3-kinase and protein kinase C activation 6, required for IgE-mediated degranulation and cytokine production by mast cells and basophils. As an initial step in exploring the utility of γT as an adjunctive treatment for atopy, we assessed the effect of γT in vitro on IgE-mediated degranulation and mediator production using a rat basophilic leukemia cell line, RBL SX-38, which stably expresses human FcεRI αβγ2. Based on promising in vitro findings, we then examined the role of consuming a γT-enriched preparation on IgE-mediated basophil activation in a proof-of-concept ex vivo study in dust mite allergic volunteers.
RBL SX-38 cells were kindly provided by Jean-Pierre Kinet from the Beth Israel Deaconess Medical Center 7. The release assay was adapted from Blanc et al 8. Cells were sensitized with 0.5 μg/ml human IgE (Abbiotec) or fresh media (MEM with 10% FBS from Hyclone) for 24 hours. The next day, 20 uM γT, vehicle (0.1% DMSO), or 1 μM cyclosporine were added and incubated for 16 hours overnight. The following morning, cells were washed with NaPIPES buffer followed by stimulation with 10 μg/ml anti-human IgE antibody (α-IgE, Bethyl Labs) for 60 minutes 7. All conditions were run in triplicate. Degranulation was assessed by β-hexosaminidase release 7; total cellular β-hexosaminidase content was quantified by lysing the cells with 0.1% Triton X-100. Supernatants were assayed in duplicate for IL-4, IL-13, and CysLTs using the Luminex 100IS Multiplex flow cytometric assay. Student's T tests were used to compare γT-treated cells to the vehicle control.
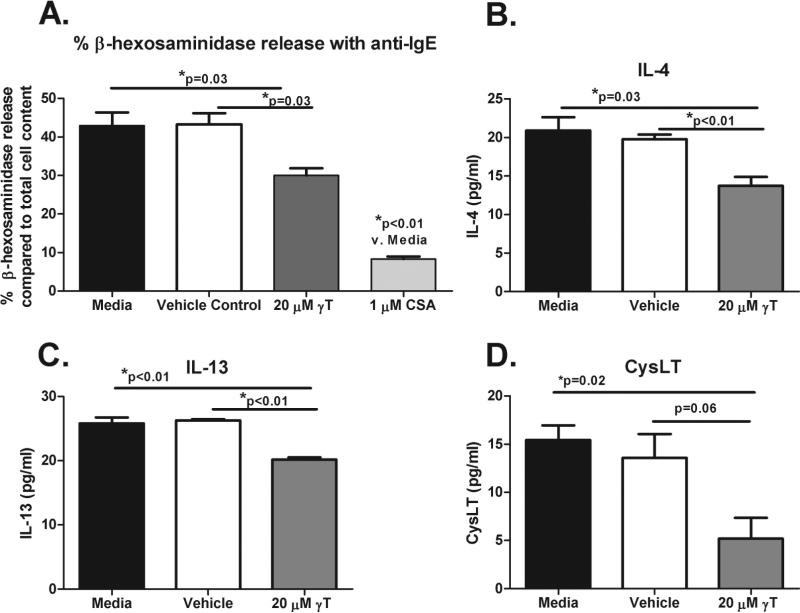
Pretreatment with 20 μM γT modestly but significantly inhibited α-IgE-stimulated β-hexosaminidase release (Figure 1a, p=0.03). Although there was a significant reduction in IL-4 & IL-13 production (p<0.01 for both) (Figure 1b-c) compared to the vehicle control, there was a non-significant reduction in CysLT production (Figure 1d, p=0.06). γT treatment did not induce cellular cytotoxicity compared to the culture media or to the vehicle control (data not shown), assessed by a LDH cytotoxicity assay (Cayman Chemicals).
Figure 1. Effect of γT on degranulation, de novo TH2 cytokines, and cysteinyl leukotrienes in a basophil cell line.
(A). % basophil degranulation (compared to total cellular content of β-hexosaminidase) with culture media & with pretreatment with vehicle control, 20 μM γT, or 1 μM cyclosporine (CSA) prior to stimulation with human α-IgE. De-novo production of IL-4 (B), IL-13 (C), and CysLTs (D) after stimulation of RBL SX-38 cells with human α-IgE. Mean and SEM are shown.
The ex vivo study was reviewed and approved by the Committee for the Protection of the Rights of Human subjects of the University of North Carolina, and registered on ClinicalTrials.gov (NCT00836368). Twenty D. farinae-allergic volunteers (ages 18-50) completed the study. The primary endpoint was %CD63high basophils as a marker of basophil activation 9 after ex vivo challenge with Der f 1, using paired testing to compare the post supplementation values to baseline values.
Each subject took 2 capsules of a γT-enriched supplement on a daily basis for 7 days provided by YASOO Health Inc. (Johnson City, TN). Each γT-enriched capsule contained 50 mg d-α-tocopherol, 240 mg d-β tocopherol plus d-δ-tocopherol, and 540 mg d-γ tocopherol 10. Blood was drawn immediately before the first supplementation dose, and six hours after the day 7 dose. Whole blood was primed with 2 ng/ml of human rIL-3 for 10 minutes. Samples were then incubated with PBS, 10 μg/ml α-IgE or Der f 1 (Greer Laboratories, Lenoir, NC) (0.1, 1, and 10 μg/ml) for 30 minutes at 37C and then placed on ice.
The percentages of CD63high and CD203chigh cells served as indices of basophil activation 9. Expression of CD63/LAMP-3 and CD203c/E-NNP3 on circulating basophils were measured by flow cytometry (BD-LSR-II flow cytometer and BD FACSDiva 6.1 software). Basophils were identified in blood as CD203c(+) cells with low side scatter within the CD45(+) leukocyte population. Using histogram analysis, ≈98 to 99% (minimum 95%) of the basophil population of the un-stimulated controls was gated to establish the baseline expression (mean fluorescent intensity) of each of the surface proteins. Basophils which shifted to the right out of the baseline gate (CD63high and CD203chigh) following stimulation with α-IgE or Der f 1 were considered activated. Flow cytometric values were corrected for mechanical manipulation of cells by subtracting the PBS control values from the α-IgE and Der f 1 values. Basophil activation was compared before and after 7 days of supplementation using Wilcoxon signed rank tests.
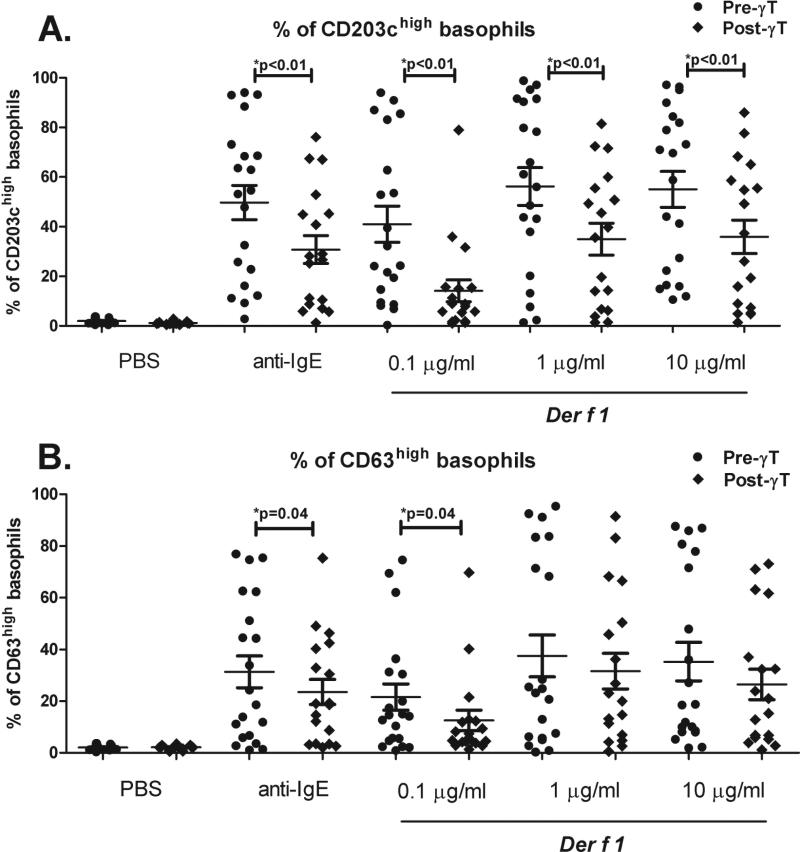
α-IgE and Der f 1 (0.1, 1, and 10 μg/ml) all significantly upregulated CD63 & CD203c on basophils compared to the PBS control (p<0.001 for all comparisons). After one week of γT-enriched supplementation, %CD203chigh basophils were significantly decreased with α-IgE and all doses of Der f 1 (p<0.01, Figure 2a); %CD63high basophils were significantly decreased with α-IgE and the 0.1 μg/ml Der f 1 dose (Figure 2b).
Figure 2. Effect of oral γT supplementation on basophil activation with dust mite allergen in 20 Der f 1-allergic volunteers.
Volunteers had positive skin prick testing to D. farinae (defined as a 3 mm wheal greater than the negative saline control), and mean Der f 1 IgE of 15.8 (SD + 21.5) IU/ml. %CD203chigh basophils (A) and %CD63high basophils (B) with α–IgE and graded doses of Der f 1. Mean and SEM are shown.
We conducted these studies to determine if γT may be a useful adjunct therapy against IgE-mediated processes. Results of in vitro studies suggested IgE-mediated basophil activation & de novo mediator production is modestly reduced with a γT concentration similar to that observed in the serum of healthy and atopic volunteers after oral supplementation with the identical γT dosing regimen 10,11. Although supplementation with α-tocopherol was previously noted to increase bleeding risk 12, this side effect has not been noted with γT supplementation. We demonstrated that oral consumption of the γT-enriched supplement by dust mite-allergic volunteers reduced ex vivo IgE-mediated upregulation of CD63 & CD203c, suggestive of reduced basophil activation 9. This open label proof-of-concept study provides rationale for further mechanistic work examining the inhibitory effect of γT on IgE-mediated activation, as well as for placebo controlled phase II studies to assess the safety and clinical impact of γT supplementation as a therapeutic intervention for allergic asthma & rhinitis.
Acknowledgements
The authors would like to thank Tatiana Quintero-Varca for her assistance with the Luminex assays, and Martha Almond, and Peg Herbst for assistance with subject recruitment. We also thank Dr. Michael Kulis for thoughtful review of the manuscript.
This publication was made possible by grants P01AT002620 from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health, K23-ES021745-01 from the NIEHS, and RO1- ES023349 from the NIEHS.
Abbreviations
- γT
γ–tocopherol
- RBL
Rat Basophilic Leukemia
- rIL-3
recombinant IL-3
- Interleukin-4, IL-4
Dermatophagoides farinae 1 antigen, Der f 1
- IL-13
Interleukin-13
- CysLTs
Cysteinyl Leukotrienes
- α-IgE
anti-human IgE antibody
Footnotes
The authors have no conflicts of interest to disclose.
Author Contributions: All authors contributed to the design of the study. Experiments were performed by Katherine Mills, John Lay, Weidong Wu and Matthew Kesic. Carole Robinette prepared the posting for ClinicalTrials.gov, recruited subjects, performed the skin testing and venipuncture, and administered the supplements. Weidong Wu, Katherine Mills, Matthew Kesic, and Michelle Hernandez analyzed the in vitro data; Katherine Mills, John Lay, and Matthew Kesic analyzed the ex vivo data. Stephen Dreskin facilitated the procurement of the RBL-SX38 cells and provided the methods for both culture and the degranulation experiments. Katherine Mills, Matthew Kesic, David Peden, and Michelle Hernandez wrote the manuscript.
References
- 1.Schafer T. Epidemiology of complementary alternative medicine for asthma and allergy in Europe and Germany. Ann Allergy Asthma Immunol. 2004;93(2 Suppl 1):S5–10. doi: 10.1016/s1081-1206(10)61481-0. [DOI] [PubMed] [Google Scholar]
- 2.Sidora-Arcoleo K, Yoos HL, McMullen A, Kitzman H. Complementary and alternative medicine use in children with asthma: prevalence and sociodemographic profile of users. J Asthma. 2007;44(3):169–75. doi: 10.1080/02770900701209640. [DOI] [PubMed] [Google Scholar]
- 3.Fogarty A, Lewis S, Weiss S, Britton J. Dietary vitamin E, IgE concentrations, and atopy. Lancet. 2000;356(9241):1573–4. doi: 10.1016/S0140-6736(00)03132-9. [DOI] [PubMed] [Google Scholar]
- 4.Litonjua AA, Rifas-Shiman SL, Ly NP, Tantisira KG, Rich-Edwards JW, Camargo CA, Jr., et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84(4):903–11. doi: 10.1093/ajcn/84.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, et al. Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin Exp Allergy. 2008;38(3):501–11. doi: 10.1111/j.1365-2222.2007.02855.x. [DOI] [PubMed] [Google Scholar]
- 6.Azzi A, Gysin R, Kempna P, Munteanu A, Negis Y, Villacorta L, et al. Vitamin E mediates cell signaling and regulation of gene expression. Ann N Y Acad Sci. 2004;1031:86–95. doi: 10.1196/annals.1331.009. [DOI] [PubMed] [Google Scholar]
- 7.Palmer GW, Dibbern DA, Jr., Burks AW, Bannon GA, Bock SA, Porterfield HS, et al. Comparative potency of Ara h 1 and Ara h 2 in immunochemical and functional assays of allergenicity. Clin Immunol. 2005;115(3):302–12. doi: 10.1016/j.clim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Blanc F, Adel-Patient K, Drumare MF, Paty E, Wal JM, Bernard H. Capacity of purified peanut allergens to induce degranulation in a functional in vitro assay: Ara h 2 and Ara h 6 are the most efficient elicitors. Clin Exp Allergy. 2009;39(8):1277–85. doi: 10.1111/j.1365-2222.2009.03294.x. [DOI] [PubMed] [Google Scholar]
- 9.MacGlashan D., Jr. Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. 2010;40(9):1365–77. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez ML, Wagner JG, Aline Kala R, Mills K, Wells HB, Alexis NE, et al. Vitamin E Gamma-tocopherol Reduces Airway Neutrophil Recruitment after Inhaled Endotoxin Challenge in Rats and in Healthy Volunteers. Free Radic Biol Med. 2013 doi: 10.1016/j.freeradbiomed.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiser J, Alexis NE, Jiang Q, Wu W, Robinette C, Roubey R, et al. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic Biol Med. 2008;45(1):40–9. doi: 10.1016/j.freeradbiomed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA : the journal of the American Medical Association. 2008;300(18):2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]