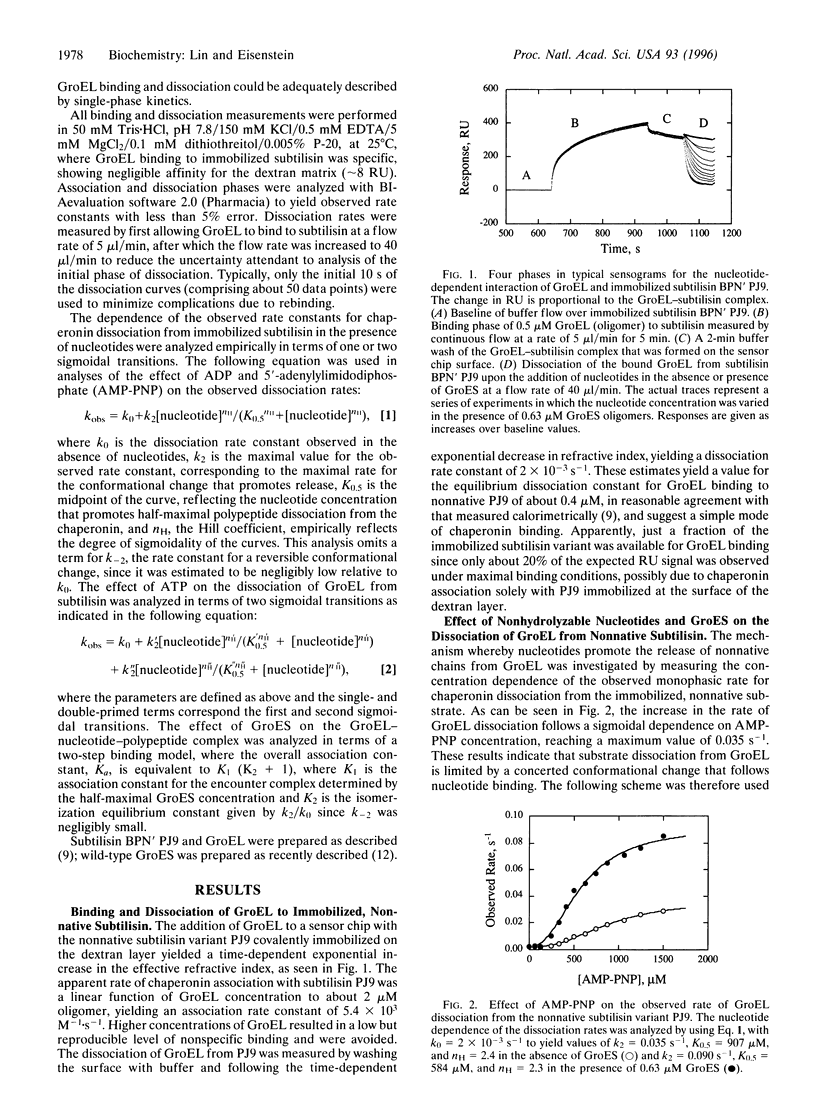
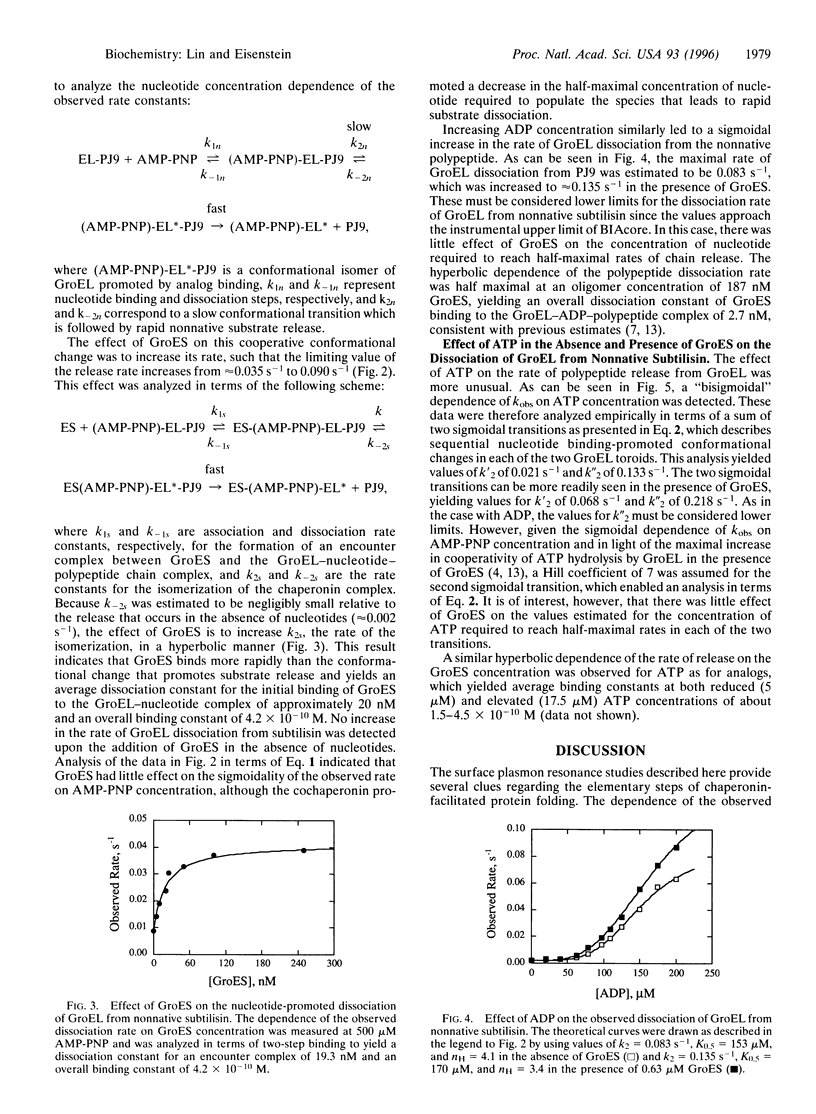
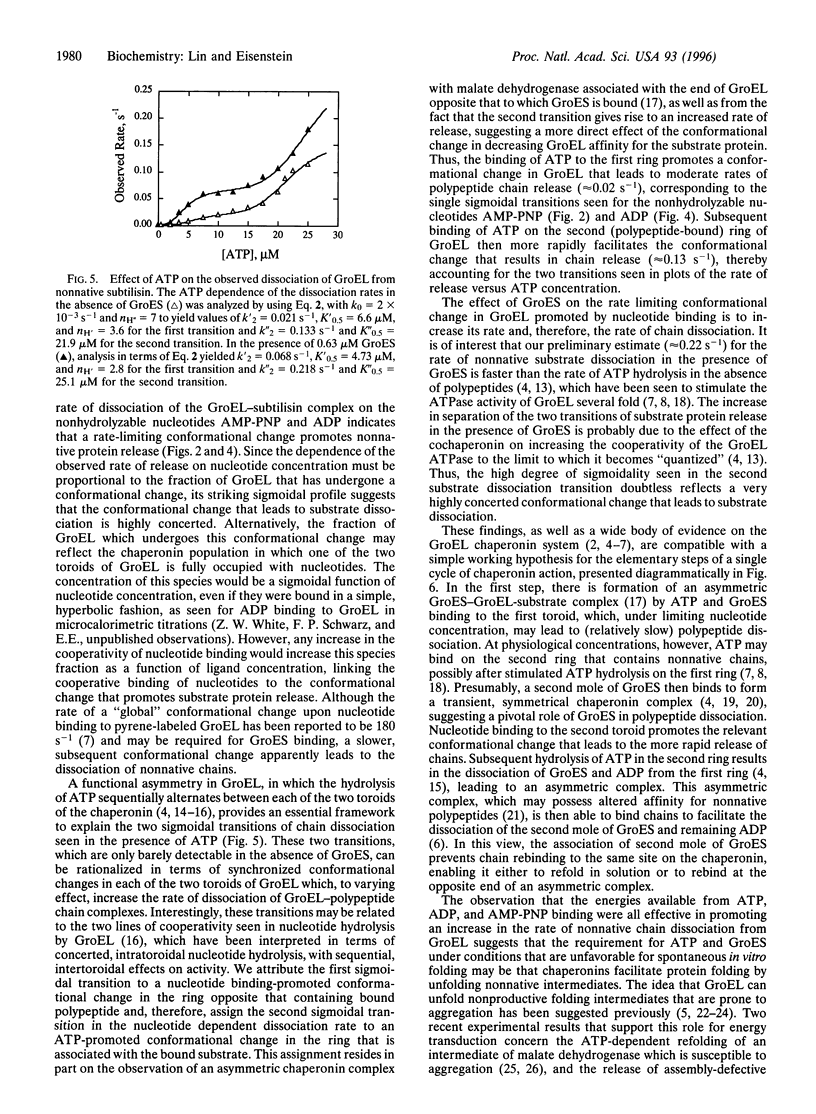
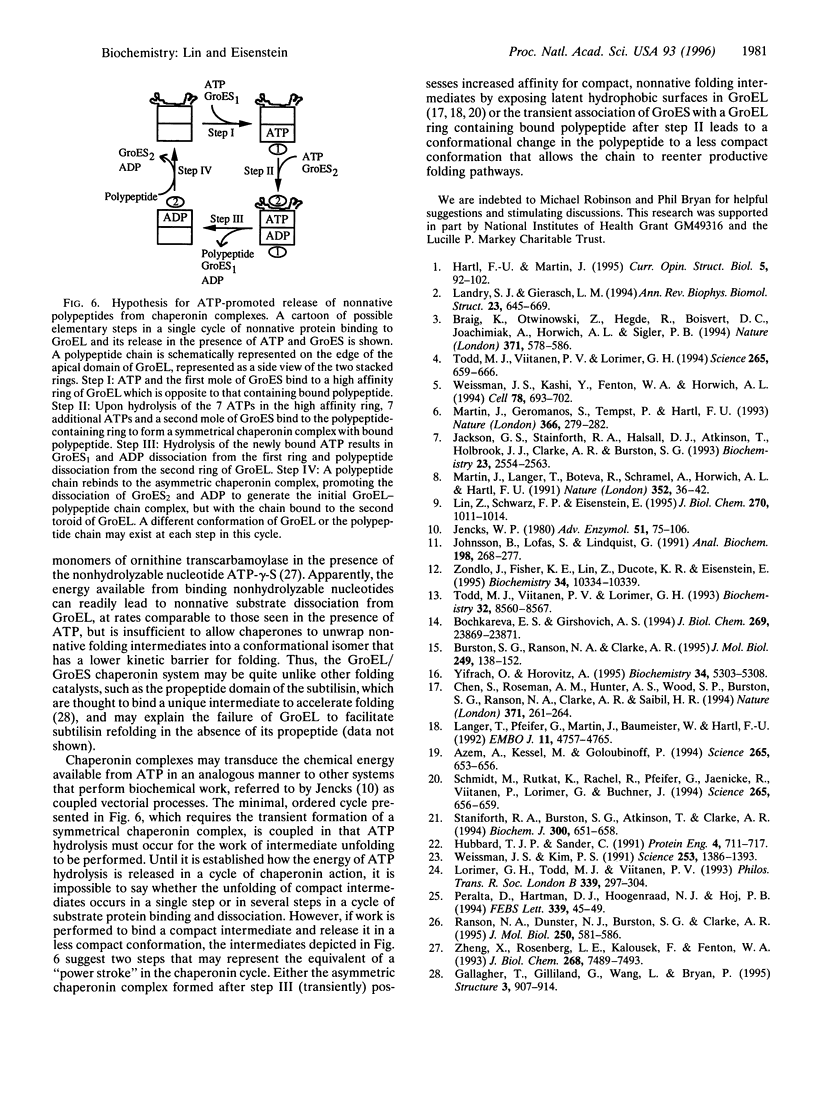
Abstract
The Escherichia coli chaperonins GroEL and GroES facilitate the refolding of polypeptide chains in an ATP hydrolysis-dependent reaction. The elementary steps in the binding and release of polypeptide substrates to GroEL were investigated in surface plasmon resonance studies to measure the rates of binding and dissociation of a normative variant of subtilisin. The rate constants determined for GroEL association with and dissociation from this variant yielded a micromolar dissociation constant, in agreement with independent calorimetric estimates. The rate of GroEL dissociation from the nonnative chain was increased significantly in the presence of 5'-adenylylimidodiphosphate (AMP-PNP), ADP, and ATP, yielding maximal values between 0.04 and 0.22 s(-1). The sigmoidal dependence of the dissociation rate on the concentration of AMP-PNP and ADP indicated that polypeptide dissociation is limited by a concerted conformational change that occurs after nucleotide binding. The dependence of the rate of release on ATP exhibited two sigmoidal transitions attributable to nucleotide binding to the distal and proximal toroid of a GroEL-polypeptide chain complex. The addition of GroES resulted in a marked increase in the rate of nonnative polypeptide release from GroEL, indicating that the cochaperonin binds more rapidly than the dissociation of polypeptides. These data demonstrate the importance of nucleotide binding-promoted concerted conformational changes for the release of chains from GroEL, which correlate with the sigmoidal hydrolysis of ATP by the chaperonin. The implications of these findings are discussed in terms of a working hypothesis for a single cycle of chaperonin action.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azem A., Kessel M., Goloubinoff P. Characterization of a functional GroEL14(GroES7)2 chaperonin hetero-oligomer. Science. 1994 Jul 29;265(5172):653–656. doi: 10.1126/science.7913553. [DOI] [PubMed] [Google Scholar]
- Bochkareva E. S., Girshovich A. S. ATP induces non-identity of two rings in chaperonin GroEL. J Biol Chem. 1994 Sep 30;269(39):23869–23871. [PubMed] [Google Scholar]
- Braig K., Otwinowski Z., Hegde R., Boisvert D. C., Joachimiak A., Horwich A. L., Sigler P. B. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature. 1994 Oct 13;371(6498):578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- Burston S. G., Ranson N. A., Clarke A. R. The origins and consequences of asymmetry in the chaperonin reaction cycle. J Mol Biol. 1995 May 26;249(1):138–152. doi: 10.1006/jmbi.1995.0285. [DOI] [PubMed] [Google Scholar]
- Chen S., Roseman A. M., Hunter A. S., Wood S. P., Burston S. G., Ranson N. A., Clarke A. R., Saibil H. R. Location of a folding protein and shape changes in GroEL-GroES complexes imaged by cryo-electron microscopy. Nature. 1994 Sep 15;371(6494):261–264. doi: 10.1038/371261a0. [DOI] [PubMed] [Google Scholar]
- Gallagher T., Gilliland G., Wang L., Bryan P. The prosegment-subtilisin BPN' complex: crystal structure of a specific 'foldase'. Structure. 1995 Sep 15;3(9):907–914. doi: 10.1016/S0969-2126(01)00225-8. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Martin J. Molecular chaperones in cellular protein folding. Curr Opin Struct Biol. 1995 Feb;5(1):92–102. doi: 10.1016/0959-440x(95)80014-r. [DOI] [PubMed] [Google Scholar]
- Hubbard T. J., Sander C. The role of heat-shock and chaperone proteins in protein folding: possible molecular mechanisms. Protein Eng. 1991 Oct;4(7):711–717. doi: 10.1093/protein/4.7.711. [DOI] [PubMed] [Google Scholar]
- Jackson G. S., Staniforth R. A., Halsall D. J., Atkinson T., Holbrook J. J., Clarke A. R., Burston S. G. Binding and hydrolysis of nucleotides in the chaperonin catalytic cycle: implications for the mechanism of assisted protein folding. Biochemistry. 1993 Mar 16;32(10):2554–2563. doi: 10.1021/bi00061a013. [DOI] [PubMed] [Google Scholar]
- Jencks W. P. The utilization of binding energy in coupled vectorial processes. Adv Enzymol Relat Areas Mol Biol. 1980;51:75–106. doi: 10.1002/9780470122969.ch2. [DOI] [PubMed] [Google Scholar]
- Johnsson B., Löfås S., Lindquist G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem. 1991 Nov 1;198(2):268–277. doi: 10.1016/0003-2697(91)90424-r. [DOI] [PubMed] [Google Scholar]
- Landry S. J., Gierasch L. M. Polypeptide interactions with molecular chaperones and their relationship to in vivo protein folding. Annu Rev Biophys Biomol Struct. 1994;23:645–669. doi: 10.1146/annurev.bb.23.060194.003241. [DOI] [PubMed] [Google Scholar]
- Langer T., Pfeifer G., Martin J., Baumeister W., Hartl F. U. Chaperonin-mediated protein folding: GroES binds to one end of the GroEL cylinder, which accommodates the protein substrate within its central cavity. EMBO J. 1992 Dec;11(13):4757–4765. doi: 10.1002/j.1460-2075.1992.tb05581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Schwartz F. P., Eisenstein E. The hydrophobic nature of GroEL-substrate binding. J Biol Chem. 1995 Jan 20;270(3):1011–1014. doi: 10.1074/jbc.270.3.1011. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Todd M. J., Viitanen P. V. Chaperonins and protein folding: unity and disunity of mechanisms. Philos Trans R Soc Lond B Biol Sci. 1993 Mar 29;339(1289):297–304. doi: 10.1098/rstb.1993.0028. [DOI] [PubMed] [Google Scholar]
- Martin J., Geromanos S., Tempst P., Hartl F. U. Identification of nucleotide-binding regions in the chaperonin proteins GroEL and GroES. Nature. 1993 Nov 18;366(6452):279–282. doi: 10.1038/366279a0. [DOI] [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Peralta D., Hartman D. J., Hoogenraad N. J., Høj P. B. Generation of a stable folding intermediate which can be rescued by the chaperonins GroEL and GroES. FEBS Lett. 1994 Feb 14;339(1-2):45–49. doi: 10.1016/0014-5793(94)80381-1. [DOI] [PubMed] [Google Scholar]
- Ranson N. A., Dunster N. J., Burston S. G., Clarke A. R. Chaperonins can catalyse the reversal of early aggregation steps when a protein misfolds. J Mol Biol. 1995 Jul 28;250(5):581–586. doi: 10.1006/jmbi.1995.0399. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Rutkat K., Rachel R., Pfeifer G., Jaenicke R., Viitanen P., Lorimer G., Buchner J. Symmetric complexes of GroE chaperonins as part of the functional cycle. Science. 1994 Jul 29;265(5172):656–659. doi: 10.1126/science.7913554. [DOI] [PubMed] [Google Scholar]
- Staniforth R. A., Burston S. G., Atkinson T., Clarke A. R. Affinity of chaperonin-60 for a protein substrate and its modulation by nucleotides and chaperonin-10. Biochem J. 1994 Jun 15;300(Pt 3):651–658. doi: 10.1042/bj3000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd M. J., Viitanen P. V., Lorimer G. H. Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science. 1994 Jul 29;265(5172):659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- Todd M. J., Viitanen P. V., Lorimer G. H. Hydrolysis of adenosine 5'-triphosphate by Escherichia coli GroEL: effects of GroES and potassium ion. Biochemistry. 1993 Aug 24;32(33):8560–8567. doi: 10.1021/bi00084a024. [DOI] [PubMed] [Google Scholar]
- Weissman J. S., Kashi Y., Fenton W. A., Horwich A. L. GroEL-mediated protein folding proceeds by multiple rounds of binding and release of nonnative forms. Cell. 1994 Aug 26;78(4):693–702. doi: 10.1016/0092-8674(94)90533-9. [DOI] [PubMed] [Google Scholar]
- Weissman J. S., Kim P. S. Reexamination of the folding of BPTI: predominance of native intermediates. Science. 1991 Sep 20;253(5026):1386–1393. doi: 10.1126/science.1716783. [DOI] [PubMed] [Google Scholar]
- Yifrach O., Horovitz A. Nested cooperativity in the ATPase activity of the oligomeric chaperonin GroEL. Biochemistry. 1995 Apr 25;34(16):5303–5308. doi: 10.1021/bi00016a001. [DOI] [PubMed] [Google Scholar]
- Zheng X., Rosenberg L. E., Kalousek F., Fenton W. A. GroEL, GroES, and ATP-dependent folding and spontaneous assembly of ornithine transcarbamylase. J Biol Chem. 1993 Apr 5;268(10):7489–7493. [PubMed] [Google Scholar]
- Zondlo J., Fisher K. E., Lin Z., Ducote K. R., Eisenstein E. Monomer-heptamer equilibrium of the Escherichia coli chaperonin GroES. Biochemistry. 1995 Aug 22;34(33):10334–10339. doi: 10.1021/bi00033a003. [DOI] [PubMed] [Google Scholar]


