Abstract
Background
Twenty-four low frequency platelet antigens (HPAs) have been implicated as immunogens in neonatal alloimmune thrombocytopenia (NAIT). We performed studies to define more fully how often these antigens trigger maternal immunization leading to NAIT.
Study design and methods
In a Phase 1 study, fathers of selected NAIT cases not resolved by serologic testing but thought to have a high likelihood of NAIT on clinical and serologic grounds were typed for low frequency HPAs (LFHPAs) by DNA sequencing. In a Phase 2 study, high-throughput methods were used to type fathers of 1067 consecutive unresolved NAIT cases for LFHPAs. Mothers of 1338 unresolved cases were also typed to assess the prevalence of LFHPAs in a population racially/ethnically similar to the fathers.
Results
In Phase 1, LFHPAs were identified in 16 of 244 fathers (6.55%). In Phase 2, LFPAs were found in only 28 of 1067 fathers (2.62%). LFHPAs were identified in 27 of 1338 maternal samples (2.01%). HPA-9bw was by far the most common LFHPA identified in the populations studied and was the only LFHPA that was significantly more common in fathers than in mothers of affected infants (P=0.02).
Conclusions
Maternal immunization against recognized LFHPAs accounts for only a small fraction of the cases of apparent NAIT not resolved by standard serologic testing. Typing of the fathers of such cases for LFHPAs is likely to be rewarding only when a maternal antibody specific for a paternal platelet glycoprotein is demonstrated and/or there is compelling clinical evidence for NAIT.
INTRODUCTION
Neonatal alloimmune thrombocytopenia (NAIT), caused by maternal antibodies directed against fetal platelet antigens1–4, occurs about once in 1000 live births and is the major cause of intracranial hemorrhage in full-term infants5–7. To provide optimal care for affected infants and for management of future pregnancies, it is important that a serologic diagnosis be made whenever possible. However, maternal HPA antibodies are identified in only 20–35% of apparent NAIT cases referred for laboratory investigation4,8,9. In about 80% of the resolved cases the antibody detected is specific for HPA-1a carried on β3 integrin (GPIIIa)4,8,10,11. Antibodies identified in the remaining cases are mainly specific for HPA-5b, -1b, -3a or 15b4,8,11,12.
Over the past two decades, individual cases of NAIT have been described in which the mother was immunized against a low frequency HPA antigen (LFHPA)4. As of this writing, a total of 24 such antigens have been identified. Each is determined by a single amino acid substitution in platelet GP IIb/IIIa, Ib/IX or Ia/IIa4,13 except for HPA-14bw, which results from an in-frame deletion of three nucleotides in the gene encoding GPIIIa14. Healthy donor platelets carrying the relevant target antigen are usually not available to confirm specificity when a maternal antibody reacts only with paternal platelets in the initial screen. Therefore, maternal immunization against a low frequency HPA antigen can easily be overlooked. To investigate the prevalence of this problem, Ghevaert et al9 collected DNA from fathers of 1054 unresolved NAIT cases identified in four European laboratories and used a Taqman-based method to type for the low frequency HPA antigens 4b, 6–8bw, 10–14bw, 16bw and 17bw. A recognized low frequency antigen was identified in only eight instances. They concluded that maternal immunization against low frequency HPA antigens is unlikely to account for more than a small fraction of suspected NAIT cases but recognized that results could have been affected by poor quality of some DNA samples (which had been stored for up to 10 years). In addition, for technical reasons, fathers were not typed for HPA-9bw, a low frequency antigen that may be particularly immunogenic15,16 and, since this study was performed, 11 new low frequency HPA antigens (HPA- 7c, and 18 through 28) have been described4,13.
To better define the prevalence of LFHPAs in a North American population and more fully define the role of these antigens in NAIT, we determined the prevalence of 22 LFHPAs in 1311 fathers of referred NAIT cases not resolved by routine serologic testing. DNA from 1338 mothers was similarly typed to estimate the normal prevalence of these antigens in a population similar to the fathers in ethnic/racial background.
METHODS
Patients
Blood samples from parents of infants suspected of having neonatal alloimmune thrombocytopenia were referred to the Platelet and Neutrophil Immunology Laboratory (PNIL) of the BloodCenter of Wisconsin (BCW) for diagnostic testing because infants were suspected on clinical grounds of having NAIT. Maternal serum was tested against paternal platelets and a typed panel of normal platelets for platelet-reactive and glycoprotein-specific antibodies as previously described17 using flow cytometry and/or modified capture ELISA (MACE)8,18,19. Genotyping for antigens HPA-1 through -6, -9 and -15 was carried out using in-house allelic discrimination assays described previously20. Diagnosis of NAIT was considered to be confirmed when a maternal antibody was identified that recognized an HPA antigen present in the father or, in rare cases, was specific for GPIV (CD36); other cases were considered to be “unresolved.” Detailed clinical information was available only on cases in which a low frequency HPA paternal antigen was identified in the Phase 1 study; histories in these cases were consistent with NAIT of varying degrees of severity. Some cases in which LFHPAs were identified in the Phase 1 study (see Results) have been described previously in a different context15,17,20.
Genotyping for low frequency variants encoding alloantigens
DNA from1478 unresolved NAIT cases (in 927 cases both parents, in 140 cases father only and in 411 cases mother only) was typed for HPA antigens (-4 through -26) by one or more of three different methods: 1) PCR amplification of selected exons and direct sequencing of the PCR products20, 2) a TaqMan OpenArray Real-time PCR platform (Applied Biosystems Life Technologies Corporation, Carlsbad, California) and 3) PCR allelic discrimination assays. All antigens identified by the latter two methods were confirmed by direct DNA sequencing. LFHPAs -27 and -28 were not examined in this study. Single nucleotide polymorphism (SNP) genotyping using the TaqMan OpenArray Genotyping system was performed as described previously21,22. Pre-designed and custom-designed genotyping assays consisting of allele-specific minor groove binding probes and PCR primer sets loaded onto individual through-holes of a metal-based array were supplied by the manufacturer (Applied Biosystems). Detailed assay information can be obtained from corresponding author upon request. Genomic DNA and TaqMan OpenArray Master Mix (Life Technologies, Carlsbad, CA) were loaded onto the arrays and PCR was performed on the GeneAmp PCR System 9700 (ABI). Arrays were imaged on an OpenArray NT Imager using the OpenArray SNP Genotyping Analysis Software (Life Technologies). Assays were validated using genomic DNA samples containing known rare SNPs when available.
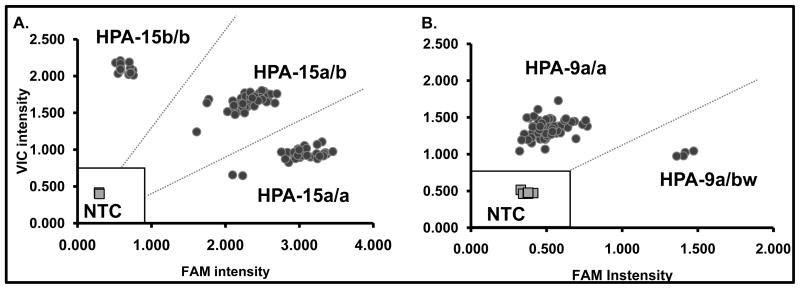
DNA samples heterozygous or homozygous for both alleles are readily encountered when typing is done for relatively common SNPs. With low frequency SNPs, however, samples homozygous for the rare allele are almost never seen. Since the OpenArray SNP genotyping software is designed to discriminate between three allelic populations (a/a, a/b and b/b), the readout was unsuitable for analysis using the standard OpenArray software. Accordingly, data were analyzed with Microsoft Excel software and a Cartesian plot in which fluorescence signal intensities of the VIC reporter (allele one) and FAM reporter (allele 2) were normalized to the internal fluorescence Rox signals. Individual reactions were discarded if the signal fell within a “no call” zone established independently for each SNP assay. This occurred whenever any of the following criteria were met; 1) the internal reference Rox signal was below the average Rox signal +/− 3SD for each that SNP, 2) the sum of VIC and FAM signal intensities was less than the average sum of VIC and FAM + 3SD of four no template control reactions or 3) both the normalized VIC and normalized FAM signal intensities were less than average normalized VIC and FAM signals +/− 3SD of no template controls. A formula was then generated for each SNP to make automatic genotype calls based on the signals of positive and negative control samples. All samples were tested in duplicate. When both duplicates tested positive for a low frequency SNP, the finding was confirmed by direct sequencing of a PCR product derived from the relevant exon. Representative OpenArray data obtained in typing for SNPs encoding HPA-15 and HPA-9 are shown in Figure 1. HPA-15a and -15b alleles have an allelic frequency of 0.455 and 0.545 respectively, resulting in three genotypic populations corresponding to HPA-15a/a, -15a/b and -15b/b. Due to the rarity of the HPA-9bw allele15, only two populations were identified corresponding to the HPA-9a/a and HPA-9a/bw genotypes.
Figure 1. TaqMan OpenArray Genotyping analysis.
Typical HPA-15 and HPA-9 results shown for 45 fathers of unresolved NAIT cases. Signal intensities of Vic versus Fam fluorophores (normalized to internal Rox signal) are plotted. Each individual is genotyped in duplicate. No template controls (NTC) are denoted by grey squares (four individual reactions throughout the array). The no call region (marked with open square) is defined as the average signal of NTCs + 3SD. A. HPA-15 genotypic analysis (black circles): The Vic fluorophore reports the HPA-15b and FAM reports HPA-15a allele. HPA-15a and -15b alleles have an allelic frequency of 0.455 and 0.545 respectively resulting in three genotypic populations corresponding to HPA-15a/a, -15a/b and -15b/b. B. HPA-9 genotypic analysis (black circles): Vic reports the HPA-9a allele and FAM reports HPA-9bw. Two populations are seen rather than three because the allelic frequency of HPA-9bw is 0.002 and an HPA-9bw/bw individual has never been encountered.
As noted, primers incorporated into the OpenArray SNP assays by Applied Biosystems Life Technologies are custom-designed using proprietary techniques to optimize the likelihood that the desired SNPs can be detected under the same conditions. In preliminary studies, we found that the customized assays failed to detect SNPs encoding the antigens HPA-8bw, -11bw, -12bw, -21bw, 22bw and 23bw. Therefore, these antigens were typed using allelic discrimination assays as described previously23. Fluorescently-labeled probes for each SNP were designed using Primer3 (http://frodo.wi.mit.edu/) software available on the world wide web24. Common sequence probes were labeled with 5′ FAM reporter dye and 3′ Iowa Black FQ quencher and mutant sequence probes were labeled with 5′ Cy5 reporter dye and 3′ Black Hole quencher dye. The reaction mixture containing Quanta PerfeCTAqPCR SuperMix, UNG, Low Rox (Quanta Biosciences, Gaithersburg, MD), primers, probes and genomic DNA isolated from peripheral blood cells15 was cycled on the Applied BioSystems 7500 (Life Technologies, Carlsbad, CA) device. Cycling conditions and probe/primer sequences are available upon request. All mutations identified were confirmed by direct sequencing of a PCR product derived from the relevant exon. Representative allelic discrimination data obtained in typing for SNPs encoding HPA-12 and HPA-21 are shown in Supplemental Figure 1.
Statistical Analyses
The prevalence of carriers for each allele in the maternal population studied was estimated as the proportion of carriers among mothers of children with suspected NAIT and non-carrier fathers. Mothers of children with carrier fathers were excluded, because they are potentially negatively selected for carrier status. Confidence intervals were calculated using the exact binomial method.
The inference for evaluating whether a low frequency allele is associated with NAIT is based on the imbalance of fathers being carriers compared to mothers using a combination of matched parents (both a father and mother of an affected child) and unmatched parents (either a father or a mother of an affected child). Among matched parents, the inference is based on a one-sided exact McNemar’s test and the confidence interval was constructed using Newcombe’s method for difference in binomial proportions with paired data25. Among unmatched parents the inference is based on a one-sided Fisher’s exact test and the confidence interval was constructed using Newcombe’s methods for difference in binomial proportions with independent data26. The combined estimate and confidence interval is then based on a weighted average of the matched and unmatched estimates proportional to the number of subjects in each group27. Exact permutation test p-values were computed as the sum of the probabilities of the possible outcomes under the null hypothesis using the mid-p method28.
Approvals
Studies involving human subjects and animals were approved by the institutional review board of the BloodCenter of Wisconsin
RESULTS
Study format
The study was conducted in two phases. In Phase 1, cases studied were ones in which serologic evidence of maternal-fetal incompatibility for common HPA antigens was not found in the standard NAIT evaluation and one or more of the following three conditions applied: mother’s serum reacted strongly with father’s platelets in flow cytometry, mother’s serum reacted with father’s GPIIb/IIIa in MACE, the clinical history was compelling for NAIT. In Phase 2, DNA from fathers of 1067 serologically unresolved cases referred consecutively for NAIT testing was typed using high throughput methods. DNA from 1338 mothers of suspected NAIT cases was similarly studied to obtain an unbiased estimate of the normal prevalence of these antigens in a population similar in its demographics to the population of fathers studied.
Phase 1 studies
DNA from 244 fathers of unresolved NAIT cases satisfying the criteria listed above was typed by direct sequencing of exons known to encode low frequency HPA antigens. For practical reasons, GPIIb/IIIa was sequenced most often because these GPs are known to carry most low frequency antigens. As shown in Table 1, low frequency antigens were identified in 16 fathers. Antigens HPA-4b, 6bw, 11bw, 12bw, 13bw, 19bw, 20bw, 21bw, 22bw, and 23bw were identified in ten individuals; six fathers were positive for HPA-9bw. The novel antigens HPA-19bw through -23bw were identified by sequencing the entire extracellular domains of GPIIb and GPIIIa in five families in which there was compelling clinical and serologic evidence for NAIT17,20.
Table 1.
Low frequency HPA alleles identified in 244 fathers in Phase 1.
| HPA | Number tested | Heterozygotes detected |
|---|---|---|
| 4b | 167 | 1 |
| 6bw | 235 | 1 |
| 7bw | 238 | 0 |
| 8bw | 233 | 0 |
| 9bw | 217 | 6 |
| 10bw | 241 | 0 |
| 11bw | 232 | 1 |
| 12bw | 223 | 1 |
| 13bw | 222 | 1 |
| 14bw | 131 | 0 |
| 16bw | 237 | 0 |
| 19bw | 1 | 1 |
| 20bw | 1 | 1 |
| 21bw | 1 | 1 |
| 22bw | 1 | 1 |
| 23bw | 1 | 1 |
Phase 2 studies
As noted, Phase 1 studies were performed in a highly selected group of NAIT cases. Phase 2 studies were done to estimate the prevalence of low frequency antigens in fathers of cases selected only because they were not resolved on the basis of maternal-fetal incompatibility for common HPA antigens. Antigens for which the OpenArray technique was found to be suitable were typed by that method because of its simplicity and relatively low cost. OpenArray was found to be unsuitable for antigens HPA-8bw, -11bw, -12bw, -21bw, -22bw, and -23bw. Therefore, SNPs encoding these markers were detected using allelic discrimination assays. The same approaches were used to identify SNPs in maternal DNA samples. For logistical and technical reasons, it was not possible to type every DNA sample for every antigen. All SNPs identified by either of the testing methods were confirmed by direct sequencing of the exon in which the SNP is located.
Results obtained in typing paternal DNA from 1067 suspected NAIT cases using the OpenArray and allelic discrimination assays are summarized in Table 2, where it can be seen that 28 paternal DNA samples were positive for the low frequency antigens HPA-4b (1 case), 6bw (2 cases), 8bw (1 case), 9bw (17 cases), 12bw (4 cases), and 21bw (3 cases).
Table 2.
Low frequency HPA antigens identified in 1067 fathers and 1338 mothers in the Phase 2 study.*
| HPA | Number tested | Hetero-zygotes detected | Number tested | Hetero-zygotes detected |
|---|---|---|---|---|
|
| ||||
| fathers | mothers | |||
| 4 | 1003 | 1 | 1240 | 2 |
| 6 | 1020 | 2 | 1274 | 5 |
| 7 | 1034 | 0 | 1298 | 0 |
| 7c | 727 | 0 | 906 | 0 |
| 8 | 949 | 1 | 1215 | 0 |
| 9 | 1030 | 17 | 1277 | 12 |
| 10 | 680 | 0 | 849 | 0 |
| 11 | 945 | 0 | 1214 | 0 |
| 12 | 952 | 4 | 1232 | 4 |
| 13 | 1041 | 0 | 1306 | 3 |
| 14 | 1047 | 0 | 1317 | 0 |
| 16 | 1049 | 0 | 1315 | 0 |
| 17 | 1031 | 0 | 1294 | 0 |
| 18 | 1015 | 0 | 1262 | 0 |
| 19 | 1009 | 0 | 1263 | 0 |
| 20 | 999 | 0 | 1261 | 0 |
| 21 | 947 | 3 | 1217 | 1 |
| 22 | 944 | 0 | 1214 | 0 |
| 23 | 945 | 0 | 1212 | 0 |
| 24 | 831 | 0 | 1043 | 0 |
| 25 | 1046 | 0 | 1308 | 0 |
| 26 | 724 | 0 | 905 | 0 |
HPA-27 and HPA-28 were not analyzed in this study.
Maternal DNA samples
Mothers and fathers of NAIT cases are likely to have similar racial/ethnic backgrounds and it is extremely unlikely that both parents will possess the same low frequency antigen, thereby biasing the representation of any particular antigen in the maternal samples. For these reasons, the prevalence of low frequency antigens identified in the maternal population is probably superior to that found in a randomly selected normal population as a basis for assessing the significance of low frequency antigens identified in the fathers. As shown in Table 2, the low frequency antigens HPA-4b (4 cases), -6bw (5 cases), -9bw (12 cases), -12bw (4 cases), -13bw (3 cases) and -21bw (one case) were identified in 27 of 1338 maternal samples.
DISCUSSION
In our Phase I study, low frequency HPA antigens were identified in 16 of 244 (6.6%) of fathers of infants suspected of having NAIT not attributable to maternal-fetal incompatibility for common HPA antigens (Table 1). As noted, NAIT was thought to be highly likely in this group on the basis of initial laboratory and clinical findings. The Phase 2 study was conducted to estimate the prevalence of low frequency antigens in fathers of serologically unresolved cases referred consecutively for NAIT testing without regard to clinical status other than the fact that NAIT was suspected. As shown in Table 2, low frequency antigens were identified in the Phase 2 study in only 28 of 1067 fathers (2.6%), most of which (17 cases, 1.6%) were accounted for by HPA-9bw. Typing for low frequency HPA antigens was carried out with DNA from 1338 mothers of unresolved NAIT cases to estimate how often low frequency antigens might be encountered by chance in a population similar in ethnic and racial make-up to the group of fathers studied. Low frequency antigens were identified in 27 (2.0%) of the mothers; 12 of these (0.90%) were HPA-9bw-positive.
The low frequency antigens HPA-4b, 6bw, 8bw, 9bw, 12bw, 13bw and 21bw were identified in one or more individuals in the Phase 2 study (Table 2). Data were analyzed to determine whether the carrier prevalence in fathers exceeded that in mothers. Only HPA-9bw was found to be significantly more common in fathers (1.65%) than in mothers (0.94%) (p=0.021) (Table 3). This finding is statistically significant (p=0.02) when HPA-9bw alone is considered, but not when a multiple-testing adjusted cutoff is used. Prevalence of the remaining low frequency SNPs (HPA-4b,-6bw,-8bw,-12bw,-13bw and-21bw) was not significantly different in the paternal and maternal populations. The estimated prevalence of each low frequency antigen (95% confidence levels) tested for in the maternal population (excluding cases where an antigen was identified in a father) is shown in Table 3, where it can be seen that the HPA-9bw allele is expected to be present by chance in 8.3 of every 1000 individuals (95% CI: 3.3–17.0). These estimates, of course, apply only to the population of mothers whose blood samples were referred for study, and not to the population at large. It is well established that the antigens HPA-4b and HPA-21bw (Asians) and 6bw (Finns and Asians) are more common in certain racial groups29–31 and it was not feasible to define the racial make-up of the study population.
Table 3.
Estimates carrier prevalence of each low frequency SNP and association with NAIT.
| Excess of paternal carriers per 1000 unresolved NAIT cases | Carrier prevalence per 1000 individuals | ||||
|---|---|---|---|---|---|
|
| |||||
| SNP | r* | P-value┼ | 95% CIǂ | p§ | 95% CI |
| HPA-4b | −3.7 | 0.934 | (−10, 3.7) | 3.6 | (0.7, 10.5) |
| HPA-6bw | −1.7 | 0.699 | (−8, 6.7) | 4.7 | (1.3, 12.0) |
| HPA-7bw | 0.0 | (−4, 6.8) | 0.0 | [0.0, 4.2) | |
| HPA-7cw | 0.0 | (−5.7, 9.3) | 0.0 | [0.0, 6.0) | |
| HPA-8bw | 1.8 | 0.126 | (−2.7, 10.8) | 0.0 | [0.0, 4.5) |
| HPA-9bw | 9.8 | 0.021 | (−0.2, 23.0) | 8.3 | (3.3, 17.0) |
| HPA-10bw | 0.0 | (−6.2, 9.1) | 0.0 | [0.0, 6.9) | |
| HPA-11bw | 0.0 | (−4.2, 7.8) | 0.0 | [0.0, 4.5) | |
| HPA-12bw | 0.0 | 0.5 | (−6.6, 9.2) | 4.9 | (1.3, 12.6) |
| HPA-13bw | −2.3 | 0.909 | (−7.5, 4.6) | 2.3 | (0.3, 8.1) |
| HPA-14bw | 0.0 | (−3.9, 6.8) | 0.0 | [0.0, 4.1) | |
| HPA-16bw | 0.0 | (−3.9, 6.8) | 0.0 | [0.0, 4.1) | |
| HPA-17bw | 0.0 | (−4.0, 6.7) | 0.0 | [0.0, 4.3) | |
| HPA-18bw | 0.0 | (−4.1, 6.4) | 0.0 | [0.0, 4.4) | |
| HPA-19bw | 0.0 | (−4.1, 6.6) | 0.0 | [0.0, 4.4) | |
| HPA-20bw | 0.0 | (−4.1, 6.6) | 0.0 | [0.0, 4.5) | |
| HPA-21bw | 1.8 | 0.188 | (−3.4, 10.7) | 1.2 | (0.0, 6.8) |
| HPA-22bw | 0.0 | (−4.3, 7.8) | 0.0 | [0.0, 4.5) | |
| HPA-23bw | 0.0 | (−4.3, 7.7) | 0.0 | [0.0, 4.5) | |
| HPA-24bw | 0.0 | (−5.1, 7.4) | 0.0 | [0.0, 5.8) | |
| HPA-25bw | 0.0 | (−3.9, 6.7) | 0.0 | [0.0. 4.1) | |
| HPA-26bw | 0.0 | (−5.7, 9.2) | 0.0 | [0.0, 6.1) | |
r is excess of fathers carriers compared to mother carriers.
P-values test the hypothesis that the excess paternal prevalence is positive, and are based on an exact permutation test.
The 95% CI does not completely match with the p-value, because it is a two-sided approximate interval, while the test is one-sided and exact.
p is carrier prevalence of each SNP per 1000 people.
The similar distribution of low frequency antigens other than HPA-9bw in the paternal and maternal populations (Table 2) favors the possibility that the antigens HPA-8bw, 12bw and 21bw were identified in some fathers by chance and were unrelated to NAIT. As an alternate means of addressing the issue of causality, we examined results obtained when maternal serum was tested against platelets from fathers who were found to possess a low frequency HPA antigen. Maternal serum reacted significantly with paternal platelets in a flow cytometric assay in 18 of 25 instances. However, Class I HLA antibodies present in 11 of the maternal samples undoubtedly explain some positive cross match results. It is also possible that in some cases ABO incompatibility produced a positive test results in flow cytometry. Immunoprecipitation (MACE) assays using maternal serum and paternal platelets were positive in only 2 of 23 cases in which the low frequency antigen identified in the father was expressed on GPIIb/IIIa. These findings differ from those made in the Phase 1 study in which 15 of 16 maternal serum samples reacted with paternal platelets in flow cytometry (2 possibly influenced by HLA) and 8 of 8 reacted with paternal GPIIb/IIIa in MACE. It is apparent that serologic findings made in the Phase 2 cases do not, in themselves, provide persuasive evidence for NAIT.
In the only previously reported study of this type, Ghevaert and colleagues determined the prevalence of 11 low frequency HPA antigens in 1054 fathers of suspected NAIT cases, 831 of which could not be accounted for by maternal antibodies against common HPA antigens9. Eight fathers were positive for the antigens HPA-6bw (4 cases), HPA-10bw (one case), HPA-11bw (one case) and HPA-12bw (two cases). Three of the HPA-6bw cases were identified in a Finnish population in which this antigen is relatively common. Cross-matches with maternal serum against paternal platelets were positive in four of the eight cases. Findings made in our study support the conclusion of Ghevaert et al that only a small fraction of NAIT cases not attributable to incompatibility for common HPA antigens can be accounted for by maternal immunization against recognized low frequency antigens. The small number of low frequency paternal HPA antigens identified in the two studies supports the view that typing of fathers for low frequency antigens should probably be limited to cases in which a common HPA antibody is not identified but the likelihood of NAIT is high as judged from initial laboratory and clinical findings. A possible exception is the antigen HPA-9bw, which was not tested for by Ghevaert et al for technical reasons9 and which was identified in 17 of 1030 fathers (1.65%) but only in 12 of 1277 mothers (0.94%) in the Phase 2 study (Table 2). Three previous reports describe 15 cases of NAIT associated with maternal-fetal incompatibility for HPA-9bw. Nearly all of the affected infants had severe thrombocytopenia and bleeding and five had intracranial hemorrhage15,16,32. In our Phase 2 study, we found that about one percent of the maternal population was HPA-9bw-positive (gene frequency about 0.5%), higher than the value of 0.3% (3 of 500) found by Norris et al in a normal Dutch population32. Thus, HPA-9bw appears to be much more common than other low frequency HPA antigens described to date. Moreover, the prevalence of NAIT cases caused by maternal-fetal incompatibility for this marker identified in two studies suggests that it may be more immunogenic than most of the other HPA antigens15,16. Although we would expect the majority of affected infants in this study whose father was HPA-9b positive to have inherited the -9b allele it was not feasible to obtain follow-up DNA samples from the infants to verify this assumption. Beyond these considerations, for reasons not fully understood, HPA-9bw antibodies can be extremely difficult to detect in standard serologic assays15,16. In light of these facts, an argument can be made that fathers of NAIT cases should be routinely typed for HPA-9bw and our own laboratory has adopted this policy. In fathers of Asian ancestry, typing for HPA-4b29,33, HPA-6bw31,33 and HPA-21bw30,34 can also be considered.
Our findings leave open the question of why maternal-fetal incompatibility for HPA is documented serologically only in a minority of cases in which NAIT appears to be likely on clinical grounds. Recent studies have shown that low avidity antibodies specific for HPA-1a that cannot be detected using conventional serology can cause clinically significant NAIT35,36. Antibodies specific for Class I HLA antigens are common in pregnancy4 and, although such antibodies are widely thought not to cause NAIT, many anecdotal reports suggest that they can do so under some circumstances4. Rarely, NAIT can be caused by high-titer antibodies specific for blood groups A and B37 or glycoprotein IV (CD36)38. Further studies of these and other potential explanations for the “diagnostic gap” in NAIT are needed.
Supplementary Material
Typical HPA-21 (A) and HPA-12 (B) results shown for 50 fathers of unresolved NAIT cases. Signal intensities of Cy5 (HPA-21bw or HPA-12bw) versus FAM (HPA-21a or HPA-12bw) fluorophores are plotted. No template controls are denoted by grey diamonds. Homozygous and heterozygous samples are denoted with black diamonds and triangles respectively. Unknowns that tested positive in the assay are denoted with grey circles. Each unknown that typed at HPA-21a/bw or HPA-12a/bw was verified with sequence analysis.
Acknowledgments
We thank Stephanie Balthazor for technical assistance. The development of Primer3 and the Primer3 web site was funded by Howard Hughes Medical Institute and by the National Institutes of Health, National Human Genome Research Institute. under grants R01-HG00257 (to David C. Page) and P50-HG00098 (to Eric S. Lander). This work was supported by Grant HL-13629 (RHA) and AHA 11GRNT7690032 (JP).
Footnotes
CONFLICTS OF INTEREST
The authors have no disclaimers to make or conflicts to disclose.
AUTHOR CONTRIBUTIONS
JP contributed to research design, oversight of laboratory studies, interpretation of results and manuscript preparation. MG contributed to oversight and performance of laboratory studies, (Figure 1, Table 1, and Table 2). DB contributed to research design, data analysis, interpretation of results and manuscript preparation (Figure 1, Table 2). SP performed laboratory studies (Supplemental Figure 1, Table 2). KH and BP provided technical guidance with all aspects of OpenArray Genotyping system. AS performed all statistical analyses. BC provided oversight to NAIT diagnostic studies and provided diagnostic data. JM provided oversight to NAIT diagnosis and communicated with referring physicians. RHA contributed to research design, oversight of laboratory studies, interpretation of results and manuscript preparation.
References
- 1.Arnold DM, Smith JW, Kelton JG. Diagnosis and management of neonatal alloimmune thrombocytopenia. Transfus Med Rev. 2008;22:255–67. doi: 10.1016/j.tmrv.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Sachs UJ. Fetal/neonatal alloimmune thrombocytopenia. Thromb Res. 2013;131 (Suppl 1):S42–6. doi: 10.1016/S0049-3848(13)70020-3. [DOI] [PubMed] [Google Scholar]
- 3.Risson DC, Davies MW, Williams BA. Review of neonatal alloimmune thrombocytopenia. J Paediatr Child Health. 2012;48:816–22. doi: 10.1111/j.1440-1754.2012.02528.x. [DOI] [PubMed] [Google Scholar]
- 4.Peterson JA, McFarland JG, Curtis BR, Aster RH. Neonatal alloimmune thrombocytopenia: pathogenesis, diagnosis and management. Br J Haematol. 2013;161:3–14. doi: 10.1111/bjh.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreyfus M, Kaplan C, Verdy E, Schlegel N, Durand-Zaleski I, Tchernia G. Frequency of immune thrombocytopenia in newborns: a prospective study. Immune Thrombocytopenia Working Group. Blood. 1997;89:4402–6. [PubMed] [Google Scholar]
- 6.Williamson LM. Screening programmes for foetomaternal alloimmune thrombocytopenia. Vox Sang. 1998;74 (Suppl 2):385–9. doi: 10.1111/j.1423-0410.1998.tb05446.x. [DOI] [PubMed] [Google Scholar]
- 7.Kjeldsen-Kragh J, Killie MK, Tomter G, Golebiowska E, Randen I, Hauge R, Aune B, Oian P, Dahl LB, Pirhonen J, Lindeman R, Husby H, Haugen G, Gronn M, Skogen B, Husebekk A. A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombocytopenia. Blood. 2007;110:833–9. doi: 10.1182/blood-2006-08-040121. [DOI] [PubMed] [Google Scholar]
- 8.Davoren A, Curtis BR, Aster RH, McFarland JG. Human platelet antigen-specific alloantibodies implicated in 1162 cases of neonatal alloimmune thrombocytopenia. Transfusion. 2004;44:1220–5. doi: 10.1111/j.1537-2995.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 9.Ghevaert C, Rankin A, Huiskes E, Porcelijn L, Javela K, Kekomaki R, Bakchoul T, Santoso S, Nutland S, Smyth DJ, Smith GA, McBride S, Watkins NA, Ouwehand WH. Alloantibodies against low-frequency human platelet antigens do not account for a significant proportion of cases of fetomaternal alloimmune thrombocytopenia: evidence from 1054 cases. Transfusion. 2009;49:2084–9. doi: 10.1111/j.1537-2995.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- 10.Mueller-Eckhardt C, Kiefel V, Grubert A, Kroll H, Weisheit M, Schmidt S, Mueller-Eckhardt G, Santoso S. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet. 1989;1:363–6. doi: 10.1016/s0140-6736(89)91733-9. [DOI] [PubMed] [Google Scholar]
- 11.McQuilten ZK, Wood EM, Savoia H, Cole S. A review of pathophysiology and current treatment for neonatal alloimmune thrombocytopenia (NAIT) and introducing the Australian NAIT registry. Aust N Z J Obstet Gynaecol. 2011;51:191–8. doi: 10.1111/j.1479-828X.2010.01270.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghevaert C, Campbell K, Walton J, Smith GA, Allen D, Williamson LM, Ouwehand WH, Ranasinghe E. Management and outcome of 200 cases of fetomaternal alloimmune thrombocytopenia. Transfusion. 2007;47:901–10. doi: 10.1111/j.1537-2995.2007.01208.x. [DOI] [PubMed] [Google Scholar]
- 13.Poles A, Wozniak MJ, Walser P, Ridgwell K, Fitzgerald J, Green A, Gilmore R, Lucas G. A V740L mutation in glycoprotein IIb defines a novel epitope (War) associated with fetomaternal alloimmune thrombocytopenia. Transfusion. 2013 Jan 10; doi: 10.1111/trf.12067. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Santoso S, Kiefel V, Richter IG, Sachs UJ, Rahman A, Carl B, Kroll H. A functional platelet fibrinogen receptor with a deletion in the cysteine-rich repeat region of the beta(3) integrin: the Oe(a) alloantigen in neonatal alloimmune thrombocytopenia. Blood. 2002;99:1205–14. doi: 10.1182/blood.v99.4.1205. [DOI] [PubMed] [Google Scholar]
- 15.Peterson JA, Balthazor SM, Curtis BR, McFarland JG, Aster RH. Maternal alloimmunization against the rare platelet-specific antigen HPA-9b (Max a) is an important cause of neonatal alloimmune thrombocytopenia. Transfusion. 2005;45:1487–95. doi: 10.1111/j.1537-2995.2005.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan C, Porcelijn L, Vanlieferinghen P, Julien E, Bianchi F, Martageix C, Bertrand G, Jallu V. Anti-HPA-9bw (Maxa) fetomaternal alloimmunization, a clinically severe neonatal thrombocytopenia: difficulties in diagnosis and therapy and report on eight families. Transfusion. 2005;45:1799–803. doi: 10.1111/j.1537-2995.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 17.Peterson JA, Gitter ML, Kanack A, Curtis B, McFarland J, Bougie D, Aster R. New low-frequency platelet glycoprotein polymorphisms associated with neonatal alloimmune thrombocytopenia. Transfusion. 2010;50:324–33. doi: 10.1111/j.1537-2995.2009.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis BR, Edwards JT, Hessner MJ, Klein JP, Aster RH. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood. 2000;96:1574–81. [PubMed] [Google Scholar]
- 19.Curtis BR, McFarland JG. Detection and identification of platelet antibodies and antigens in the clinical laboratory. Immunohematology. 2009;25:125–35. [PubMed] [Google Scholar]
- 20.Peterson JA, Pechauer SM, Gitter ML, Kanack A, Curtis BR, Reese J, Kamath VM, McFarland JG, Aster RH. New platelet glycoprotein polymorphisms causing maternal immunization and neonatal alloimmune thrombocytopenia. Transfusion. 2012;52:1117–24. doi: 10.1111/j.1537-2995.2011.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopp K, Weber K, Bellissimo D, Johnson ST, Pietz B. High-throughput red blood cell antigen genotyping using a nanofluidic real-time polymerase chain reaction platform. Transfusion. 2010;50:40–6. doi: 10.1111/j.1537-2995.2009.02377.x. [DOI] [PubMed] [Google Scholar]
- 22.Yeung EH, Zhang C, Chen J, Bowers K, Hu FB, Kang G, Qi L. Polymorphisms in the neuropeptide Y gene and the risk of obesity: findings from two prospective cohorts. J Clin Endocrinol Metab. 2011;96:E2055–62. doi: 10.1210/jc.2011-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan L, Pei B, Li Q. Multicolor real-time polymerase chain reaction genotyping of six human platelet antigens using displacing probes. Transfusion. 2007;47:1637–42. doi: 10.1111/j.1537-2995.2007.01335.x. [DOI] [PubMed] [Google Scholar]
- 24.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 25.Newcombe RG. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med. 1998;17:2635–50. [PubMed] [Google Scholar]
- 26.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–90. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 27.Choi SC, Stablein DM. Practical tests for comparing two proportions with incomplete data. Applied Statistics. 1982;31:256–62. [Google Scholar]
- 28.Lancaster HO. Significance Tests in Discrete Distributions. Journal of the American Statistical Association. 1961;56:223–34. [Google Scholar]
- 29.Kupatawintu P, Nathalang O, ROC, Patmasiriwat P. Gene frequencies of the HPA-1 to 6 and Gov human platelet antigens in Thai blood donors. Immunohematology. 2005;21:5–9. [PubMed] [Google Scholar]
- 30.Peterson JA, Pechauer SM, Gitter ML, Szabo A, Curtis BR, Aster RH. The human platelet antigen-21bw is relatively common among Asians and is a potential trigger for neonatal alloimmune thrombocytopenia. Transfusion. 2012;52:915–6. doi: 10.1111/j.1537-2995.2011.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka S, Taniue A, Nagao N, Tomita T, Ohnoki S, Shibata H, Okubo Y, Yamaguchi H, Shibata Y. Genotype frequencies of the human platelet antigen, Ca/Tu, in Japanese, determined by a PCR-RFLP method. Vox Sang. 1996;70:40–4. doi: 10.1111/j.1423-0410.1996.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 32.Noris P, Simsek S, Bruijne-Admiraal LG, Porcelijn L, Huiskes E, van der Vlist GJ, van Leeuwen EF, van der Schoot CE, dem Borne AE. Max(a), a new low-frequency platelet-specific antigen localized on glycoprotein IIb, is associated with neonatal alloimmune thrombocytopenia. Blood. 1995;86:1019–26. [PubMed] [Google Scholar]
- 33.Feng ML, Liu DZ, Shen W, Wang JL, Guo ZH, Zhang X, Du KM, Qian KC, Zhao TM. Establishment of an HPA-1- to -16-typed platelet donor registry in China. Transfus Med. 2006;16:369–74. doi: 10.1111/j.1365-3148.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- 34.Koh Y, Ishii H, Amakishi E, Hayashi T, Matsuyama N, Fukumori Y, Hirayama F, Shimizu J, Nakauchi S, Kawa K. The first two cases of neonatal alloimmune thrombocytopenia associated with the low-frequency platelet antigen HPA-21bw (Nos) in Japan. Transfusion. 2012;52:1468–75. doi: 10.1111/j.1537-2995.2011.03491.x. [DOI] [PubMed] [Google Scholar]
- 35.Peterson JA, Kanack A, Nayak D, Bougie DW, McFarland JG, Curtis BR, Aster RH. Prevalence and clinical significance of low-avidity HPA-1a antibodies in women exposed to HPA-1a during pregnancy. Transfusion. 2013;53:1309–18. doi: 10.1111/j.1537-2995.2012.03903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakchoul T, Kubiak S, Krautwurst A, Roderfeld M, Siebert HC, Bein G, Sachs UJ, Santoso S. Low-avidity anti-HPA-1a alloantibodies are capable of antigen-positive platelet destruction in the NOD/SCID mouse model of alloimmune thrombocytopenia. Transfusion. 2011;51:2455–61. doi: 10.1111/j.1537-2995.2011.03171.x. [DOI] [PubMed] [Google Scholar]
- 37.Curtis BR, Fick A, Lochowicz AJ, McFarland JG, Ball RH, Peterson J, Aster RH. Neonatal alloimmune thrombocytopenia associated with maternal-fetal incompatibility for blood group B. Transfusion. 2008;48:358–64. doi: 10.1111/j.1537-2995.2007.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtis BR, Ali S, Glazier AM, Ebert DD, Aitman TJ, Aster RH. Isoimmunization against CD36 (glycoprotein IV): description of four cases of neonatal isoimmune thrombocytopenia and brief review of the literature. Transfusion. 2002;42:1173–9. doi: 10.1046/j.1537-2995.2002.00176.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Typical HPA-21 (A) and HPA-12 (B) results shown for 50 fathers of unresolved NAIT cases. Signal intensities of Cy5 (HPA-21bw or HPA-12bw) versus FAM (HPA-21a or HPA-12bw) fluorophores are plotted. No template controls are denoted by grey diamonds. Homozygous and heterozygous samples are denoted with black diamonds and triangles respectively. Unknowns that tested positive in the assay are denoted with grey circles. Each unknown that typed at HPA-21a/bw or HPA-12a/bw was verified with sequence analysis.