Abstract
Obesity is a risk factor for various cardiovascular diseases including hypertension, atherosclerosis, and myocardial infarction. Recent studies aimed at understanding the microenvironment of adipose tissue and its impact on systemic metabolism have shed light on the pathogenesis of obesity-linked cardiovascular diseases. Adipose tissue functions as an endocrine organ by secreting multiple immune-modulatory proteins known as adipokines. Obesity leads to increased expression of pro-inflammatory adipokines and diminished expression of anti-inflammatory adipokines, resulting in the development of a chronic, low-grade inflammatory state. This adipokine imbalance is thought to be a key event in promoting both systemic metabolic dysfunction and cardiovascular disease. This review will focus on the adipose tissue microenvironment and the role of adipokines in modulating systemic inflammatory responses that contribute to cardiovascular disease.
Keywords: Cardiovascular disease, Adiponectin, Sfrp5, Leptin, TNFα
Introduction
Obesity and associated metabolic disorders are becoming major health care concerns around the world. It is estimated that over 60% of adults and 30% of children are overweight in the USA, and if trends continue more than 50% of the world’s adult population will be overweight in a few decades [1–3]. Obesity and its comorbidities have a devastating effect on vascular function and create conditions that favor cardiovascular disease. Obesity promotes cardiovascular disease via many mechanisms including ectopic lipid deposition, hyperglycemia, and the development of a procoagulant state, to name a few. This review will focus on how obesity influences the production in the adipose tissue of pro- and anti-inflammatory cytokines, referred to as adipokines, which contribute to the development of metabolic and cardiovascular diseases.
Obesity-induced changes in adipose tissue microenvironment
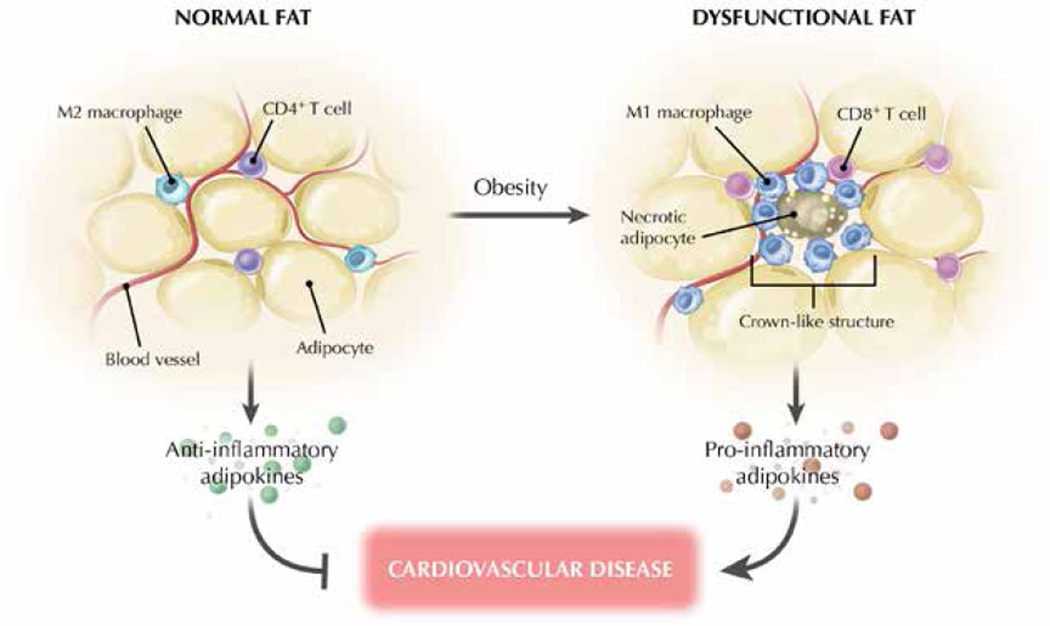
To understand how obesity has an impact on cardiovascular function, it is important to first focus on obesity-induced changes in the microenvironment of adipose tissue (Fig. 1). The excess of caloric intake leads to an expansion of the adipose tissue that is initially driven by an increase in the number of adipocytes (adipocyte hyperplasia) mediated by the recruitment and proliferation of adipogenic progenitors [4–7]. This hyperplastic response is severely blunted with age [8], so the sustained exposure to excessive energy intake ultimately leads to an increase in adipocyte size (adipocyte hypertrophy) that compromises the functionality of the adipose tissue [6, 9]. In advanced obesity, lipid-laden hypertrophied adipocytes undergo necrotic and/or apoptotic cell death, contributing to the recruitment of inflammatory cells and to adipose tissue dysfunction [10–12].
Figure 1. Obesity-linked changes in adipose tissue composition.
Obesity can promote changes in adipose tissue and promote the transition to a metabolically dysfunctional phenotype. As the body develops obesity, adipocytes undergo hypertrophy due to the increased storage of triglycerides. Macrophages in lean fat express markers of a M2 or “alternatively activated” state, whereas obesity leads to recruitment and accumulation of a M1 or “classically activated” state with macrophages and CD8+ T cells in adipose tissue. Metabolically dysfunctional adipose tissue is indicated by the presence of crown-like histological structures that represent activated M1-like macrophages surrounding a necrotic adipocyte and CD4+ T cells. Anti-inflammatory adipokines, such as adiponectin are preferentially produced by lean adipose tissue, whereas high levels of pro-inflammatory factors are produced in obese states.
Whereas adipose tissue is mainly composed of adipocytes, other cell types, including lymphocytes, macrophages, fibroblasts, and vascular cells, also appear to have important roles in controlling the functional status of this tissue. Obesity leads to major changes in the cellular composition of adipose tissue and also modulates the phenotype of individual cells within this tissue. For example, adipose tissue from obese organisms is infiltrated by a large number of macrophages, leading to increases in both absolute macrophage number and the relative level of macrophage-to-adipocyte ratio. Macrophage recruitment to adipose tissue is associated with systemic inflammation and insulin resistance [13, 14]. In addition to this quantitative change, the macrophage phenotype is also altered by the obese state. The M1/M2 concept is a convenient means for classifying the inflammatory status of the macrophage. Macrophages that accumulate in adipose tissue of obese organisms tend to express genes associated with a M1-like or “classically activated” phenotype. In contrast, adipose tissue macrophages from lean organisms tend to express genes associated with a M2-like or “alternatively activated” phenotype [15]. Stimulation with T helper 1 (TH1)-type cytokines, including interferon-γ, or bacterial products will promote the M1-like phenotype in macrophages. M1 macrophages produce pro-inflammatory cytokines, such as tumor necrosis factor (TNF)α, express inducible nitric oxide synthase (iNOS), and produce high levels of reactive oxygen and nitrogen intermediates [16]. This class of macrophages is typically associated with inflammation and tissue destruction. On the other hand, M2-like macrophages preferentially express anti-inflammatory cytokines, such as interleukin (IL)-10, and the enzyme arginase-1, which inhibits iNOS activity. These types of macrophages tend to be associated with wound healing, angiogenesis, and the resolution of inflammation [16]. It is believed that M1-like macrophages promote insulin resistance, whereas M2-like macrophages protect against obesity-induced insulin resistance [17]. Supporting this notion, ablation of CD11c-positive, M1-like macrophages normalizes insulin sensitivity in obese mice [18].
Another distinctive feature of adipose tissue from obese organisms is the presence of “crown-like” structures in histological sections. These features represent macrophages that surround dead or dying adipocytes [10, 11]. Obese subjects lacking crown-like structures exhibit better metabolic control, diminished inflammatory gene expression, and reduced cardiovascular risk than body mass-matched individuals who display this histological feature [19]. On the other hand, the number of crown-like structures in adipose tissue is correlated with inflammation and insulin resistance in metabolic syndrome patients [10, 19]. Typically macrophages function to rapidly remove dead cell debris prior to the disruption of their membranes. Thus, it is tempting to speculate that the presence of crown-like structures signifies a breakdown in the phagocytic process in adipose tissue, thereby exacerbating the pro-inflammatory state.
Obesity also influences the subsets of T cells that are present in adipose tissue, where they appear to function in the regulation of macrophage phenotype. CD4+ regulatory T cells and TH2-polarized cells are found in higher abundance in the adipose tissue of lean mice, and these cells contribute to the maintenance of adipose tissue function and insulin sensitivity, in part through promoting an anti-inflammatory alternative activation of macrophages [20, 21]. On the other hand, under conditions of obesity, the accumulation of CD8+ effector T cells and CD4+ TH1 cells in the adipose tissue will generate TH1 signals and initiate the recruitment and activation of macrophages, perpetuating the pro-inflammatory cascade that is associated with insulin resistance [21, 22]. Thus, obesity-induced alterations in the balance of TH1- and TH2-type signals are likely to influence macrophage recruitment and phenotype in adipose tissue, thereby generating either a pathogenic or a protective environment. B cells also appear to have a pivotal role in obesity-induced adipose tissue inflammation, promoting T cell and macrophage activation and contributing to insulin resistance [23]. Similarly, mast cells have been reported to accumulate in obese adipose tissue before the appearance of macrophages, and studies in mast-cell deficient mice suggest a role for this cell-type in obesity-associated metabolic dysfunction [24]. Neutrophil infiltration and activation also contributes to adipose tissue dysfunction through the action of neutrophil elastase [25, 26]. Finally, it has been reported that eosinophils in adipose tissue promote alternative activation of macrophages and glucose tolerance through the production of the TH2 cytokine IL-4 [27]. Therefore, the status of immune cells in adipose tissue is an area of intense investigation, and a better understanding of the interplay between inflammation and metabolism is warranted.
Of particular relevance to the subject of this review, a number of provocative studies have made the case that obesity is also associated with changes in vascular structure and function within adipose tissue. Several studies in patients and animal models have shown that obesity causes capillary rarefaction in adipose tissue, and this leads to localized hypoxia [28–31]. In contrast with these studies, a recent report showed that obese human subjects display increased rather than decreased oxygen tension in adipose tissue, despite reduced adipose tissue blood flow [32]. However, it must be noted that, while suggesting an overall hyperoxic state, this latter study does not rule out the possibility of localized hypoxic areas within adipose tissue, and therefore further studies are required in this field. Regardless, the various studies coincide in their findings of decreased adipose tissue capillarization, which may limit nutrient delivery and contribute to insulin resistance. Alternatively, this vascular dysfunction could contribute to adipose tissue inflammation by promoting ischemia-induced adipocyte necrosis, followed by macrophage recruitment and activation. Recent studies with genetically engineered mice have provided conclusive evidence of the role of adipose tissue vascularization in obesity-associated metabolic dysfunction. Experiments with mice overexpressing vascular endothelial growth factor A (VEGF-A) in adipocytes show that increased VEGF-mediated angiogenesis in white adipose tissue can attenuate some of the metabolic effects of diet-induced obesity, such as insulin resistance and hepatic steatosis [33–35]. Conversely, adipocyte-restricted deletion of VEGF-A results in scarce adipose tissue vascularization, which leads to increased adipose tissue inflammation and systemic insulin resistance and glucose intolerance, as well as hepatic steatosis [35]. Furthermore, it has been reported that hypoxia-inducible factor 1α (HIF1α) – a master regulator of VEGF expression – is upregulated during obesity-associated adipose tissue expansion [36]. Interestingly, various experiments in genetically engineered mice suggest that HIF1α does not influence adipose tissue capillarization, but promotes adipose tissue expansion, inflammation, and fibrosis, leading to systemic metabolic dysfunction [37–40].
The phenotypic changes in adipose tissue that result from obesity are also thought to induce vascular endothelial cell activation. Because activated endothelial cells express adhesion molecules and chemotactic factors that contribute to the recruitment of inflammatory cells, it is reasonable to speculate that obesity promotes a vicious cycle of endothelial activation and tissue inflammation that contributes to adipose tissue dysfunction. Supporting this notion, it has been shown that endothelial cells within the adipose tissue of obese mice express higher levels of adhesion molecules such as P-selectin, E-selectin, and intercellular adhesion molecule 1 (ICAM-1). Moreover, administration of anti-ICAM-1 antibody to obese mice prevents leukocyte-endothelium interactions and macrophage infiltration into adipose tissue [41]. In addition, adipose tissue inflammation may also promote a state of systemic endothelial cell activation through the endocrine actions of inflammatory adipokines such as TNFα (see below), which could contribute to obesity-associated cardiovascular disease.
The adipokine concept
Accumulating evidence from animal models indicates that the pathogenesis of obesity-related metabolic dysfunction involves the development of a systemic, low-grade inflammatory state [42, 43]. Epidemiological studies have also demonstrated a connection between metabolic disease and a low-grade inflammation. For example, increasing adipose mass in obese women is associated with increased serum levels of the pro-inflammatory marker C-reactive protein (CRP) [44]. Furthermore, high levels of CRP and IL-6, are predictive of the development of type 2 diabetes [44, 45]. In contrast, weight loss leads to reductions in circulating levels of CRP and IL-6 [46]. These findings strongly suggest that obesity is highly associated with chronic low-grade inflammation, and it is believed that this obesity-linked inflammatory state is due to changes in the expression of cytokines by adipose tissue. Over the past two decades, it has become apparent that adipose tissue is a source of secreted immunomodulatory proteins, and that these adipokines act as modulators of metabolic and cardiovascular processes. While adipose tissue is mainly found in visceral and subcutaneous depots, it is also widely dispersed throughout the body. Other depot sites that may be particularly relevant in influencing cardiovascular disease include epicardial, perivascular, and pulmonary adipose tissue. Whilst adipose tissue depots differ from one another based upon their relative levels of adipokine production, obesity will generally favor the production of pro-inflammatory adipokines regardless of depot site location [47–49]. Interestingly, studies in mice suggest that aging in the absence of diet-induced obesity also induces the expression of proinflammatory adipokines, such as TNFα and IL-6 in visceral adipose tissue [50, 51].
Most adipokines identified to date are pro-inflammatory and they are upregulated in the obese state. Under conditions of obesity, these adipokines function to promote metabolic and cardiovascular diseases. Pro-inflammatory adipokines include TNFα leptin, IL-6, resistin, RBP4, lipocalin 2, IL-18, ANGPTL2, and others. Here, as examples, we will only discuss TNFα and leptin, but a partial list of other pro-inflammatory adipokines and their cardiovascular actions can be found in Table 1 and Ref. [52]. In addition to the numerous pro-inflammatory adipokines, adipose tissue also secretes a smaller number of anti-inflammatory factors. These factors include adiponectin, that has been the subject of intense investigation [53, 54], and SFRP5, that has been recently identified as an adipokine with anti-inflammatory activities [55].
Table 1.
Adipokines and Cardiovascular Function.
| Adipokine | Inflammation | Cardiovascular function(s) | Reference |
|---|---|---|---|
| Leptin | Pro-inflammatory | See text | |
| Resistin | Pro-inflammatory | Pro-atherogenic, subclinical marker of atherosclerosis, marker of heart failure, predictive of MI and stroke risk |
[168–176] |
| RBP4 | Pro-inflammatory | subclinical marker of atherosclerosis | [177–181] |
| Lipocalin2 | Pro-inflammatory | Unknown | [182,183] |
| ANGPTL2 | Pro-inflammatory | Unknown | [184] |
| TNF | Pro-inflammatory | See text | |
| IL-6 | Pro-inflammatory | Pro-atherogenic, predictive of MI risk | [185–187] |
| Adiponectin | Anti-inflammatory | See text | |
| SFRP5 | Anti-inflammatory | See text | |
| adipolin | Anti-inflammatory | Unknown | [188] |
| Omentin | Anti-inflammatory | Pro-angiogenic, inhibition of vascular inflammation, subclinical marker of atherosclerosis |
[189,190] |
RBP4, retinol-binding protein4; ANGPT2, angiopoietin-like protein2; TNF, tumor necrosis factor; IL, interleukin; MI, myocardial infarction.
In this regard, it is difficult to discern whether the increase in cardiovascular disease associated with an adipokine imbalance is due to a paracrine mechanism, i.e. the local release of pro-inflammatory factors from epicardial or perivascular adipose tissue, or an endocrine mechanism that is reflected by an increase in serum adipokine levels. One study that shed light on this question performed experiments involving perivascular adipose tissue transplantation between lean and obese mice [56]. This study provided evidence that the perivascular location of adipose tissue is critical in its ability to promote pathological vascular remodeling and that these effects could be attributed to alterations in the levels of adiponectin, an adipokine that will be discussed later.
Pro-inflammatory adipokine TNFα
TNFα is a pro-inflammatory cytokine that is predominantly produced by monocytes/macrophages and is involved in many inflammatory diseases. In 1993, TNFα was found to be induced in adipose tissue in models of diabetes and obesity, providing the initial evidence for a link between inflammation and obesity [57]. TNFα expression is upregulated in the adipose tissue and the serum of patients with obesity, whereas weight loss in obese individuals is associated with a decrease in TNFα levels [58, 59]. TNFα levels also correlate with insulin resistance [60]. Mechanistically, TNFα contributes to insulin resistance by inhibiting the activating phosphorylation of the insulin receptor and insulin receptor substrate 1 (IRS1) in muscle and adipose tissues [61]. In animal models of obesity, the inhibition of TNFα leads to an enhancement of insulin signaling in muscle and adipose tissue [57, 61, 62].
TNFα has numerous pathophysiological roles in the cardiovascular system. TNFα levels are increased by acute and chronic ischemic injuries and under conditions of heart failure in humans as well as animals [63]. As with obesity, aging is associated with a chronic low-grade increase in TNFα levels [50, 64]. TNFα acts on monocytes/macrophages, vascular endothelial cells, and smooth muscle cells to induce the expression of many pro-inflammatory, pro-coagulant, and proliferative genes, contributing to atherosclerosis development in animal models [63, 65–69]. In vascular smooth muscle cells, TNFα induces migration, proliferation, and apoptosis [70–72], which are cellular processes of major relevance in vascular pathologies. TNFα also induces the rapid expression of adhesion molecules such as E-selectin, vascular cell adhesion molecule-1, and ICAM-1 in endothelial cells [63, 73].
TNFα can have deleterious or beneficial effects on the heart in models of ischemia-reperfusion injury and heart failure depending on the amount of TNFα and the duration of exposure [63, 74]. Studies aimed at investigating the effects of TNFα neutralization for the treatment of cardiac disease have led to inconsistent results in experimental models and clinical trials. Some, but not all, animal studies have demonstrated that TNFα neutralization or blockade of TNFα receptor (TNFR) can attenuate myocardial damage in animal models of ischemia/reperfusion and heart failure [63]. In contrast, most clinical studies have failed to document the efficacy of TNFα antagonists [63]. The potential reasons for these discrepant findings are manifold. Contributing to this complexity, TNFα exerts its effects via two different receptors, TNFR1 and TNFR2, which exhibit different patterns of expression and induce distinct signaling pathways and cellular processes. TNFR1 is constitutively expressed in most cell types except erythrocytes, whereas expression of TNFR2 is highly regulated and is typically higher in immune cells [75, 76]. Both the pro-inflammatory and the proapoptotic actions of TNFα are largely mediated through TNFR1. In contrast, TNFR2 mediates the activation of the protective JAK/STAT3 pathway and is believed to promote tissue repair and angiogenesis [63, 76].
Role of leptin in metabolic and cardiovascular disease
The adipokine leptin is the product of the obese gene (ob), that was identified in ob/ob mice through positional cloning [77]. Leptin regulates feeding behavior, and mice that lack leptin show hyperphagia, obesity, and insulin resistance. The delivery of leptin to ob/ob mice reverses these phenotypes [78]. Notably, leptin has been shown to be effective at improving metabolic dysfunction in patients with lipodystrophy or congenital leptin deficiency [79, 80]. However, circulating leptin levels typically correlate with adipose tissue mass, and obese humans and rodents have elevated levels of leptin without the expected anorexic responses [78], suggesting that leptin resistance is common in obesity. A number of mechanisms may contribute to leptin resistance. The anorexic effects of leptin result from its actions in the hypothalamus, which are mediated by its binding to the leptin receptor b (LRb) and the activation of JAK2/STAT3 signaling. In obesity, this signaling pathway is blocked through different mechanisms, leading to leptin resistance. One of the major cellular mechanisms contributing to this phenomenon is the STAT3-mediated induction of the inhibitory suppressor of cytokine signaling 3 (SOCS3) protein, which binds the phosphorylated Tyr985 residue in LRb, impairing leptin-induced signaling [81, 82]. Conclusive evidence from animal studies supports a primary role for SOCS3 in leptin resistance [83, 84]. Additional mechanisms may include LRb binding to the tyrosine phosphatase SHP-2, and activation of the tyrosine phosphatase PTP1B, which dephosphorylates Jak2 and thus diminishes LRb signaling (reviewed in [85]). In addition, it has been suggested that obesity-induced endoplasmic reticulum stress in the hypothalamus also plays a central role in leptin resistance [86].
Leptin is structurally similar to the helical cytokine family that includes IL-2. Inflammatory stimuli increase leptin levels in adipose tissue and in serum [87] and leptin acts on multiple types of immune cells, such as monocytes/macrophages, neutrophils, and T cells, to promote the release of inflammatory cytokines [88–91]. In T cells, leptin specifically increases the production of TH1-type cytokines, and suppresses production of the TH2-type cytokine IL-4 [88], thereby polarizing T cells toward a TH1 cell phenotype. Consistent with these observations, leptin deficiency protects against liver damage in models of T cell-mediated hepatitis and autoimmune encephalomyelitis [88, 92, 93]. Thus, it is generally accepted that leptin acts as a pro-inflammatory adipokine. Numerous studies have indicated that leptin plays an important role in cardiovascular diseases. In humans, circulating leptin levels are increased after myocardial infarction [94]. Similarly, leptin levels are also increased in heart failure patients independent of body mass [95], and mechanical unloading reverses this increase [96]. Leptin-deficient mice display greater cardiac hypertrophy [97], increased mortality in a model of viral myocarditis [98], and greater cardiac remodeling in response to chronic ischemic injury [99]. Leptin receptor deficiency also leads to a reduction in ischemia-induced revascularization that is associated with the impaired induction of angiogenesis-regulatory factors in the ischemic tissue [100]. In many of these cases, it has been shown that leptin repletion can reverse the deleterious effects of leptin deficiency. However, it is difficult to ascertain whether leptin depletion or repletion has a direct impact on these changes in cardiovascular function because these manipulations also affect feeding behavior, adipose tissue mass, and numerous systemic metabolic properties. Whereas leptin can activate protective JAK-STAT and AMPK signaling pathways in cardiovascular tissues, the interpretation of experimental studies on leptin that employ ob/ob mice or diet-induced obese mice are difficult to interpret due to secondary metabolic defects that result from hyperphagia (e.g. hyperglycemia, hyperinsulinemia, insulin resistance) or the development of leptin resistance, respectively. Therefore, studies of cardiovascular disease in mice where the leptin receptor is conditionally ablated in specific cardiac, vascular, or immune cell types may be required to definitely address these issues.
The anti-inflammatory adipokine adiponectin
Adiponectin (also known as ACRP30, AdipoQ, and apM1) was identified as an adipocyte-specific adipokine approximately 15 years ago [101–103]. Adiponectin typically circulates at high levels (3 to 30 µg/ml) in the blood [104], and is markedly downregulated in obese human subjects. Furthermore, plasma adiponectin level is a good predictor of the risk of type 2 diabetes [105, 106]. Adiponectin is synthesized at highest levels by functional adipocytes found in lean organisms but its expression is downregulated in the dysfunctional adipocytes that are associated with obesity. Consistent with this notion, adiponectin expression by adipocytes is inhibited by pro-inflammatory cytokines, hypoxia, and oxidative stress [53, 105, 106], conditions associated with the adipose tissue milieu of obese organisms.
Evidence from experimental models indicates that adiponectin protects against obesity-linked metabolic disease. The acute administration of adiponectin leads to an improvement in metabolic parameters in a mouse model of obesity [107]. Conversely, adiponectin-deficient mice develop greater insulin resistance when placed on a high calorie diet [108, 109], whereas the transgenic overexpression of adiponectin in ob/ob mice improves metabolic parameters independently of weight loss [110]. The beneficial effects of adiponectin on insulin sensitivity appear to be mediated in part by its ability to activate AMPK signaling axis in metabolically important tissues [111, 112]. AMPK activation by adiponectin is thought to involve its interaction with the cell surface receptors adiponectin receptor 1 and adiponectin receptor 2 (AdipoR1 and AdipoR2, respectively) [113–115]. In addition, studies suggest that the actions of adiponectin may also be mediated through additional receptors including PAQR3 (renamed AdipoR3) [116], AdipoRX [117], and T-cadherin [118].
Many lines of evidence show that adiponectin has anti-inflammatory functions. Plasma adiponectin levels are negatively correlated with patient CRP levels [104], and adiponectin-deficient mice have higher levels of TNFα in adipose tissue and blood [108]. Despite morbid obesity, transgenic overexpression of adiponectin in ob/ob mice leads to marked improvement in glucose metabolism, which is accompanied by reductions in macrophage infiltration and TNFα expression in adipose tissue [110]. Adiponectin-mediated modulation of macrophage phenotype contributes to its role in controlling inflammation. Macrophages isolated from adiponectin-deficient mice show increased expression of pro-inflammatory M1 markers and decreased expression of anti-inflammatory M2 markers [119]. Conversely, the overexpression of adiponectin in mice stimulates macrophage M2 marker expression. Adiponectin inhibits the transformation of macrophages into foam cells by reducing intracellular cholesteryl ester content [120]. Adiponectin also inhibits Toll-like receptor-mediated nuclear factor- κB activation and stimulates production of the anti-inflammatory cytokine IL-10 by macrophages [121, 122]. Given the importance of TNFα expression and macrophage infiltration in adipose tissue dysfunction [13, 14, 57], the ability of adiponectin to suppress these pro-inflammatory events may be an important feature in its ability to prevent obesity-associated metabolic dysfunction.
Adiponectin is structurally similar to the collectin family of proteins that include complement factor C1q and surfactant proteins A and D. Similar to these abundantly expressed collectin proteins, adiponectin can form high molecular mass oligomers through its ability to multimerize via interactions through its collagen-like domain [53, 105]. A property shared by many collectin proteins is their ability to bind to apoptotic cells and facilitate their uptake by macrophages, a feature that is also exhibited by adiponectin [123]. Macrophages in adiponectin-deficient mice display a reduced ability to clear apoptotic debris when challenged by an overload of dead cells. Because phagocytosis of early apoptotic cells will promote an M2-like phenotype in macrophages [124], these data suggest adiponectin’s collectin-like function contributes to its anti-inflammatory actions. Finally, it should be noted that adiponectin levels are elevated in autoimmune and chronic inflammatory diseases [125]. While the molecular basis for adiponectin upregulation under these conditions is unknown, it may represent a compensatory response because adiponectin has been shown to ameliorate auto-immunity in a mouse model of lupus [126, 127].
Cardiovascular effects of adiponectin
Low serum levels of adiponectin have been associated with coronary artery disease [128], hypertension [129], left ventricular hypertrophy [130], and a greater risk of myocardial infarction [131]. Numerous experimental studies have shown that adiponectin exerts protective actions on cardiovascular cell types including vascular endothelial cells, smooth muscle cells, and cardiac myocytes, and adiponectin-deficient mice display worse outcomes in various models of cardiovascular disease. Importantly, mouse models of adiponectin-deficiency display normal body and fat mass and normal metabolic parameters when fed a normal chow diet. Thus, the cardiovascular actions of adiponectin can be studied independently of its metabolic regulatory effects.
Adiponectin has been shown to exert many vasculoprotective and angiogenic properties. Studies with adiponectin-deficient mice have demonstrated that adiponectin promotes revascularization of ischemic limbs [132, 133], and protects against cerebral ischemia-reperfusion [134]. Furthermore, adiponectin inhibits experimental injury-induced neointimal hyperplasia [135]. Adiponectin-deficient mice also develop enhanced salt-induced hypertension due in part to a reduction of endothelial nitric oxide synthase (eNOS) activity [136], and display impaired endothelial cell-dependent vasodilatory responses when fed an atherogenic diet [137]. Finally, some studies, but not all, have shown that adiponectin overexpression inhibits atherosclerotic lesion formation, whereas adiponectin-deficiency leads to augmented atherosclerosis [138–141]. Mechanistically, many of these protective actions of adiponectin are linked to its beneficial effects on endothelial cell function, which are mediated to a great extent by its ability to stimulate NO production through AMPK-dependent activation of eNOS [142– 144]. In addition, adiponectin induces the expression of PGI2 – an autacoid that promotes vascular function [145] – and prevents TNFα-induced endothelial cell activation [146, 147]. Furthermore, adiponectin has been shown to inhibit proliferation and migration of vascular smooth muscle cells through direct inhibition of PDGF-BB [148], and to promote the differentiation of vascular smooth muscle cells via repression of mammalian target of rapamycin complex 1 (mTORC1) and FoxO4 [149].
Various studies have evaluated adiponectin’s actions on the heart. Adiponectin inhibits pressure overload or angiotensin II-induced cardiac hypertrophy, at least in part, through its ability to activate AMPK signaling in myocytes [132, 150]. Adiponectin protects the heart from ischemic reperfusion injury [151, 152], and it has been shown to be protective in models of systolic and diastolic heart failure [153, 154].
While AdipoR1 and AdipoR2 are widely accepted as the main receptors accountable for the metabolic actions of adiponectin [113, 114], few studies have investigated adiponectin receptors in cardiovascular tissues. In this regard, T-cadherin, a GPI-anchored adiponectin-binding protein [118], was recently shown to be essential for the cardioprotective and pro-revascularization actions of adiponectin [155, 156]. T-cadherin is highly expressed in the plasma membrane of heart, skeletal muscle, and vascular tissue [157], where it co-localizes with adiponectin [155, 156]. Of note, T-cadherin-deficient mice phenocopy adiponectin-deficient mice in experimental models of chronic and acute cardiac injury [155] and chronic limb ischemia [156], strongly supporting the role of T-cadherin in mediating the effects of adiponectin in the cardiovascular system. While T-cadherin lacks an intracellular domain and thus is unlikely to have a direct effect on intracellular signaling, it has been proposed to be essential for the recruitment of adiponectin to cardiovascular tissues. Supporting this notion, adiponectin is absent from the heart, vascular endothelium, and skeletal muscle in T-cadherin-deficient mice [155, 156, 158]. In addition, T-cadherin-deficient mice exhibit significantly elevated serum levels of adiponectin, further supporting the impaired recruitment of adiponectin to cardiovascular tissues in these mice. Conversely, adiponectin-deficient mice have reduced tissue expression of T-cadherin suggesting a regulatory axis between these proteins [155, 156, 158].
Sfrp5
A recent study has identified Sfrp5, a soluble modulator of Wnt proteins, as an adipokine with anti-inflammatory properties that protects against metabolic dysfunction [55]. Sfrp5 is expressed at highest levels in white adipose tissue of mice. Its expression in adipose tissue is down-regulated in various models of rodent obesity and in the visceral adipose tissue of metabolically dysfunctional obese patients that display crownlike structures. Sfrp5 functions to antagonize noncanonical Wnt5a signaling. Wnt5a is upregulated in fat depots of obese rodents and, as discussed below, it functions to promote inflammatory responses involving macrophages.
Sfrp5-deficient mice are metabolically normal when maintained on a regular diet, but display impaired glucose metabolism and increased fatty liver disease when fed a high caloric diet [55]. The enhanced metabolic dysfunction caused by Sfrp5-deficiency is associated with an increased adipose tissue accumulation of macrophages and increased production of pro-inflammatory cytokines. Mechanistically, Sfrp5 functions to suppress Wnt5a–mediated activation of JUN N-terminal kinase 1 (JNK1) in adipose tissue, and deletion of JNK1 in Sfrp5-deficient mice reverses the metabolic and inflammatory phenotypes. Thus, the increases in obesity-induced adipose tissue inflammation and metabolic dysfunction caused by overactivation of JNK1 signaling in Sfrp5-deficient mice, consistent with the well-described role for JNK1 in regulating insulin resistance and fat inflammation [159–162].
The role of the Sfrp5/Wnt5a regulatory system in cardiovascular disease is relatively unexplored, but a number of recent studies suggest that this may be an interesting area of investigation. It has been shown that Wnt5a functions as a macrophage effector molecule that promotes inflammation in response to microbial infection via the activation of noncanonical Wnt signaling [163, 164]. With regard to cardiovascular actions, Wnt5a promotes inflammation in endothelial cells [165], and noncanonical Wnt5a signaling has been shown to suppress angiogenesis through the upregulation of Flt1, an endogenous VEGF inhibitor produced by myeloid cells [166]. Finally, Wnt5a expression has been detected in mouse and human atherosclerotic lesions [165, 167]. Thus, it is tempting to speculate that Sfrp5 deficiency, as occurs in obesity, leads to runaway Wnt5a signaling favoring a chronic inflammatory state that promotes cardiovascular disease. Further studies are required to definitively address the role of Sfrp5/Wnt5a signaling in regulation of obesity-linked inflammatory cardiovascular disorders.
Conclusions
Adipose tissues produce various adipokines that function to regulate the microenvironment of adipose tissue and communicate with the brain, heart, vasculature, liver, and muscle. These adipokines have either pro-inflammatory or anti-inflammatory activities, and their balance is critical in maintaining systemic homeostasis. Obesity-induced adipose tissue dysfunction leads to dysregulated adipokine production that has both local and systemic effects on inflammatory cells. This adipokine imbalance leads to the development of a low-grade, chronic inflammatory state that contributes to the development of metabolic and cardiovascular diseases.
Acknowledgments
Funding sources
Funded by the U.S. National Institutes of Health grants (AG34972, HL86785, AG15052, and HL81587) to KW. JJF was supported by a Postgraduate Studies Grant sponsored by the Ramon Areces Foundation.
Footnotes
Disclosures
None.
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5:299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 5.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 6.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faust IM, Johnson PR, Stern JS, Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978;235:E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- 8.Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp Gerontol. 2007;42:463–471. doi: 10.1016/j.exger.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm Metab Res Suppl. 1988;19:26–29. [PubMed] [Google Scholar]
- 10.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. (2nd) 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 13.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 17.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c–positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 23.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansuy-Aubert V, Zhou QL, Xie X, Gong Z, Huang JY, Khan AR, Aubert G, Candelaria K, Thomas S, Shin DJ, Booth S, Baig SM, Bilal A, Hwang D, Zhang H, Lovell-Badge R, Smith SR, Awan FR, Jiang ZY. Imbalance between neutrophil elastase and its inhibitor alpha1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 2013;17:534–548. doi: 10.1016/j.cmet.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 30.Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab. 2010;95:4052–4055. doi: 10.1210/jc.2009-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clement K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 33.Sun K, Asterholm IW, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci U S A. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, Roca C, Ramos D, Pujol A, Riu E, Ruberte J, Bosch F. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012 doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sung HK, Doh KO, Son JE, Park JG, Bae Y, Choi S, Nelson SM, Cowling R, Nagy K, Michael IP, Koh GY, Adamson SL, Pawson T, Nagy A. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013;17:61–72. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Corvera S, Czech MP. Tensions rise and blood flows over dysfunctional fat. Circulation. 2011;124:13–16. doi: 10.1161/CIRCULATIONAHA.111.037804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60:2484–2495. doi: 10.2337/db11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnan J, Danzer C, Simka T, Ukropec J, Walter KM, Kumpf S, Mirtschink P, Ukropcova B, Gasperikova D, Pedrazzini T, Krek W. Dietary obesity-associated Hif1alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev. 2012;26:259–270. doi: 10.1101/gad.180406.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Lam KS, Ye H, Chung SK, Zhou M, Wang Y, Xu A. Adipose tissue-specific inhibition of hypoxia-inducible factor 1{alpha} induces obesity and glucose intolerance by impeding energy expenditure in mice. J Biol Chem. 2010;285:32869–32877. doi: 10.1074/jbc.M110.135509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 43.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 45.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 46.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 47.Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18:884–889. doi: 10.1038/oby.2009.443. [DOI] [PubMed] [Google Scholar]
- 48.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 49.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- 51.Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 2009;64:723–730. doi: 10.1093/gerona/glp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 55.Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–911. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 57.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 58.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D∧Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 60.Hivert MF, Sullivan LM, Fox CS, Nathan DM, D∧Agostino RB, Sr, Wilson PW, Meigs JB. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab. 2008;93:3165–3172. doi: 10.1210/jc.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest. 1994;94:1543–1549. doi: 10.1172/JCI117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 63.Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Bruunsgaard H, Skinhoj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121:255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, Saito K, Sekikawa K, Seishima M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180:11–17. doi: 10.1016/j.atherosclerosis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 66.Bergh N, Ulfhammer E, Glise K, Jern S, Karlsson L. Influence of TNF-alpha and biomechanical stress on endothelial anti- and prothrombotic genes. Biochem Biophys Res Commun. 2009;385:314–318. doi: 10.1016/j.bbrc.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 67.Boesten LS, Zadelaar AS, van Nieuwkoop A, Gijbels MJ, de Winther MP, Havekes LM, van Vlijmen BJ. Tumor necrosis factor-alpha promotes atherosclerotic lesion progression in APOE*3-Leiden transgenic mice. Cardiovasc Res. 2005;66:179–185. doi: 10.1016/j.cardiores.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 68.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 69.Xiao N, Yin M, Zhang L, Qu X, Du H, Sun X, Mao L, Ren G, Zhang C, Geng Y, An L, Pan J. Tumor necrosis factor-alpha deficiency retards early fatty-streak lesion by influencing the expression of inflammatory factors in apoE-null mice. Mol Genet Metab. 2009;96:239–244. doi: 10.1016/j.ymgme.2008.11.166. [DOI] [PubMed] [Google Scholar]
- 70.Boyle JJ, Weissberg PL, Bennett MR. Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler Thromb Vasc Biol. 2003;23:1553–1558. doi: 10.1161/01.ATV.0000086961.44581.B7. [DOI] [PubMed] [Google Scholar]
- 71.Goetze S, Kintscher U, Kaneshiro K, Meehan WP, Collins A, Fleck E, Hsueh WA, Law RE. TNFalpha induces expression of transcription factors c-fos, Egr-1, and Ets-1 in vascular lesions through extracellular signal-regulated kinases 1/2. Atherosclerosis. 2001;159:93–101. doi: 10.1016/s0021-9150(01)00497-x. [DOI] [PubMed] [Google Scholar]
- 72.Goetze S, Xi XP, Kawano Y, Kawano H, Fleck E, Hsueh WA, Law RE. TNF-alpha-induced migration of vascular smooth muscle cells is MAPK dependent. Hypertension. 1999;33:183–189. doi: 10.1161/01.hyp.33.1.183. [DOI] [PubMed] [Google Scholar]
- 73.Slowik MR, De Luca LG, Fiers W, Pober JS. Tumor necrosis factor activates human endothelial cells through the p55 tumor necrosis factor receptor but the p75 receptor contributes to activation at low tumor necrosis factor concentration. Am J Pathol. 1993;143:1724–1730. [PMC free article] [PubMed] [Google Scholar]
- 74.Kleinbongard P, Schulz R, Heusch G. TNFalpha in myocardial ischemia/reperfusion, remodeling and heart failure. Heart Fail Rev. 2011;16:49–69. doi: 10.1007/s10741-010-9180-8. [DOI] [PubMed] [Google Scholar]
- 75.Kadokami T, McTiernan CF, Kubota T, Frye CS, Feldman AM. Sex-related survival differences in murine cardiomyopathy are associated with differences in TNF-receptor expression. J Clin Invest. 2000;106:589–597. doi: 10.1172/JCI9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 78.Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutr Rev. 1998;56:s38–s46. doi: 10.1111/j.1753-4887.1998.tb01685.x. discussion s54–75. [DOI] [PubMed] [Google Scholar]
- 79.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 80.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O∧Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 82.Dunn SL, Bjornholm M, Bates SH, Chen Z, Seifert M, Myers MG., Jr Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and suppressor of cytokine signaling 3. Mol Endocrinol. 2005;19:925–938. doi: 10.1210/me.2004-0353. [DOI] [PubMed] [Google Scholar]
- 83.Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 84.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 85.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 86.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 87.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 89.Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 90.Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S. Leptin enhances CC-chemokine ligand expression in cultured murine macrophage. Biochem Biophys Res Commun. 2009;384:311–315. doi: 10.1016/j.bbrc.2009.04.121. [DOI] [PubMed] [Google Scholar]
- 91.Zarkesh-Esfahani H, Pockley AG, Wu Z, Hellewell PG, Weetman AP, Ross RJ. Leptin indirectly activates human neutrophils via induction of TNF-alpha. J Immunol. 2004;172:1809–1814. doi: 10.4049/jimmunol.172.3.1809. [DOI] [PubMed] [Google Scholar]
- 92.Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, Fantuzzi G. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci U S A. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matarese G, Di Giacomo A, Sanna V, Lord GM, Howard JK, Di Tuoro A, Bloom SR, Lechler RI, Zappacosta S, Fontana S. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 2001;166:5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- 94.Fujimaki S, Kanda T, Fujita K, Tamura J, Kobayashi I. The significance of measuring plasma leptin in acute myocardial infarction. J Int Med Res. 2001;29:108–113. doi: 10.1177/147323000102900207. [DOI] [PubMed] [Google Scholar]
- 95.Schulze PC, Biolo A, Gopal D, Shahzad K, Balog J, Fish M, Siwik D, Colucci WS. Dynamics in insulin resistance and plasma levels of adipokines in patients with acute decompensated and chronic stable heart failure. J Card Fail. 2011;17:1004–1011. doi: 10.1016/j.cardfail.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McGaffin KR, Moravec CS, McTiernan CF. Leptin signaling in the failing and mechanically unloaded human heart. Circ Heart Fail. 2009;2:676–683. doi: 10.1161/CIRCHEARTFAILURE.109.869909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barouch LA, Berkowitz DE, Harrison RW, O∧Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 98.Kanda T, Takahashi T, Kudo S, Takeda T, Tsugawa H, Takekoshi N. Leptin deficiency enhances myocardial necrosis and lethality in a murine model of viral myocarditis. Life Sci. 2004;75:1435–1447. doi: 10.1016/j.lfs.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 99.McGaffin KR, Zou B, McTiernan CF, O∧Donnell CP. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovasc Res. 2009;83:313–324. doi: 10.1093/cvr/cvp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schiekofer S, Galasso G, Sato K, Kraus BJ, Walsh K. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arterioscler Thromb Vasc Biol. 2005;25:1603–1609. doi: 10.1161/01.ATV.0000171994.89106.ca. [DOI] [PubMed] [Google Scholar]
- 101.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 102.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 103.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 104.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 105.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 106.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 107.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 109.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 110.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 113.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 114.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 115.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 116.Garitaonandia I, Smith JL, Kupchak BR, Lyons TJ. Adiponectin identified as an agonist for PAQR3/RKTG using a yeast-based assay system. J Recept Signal Transduct Res. 2009;29:67–73. doi: 10.1080/10799890902729456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 118.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 121.Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, Hanazawa S, Yamashita Y. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6826. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 122.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 123.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 125.Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013;64:1–10. doi: 10.1016/j.cyto.2013.06.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Parker J, Menn-Josephy H, Laskow B, Takemura Y, Aprahamian T. Modulation of lupus phenotype by adiponectin deficiency in autoimmune mouse models. J Clin Immunol. 2011;31:167–173. doi: 10.1007/s10875-010-9486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aprahamian T, Bonegio RG, Richez C, Yasuda K, Chiang LK, Sato K, Walsh K, Rifkin IR. The peroxisome proliferator-activated receptor gamma agonist rosiglitazone ameliorates murine lupus by induction of adiponectin. J Immunol. 2009;182:340–346. doi: 10.4049/jimmunol.182.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 129.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 130.Hong SJ, Park CG, Seo HS, Oh DJ, Ro YM. Associations among plasma adiponectin, hypertension, left ventricular diastolic function and left ventricular mass index. Blood Press. 2004;13:236–242. doi: 10.1080/08037050410021397. [DOI] [PubMed] [Google Scholar]
- 131.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 132.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 133.Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, Ohashi T, Kihara S, Maeda N, Walsh K, Ouchi N, Murohara T. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–1724. doi: 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, Shin HK, Moskowitz MA, Ouchi N. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117:216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- 135.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular. stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 136.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 137.Li R, Wang WQ, Zhang H, Yang X, Fan Q, Christopher TA, Lopez BL, Tao L, Goldstein BJ, Gao F, Ma XL. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am J Physiol Endocrinol Metab. 2007;293:E1703–E1708. doi: 10.1152/ajpendo.00462.2007. [DOI] [PubMed] [Google Scholar]
- 138.Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, Libby P. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res. 2008;102:218–225. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
- 139.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 140.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 141.Nawrocki AR, Hofmann SM, Teupser D, Basford JE, Durand JL, Jelicks LA, Woo CW, Kuriakose G, Factor SM, Tanowitz HB, Hui DY, Tabas I, Scherer PE. Lack of association between adiponectin levels and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2010;30:1159–11565. doi: 10.1161/ATVBAHA.109.195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 144.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa TO, Herschman HR, Kihara S, Walsh K. Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol. 2009;29:3487–3499. doi: 10.1128/MCB.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 147.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 148.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 149.Ding M, Xie Y, Wagner RJ, Jin Y, Carrao AC, Liu LS, Guzman AK, Powell RJ, Hwa J, Rzucidlo EM, Martin KA. Adiponectin induces vascular smooth muscle cell differentiation via repression of mammalian target of rapamycin complex 1 and FoxO4. Arterioscler Thromb Vasc Biol. 2011;31:1403–1410. doi: 10.1161/ATVBAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 151.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 152.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sam F, Duhaney TA, Sato K, Wilson RM, Ohashi K, Sono-Romanelli S, Higuchi A, De Silva DS, Qin F, Walsh K, Ouchi N. Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology. 2010;151:322–331. doi: 10.1210/en.2009-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, Sam F, Ouchi N, Walsh K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–1074. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342–4352. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Parker-Duffen JL, Nakamura K, Silver M, Kikuchi R, Tigges U, Yoshida S, Denzel MS, Ranscht B, Walsh K. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem. 2013;288:24886–24897. doi: 10.1074/jbc.M113.454835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ivanov D, Philippova M, Antropova J, Gubaeva F, Iljinskaya O, Tararak E, Bochkov V, Erne P, Resink T, Tkachuk V. Expression of cell adhesion molecule T-cadherin in the human vasculature. Histochem Cell Biol. 2001;115:231–242. doi: 10.1007/s004180100252. [DOI] [PubMed] [Google Scholar]
- 158.Hebbard LW, Garlatti M, Young LJ, Cardiff RD, Oshima RG, Ranscht B. T-cadherin supports angiogenesis and adiponectin association with the vasculature in a mouse mammary tumor model. Cancer Res. 2008;68:1407–1416. doi: 10.1158/0008-5472.CAN-07-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 161.Vallerie SN, Furuhashi M, Fucho R, Hotamisligil GS. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS One. 2008;3:e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 163.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28:504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 164.Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- 165.Kim J, Kim J, Kim DW, Ha Y, Ihm MH, Kim H, Song K, Lee I. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J Immunol. 2010;185:1274–1282. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 166.Stefater JA, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, Wills-Karp M, Pollard JW, Yamaguchi T, Ferrara N, Gerhardt H, Lang RA. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. (3rd) 2011;474:511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Christman MA, Goetz DJ, Dickerson E, McCall KD, Lewis CJ, Benencia F, Silver MJ, Kohn LD, Malgor R. Wnt5a is expressed in murine and human atherosclerotic lesions. Am J Physiol Heart Circ Physiol. (2nd) 2008;294:H2864–H2870. doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- 168.Burnett MS, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182:241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 169.Kawanami D, Maemura k, Takeda K, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 170.Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, Mickle DA. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 171.Robertson SA, Rae CJ, Graham A. Induction of angiogenesis by murine resistin: putative role of PI3-kinase and NO-dependent pathways. Regul Pept. 2009;152:41–47. doi: 10.1016/j.regpep.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 172.Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, Yao Q, Chen C. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146–157. doi: 10.1016/j.cardiores.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 173.Weikert C, Westphal S, Berger K, Dierkes J, Mohlig M, Spranger J, Rimm EB, Willich SN, Boeing H, Pischon T. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. J Clin Endocrinol Metab. 2008;93:2647–2653. doi: 10.1210/jc.2007-2735. [DOI] [PubMed] [Google Scholar]
- 174.Rajpathak SN, Kaplan RC, Wassertheil-Smoller S, Cushman M, Rohan TE, McGinn AP, Wang T, Strickler HD, Scherer PE, Mackey R, Curb D, Ho GY. Resistin, but not adiponectin and leptin, is associated with the risk of ischemic stroke among postmenopausal women: results from the Women∧s Health Initiative. Stroke. 2011;42:1813–1820. doi: 10.1161/STROKEAHA.110.607853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Frankel DS, Vasan RS, D∧Agostino RB, Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cho Y, et al. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J Am Coll Cardiol. 2011;57:99–109. doi: 10.1016/j.jacc.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 177.Cabre A, Lazaro I, Girona J, Manzanares J, Marimon F, Plana N, Heras M, Masana L. Retinol-binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J Intern Med. 2007;262:496–503. doi: 10.1111/j.1365-2796.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- 178.Ingelsson E, Lind L. Circulating retinol-binding protein 4 and subclinical cardiovascular disease in the elderly. Diabetes Care. 2009;32:733–735. doi: 10.2337/dc08-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ingelsson E, Sundstrom J, Melhus H, Michaelsson K, Berne C, Vasan RS, Riserus U, Blomhoff R, Lind L, Arnlov J. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis. 2009;206:239–244. doi: 10.1016/j.atherosclerosis.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 180.Mohapatra J, Sharma M, Acharya A, Pandya G, Chatterjee A, Balaraman R, Jain MR. Retinol-binding protein 4 : a possible role in cardiovascular complications. Br J Pharmacol. 2011;164:1939–1948. doi: 10.1111/j.1476-5381.2011.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, Aryal P, Kiernan UA, Phillips DA, Maruyama H, Kraus BJ, Usheva A, Davis RJ, Smith U, Kahn BB. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol. 2012;32:2010–2019. doi: 10.1128/MCB.06193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–2540. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 183.Zhang J, Wu Y, Zhang Y, Leroith D, Bernlohr DA, Chen X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol. 2008;22:1416–1426. doi: 10.1210/me.2007-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tabata M, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10:178–188. doi: 10.1016/j.cmet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 185.Elhage R, Clamens S, Besnard S, Mallat Z, Tedgui A, Arnal J, Maret A, Bayard F. Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17beta-estradiol in apolipoprotein E-deficient mice. Atherosclerosis. 2001;156:315–320. doi: 10.1016/s0021-9150(00)00682-1. [DOI] [PubMed] [Google Scholar]
- 186.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 187.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 188.Enomoto T, Ohashi K, Shibata R, Higuchi A, Maruyama S, Izumiya Y, Walsh K, Murohara T, Ouchi N. Adipolin/C1qdc2/CTRP12 protein functions as an adipokine that improves glucose metabolism. J Biol Chem. 2011;286:34552–34558. doi: 10.1074/jbc.M111.277319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–343. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 190.Maruyama S, Shibata R, Kikuchi R, Izumiya Y, Rokutanda T, Araki S, Kataoka Y, Ohashi K, Daida H, Kihara S, Ogawa H, Murohara T, Ouchi N. Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism. J Biol Chem. 2012;287:408–417. doi: 10.1074/jbc.M111.261818. [DOI] [PMC free article] [PubMed] [Google Scholar]