Abstract
Lacosamide is an anticonvulsant hypothesized to enhance slow inactivation of neuronal Na+ channels for its therapeutic action. Cardiac Na+ channels display less and incomplete slow inactivation, but their sensitivity toward lacosamide remains unknown. We therefore investigated the action of lacosamide in human cardiac Nav1.5 and Nav1.5-CW inactivation-deficient Na+ channels. Lacosamide showed little effect on hNav1.5 Na+ currents at 300 µM when cells were held at −140 mV. With 30-second conditioning pulses from −90 to −50 mV; however, hNav1.5 Na+ channels became sensitive to lacosamide with IC50 (50% inhibitory concentration) around 70–80 µM. Higher IC50 values were found at −110 and −30 mV. The development of lacosamide block at −70 mV was slow in wild-type Na+ channels (τ; 8.04 ± 0.39 seconds, n = 8). This time constant was significantly accelerated in hNav1.5-CW inactivation-deficient counterparts. The recovery from lacosamide block at −70 mV for 10 seconds was relatively rapid in wild-type Na+ channels (τ; 639 ± 90 milliseconds, n = 8). This recovery was accelerated further in hNav1.5-CW counterparts. Unexpectedly, lacosamide elicited a time-dependent block of persistent hNav1.5-CW Na+ currents with an IC50 of 242 ± 19 µM (n = 5). Furthermore, both hNav1.5-CW/F1760K mutant and batrachotoxin-activated hNav1.5 Na+ channels became completely lacosamide resistant, indicating that the lacosamide receptor overlaps receptors for local anesthetics and batrachotoxin. Our results together suggest that lacosamide targets the intermediate preopen and open states of hNav1.5 Na+ channels. Lacosamide may thus track closely the conformational changes at the hNav1.5-F1760 region along the activation pathway.
Introduction
Voltage-gated Na+ channels are responsible for the generation of action potentials in excitable membranes. The Na+ channel protein consists of one large α-subunit and one or two smaller auxiliary β-subunits (Catterall, 2012). In mammals, there are nine α-subunit (Nav1.1 to Nav1.9) and four β-subunit (β1–β4) isoforms. The Na+ channel α-subunit contains four homologous domains (D1–D4), each with six transmembrane segments (S1–S6). The α-subunit alone can form a functional Na+ channel in mammalian cells with fast and slow inactivation gating (Cummins and Sigworth, 1996; Wang and Wang, 1996). Because of their functional importance, voltage-gated Na+ channels have been linked to many disorders such as chronic pain, myotonia, arrhythmia, and epilepsy. Not surprisingly, voltage-gated Na+ channels have also been the primary target of various therapeutics, such as local anesthetics (LAs), class 1 antiarrhythmics, and anticonvulsants.
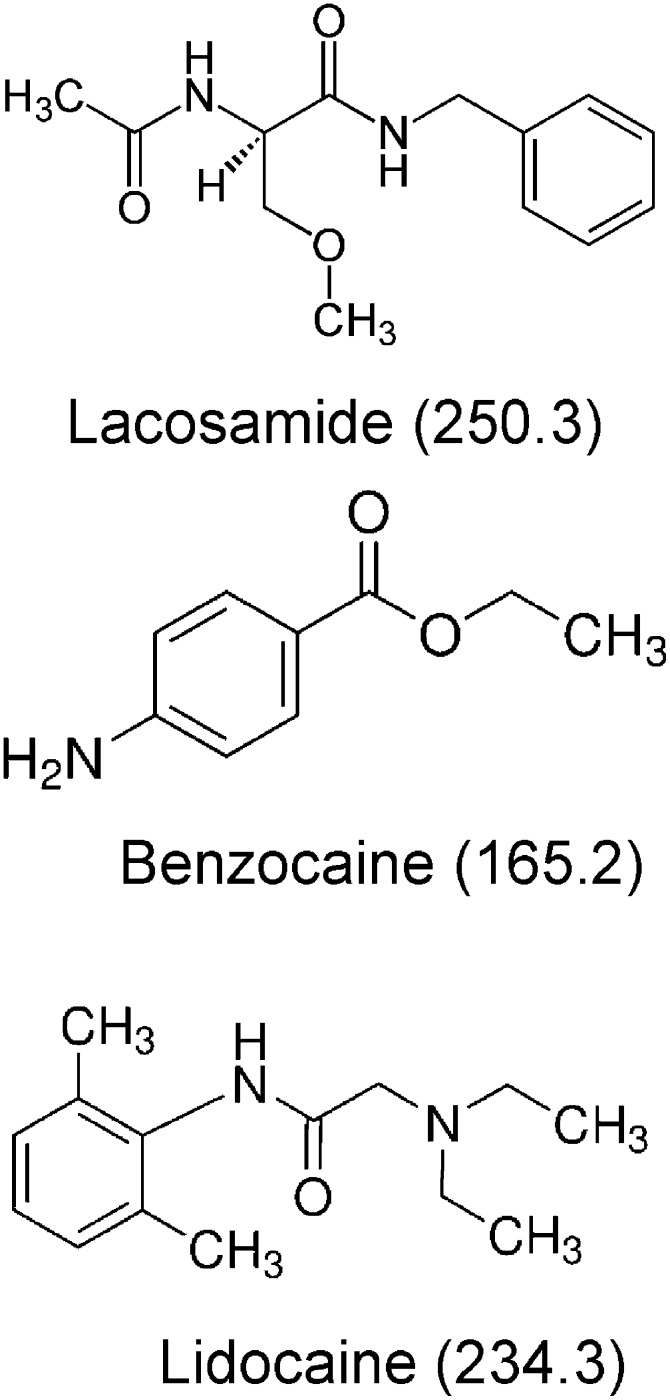
Lacosamide is a Food and Drug Administration–approved anticonvulsant, currently used as an antiepileptic drug for adjunctive therapy of partial onset seizures (Halasz et al., 2009). This drug is taken orally at 200 or 400 mg/day. The clinical relevant concentrations of lacosamide range from 32 to 100 µM (Errington et al., 2008). With regard to comorbidities, lacosamide has no approved indications outside of partial epilepsy, but there are clinical trials supporting its efficacy in painful diabetic neuropathy. As a result, it may be a drug that could be considered for patients with epilepsy who also suffer this comorbidity. Recent studies on cortical neurons have found that lacosamide acts differently from many well characterized anticonvulsants, including carbamazepine, lamotrigine, and phenytoin (Errington et al., 2006). Instead of targeting fast inactivation as traditional anticonvulsants and LAs (Hille, 1977), lacosamide supposedly enhances slow inactivation of neuronal Na+ channels for its efficacy (Errington et al., 2008). Lacosamide is a functionalized amino acid and carries no charge in the aqueous solution. Despite a common benzene ring, lacosamide does not resemble much the structure of neutral LA benzocaine or lidocaine with a tertiary amine (Fig. 1).
Fig. 1.
Chemical structures of lacosamide, benzocaine, and lidocaine: both lacosamide and benzocaine are neutral compounds, whereas lidocaine contains a tertiary amine moiety that carries a positive charge when protonated. The molecular masses in Daltons are included in parentheses.
We are interested in the action of lacosamide with respects to its selective block of slow-inactivated Na+ channels. There are only a handful of drugs that are implicated with this selective mode of action (McNulty et al., 2006; Jo and Bean, 2011). The goal of this investigation is to study the lacosamide action on human cardiac Nav1.5 Na+ channels and, if possible, to locate the binding site for lacosamide. The effects of lacosamide on neuronal Na+ channel isoforms have been investigated previously using Nav1.3, Nav1.7, and Nav1.8 Na+ channels (Sheets et al., 2008). Human cardiac Na+ channels (hNav1.5) display less and incomplete slow inactivation compared with these neuronal counterparts (Richmond et al., 1998; O'Reilly et al., 1999). This incomplete slow inactivation phenotype of cardiac Na+ channels allows sustained firing of rhythmic action potentials in the heart. We asked whether such a phenotype will lessen the block of human cardiac Na+ channels by lacosamide. In addition, we asked whether hNav1.5-CW inactivation-deficient mutant Na+ channels (Wang et al., 2013) become more sensitive to lacosamide block. This question was raised from the fact that Na+ channels with an impaired fast inactivation are known to have enhanced slow inactivation (Rudy, 1978; Wang et al., 2003). Finally, we asked whether the lacosamide receptor overlaps the receptors for LAs and batrachotoxin (BTX) within the Na+ permeation pathway of this cardiac Na+ channel isoform. To our surprise, our findings appear to deviate from the current hypothesis considerably and support an alternative view of a slow lacosamide action along the activation pathway of hNav1.5 cardiac Na+ channels.
Materials and Methods
Culture and Transient Transfection of Human Embryonic Kidney Cells.
Human embryonic kidney cells (HEK293) were purchased from American Type Culture Collection (Manassas, VA). Cultured HEK293 cells were plated on 30-mm culture dishes and maintained at 37°C in a 5% CO2 incubator in Dulbecco’s modified Eagle’s medium (Life Technologies, Rockville, MD) containing 10% fetal bovine serum (HyClone, Logan, UT) and 1% penicillin and streptomycin solution (Sigma-Aldrich, St. Louis, MO). HEK293 cells expressing hNav1.5-CW/F1760K mutant Na+ channels along with β1-subunit and CD8 surface antigen were prepared by transient transfection via a calcium phosphate precipitation method and used within 2–4 days after transfection (Wang et al., 2003). Cells that expressed the CD8, as determined by antibody-coated beads (Dynabeads M-450 CD8; Life Technologies), were selected for patch-clamp experiments.
Preparation of HEK293 Stable Cell Lines Expressing Inactivation-Deficient hNav1.5-CW and hNav1.5 Wild-Type Na+ Currents.
Cells that stably expressed inactivation-deficient hNav1.5-CW Na+ currents were prepared as described previously (Wang et al., 2013). The inactivation-deficient hNav1.5-CW clone has double mutations (L409C/A410W) at the D1S6 region. Cells that stably expressed hNav1.5 wild-type Na+ currents were also isolated according to the same method. Briefly, HEK293 cells were transfected with the wild-type hNav1.5 clone by a calcium phosphate precipitation method. One day after transfection, HEK293 cells were treated with 1.0 mg/ml G418 (Invitrogen, Carlsbad, CA) in 100-mm culture dishes. One colony expressing large hNav1.5 Na+ currents was selected, expanded, frozen, and stored in liquid nitrogen. Frozen cells were thawed and plated at very low density in 100-mm dishes for a second colony-selection procedure without G418. Seven colonies were picked by glass cylinders. Cells from one colony that stably expressed a high level of Na+ currents were expanded, frozen, and stored in liquid nitrogen. Cells from this stable cell line were used in the present study without G418 added in the medium.
Electrophysiology, Data Acquisition, and Statistics.
HEK293 cells were plated in 30-mm dishes, which were subsequently used as recording chambers. Individual cells were perfused with an extracellular solution containing (in mM) 65 NaCl, 85 choline-Cl, 2 CaCl2, and 10 HEPES (titrated with tetramethylammonium-OH to pH 7.4). The pipette (intracellular) solution consisted of (in mM) 100 NaF, 30 NaCl, 10 EGTA, and 10 HEPES (titrated with cesium-OH to pH 7.2). Lacosamide was purchased from Selleckchem (Houston, TX), and the drug was dissolved in dimethylsulfoxide (DMSO) at 100 mM and stored at 4°C. Final lacosamide concentrations from 30 µM to 1 mM were made by serial dilution. BTX was dissolved in DMSO at a stock concentration of 0.5 mM. BTX was added into the pipette solution to a final concentration of 5 µM. DMSO (up to 1%) in the bath or in the pipette solution had little effect on Na+ currents.
The whole-cell configuration of a patch-clamp technique (Hamill et al., 1981) was used to record Na+ currents in HEK293 cells at room temperature (22 ± 2°C). Electrode resistance ranged from 0.4 to 0.6 MΩ. Command voltages were elicited with pCLAMP9 software and delivered by Axopatch 200B (Molecular Devices, Inc., Sunnyvale, CA). Cells were held at –140 mV holding potential and dialyzed for 10 to 15 minutes before recording. The capacitance and leak currents were cancelled with the patch-clamp device and by P/−4 subtraction. Access resistance was about 1 MΩ under the whole-cell configuration; series resistance compensation of ≥95% usually resulted in voltage errors of ≤3 mV at +50 mV. The threshold for the activation of hNav1.5 and hNav1.5-CW Na+ currents was the same around −70 mV (Wright et al., 1997; Wang et al., 2013). Dose-response studies were typically performed at a test pulse of +50 mV for the outward Na+ currents. Such recordings allowed us to avoid the complication of series resistance artifacts and to circumvent inward Na+ ion loading (Cota and Armstrong, 1989). Extracellular solutions with or without drug were applied via a series of narrow-bored capillary tubes positioned within 300 µm from the cell. Curve fitting was performed by Microcal Origin (Northampton, MA) with a Hill equation
for dose-response studies or with a single exponential function for the development of lacosamide block and its recovery. The IC50 value corresponds to the 50% inhibitory concentration, and n corresponds to the Hill coefficient. An unpaired Student’s t test was used to evaluate estimated parameters (mean ± S.E.M. or fitted value ± S.E. of the fit); P values of <0.05 were considered statistically significant.
Results
Minimal Tonic Block of Resting hNav1.5 Na+ Channels by Lacosamide at the −140 mV Holding Potential.
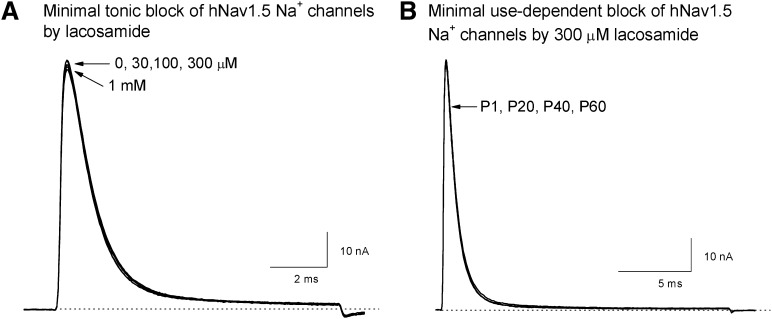
Wild-type hNav1.5 Na+ currents were relatively insensitive to lacosamide block when the cells were depolarized to +50 from −140 mV holding potential. Figure 2A shows that lacosamide from 30 µM to 1 mM has very little effect on hNav1.5 wild-type Na+ currents. The peak Na+ current amplitude remained about the same, and the current kinetics were not changed. This result demonstrates that the resting state of hNav1.5 channel at −140 mV holding potential does not interact tonically with lacosamide. In addition, lacosamide at 300 µM failed to block hNav1.5 Na+ currents even with 60 repetitive pulses to +50 mV for 20 milliseconds (Fig. 2B; at 5 Hz). This lack of tonic and use-dependent block of Na+ currents by lacosamide is quite unique, because traditional anticonvulsants and LAs typically elicit both types of block.
Fig. 2.
Minimal tonic and use-dependent block of wild-type hNav1.5 Na+ channels by lacosamide. (A) When HEK293 cells were held at −140 mV holding potential, little or no tonic block of hNav1.5 Na+ currents was found after a 10-millisecond depolarization to +50 mV at various lacosamide concentrations. The current trace without drug was included (trace 0) and superimposed with those at 30, 100, or 300 µM, or 1 mM lacosamide. (B) Repetitive pulses (+50 mV for 20 milliseconds) failed to elicit use-dependent block of hNav1.5 Na+ currents. Superimposed traces of Na+ currents corresponding to pulse P1, P20, P40, and P60 were shown. Repetitive pulses were applied at a frequency of 5 Hz. This experiment was repeated in 5 different cells with similar results.
Block of hNav1.5 Na+ Channels by Lacosamide at Various Membrane Potentials.
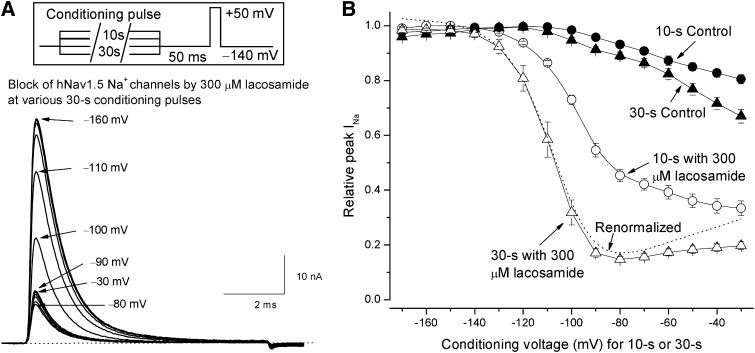
To determine the voltage dependence of lacosamide block, we used a pulse protocol with a 30-second conditioning pulse ranging from −170 to −30 mV as shown in Fig. 3A, inset. To limit cumulative effects of slow inactivation, pulses were applied at a 60-second interval. Similar conditioning protocols but with a 10-second pulse were applied in previous studies on lacosamide (Errington et al., 2008; Sheets et al., 2008; Niespodziany et al., 2013). Representative traces of superimposed Na+ currents recorded at the test pulse showed that lacosamide at 300 µM elicited significant block of Na+ channels under conditioning pulses ≥ −120 mV (Fig. 3A). Relative block of peak Na+ currents by lacosamide was measured, normalized by the maximal peak value, and plotted against the conditioning voltage as shown in Fig. 3B (▵). Control records measured before the drug treatment showed little changes of peak Na+ currents with the conditioning pulses from −170 to −100 mV. However, the control peak Na+ currents began to decrease progressively with the conditioning pulses from −90 to −30 mV (Fig. 3B, ▴), probably because of the slow inactivation of hNav1.5 Na+ channels. With 300 µM lacosamide present, the reduction of peak Na+ currents began at −130 mV and reached the maximal reduction around −90 to −70 mV when a 30-second conditioning pulse was applied (Fig. 3A, ▵). At more depolarized voltages from −60 to −30 mV, the drug-treated data showed a small but steady relief of peak current block, whereas the control counterparts continued to decrease (▴). To account for the decrease of the peak current in control, we also recalculated the lacosamide block by normalization (dashed line). For comparison, we included the lacosamide block at various conditioning pulses with a shorter duration of 10 seconds (Fig. 3B, ○). Interestingly, with a 30-second conditioning pulse, the voltage-dependence of lacosamide block was leftward shifted considerably (▵ versus ○) as if the block was greatly enhanced by a prolonged conditioning pulse.
Fig. 3.
Block of hNav1.5 Na+ channels by lacosamide at various conditioning pulses. (A) Representative hNav1.5 Na+ currents were superimposed after various 30-second conditioning pulses were applied. Some conditioning pulses were labeled. The peak currents were reduced progressively, reaching their minimum at −80 mV and then reversed. The inset shows the pulse protocol. An interpulse (50 milliseconds at −140 mV) was inserted so that the fast-inactivated Na+ channels could return to their resting state before the final test pulse to +50 mV for 10 milliseconds. Cumulative accumulation of slow inactivation was limited by applying the conditioning pulse once every 60 seconds. (B) The voltage-dependent block of hNav1.5 Na+ channels was measured in the presence (▵, n = 8) or absence of 300 µM lacosamide (control; ▴, n = 8) at various conditioning voltages lasting for 30 seconds. The dashed line represents the normalized data of 300 µM lacosamide to account for the decrease of the peak current in control (i.e., divided by the control value at a given voltage). For comparison, data were also obtained using the same pulse protocol, except a 10-second conditioning pulse was applied in the presence (○, n = 8) or absence of 300 µM lacosamide (control; ●, n = 8). The block of peak currents is far greater when a 30-second conditioning pulse was applied.
Dose-Response Curves of hNav1.5 Wild-Type Na+ Channels by Lacosamide.
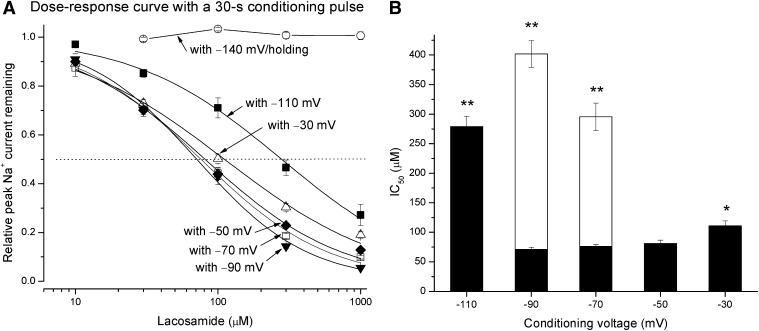
To determine the dose-response curve at various lacosamide concentrations, we used a single 30-second conditioning pulse. Figure 4A shows that lacosamide up to 1 mM elicited no block of the peak Na+ currents (○, n = 8) when the cells were held at −140 mV (also see Fig. 2A for current traces). At this holding potential, most Na+ channels were in their resting state. In contrast, with a −70 mV conditioning pulse lasting for 30 seconds, lacosamide elicited significant block of the peak Na+ currents at the test pulse (□). The estimated IC50 value for lacosamide block was 75.9 ± 3.4 µM, with a Hill coefficient of 0.97 ± 0.04 (n = 8). Under our ionic conditions, hNav1.5 wild-type Na+ channels were activated around −70 mV (Wright et al., 1997). At this potential (−70 mV for 30 seconds), most Na+ channels reached their intermediate preopen states initially, and some eventually entered their open, fast- and/or slow-inactivated states. In addition, we measured and compared other dose-response curves under separate conditioning pulses ranging from −110 to −30 mV against the lacosamide concentrations (Fig. 4A). Figure 4B (filled bar) shows the estimated IC50 values measured at different conditioning voltages ranging from −110 to −30 mV that lasted for 30 seconds. This plot validated the block of peak Na+ currents after a 30-second conditioning pulse by a single lacosamide dose (Fig. 3B). The dose-response curve also took into the consideration of the control peak current during curve construction. This process was equivalent to the renormalized curve shown in Fig. 3B (dashed line). The maximal lacosamide block occurred around −90 to −50 mV (IC50; ∼70–80 µM) and then a small but statistically significant relief of block followed at −30 mV (∼110 µM; Fig. 4B). In addition, we also measured the IC50 values with a conditioning pulses at −90 and −70 mV that lasted for 10 seconds. These results were included in Fig. 4B (unfilled bar) for comparison. Earlier studies in literature were all performed with a 10-second conditioning pulse because severe slow inactivation occurred in most Na+ channel isoforms. Thus, lacosamide appears significantly more potent when a longer conditioning pulse was applied near the activation threshold of hNav1.5 Na+ channels (Fig. 4B). Could this occur because lacosamide interacts with Na+ channels in a very slow manner? This possibility was investigated as described next.
Fig. 4.
Dose-response curves under various conditioning voltages. (A) Under a given 30-second conditioning voltage, hNav1.5 Na+ currents were recorded at various lacosamide concentrations. Peak currents were measured, normalized with respect to the control peak amplitude without drug, and plotted against drug concentration. The curves were best fitted by a Hill equation with IC50 and Hill coefficient (in brackets) values of 279.0 ± 17.4 µM [0.84 ± 0.05], 71.0 ± 3.7 µM [1.12 ± 0.06], 75.9 ± 3.4 µM [0.97 ± 0.04], 81.1 ± 5.6 µM [0.90 ± 0.05], and 110.9 ± 8.5 µM [0.77 ± 0.05] corresponding to conditioning pulses of −110, −90, −70, −50, and −30 mV, respectively (n = 8). The dotted line corresponds to 50% of current remaining. (B) IC50 values were plotted against conditioning voltages for comparison. The IC50 values at −90, −70, and −50 mV are not significantly different from each other (P > 0.05). However, IC50 value at −30 mV is significantly greater than that at −50 mV (*P < 0.05), whereas IC50 value at −110 mV is also greater than that at −90 mV (**P < 0.01). Also included are IC50 value measured with −90 and −70 mV conditioning voltages that lasted for 10 seconds (n = 8). These values are significantly higher than those with same conditioning voltages that last for 30 seconds (**P < 0.01).
Development of Lacosamide Block at −70 mV and Its Recovery at −140 mV.
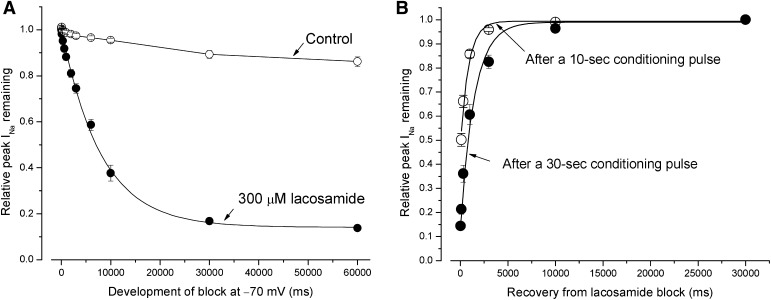
To determine the development of lacosamide block, we applied a conditioning pulse at −70 mV with a duration varying from 0 to 60 seconds. Figure 5A shows that the development of lacosamide block at 300 µM follows a single exponential function with a time constant of 8.04 ± 0.39 seconds (n = 8; ●). Without lacosamide, the peak Na+ currents were reduced but only moderately (14 ± 2% at 60 seconds, ○; n = 8, control). Clearly, lacosamide interacts slowly with Na+ channels at −70 mV.
Fig. 5.
The development of lacosamide block at −70 mV in wild-type hNav1.5 Na+ channels and its recovery. (A) The development of block in hNav1.5 Na+ channels induced by 300 µM was determined using a conditioning pulse of −70 mV with a duration ranging from 0 to 60 seconds. A final test pulse (+50 mV for 10 milliseconds) was applied after an interpulse (50 milliseconds at −140 mV) to record the relative peak Na+ current remaining. The development of block could be best fitted by a single exponential decay with a time constant of 8.04 ± 0.39 seconds (n = 8; ●). The relative peak Na+ currents were little changed in the absence of drug (○; control). (B) The recovery from block by 300 µM lacosamide occurred at −70 mV for 10 seconds (○), and 30 seconds (●) were measured after the voltage was switched back to −140 mV holding potential, with a recovery duration ranging from 0 to 30 seconds. The relative peak Na+ currents were then determined by a final test pulse (+50 mV for 10 milliseconds). The time course for recovery was fitted by an exponential function with a time constant of 639 ± 90 (n = 8) and 1457 ± 121 milliseconds (n = 8) for a 10-second and a 30-second conditioning pulse at −70 mV, respectively (solid fitted lines).
To determine the recovery from lacosamide block, we applied a conditioning pulse at −70 mV for either 10 or 30 seconds and then switched the membrane voltage to −140 mV holding potential, with a duration ranging from 30 milliseconds to 30 seconds before applying a test pulse to +50 mV. Figure 5B shows that the recovery from lacosamide block is surprisingly rapid at −140 mV. The time course followed an exponential function, with a time constant of 639 ± 90 milliseconds (n = 8) after a 10-second conditioning pulse at −70 mV. Interestingly, with a −70-mV conditioning pulse that lasted for 30 seconds, the recovery time course was slowed by more than twofold with a time constant of 1.46 ± 0.12 seconds (n = 6) (Fig. 4B). This result is still in sharp contrast with a previous report that found that the half-life for recovery from lacosamide block was 6.71 seconds (or τ ≈ 9.66 second) (Errington et al., 2008).
It is noteworthy that the measurement of the block by lacosamide is quite difficult. First, the conditioning pulse as long as 10 seconds was not enough for lacosamide to reach the steady-state block of wild-type hNav1.5 Na+ channels. A steady-state block of hNav1.5 Na+ channels may be reached only when a 30-second duration of the conditioning pulse was applied (Fig. 4A). Second, the 50-millisecond interpulse between the test pulse and the conditioning pulse might allow a small fraction of lacosamide to escape from its receptor (Fig. 4B). Third, the recovery time course is notably dependent on the duration of the conditioning pulse. This phenomenon implies that the conventional holding potential (i.e., −120 to −80 mV) will elicit the tonic block of lacosamide inadvertently and in a time-dependent manner.
Block of Inactivation-Deficient hNav1.5-CW Na+ Channels by Lacosamide at Various Membrane Potentials.
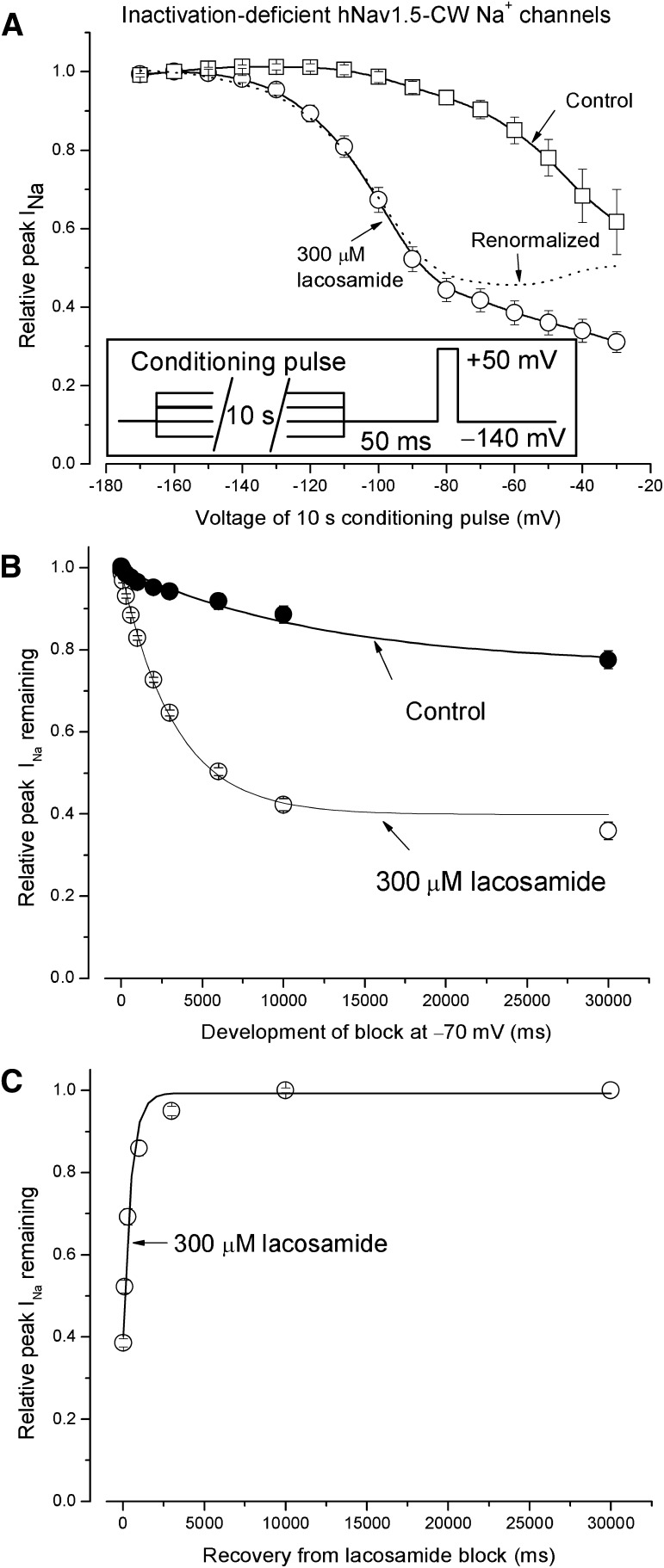
To study whether lacosamide blocks the inactivation-deficient Na+ channel, we used the hNav1.5-CW cell line that expressed robust inactivation-deficient cardiac Na+ currents. Figure 6A shows the voltage-dependent block of inactivation-deficient hNav1.5-CW by 300 µM lacosamide at various membrane potentials ranging from −170 to −30 mV (inset). The duration of the conditioning pulse was set at 10 seconds because of severe slow inactivation of hNav1.5-CW Na+ channels. In the absence of drug we observed a larger decrease in peak Na+ currents, with a conditioning pulse ranging from −100 to −30 mV (□) than that for wild-type counterparts (Fig. 3A). In the presence of 300 µM lacosamide, the block started around −120 mV and became progressively more prominent as the membrane voltage was more depolarized (○). These results are similar to those found in wild-type hNav1.5 Na+ channels, with conditioning pulses lasting for 10 seconds (Fig. 6A, ○, versus Fig. 3B, ○). The dashed line represents the lacosamide block after renormalization of peak Na+ currents remaining (○) with the control (□) as shown in Fig. 3. The resulting curve implies that the block of hNav1.5-CW Na+ channels by lacosamide reaches its maximal around −70 mV (with 10-second duration) and then decreases progressively up to −30 mV, which is comparable to the voltage-dependent block observed in wild-type hNav1.5 Na+ channels (Fig. 6A, dashed line, versus Fig. 3B, dashed line).
Fig. 6.
Block of inactivation-deficient hNav1.5-CW Na+ channels by lacosamide at various membrane potentials. (A) The voltage-dependent block was measured at various conditioning pulses in the presence (○) or absence of 300 µM lacosamide (□). The inset shows the pulse protocol. Without drug, the peak current amplitude decreased considerably from −100 to −30 mV, probably because of the enhanced slow inactivation in inactivation-deficient hNav1.5-CW mutant Na+ channels. (B) The development of block by 300 µM lacosamide at −70 mV was recorded using hNav1.5-CW Na+ channels and plotted against the time (○). The solid line was the best fitted of the data by a single exponential function with a time constant of 3.28 ± 0.12 seconds (n = 8). Without drug, the time constant was 12.5 ± 3.7 seconds. (C) The recovery from the lacosamide block at −70 mV for 10 seconds was measured at the holding potential −140 mV, with a duration ranging from 0 to 30 seconds before a test pulse (+50 mV for 10 milliseconds) was applied. The relative peak currents were then plotted against the time, and the data were fitted by an exponential function with a time constant of 476 ± 38 milliseconds (n = 8; r2 = 0.99).
Figure 6B shows the time course of the development of block induced by 300 µM lacosamide in hNav1.5-CW mutant Na+ channels. Without drug, the relative peak Na+ currents were reduced with a slow τ value of 12.5 ± 3.7 seconds (n = 8), likely due to the enhanced slow inactivation inasmuch as this reduction was more pronounced than that found in wild-type Na+ channels (Fig. 3B). With 300 µM lacosamide present, the currents were blocked after a τ value of 3.28 ± 0.12 seconds (n = 8), which was significantly faster than that found in the wild-type hNav1.5 Na+ channels (8.04 ± 0.39 seconds; P < 0.05). The time course of the recovery from lacosamide block was measured as shown in Fig. 6C. The data were fitted by a single exponential function, with a τ value of 476 ± 38 milliseconds (n = 8). This time constant was significantly faster than that in hNav1.5 wild-type Na+ channels (639 ± 90 milliseconds; n = 8; P < 0.05) after a 10-second/−70-mV conditioning pulse.
Block of Peak and Persistent Late hNav1.5-CW Na+ Currents by Lacosamide.
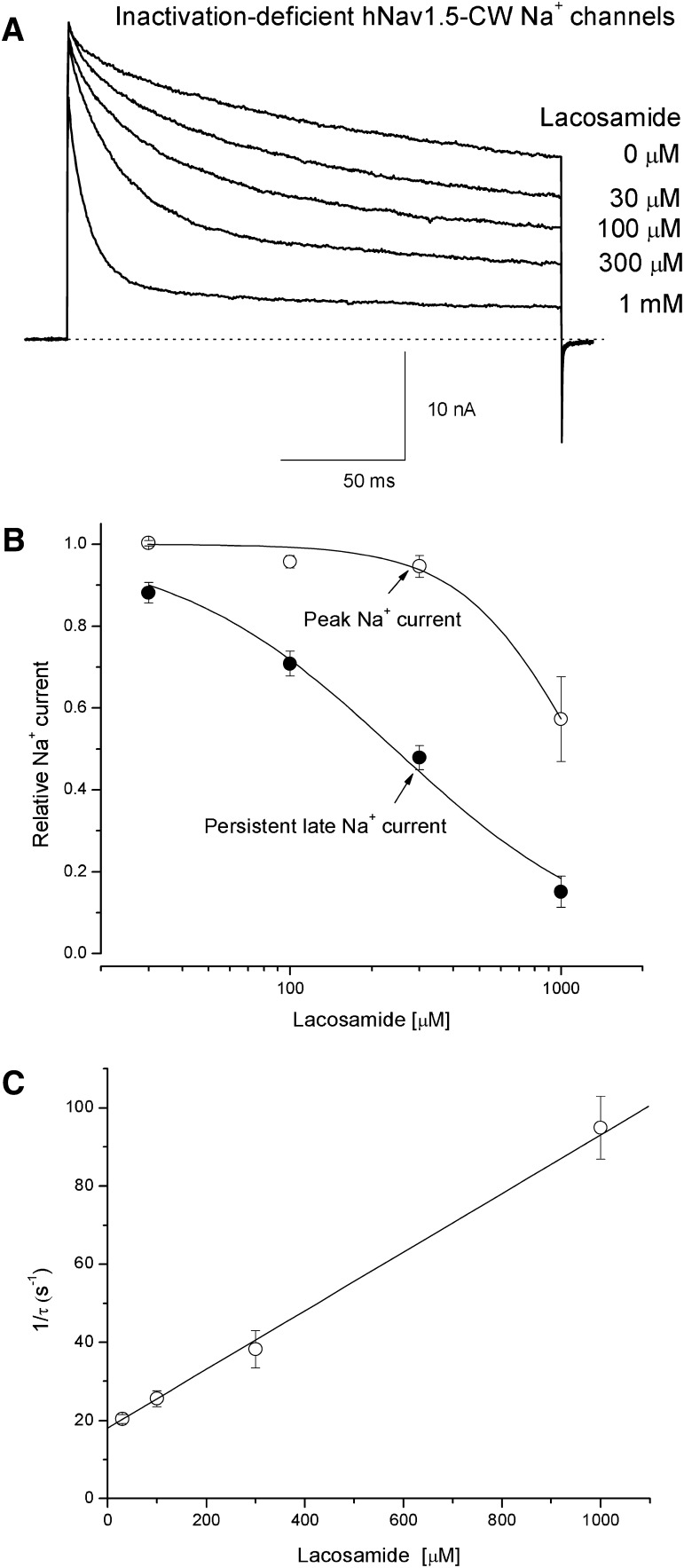
Figure 7A shows superimposed traces of hNav1.5-CW Na+ currents in the absence (0 µM) and presence of lacosamide ranging from 30 µM to 1 mM. We found that peak Na+ currents were reduced far less than persistent late Na+ currents. Furthermore, the higher the concentration of lacosamide, the more conspicuous the time-dependent block was (Fig. 7A). This phenomenon is indicative of an open-channel block of inactivation-deficient cardiac Na+ channels by lacosamide. Figure 7B shows the dose-response curve for the block of persistent late Na+ currents (●). The IC50 value was estimated 242 ± 19 µM with a Hill coefficient of 1.05 ± 0.09 (n = 5). This Hill coefficient is close to unity, suggesting that one lacosamide molecule blocks one open Na+ channel. In comparison, the IC50 value for the block of peak Na+ currents was estimated 1.16 ± 0.06 mM (○; n = 5). This estimated value should not be considered as the accurate IC50 for the resting-channel block of lacosamide at −140 mV, because most of current reduction at 1 mM lacosamide may be due to the rapid open-channel block occurred during the Na+ channel activation. At lower concentrations of lacosamide (e.g., 300 µM, Fig. 7B), such contamination was less severe. This notion is consistent with results found in wild-type hNav1.5 channels (Fig. 2), where fast inactivation appears to protect the block of the transient open state during channel activation. The time-dependent block (Fig. 7A) followed an exponential decay with a single time constant (τ). The plot of 1/τ versus the concentration of lacosamide was best fitted by a linear equation where the y-intercept (18.0) represents the value of koff (in s−1) and the slope (0.075) represents the value of kon (in µM−1/s−1) (Fig. 7C). The apparent dissociation constant for the open-channel block of lacosamide was calculated as KD = koff/kon= 240 µM. This KD value is nearly identical to the IC50 value measured in the dose-response curve (Fig. 7B, ●).
Fig. 7.
Time-dependent block of inactivation-deficient hNav1.5-CW Na+ currents by lacosamide. (A) Inactivation-deficient Na+ currents were elicited by a depolarization to +50 mV for 200 milliseconds and superimposed in the absence and presence of various concentrations of lacosamide. The corresponding concentrations were labeled near the traces. The holding potential was set at −140 mV. (B) The dose-response curves were constructed for the block of peak (○) and the persistent late Na+ currents (●). The peak currents and the persistent late Na+ currents (near the end of 200- millisecond pulse) as shown in A were measured, normalized with the control amplitude, and plotted against the concentrations. Data were fitted by a Hill equation with IC50 and Hill coefficient values (in brackets) of 1.16 ± 0.06 mM [2.00 ± 0.30] and 242 ± 19 µM [1.05 ± 0.09] for the block of peak and persistent late Na+ currents, respectively (n = 5). (C) The time-dependent block of Na+ currents shown in A was measured, normalized with the control currents, graphed against time, and fitted by an exponential decay function with a time constant (τ). The 1/τ values were then plotted against the concentrations of lacosamide and best fitted by a linear function. The y-intercept (18.0 ± 1.1 s−1) and the slope (75.1 ± 7.6 mM−1/s−1, n = 5) represent the off-rate and the on-rate of lacosamide, respectively.
Block of Inactivation-Deficient Na+ Channels by Lacosamide Near the Activation Threshold.
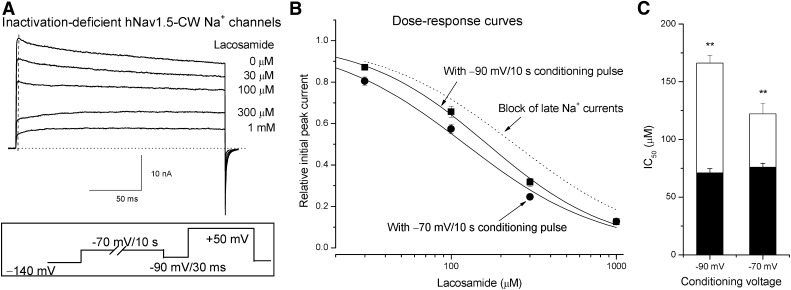
We recorded the hNav1.5-CW Na+ current traces immediately after the block occurred during a conditioning pulse of −70 mV for 10 seconds. Figure 8A shows the superimposed current traces in the absence and presence of lacosamide ranging from 30 µM to 1 mM. A test pulse to +50 mV was applied after the conditioning pulse to evoke hNav1.5-CW Na+ currents. The pulse protocol is shown in Fig. 8A (inset). A 30-millisecond gap at −90 mV was inserted between the conditioning and the test pulse to reset the current baseline (dotted line). We observed that as the concentrations of lacosamide were raised, the initial peak currents were reduced more and more (Fig. 8A, dashed line). However, there was no evidence of an apparent time-dependent block as observed in Fig. 7A when the cell was held at −140 mV. At concentrations of 300 µM and 1 mM, the Na+ currents appeared rapidly during channel activation (due to the rapid activation of drug-free hNav1.5-CW Na+ channels), followed by a slow rising phase, and then leveled off eventually during the 200-milliseond pulse (due to the slow relaxation of the lacosamide-bound hNav1.5-CW Na+ channels). At a concentration of 100 µM lacosamide, the block at the initial rapid rising phase was 57.1%, whereas at the end of the pulse the block was 68.2%. At a concentration of 30 µM, the block at the peak was 78.6%, whereas at the end of the pulse the block was 84.0%. This observation in the drug dissociation during the 200-millisecond pulse suggests that the block at −70 mV is more pronounced than that at the test pulse of +50 mV (see Fig. 6A). Figure 8B shows the dose-response curve for the block of the initial peak Na+ currents after the conditioning pulse of −70 mV for 10 seconds. The IC50 was estimated 122 ± 9 µM, with a Hill coefficient of 1.05 ± 0.09 (n = 7). This value was twofold smaller than the open-channel block that occurred at +50 mV (Fig. 8B, dashed line for block of late Na+ currents). In addition, we determined the block of hNav1.5-CW Na+ channels with a conditioning pulse of −90 mV for 10 seconds (Fig. 8B), which yielded an IC50 of 166 ± 7 µM and a Hill coefficient of 1.16 ± 0.05 (n = 9). This IC50 value was significantly larger than the IC50 value determined at −70 mV (122 µM; P < 0.05). It is noteworthy that the block induced by lacosamide in hNav-CW mutant channels reached its steady state after a −70-mV conditioning pulse that lasted for 10 seconds (Fig. 6B), whereas in wild-type it requires a longer conditioning pulse that lasted for 30 seconds (Fig. 5A). Under conditions that reached the steady-state block, hNav1.5-CW inactivation-deficient Na+ channels (although with enhanced slow inactivation) appeared to have significantly less affinity (122 µM at −70 mV for 10 seconds) toward lacosamide than that of wild-type counterparts (75.9 µM at −70 mV for 30 seconds; P < 0.01) (Fig. 8C, open versus filled bar).
Fig. 8.
Dose-response block of inactivation-deficient hNav1.5-CW Na+ channels by lacosamide at −70 mV. (A) Superimposed inactivation-deficient hNav1.5-CW Na+ currents were recorded at a test pulse of +50 mV for 200 milliseconds in the absence and presence of various concentrations of lacosamide. The inset shows the pulse protocol with a conditioning pulse of −70 mV for 10 seconds, which was applied so that the drug could interact with the intermediate preopen states of hNav1.5-CW mutant Na+ channels. An interpulse (−90 mV for 30 milliseconds) was inserted to remove residual Na+ currents at −70 mV before the test pulse was applied. The Na+ current amplitude of the initial rapid rising phase before reaching the peak was measured, normalized with respect to the control peak current amplitude (i.e., at the dashed vertical line), and plotted against the concentrations of lacosamide (B; ●). The data were fitted with a Hill equation with an IC50 and Hill coefficient (in brackets) of 122 ± 9 µM [1.05 ± 0.09] (n = 7). The dose-response data with a conditioning pulse of −90 mV for 10 seconds (▪) was obtained in the same manner but without an interpulse, and was fitted by a Hill equation with an IC50 and Hill coefficient (in brackets) of 166 ± 7 µM [1.16 ± 0.05] (n = 9). This IC50 value is significantly larger than that measured at −70 mV (P < 0.01). (C) The IC50 values of inactivation-deficient hNav1.5-CW Na+ channels were plotted against conditioning voltage (open bar) and each was compared with the IC50 value of wild-type with a conditioning pulse that lasted for 30 seconds (filled bar; **P < 0.01).
Voltage Dependence of Open-Channel Block as Determined by Ramp Na+ Currents.
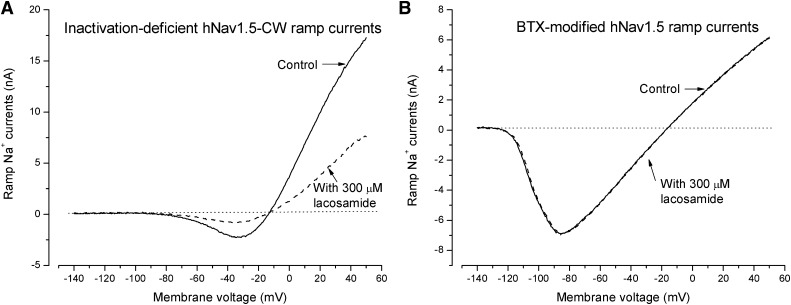
A ramp stimulus with a 1-second duration from −140 to +50 mV was used to generate inactivation-deficient hNav1.5-CW ramp Na+ currents. The presence of the ramp Na+ currents during a 1-second stimulus was due to the lack of fast inactivation of hNav1.5-CW Na+ channels. Figure 9A shows the ramp currents without (control) and with 300 µM lacosamide. Without lacosamide, the inward ramp Na+ currents appeared around the activation threshold (∼−70 mV), reached maximal inward currents around −30 mV, reversed to outward around −10 mV, and increased continuously up to +50 mV. With 300 µM lacosamide present, the majority of inward and outward ramp currents were inhibited equally as if the rapid open-channel block remained constant (∼60%) from −70 to +50 mV. The block of preopen states by lacosamide was limited because of the slow time course of such block. Evidently, the direction of Na+ flux did not influence the level of open-channel block induced by neutral lacosamide.
Fig. 9.
Block of inactivation-deficient hNav1.5-CW ramp Na+ currents by lacosamide. (A) Superimposed ramp Na+ currents of inactivation-deficient hNav1.5-CW Na+ channels were generated by a 1-second ramp stimulus from −140 to +50 mV in the absence (control) and presence of 300 µM lacosamide (dashed line). The inward Na+ currents appeared at the activation threshold near −75 mV and reversed to the outward direction at the reversal potential of approximately −10 mV. About 60% of currents were blocked at −30 and +50 mV, where the inward and outward Na+ currents reached the maximal values. (B) Superimposed ramp currents of BTX-modified hNav1.5 wild-type Na+ channels were elicited by the same pulse protocol as in A without (control) and with 300 µM lacosamide present (dashed line). One thousand repetitive pulses (+50 mV for 20 milliseconds at 2 Hz) were applied earlier to facilitate BTX binding with wild-type hNav1.5 Na+ channels. These BTX-modified ramp Na+ currents were completely resistant to 300 µM lacosamide. The inward BTX-modified ramp Na+ currents appeared around −120 mV because of the leftward shift of the activation threshold by BTX.
The Receptor for Lacosamide Versus the Receptor for BTX.
To probe the possible lacosamide binding site, we first determined whether the block of lacosamide remained unchanged in the BTX-activated Na+ channels. We previously reported that BTX when bound eliminates both fast and slow inactivation of wild-type hNav1.5 Na+ channels (Wang et al., 2007). As required, repetitive pulses were first applied to facilitate BTX binding to open hNav1.5 Na+ channels. Figure 9B shows that BTX-activated wild-type hNav1.5 ramp Na+ currents could be detected from −120 to +50 mV (solid line), but these currents were completely resistant to 300 µM lacosamide (dashed line). Because of the leftward shift in the Na+ channel activation by BTX, the ramp Na+ currents appeared at a threshold around −120 mV. Evidently, lacosamide fails to block the BTX-activated hNav1.5 Na+ channels, suggesting that BTX prevents the binding of lacosamide. Such resistance is consistent with the notion that the receptor for lacosamide overlaps the BTX receptor along the permeation pathway, and, when bound, lacosamide occludes the Na+ permeation pathway directly.
The Receptor for Lacosamide Versus the Receptor for LAs.
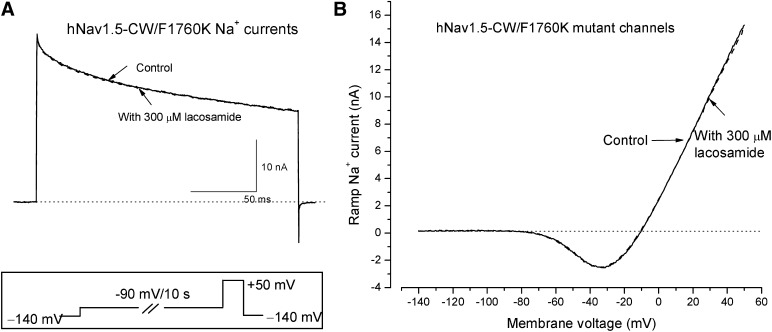
To explore further whether the open-channel block of hNav1.5-CW Na+ currents by lacosamide is through the permeation pathway, we used the F1760K mutant channel to address this question. The phenylalanine reside F1760 at the D4S6 region is known to face the inner cavity of the permeation pathway when the channel is activated, and this residue is the most critical one for the LA block of Na+ currents (Ahern et al., 2008). Interestingly, hNav1.5-CW/F1760K Na+ currents were also completely resistant to lacosamide; little or no block of these currents was observed at 300 µM (Fig. 10). This drug resistance occurred in hNav1.5-CW-F1760K inactivation-deficient Na+ currents either when the conditioning pulse of −90 mV was applied for 10 seconds before the test pulse (Fig. 10A; n = 6) or when the cells were held at −140 mV without a conditioning pulse. No time-dependent block was observed in either case. In addition, hNav1.5-CW/F1760K inactivation-deficient ramp Na+ currents found between −70 to +50 mV were also completely resistant to 300 µM lacosamide (Fig. 10B). Evidently, the phenylalanine residue at position hNav1.5-CW/F1760 is essential for lacosamide block of both the intermediate preopen and open Na+ channel.
Fig. 10.
Lacosamide-resistant inactivation-deficient hNav1.5-CW/F1760K mutant Na+ channels. (A) Superimposed inactivation-deficient hNav1.5-CW/F1760K mutant Na+ currents were generated by a +50 mV pulse for 20 milliseconds in the absence (control) and presence of 300 µM lacosamide (dashed line). A conditioning pulse of −90 mV for 10 second was applied first (inset), which allowed the drug to interact with the intermediate preopen states of the mutant Na+ channels. Notice that hNav1.5-CW/F1760K mutant Na+ currents were completely resistant to 300 µM lacosamide. (B) Superimposed ramp currents were generated by a 1-second ramp stimulus in the absence (control) and presence of 300 µM lacosamide (dashed line) as described in Fig. 8A. These hNav1.5-CW/F1760K mutant ramp currents also appeared completely resistant to 300 µM lacosamide.
Discussion
We characterized the action of lacosamide in hNav1.5 wild-type and inactivation-deficient Na+ channels. Our findings suggest that 1) the lacosamide binding site may be within the permeation pathway, because the drug elicits a conspicuous time-dependent block of persistent late Na+ currents; 2) lacosamide may bind to the intermediate pre-open states near the activation threshold of Na+ channels, but the development of this block is slow and the recovery from such block is rapid; 3) both fast and slow inactivation of Na+ channels may modulate lacosamide binding; and 4) the receptor for lacosamide overlaps the receptors for BTX and LAs. These notions clearly contradict with the existing hypothesis that lacosamide selectively enhances slow inactivation of Na+ channels (Errington et al., 2008; Sheets et al., 2008). One explanation is that the action of lacosamide in hNav1.5 cardiac Na+ channels is different from that in neuronal counterparts. Alternatively, because the block of the preopen states of Na+ channels by lacosamide develops slowly, it may have tricked us into believing that slow inactivation plays a pivotal role. This possibility was discussed (Errington et al., 2008) as one of several binding mechanisms for lacosamide.
Lacosamide Is an Open-Channel Blocker.
The time-dependent block provides a clear clue that lacosamide blocks the permeation pathway while the channel is in its open state. However, the on-rate for lacosamide block of the open Na+ channel is very different from that for class 1 antiarrhythmic flecainide. For lacosamide, the on- and off-rates at +50 mV are 7.5 × 104 M−1/s−1 and 18 s−1, respectively (Fig. 7C). For flecainide, the on- and the off-rates are 14.6 × 106 M−1/s−1 and 6.6 s−1, respectively (Wang et al., 2013). The slower on-rate for lacosamide binding is likely because of the absence of a positive charge of lacosamide in aqueous solution. Previous studies have indicated that a charged LA molecule has an 80-fold faster on-rate than its neutral counterpart in BTX-activated Na+ channels (Nettleton and Wang, 1990). There is no evidence that lacosamide block of the open Na+ channel is voltage dependent, probably because lacosamide carries no positive charge. This slow on-rate also explains that lacosamide elicits no use-dependent block of hNav1.5 Na+ channels during repetitive pulse because of their brief open dwell time (Fig. 2B).
Lacosamide Blocks Preopen States of Na+ Channels Preferentially, Albeit with a Slow Time Course.
The notion that lacosamide blocks preopen Na+ channels preferentially is more complicated. When activation of Na+ channels occurs rapidly during a test pulse of +50 mV, there is no evidence that lacosamide binds to hNav1.5 wild-type Na+ channels (Fig. 2). Such a result indicates that lacosamide does not interact with the resting state of hNav1.5 Na+ channels when the cell is held at −140 mV. On the other hand, if a conditioning pulse is allowed to last for 10 or 30 seconds near the activation threshold, there will be ample chances for lacosamide to bind with the pre-open states. With such a pulse protocol, the maximal block induced by lacosamide occurs between −90 and −50 mV (Figs. 3B and 4A). For hNav1.5 wild-type Na+ channels the development of block by lacosamide at −70 mV is extremely slow, with a τ value of 8.04 seconds. For inactivation-deficient hNav1.5-CW Na+ channels, the τ value is 3.28 seconds. This result implies that fast inactivation hinders the development of lacosamide block, as if the lacosamide receptor is guarded by the fast-inactivation gate (Starmer, 1987). In contrast, the recovery from lacosamide block of hNav1.5-CW Na+ channels is quite rapid, with a τ value of 476 milliseconds (Fig. 6C). The τ value is 639 milliseconds for wild-type hNav1.5 Na+ channels (Fig. 5B; 10-second pulse). This acceleration in inactivation-deficient hNav1.5-CW Na+ channels suggests that lacosamide once bound stays at its receptor longer when the channel is fast inactivated, as if the lacosamide receptor is also modulated by the fast-inactivation gate (Hille, 1977).
Moreover, slow inactivation may likewise modulate lacosamide binding. First, the recovery from lacosamide block in wild-type Na+ channels after a 30-second conditioning pulse is slower than that after a 10-second conditioning pulse, suggesting that the lacosamide receptor once occupied by its ligand may undergo additional slow conformational changes. The origin for these changes is unclear but could be due to slow inactivation gating. Second, the level of lacosamide block at −90 mV appears greater than that at −30 mV (Fig. 4), where slow inactivation occurs significantly during a 30-second conditioning pulse. This would suggest that, like fast inactivation, slow inactivation may also protect hNav1.5 Na+ channels from lacosamide binding.
Lacosamide Receptor Overlaps BTX and LA Receptors.
The facts that BTX-modified hNav1.5 Na+ channels and hNav1.5-CW/F1760K Na+ channels become resistant to lacosamide block suggest that the lacosamide binding site overlaps receptors for BTX and LAs. It is known that BTX abolishes both fast and slow inactivation of hNav1.5 cardiac Na+ channels (Wang et al., 2007) and keeps hNav1.5 channels open persistently. Such action may render the BTX-activated Na+ channels resistant to lacosamide through an allosteric site. However, both BTX and LA receptors are located on multiple S6 segments and they overlap one another, including at the F1760 residue (Linford et al., 1998; Wang and Wang, 2003).
The positive charge of protonated LAs interacts with π electrons on the benzene ring of F1760 (Ahern et al., 2008). Such interaction could not take place for benzocaine or lacosamide, because both drugs do not carry a positive charge (Fig. 1). An alternative binding could occur through the π stacking interaction between F1760 and the benzene ring of these neutral drugs. This alternative binding has been suggested in a closed Na+ channel (Bruhova et al., 2008). To reach F1760 in a closed preopen Na+ channel, lacosamide may need to pass laterally through the interfaces of S6 segments that are lining the inner cavity. This drug entryway may resemble the lateral pore fenestration found in closed bacterial Na+ channels (Payandeh et al., 2012).
How Does Lacosamide Block the Na+ Channel Permeation Pathway?
Because F1760 is critical for lacosamide binding, we suggest that during channel activation, the spatial arrangement of F1760 is altered as D4S6 moves from its resting to its open configuration through asynchronous movements of voltage sensors (Goldschen-Ohm et al., 2013). Before Na+ channels reach their activation threshold, lacosamide could access its receptor albeit with a slow time course. When a 30-second conditioning pulse of −90 mV is applied (Fig. 3A), such interactions between preopen Na+ channels and lacosamide could indeed produce a maximal block of hNav1.5 Na+ channels. This notion is consistent with the hypothesis that the D4S6 segment undergoes a conformational change upon depolarization that exposes the previously buried/hidden face of F1760 to the permeation pathway (Pless et al., 2011). Evidently, lacosamide can track the conformational changes of its receptor along the activation pathway as described for benzocaine and lidocaine (Vedantham and Cannon, 1999; Wang et al., 2004). As a result, the aberrant window currents that cause seizures (Spampanato et al., 2004) may be particularly susceptible to lacosamide block, because Na+ channels transition repetitively between pre-open and open states in chronically depolarized conditions.
Physiologic Significance of Lacosamide Action in Human Cardiac Na+ Channels.
The potency of lacosamide in hNav1.5 Na+ channels is comparable with that found in neuronal counterparts. An overdose of lacosamide (>100 µM) could therefore have considerable impact on cardiac Na+ channels and may trigger the cardiac dysfunctions reported recently (Degiorgio, 2010; Nizam et al., 2011; Chinnasami et al., 2013). On the other hand, lacosamide within its therapeutic concentration may be beneficial for patients who also suffer from aberrant cardiac window Na+ currents (Bennett, 2000; Belardinelli et al., 2006). Cautions should be taken in extrapolating our results in terms of lacosamide action in vivo, because cardiomyocytes likely display different posttranslational modifications of hNav1.5 Na+ channels and express multiple β-subunit isoforms (Maier et al., 2004) that are tightly associated with the α-subunit.
Acknowledgments
The authors thank Ms. Gabriella Russell for excellent assistance. The authors are grateful to Dr. Augustus Grant (Duke University, School of Medicine, Durham, NC) for providing the hNav1.5-pcDNA3 plasmid and to the late Dr. John Daly (Bethesda, MD) for providing batrachotoxin.
Abbreviations
- BTX
batrachotoxin
- DMSO
dimethylsulfoxide
- HEK
human embryonic kidney
- hNav1.5
human cardiac Na+ channel
- hNav1.5-CW
human cardiac Nav1.5 Na+ channel with L409C/A410W mutations
- LAs
local anesthetics
Authorship Contributions
Participated in research design: G. K. Wang, S.-Y. Wang.
Conducted experiments: G. K. Wang, S.-Y. Wang.
Performed data analysis: G. K. Wang, S.-Y. Wang.
Wrote or contributed to the writing of the manuscript: G. K. Wang, S.-Y. Wang.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM094152].
References
- Ahern CA, Eastwood AL, Dougherty DA, Horn R. (2008) Electrostatic contributions of aromatic residues in the local anesthetic receptor of voltage-gated sodium channels. Circ Res 102:86–94 [DOI] [PubMed] [Google Scholar]
- Belardinelli L, Shryock JC, Fraser H. (2006) Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart 92 (Suppl 4):iv6–iv14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett PB. (2000) Long QT syndrome: biophysical and pharmacologic mechanisms in LQT3. J Cardiovasc Electrophysiol 11:819–822 [DOI] [PubMed] [Google Scholar]
- Bruhova I, Tikhonov DB, Zhorov BS. (2008) Access and binding of local anesthetics in the closed sodium channel. Mol Pharmacol 74:1033–1045 [DOI] [PubMed] [Google Scholar]
- Catterall WA. (2012) Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol 590:2577–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnasami S, Rathore C, Duncan JS. (2013) Sinus node dysfunction: an adverse effect of lacosamide. Epilepsia 54:e90–e93 [DOI] [PubMed] [Google Scholar]
- Cota G, Armstrong CM. (1989) Sodium channel gating in clonal pituitary cells. The inactivation step is not voltage dependent. J Gen Physiol 94:213–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Sigworth FJ. (1996) Impaired slow inactivation in mutant sodium channels. Biophys J 71:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degiorgio CM. (2010) Atrial flutter/atrial fibrillation associated with lacosamide for partial seizures. Epilepsy Behav 18:322–324 [DOI] [PubMed] [Google Scholar]
- Errington AC, Coyne L, Stöhr T, Selve N, Lees G. (2006) Seeking a mechanism of action for the novel anticonvulsant lacosamide. Neuropharmacology 50:1016–1029 [DOI] [PubMed] [Google Scholar]
- Errington AC, Stöhr T, Heers C, Lees G. (2008) The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol Pharmacol 73:157–169 [DOI] [PubMed] [Google Scholar]
- Goldschen-Ohm MP, Capes DL, Oelstrom KM, Chanda B. (2013) Multiple pore conformations driven by asynchronous movements of voltage sensors in a eukaryotic sodium channel. Nat Commun 4:1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halász P, Kälviäinen R, Mazurkiewicz-Beldzińska M, Rosenow F, Doty P, Hebert D, Sullivan T, SP755 Study Group (2009) Adjunctive lacosamide for partial-onset seizures: Efficacy and safety results from a randomized controlled trial. Epilepsia 50:443–453 [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100 [DOI] [PubMed] [Google Scholar]
- Hille B. (1977) Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol 69:497–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Bean BP. (2011) Inhibition of neuronal voltage-gated sodium channels by brilliant blue G. Mol Pharmacol 80:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford NJ, Cantrell AR, Qu Y, Scheuer T, Catterall WA. (1998) Interaction of batrachotoxin with the local anesthetic receptor site in transmembrane segment IVS6 of the voltage-gated sodium channel. Proc Natl Acad Sci USA 95:13947–13952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. (2004) Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation 109:1421–1427 [DOI] [PubMed] [Google Scholar]
- McNulty MM, Kyle JW, Lipkind GM, Hanck DA. (2006) An inner pore residue (Asn406) in the Nav1.5 channel controls slow inactivation and enhances mibefradil block to T-type Ca2+ channel levels. Mol Pharmacol 70:1514–1523 [DOI] [PubMed] [Google Scholar]
- Nettleton J, Wang GK. (1990) pH-dependent binding of local anesthetics in single batrachotoxin-activated Na+ channels. Cocaine vs. quaternary compounds. Biophys J 58:95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niespodziany I, Leclère N, Vandenplas C, Foerch P, Wolff C. (2013) Comparative study of lacosamide and classical sodium channel blocking antiepileptic drugs on sodium channel slow inactivation. J Neurosci Res 91:436–443 [DOI] [PubMed] [Google Scholar]
- Nizam A, Mylavarapu K, Thomas D, Briskin K, Wu B, Saluja D, Wong S. (2011) Lacosamide-induced second-degree atrioventricular block in a patient with partial epilepsy. Epilepsia 52:e153–e155 [DOI] [PubMed] [Google Scholar]
- O’Reilly JP, Wang SY, Kallen RG, Wang GK. (1999) Comparison of slow inactivation in human heart and rat skeletal muscle Na+ channel chimaeras. J Physiol 515:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Gamal El-Din TM, Scheuer T, Zheng N, Catterall WA. (2012) Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature 486:135–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pless SA, Galpin JD, Frankel A, Ahern CA. (2011) Molecular basis for class Ib anti-arrhythmic inhibition of cardiac sodium channels. Nat Commun 2:351. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Featherstone DE, Hartmann HA, Ruben PC. (1998) Slow inactivation in human cardiac sodium channels. Biophys J 74:2945–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. (1978) Slow inactivation of the sodium conductance in squid giant axons. Pronase resistance. J Physiol 283:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets PL, Heers C, Stoehr T, Cummins TR. (2008) Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide], lidocaine, and carbamazepine. J Pharmacol Exp Ther 326:89–99 [DOI] [PubMed] [Google Scholar]
- Spampanato J, Kearney JA, de Haan G, McEwen DP, Escayg A, Aradi I, MacDonald BT, Levin SI, Soltesz I, Benna P, et al. (2004) A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction. J Neurosci 24:10022–10034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer CF. (1987) Theoretical characterization of ion channel blockade. Competitive binding to periodically accessible receptors. Biophys J 52:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedantham V, Cannon SC. (1999) The position of the fast-inactivation gate during lidocaine block of voltage-gated Na+ channels. J Gen Physiol 113:7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GK, Russell G, Wang SY. (2013) Persistent human cardiac Na (+) currents in stably transfected mammalian cells: Robust expression and distinct open-channel selectivity among Class 1 antiarrhythmics. Channels (Austin) 7:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-Y, Bonner K, Russell C, Wang GK. (2003) Tryptophan scanning of D1S6 and D4S6 C-termini in voltage-gated sodium channels. Biophys J 85:911–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-Y, Mitchell J, Moczydlowski E, Wang GK. (2004) Block of inactivation-deficient Na+ channels by local anesthetics in stably transfected mammalian cells: evidence for drug binding along the activation pathway. J Gen Physiol 124:691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-Y, Tikhonov DB, Mitchell J, Zhorov BS, Wang GK. (2007) Irreversible block of cardiac mutant Na+ channels by batrachotoxin. Channels (Austin) 1:179–188 [DOI] [PubMed] [Google Scholar]
- Wang S-Y, Wang GK. (2003) Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell Signal 15:151–159 [DOI] [PubMed] [Google Scholar]
- Wang S-Y, Wang GK. (1996) Slow inactivation of muscle mu1 Na+ channels in permanently transfected mammalian cells. Pflugers Arch 432:692–699 [DOI] [PubMed] [Google Scholar]
- Wright SN, Wang S-Y, Kallen RG, Wang GK. (1997) Differences in steady-state inactivation between Na channel isoforms affect local anesthetic binding affinity. Biophys J 73:779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]