Abstract
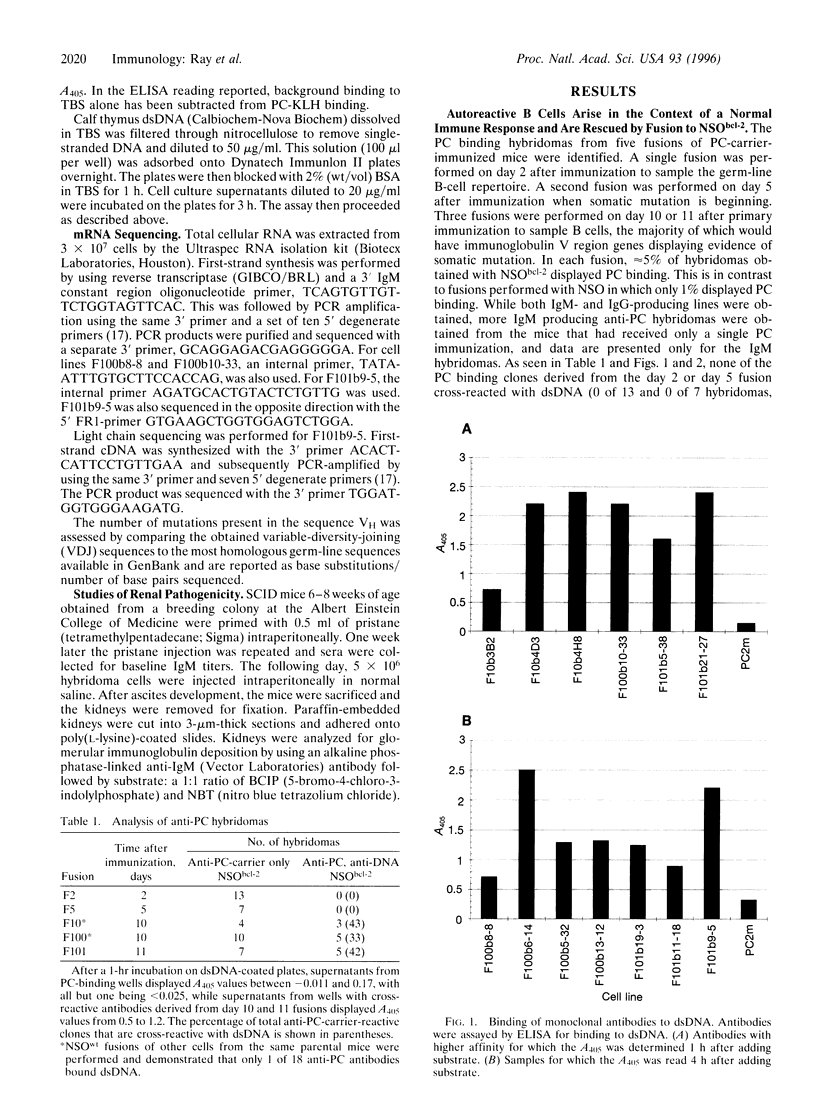
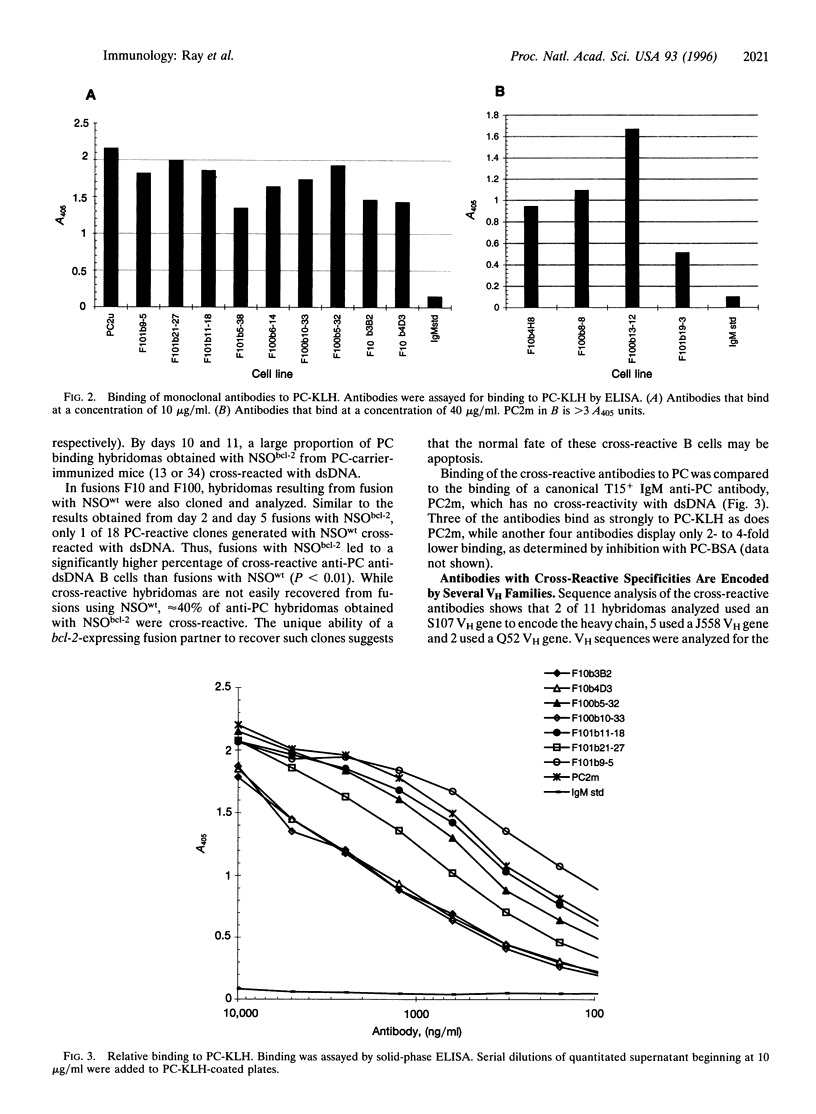
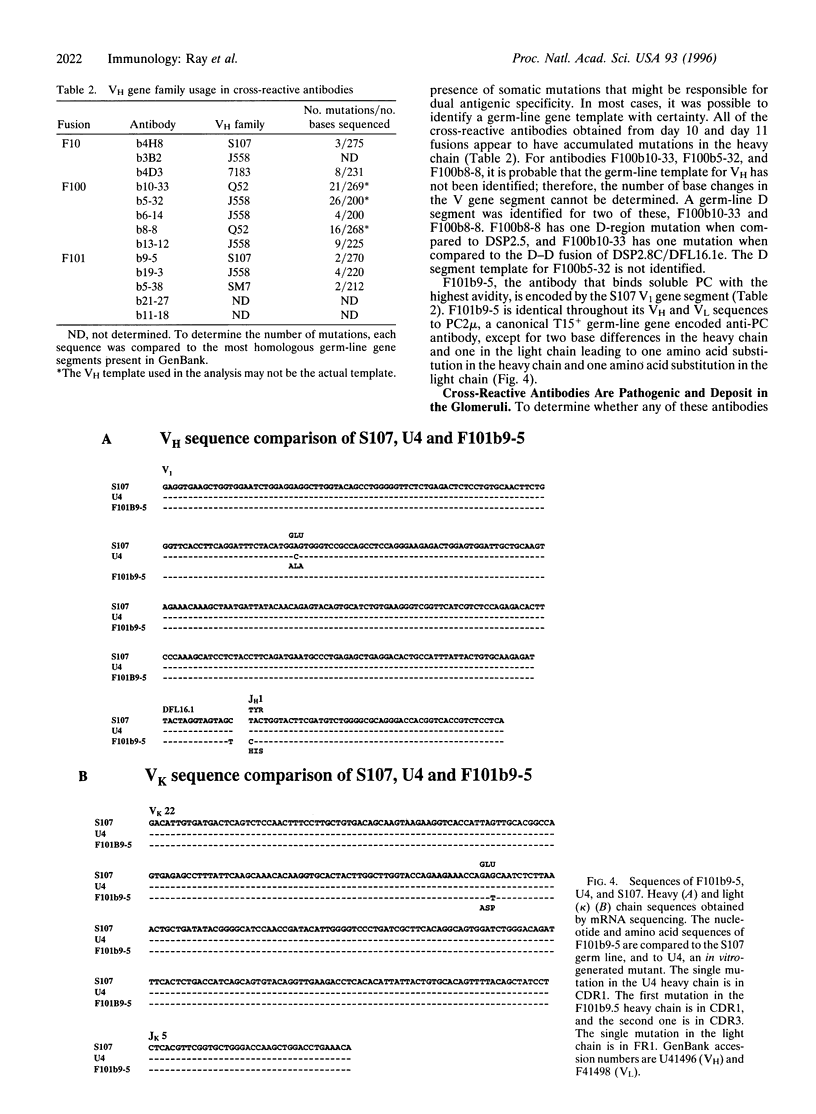
The immune system's ability to distinguish self and nonself is essential for both host defense against foreign agents and protection of self-antigens from autoimmune destruction. Such discrimination is complicated by extensive structural homology shared between foreign and self antigens. One hypothesis to explain the development of an autoimmune response is that some B cells activated by foreign antigen acquire, through somatic mutation, specificity for both the eliciting foreign antigen and self antigen. If such clones arise frequently, there must be a mechanism for their elimination. We have analyzed the extent of autoreactivity arising in a nonautoimmune host during the response to a foreign antigen. To overcome the process of apoptosis in primary B cells that might routinely eliminate autoreactive clones, we generated B-cell hybridomas from spleen cells of immunized mice by using a fusion partner constitutively expressing bcl-2. Multiple lines were obtained that recognize simultaneously the hapten phosphorylcholine and the self antigen double-stranded DNA. This dual specificity was not present early but was detected by day 10 after immunization. Some of these cross-reactive antibodies deposit in kidneys in a pattern similar to what is seen in autoimmune disease. These results demonstrate that autoantibodies arise at a high frequency as part of a response to foreign antigen. It has previously been shown that autoreactivity is regulated by central deletion; these data demonstrate a need for negative selection in peripheral lymphoid organs also, to regulate autoantibodies acquiring their self-specificity by somatic mutation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnefoy J. Y., Henchoz S., Hardie D., Holder M. J., Gordon J. A subset of anti-CD21 antibodies promote the rescue of germinal center B cells from apoptosis. Eur J Immunol. 1993 Apr;23(4):969–972. doi: 10.1002/eji.1830230432. [DOI] [PubMed] [Google Scholar]
- Borghesi C., Nicoletti C. Increase of cross(auto)-reactive antibodies after immunization in aged mice: a cellular and molecular study. Int J Exp Pathol. 1994 Apr;75(2):123–130. [PMC free article] [PubMed] [Google Scholar]
- Chang S. P., Brown M., Rittenberg M. B. Immunologic memory to phosphorylcholine. II. PC-KLH induces two antibody populations that dominate different isotypes. J Immunol. 1982 Feb;128(2):702–706. [PubMed] [Google Scholar]
- Claflin J. L., Lieberman R., Davie J. M. Clonal nature of the immune response to phosphorylcholine. I. Specificity, class, and idiotype of phosphorylcholine-binding receptors on lymphoid cells. J Exp Med. 1974 Jan 1;139(1):58–73. doi: 10.1084/jem.139.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. R., Young D. B. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991 Apr;12(4):105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Diamond B., Katz J. B., Paul E., Aranow C., Lustgarten D., Scharff M. D. The role of somatic mutation in the pathogenic anti-DNA response. Annu Rev Immunol. 1992;10:731–757. doi: 10.1146/annurev.iy.10.040192.003503. [DOI] [PubMed] [Google Scholar]
- Diamond B., Scharff M. D. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Munaín C., De Diego J. L., Alcina A., Fresno M. A Trypanosoma cruzi membrane protein shares an epitope with a lymphocyte activation antigen and induces crossreactive antibodies. J Exp Med. 1992 Jun 1;175(6):1473–1482. doi: 10.1084/jem.175.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder M. J., Wang H., Milner A. E., Casamayor M., Armitage R., Spriggs M. K., Fanslow W. C., MacLennan I. C., Gregory C. D., Gordon J. Suppression of apoptosis in normal and neoplastic human B lymphocytes by CD40 ligand is independent of Bc1-2 induction. Eur J Immunol. 1993 Sep;23(9):2368–2371. doi: 10.1002/eji.1830230948. [DOI] [PubMed] [Google Scholar]
- Johnson J. G., Jemmerson R. Tolerance induction in resting memory B cells specific for a protein antigen. J Immunol. 1992 May 1;148(9):2682–2689. [PubMed] [Google Scholar]
- Katz J. B., Limpanasithikul W., Diamond B. Mutational analysis of an autoantibody: differential binding and pathogenicity. J Exp Med. 1994 Sep 1;180(3):925–932. doi: 10.1084/jem.180.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettleborough C. A., Saldanha J., Ansell K. H., Bendig M. M. Optimization of primers for cloning libraries of mouse immunoglobulin genes using the polymerase chain reaction. Eur J Immunol. 1993 Jan;23(1):206–211. doi: 10.1002/eji.1830230132. [DOI] [PubMed] [Google Scholar]
- Knox K. A., Gordon J. Protein tyrosine phosphorylation is mandatory for CD40-mediated rescue of germinal center B cells from apoptosis. Eur J Immunol. 1993 Oct;23(10):2578–2584. doi: 10.1002/eji.1830231030. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2: a repressor of lymphocyte death. Immunol Today. 1992 Aug;13(8):285–288. doi: 10.1016/0167-5699(92)90037-8. [DOI] [PubMed] [Google Scholar]
- Lawson C. M., O'Donoghue H. L., Reed W. D. Mouse cytomegalovirus infection induces antibodies which cross-react with virus and cardiac myosin: a model for the study of molecular mimicry in the pathogenesis of viral myocarditis. Immunology. 1992 Mar;75(3):513–519. [PMC free article] [PubMed] [Google Scholar]
- Levy Y., Brouet J. C. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994 Jan;93(1):424–428. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpanasithikul W., Ray S., Diamond B. Cross-reactive antibodies have both protective and pathogenic potential. J Immunol. 1995 Jul 15;155(2):967–973. [PubMed] [Google Scholar]
- Linton P. J., Rudie A., Klinman N. R. Tolerance susceptibility of newly generating memory B cells. J Immunol. 1991 Jun 15;146(12):4099–4104. [PubMed] [Google Scholar]
- Liu Y. J., Mason D. Y., Johnson G. D., Abbot S., Gregory C. D., Hardie D. L., Gordon J., MacLennan I. C. Germinal center cells express bcl-2 protein after activation by signals which prevent their entry into apoptosis. Eur J Immunol. 1991 Aug;21(8):1905–1910. doi: 10.1002/eji.1830210819. [DOI] [PubMed] [Google Scholar]
- Lötscher M., Amstutz H., Heusser C. H., Blaser K. Specific VDJ gene combinations contribute to the specificity of memory antibodies to the phosphorylcholine hapten. J Immunol. 1992 Jun 1;148(11):3631–3635. [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Pulendran B., Kannourakis G., Nouri S., Smith K. G., Nossal G. J. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995 May 25;375(6529):331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- Ray S., Diamond B. Generation of a fusion partner to sample the repertoire of splenic B cells destined for apoptosis. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5548–5551. doi: 10.1073/pnas.91.12.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokat K. M., Goodnow C. C. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995 May 25;375(6529):334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore M. P., Rittenberg M. B. Clonal diversity, somatic mutation, and immune memory to phosphocholine-keyhole limpet hemocyanin. J Immunol. 1989 Dec 15;143(12):4123–4133. [PubMed] [Google Scholar]
- Ternynck T., Bleux C., Gregoire J., Avrameas S., Kanellopoulos-Langevin C. Comparison between autoantibodies arising during Trypanosoma cruzi infection in mice and natural autoantibodies. J Immunol. 1990 Feb 15;144(4):1504–1511. [PubMed] [Google Scholar]
- Thorbecke G. J., Amin A. R., Tsiagbe V. K. Biology of germinal centers in lymphoid tissue. FASEB J. 1994 Aug;8(11):832–840. doi: 10.1096/fasebj.8.11.8070632. [DOI] [PubMed] [Google Scholar]
- Unni K. K., Holley K. E., McDuffie F. C., Titus J. L. Comparative study of NZB mice under germfree and conventional conditions. J Rheumatol. 1975 Mar;2(1):36–44. [PubMed] [Google Scholar]
- Zhao K. S., Wang Y. F., Guéret R., Weksler M. E. Dysregulation of the humoral immune response in old mice. Int Immunol. 1995 Jun;7(6):929–934. doi: 10.1093/intimm/7.6.929. [DOI] [PubMed] [Google Scholar]
- Zupo S., Rugari E., Dono M., Taborelli G., Malavasi F., Ferrarini M. CD38 signaling by agonistic monoclonal antibody prevents apoptosis of human germinal center B cells. Eur J Immunol. 1994 May;24(5):1218–1222. doi: 10.1002/eji.1830240532. [DOI] [PubMed] [Google Scholar]



