Abstract
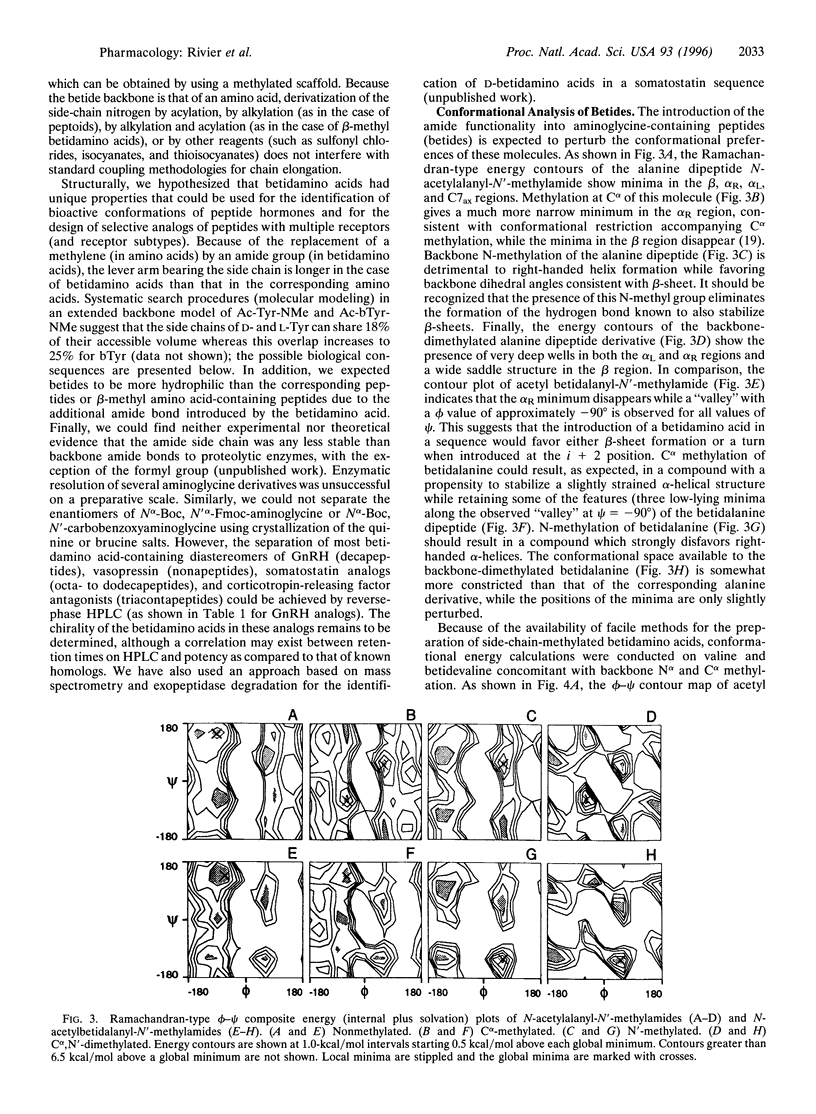
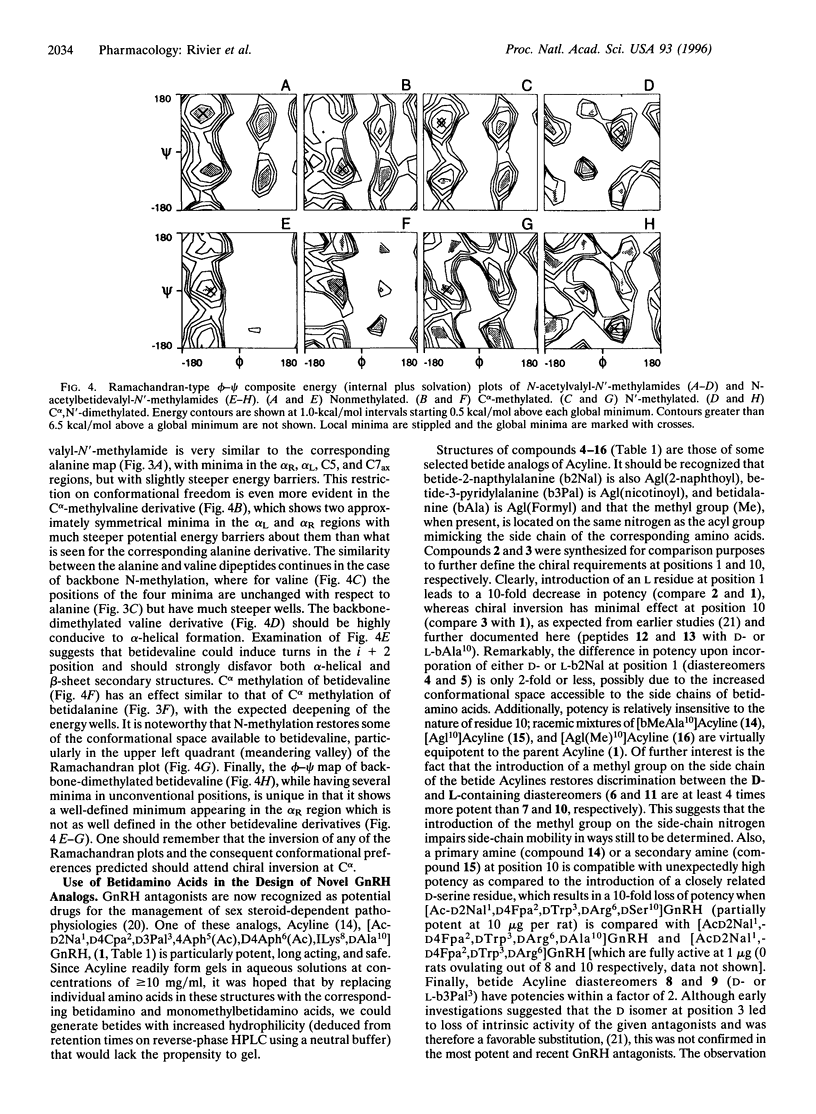
Betidamino acids (a contraction of "beta" position and "amide") are N'-monoacylated (optionally, N'-monoacylated and N-mono- or N,N'-dialkylated) aminoglycine derivatives in which each N'acyl/alkyl group may mimic naturally occurring amino acid side chains or introduce novel functionalities. Betidamino acids are most conveniently generated on solid supports used for the synthesis of peptides by selective acylation of one of the two amino functions of orthogonally protected aminoglycine(s) to generate the side chain either prior to or after the elongation of the main chain. We have used unresolved Nalpha-tert-butyloxycarbonyl-N'alpha-fluorenylmethoxycarbonyl++ + aminoglycine, and Nalpha-(Nalpha-methyl)-tert-butyloxycarbonyl-N'alpha-fluo renylmethoxycarbonyl aminoglycine as the templates for the introduction of betidamino acids in Acyline [Ac-D2Nal-D4Cpa-D3Pal-Ser-4Aph(Ac)-D4Aph(A c)-Leu-Ilys-Pro-DAla-NH2, where 2Nal is 2-naphthylalanine, 4Cpa is 4-chlorophenylalanine, 3Pal is 3-pyridylalanine, Aph is 4-aminophenylalanine, and Ilys is Nepsilon-isopropyllysine], a potent gonadotropin-releasing hormone antagonist, in order to test biocompatibility of these derivatives. Diasteremneric peptides could be separated in most cases by reverse-phase HPLC. Biological results indicated small differences in relative potencies (<5-fold) between the D and L nonalkylated betidamino acid-containing Acyline derivatives. Importantly, most betide diastereomers were equipotent with Acyline. In an attempt to correlate structure and observed potency, Ramachandran-type plots were calculated for a series of betidamino acids and their methylated homologs. According to these calculations, betidamino acids have access to a more limited and distinct number of conformational states (including those associated with alpha-helices, beta-sheets, or turn structures), with deeper minima than those observed for natural amino acids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corbin A., Beattie C. W. Ihibition of the pre-ovulatory proestrous gonadotropin surge, ovulation and pregnancy with a peptide analogue of luteinizing hormone releasing hormone. Endocr Res Commun. 1975;2(1):1–23. doi: 10.3109/07435807509053836. [DOI] [PubMed] [Google Scholar]
- Karten M. J., Rivier J. E. Gonadotropin-releasing hormone analog design. Structure-function studies toward the development of agonists and antagonists: rationale and perspective. Endocr Rev. 1986 Feb;7(1):44–66. doi: 10.1210/edrv-7-1-44. [DOI] [PubMed] [Google Scholar]
- Maple J. R., Dinur U., Hagler A. T. Derivation of force fields for molecular mechanics and dynamics from ab initio energy surfaces. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5350–5354. doi: 10.1073/pnas.85.15.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall G. R., Clark J. D., Dunbar J. B., Jr, Smith G. D., Zabrocki J., Redlinski A. S., Leplawy M. T. Conformational effects of chiral alpha,alpha-dialkyl amino acids. I. C-terminal tetrapeptides of emerimicin containing alpha-ethylalanine. Int J Pept Protein Res. 1988 Dec;32(6):544–555. doi: 10.1111/j.1399-3011.1988.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Moran E. J., Wilson T. E., Cho C. Y., Cherry S. R., Schultz P. G. Novel biopolymers for drug discovery. Biopolymers. 1995;37(3):213–219. doi: 10.1002/bip.360370305. [DOI] [PubMed] [Google Scholar]
- Rivier J. E., Jiang G., Porter J., Hoeger C. A., Craig A. G., Corrigan A., Vale W., Rivier C. L. Gonadotropin-releasing hormone antagonists: novel members of the azaline B family. J Med Chem. 1995 Jul 7;38(14):2649–2662. doi: 10.1021/jm00014a017. [DOI] [PubMed] [Google Scholar]
- Simon R. J., Kania R. S., Zuckermann R. N., Huebner V. D., Jewell D. A., Banville S., Ng S., Wang L., Rosenberg S., Marlowe C. K. Peptoids: a modular approach to drug discovery. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9367–9371. doi: 10.1073/pnas.89.20.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. B., 3rd, Hirschmann R., Pasternak A., Akaishi R., Guzman M. C., Jones D. R., Keenan T. P., Sprengeler P. A., Darke P. L., Emini E. A. Design and synthesis of peptidomimetic inhibitors of HIV-1 protease and renin. Evidence for improved transport. J Med Chem. 1994 Jan 21;37(2):215–218. doi: 10.1021/jm00028a001. [DOI] [PubMed] [Google Scholar]


