Abstract
The vast majority of new HIV infections result from relatively inefficient transmission1,2 of the virus across mucosal surfaces during sexual intercourse3. A consequence of this inefficiency is that small numbers of transmitted founder viruses initiate most heterosexual infections4. This natural bottleneck to transmission has stimulated efforts to develop interventions aimed at blocking this step of the infection process5. Despite the promise of this strategy, clinical trials of pre-exposure prophylaxis have had limited degrees of success in humans, due in part to lack of adherence to the recommended pre-exposure treatment regimens6,7. In contrast, a number of existing vaccines elicit systemic immunity that protects against mucosal infections, such as the vaccines for influenza8 and HPV9. We recently demonstrated the ability of vectored immunoprophylaxis (VIP) to prevent intravenous transmission of HIV using broadly neutralizing antibodies10. Here we demonstrate that VIP is capable of protecting humanized mice from intravenous as well as vaginal challenge with diverse viral strains, despite repeated exposures. Moreover, animals receiving VIP that expresses a modified VRC07 antibody were completely resistant to repetitive intravaginal challenge by a heterosexually transmitted founder HIV strain11, suggesting that VIP may be effective in preventing vaginal transmission of HIV between humans.
Keywords: HIV, antibody, prophylaxis, vaccine, AAV, humanized mice, T lymphocyte, VIP, engineered immunity, mucosal transmission
The description of numerous broadly neutralizing HIV antibodies has invigorated strategies aimed at eliciting similar antibodies in naïve patients12. In addition, it has been proposed that such antibodies could serve to prevent the transmission of HIV if administered to patients by passive transfer. We and others have described the use of adeno-associated virus (AAV) vectors to deliver the genes encoding such antibodies or immunoadhesins to muscle tissues, thereby enabling their long-term, systemic production, and have demonstrated the effectiveness of such a strategy to prevent intravenous transmission of SIV13 or laboratory strains of HIV10. However, it remains to be shown whether such an approach can be effective against HIV transmission between humans, where transmission typically occurs across mucosal surfaces by HIV strains that are distinct from the laboratory strains currently used to test interventions.
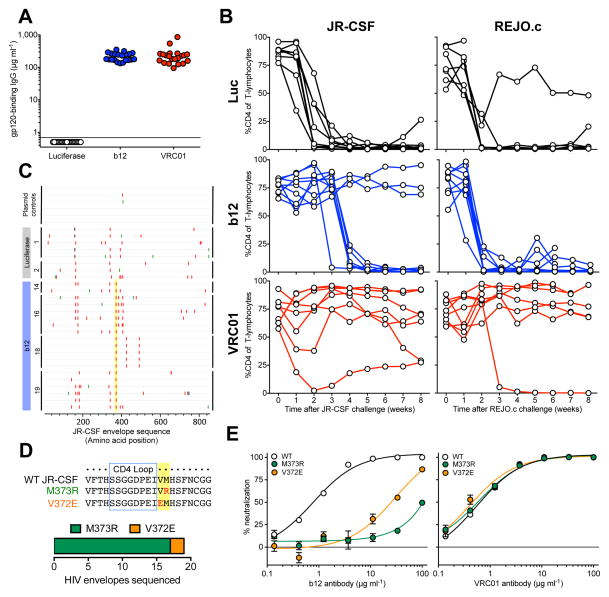
To explore this question, we determined the ability of VIP to prevent intravenous transmission of both JR-CSF, a CCR5-tropic primary isolate14, as well as REJO.c, a CCR5-tropic transmitted molecular founder strain11. AAV vectors were administered intramuscularly to establish groups of mice expressing high levels of human b12 IgG, VRC01 IgG, or luciferase as a negative control (Fig. 1a). Following human peripheral blood mononuclear cell (PBMC) administration and engraftment, humanized mice were challenged intravenously with either JR-CSF or REJO.c (Fig. 1b). With either virus challenge, we observed robust depletion of CD4 cells in animals expressing luciferase. Similarly, all b12-expressing mice challenged with REJO.c exhibited CD4 cell depletion, consistent with the previously observed resistance of this strain to b12 in vitro (Supplemental Fig. 1). Unexpectedly, only three of the eight animals expressing b12 exhibited CD4 cell protection following JR-CSF challenge (Fig. 1b). To determine whether viral escape was responsible for the loss of CD4 cells observed in the remaining animals, we sequenced the viral envelope from mice exhibiting CD4-cell depletion and compared them to the known wildtype sequence of JR-CSF (Fig. 1c). Interestingly, envelope sequences obtained from mice expressing b12 antibody exhibited many of the same common mutations found in luciferase control animals, but also contained additional unique mutations at JR-CSF Env residues V372 or M373 (numbered relative to the HXB2 reference strain), both of which have been previously implicated in escape from b12 neutralization15 (Fig. 1d). To determine whether these mutations were responsible for the in vivo escape of JR-CSF from b12, we engineered each individual mutation into otherwise wildtype JR-CSF and tested the sensitivity of the resulting viral stocks to either b12 or VRC01 in vitro (Fig. 1e). Interestingly, we found that either mutation enabled nearly complete resistance to b12 but neither had an effect on VRC01 neutralization. This was despite both antibodies targeting the CD4 binding site (CD4bs) of envelope, and likely results from their distinct modes of CD4bs recognition16 (Supplemental Fig. 2). When humanized mice expressing VRC01 were challenged with JR-CSF or REJO.c, all animals, except a single REJO.c challenged mouse, showed at least partial protection of CD4 cells (Fig. 1b). The single VRC01-expressing mouse which lost all CD4 cells exhibited no detectable viral load at any time point tested and efforts to amplify envelope sequences from either plasma viral RNA or genomic DNA were unsuccessful (data not shown). Consequently, we suspect that this mouse lost CD4 cells for reasons unrelated to HIV challenge. Together, these results demonstrate that mice can be protected against CCR5-tropic HIV strains by VRC01, but that the b12 monoclonal antibody, which provided robust protection against the CXCR4-tropic NL4-3 strain10, is easily escaped by the CCR5-tropic JR-CSF strain.
Figure 1. VIP protects against CD4 cell depletion in humanized mice resulting from challenge with CCR5-tropic or transmitted founder HIV strains.
(a) Quantitation of human antibodies in serum one day prior to challenge, 3 weeks after adoptive transfer of human PBMCs and 13 weeks after intramuscular administration of 1×1011 GC of AAV encoding either luciferase, b12, or VRC01-IgG as detected by a gp120-specific ELISA to determine the fraction of human IgG capable of binding HIV (n=21). (b) CD4 cells as a percentage of CD3 positive T-lymphocytes in the peripheral blood of PBMC-NSG humanized mice expressing luciferase (top), b12 (center) or VRC01 (bottom) following intravenous challenge with either JR-CSF (left) or REJO.c (right) strains of HIV as measured by flow cytometry. (c) Highlighter plot of envelope sequences amplified from control JR-CSF plasmid, or spleen genomic DNA from infected luciferase- or b12-expressing humanized mice as compared to parental JR-CSF sequence. Colored marks along each line indicate the position of missense mutations, silent mutations or gaps in alignment in red, green or grey respectively. Yellow highlight denotes mutations commonly identified among sequences isolated from antibody but not luciferase-expressing animals. (d) (top) Alignment of envelope sequences from wildtype compared to M373R, or V372E mutant JR-CSF demonstrating the location of mutations relative to the CD4 binding loop. (bottom) Relative frequency of the two observed escape mutations obtained from four independent b12-expressing mice. (e) In vitro neutralization assays performed using the TZM-bl cell line infected with either wildtype or the indicated mutant strain of JR-CSF in the presence of serial dilutions of either b12 (left) or VRC01 (right) (n=3). Each plot contains data generated from one individual experiment, and each experiment was performed once with the indicated number of mice.
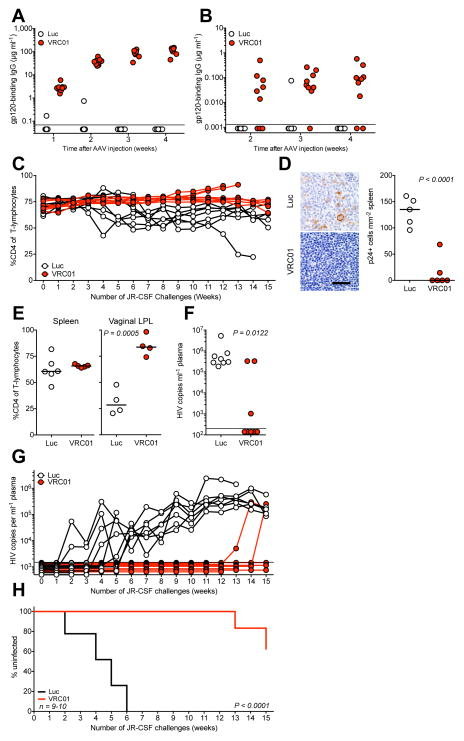
While these results show that VRC01 is capable of preventing the intravenous transmission of multiple strains, the predominant route of HIV infection worldwide is via heterosexual contact3. The BLT humanized mouse model, in contrast to huPBMC-NSG mice, exhibits extensive engraftment of human immune cells into mucosal tissues, allowing for HIV transmission to occur across mucosal surfaces17. To create a model of heterosexual transmission that better reflects the stochastic nature of human transmission, we modified the established high-dose, single vaginal challenge model in bone-liver-thymus (BLT) humanized mice18 to implement a repetitive, non-abrasive, low-dose viral challenge similar to those utilized in non-human primates19. Following administration of VIP encoding VRC01 to BLT mice, we observed production of human IgG specific for HIV gp120 at over 100 μg ml−1 in serum, while a luciferase-encoding vector produced no specific antibody (Fig. 2a). To determine the concentration of antibody reaching the challenge site, we analyzed vaginal wash fluid by ELISA and detected nearly 100 ng ml−1 of VRC01 four weeks post-AAV injection and one day prior to the first challenge (Fig. 2b). We believe this to be a minimum estimate of the concentration because of the uncertainty of the dilution resulting from washing a small volume of vaginal mucus. We challenged mice weekly via intravaginal administration of JR-CSF and collected blood samples to monitor CD4 depletion and viral load. We observed limited but detectable depletion of CD4 cells in mice expressing luciferase but steady or rising CD4 ratios in mice expressing VRC01 (Fig. 2c). At the conclusion of the study, we subjected spleens from both groups to immunohistochemistry and observed a substantial number of p24 expressing cells in the spleens of luciferase control mice, which were largely absent from mice expressing VRC01 (Fig. 2d and Supplemental Fig. 3). While we observed very limited depletion of CD4 cells in the spleen by FACS, we found significant depletion of CD4 cells in the vaginal lamina propria of luciferase-expressing mice as compared to VRC01-expressing mice (Fig. 2e). Serum samples collected at terminal time points demonstrated that all luciferase-expressing mice had become infected while five of eight VRC01-expressing mice exhibited no detectable virus using an ultrasensitive clinical viral load assay (Fig. 2f). To determine the time course of infection, we subjected longitudinal serum samples to a viral load assay with reduced sensitivity and observed infection of control mice with a mean of 4.25 ± 1.32 challenges (Fig. 2g). In contrast, this assay indicated that only two VRC01 mice became infected over the duration of the experiment, and this occurred only after 13 or 15 exposures, indicating that VIP expressing VRC01 substantially reduced the risk of infection (Fig. 2h).
Figure 2. VIP prevents mucosal transmission of CCR5-tropic HIV following repetitive intravaginal challenge.
(a) Quantitation of gp120-binding human antibody in BLT humanized mice at the indicated times following administration of 1×1011 GC of AAV encoding either luciferase or VRC01-IgG prior to challenge as measured by ELISA (n=9–10). Limit of detection = 70 ng ml−1. (b) Quantitation of gp120-binding human antibody in vaginal wash samples taken from BLT humanized mice at the indicated times following administration of 1×1011 GC of AAV encoding either luciferase or VRC01-IgG prior to challenge as measured by ELISA (n=9–10). Limit of detection = 1.3 ng ml−1. (c) CD4 cells as a percentage of CD3 positive T-lymphocytes in the peripheral blood of BLT humanized mice expressing luciferase (white) or VRC01 (red) throughout weekly intravaginal challenges with JR-CSF as measured by flow cytometry. (d) (left) HIV p24 detection by immunohistochemical (IHC) staining of representative sections taken from spleens of challenged animals. Scale bar represents 40 micrometers. (right) Quantitation of IHC staining of spleen denoting the relative frequency of p24-expressing cells in spleens of challenged animals. (e) CD4 cells as a percentage of CD3 positive T-lymphocytes in mouse spleen (left) or vaginal lamina propria (right) tissues after repetitively challenge as detected by flow cytometry. (f) HIV viral load detected in plasma of mice at the time of sacrifice of repetitively challenged BLT mice as detected by the Abbott RealTime HIV-1 Viral Load Assay. Limit of detection = 200 copies ml−1. (g) HIV viral load in plasma throughout weekly intravaginal challenge of BLT mice as detected by an in-house viral load assay. Limit of detection = 1500 copies ml−1. (h) Fraction of uninfected mice over the course of repetitive intravaginal challenge. Positive infection defined by two consecutive viral load measurements above the limit of detection (n=9–10). Statistics for grouped comparisons calculated using either a one or two-tailed t-test. Statistics for Kaplan-Meier analysis calculated by log rank test. Samples from mice exhibiting fewer than 20 CD3 positive cells were excluded from this analysis. Each plot contains data generated from one individual experiment, and each experiment was performed once with the indicated number of mice.
Since the available repertoire of broadly neutralizing antibodies against HIV has dramatically expanded since our original study, we set out to determine the minimum protective dose of the recently-isolated antibodies to ascertain their in vivo potency. Mice were given decreasing doses of AAV vectors encoding each antibody, or a luciferase-encoding AAV as a control, to establish groups of animals expressing a range of antibody concentrations (Supplemental Fig. 4). After administration and engraftment of human PBMCs, mice were challenged intravenously with 10 ng p24 of NL4-3 and monitored weekly for CD4 decline. As summarized in Table 1, we observed protection of humanized mice with a number of antibodies at concentrations as low as 350 ng ml−1 (Supplemental Fig. 5). Factoring together the activity we observed and the published breadth of each antibody, we selected the recently-described VRC07 antibody containing a G54W mutation20,21 for further study.
Table 1.
Protection against NL4-3 challenge in vivo by the indicated antibody concentration (μg ml−1)
| Vector | Dose administered (GC) | ||||
|---|---|---|---|---|---|
|
| |||||
| 1×1011 | 5×1010 | 2.5×1010 | 1.25×1010 | 6.25×109 | |
| 3BNC117 | 24.41 | 7.45 | 2.43 | 0.46 | 0.21 |
| 12A12 | 22.87 | 7.30 | 2.59 | 0.43 | 0.12 |
| VRC-PG04 | 31.64 | 20.49 | 5.25 | 1.38 | 0.35 |
| VRC07 | 131.42 | 65.82 | 26.51 | 5.76 | 0.39 |
| VRC07G54W | 73.51 | 35.00 | 15.30 | 0.68 | 0.69 |
| NIH45-46G54W | 39.24 | 13.58 | 0.87 | 0.46 | 0.05 |
| PGT121* | 256.19 | 108.51 | 70.60 | 13.70 | 2.89 |
| PGT128* | 50.34 | 36.26 | 49.46 | 18.97 | 4.96 |
| PG9 | 390.61 | 263.42 | 134.81 | 26.32 | 7.58 |
Blue: Protected, Yellow: Partial protection, Red: Not protected;
Antibody demonstrated no neutralization activity in vitro against NL4-3 strain,
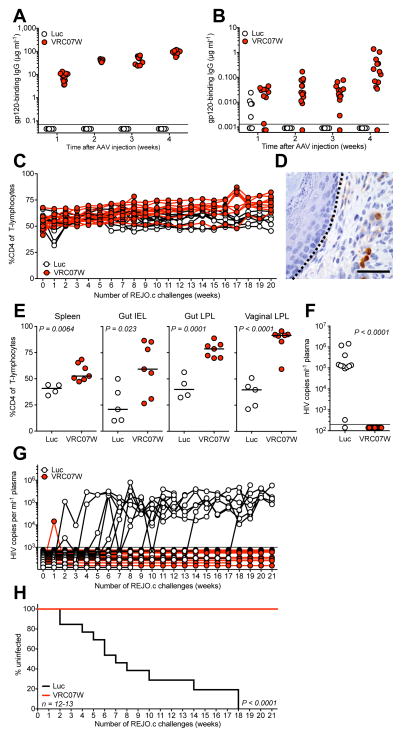
The bottleneck that occurs during mucosal transmission of HIV between humans appears to result in the selection of strains with unique properties that may enhance infectivity22. To determine the potential for VRC07G54W to prevent heterosexual transmission, we conducted a second repetitive challenge study with the REJO.c transmitted molecular founder strain of HIV11. VRC07G54W was expressed at concentrations similar to those of VRC01, achieving nearly 100 μg ml−1 in the serum within four weeks of intramuscular injection of the vector (Fig. 3a). Interestingly, we observed levels of VRC07G54W in the vaginal mucosa that approached 1 μg ml−1 four weeks post-AAV injection and one day prior to the first challenge (Fig. 3b). Following the initiation of weekly intravaginal challenges with REJO.c, we observed that peripheral blood CD4 cells were relatively unperturbed in mice expressing luciferase, but demonstrated a gradual rise in mice expressing VRC07G54W (Fig. 3c). At the conclusion of the experiment, we analyzed vaginal tissues by immunohistochemistry and only observed vaginal lamina propria lymphocytes displaying HIV p24 antigen in mice expressing luciferase, suggesting that a local infection was taking place at the site of challenge (Fig. 3d). FACS analysis of splenic tissue demonstrated a modest but statistically significant reduction in the level of CD4 cells (Fig. 3e). Interestingly, we observed significant differences among CD4 populations within the gut intraepithelial and lamina propria lymphocytes as well as in the vaginal lamina propria, suggesting that VRC07G54W was able to protect CD4 cells in mucosal tissues which are typically depleted during the initial phase of HIV infection in patients23 (Fig. 3e and Supplemental Fig. 6). In agreement with this, the ultrasensitive clinical viral load assay detected infection in nearly all luciferase control animals whereas none of the mice producing VRC07G54W exhibited detectable virus in plasma despite the 21 consecutive weekly challenges with REJO.c (Fig. 3f). To determine the number of vaginal exposures necessary for infection in this model, we quantified the viral load in longitudinal plasma samples using the less sensitive method described above (Fig. 3g). Luciferase expressing control mice became infected by REJO.c with a mean of 7.45 ± 3.31 challenges. However, in one case, just 2 challenges sufficed to initiate an infection, while in another animal, infection required 17 challenges. The two control animals that failed to become infected exhibited declining health during the course of the experiment and were euthanized after 8 or 14 challenges. Remarkably, none of the mice expressing VRC07G54W exhibited a sustained viral load above 1000 copies ml−1 throughout the course of the experiment, despite 21 consecutive exposures (Fig. 3h).
Figure 3. VIP prevents mucosal transmission of transmitted founder HIV following repetitive intravaginal challenge.
(a) Quantitation of gp120-binding human antibody in serum taken from BLT humanized mice at the indicated times following administration of 1×1011 GC of AAV encoding either luciferase or VRC07G54W-IgG prior to challenge as measured by ELISA (n=12–13). Limit of detection = 70 ng ml−1. (b) Quantitation of gp120-binding human antibody in vaginal wash samples taken from BLT humanized mice at the indicated times following administration of 1×1011 GC of AAV encoding either luciferase or VRC07G54W-IgG prior to challenge as measured by ELISA (n=12–13). Limit of detection = 1.3 ng ml−1. (c) CD4 cells as a percentage of CD3 positive T-lymphocytes in the peripheral blood of BLT humanized mice expressing luciferase (white) or VRC07G54W (red) throughout weekly intravaginal challenges with REJO.c as measured by flow cytometry. (d) HIV p24 detection by immunohistochemical (IHC) staining of a representative section taken from vaginal tissue of a REJO.c infected, luciferase expressing animal. Dashed line represents interface between epithelium (left) and lamina propria (right) demonstrating infected lamina propria lymphocytes. Scale bar represents 40 micrometers. (e) CD4 cells as a percentage of CD3 positive T-lymphocytes in mouse spleen, gut intraepithelial, gut lamina propria or vagina lamina propria lymphocytes tissues after repetitive challenge animals as detected by flow cytometry. (f) HIV viral load detected in plasma of mice at the time of sacrifice of repetitively challenged BLT mice as detected by the Abbott RealTime HIV-1 Viral Load Assay. Limit of detection = 200 copies ml−1. (g) HIV viral load detected in plasma throughout weekly intravaginal challenge of BLT mice as detected by an in-house viral load assay. Limit of detection = 1000 copies ml−1. (h) Fraction of uninfected mice over the course of repetitive intravaginal challenge. Positive infection defined by two consecutive viral load measurements above the limit of detection (n=12–13). Statistics for grouped comparisons calculated using either a one or two-tailed t-test. Statistics for Kaplan-Meier analysis calculated by log rank test. Each plot contains data generated from one individual experiment, and each experiment was performed once with the indicated number of mice.
Taken together, our results suggest that providing broadly neutralizing antibodies through VIP is capable of protecting humanized mice against infection by strains of HIV similar to those responsible for human transmission. Interestingly, we detected viral escape from antibody neutralization despite our use of virus produced from transfection of molecular clones that would not be expected to result in a diverse virus stock containing many pre-existing mutations. We hypothesize that limited viral replication may occur in vivo—perhaps locally in the mucosa— despite the presence of neutralizing antibodies and this might allow for selection of resistant strains. While we observed rapid CD4 decline in huPBMC-NSG control mice challenged with HIV, the kinetics of CD4 depletion following infection appeared to be substantially slower in BLT control mice. We hypothesize that this difference may be a result of lower levels of xenogenic activation of CD4 T-cells that develop in BLT model and the regeneration of T-cells from engrafted stem cells. Despite the IgG1 isotype expressed by our vectors, we found that VIP produced antibodies that reached the vaginal mucosa. It is unclear whether this mucosal antibody alone was sufficient to prevent transmission, or whether protection also required the high concentrations of circulating antibody present in our mice. Our results demonstrate that repetitive challenge with a transmitted founder strain can be used to mimic the inefficient nature of vaginal HIV transmission in humans and highlight the utility of such a model as a relatively low-cost approach towards testing novel prophylactic interventions. However, it is important to note that in addition to the significant anatomical differences between mice and humans, the existing BLT humanized mouse model does not entirely recapitulate a functional human immune system. Despite these limitations, it seems reasonable to examine whether a sufficiently high circulating concentration of broadly neutralizing antibody might substantially reduce the probability of sexual transmission of HIV between humans.
Online Methods
Statistics, sample size selection and exclusion criteria
All experimental group sizes were chosen to ensure adequate statistical power despite the highly variable nature of the studies performed. No animals were excluded, regardless of their level of human cell engraftment. However, Individual samples taken during the course of experiments measured by flow cytometry that exhibited fewer than 20 detectable human CD3+ cells were excluded from analysis to reduce variability. Groups of animals were chosen in consecutive order and were not randomized prior to commencing the study. Animal studies were not performed in a blinded fashion.
Mouse strains
Immunodeficient male, NOD/SCID/γc (NSG) mice were obtained from the Jackson Laboratory at 4–6 weeks of age. BLT mice were immunodeficient female NOD/SCID/γc (NSG) mice obtained from the UCLA breeding colony at 4–6 weeks of age and transplanted with human fetal liver and thymus tissue under the kidney capsule by the UCLA CFAR humanized mouse core laboratory24. Animal experiments were conducted in compliance with all relevant ethical regulations and were approved by the California Institute of Technology IACUC committee.
AAV virus production and administration
AAV virus encoding luciferase, or broadly neutralizing monoclonal antibodies were produced as previously described10. AAV intramuscular injection was performed as previously described10. Briefly, aliquots of previously titered viruses were thawed slowly on ice and diluted to achieve the predetermined dose in a 40 μl volume. A single 40 μl injection was administered into the gastrocnemius muscle of humanized NSG mice with a 28G insulin syringe. At various times after vector administration, mice were bled to determine antibody concentration in serum.
Antibody quantification by ELISA
For detection of gp120-binding IgG, ELISA plates were coated with 0.04 μg HIV-1 gp120MN protein (Protein Sciences) per well overnight at 4C. Plates were blocked with 1% BSA (KPL) in TBS for 2 h at room temperature. Samples were incubated in TBST containing 1% BSA (KPL) overnight at 4 °C, before incubation with HRP-conjugated goat anti-human IgG-Fc antibody (Bethyl, Catalog #A80-104A) for 30 minutes at room temperature. Samples were detected by TMB Microwell Peroxidase Substrate System (KPL). A standard curve was generated using either purified VRC01 or VRC07G54W protein as appropriate for the samples.
In vitro susceptibility assay
HIV was produced via transient transfection of 293T cells with plasmids encoding NL4-3, JR-CSF, or REJO.c (AIDS Reagent program) followed by collection of supernatant. Supernatants were titered by p24 ELISA (Perkin-Elmer) to quantify viral concentration. To perform the assay, TZM-bl cells were suspended in media containing 75 μg ml−1 DEA-Dextran at 200,000 cells ml−1 and two times the final concentration of either b12 or VRC01 antibody. Following one hour of incubation at 37 °C, cell and antibody mixtures were plated and combined with an equal volume of media containing 27.75 ng p24 of each virus per well (in triplicate) to achieve the final concentration of antibody and allowed to infect overnight. The following day, luciferase expression was determined by adding Britelite reagent to each well, incubating for two minutes prior to transferring to an opaque 96-well plate for reading by an automated luminometer (VICTOR).
Intravenous challenge of humanized mice
Humanized NSG mice were produced as previously described10. 24 h before intravenous HIV challenge, blood samples were obtained from the mice and subjected to flow cytometry to determine the baseline CD4/CD8 ratio and ELISA for antibody quantification. The following day, mice were intravenously challenged with 10 ng p24 of NL4-3, JR-CSF or REJO.c, diluted in PBS to a volume of 50 μl. Blood samples of the infected mice were obtained weekly to determine the CD4/CD8 ratio by flow cytometry.
Flow cytometry
Blood samples were centrifuged at 1,150 g for 5 minutes at room temperature to separate the plasma from the cell pellets. Plasma was removed and frozen at −20 °C for future analysis. The cell pellets were re-suspended in 1.1 ml of 1X RBC lysis buffer (Biolegend) and incubated on ice for 10 minutes to remove the red blood cells. After lysis, samples were pelleted at 1,150 g in a microcentrifuge for 5 minutes at room temperature, and then stained with 65 μl of an antibody cocktail containing 5 μl anti-human CD3-FITC, 5 μl anti-human CD4-PE, 5 μl anti-human CD8a-APC antibodies (Biolegend, Catalog #300406, 300508, 301014 respectively) and 50 μl of phosphate buffered saline supplemented with 2% fetal bovine serum (PBS+). Samples were washed once with 1 ml PBS+ and pelleted again at 1,150 g for 5 minutes. Pelleted cells were re-suspended in 200 μl of PBS+ supplemented with 2 μg ml−1 propidium iodine (Invitrogen) and analyzed on MACSQuant flow cytometer (Miltenyi Biotec). Samples were first gated by CD3 expression before determining the ratio of CD4 to CD8 cells within this subset. Samples containing fewer than 20 CD3+ events were excluded from the analysis.
Identification of HIV envelope mutations
Mouse spleens were harvested for genomic DNA isolation. Nested PCR was performed for the Env gene. Primers used in these reactions were 5′ GCAATAATTGTGTGGTCCATAGTACTCATAGAATATAGGA and 3′ CCCTATCTGTTGCTGGCTCAGCTCGTC for the first round, and 5′ AAAATAGATAGGTTAATTGATAAAATAAGAGAGAGAGCAGAAGACAG and 3′ TCATTCTTTCCCTTACAGTAGACCATCCAGGC for second round, targeting the Env gene of HIV JR-CSF strain. For the 1st round PCR reaction, 100–300 ng of genomic DNA was used as the template for amplification by KOD Xtreme Hot Start DNA polymerase (EMD) using the following cycling conditions: one cycle at 95 °C for 2 minutes, twenty cycles of 98 °C for 10 seconds, 65 °C for 30 seconds and 70 °C for 3 minutes. For the 2nd round PCR reaction, 1 μl of the 1st round PCR product was used as template for re-amplification by KOD Hot Start Master mix (EMD) using the following cycling conditions: one cycle at 94 °C for 2 minutes, thirty cycles of 98 °C for 5 seconds and 68 °C for 3 minutes and 30 seconds. The 2nd round PCR product was purified by agarose gel electrophoresis and gel extraction (Bioland) and the product was cloned by homologous recombination into an appropriate JR-CSF parental backbone vector using the In-Fusion HD Cloning kit (Clontech). The product was transformed into DH5α competent cells and positive clones were selected for standard sequencing.
Construction of point-directed envelope mutants
Individual mutations selected for further study were introduced into the parental vector expressing the molecular clone using overlapping PCR with primers incorporating the desired change. Following amplification of the Env gene with the primers described above using KOD Hot Start Master mix, the PCR product was purified by gel extraction (Bioland) and cloned by homologous recombination into the appropriate recipient parental backbone vector using the In-Fusion HD Cloning kit (Clontech). The ligation product was transformed into DH5α and positive clones were selected for standard sequencing to confirm successful introduction of the desired mutation.
In vitro neutralization assay
To compare the sensitivity of point-mutant viruses to b12 and VRC01 antibody neutralization, we produced each mutant by transient transfection of 293T cells and virus in the supernatants were titered by TCID50 assay in TZM-bl cells. Neutralzation assays were performed by mixing 250 TCID50 of HIV virus in 50 μl with three-fold serial dilutions of each antibody and incubation at 37 °C for 1 hour. Following the incubation, 10,000 TZM-bl cells with 75 μg ml−1 of DEAE-Dextran (Sigma) were added to each well, and incubated at 37 °C for 48 hours. Cells were then lysed using BriteLite plus (PerkinElmer) and and assayed for luciferase expression using a VICTOR3 luminometer (PerkinElmer). Percentage neutralization was determined by calculating the difference in luminescence between test wells (cells with virus and antibody) and cell control wells (cells only) and dividing this value by the difference between the virus control wells (cells with virus) and cell control wells.
Repetitive mucosal challenge experiments
Following AAV administration to BLT mice, blood samples and vaginal washes were obtained weekly by retro-orbital bleeding and by rinsing the vaginal vault with 20 μl of PBS. 4 weeks after AAV administration, the BLT mice were challenged with 50 ng p24 of JR-CSF or 16 ng p24 of REJO.c. in a volume of 20 μl. Virus challenge was performed non-abrasively, by placing isofluorane anesthetized mice in a supine position and elevating the posterior of the animal prior to shallow insertion of the pipet loaded with virus into the vaginal vault. Virus was administered slowly and following removal of the pipet, mice were maintained in a supine position for five minutes to prevent loss of the virus. This vaginal challenge protocol was repeated weekly until the conclusion of the experiment. Mice were bled prior to vaginal challenge each week to obtain samples for CD4 determination and viral load.
IEL and LPL isolation
Following harvest of spleen, gut and genital tract from BLT mice, fecal material and mucus was removed from the mouse gut and female genital tract by gentle scraping with forceps and tissues were cut into 0.5cm fragments and rinsed with cold PBS. Cleaned gut and genital pieces were incubated twice in freshly prepared cell dissociation solution (Ca2+ and Mg2+ free 1x HBSS, 5 mM EDTA, 10 mM HEPES) for 20 minutes at 37 °C with slow rotation at 100 rpm. This fraction was filtered through a 70 μm cell strainer (BD) and considered to contain the intraepithelial lymphocytes (IEL). The remainder of the tissues were then incubated twice in freshly prepared lamina propria lymphocyte (LPL) isolation solution (25 ml C10 medium with 0.5 mg ml−1 type II collagenase for the gut, and 12.5 ml C10 medium with 0.5 mg ml−1 type II collagenase, 0.1 mg ml−1 type I DNase, 25 mM HEPES, and 5 mM β-mercaptoethanol for the genital tract) for 30 minutes at 37 °C with slow rotation at 100rpm. After each incubation, tissues were vortexed for 30 seconds, and drained onto clean metal mesh screens over clean 10 cm dishes to collect supernatants. This fraction was filtered through a 40 μm cell strainer (BD) and considered to contain the lamina propria lymphocytes (LPL). Cells were further purified by suspension in 10 ml 40% percoll solution (13.2 ml 100% percoll with 19.8 ml D10 medium) prior to underlay of 5 ml of 80% percoll (13.2 ml 100% percoll with 3.3 ml 1X PBS) and these gradients were centrifuge at 2,500 rpm for 20 minutes at 20 °C. Cells at the interface were washed in cold PBS prior to antibody staining for flow cytometry.
Viral load test by quantitative reverse transcription PCR
Viral RNA was extracted using the QIAamp viral RNA mini kit (Qiagen). Each RNA sample was treated with 0.04 U of heparinase I (Sigma-Aldrich) at room temperature for 40 minutes, and then treated with 2 U of Turbo DNase (Ambion) at 37 °C for 30 minutes, following by heat inactivation at 75 °C for 10 minutes. 10 μl of the treated RNA was used in a 20 μl quantitative reverse transcription PCR reaction with the qScript One-Step Fast qRT-PCR kit (Quanta Biosciences), Taqman probe (5′-/56-FAM/CCC ACC AAC AGG CGG CCT TAA CTG/36-TAMSp/-3′) (IDT) and primers designed targeting the Pol gene of JR-CSF (5′ CAATGGCAGCAATTTCACCA and 3′ GAATGCCAAATTCCTGCTTGA) or REJO.c strain (5′ CAATGGCCCCAATTTCATCA and 3′ GAATGCCGAATTCCTGCTTGA). Samples were run in triplicate on an Eppendorf Realplex4 Mastercycler (Eppendorf). The following cycling conditions were used: one cycle of 49 °C for 5 minutes, one cycle of 95 °C for 30 seconds, 55 cycles of 95 °C for 3 seconds and 60 °C for 1 minute. Virus titer was determined by comparison with a standard curve generated using RNA extracted from a serially diluted mixture of commercially titered viral stock and pure mouse serum.
Histological staining for HIV p24
Spleens were removed from mice and kept in 10% neutral buffered formalin for 24 h, and transferred to 70% ethanol until standard paraffin embedding and processing. 4 μm thick sections were taken and immunohistochemical staining was performed for HIV p24 detection using the Kal-1 murine monoclonal antibody and standard antigen retrieval techniques. The slides were examined using the Olympus BX51 light microscope, and images were obtained using a SPOT Insight Digital Camera (Diagnostic Instruments).
Supplementary Material
Acknowledgments
The authors wish to acknowledge Gary Nabel (Sanofi-Pasteur) and John Mascola (NIH Vaccine Research Center) for VRC01, VRC-PG04, VRC07 and VRC07G54W expression plasmids and proteins, Dennis Burton (Scripps) for b12, PG9, PGT121 and PGT128 expression plasmids, Michelle Nussenzweig (Rockefeller) for 3BNC117, 12A12 expression plasmids and Pamela Bjorkman (Caltech) for NIH45-46W expression plasmid. We also thank the Caltech Protein Expression Center for providing purified antibodies. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pYK-JRCSF from Dr. Irvin SY Chen and Dr. Yoshio Koyanagi, pREJO.c/2864 from Dr. John Kappes and Dr. Christina Ochsenbauer and TZM-bl cells from Dr. John Kappes and Dr. Xiaoyun Wu. The authors wish to thank J. Kim, D. Majumdar, M. Mann and A. So, for their helpful comments, and other members of the Baltimore lab as well as R. Cortado and S. Shimizu in the An lab for their assistance in carrying out this work. Preparation of human CD34+ cells, tissue procurement and BLT mice were supported by the UCLA Center for AIDS Research (CFAR) AI028697. A.B.B. is supported by the National Institute of Allergy and Infectious Disease (NIAID) Career Transition Award 1K22AI102769. D.S.R. was a Sidney Kimmel Scholar supported by the Sidney Kimmel Foundation for Cancer Research (Translational Award SKF-11-013) and is supported by career development award 1K08CA133521 from the NIH. D.S.A. is supported by the NIAID grant 1R01AI100652-01A1. This project was supported by the National Institutes of Health (HHSN266200500035C) through a contract from the NIAID and by the Joint Center for Translational Medicine.
Footnotes
Author Contributions
A.B.B. and D.B. conceived the study, A.B.B. designed the experiments. D.S.A. offered suggestions for the experiments and provided the BLT humanized mice. A.B.B., Y.O., C.M.H., J.C. and S.M.N. carried out experiments. A.B.B., Y.O., C.M.H., J.C. and S.M.N. analyzed the data. D.S.R. performed immunohistochemistry and analysis. A.B.B. and D.B. wrote the paper with contributions from all authors.
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article at www.nature.com/nm.
References
- 1.Gray RH, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 2.Pilcher CD, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J INFECT DIS. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 3.Kilmarx PH. Global epidemiology of HIV. Current Opinion in HIV and AIDS. 2009;4:240–246. doi: 10.1097/COH.0b013e32832c06db. [DOI] [PubMed] [Google Scholar]
- 4.Salazar-Gonzalez JF, et al. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. Journal of Virology. 2008;82:3952–3970. doi: 10.1128/JVI.02660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdool Karim Q, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Damme L, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrazzo MJ, et al. Pre-exposure prophylaxis for HIV in women: Daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE study (MTN-003) 20th Conference on Retroviruses and Opportunistic Infections. 2013 [Google Scholar]
- 8.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2011 doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 9.Romanowski B. Long term protection against cervical infection with the human papillomavirus: review of currently available vaccines. Hum Vaccin. 2011;7:161–169. doi: 10.4161/hv.7.2.13690. [DOI] [PubMed] [Google Scholar]
- 10.Balazs AB, et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2011;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochsenbauer C, et al. Generation of Transmitted/Founder HIV-1 Infectious Molecular Clones and Characterization of their Replication Capacity in CD4 T-Lymphocytes and Monocyte-derived Macrophages. Journal of Virology. 2011 doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson PR, et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Vol. 15. Nature Publishing Group; 2009. pp. 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyanagi Y, et al. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 15.Poignard P, et al. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity. 1999;10:431–438. doi: 10.1016/s1074-7613(00)80043-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhou T, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Z, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204:705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denton PW, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nature Medicine. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diskin R, et al. Increasing the Potency and Breadth of an HIV Antibody by Using Structure-Based Rational Design. Science. 2011 doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon Y, et al. Structure-guided modification and optimization of antibody VRC07. Retrovirology. 2012 [Google Scholar]
- 22.Parrish NF, et al. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci USA. 2013;110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim SG, et al. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin Exp Immunol. 1993;92:448–454. doi: 10.1111/j.1365-2249.1993.tb03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melkus MW, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nature Medicine. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.