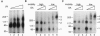Abstract
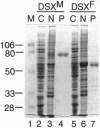
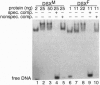
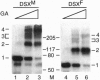
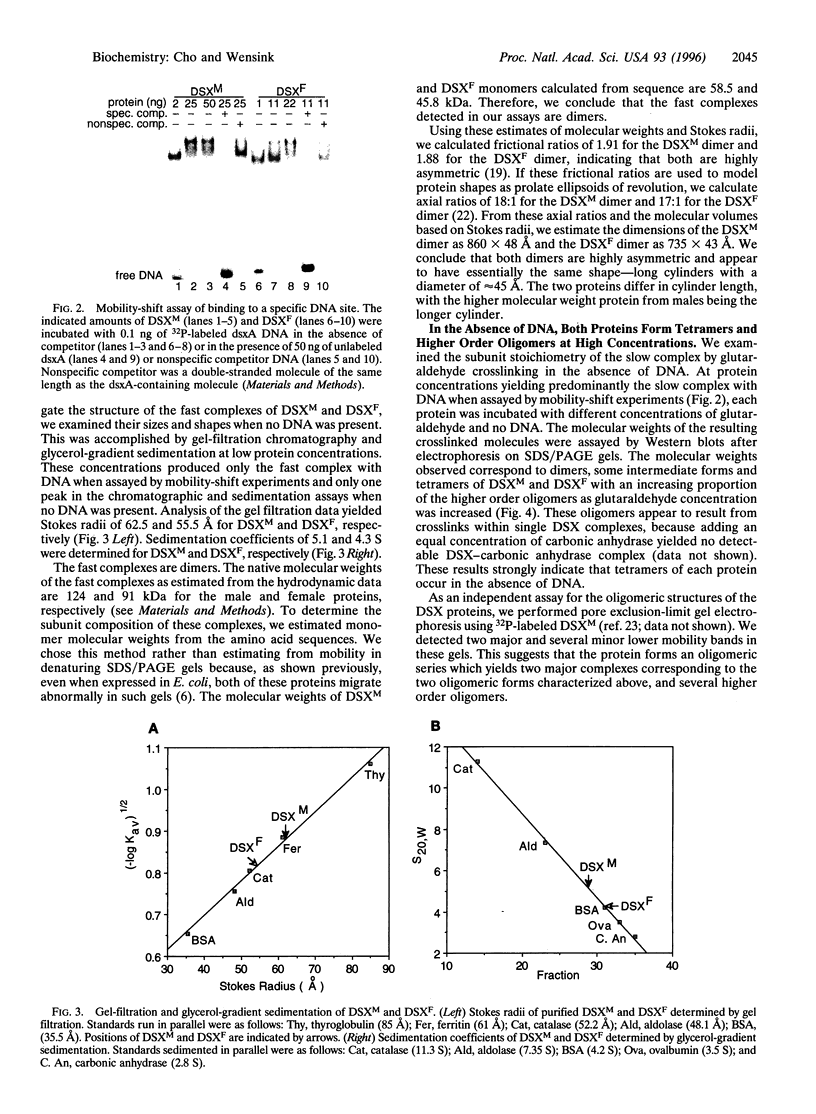
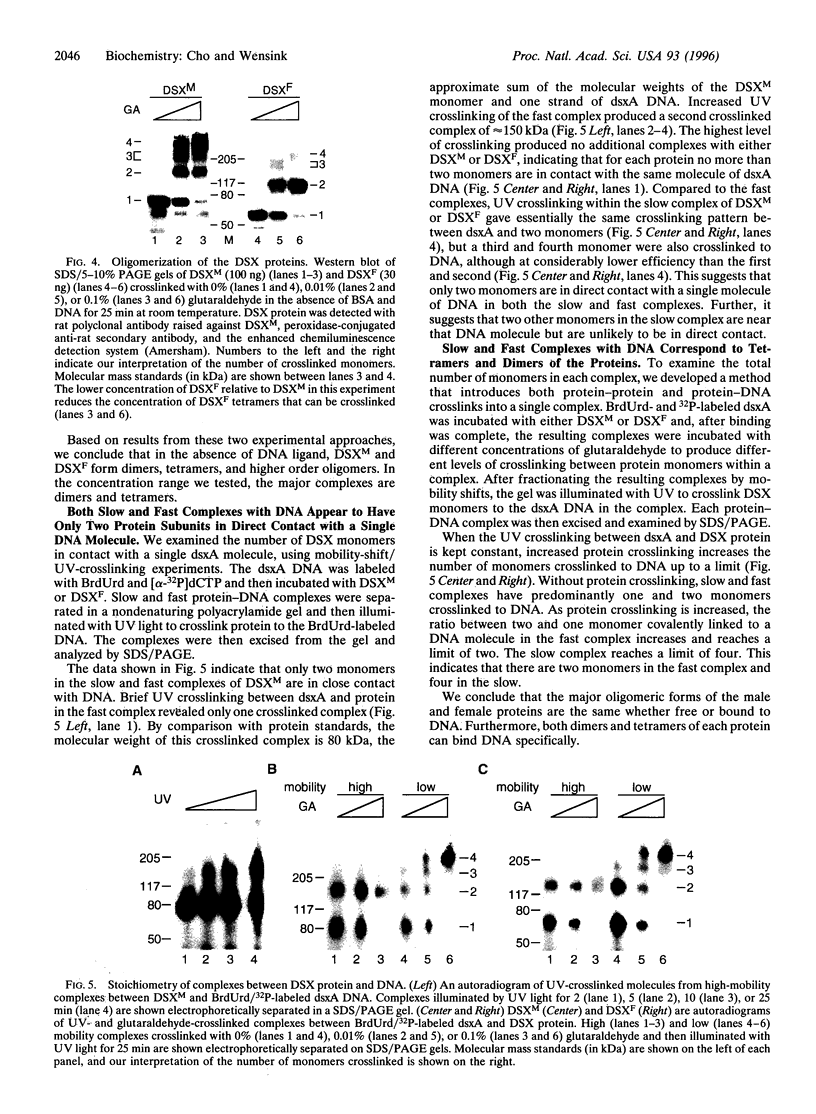
The double sex gene (dsx) encodes two proteins, DSX(M) and DSX(F), that regulate sex-specific transcription in Drosophila. These proteins bind target sites in DNA from which the male-specific DSX(M) represses and the female-specific DSX(F) activates transcription of yolk protein (Yp) genes. We investigated the physical properties of these DSX proteins, which are identical in their amino-terminal 397 residues but are entirely different in their carboxyl-terminal sequences (DSX(F), 30 amino acids; DSX(M), 152 amino acids). DSX(M) and DSX(F) were overexpressed in cultured insect cells and purified to near homogeneity. Gel filtration chromatography and glycerol gradient sedimentation showed that at low concentrations both proteins are dimers of highly asymmetrical shape. The axial ratios are approximately 18:1 (DSX(M), 860 X 48 angstroms; DSX(F), 735 X 43 angstroms). At higher concentrations, the proteins form tetramers. Through use of a novel, double crosslinking assay (protein-DNA plus protein-protein), we demonstrated that a DNA regulatory site binds to both monomers of the DSX dimer and to only two monomers of the tetramer. Furthermore, binding another DNA molecule to what we presume is the second and identical site in the tetramer dramatically shifts the equilibrium from tetramers to dimers. These oligomerization and DNA binding properties are indistinguishable between the male and female proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrein H., Hedley M. L., Maniatis T. The role of specific protein-RNA and protein-protein interactions in positive and negative control of pre-mRNA splicing by Transformer 2. Cell. 1994 Feb 25;76(4):735–746. doi: 10.1016/0092-8674(94)90512-6. [DOI] [PubMed] [Google Scholar]
- An W., Wensink P. C. Integrating sex- and tissue-specific regulation within a single Drosophila enhancer. Genes Dev. 1995 Jan 15;9(2):256–266. doi: 10.1101/gad.9.2.256. [DOI] [PubMed] [Google Scholar]
- Belote J. M., Handler A. M., Wolfner M. F., Livak K. J., Baker B. S. Sex-specific regulation of yolk protein gene expression in Drosophila. Cell. 1985 Feb;40(2):339–348. doi: 10.1016/0092-8674(85)90148-5. [DOI] [PubMed] [Google Scholar]
- Burtis K. C., Baker B. S. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989 Mar 24;56(6):997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Burtis K. C., Coschigano K. T., Baker B. S., Wensink P. C. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 1991 Sep;10(9):2577–2582. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis K. C. The regulation of sex determination and sexually dimorphic differentiation in Drosophila. Curr Opin Cell Biol. 1993 Dec;5(6):1006–1014. doi: 10.1016/0955-0674(93)90085-5. [DOI] [PubMed] [Google Scholar]
- Carey J. Gel retardation. Methods Enzymol. 1991;208:103–117. doi: 10.1016/0076-6879(91)08010-f. [DOI] [PubMed] [Google Scholar]
- Coschigano K. T., Wensink P. C. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 1993 Jan;7(1):42–54. doi: 10.1101/gad.7.1.42. [DOI] [PubMed] [Google Scholar]
- Delphin C., Cahen P., Lawrence J. J., Baudier J. Characterization of baculovirus recombinant wild-type p53. Dimerization of p53 is required for high-affinity DNA binding and cysteine oxidation inhibits p53 DNA binding. Eur J Biochem. 1994 Jul 15;223(2):683–692. doi: 10.1111/j.1432-1033.1994.tb19041.x. [DOI] [PubMed] [Google Scholar]
- Erdman S. E., Burtis K. C. The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. EMBO J. 1993 Feb;12(2):527–535. doi: 10.1002/j.1460-2075.1993.tb05684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M. Sex differentiation: the role of alternative splicing. Curr Opin Genet Dev. 1992 Apr;2(2):299–303. doi: 10.1016/s0959-437x(05)80288-6. [DOI] [PubMed] [Google Scholar]
- Mitsis P. G., Wensink P. C. Purification and properties of yolk protein factor I, a sequence-specific DNA-binding protein from Drosophila melanogaster. J Biol Chem. 1989 Mar 25;264(9):5195–5202. [PubMed] [Google Scholar]
- O'Donnell K. H., Wensink P. C. GAGA factor and TBF1 bind DNA elements that direct ubiquitous transcription of the Drosophila alpha 1-tubulin gene. Nucleic Acids Res. 1994 Nov 11;22(22):4712–4718. doi: 10.1093/nar/22.22.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., McCaslin D. R. Determination of protein molecular weight in complexes with detergent without knowledge of binding. Methods Enzymol. 1985;117:41–53. doi: 10.1016/s0076-6879(85)17005-9. [DOI] [PubMed] [Google Scholar]
- Sarge K. D., Murphy S. P., Morimoto R. I. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993 Mar;13(3):1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]