Abstract
GH is an important regulator of body growth and composition as well as numerous other metabolic processes. In particular, liver plays a key role in the GH/IGF-I axis, because the majority of circulating “endocrine” IGF-I results from GH-stimulated liver IGF-I production. To develop a better understanding of the role of liver in the overall function of GH, we generated a strain of mice with liver-specific GH receptor (GHR) gene knockout (LiGHRKO mice). LiGHRKO mice had a 90% decrease in circulating IGF-I levels, a 300% increase in circulating GH, and significant changes in IGF binding protein (IGFBP)-1, IGFBP-2, IGFBP-3, IGFBP-5, and IGFBP-7. LiGHRKO mice were smaller than controls, with body length and body weight being significantly decreased in both sexes. Analysis of body composition over time revealed a pattern similar to those found in GH transgenic mice; that is, LiGHRKO mice had a higher percentage of body fat at early ages followed by lower percentage of body fat in adulthood. Local IGF-I mRNA levels were significantly increased in skeletal muscle and select adipose tissue depots. Grip strength was increased in LiGHRKO mice. Finally, circulating levels of leptin, resistin, and adiponectin were increased in LiGHRKO mice. In conclusion, LiGHRKO mice are smaller despite increased local mRNA expression of IGF-I in several tissues, suggesting that liver-derived IGF-I is indeed important for normal body growth. Furthermore, our data suggest that novel GH-dependent cross talk between liver and adipose is important for regulation of adipokines in vivo.
The GH receptor (GHR) gene-disrupted mouse (GHR−/−) was generated nearly 20 years ago (1) and exhibits several striking and clinically relevant phenotypes that have greatly enhanced our understanding of the GH/IGF-I axis (2). These mice are dwarf and GH insensitive resulting in low serum IGF-I and increased levels of GH. They are also obese, which is mainly due to enlargement of the sc fat depots (3), and have a unique adipokine profile with elevated levels of leptin, adiponectin, and resistin (4). In addition, GHR−/− mice are extremely insulin sensitive (5), which is presumably due to the loss of GH's diabetogenic (or antiinsulin) activities. Remarkably, these dwarf mice are long lived (6) and currently hold the record for the longest-lived laboratory mouse (2). The reason for the enhanced longevity is not fully understood, but there is evidence of improved health of multiple cellular and organ systems along with decreased rates of diabetes and cancer (7–13). Resistance to diabetes and cancer has also been reported in Ecuadorian patients with Laron syndrome, who are similar to the GHR−/− mice in that they are GH insensitive with decreased IGF-I, elevated GH, and obesity and enhanced insulin sensitivity (14). Additionally, reports of decreased rates of cancer have been shown for patients with congenital GH deficiency (15, 16).
Although GHR−/− mice have been useful for uncovering many global physiological effects of GH, the contributions of GH signaling in individual tissues has been more difficult to determine. Fortunately, the development of Cre/Lox recombination technology allows for in vivo analysis of the function of individual genes/proteins within a select tissue (17, 18). To date, this technology has been used in 6 reports that have evaluated the effects of tissue-specific GHR gene disruption in various tissues (19–24). Because the liver is thought to play a key role in the GH/IGF-I axis, liver-specific GHR knockout (KO) or deficient mice (termed GHR liver-deficient [GHRLD] mice) were the first to be reported (19). This initial publication reports a 4-fold increase in serum GH levels despite a more than 90% reduction in circulating IGF-I. Moreover, GHRLD mice have impaired glucose homeostasis as well as liver steatosis. Of particular interest, these mice have no change in body size or body composition, which suggests that liver-derived IGF-I, accounting for most circulating IGF-I, is not an essential mediator of body growth. However, because this initial report focused on lipid metabolism and no other studies have been performed in these mice, many important variables have yet to be assessed. For example, levels of local IGF-I are only reported for a single tissue, liver, and tissue weights are only reported for a few select tissues. Because these mice have significantly elevated levels of GH, extrahepatic tissues (with functional GHR) would exist in an acromegalic state with higher than normal levels of GH signaling and local IGF-I production. In turn, this could impact body size or at least the size of individual tissues. Measures of local IGF-I and a comprehensive evaluation of tissue size are essential for proper interpretation of body size results in liver-specific GHR-deficient mice. Thus, to develop a more comprehensive picture of the role of GHR in liver on physiology and metabolism, we generated a separate strain of mice with liver-specific GHR gene KO (LiGHRKO) mice. With the LiGHRKO mice, we have evaluated body weight and body composition over time, mass of selected tissues, serum adipokine and IGF binding protein (IGFBP) levels, tissue-specific IGF-I mRNA levels, as well as metabolic and physiological parameters, including measures of energy expenditure, strength, and endurance. Furthermore, we report data for both males and females uncovering important sex-specific differences in the variables measured.
Materials and Methods
LiGHRKO mouse production
Mice carrying the GHR “floxed” allele were generated as described previously (24). Liver tissue-specific GHR−/− mice (FFCx) and floxed littermate controls (FFxx) were generated by breeding conditional floxed GHRflox/flox mice to B6.Cg-Tg(alb-cre)21Mgn/J mice purchased from The Jackson Laboratory. B6.Cg-Tg(alb-cre)21Mgn/J mice are transgenic for the bacteriophage PI Cre-recombinase gene with expression under the control of the mouse albumin enhancer/promoter (25).
Mice were housed 2–4 per cage and given ad libitum access to water and standard laboratory rodent chow, in which 14% of energy is from fat, 60% from carbohydrates, and 26% from protein (ProLab RMH 3000). The cages were maintained in a temperature-controlled room (22°C) and exposed to a 14-hour light, 10-hour dark cycle. All procedures were approved by the Ohio University Institutional Animal Care and Use Committee and fully complied with all federal, state, and local policies.
Validation of liver-specific deletion of GHR
Liver specificity for the disruption of exon 4 within the GHR gene was verified by PCR in liver, white adipose tissue (WAT), brown adipose tissue (BAT), skeletal muscle, heart, kidney, spleen, and brain tissues collected from LiGHRKO and floxed control mice. DNA isolated from livers from global EIIaGHRKO mice and floxed littermate controls were used as positive and negative controls, respectively. B6.FVB-Tg(EIIa-cre)-C5379Lmgd/J mice are transgenic for the bacteriophage PI Cre-recombinase gene under the control of the adenovirus EIIa promoter, which results in widespread Cre expression in the early mouse embryo before implantation in the uterine wall (26). Tissue for DNA validation was used from EIIaGHRKO mice instead of GHR−/− mice, because the structure of the engineered floxed gene was similar between EIIaGHRKO and LiGHRKO mice but distinct from that used to generate the GHR−/− mice. EIIaGHRKO mice were generated by breeding B6.FVB-Tg(EIIa-cre)C5379Lmgd/J mice (purchased from The Jackson Laboratory) to GHRflox/flox mice. PCR was performed using primers that amplify the region containing exon 4 of the GHR gene (5′-TCAGAACGTGGAACATCTTCAG-3′ and 5′-CGGACATTGCATCTGTGATT-3′).
Hepatic GHR protein levels were determined by Western blot analysis as previously described (24).
Real-time RT-PCR
GHR mRNA expression levels were determined by real-time RT-PCR as previously described (24). For IGF-I RNA levels, real-time RT-PCR conditions and calculations were performed as previously described (27, 28). For hepatic gene expression, real-time PCR was performed as described previously (29).
Body composition measurements
Body weight and body composition were measured over time starting at 2 months until 22 months of age (n = 13–19/group per sex) using a Bruker Minispec mq NMR analyzer as previously described (3, 30).
Tissue collection, liver, and WAT analysis
A total of 63 mice (n = 15–16/group per sex) were killed at 6 months of age starting at 9 am after a 12-hour fast. Before dissection, mice were placed in a CO2 chamber until unconscious, after which blood was collected from the orbital sinus. This serum collected at dissection was used for the assays described below in blood measurements. Immediately after blood collection, the mice were then killed by cervical dislocation. Skeletal muscle (gastrocnemius and quadriceps), liver, BAT (interscapular), 4 white adipose depots (sc, retroperitoneal, mesenteric, and perigonadal), kidney, brain, heart, spleen, and lung were collected, weighed, flash frozen in liquid nitrogen, and stored at −80°C.
Liver was dissected, weighed, and a portion flash frozen in liquid nitrogen and kept at −80°C until processing for determining triglyceride (TG) levels, whereas the other portion was fixed in 10% formalin, embedded in paraffin, and processed for histology. For determining hepatic TG, tissues were thawed for extraction and triacylglycerol levels measured as described previously (31). For histology of liver samples, hematoxylin and eosin (H&E)-stained sections (2 sections per sample) of paraffin-embedded liver samples were examined using a Nikon Eclipse E600 microscope under ×200 magnification.
Blood measurements
Total IGF-I was determined using an IGF-I (mouse, rat) ELISA kit (22-IG1MS-E01; ALPCO Diagnostics). GH was determined using a rat/mouse GH ELISA kit (EZRMGH-45K; Millipore). Total and high molecular weight adiponectin levels were measured using an adiponectin ELISA kit (47-ADPMS-E01) from ALPCO Diagnostics. Insulin, C-peptide, leptin, resistin, and gastric inhibitory polypeptide were measured using a Mouse Metabolic Panel (MMHMAG-44K; Millipore). IGFBP-1, IGFBP-2, IGFBP-3, IGFBP-5, IGFBP-6, and IGFBP-7 were measured using the Mouse IGF Binding Protein MAGNETIC Bead Panel (catalog no. MIGFBPMAG-43; Millipore). All Millipore Milliplex kits were analyzed using a Milliplex 200 Analyzer (Millipore). All measurements were performed in accordance with the manufacturer's instructions.
Analysis of mouse metabolic, strength, and endurance phenotypes
Mice (male and female, 21–22 mo old) were sent to Mayo Clinic and were individually housed at 22 ± 0.5°C on a 12-hour light, 12-hour dark cycle (n = 6–8/group per sex). Mice had ad libitum access to standard chow and water throughout the analyses. Treadmill endurance was performed on an Exer-6M Treadmill from Columbus Instruments. Mice were acclimated for 3 days before testing for 5 minutes at a speed of 5 m/min. For the experiment, mice began at a speed of 5 m/min, which was increased at a rate of 2 m/min every 2 minutes until exhaustion. Mass (kg) and total distance run (m) were used to calculate total work (kJ) through the following formula: mass (kg) × g (9.8 m/s2) × distance (m) × sin(θ) (with an incline of θ = 5°). Forelimb strength (N-F/kg lean body mass [lbm]) was determined using a grip strength meter from Columbus Instruments. Mice were acclimated for 3 days before testing. Metabolic analyses, including indirect calorimetry, activity, and food intake, were measured using the Oxymax System from Columbus Instruments. All data were normalized to lbm, which was determined within 3 days of measurement unless otherwise stated. All procedures were in accordance with the Mayo Clinic Institutional Animal Care and Use Committee.
Statistical analysis
All values are given as means ± SE. Statistics were performed using SPSS version 14.0. The two-tailed unpaired Student's t test was used to assess the significance of difference between 2 sets of data. For comparison of longitudinal data, including body weight, fat mass, and lean mass over time, repeated measures ANOVA was used. Differences were considered to be statistically significant when P < .05.
Results
LiGHRKO mice
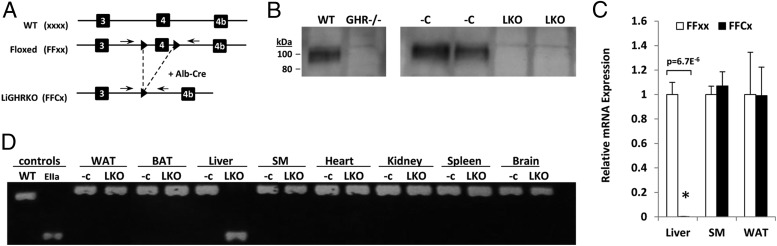
Disruption of the GHR gene in liver was achieved by breeding B6.Cg-Tg(alb-cre)21Mgn/J mice to floxed mice with LoxP sites flanking exon 4 of the GHR (GHRflox/flox) to produce LiGHRKO (FFCx) mice and floxed littermate controls (FFxx) as depicted in Figure 1A. Absence of GHR protein in liver was determined by Western blot analysis (Figure 1B). Quantification of RNA by real-time RT-PCR showed that GHR mRNA levels were reduced more than 99% in livers of LiGHRKO mice compared with controls (P = 6 × 10−6), whereas no change was observed in skeletal muscle (P = .6) or epididymal WAT (P = 1.0) (Figure 1C). Verification that disruption of the GHR gene was specific to liver was determined using PCR, which revealed that recombination was specific to liver (Figure 1D).
Figure 1.
Disruption of GHR in liver of LiGHRKO mice. A, LiGHRKO mice were generated by crossing transgenic mice that express Cre recombinase under the control of the albumin promoter/enhancer (Alb-Cre) to mice with a floxed exon 4 of the GHR. Small black triangles depict the LoxP sites with dashed lines showing the removal of exon 4 in liver with Alb-Cre. The arrows above the DNA strands indicate the location of the PCR primers (PCR is shown later in C). B, Western blot analysis of GHR protein. The presence or absence of GHR in livers from wild-type control and global GHR−/− mice is shown in the first 2 lanes on the left, whereas livers of floxed control mice (−C) and LiGHRKO mice (LKO) is shown on the right 4 lanes, respectively. C, Quantification of GHR mRNA levels in liver, skeletal muscle, and WAT in floxed controls (n = 6; white bars) and LiGHRKO mice (n = 6; black bars) was performed by real-time RT-PCR. D, DNA isolated from livers from floxed controls and global EIIaGHRKO mice were used as negative and positive controls, respectively, for PCR analysis (first 2 lanes on the left). DNAs isolated from WAT, BAT, liver, skeletal muscle, heart, kidney, spleen, and brain tissues were analyzed by PCR using primers that flank the 2 LoxP sites in floxed (−C) control mice or the single LoxP site in LiGHRKO (LKO) (primers locations are shown in A). Values are represented as mean ± SEM. All P values are indicated, and only those less than 0.05 were considered significant; LiGHRKO (FFCx) vs control (FFxx). SM, skeletal muscle; WT, wild-type.
GH, IGF-I, and IGFBP
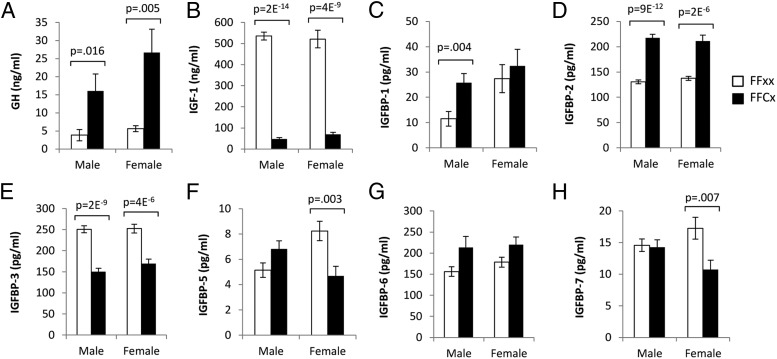
The GH/IGF-I axis was altered markedly in both sexes of LiGHRKO mice vs controls (Figure 2). GH levels (Figure 2A) were increased more than 300% in both male (P = .02) and female (P = .005) LiGHRKO mice, whereas IGF-I levels were decreased by 91% in male LiGHRKO (P = 2 × 10−14) and 87% in LiGHRKO female (87% decrease; P = 4 × 10−9) mice (Figure 2B). IGFBP-3 was decreased in both sexes of LiGHRKO mice (40% decrease, P = 2 × 10−9 in males; 33% decrease, P = 4 × 10−6 in females) (Figure 2D), whereas IGFBP-2 was increased in both sexes (67% increase, P = 9 × 10−12 in males; 54% increase, P = 2 × 10−6 in females) compared with controls (Figure 2C). Other binding proteins showed sex-specific alterations. IGFBP-1 was significantly increased in male LiGHRKO mice (123% increase, P = .004) but not in females (Figure 2C), whereas IGFBP-5 and IGFBP-7 were significantly decreased in females (43%, P = .003 and 38%, P = .007, respectively) but not males (Figure 2, F and H). IGFBP-6 levels showed no significant changes in LiGHRKO mice (Figure 2G) compared with controls.
Figure 2.
The GH/IGF-I axis is markedly altered in male and female LiGHRKO mice compared with controls. A–H, Serum was collected at 6 months of age and used to determine levels of GH, IGF-I, and IGFBPs in male and female LiGHRKO (black bars; n = 9–16/group per sex) and control (white bars; n = 10–16/group per sex) mice. Levels of circulating (A) GH, (B) IGF-I, (C) IGFBP-1, (D) IGFBP-2, (E) IGFBP-3, (F) IGFBP-5, (G) IGFBP-6, and (H) IGFBP-7 are shown. All values are represented as mean ± SEM. P values that were considered significant (P < .05) are indicated; LiGHRKO (FFCx) vs control (FFxx).
Body weight, body composition, and body length
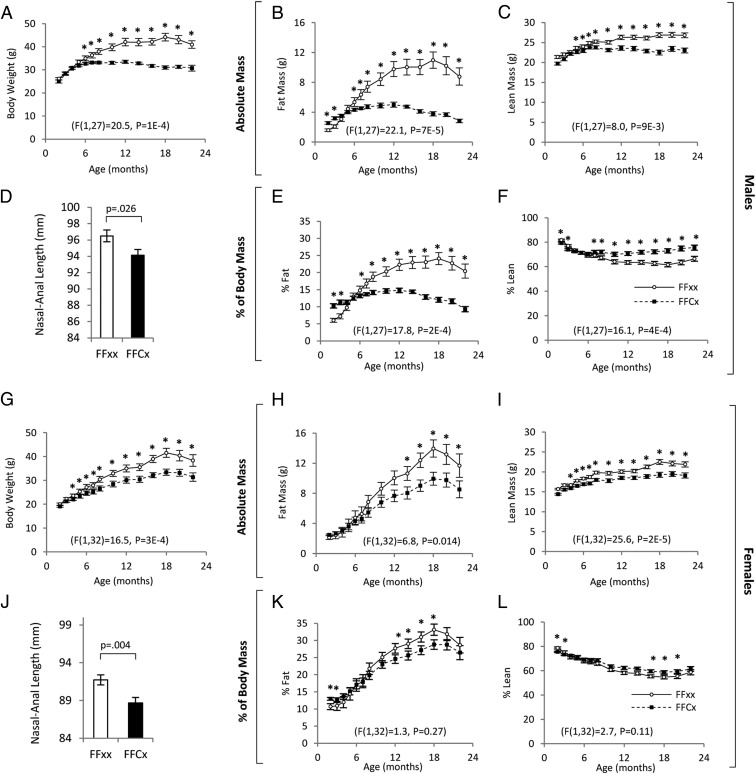
Body weights from 2 to 22 months of age were significantly decreased in male and female LiGHRKO mice compared with controls (Figure 3, A and G). The difference became significant at 6 months of age in males and 4 months of age in females. Body lengths were significantly decreased in LiGHRKO mice in males (P = .026) and females (P = .004) at 6 months of age (Figure 3, D and J, respectively). Longitudinal measures of body composition showed that male LiGHRKO mice had increased percent body fat compared with controls before 4 months of age followed by a progressive decrease in body fat compared with controls with advanced age (Figure 3, E and K). The data in females showed a similar trend, but the increase in percent body fat did not reach significance until 12 months of age, and the reduced adiposity in LiGHRKO mice compared with controls was much more pronounced in males vs females at most adult time points. Absolute values for body composition over time showed that both male and female LiGHRKO mice had significant reductions to fat (Figure 3, B and H) and lean (Figure 3, C and I) mass.
Figure 3.
Disruption of GHR in liver significantly decreases body size and alters body composition. Body weight in males (A) and females (G) are shown for LiGHRKO (FFCx; black boxes) and controls (FFxx; white circles). Absolute fat mass (B and H), and absolute lean mass (C and I) values are shown for males and females, respectively. Male (D) and female (J) body lengths were measured after euthanasia at 6 months of age for LiGHRKO (FFCx; black bars) and controls (FFxx; white bars). Percent fat mass (E and K) and percent lean mass (F and L) are shown for males and females, respectively. All values are represented as mean ± SEM. For body weight and body composition over time, repeated measures ANOVA were used for comparison between genotypes over time, and results are given at the bottom of each line graph. For individual time points, Student's t tests were performed; *, P < .05 indicates significance for a given time point.
Organ size, liver TG content, and liver histology
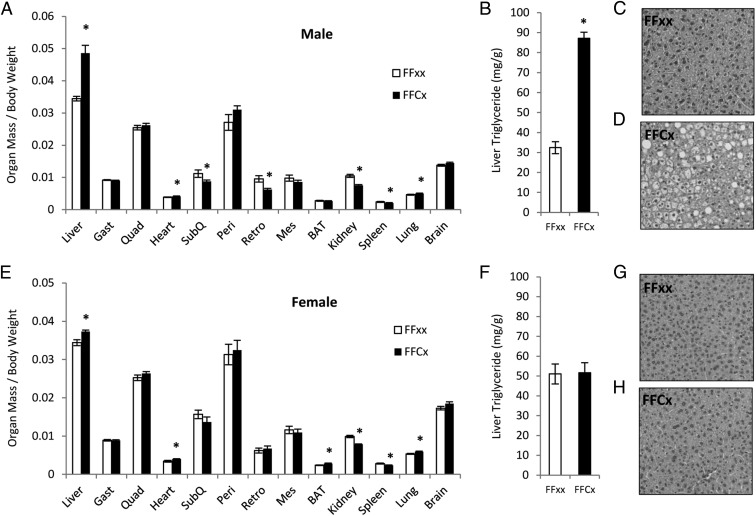
Disruption of GHR in liver significantly decreased the weights of several organs at 6 months of age (Figure 4). The relative size (organ mass/body mass) of kidneys and spleens were significantly smaller in both male and female LiGHRKO mice, while retroperitoneal WAT depots were significantly decreased in male but not in female LiGHRKO mice. The relative size of hearts, lungs, and livers were significantly larger in LiGHRKO mice for both sexes, while BAT was increased in female LiGHRKO mice. Analysis of hepatic tissues revealed that liver TG content was significantly increased in males (Figure 4B) but not in females (Figure 4F). The increased TG content in hepatic tissue of males was confirmed by visual examination of H&E-stained sections of liver tissue of LiGHRKO mice (Figure 4D) compared with controls (Figure 4C). No histological difference was apparent in livers from females (Figure 4, G and H).
Figure 4.
Organ size relative to body weight and liver TG content. Organ weights were measured at 6 months of age in male (A) and female (E) control (white bars; n = 15–16/group per sex) and LiGHRKO (black bars; n = 15–16/group per sex) mice and are reported relative to body weight. Liver, skeletal muscle (gastrocnemius, quadriceps), heart, WAT (sc, perigonadal, retroperitoneal, mesenteric), BAT, kidney, spleen, lung, and brain weights are shown (A and E). Liver TG content was determined in hepatic tissue from male (B) and female (F) LiGHRKO and control mice (n = 15–16/group per sex). H&E staining was also performed on hepatic tissue from male (C and D) and female (G and H) control and LiGHRKO mice, respectively. Values in A, B, E, and F are represented as mean ± SEM (n = 15–16/group per sex). *, P < .05; LiGHRKO (FFCx) vs control (FFxx). Gast, gastrocnemius; Quad, quadriceps; SubQ, sc; peri, perigonadal; retro, retroperitoneal; Mes, mesenteric.
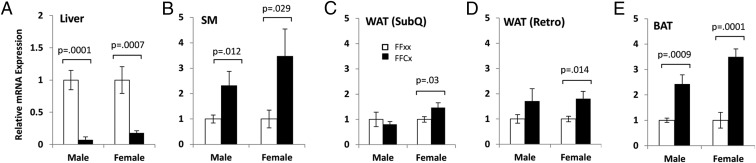
IGF-I mRNA expression
IGF-I mRNA levels in liver were significantly decreased by 93% in male and 82% in female LiGHRKO mice compared with controls (Figure 5A). In order to determine whether local IGF-I levels were elevated in tissues other than liver due to increased circulating GH, IGF-I mRNA expression levels were quantified using real-time RT-PCR (Figure 5). IGF-I mRNA expression in skeletal muscle was significantly increased in LiGHRKO mice compared with controls by 150% in males and 250% in females (Figure 5B). For sc and retroperitoneal WAT depots (Figure 5, C and D, respectively), IGF-I mRNA expression was increased by 60% in female LiGHRKO mice compared with controls, whereas no difference was observed in males. IGF-I mRNA levels were significantly increased in BAT of male (140% increase) and female (250% increase) LiGHRKO mice compared with controls.
Figure 5.
Local expression of IGF-I mRNA in liver, muscle, and adipose tissue. A–E, IGF-I mRNA expression levels in various tissues from control (white bars) and LiGHRKO (black bars) mice are shown for both sexes at 9 months of age. Liver (A), skeletal muscle (B), sc WAT depot (C), retroperitoneal WAT depot (D), and BAT tissue (E) were assayed as indicated. Values in A–E are represented as mean ± SEM (n = 5–8/group per sex). P values that were considered significant (P < .05) are indicated, LiGHRKO (FFCx) vs control (FFxx). SM, skeletal muscle; SubQ, sc; Retro, retroperitoneal.
Glucose metabolism, circulating adipokine, and cytokine levels
Blood glucose levels were significantly increased in LiGHRKO mice compared with controls in both sexes during fasted and nonfasted states (see Table 1). Fasting serum insulin and C-peptide levels were significantly increased in males, whereas only C-peptide levels were increased in females. Circulating levels of resistin, total adiponectin, and high molecular weight adiponectin were increased in both sexes of LiGHRKO mice compared with controls. Leptin levels were increased in female LiGHRKO mice, whereas elevated levels of leptin in male LiGHRKO mice did not reach statistical significance (P = .057). Adipsin levels were significantly increased in female but not male LiGHRKO mice. The inflammatory cytokine, IL-6, was significantly increased in both sexes, whereas monocyte chemotactic protein-1 was increased in males but not females of LiGHRKO mice compared with controls.
Table 1.
Glucuse Metabolism and Circulating Adipokine/Cytokine Levels in Male and Female LiGHRKO (FFCx) and Control (FFxx) Mice at 6 Months of Age
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| FFxx | FFCx | P value | FFxx | FFCx | P value | |
| Glucose | ||||||
| Fasting glucose, n = 15–16 (mg/dL) | 154 ± 6 | 188 ± 8 | .003a | 130 ± 8 | 158 ± 10 | .037a |
| Fed glucose, n = 15–16 (mg/dL) | 194 ± 5 | 219 ± 6 | .005a | 180 ± 5 | 200 ± 7 | .021a |
| Insulin and C-peptide | ||||||
| Insulin, n = 15–16 (pg/mL) | 1002 ± 133 | 1659 ± 146 | .002a | 491 ± 103 | 663 ± 90 | .215 |
| C-peptide, n = 15–16 (pg/mL) | 1507 ± 215 | 2763 ± 304 | .002a | 1162 ± 173 | 1725 ± 185 | .034a |
| Adipokines/cytokines | ||||||
| Leptin, n = 15–16 (pg/mL) | 5248 ± 819 | 7260 ± 582 | .057 | 4420 ± 680 | 7098 ± 822 | .019a |
| Resistin, n = 15–16 (pg/mL) | 9931 ± 1062 | 18 028 ± 1022 | 7E-06a | 12 060 ± 1112 | 20 254 ± 1595 | 3E-04a |
| Total adiponectin, n = 15–16 (pg/mL) | 23 267 ± 1122 | 35 619 ± 1674 | 1E-06a | 50 102 ± 3961 | 71 604 ± 5095 | .004a |
| HMW adiponectin, n = 15–16 (pg/mL) | 5632 ± 551 | 9928 ± 1003 | .001a | 15 296 ± 1863 | 22 472 ± 2552 | .036a |
| HMW/total, n = 15–16 (pg/mL) | 0.23 ± 0.01 | 0.27 ± 0.01 | .055 | 0.32 ± 0.05 | 0.35 ± 0.06 | .780 |
| Adipsin, n = 15–16 (pg/mL) | 1779 ± 104 | 2037 ± 166 | .217 | 1706 ± 128 | 2821 ± 256 | .002a |
| IL-6, n = 15–16 (pg/mL) | 80 ± 15 | 151 ± 27 | .029a | 55 ± 7 | 80 ± 9 | .031a |
| MCP-1, n = 15–16 (pg/mL) | 112 ± 16 | 228 ± 18 | 5E-05a | 188 ± 25 | 220 ± 15 | .282 |
Abbreviation: HMW, high molecular weight.
Significance with P values given.
Analysis of mouse metabolic, strength, and endurance phenotypes
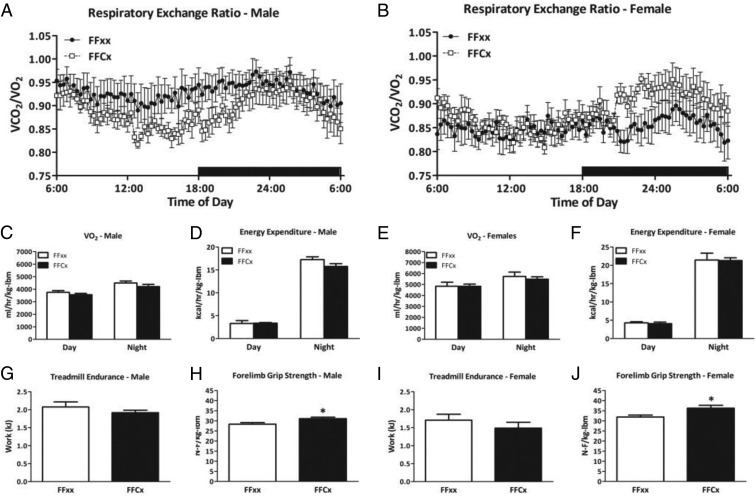
LiGHRKO mice showed no change in respiratory exchange rate compared with controls (Figure 6, A and B). Day and night values for volume of oxygen consumed normalized to lbm did not differ between LiGHKO and controls in males or females (Figure 6, C and E, respectively). Similarly, day and night values for energy expenditure normalized to lbm did not differ between LiGHRKO and controls (Figure 6, D and F). Other measures, such as food intake adjusted for body weight and volume of carbon dioxide expired per lbm, did not differ between LiGHRKO and controls in either sex (data not shown). Treadmill endurance was also unchanged between LiGHRKO mice compared with controls in both sexes (Figure 6, G and I). Interestingly, forelimb grip strength was significantly increased in male and female LiGHRKO mice compared with controls (Figure 6, H and J).
Figure 6.
Indirect calorimetry, endurance, and grip strength. A and B, Respiratory exchange ratios over a 24-hour period are shown for male (A) and female (B) LiGHRKO (white boxes) and controls (black boxes). Day and night values for VO2 values normalized to lbm are shown for LiGHRKO (black bars) and controls (white bars) for (C) males and (E) females. Day and night values for energy expenditure normalized to lbm values are shown for males (D) and females (F). Treadmill endurance in (G) males and (I) females, and forelimb grip strength in (H) males and (J) females are shown for LiGHRKO and controls. All values are represented as mean ± SEM (n = 6–8/group per sex). *, P < .05; LiGHRKO (FFCx) vs control (FFxx).
Hepatic mRNA expression
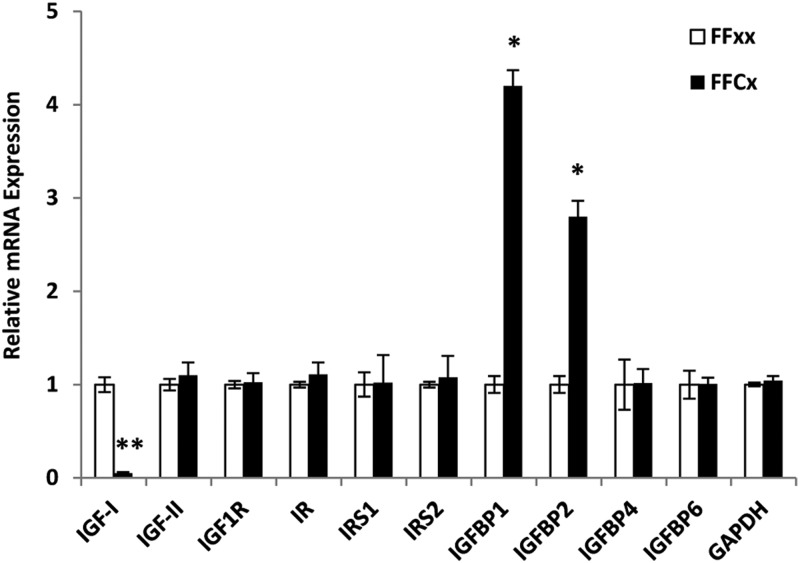
The relative mRNA expression levels of several IGF and insulin-related genes were determined in the livers of male LiGHRKO mice and controls (Figure 7). As expected and as a control, IGF-I mRNA levels were significantly decreased in LiGHRKO mice. Message levels for IGFBP-1 and IGFBP-2 were significantly increased in hepatic tissue, whereas IGFBP-4 and IGFBP-6 were unchanged. Hepatic mRNA expression of IGF-2, IGF-I receptor, insulin receptor, insulin receptor substrate-1, and insulin receptor substrate-2 were all unchanged between LiGHRKO and controls.
Figure 7.
Hepatic mRNA expression levels of several IGF and insulin-related genes in male LiGHRKO mice and controls. mRNA expression levels of various genes in liver tissue from control (white bars) and LiGHRKO (black bars) mice are shown for males at 9 months of age. IGF-IR, IGF-I receptor; IR, insulin receptor; IRS1, insulin receptor substrate-1; IRS2, insulin receptor substrate-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. All values are represented as mean ± SEM (n = 6/group). *, P < .01; **, P < .001; LiGHRKO (FFCx) vs control (FFxx).IR
Discussion
We show here that disruption of GHR specifically in liver results in significantly decreased adult body size, as evidenced by reductions in body weight and body length in both sexes of LiGHRKO mice. As would be expected, LiGHRKO mice have decreased circulating IGF-I and elevated GH levels. As a consequence of elevated GH, local IGF-I mRNA expression in skeletal muscle and select adipose tissue depots is increased in LiGHRKO mice. Thus, even in the face of elevated local IGF-I, the loss of hepatic-derived endocrine IGF-I is sufficient to decrease adult body weight and body length. Furthermore, despite the smaller body size in LiGHRKO mice, grip strength/lbm is increased in both sexes. Our inclusion of both sexes also reveals an important sex-specific difference in hepatic lipid accumulation after disrupting of GH action in liver, with fatty liver being limited to males. Interestingly, LiGHRKO mice display a body composition profile over time that is similar to GH transgenic mice where superphysiological levels of GH results in increased adiposity in early life and decreased adiposity in later life compared with controls (32). Another interesting finding is that removal of GHR in liver produces an adipokine profile that is more similar to global GHR−/− mice (33, 34) than when GHR is removed specifically in adipose tissue (24), suggesting that GH action on liver has more of an effect on adipokine production than direct GH action on adipose tissue. Taken together, these data strongly suggest that liver-derived endocrine IGF-I is indeed important for growth and metabolism throughout life. Furthermore, our data suggest that GH-dependent cross talk between liver and adipose is important for in vivo regulation of adipokines.
The GH/IGF-I axis accounts for most postnatal growth. More specially, by using IGF-I-null, GHR-null, and IGF-I/GHR double null mice, Lupu et al (35) estimate that the GH/IGF-I axis accounts for 83% of postnatal growth with 14% attributed to GH alone, 35% to IGF-I alone, and 34% to overlapping GH/IGF-I function while 17% is unrelated to the GH/IGF-I axis. Thus, GH and IGF-I have distinct yet overlapping functions relative to mouse growth. One of the major findings from our current study is the apparent importance of liver-derived IGF-I for growth. This topic has been debated over the past 15 years. In 1999, 2 separate liver-specific IGF-I-deficient mouse lines (referred to as “LID” mice [36] and “LI-IGF-I−/−” mice [37]) led the authors to suggest that liver-derived endocrine IGF-I is not important for normal body growth (36, 37). However, later studies in these same mouse lines show that skeletal and bone growth are decreased (38, 39). Using an opposite approach by starting with IGF-I-null mice and then “knocking-in” endogenous liver-specific expression of IGF-I, liver-derived endocrine IGF-I was shown to be important for normal growth accounting for 30% of body growth (40). In 2009, Fan et al (19) created the first GHR liver-specific KO mice (GHRLD) and reported that there are no significant changes in body size. Using a similar strategy to Fan et al, we generated a separate line of LiGHRKO mice and observe different results from GHRLD mice, because we show a decrease in body weight and body length. In agreement with our data, mice with liver-specific deletion of Janus kinase 2 (JAK2) (mice referred to as liver specific Janus Kinase 2 knockout or JAK2L) or signal transducer and activator of transcription 5 (STAT5), both of which are central within the GHR signaling cascade, show decreased body size (41–44). Based on our long-term measures of body weight and composition, a likely explanation for the size discrepancies among these reported mouse lines is the age at which body size measurements were taken. That is, we observed no changes in body weight until 2 and 4 months of age for females and males, respectively. Likewise, LI-IGF1−/− and JAK2L mice do not differ in body weight until later in life (41, 45), indicating the importance of age when interpreting the effects of endocrine IGF-I on body size (discussed here) and body composition (discussed below) in these liver-specific gene-disrupted mouse lines.
IGFBPs play an important role in the GH/IGF-I axis by acting as carriers of IGF-I, modulating IGF-I bioavailability and activity. IGFBPs are also thought to possess additional activities independent of IGF-I. As reported previously (19), IGFBP-3 is significantly decreased in males with tissue-specific removal of GHR in liver. In our study, we expand on these findings and show that this decrease in IGFBP-3 occurs in both males and females. IGFBP-3 is also decreased in LID mice (38, 46) but not in JAK2L mice (41). Although JAK2 is a main signaling molecule for GH, it is also activated by several other cytokines and growth factors, including interferon-γ, IL-3, IL-4, IL-5, IL-6, IL-11, IL-12, IL-13, granulocyte-macrophage colony-stimulating factor, erythropoietin, thyroid peroxidase, granulocyte colony-stimulating factor, prolactin, and leptin (47), which may account for this discrepancy. Not reported previously are measures of other IGFBPs at the protein level in circulation after liver-specific removal of GHR, IGF-I, JAK2, or STAT5. However, Sjögren et al (45) have reported hepatic mRNA expression levels of IGFBP-1, IGFBP-2, and IGFBP-3 in LI-IGF-I−/− mice and mRNA levels for all 3 binding proteins are decreased, which is in contrast to our data. The difference is likely due the fact that LI-IGF-I−/− (as well as LID) mice have fully functional GHR in the liver; thus, LI-IGF-I−/− and LID mice can respond to the elevated GH levels, whereas LiGHRKO mice cannot. Another difference is that we measured protein levels in circulation, whereas Sjögren et al measured hepatic mRNA expression levels, which do not necessarily translate to circulating protein levels. In our study, IGFBP-2 is the only other IGFBP that is significantly altered (increased) in both sexes in LiGHRKO mice. Because GH deficiency and decreased circulating IGF-I are associated with increased IGFBP-2 (48) and liver is a major site of IGFBP-2 production, the hepatic GH resistance and subsequent decreased circulating IGF-I likely accounts for this increase. The implications of IGFBP-2 on long-term health are both positive and negative. On one hand, IGFBP-2 has been shown to protect against obesity and insulin resistance when overexpressed in mice (49, 50) and is also elevated in long-lived Snell mice (51). On the other hand, IGBP-2 is proposed to have an IGF-I-independent ability to stimulate growth (52), to promote angiogenesis (53), and to be positively associated with certain types of cancer (54, 55). Thus, the long-term impact of elevated IGFBP-2 in these mice is in conflict. The other IGFBPs show no change (IGFBP-6) or sex-specific alterations (IGFBP-1,IGFBP-5, and IGFBP-7). Regardless, our data show a central role of GH action in the liver for circulating levels of IGFBPs.
Fan et al (19) have previously shown that removal of GHR in liver of male mice results in fatty liver. This has also been shown when IGF-I or JAK2 are removed specifically in the liver (41, 42, 56, 57). However, to our knowledge, no study has reported data in both males and females separately, because only males were analyzed or male and female data were combined in these previous studies (19, 41, 42, 57). Therefore, our analysis, which included both males and females, is unique and reveals that liver steatosis is limited to male LiGHRKO mice. A sex-specific effect on liver steatosis is well documented in the literature (58–61), with wild-type females being more susceptible than wild-type males for the C57BL/6 mouse stock (62). Similarly, our data in the control mice show that females started with a higher level of TG in the livers compared with males. However, females are protected from further increases in hepatic TG accumulation with liver-specific disruption of GHR, whereas males are more susceptible to hepatic TG accumulation. One obvious explanation would be that females have higher levels of estrogen, which blocks GH action at multiple levels ranging from the pituitary secretion of GH to GH signaling at the level of STAT5 within the liver itself (63). Thus, sex hormones may account for these differences. Further studies are needed to uncover the mechanisms of this sex-specific effect.
Data for body composition in the various liver-specific models mentions above are conflicting. Results for GHRLD mice are reported to be unchanged (19). In JAK2L mice, Sos et al (41) report a decrease in body fat using male/female combined data, whereas Nordstrom et al (57) and Shi et al (42) report no change in body fat or body mass index, respectively. LI-IGF-I−/− mice are reported to have decreased fat mass (45, 64), whereas LID mice have no change in fat mass (65). Because measures of body composition in GHRLD, JAK2L, LI-IGF-I−/−, and LID mice provide conflicting results, and because body size has been shown to be age- and sex dependent, it was valuable for us to report longitudinal measurements in this large cohort of male and female LiGHRKO as compared with control mice. Interestingly, LiGHRKO mice have increased body fat at early ages (2–3 mo of age) compared with controls but greatly reduced body fat in adulthood. These results are strikingly similar to body composition measurements reported for bovine GH transgenic (bGH) mice (32), where fat mass is also increased at early ages and decreased at later ages relative to controls. Thus, based on the only 2 studies that have done longitudinal body composition measurements in 2 separate mouse lines with elevated GH (bGH and LiGHRKO mice) and in both sexes, these data strongly suggest that elevated GH is responsible for this unique pattern of fat mass accumulation. Based on GH's divergent activities on preadipocytes (proliferative) (66, 67) and adipocytes (lipolytic and antilipogenic) (68, 69), we hypothesize that elevated levels of GH in LiGHRKO (and bGH) mice increases adiposity at early ages by increasing the number of adipocytes via proliferation and differentiation of preadipocytes, whereas the decrease in adiposity at later ages is due to GH's lipolytic and antilipogenic properties eventually overtaking the early increase in proliferation. Further studies assessing the pool of preadipocytes precursor cells, measuring adipocyte number vs size, as well as measures of lipolysis/lipogenesis in WAT from these mouse lines at young vs older ages are warranted.
Another curious finding was that grip strength/lbm was significantly increased in both male and female LiGHRKO mice. Although the notion that circulating GH and IGF-I can increase muscle strength remains controversial (70), it is generally believed that if there is indeed such an effect, that IGF-I is likely responsible via increased muscle size (nicely reviewed in Ref. 70). Consequently, the reduction in skeletal muscle size in LiGHRKO would be expected due to their decreased endocrine IGF-I. However, the increased muscle strength/lbm in this mouse line is a novel finding and suggests that although endocrine IGF-I may be important for muscle size, GH (or local IGF-I) may have a more significant impact on the strength of the muscle. Recent evidence that GH plays an important role in myofiber development may explain why the LiGHRKO had significantly greater grip strength/lbm. Mavalli et al (20) showed that skeletal muscle-specific removal of GHR changes fiber type (decreases the proportion of type I fibers, increases the proportion of type II fibers) and results in decreased grip strength. Furthermore, elevated levels of GH have been shown to increase proportion of type I/type II fibers in bGH mice (71). Thus, it is reasonable to suggest that increased grip strength in LiGHRKO mice is due to an increased type I/type II fiber ratio.
One of the most interesting findings in this study is the serum adipokine profile. LiGHRKO mice have significant alterations in adipokines, including leptin, adiponectin, and resistin, all of which are increased in LiGHRKO mice. Similarly, LID mice have elevated leptin levels (65), and JAK2L also have elevated leptin and adiponectin (57) with resistin levels not reported. In particular, the high levels of leptin in these liver-specific mouse lines are unexpected. Leptin levels are consistently and positively correlated with adiposity in most other studies of mice with modifications of the GH/IGF-I axis (4, 34, 72), with the exception of mouse lines with liver-specific disruption of GH action. More intriguing is the fact that all 3 adipokines follow the same trend as seen in global GHR−/− mice (3, 34), even though the global GHR−/− mice have increases in fat mass, specific enlargement of sc depots, and larger cell sizes at this same age (3, 33). Thus, there appears to be a disconnect between adiposity measures and adipokine output by adipose tissue when GHR signaling is disrupted in the liver. Interestingly, this disconnect occurs by global disruption of the GHR (3, 72), by liver-specific disruption of the GHR or by liver-specific disruption of JAK2 (57), and suggests that GH signaling in the liver influences adipokine production in vivo. Moreover, data generated in mice with fat-specific disruptions to GHR using the adipocyte protein 2 promoter (24) or JAK2 using the adiponectin promoter (57) implicate GH signaling in a tissue other than adipose as being responsible for the unique adipokine profile observed in the global GHR−/− mice, because removal of GHR or JAK2 specifically in adipose tissue does not replicate the adipokine profile in GHR−/− mice. On the other hand, because liver-specific removal of GHR or JAK2 does replicate the adipokine profiles observed in GHR−/− mice, it appears that liver influences adipokine output in a GH-dependent manner independent of adiposity. Further studies are needed to identify the liver-derived factor(s) that negatively influence adipokine production in adipose tissue with GHR or JAK2 disruption.
In conclusion, the current study provides a comprehensive assessment of mice with liver-disrupted GHR. We provide evidence that GH action in liver plays an important role in body size and body composition throughout life. Furthermore, our data suggest that novel GH-dependent cross talk between liver and adipose is important for regulating adipokines in vivo. Thus, LiGHRKO mice have a unique growth and adiposity profile and provide further evidence of the importance of the liver in the GH/IGF-I axis and growth.
Acknowledgments
This work was supported by the State of Ohio's Eminent Scholar Program that includes a gift from Milton and Lawrence Goll, by the AMVETS, by the Diabetes Institute at Ohio University, and by the Polish Ministry of Science and Higher Education Grant NN401042638. J.L.K. is supported by National Institutes of Health (NIH) Grant P01AG031736. M.M.M. is supported by National Institute on Aging Grant AG032290. The floxed GHR mouse strain used for this research project was generated by the trans-NIH Knock-Out Mouse Project (KOMP) and obtained from the KOMP Repository. NIH grants to Velocigene at Regeneron, Inc (U01HG004085) and the CSD (Children's Hospital Oakland Research Institute [CHORI], Sanger Institute, and University of California [UC] Davis) Consortium (U01HG004080) funded the generation of gene-targeted ES cells for 8500 genes in the KOMP Program and archived and distributed by the KOMP Repository at UC Davis and CHORI (U42RR024244).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- bGH
- bovine GH transgenic
- GHR
- GH receptor
- GHRLD
- GHR liver-deficient
- H&E
- hematoxylin and eosin
- IGFBP
- IGF binding protein
- JAK2
- Janus kinase 2
- JAK2L
- liver specific Janus kinase 2 knockout
- KO
- knockout
- lbm
- lean body mass
- LiGHRKO
- liver-specific GHR gene KO
- STAT5
- signal transducer and activator of transcription 5
- TG
- triglyceride
- WAT
- white adipose tissue.
References
- 1. Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA. 1997;94:13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. List EO, Sackmann-Sala L, Berryman DE, et al. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR−/−) mouse. Endocr Rev. 2011;32:356–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berryman DE, List EO, Palmer AJ, et al. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berryman DE, List EO, Sackmann-Sala L, Lubbers E, Munn R, Kopchick JJ. Growth hormone and adipose tissue: beyond the adipocyte. Growth Horm IGF Res. 2011;21:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dominici FP, Arostegui Diaz G, Bartke A, Kopchick JJ, Turyn D. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J Endocrinol. 2000;166:579–590 [DOI] [PubMed] [Google Scholar]
- 6. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810 [DOI] [PubMed] [Google Scholar]
- 7. Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci. 2006;61:562–567 [DOI] [PubMed] [Google Scholar]
- 8. Egecioglu E, Andersson IJ, Bollano E, et al. Growth hormone receptor deficiency in mice results in reduced systolic blood pressure and plasma renin, increased aortic eNOS expression, and altered cardiovascular structure and function. Am J Physiol Endocrinol Metab. 2007;292:E1418–E1425 [DOI] [PubMed] [Google Scholar]
- 9. Bellush LL, Doublier S, Holland AN, Striker LJ, Striker GE, Kopchick JJ. Protection against diabetes-induced nephropathy in growth hormone receptor/binding protein gene-disrupted mice. Endocrinology. 2000;141:163–168 [DOI] [PubMed] [Google Scholar]
- 10. Wang M, Miller RA. Fibroblasts from long-lived mutant mice exhibit increased autophagy and lower TOR activity after nutrient deprivation or oxidative stress. Aging Cell. 2012;11:668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–E29 [DOI] [PubMed] [Google Scholar]
- 12. Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147:2801–2808 [DOI] [PubMed] [Google Scholar]
- 13. Ikeno Y, Hubbard GB, Lee S, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 2011;3:70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164:485–489 [DOI] [PubMed] [Google Scholar]
- 16. Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res. 2007;17:54–57 [DOI] [PubMed] [Google Scholar]
- 17. Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods. 1998;14:381–392 [DOI] [PubMed] [Google Scholar]
- 18. Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci USA. 1988;85:5166–5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan Y, Menon RK, Cohen P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284:19937–19944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mavalli MD, DiGirolamo DJ, Fan Y, et al. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J Clin Invest. 2010;120:4007–4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vijayakumar A, Wu Y, Sun H, et al. Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes. 2012;61:94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu Y, Liu C, Sun H, et al. Growth hormone receptor regulates β cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J Clin Invest. 2011;121:2422–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu C, Kumar PA, Sun J, et al. Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity. J Biol Chem. 2013;288:15725–15735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. List EO, Berryman DE, Funk K, et al. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27:524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Postic C, Shiota M, Niswender KD, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315 [DOI] [PubMed] [Google Scholar]
- 26. Lakso M, Pichel JG, Gorman JR, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masternak MM, Al-Regaiey KA, Bonkowski MS, Panici JA, Bartke A. Effect of every other day feeding diet on gene expression in normal and in long-lived Ames dwarf mice. Exp Gerontol. 2005;40:491–497 [DOI] [PubMed] [Google Scholar]
- 28. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005;60:1238–1245 [DOI] [PubMed] [Google Scholar]
- 29. Sun LY, D'Ercole AJ. Insulin-like growth factor-I stimulates histone H3 and H4 acetylation in the brain in vivo. Endocrinology. 2006;147:5480–5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. List EO, Palmer AJ, Berryman DE, Bower B, Kelder B, Kopchick JJ. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia. 2009;52:1647–1655 [DOI] [PubMed] [Google Scholar]
- 31. Salmon DM, Flatt JP. Effect of dietary fat content on the incidence of obesity among ad libitum fed mice. Int J Obes. 1985;9:443–449 [PubMed] [Google Scholar]
- 32. Palmer AJ, Chung MY, List EO, et al. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology. 2009;150:1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318 [DOI] [PubMed] [Google Scholar]
- 34. Lubbers ER, List EO, Jara A, et al. Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity? J Endocrinol. 2013;216:363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162 [DOI] [PubMed] [Google Scholar]
- 36. Yakar S, Liu JL, Stannard B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sjögren K, Liu JL, Blad K, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA. 1999;96:7088–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yakar S, Rosen CJ, Beamer WG, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sjögren K, Sheng M, Movérare S, et al. Effects of liver-derived insulin-like growth factor I on bone metabolism in mice. J Bone Miner Res. 2002;17:1977–1987 [DOI] [PubMed] [Google Scholar]
- 40. Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A. The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci USA. 2008;105:19378–19383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sos BC, Harris C, Nordstrom SM, et al. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J Clin Invest. 2011;121:1412–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shi SY, Martin RG, Duncan RE, et al. Hepatocyte-specific deletion of Janus kinase 2 (JAK2) protects against diet-induced steatohepatitis and glucose intolerance. J Biol Chem. 2012;287:10277–10288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Engblom D, Kornfeld JW, Schwake L, et al. Direct glucocorticoid receptor-Stat5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Dev. 2007;21:1157–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Friedbichler K, Themanns M, Mueller KM, et al. Growth-hormone-induced signal transducer and activator of transcription 5 signaling causes gigantism, inflammation, and premature death but protects mice from aggressive liver cancer. Hepatology. 2012;55:941–952 [DOI] [PubMed] [Google Scholar]
- 45. Sjögren K, Jansson JO, Isaksson OG, Ohlsson C. A model for tissue-specific inducible insulin-like growth factor-I (IGF-I) inactivation to determine the physiological role of liver-derived IGF-I. Endocrine. 2002;19:249–256 [DOI] [PubMed] [Google Scholar]
- 46. Wu Y, Cui K, Miyoshi K, et al. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63:4384–4388 [PubMed] [Google Scholar]
- 47. Quintás-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10:127–140 [DOI] [PubMed] [Google Scholar]
- 48. Smith WJ, Nam TJ, Underwood LE, Busby WH, Celnicker A, Clemmons DR. Use of insulin-like growth factor-binding protein-2 (IGFBP-2), IGFBP-3, and IGF-I for assessing growth hormone status in short children. J Clin Endocrinol Metab. 1993;77:1294–1299 [DOI] [PubMed] [Google Scholar]
- 49. Wheatcroft SB, Kearney MT, Shah AM, et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56:285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hedbacker K, Birsoy K, Wysocki RW, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11–22 [DOI] [PubMed] [Google Scholar]
- 51. Dozmorov I, Galecki A, Chang Y, Krzesicki R, Vergara M, Miller RA. Gene expression profile of long-lived snell dwarf mice. J Gerontol A Biol Sci Med Sci. 2002;57:B99–B108 [DOI] [PubMed] [Google Scholar]
- 52. Kawai M, Breggia AC, DeMambro VE, et al. The heparin-binding domain of IGFBP-2 has insulin-like growth factor binding-independent biologic activity in the growing skeleton. J Biol Chem. 2011;286:14670–14680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Das SK, Bhutia SK, Azab B, et al. MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res. 2013;73:844–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Foulstone EJ, Zeng L, Perks CM, Holly JM. Insulin-like growth factor binding protein 2 (IGFBP-2) promotes growth and survival of breast epithelial cells: novel regulation of the estrogen receptor. Endocrinology. 2013;154:1780–1793 [DOI] [PubMed] [Google Scholar]
- 55. Guo C, Lu H, Gao W, et al. Insulin-like growth factor binding protein-2 level is increased in blood of lung cancer patients and associated with poor survival. PLoS One. 2013;8:e74973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haluzik M, Yakar S, Gavrilova O, Setser J, Boisclair Y, LeRoith D. Insulin resistance in the liver-specific IGF-1 gene-deleted mouse is abrogated by deletion of the acid-labile subunit of the IGF-binding protein-3 complex: relative roles of growth hormone and IGF-1 in insulin resistance. Diabetes. 2003;52:2483–2489 [DOI] [PubMed] [Google Scholar]
- 57. Nordstrom SM, Tran JL, Sos BC, Wagner KU, Weiss EJ. Disruption of JAK2 in adipocytes impairs lipolysis and improves fatty liver in mice with elevated GH. Mol Endocrinol. 2013;27:1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pinidiyapathirage MJ, Dassanayake AS, Rajindrajith S, et al. Non-alcoholic fatty liver disease in a rural, physically active, low income population in Sri Lanka. BMC Res Notes. 2011;4:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang H, He SM, Sun J, et al. Prevalence and etiology of abnormal liver tests in an adult population in Jilin, China. Int J Med Sci. 2011;8:254–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fernandes MT, Ferraro AA, de Azevedo RA, Fagundes Neto U. Metabolic differences between male and female adolescents with non-alcoholic fatty liver disease, as detected by ultrasound. Acta Paediatr. 2010;99:1218–1223 [DOI] [PubMed] [Google Scholar]
- 61. Haentjens P, Massaad D, Reynaert H, et al. Identifying non-alcoholic fatty liver disease among asymptomatic overweight and obese individuals by clinical and biochemical characteristics. Acta Clin Belg. 2009;64:483–493 [DOI] [PubMed] [Google Scholar]
- 62. Spruss A, Henkel J, Kanuri G, et al. Female mice are more susceptible to nonalcoholic fatty liver disease: sex-specific regulation of the hepatic AMP-activated protein kinase-plasminogen activator inhibitor 1 cascade, but not the hepatic endotoxin response. Mol Med. 2012;18:1346–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fernandez-Perez L, Guerra B, Diaz-Chico JC, Flores-Morales A Estrogens regulate the hepatic effects of growth hormone, a hormonal interplay with multiple fates. Front Endocrinol (Lausanne). 2013;4:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Svensson J, Sjogren K, Faldt J, et al. Liver-derived IGF-I regulates mean life span in mice. PLoS One. 2011;6:e22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yakar S, Setser J, Zhao H, et al. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest. 2004;113:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nam SY, Lobie PE. The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes Rev. 2000;1:73–86 [DOI] [PubMed] [Google Scholar]
- 67. Blüher S, Kratzsch J, Kiess W. Insulin-like growth factor I, growth hormone and insulin in white adipose tissue. Best Pract Res Clin Endocrinol Metab. 2005;19:577–587 [DOI] [PubMed] [Google Scholar]
- 68. Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152–177 [DOI] [PubMed] [Google Scholar]
- 69. Berryman DE, Glad CA, List EO, Johannsson G. The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2013;9:346–356 [DOI] [PubMed] [Google Scholar]
- 70. Chikani V, Ho KK. Action of GH on skeletal muscle function: molecular and metabolic mechanisms. J Mol Endocrinol. 2014;52:R107–R123 [DOI] [PubMed] [Google Scholar]
- 71. Schuenke MD, Kopchick JJ, Hikida RS, Kraemer WJ, Staron RS. Effects of growth hormone overexpression vs. growth hormone receptor gene disruption on mouse hindlimb muscle fiber type composition. Growth Horm IGF Res. 2008;18:479–486 [DOI] [PubMed] [Google Scholar]
- 72. Vijeyta F. Effects of Growth Hormone on Circulating Resistin Levels in Mice. Athens, OH: Ohio University Masters Thesis; 2012:1–141 [Google Scholar]