Abstract
Introduction
Platelet refrigeration decreases the risk of bacterial contamination and may preserve function better than standard-of-care room temperature storage. Benefits could include lower transfusion-related complications, decreased costs, improved hemostasis in acutely bleeding patients, and extended shelf-life. In this study, we compared the effects of 22°C and 4°C storage on the functional and activation status of apheresis platelets (APs).
Methods
APs (n = 5 per group) were stored for 5 days at 22°C with agitation (RT) versus at 4°C with agitation (4C+AG) and without (4C). Measurements included platelet counts, mean platelet volume, blood gas analytes, aggregation response, thromboelastography, TxB2 and sCD40L release, activation markers and microparticle formation.
Results
Sample pH levels were within acceptable limits for storage products (pH 6.2-7.4). Platelet glucose metabolism (P < 0.05), aggregation response (ADP: RT 0; 4C+AG 5.0 ± 0.8; 4C 5.6 ± 0.9; P < 0.05), and clot strength (MA: RT 58 ± 2; 4C+AG 63 ± 2; 4C 67 ± 2; P < 0.05) were better preserved at 4°C compared to RT storage. Refrigerated samples were more activated compared to RT (P < 0.05), although TxB2 (P < 0.05) and sCD40L release (P < 0.05) were higher at RT. Agitation did not improve the quality of 4°C-stored samples.
Conclusion
AP stored at 4°C maintain more viable metabolic characteristics, are hemostatically more effective, and release fewer pro-inflammatory mediators than AP stored at RT over 5 days. Given the superior bacteriologic safety of refrigerated products, these data suggest that cold-stored platelets may improve outcomes for acutely bleeding patients.
Keywords: Hemostasis, coagulation, aggregation, clot strength, cold storage, activation
Introduction
Current blood-banking recommendations are that platelets be stored in incubators at 22°Celsius (°C), with gentle agitation for no longer than 5 days (1). This limited shelf-life is necessary due to the risk of bacterial contamination that leads to life-threatening transfusion-related infections (2-4). These storage practices also result in a platelet storage lesion that is associated with a decline in platelet hemostatic function (5).
Cold storage at 4°C could prolong shelf-life by diminishing the risk of bacterial sepsis, decreasing platelet metabolism, and maintaining functionality. Transportation of 4°C platelets would be convenient because the infrastructure for other refrigerated blood products, such as red blood cells (RBCs), is already in place (6). Cold platelets may be a better hemostatic product. Several in vitro studies from the 1970s showed that refrigeration of platelets results in better metabolic and functional responses such as minimal lactate accumulation, better aggregation response, and adhesion to subendothelium (7-9). In vivo human studies have shown that 4°C platelets function better than room temperature (RT) platelets in reducing the bleeding times in thrombocytopenic patients, aspirin-treated volunteers, and in aplastic thrombocytopenic patients shortly after transfusion (10-12).
Despite promising in vitro and in vivo studies in controlling acute bleeding, the practice of cold storage for transfusion was abandoned during the 1970s due to the belief that clinically effective platelets should be both hemostatically functional and survive in circulation for several days as indicated for prophylactic transfusion. It was shown by Murphy and Gardner that platelets stored for up to 18 hours in cold (2-4°C) show decreased recovery and survival upon transfusion compared to their RT (22-24°C) counterparts, i.e., the lifespan (t1/2) of cold- and RT-stored platelets are 1.3 and 3.9 days, respectively (13). Consequently, when transfused to thrombocytopenic patients, cold stored platelets are as effective as RT-stored platelets at 24 h post-transfusion though not at 72 h suggesting cold storage may be acceptable for therapeutic transfusion (i.e., therapy for active hemorrhage) (14). In recent years, Hoffmeister and colleagues have shown that platelet clearance and function may be entirely separate attributes, and that appropriate modification (such as glycosylation) may prolong platelet survival in circulation (15-17). Concurrent reports from several groups have confirmed that platelets stored at 4°C are “healthier” than platelets stored at room temperature (18). Together, these studies have prompted the reconsideration of cold stored platelets as a transfusion product to treat bleeding patients (18-20). We have recently shown that cold storage of platelet rich plasma (PRP) enhances platelet activation and aggregation under shear, and cold storage of platelets in whole blood improves their performance in a panel of functional assays compared to RT-stored platelets (21-22).
In this study, we characterized the effects of storage at both 22°C and 4°C for up to 5 days with apheresis platelets (AP) using functional and activation assays. It is particularly important to evaluate the effect of storage on AP since apheresis is the most common method of platelet isolation with 80% use in the United States and 50% in Europe, and it has been shown that the method of preparation of platelet concentrate (i.e., buffy coat, apheresis or PRP) is a determinant of platelet storage lesion (23-27). We hypothesized that apheresis platelets stored at 4°C would demonstrate more viable metabolic characteristics, perform better in functional tests, form stronger clots, and release fewer inflammatory mediators compared to platelets stored at 22°C.
Materials and Methods
Reagents and Suppliers
Calcium and kaolin used in thromboelastography were obtained from Haemonetics Corp (Braintree, MA). Multiplate agonists were obtained from Diapharma (West Chester, OH). FITC-conjugated bovine lactadherin was purchased from Haematologic Technologies, Inc (Essex Junction, VT). Gibco 1× Hanks Balanced Salt Solution (HBSS) without calcium and magnesium was obtained from Invitrogen Life Technologies (Carlsbad, CA). The following anti-human monoclonal mouse antibodies were purchased from BD Biosciences (San Jose, CA): APC-conjugated anti-CD62P (P-selectin), clone AK-4; PE conjugated anti-CD 154 (CD40 ligand or CD40L), clone TRAP1. PerCP conjugated mouse monoclonal anti-CD42b (glycoprotein Ib receptor, clone HIP-1 was purchased from BioLegend (San Diego, CA). For absolute particle counts TruCount tubes from BD Biosciences were utilized. Thromboxane B2 EIA kits were purchased from Cayman Chemicals (Ann Arbor, MI) and Human soluble CD40L (sCD40L) Extra Sensitive Platinum enzyme-linked immunosorbent assay (ELISA) kits were purchased from eBioscience (Vienna, Austria). CG4+ and CHEM8+ cartridges were purchased from Abbott Laboratories (Abbott Park, IL).
Platelet Storage and Handling
Single AP units were collected in acid-citrate-dextrose (ACD)-plasma from healthy donors (n=5) using a Trima Accel Automated Blood Collection System, Terumo BCT (Lakewood, CO) under a protocol reviewed and approved by the US Army Medical Research and Materiel Command Institutional Review Board and in accordance with the approved protocol. 10 ml aliquots were obtained from the donor bag and sterilely transferred into 15-ml BCSI mini-bags (Seattle, WA) for storage, with one mini-bag used for each time point and condition. The mini-bags were stored for 5 days in one of the following conditions: RT in a Food and Drug Administration (FDA)-approved platelet incubator with agitation (RT); in a walk-in refrigerator at 4°C with agitation (4C+AG) or without agitation (4C). Platelet function, characteristics, and release markers were analyzed for all stored samples on the day of collection (Baseline or Day 1), Day 3, and Day 5. Samples stored at 4°C were allowed to come to RT for 30 minutes and gently massaged before testing commenced. AP was centrifuged for 10 minutes at 3000×g to obtain platelet-poor plasma (PPP) where applicable. For RBC reconstitution, whole blood was collected in ACD vacutainer tubes, spun at 3500 × g for 10 minutes, and the plasma and buffy coat layers were carefully aspirated from the RBCs.
Laboratory equipment and measurements
All tests were performed at the U.S. Army Institute of Surgical Research at Joint Base San Antonio/FT Sam Houston according to routine protocols. Platelet count and mean platelet volume (MPV) were determined in duplicate using a standard cell counter (Coulter Ac-T diff2 hematology system, Beckman Coulter, Brea, CA). Mean platelet component was determined using an Advia 120 Haematology System (Bayer Diagnostics, Tarrytown, NY). Blood pH, sodium (mM), potassium (mM), chloride (mM), glucose (mg/dL), pCO2 (mmHg), pO2 (mmHg), bicarbonate (mM), and lactate (mM) were determined using an i-STAT point-of-care device (Abbot Labs, Abbott Park, IL). Thromboelastography (TEG) was performed using a TEG5000 device (Haemoscope Corp., Niles, IL) with kaolin activation. Aggregation was conducted using a Multiplate impedance aggregometer (Verum Diagnostica, Munich, Germany) with adenosine diphosphate (ADP), collagen, and thrombin receptor activating peptide (TRAP) as agonists. Platelet markers were assessed by flow cytometry with BD FacsCanto I and microparticle counts were determined using a BD FacsCanto II, RUO flow cytometer equipped with a FSC-PMT capable of detecting particles down to 200 nm. Particles were considered microparticles if their sizes fell within the range of 200-1000 nm.
Blood gas analysis
Blood gas and chemistry analyses in fresh and stored AP were performed using CG4+ and CG8+ cartridges, respectively. PPP samples (100 μl/cartridge) were obtained to test for metabolic parameters.
Thromboelastography (TEG)
All samples were tested as previously described (28). Briefly, samples were recalcified by the addition of CaCl2 (0.2M, 30 μL) to a TEG sample cup followed by kaolin-treated AP (330 μl). All tests were performed at 37°C in duplicate.
Impedance aggregometry
Impedance aggregometry was performed according to established methods (28). Briefly, 300 μl of AP diluted with PPP to a concentration of 250×103 platelets/μl was incubated with 300 μl of 3mM NaCl2/CaCl2 solution (Verum Diagnostica). After incubation, 20 μl of agonist (6.5 μM ADP, 3.2 μg/ml Collagen, or 32 μM TRAP) was added. The tests were carried out for 6 minutes in duplicate, and area under the curve (AUC) was reported. Aggregation was also performed in the presence of RBCs with a platelet count of 300×103/μl and 40% hematocrit for 12 minutes.
Surface receptor expression
Geometric mean fluorescence intensity (GMFI) and abundance (% positive) was determined for the following platelet markers: CD42b (glycoprotein Ib receptor or GPIb), CD62P (P-selectin), lactadherin (phosphatidylserine surface expression), and CD154 (CD40L surface expression molecule). Ten thousand (10,000) events per sample were recorded for each marker, and forward and side scatter characteristics were used to identify the platelet population. For microparticle analysis, absolute particle counts were determined using TruCount tubes (BD Biosciences).
ELISA
Commercially available kits were used to assess sCD40L and thromboxane B2 (TxB2) levels released into plasma during storage. PPP was stored at -80°C until analysis by ELISA. All plasma samples were diluted before testing to ensure that the concentrations fell within the standard range prescribed by the manufacturer.
Statistics
The data collected were analyzed by one-way ANOVA for repeated measures with a post-hoc Bonferroni adjustment for pairwise comparisons. Significance from baseline (Day 1) and between groups was determined when P<0.05. Data in tables are mean ± SD, and data in graphs are mean ± SEM, unless otherwise stated. Microsoft Excel (Microsoft Corp., Redmond, WA) was used to manage data and analysis was performed using the statistical software JMP (Version 10, SAS Institute Inc., Cary, NC).
Results
Platelet Count, MPV and MPC
We obtained platelet counts for all AP samples to assess loss of single platelets during storage in minibags as described in the Methods section (Table 1A). We observed a significant drop in the platelet count in both 4C+AG and 4C samples by Day 5 (P < 0.05), along with macroscopic platelet clumping, but the platelet number was unchanged in RT samples. The decrease in platelet number in 4°C samples on Day 5 compared to fresh platelets (Day 1 or baseline) was ∼20%; however platelet counts were stable and the clumping effect was abolished when refrigerated platelets were stored in full-sized bags (Table 1B). MPV remained unchanged in both RT and 4°C samples suggesting no significant change in the size of single platelets. The mean platelet component (MPC) decreased significantly after 5 day RT storage (P < 0.05) suggesting platelet degranulation due to RT storage. Platelets stored at 4°C maintained MPC. Stored platelet samples were visually inspected for swirling according to the guidelines set by Bertolini (29). RT platelets maintained their ability to swirl at Day 5 of storage, while swirling in 4°C-stored samples was nonexistent, most likely due to the shape change that occurs during storage at 4°C (30).
Table 1.
A. Platelet count ×103, mean platelet volume (MPV), and mean platelet component (MPV) during storage in minibags (research use only). B. Platelet count ×103 of apheresis platelets stored in full-sized FDA-approved platelet storage bags (n = 3).
| A | Baseline | RT | 4C | 4C+AG | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Day 1 | Day 3 | Day 5 | Day 3 | Day 5 | Day 3 | Day 5 | |
| Count (×103 plts/μl) | 1084±47 | 1019±66 | 924±149 | 815±152 | 727±153* | 854±103 | 687±147* |
| MPV (fl) | 7.0±0.2 | 6.9±0.1 | 7.0±0.2 | 6.9±0.4 | 6.9±0.3 | 7.0±0.4 | 6.8±0.3 |
| MPC (g/dL) | 25±1.8 | - | 20±1.7* | - | 26±2.3 | - | - |
|
| |||||||
| Values (mean ± SD) significant from Baseline represented by *(P < 0.05) | |||||||
| B | Baseline | RT | 4C |
|---|---|---|---|
|
| |||
| Day 1 | Day 5 | Day 5 | |
| Count (×103 plts/μl) | 1192±445 | 1187±51 | 1123±40 |
Values (mean ± SD) were not significantly different (P ≥ 0.05)
Platelet metabolism
We measured the pH and blood gases in stored platelet samples using i-STAT, and the results are tabulated in Table 2. All samples maintained acceptable levels of pH, i.e., between 6.2 and 7.4 (31). We observed an increase in lactate production and glucose consumption in both RT- and 4°C-stored platelets. The lactate levels in RT-stored platelets increased by ∼300% and ∼600%, and glucose levels dropped by 8% and 25% by Day 3 and Day 5, respectively (P < 0.05). In comparison, the lactate levels in 4°C-stored platelets increased only by 150% and 225% by Day 3 and Day 5, respectively (P < 0.05), and the glucose levels did not change significantly. Correspondingly, more bicarbonate was consumed and carbon dioxide released in RT- than 4°C-stored platelets (P < 0.05). The dissolved oxygen levels were comparable and remained high, suggesting adequate gas exchange during the storage period. The glucose consumption and lactate production rates were 3- and 2-fold higher, respectively, during RT storage than during cold storage over 5 days (Table 3). There was no difference between 4°C platelets stored under non-agitated or agitated conditions. Thus, these parameters suggest that 4°C-stored platelets show a significant decrease (more than 2-3 fold) in metabolic rate compared to RT-stored platelets over 5-day storage. A small, but significant difference in sodium and chloride levels was observed at Day 5 in RT samples, and in potassium levels in 4C and 4C+AG samples.
Table 2.
Metabolic and electrolyte levels in plasma during storage.
| Baseline | RT | 4C | 4C+AG | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Day 1 | Day 3 | Day 5 | Day 3 | Day 5 | Day 3 | Day 5 | |
| pH | 7.24±.07 | 7.45±.16 | 7.28±.08 | 7.45±.21 | 7.44±.13 | 7.48±.25 | 7.44±.15 |
| Lactate (mg/dL) | 1.77±0.6 | 6.85±0.8* | 12.87±2.1* | 4.51±0.6* | 5.67±0.3*† | 4.83±0.7* | 6.05±0.4*† |
| Glucose (mg/dL) | 321.5±8.1 | 294.0±22.1* | 238.3±42.5* | 310.2±14.2* | 307.5±25.0*† | 312.4±28.1* | 303.5±24.6*† |
| Bicarbonate (mM) | 18.04±1.6 | 10.06±2.9* | 6.27±0.9* | 14.00±1.7*δ | 12.78±0.8*† | 14.01±2.7* δ | 12.38±1.0*† |
| pCO2 (mmHg) | 38.5±4.7 | 13.8±1.5* | 13.1±.25* | 24.1±4.2* δ | 19.5±4.4*† | 22.6±5.6* δ | 18.7±4.7*† |
| pO2 (mmHg) | 91.8±9.7 | 113±17.8 | 132±41.2 | 120.4±32.7 | 139.8±26.8*† | 116.6±31.0 | 128.0±29.3† |
| Sodium (mM) | 137.5±1 | 139.5±3.7 | 140.25±1.3* | 137.4±1.7 | 136.8±.96† | 137.8±1.6 | 136.5±1.3† |
| Potassium (mM) | 3.24±.18 | 3.34±.23 | 3.45±.31 | 3.52±.19* | 3.60±.25* | 3.52±.25* | 3.55±.27*† |
| Chloride (mM) | 97.6±.89 | 101.3±4.9 | 104.8±1.3* | 99.2±2.8 | 99.7±1.2† | 99.0±2.2 | 98.8±1.7† |
Values (mean ± SD) significant from Baseline represented by (P < 0.05)
Values significant from Day 3 RT represented by (P < 0.05)
Values significant from Day 5 RT represented by (P < 0.05)
Table 3.
Glucose consumption and lactate production rates calculated from the measurements in Table 1.
| RT | 4C | 4C+AG | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Day 3 | Day 5 | Day 3 | Day 5 | Day 3 | Day 5 | |
| Glucose consumption from Baseline (μmol/1012 platelets/hr) | 30.6±7.9 | 75.6±25.0 | 17.1±11.3 | 25.3±3.6* | 14.8±7.1* | 29.4±9.8* |
| Lactate production from Baseline (μmol/1012 platelets/hr) | 71.8±7.2 | 97.6±23.0 | 36.1±5.1* | 40.0±8.6* | 40.3±4.9* | 44.6±8.5* |
Values (mean ± SD) significant from corresponding RT samples represented by (P < 0.05)
Platelet aggregation
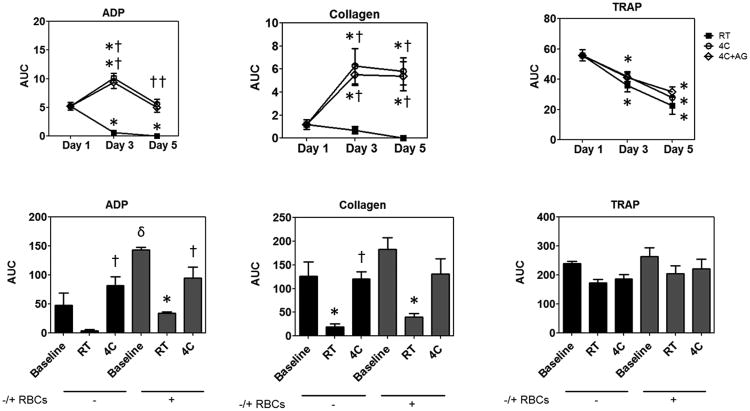
Next, we tested the aggregation response of fresh and stored platelets to physiologically-relevant agonists including ADP, collagen, and TRAP (Figure 1). Cold-stored platelets aggregate significantly better than both RT-stored and fresh platelets when stimulated with ADP (Figure 1A) and collagen (Figure 1B). However, in the case of TRAP (Figure 1C), all storage products decreased compared to fresh platelets, and no differences were seen between groups. There was no difference between 4°C platelets stored under non-agitated or agitated conditions. These data show that 4°C-stored platelets are more responsive to activating stimuli than RT-stored platelets.
Figure 1.
Aggregation of platelets without (A, B, C) and with RBCs (D, E, F) when stimulated with (A, D) ADP, (B, E) Collagen, and (C, F) TRAP. Bar graphs D, E, and F are comparisons between Baseline versus Day 5 RT and 4C AP. Treatment conditions are represented as follows: RT =
 ; 4C =
; 4C =
 ; 4C+AG =
; 4C+AG =
 . Area under the curve (AUC) are represented as mean ± SEM. Differences compared to baseline (*), compared to RT (†), and between samples without RBCs versus with (δ) are shown if results from both the one-way ANOVA for repeated measures and the post hoc Bonferroni test comparisons are significant (P < 0.05). (Note: - RBC AUC values are higher in graphs D-F compared to A-C because aggregation was measured over 12 minutes instead of 6.)
. Area under the curve (AUC) are represented as mean ± SEM. Differences compared to baseline (*), compared to RT (†), and between samples without RBCs versus with (δ) are shown if results from both the one-way ANOVA for repeated measures and the post hoc Bonferroni test comparisons are significant (P < 0.05). (Note: - RBC AUC values are higher in graphs D-F compared to A-C because aggregation was measured over 12 minutes instead of 6.)
We also evaluated the effect of RBC on aggregation response by adding 40% RBC to platelets stored for 5 days (Figure 1D-E). The baseline AUC is higher than in Figure 1A-C because aggregation was monitored for 12 minutes instead of 6. We observed that the addition of RBC significantly increased the response of fresh platelets to ADP. The response of RT platelets to ADP and collagen stimulation again declined significantly both with and without RBCs compared to baseline, while the response of cold-stored platelets was not enhanced by the addition of RBC. Responses of Day 5 4C samples to ADP and collagen stimulation over 12 minutes were similar to baseline. Unlike the six minute data, the 4C collagen response was not enhanced at Day 5 compared to fresh. Decreases in TRAP aggregation over time were not significant in 12-minute aggregation tests for any group, and responses did not were not changed by the addition of RBC.
Clot formation and strength
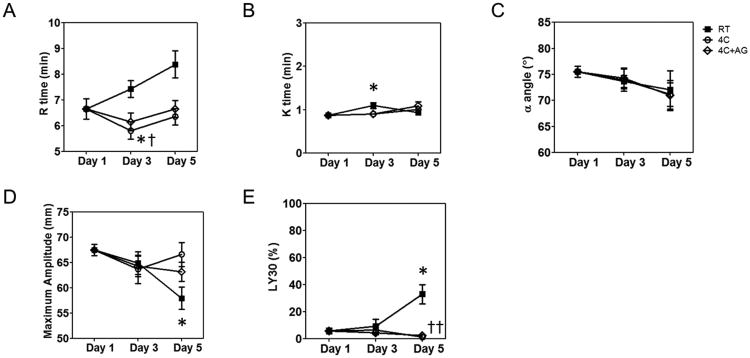
We tested the kinetics of formation, strength, and stability of clots formed from fresh or stored platelets using thromboelastography. TEG measures time to initial fibrin formation (reaction time or R), time to clot formation (clot time or K), rate of clot formation (α angle), clot strength (maximum amplitude or MA), and clot lysis at 30 minutes (LY30). As can be seen in Figure 2A, the time to initial fibrin formation, R time, was significantly faster with storage at 4°C without agitation, resulting in faster clot formation at Day 3 both compared to baseline and RT storage. The clot generation time, K, for the RT samples was statistically different from baseline at Day 3, but differences were clinically unimportant (Figure 2B). The α-angle was not significantly changed by storage method or duration (Figure 2C). The clot strength, as measured by MA, decreased significantly on Day 5 for RT-stored platelets but remained unaffected in 4°C-stored platelets (Figure 2D). The clot lysis, as measured by LY30, increased significantly after 5 days for RT-stored platelets, but remained constant for 4°C-stored platelets (Figure 2E). With the exception of the Day 3 R values, agitation did not alter any of the TEG parameters during storage at 4°C. Taken together, the TEG assay clearly demonstrates that 4°C-stored platelets retain the ability to support normal clotting, comparable to fresh platelets even after 5 days of storage, whereas RT-stored platelets form clots that are weak and susceptible to lysis. Conversely, cold storage enhanced initiation of fibrin formation.
Figure 2.
Measurement of clot properties by thromboelastography. (A) R time; (B) K time; (C) α angle; (D) Maximum amplitude. Treatment conditions are represented as follows: RT =
 ; 4C =
; 4C =
 ; 4C+AG =
; 4C+AG =
 . Data are represented as mean ± SEM. Differences from Baseline (*) and between treatment groups (†) are shown if results from both the one-way ANOVA for repeated measures and the post-hoc Bonferroni test comparisons are significant (P<0.05).
. Data are represented as mean ± SEM. Differences from Baseline (*) and between treatment groups (†) are shown if results from both the one-way ANOVA for repeated measures and the post-hoc Bonferroni test comparisons are significant (P<0.05).
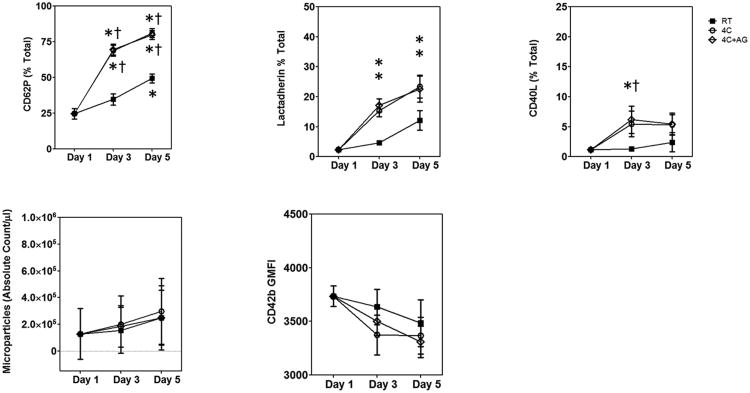
Platelet activation marker expression
We measured the levels of receptors expressed on activated platelets including P-selectin, lactadherin binding to exposed phosphotidylserine (PS), and CD40L (Figure 3 A-C). We observed a significant increase in the percentage of platelets expressing P-selectin (CD62P) over storage duration in both RT and 4°C-stored platelets. The 4°C-stored platelets showed a greater increase in CD62P expression (Figure 3A) compared to RT-stored platelets. PS exposure, as measured by lactadherin binding (Figure 3B), also increased with storage duration at 4°C. We used lactadherin instead of annexin V since lactadherin binding does not require Ca+2 and has higher sensitivity (32). The CD40L expression on platelet surfaces were higher with cold storage and agitation on day 3 compared to baseline and RT conditions (Figure 3C). Changes in microparticle count and CD42b surface expression were not significant either over time or between cold and RT-stored samples (Figure 3D-E).
Figure 3.
Estimation of surface receptor levels by flow cytometry. (A) P-Selectin; (B) lactadherin binding (PS exposure); (C) CD40L expression; (D) Microparticle release; and (E) GP Ibα. Treatment conditions are represented as follows: RT =
 ; 4C =
; 4C =
 ; 4C+AG =
; 4C+AG =
 . The expression levels are represented as mean±SEM. Differences from Baseline (*) and between treatment groups (†) are shown if results from both the one-way ANOVA for repeated measures and the post-hoc Bonferroni test comparisons are significant (P<0.05).
. The expression levels are represented as mean±SEM. Differences from Baseline (*) and between treatment groups (†) are shown if results from both the one-way ANOVA for repeated measures and the post-hoc Bonferroni test comparisons are significant (P<0.05).
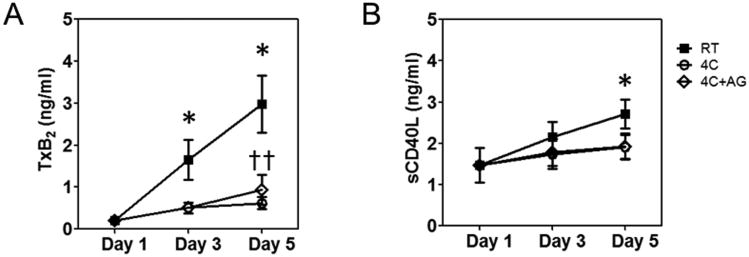
Release of pro-inflammatory mediators
When platelets become activated, they release their granular contents and other metabolites into plasma. We tested the levels of sCD40L and TxB2 released by platelets into plasma during storage (Figure 4). TxB2 is the stable end product of a metabolite of the arachidonic acid pathway, TxA2, which intracellularly amplifies signals from other agonists such as ADP or thrombin. RT-stored platelets released 4-fold more TxB2 than 4°C-stored platelets after 5 days of storage while the latter platelets did not release any significant quantities of TxB2 (Figure 4A). Soluble CD40L is a protein expressed in platelets that is known to play a role in inflammation, thrombosis, and restenosis (33). CD40L translocates from inside to the surface of agonist-activated platelets, and is shed from the platelet surface as sCD40L. RT-stored samples released slightly more sCD40L into plasma during storage compared to baseline on Day 5 (Figure 4B).
Figure 4.
ELISA quantification of soluble factors released by platelets. (A) TxB2, and (B) sCD40L. Treatment conditions are represented as follows: RT =
 ; 4C =
; 4C =
 ; 4C+AG =
; 4C+AG =
 . The concentrations are represented as mean ± SEM. Differences from Baseline (*) and between treatment groups (†) are shown if results from both the one-way ANOVA for repeated measures and the post-hoc Bonferroni test comparisons are significant (P<0.05).
. The concentrations are represented as mean ± SEM. Differences from Baseline (*) and between treatment groups (†) are shown if results from both the one-way ANOVA for repeated measures and the post-hoc Bonferroni test comparisons are significant (P<0.05).
Discussion
In this work, we demonstrated that apheresis platelets stored at 4°C are superior in metabolic and functional assays to platelets stored under standard blood banking conditions at RT. Furthermore, we find that agitation does not significantly influence the function of the 4°C-stored product. Our results suggest that 4°C-stored apheresis platelets would perform better to stop acute hemorrhage. Additional benefits of refrigeration would include decreased risk of bacterial contamination and simplified transport and storage because other blood products must be maintained at 4°C (2-4). Cold platelets may be a viable alternative to RT-stored platelets for therapeutic, as opposed to prophylactic, transfusion when immediate hemostasis is required.
As platelets stored in plasma metabolize glucose via the glycolytic pathway, they produce lactate and free hydrogen ions, which are buffered by bicarbonate in plasma to yield carbon dioxide and water (34). Glucose levels in 4°C-stored platelets did not change over time, consistent with decreased metabolic rates in the refrigerated samples. Similarly, changes in lactate and bicarbonate levels were also small. A similar effect was described in platelet concentrates from PRP stored at 4°C for up to 3 days (8). Stable pH levels correlate with preserved platelet viability, thus the nearly constant pH measurements in the stored 4°C samples indicate that platelet quality is better maintained during refrigeration. In contrast, the RT-stored samples demonstrated a precipitous drop in glucose levels, increased lactate levels, and a corresponding decrease in bicarbonate levels, consistent with the increased metabolic rates previously described during RT storage (31, 35). The glucose consumption and lactate production rates during RT storage were comparable to those reported by Dumont et al (31). Surprisingly, despite better metabolic indices, the platelet counts during 4°C storage were significantly lower compared to RT storage suggesting either adhesion to the bag, clumping or cell death. This loss was abolished when platelets were stored in larger, standard-sized bags, suggesting that higher surface-to-volume ratio in the smaller bags may contribute to the reduction in platelet count.
The various markers of platelet activation including CD62P expression, PS exposure, and expression of CD40L increased during 4°C storage, but only CD62P expression was greater at RT. A similar difference in CD62P expression and PS exposure between 4°C and RT storage has been reported for PRP (36). PS exposure and the release of α-granule contents during RT storage is correlated with the loss in mitochondrial membrane potential and apoptosis due to depletion of glucose and lactate accumulation that occurs from continuous metabolic activity (37-38). These markers represent different molecular events of activation: CD62P is released from platelet α-granules; PS is externalized from the inner leaflet of the platelet membrane; and CD40L is a pro-inflammatory mediator translocated to the platelet membrane. All were higher in 4°C-stored platelets compared to RT despite significantly reduced metabolic rates. In addition, RT storage, but not cold storage, results in platelet degranulation which has been correlated with activation (39). These data suggest that distinct mechanisms of activation are triggered at each storage temperature tested. While platelet activation at RT is related to aging or senescence, we speculate that cold-induced biophysical changes such as membrane rafting, GP Ib receptor clustering, and cytoskeletal rearrangement may trigger a set of downstream biochemical pathways distinct from ageing that result in platelet activation at 4°C (15, 40-43).
In contrast, we found that RT-stored platelets release more pro-inflammatory mediators such as sCD40L and TxA2 (measured as the more stable downstream product, TxB2), possibly due to platelet degranulation. Elevated release of sCD40L at RT has been previously described and may play a potential role in adverse transfusion reactions (44-45). TxA2 is produced by the oxidation of arachidonic acid during platelet activation and is in itself a potent activator of platelets, thus may result in inappropriate coagulation upon transfusion (46). In essence, a decrease in soluble inflammatory mediators suggests that cold stored platelets may be safer for transfusion than RT-stored platelets and may result in fewer adverse events such as transfusion-related acute lung injury (TRALI). Low TxB2 release from 4°C-stored platelets indicates that arachidonic acid is relatively preserved, suggesting that the dynamic range of platelet response to physiologic agonists after transfusion is similarly preserved and may explain our aggregometry results.
Circulating platelets are in a state of low activation but high responsiveness. The stimulation of fresh platelets with suboptimal concentrations of natural agonists activates the platelets but also decreases their response to subsequent stimulation with the same or different agonists (47-49). Both RT and 4°C storage activate platelets, but this activation leads to variable responses to chemical agonists. The markedly reduced ability of RT-stored platelets to aggregate upon stimulation with ADP, collagen and TRAP is concordant with previous studies of platelets obtained via both the buffy coat and apheresis methods (50-51). This phenomenon has been attributed to senescence due to progressive loss in energy generating machinery (52). In contrast, 4°C-stored platelets aggregate as well as fresh platelets to ADP and collagen, possibly due to cold-induced platelet activation. This trend was maintained even after the addition of RBC to mimic the physiological environment. Together, our data suggests that activation due to storage at 4°C ‘primes’ the platelet to a state of heightened responsiveness without compromising the innate response to stimuli. Thus, it appears that the activation state attained by platelets due to 4°C storage may be different from activation states associated with either RT storage or prior activation with chemical stimuli. The similar decline in aggregation response to TRAP activation for both storage temperatures may be due to a loss of thrombin receptors (53).
The superior ability of 4°C-stored platelets to aggregate when stimulated with chemical agonists is accompanied by improved clot strength and stability. While RT storage negatively impacts clot strength and enhances fibrinolysis compared to fresh platelets, significant loss of function was not seen in 4°C-stored samples. Specifically, TEG analysis indicates that despite pre-activation, RT-stored platelets result in the formation of weaker clots that are less resistant to fibrinolysis, probably due to low thrombin generation levels (54). The maintenance of hemostatic function in 4°C-stored platelets, as estimated by TEG, is in line with previous reports (20). Although TEG measures global coagulation, the contribution of platelets is reflected predominantly in MA. In this study, the MA values confirm that platelet integrity and function are well preserved during cold storage without significantly changing AP clot strength.
We stored platelets at 4°C both with and without the method of agitation currently employed by blood banks during standard RT storage. Our assays did not indicate that agitation during cold storage has a significant role in maintaining platelet health and function. Conversely, RT-stored platelets must be agitated either continuously or intermittently every 24 hours to prevent poor gas exchange, acidosis (pH<6), loss of normal morphology, and low platelet recovery (55-57). Thus cold storage may reduce storage costs and minimize space by eliminating the need for platelet agitators during storage and transportation to remote locations. Furthermore, the cost of 4°C stored platelets could be further lowered if reduced need for bacterial testing is proven.
In summary, our data demonstrates that cold-stored platelets have better functional competence and fewer inflammatory mediators than RT-stored platelets. Despite a few promising human trials in the 1970's, platelet refrigeration has been abandoned because of shorter circulation time post transfusion. Platelet utilization is shifting due to clinical practice guidelines that recommend 1:1:1 transfusion ratios for massive transfusion trauma protocols. The medical environment in which prophylactic transfusions predominated is giving way to one in which active bleeding is frequently the target of therapy. When treating severe hemorrhage, the superior hemostatic effectiveness of cold platelets may actually make them the better choice. Refrigerated platelets were the standard of care for more than a decade. Our results, along with a growing body of evidence, suggest the need to seriously consider the re-instatement of cold-stored platelets as a widely-available therapeutic product.
Acknowledgments
We thank Ron Bryant for collecting apheresis platelets.
Sources of support 1. U.S. Army Medical Research and Materiel Command; 2. National Institutes of Health (NIGMS MBRS-RISE GM060655)
List of Abbreviations
- RT
room temperature
- 4C
storage at 4 °Celsius
- 4C+AG
storage at 4 °Celsius with gentle agitation
- AP
apheresis platelets
- ADP
adenosine diphosphate
- TRAP
thrombin receptor activating peptide
- CD62P
P-selectin
- CD40L
CD40 ligand
- sCD40L
soluble CD40 ligand
- CD154
CD40L surface expression molecule
- CD42b
glycoprotein Ib receptor
- GPIb
glycoprotein Ib
- TxB2
Thromboxane B2
- RBC
red blood cell
- HBSS
Hanks Balanced Salt Solution
- ELISA
enzyme-linked immunosorbent assay
- ACD
acid-citrate-dextrose
- PPP
platelet-poor plasma
- FDA
Food and Drug Administration
- MPV
mean platelet volume
- MPC
mean platelet component
- TEG
Thromboelastography
- GMFI
Geometric mean fluorescence intensity
- AUC
area under the curve
- R
reaction time (time to initial fibrin formation)
- K
time to clot formation
- α angle
alpha angle (rate of clot formation)
- MA
maximum amplitude (clot strength)
- LY30
clot lysis at 30 minutes
- TRALI
transfusion-related acute lung injury
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest relevant to this manuscript.
The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
This study was conducted under a protocol reviewed and approved by the US Army Medical Research and Materiel Command Institutional Review Board and in accordance with the approved protocol.
References
- 1.Stroncek DF, Rebulla P. Platelet transfusions. Lancet. 2007;370(9585):427–38. doi: 10.1016/S0140-6736(07)61198-2. [DOI] [PubMed] [Google Scholar]
- 2.Brecher ME, Hay SN, Rothenberg SJ. Evaluation of a new generation of plastic culture bottles with an automated microbial detection system for nine common contaminating organisms found in PLT components. Transfusion. 2004;44(3):359–63. doi: 10.1111/j.1537-2995.2003.00617.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuehnert MJ, Roth VR, Haley NR, Gregory KR, Elder KV, Schreiber GB, Arduino MJ, Holt SC, Carson LA, Banerjee SN, Jarvis WR. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion. 2001;41(12):1493–9. doi: 10.1046/j.1537-2995.2001.41121493.x. [DOI] [PubMed] [Google Scholar]
- 4.Currie LM, Harper JR, Allan H, Connor J. Inhibition of cytokine accumulation and bacterial growth during storage of platelet concentrates at 4 degrees C with retention of in vitro functional activity. Transfusion. 1997;37(1):18–24. doi: 10.1046/j.1537-2995.1997.37197176946.x. [DOI] [PubMed] [Google Scholar]
- 5.Seghatchian J, Krailadsiri P. Platelet storage lesion and apoptosis: are they related? Transfus Apher Sci. 2001;24(1):103–5. doi: 10.1016/s0955-3886(00)00134-x. [DOI] [PubMed] [Google Scholar]
- 6.Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370(9585):415–26. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- 7.McGill M, Brindley DC. Effects of storage on platelet reactivity to arterial subendothelium during blood flow. J Lab Clin Med. 1979;94(2):370–80. [PubMed] [Google Scholar]
- 8.Rock G, Figueredo A. Metabolic changes during platelet storage. Transfusion. 1976;16(6):571–9. doi: 10.1046/j.1537-2995.1976.16677060241.x. [DOI] [PubMed] [Google Scholar]
- 9.Shively JA, Gott CL, De Jongh DS. The effect of storage on adhesion and aggregation of platelets. Vox Sang. 1970;18(3):204–15. doi: 10.1111/j.1423-0410.1970.tb01450.x. [DOI] [PubMed] [Google Scholar]
- 10.Becker GA, Tuccelli M, Kunicki T, Chalos MK, Aster RH. Studies of platelet concentrates stored at 22 C and 4 C. Transfusion. 1973;13(2):61–8. doi: 10.1111/j.1537-2995.1973.tb05442.x. [DOI] [PubMed] [Google Scholar]
- 11.Slichter SJ, Harker LA. Preparation and Storage of Platelet Concentrates. Transfusion. 1976;16(1):8–12. doi: 10.1046/j.1537-2995.1976.16176130842.x. [DOI] [PubMed] [Google Scholar]
- 12.Valeri CR. Circulation and hemostatic effectiveness of platelets stored at 4 C or 22 C: studies in aspirin-treated normal volunteers. Transfusion. 1976;16(1):20–3. doi: 10.1046/j.1537-2995.1976.16176130832.x. [DOI] [PubMed] [Google Scholar]
- 13.Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability--deleterious effect of refrigerated storage. N Engl J Med. 1969;280(20):1094–8. doi: 10.1056/NEJM196905152802004. [DOI] [PubMed] [Google Scholar]
- 14.Filip DJ, Aster RH. Relative hemostatic effectiveness of human platelets stored at 4 degrees and 22 degrees C. J Lab Clin Med. 1978;91(4):618–24. [PubMed] [Google Scholar]
- 15.Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, von Andrian UH, Wagner DD, Stossel TP, Hartwig JH. The clearance mechanism of chilled blood platelets. Cell. 2003;112(1):87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmeister KM, Josefsson EC, Isaac NA, Clausen H, Hartwig JH, Stossel TP. Glycosylation restores survival of chilled blood platelets. Science. 2003;301(5639):1531–4. doi: 10.1126/science.1085322. [DOI] [PubMed] [Google Scholar]
- 17.Josefsson EC, Gebhard HH, Stossel TP, Hartwig JH, Hoffmeister KM. The macrophage alphaMbeta2 integrin alphaM lectin domain mediates the phagocytosis of chilled platelets. J Biol Chem. 2005;280(18):18025–32. doi: 10.1074/jbc.M501178200. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman RM. Uncommon cold: could 4 degrees C storage improve platelet function? Transfusion. 2005;45(9):1407–12. doi: 10.1111/j.1537-2995.2005.00544.x. [DOI] [PubMed] [Google Scholar]
- 19.Egidi MG, D'Alessandro A, Mandarello G, Zolla L. Troubleshooting in platelet storage temperature and new perspectives through proteomics. Blood Transfus. 2010;8(3):s73–81. doi: 10.2450/2010.012S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stiegler G, Fischer G, Ramanathan G, Bencur P, Weigel G, Mannhalter C. P-selectin mRNA is maintained in platelet concentrates stored at 4 degrees C. Transfusion. 2009;49(5):921–7. doi: 10.1111/j.1537-2995.2008.02073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery RK, Reddoch KM, Evani SJ, Cap AP, Ramasubramanian AK. Enhanced shear-induced platelet aggregation due to low-temperature storage. Transfusion. 2013;53(7):1520–30. doi: 10.1111/j.1537-2995.2012.03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pidcoke HF, McFaul SJ, Ramasubramanian AK, Parida BK, Mora AG, Fedyk CG, Valdez-Delgado KK, Montgomery RK, Reddoch KM, Rodriguez AC, Aden JK, Jones JA, Bryant RS, Scherer MR, Reddy HL, Goodrich RP, Cap AP. Primary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over time. Transfusion. 2013;53(1):137S–149S. doi: 10.1111/trf.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bock M, Rahrig S, Kunz D, Lutze G, Heim MU. Platelet concentrates derived from buffy coat and apheresis: biochemical and functional differences. Transfus Med. 2002;12(5):317–24. doi: 10.1046/j.1365-3148.2002.00392.x. [DOI] [PubMed] [Google Scholar]
- 24.Krailadsiri P, Seghatchian J. Are all leucodepleted platelet concentrates equivalent? Comparison of Cobe LRS Turbo, Haemonetics MCS+ LD, and filtered pooled buffy-coat-derived platelets. Vox Sang. 2000;78(3):171–5. doi: 10.1159/000031176. [DOI] [PubMed] [Google Scholar]
- 25.Metcalfe P, Williamson LM, Reutelingsperger CP, Swann I, Ouwehand WH, Goodall AH. Activation during preparation of therapeutic platelets affects deterioration during storage: a comparative flow cytometric study of different production methods. Br J Haematol. 1997;98(1):86–95. doi: 10.1046/j.1365-2141.1997.1572983.x. [DOI] [PubMed] [Google Scholar]
- 26.Sloand EM, Yu M, Klein HG. Comparison of random-donor platelet concentrates prepared from whole blood units and platelets prepared from single-donor apheresis collections. Transfusion. 1996;36(11-12):955–9. doi: 10.1046/j.1537-2995.1996.36111297091737.x. [DOI] [PubMed] [Google Scholar]
- 27.Vassallo RR, Murphy S. A critical comparison of platelet preparation methods. Curr Opin Hematol. 2006;13(5):323–30. doi: 10.1097/01.moh.0000239703.40297.a5. [DOI] [PubMed] [Google Scholar]
- 28.Bochsen L, Johansson PI, Kristensen AT, Daugaard G, Ostrowski SR. The influence of platelets, plasma and red blood cells on functional haemostatic assays. Blood Coagul Fibrinolysis. 2011;22(3):167–75. doi: 10.1097/MBC.0b013e3283424911. [DOI] [PubMed] [Google Scholar]
- 29.Bertolini, Murphy S. A Multicenter Evaluation of Reproducibility of Swirling in Platelet Concentrates. Transfusion. 1994;34(9):796–801. doi: 10.1046/j.1537-2995.1994.34994378282.x. [DOI] [PubMed] [Google Scholar]
- 30.Mathai J, Resmi KR, Sulochana PV, Sathyabhama S, Baby Saritha G, Krishnan LK. Suitability of measurement of swirling as a marker of platelet shape change in concentrates stored for transfusion. Platelets. 2006;17(6):393–6. doi: 10.1080/09537100600757695. [DOI] [PubMed] [Google Scholar]
- 31.Dumont LJ, VandenBroeke T. Seven-day storage of apheresis platelets: report of an in vitro study. Transfusion. 2003;43(2):143–50. doi: 10.1046/j.1537-2995.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 32.Albanyan AM, Murphy MF, Rasmussen JT, Heegaard CW, Harrison P. Measurement of phosphatidylserine exposure during storage of platelet concentrates using the novel probe lactadherin: a comparison study with annexin V. Transfusion. 2009;49(1):99–107. doi: 10.1111/j.1537-2995.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 33.Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106(8):896–9. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 34.Baker JM, Candy DJ, Hawker RJ. Influences of pH on human platelet metabolism. Platelets. 2001;12(6):333–42. doi: 10.1080/09537100120078412. [DOI] [PubMed] [Google Scholar]
- 35.Kilkson H, Holme S, Murphy S. Platelet metabolism during storage of platelet concentrates at 22 degrees C. Blood. 1984;64(2):406–14. [PubMed] [Google Scholar]
- 36.Babic AM, Josefsson EC, Bergmeier W, Wagner DD, Kaufman RM, Silberstein LE, Stossel TP, Hartwig JH, Hoffmeister KM. In vitro function and phagocytosis of galactosylated platelet concentrates after long-term refrigeration. Transfusion. 2007;47(3):442–51. doi: 10.1111/j.1537-2995.2007.01134.x. [DOI] [PubMed] [Google Scholar]
- 37.Albanyan AM, Harrison P, Murphy MF. Markers of platelet activation and apoptosis during storage of apheresis- and buffy coat-derived platelet concentrates for 7 days. Transfusion. 2009;49(1):108–17. doi: 10.1111/j.1537-2995.2008.01942.x. [DOI] [PubMed] [Google Scholar]
- 38.Bertino AM, Qi XQ, Li J, Xia Y, Kuter DJ. Apoptotic markers are increased in platelets stored at 37 degrees C. Transfusion. 2003;43(7):857–66. doi: 10.1046/j.1537-2995.2003.t01-4-00431.x. [DOI] [PubMed] [Google Scholar]
- 39.Macey MG, Carty E, Webb L, Chapman ES, Zelmanovic D, Okrongly D, Rampton DS, Newland AC. Use of mean platelet component to measure platelet activation on the ADVIA 120 haematology system. Cytometry. 1999;38(5):250–5. doi: 10.1002/(sici)1097-0320(19991015)38:5<250::aid-cyto8>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- 40.Gousset K, Tsvetkova NM, Crowe JH, Tablin F. Important role of raft aggregation in the signaling events of cold-induced platelet activation. Biochim Biophys Acta. 2004;1660(1-2):7–15. doi: 10.1016/j.bbamem.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Rumjantseva V, Grewal PK, Wandall HH, Josefsson EC, Sorensen AL, Larson G, Marth JD, Hartwig JH, Hoffmeister KM. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15(11):1273–80. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White JG, Rao GH. Microtubule coils versus the surface membrane cytoskeleton in maintenance and restoration of platelet discoid shape. Am J Pathol. 1998;152(2):597–609. [PMC free article] [PubMed] [Google Scholar]
- 43.Gitz E, Koekman CA, Van den Heuvel DJ, Deckmyn H, Akkerman JW, Gerritsen HC, Urbanus RT. Improved platelet survival after cold storage by prevention of glycoprotein Ibα clustering in lipid rafts. Haematologica. 2012;97(12):e70–2. doi: 10.3324/haematol.2012.066290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufman J, Spinelli SL, Schultz E, Blumberg N, Phipps RP. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5(4):788–96. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]
- 45.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108(7):2455–62. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul BZ, Daniel JL, Kunapuli SP. Platelet shape change is mediated by both calcium-dependent and -independent signaling pathways. Role of p160 Rho-associated coiled-coil-containing protein kinase in platelet shape change. J Biol Chem. 1999;274(40):28293–300. doi: 10.1074/jbc.274.40.28293. [DOI] [PubMed] [Google Scholar]
- 47.Baurand A, Eckly A, Hechler B, Kauffenstein G, Galzi JL, Cazenave JP, Leon C, Gachet C. Differential regulation and relocalization of the platelet P2Y receptors after activation: a way to avoid loss of hemostatic properties? Mol Pharmacol. 2005;67(3):721–33. doi: 10.1124/mol.104.004846. [DOI] [PubMed] [Google Scholar]
- 48.Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood. 2005;105(9):3552–60. doi: 10.1182/blood-2004-07-2893. [DOI] [PubMed] [Google Scholar]
- 49.Peerschke EI. Ca+2 mobilization and fibrinogen binding of platelets refractory to adenosine diphosphate stimulation. J Lab Clin Med. 1985;106(2):111–22. [PubMed] [Google Scholar]
- 50.Curvers J, van Pampus EC, Feijge MA, Rombout-Sestrienkova E, Giesen PL, Heemskerk JW. Decreased responsiveness and development of activation markers of PLTs stored in plasma. Transfusion. 2004;44(1):49–58. doi: 10.1111/j.0041-1132.2004.00628.x. [DOI] [PubMed] [Google Scholar]
- 51.Rinder HM, Murphy M, Mitchell JG, Stocks J, Ault KA, Hillman RS. Progressive platelet activation with storage: evidence for shortened survival of activated platelets after transfusion. Transfusion. 1991;31(5):409–14. doi: 10.1046/j.1537-2995.1991.31591263195.x. [DOI] [PubMed] [Google Scholar]
- 52.Holme S, Heaton A. In-Vitro Platelet Aging at 22-Degrees-C Is Reduced Compared to in-Vivo Aging at 37-Degrees-C. Br J Haematol. 1995;91(1):212–218. doi: 10.1111/j.1365-2141.1995.tb05272.x. [DOI] [PubMed] [Google Scholar]
- 53.Schlagenhauf A, Kozma N, Leschnik B, Wagner T, Muntean W. Thrombin receptor levels in platelet concentrates during storage and their impact on platelet functionality. Transfusion. 2012;52(6):1253–1259. doi: 10.1111/j.1537-2995.2011.03475.x. [DOI] [PubMed] [Google Scholar]
- 54.Svendsen MS, Rojkjaer R, Kristensen AT, Salado-Jimena JA, Kjalke M, Johansson PI. Impairment of the hemostatic potential of platelets during storage as evaluated by flow cytometry, thrombin generation, and thrombelastography under conditions promoting formation of coated platelets. Transfusion. 2007;47(11):2057–2065. doi: 10.1111/j.1537-2995.2007.01430.x. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell SG, Hawker RJ, Turner VS, Hesslewood SR, Harding LK. Effect of Agitation on the Quality of Platelet Concentrates. Vox Sang. 1994;67(2):160–165. doi: 10.1111/j.1423-0410.1994.tb01652.x. [DOI] [PubMed] [Google Scholar]
- 56.Skripchenko A, Myrup A, Thompson-Montgomery D, Awatefe H, Moroff G, Wagner SJ. Periods without agitation diminish platelet mitochondrial function during storage. Transfusion. 2010;50(2):390–399. doi: 10.1111/j.1537-2995.2009.02450.x. [DOI] [PubMed] [Google Scholar]
- 57.Snyder EL, Bookbinder M, Kakaiya R, Ferri P, Kiraly T. 5-Day Storage of Platelet Concentrates in Clx Containers - Effect of Type of Agitation. Vox Sang. 1983;45(6):432–437. doi: 10.1111/j.1423-0410.1983.tb01940.x. [DOI] [PubMed] [Google Scholar]