Abstract
To understand the molecular and neural mechanisms underlying alcohol addiction, many models ranging from vertebrates to invertebrates have been developed. In Drosophila melanogaster, behavioral paradigms from assaying acute responses to alcohol, to behaviors more closely modeling addiction, have emerged in recent years. However, both the CAFÉ assay, similar to a 2-bottle choice consumption assay, as well as conditioned odor preference, where ethanol is used as the reinforcer, are labor intensive and have low throughput. To address this limitation, we have established a novel ethanol consumption preference assay, called FRAPPÉ, which allows for fast, high throughput measurement of consumption in individual flies, using a fluorescence plate reader. We show that naïve flies do not prefer to consume ethanol, but various pre-exposures, such as ethanol vapor or voluntary ethanol consumption, induce ethanol preference. This ethanol-primed preference is long lasting and is not driven by calories contained in ethanol during the consumption choice. Our novel experience-dependent model of ethanol preference in Drosophila – a highly genetically tractable organism – therefore recapitulates salient features of human alcohol abuse and will facilitate the molecular understanding of the development of alcohol preference.
Keywords: Addiction, alcohol, Drosophila, genetics, model organism, self-administration
INTRODUCTION
Alcohol abuse and alcoholism are afflictions that come with high individual and social costs (World Health Organization 2004). Though the understanding of the molecular mechanisms of alcoholism and its risk factors have increased significantly over recent years, many of the contributing factors to high alcohol consumption remain to be elucidated. By some estimates, more than half of the risk for alcoholism can be attributed to genetic predisposition (Gelernter & Kranzler 2009). Therefore, model organisms that are highly amenable to fast and comprehensive genetic analysis are of considerable value in the identification and characterization of genes and signaling pathways regulating alcohol drinking.
The vinegar fly, Drosophila melanogaster, has been used for over a dozen years to study the genes modulating ethanol-induced behaviors (Rodan & Rothenfluh 2010; Kaun et al. 2012). At low doses of ethanol exposure, flies show locomotion hyperactivity and disinhibition (Wolf et al. 2002; Lee et al. 2008), while high doses lead to loss of righting reflex and sedation (Rothenfluh et al. 2006). In addition to monitoring naïve flies’ behavioral responses to an acute ethanol exposure, a paradigm more relevant to addiction was developed recently (Devineni & Heberlein 2009). In this 2-bottle choice paradigm called CAFÉ, for capillary feeder, flies can choose to feed from two capillaries containing liquid food (sucrose/yeast extract) with or without 15% ethanol. After the first day flies show a slight preference for the capillary containing food and ethanol, but over the course of 3–5 days flies develop a clear preference of 2:1 for the food with ethanol, over the food without (Devineni & Heberlein 2009).
The CAFÉ assay represents a major advance in modeling addiction-like behaviors in flies, but it is associated with significant amounts of hands-on time for each genotype assayed. This is a considerable drawback for the implementation of high-throughput screens, which have been a hallmark of Drosophila research (Bellen et al. 2010). To improve on the workload associated with the CAFÉ assay, we have developed a novel ethanol consumption assay we have termed FRAPPÉ, for fluorometric reading assay of preference primed by ethanol. The cornerstone of this new assay is the precise reading of volumes ingested from two food solutions labeled with fluorophores, allowing sensitive measurement of less than 5 nL ingested and parametric determination of a preference index (PI) in individual flies. Using the FRAPPÉ, we show that in an acute choice between sucrose with or without 15% ethanol, naïve flies do not show preference. Following various ethanol vapor pre-exposures, however, they do display significant ethanol consumption preference. The preference induced by an ethanol pre-exposure is long lasting and can also be obtained by ethanol pre-feeding, even when ethanol is provided as a choice, and not as the sole food source. Lastly, we show that the consumption preference for ethanol induced by a pre-exposure cannot be accounted for solely by the caloric content of ethanol, suggesting a pharmacodynamic action of the drug on the central nervous system. The FRAPPÉ thus represents a novel, high-throughput ethanol preference assay that models numerous aspects of human addiction, including a triggering experience, long-lasting persistence, and voluntary ingestion of the drug.
MATERIALS AND METHODS
Flies
Behavioral experiments were performed with male w1118 Berlin flies, which were raised at 25°C and 70% humidity on standard cornmeal/molasses food. Flies were 1–5 days of age at the start of the experiments.
Booz-o-mat exposure
The day before ethanol vapor exposure, male flies were collected in groups of 30 and put on unyeasted food. The following day, flies were transferred into the booz-o-mat apparatus for a 20-minute exposure at desired ethanol to air ratio (E/A) as described (Wolf et al. 2002). Flies were placed back into unyeasted food vials for 4 hrs to recover, and were then transferred to vials filled with 0.7% agar solution (for hydration). These vials were placed into a 25°C/70% humidity incubator for an 18-hour food deprivation.
Ethanol consumption preference
All ethanol preference experiments were conducted with a 10–15 minute choice of 60 mM sucrose with 15% ethanol vs. 60 mM sucrose unless otherwise stated (Fig. 2, and Fig. 6a,b). Access duration was limited to prevent dye loss via excretion associated with longer feeding times (data not shown). Because flies drink in long, uninterrupted bouts, with little well-to-well movement, if presented with high-sucrose foods (PSP & AR, in preparation), the sucrose concentration was kept low to increase sampling from different wells and to increase the probability that concentrations consumed reflected a true preference of individual flies. This in turn required that flies were food-deprived for 14–18 hours, to ensure large enough quantities of ingestion for accurate FRAPPÉ measurements. Using fluorescent dyes, 0.005% rhodamine B (Acros Organics) and 0.003% fluorescein sodium salt (Sigma-Aldrich Co.), color counter-balanced solutions were made. In one plate the ethanol containing solution was colored with rhodamine B, and the sucrose-only solution with fluorescein, with opposite color pairing in the counter-balanced one. This eliminated potential color bias in the preference assay. After a 10–15 minute feeding period, flies were placed in 15 mL Falcon tubes, frozen in dry ice, and vortexed to shear legs, heads, and wings from the torsos/abdomen body core. The cores were then individually placed into the wells of a 96 well flat-bottom tissue culture plate containing 50 μl of water to keep flies centered in the excitation beam, and to increase the likelihood of their ventral side facing up; ventral being the side with the least dark cuticle, thus minimizing absorption of fluorophore emission. These whole fly core measurements correlated well with readings from flies after homogenization, centrifugation, and supernatant reading in the Fluoroskan (Fig. S1). Using Ascent Software v2.6, fluorescence data was then collected in a Fluoroskan Ascent FL2.4 plate reader for rhodamine B at excitation/emission wavelengths of 542/591 nm and at 485/527 nm for fluorescein. After taking five separate measurements, which were each followed by a ‘shake’ step (to maximize the chance of the ventral side of fly cores facing up, thus minimizing cuticle absorption, see Fig. S2), the maximum fluorescence values were recorded for each well. Rhodamine B values were multiplied by 3.23 to convert to nL consumed, while the maximum fluorescein values were multiplied by 1.74, with both conversion factors empirically determined using correlations with NanoDrop spectrophotometer readings. Overall, our individual fly Fluoroskan readings agree well with individual readings from flies fed FD&C Blue #1 and measured for volumes ingested in a NanoDrop photospectrometer (Fig. S3). The sensitivity of this fluorometric assay is less than 3 nL, but we excluded flies drinking less than 10 nL to increase the likelihood of ingestion from both solutions. A PI for each individual fly was calculated (nL consumed of sucrose/ethanol – nL consumed of sucrose)/(nL consumed of sucrose/ethanol + nL consumed of sucrose), ranging from +1 = total preference, to −1 = total avoidance.
Figure 2.
Pre-exposed flies tested 24 hours later show ethanol preference to various ethanol concentrations. (a) Naïve flies avoid high (≥ 15%) ethanol concentrations. Statistics: 5%: 0.06, 49, 0.98, 0; 10%: 0.06, 57, 0.89, 1; 15%: −0.17, 168, 0.07, −15; 20%: −0.40, 43, 0.018, −11; 25%: −0.30, 92, 0.004, −18. (b) Flies pre-exposed to 80/70 E/A show ethanol preference for ≥ 10% ethanol. Statistics for 5%: −0.24, 79, 0.008, −14; 10%: 0.29, 36, 0.021, 9; 15%: 0.47, 44, 0.003, 15; 20%: 0.37, 73, 0.033, 11; 25%: 0.53, 71, <0.001, 20. (c) Pre-exposure to a high ethanol dose also causes ethanol preference for ≥ 10% ethanol. Statistics for 5%: 0.17, 93, 0.14, 8; 10%: 0.24, 88, 0.011, 16; 15%: 0.69, 144, <0.001, 61; 20%: 0.72, 148, <0.001, 62; 25%: 0.78, 120, <0.001, 57.
Figure 6.
Ethanol consumption preference is not driven by ethanol calories. (a) Pre-exposed flies given a choice between sucrose+15% ethanol vs. sucrose+1.7M sorbitol (a tasteless but caloric sugar for flies) will prefer ethanol. Note that the two solutions to choose from are nominally isocaloric. Statistics for 0: −0.22, 200, 0.31, −11; 80: 0.60, 253, <0.001, 102. (b) In the absence of ethanol, flies strongly prefer the sorbitol-containing sucrose. Statistics for 0: −0.64, 48, 0.005, −18; 80: −0.87, 64, <0.001, −37. (c) Unlike sucrose and sorbitol, ethanol provides minimal calories for survival, and by 4 days on 15% ethanol alone, all flies die. Isocaloric sugars offered in parallel were 600mM sucrose, or 1.7 M sorbitol (p < 0.001, chi-square = 210, for ethanol vs. sorbitol survival curve, Gehan-Breslow-Wilcoxon Test, n ≥ 149 flies per condition). (d) 15% Ethanol also provided no usable calories when offered together with 60 mM sucrose, and flies die as quickly as with 60 mM sucrose alone. 1.7 M sorbitol significantly extends the survival when added to 60 mM sucrose (p < 0.001, chi-square = 168, for ethanol vs. sorbitol survival curve, Gehan-Breslow-Wilcoxon Test, n ≥ 150 flies per condition).
Ethanol Sedation/Tolerance
Ethanol sedation and tolerance experiments on groups of 20 flies were carried out using the loss-of-righting reflex (LORR) test as described previously (Rothenfluh et al. 2006). For tolerance, flies pre-exposed 24 hours prior, were subjected to a 110/40 ethanol/air (E/A) challenge dose, and the time to 50% sedation/LORR (ST-50) determined.
Feeding experiments
Flies were pre-fed in a modified CAFE assay over a span of three days in rectangular 4 well plates (127.8×85.5 mm, Thermo Scientific; Fig. 5). Food was provided in 0.2 mL PCR tubes with a 27G needle hole at the bottom for drinking access, a 27G hole atop for pressure equilibration, and a 25G hole atop for filling with solution. 50 flies per well had access to 2 food tubes (5% sucrose/5% powdered yeast extract) and 2 water tubes. For some groups, the food solution was supplemented with 15% ethanol. After 3 days of feeding, flies were food deprived on 0.7% agar for 14 hours, and ethanol-consumption preference measured as described above.
Figure 5.
Voluntary ethanol consumption induces ethanol preference. (a) Experimental design. (b) Flies were fed for 3 days with either 5% sucrose/5% powdered yeast extract (labeled “food”), or sucrose/yeast extract/15% ethanol (labeled as “food + 15% E”), or allowed to choose between those two solutions (labeled as “food + 15% E OR food”). Flies that had prior access to ethanol developed subsequent consumption preference in the FRAPPÉ, while flies that ate ethanol-less food only did not. Statistics for food: 0.04, 140, 0.43, 5; food+ethanol: 0.50, 100, 0.007, 21; food or food+ethanol: 0.37, 187, <0.001, 33.
The same setup was utilized to determine survival on different carbohydrates (Fig. 6c). Wells included a piece of 3MM filter paper for contrast making dead flies easily visible Calories were balanced in the different wells calculating from 4 kcal/g (sucrose), 7 kcal/g (ethanol), and 2.6 kcal/g (sorbitol).
Statistics
Statistics were calculated using Prism5 for Mac OSX (GraphPad Software). Preference indices for a given group were not distributed normally (D’Agostino/Pearson omnibus normality test), and were plotted as medians with quartile boxes and 10–90% whiskers. They were tested for preference/avoidance (i.e. PI <> 0) using the Wilcoxon Signed Rank Test. The legend in each figure of PIs includes the median, number of flies, p value for preference (PI <> 0), and the Wilcoxon Signed Rank Test sum for each group. Whenever no preference/avoidance was found, we made sure that at least an n of 50 flies was assayed, for adequate statistical power. Differences between PIs were queried using the Mann-Whitney U Test.
RESULTS
Flies show experience-dependent ethanol consumption preference
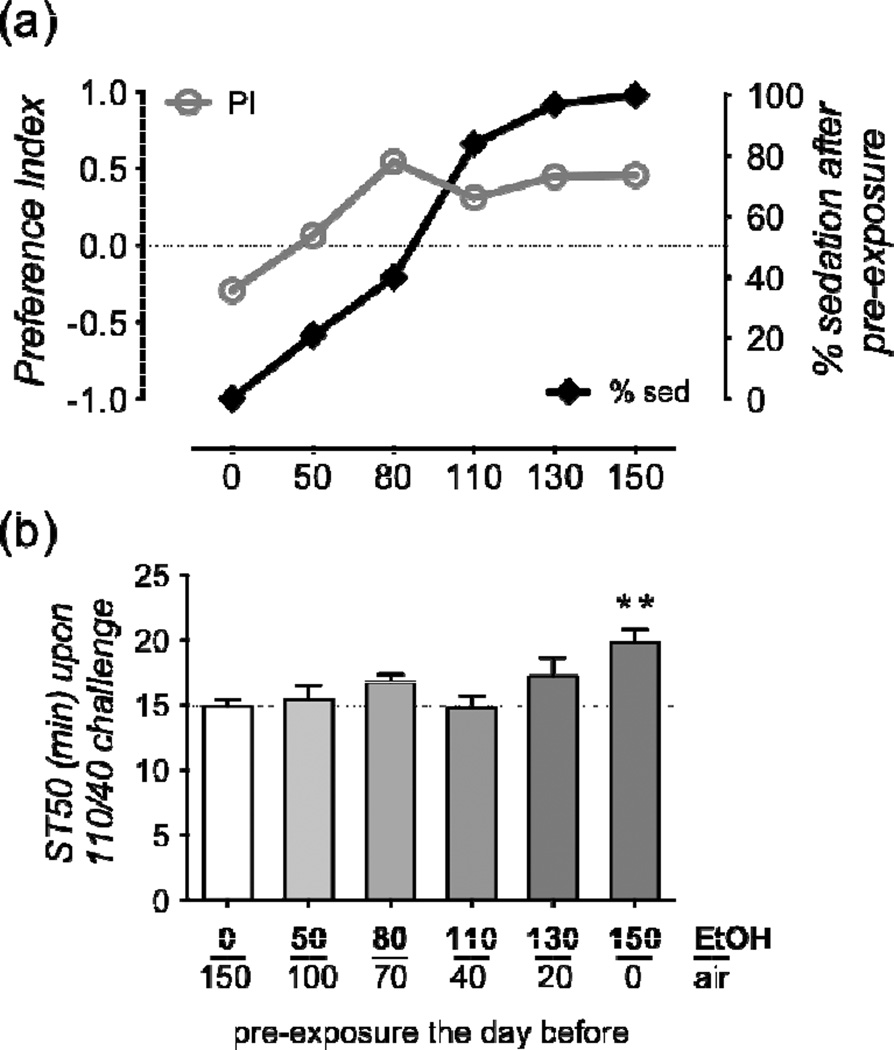
In the 2-bottle CAFÉ assay, flies are allowed to choose between two food/yeast extract mixtures, one of which contains an addition of 15% ethanol. They are then observed over the span of about 5 days, and consumption preference is measured for the ethanol-containing food mix. In two reports, preference for the ethanol-containing mix seemed to increase slightly over the 5 days assayed (Devineni & Heberlein 2009; Pohl et al. 2012). We wondered whether this increase reflected experience-dependent development of ethanol preference. The CAFÉ assay is based on one reading of consumption per day, thus it is difficult to know whether the first day’s reading should be considered coming from naïve flies, or whether one day constitutes significant ethanol-drinking experience. To directly test whether inexperienced, ethanol-naïve Drosophila showed innate ethanol consumption preference, or whether prior experience was necessary for the development of preference, we established a new consumption assay based on fluorometric readings of food volumes consumed by individual flies. In this assay, termed FRAPPÉ (Fig. 1), 30 flies were allowed 10–15 min access to a 60-well plate containing 60 mM sucrose with or without 15% ethanol. Significant consumption of at least 10 nL per fly was ensured with a prior period of food-deprivation (see Materials and Methods for more details). To determine if ethanol preference was experience dependent, we pre-exposed the flies to increasing doses of vaporized ethanol the day before the consumption choice. Figure 1c shows that naïve flies exposed to air (zero parts ethanol) showed a mild aversion to 15% ethanol (PI < 0) the day following the 20-minute mock exposure. This aversion gradually switched over to preference, as the ethanol pre-exposure increased to 80/70 E/A. At higher doses, flies showed preference indistinguishable from 80/70 pre-exposed flies. These results suggest that ethanol consumption preference in Drosophila is experience dependent.
Figure 1.
The FRAPPÉ, an experience-dependent ethanol consumption preference assay in Drosophila. (a) Schematic of the experimental design. (b) Schematic of the consumption plate, where flies chose between 60 mM sucrose and 60 mM sucrose + 15% ethanol after 14–18 hours of food deprivation. The food is labeled with (counter-balanced) fluorescent dyes. (c) Preference index of flies pre-exposed the day before for 20 min to the indicated vaporized ethanol/air (E/A) pressures. Data shown here, as in subsequent preference graphs, are medians, with quartile bars, and 10–90th percentile whiskers. Mock exposed flies (i.e. no ethanol) show mild aversion (PI < 0) to the ethanol-containing food the day after exposure, while flies exposed to 80/70 E/A or higher show significant preference (for this, and following preference graphs, a: p < 0.05 of the indicated group being different from 0, Wilcoxon Signed Rank Test, b: p < 0.01, c: p < 0.001 for each group, where one fly is an n of one.) Statistics for each group from left to right including median, number of flies, p value for preference/avoidance, Sign Test sum (as in following legends) were as follows for 0: −0.29, 130, 0.03, −18; 20: −0.15, 98, 0.42, −5; 50: 0.07, 90, 0.67, 3; 80: 0.54, 144, <0.001, 54; 110: 0.31, 174, <0.001, 37; 130: 0.45, 223, <0.001, 58; 150: 0.46, 107, <0.001, 31.
Next, we sought to investigate which ethanol concentrations pre-exposed flies would prefer to consume. Naïve, unexposed flies showed aversion to ≥ 15% ethanol (Fig. 2a), while flies pre-exposed to 80/70 or 150/0 E/A mixtures for 20 minutes the day before showed ethanol preference at ≥10% ethanol (Fig. 2b,c). These combined results for both ethanol-exposed groups suggests that pre-exposure to a threshold level of ethanol results in a preference that generalizes across various ethanol concentrations.
Experience-dependent ethanol preference is long lasting
We next sought to investigate the longevity of the ethanol consumption preference induced by a prior ethanol exposure. We exposed flies to a single, 20 min dose of 80/70 ethanol/air (E/A), or ‘mock-exposed’ them to a dose of 0/150, and assayed ethanol preference 3, 5, or 8 days later. Flies that were given a one-time pre-exposure showed stable ethanol preference that was observable 3, 5, and even 8 days following exposure (Fig. 3b). Mock-exposed flies did not show a preference 8 days after mock exposure, suggesting that preference is experience-dependent and long lasting, and not a function of the flies’ age.
Figure 3.
Ethanol pre-exposure causes long-lasting preference. (a) Experimental design. Flies were exposed to one dose of 80/70 E/A (or 0/150 mock exposed), and then assayed the indicated number of days later for their ethanol consumption preference. (b) Even 8 days after a one-time exposure, flies still show ethanol preference, while mock exposed flies still avoid ethanol (*** p < 0.001, U = 2056, for 8 days after 80/70 vs. 0/150 exposure, Mann-Whitney U Test). Statistics for 3d: 0.38, 36, 0.009, 9; 5d: 0.45, 55, <0.001, 19; 8d: 0.40, 67, 0.009, 15; 8d mock: −0.31, 100, <0.001, −23.
Behavioral correlates with ethanol consumption preference
To study a possible relationship between sedation during pre-exposure and subsequent consumption preference, we exposed flies for 20 minutes to ethanol/air pressures ranging from 0/150 to 150/0 E/A and determined the percentage of flies sedated by that exposure. We then assessed ethanol preference the following day. Figure 4a shows that sedation steadily increased as a function of the ethanol/air exposure pressure, but preference reached a plateau at 80/70 E/A. While 80/70 and 150/0 E/A pre-exposure caused the same level of ethanol consumption preference, less than half the flies were sedated after a 80/70 pre-exposure, compared to 100% after 150/0 (Fig. 4a). These data suggest that sedation during the pre-exposure is neither necessary for, nor detrimental to subsequent ethanol-preference. Note that flies pre-exposed from 80/70 to 150/0 all undergo a phase of ethanol-induced hyperactivity prior to sedation. In this experiment, we therefore have not isolated sedation as the sole behaviorally relevant experience that the flies undergo.
Figure 4.
Ethanol consumption preference does not require pre-exposure sedation, or induction of rapid tolerance. Note that the same X-axis applies to both panels. (a) 20 minute pre-exposure leads to levels of sedation (black line, right axis, n ≥ 90 per dose), which steadily increase as a function of the ethanol/air exposure pressure. Ethanol consumption preference 24 hours later, reaches a peak at 80/70 (where only 41% of flies sedate) and does not increase further (grey line, left axis, medians re-plotted from Figure 1c). (b) 24 hours after pre-exposure, a different set of flies shows that only pre-exposure to 150/0 E/A causes increased time to sedation (i.e., tolerance) compared to mock exposed flies (** p < 0.01, q = 3.9, Dunnett’s post-hoc multiple comparison test of all groups vs. 0/150 mock exposure. n = 6 groups of 20 flies per group.)
To test whether ethanol consumption preference correlates with sedation-tolerance at the time of consumption choice, groups of flies were pre-exposed to different ethanol/air pressures and on the next day their ST-50 (time until 50% of flies sedate) to a 110/40 E/A challenge dose was determined. Only the 150/0 pre-exposure caused measurable sedation tolerance 24 hours later when compared to mock-exposed flies (Fig. 4b). Since lower doses of pre-exposure cause consumption preference, but not sedation tolerance one day later, we conclude that tolerance to the sedating effects of ethanol at the time of consumption is not required for ethanol-induced consumption preference.
Different routes of pre-exposure induce consumption preference
In the experiments outlined above, the preference-inducing pre-exposure was vaporized ethanol, which the flies passively, and involuntarily breathed. While vaporized ethanol can cause dependence, lead to withdrawal (Goldstein & Pal 1971) and also to increased consumption in rodents (Roberts et al. 2000), we still wished to investigate whether a more voluntary route of pre-exposure to ethanol might induce consumption preference. To test this, we first allowed flies ad libitum access to liquid food (sucrose/yeast extract) and water in a CAFÉ-like feeding chamber. After three days of feeding in these conditions, flies did not develop ethanol consumption preference in the FRAPPÉ assay (Fig. 5b). Second, when we changed the pre-feeding solution to sucrose/yeast extract/15% ethanol, i.e. the only food available to the flies for the three days of pre-feeding contained ethanol, they did develop ethanol consumption preference in the FRAPPÉ choice assay (Fig. 5b). Third, we offered flies both food solutions simultaneously, one of them containing 15% ethanol, thereby giving the flies the choice to consume ethanol or not for the three days of pre-feeding. After this choice, flies also developed ethanol consumption preference (Fig. 5c). We thus show that multiple routes of ethanol exposure are capable of inducing ethanol consumption preference, including voluntary ethanol consumption itself.
Experience-dependent ethanol preference is independent of caloric content
Even though naïve flies showed slight aversion, or no preference for ethanol (Fig. 2a), they might develop a “taste for” ethanol because of its high caloric content, especially in the voluntary setting of the CAFÉ assay (as suggested by Pohl et al. 2012). It seems less likely that passive exposure to ethanol vapor exposure could be reinforcing for its caloric value. Nevertheless, we decided to directly test the effect of ethanol calories in our consumption preference.
To do this, we offered pre-exposed flies a choice of isocaloric solutions, one containing ethanol, and the other one containing sorbitol, which is tasteless to flies but provides calories (Burke & Waddell 2011; Fujita & Tanimura 2011; Stafford et al. 2012). Mock-exposed flies showed no preference for either sucrose/ethanol vs. sucrose/sorbitol, whereas flies that were pre-exposed to 80/70 E/A the previous day showed strong ethanol consumption preference (Fig. 6a). Both pre- and mock-exposed flies were able to detect the calories provided by sorbitol, and preferred to drink from sucrose with sorbitol, as opposed to sucrose alone solution (Fig. 6b). This suggests that ethanol pre-exposure induced ethanol consumption preference, but it did not alter pre-existing sorbitol preference. Since naïve flies showed no ethanol preference (see Fig. 2a), but did show sorbitol preference (Fig. 6b), it was a bit surprising that they only showed a trend towards sorbitol preference when offered together with ethanol (Fig. 6a). This would suggest some interaction of ethanol and sorbitol for flies’ choice, the nature of which is not clear. To directly test whether ethanol contains metabolically useful calories, we performed an experiment in which we compared the survival of flies maintained on isocaloric sucrose, sorbitol, or ethanol. Flies maintained on 1.7 M sorbitol as their only food source showed 12% death after eight days, which was not significantly different from the 5% death observed in flies kept on isocaloric 600 mM sucrose. Conversely, flies on isocaloric 15% ethanol all died within 5 days (Fig 6c). To make sure that there was no confounding interaction of ethanol or sorbitol with sucrose (which was used at 60 mM in Figures 6 a,b), we repeated the experiment by adding 60 mM sucrose to ethanol, and sorbitol. This prolonged survival on ethanol with 60 mM sucrose, compared to ethanol alone, and after 8 days 17% of flies were still alive. However, this was still significantly less survival than flies on sorbitol with 60 mM sucrose showed (88% survival after 8 days), arguing that sorbitol provides significantly more metabolically useful calories to flies than ethanol. The data in Figure 6d also show that flies on ethanol and 60 mM sucrose survived longer than on ethanol alone (suggesting that even 60 mM sucrose provides significant calories to extend survival), but that survival on ethanol with 60 mM sucrose was no different from survival on 60 mM sucrose alone. Together these data indicate that 15% ethanol is neither toxic, nor caloric to flies, and they are very similar to what has previously been shown by Xu et al. (2012). Ethanol consumption preference is therefore not driven by caloric value of ethanol, but more likely by the drug’s pharmacodynamic properties.
DISCUSSION
In this paper we describe a novel assay, termed FRAPPÉ, to measure experience-dependent ethanol consumption preference in Drosophila. Up to now, consumption preference in flies has been measured with the CAFÉ assay, a 2-bottle choice paradigm that has limited throughput (Devineni & Heberlein 2009). Since flies have been a long-standing model organism for genetic screens (Bellen et al. 2010; Rodan & Rothenfluh 2010), we sought to improve on this limitation and have developed a novel assay that measures the consumption preference index of individual flies in a fluorescence plate reader, thus enabling fast screening of large numbers on flies.
Two types of assays in Drosophila model features of addiction most closely: First, the CAFÉ assay measures consumption preference for an ethanol-containing food in groups of (Devineni & Heberlein 2009; Pohl et al. 2012), or even individual flies (Xu et al. 2012). In this assay, the flies’ preference tends to increase over the course of the 5-day experiment. When consumption is followed by a period of 3 days of forced abstinence, the flies immediately return to their acquired high preference right away, suggestive of relapse-like behavior (Devineni & Heberlein 2009). Furthermore, flies will acquire preference even for a bitter-tasting quinine solution, which is normally aversive, when added to the ethanol/food mixture, arguing that they are motivated to overcome aversion in order to consume ethanol-containing food (Devineni & Heberlein 2009). The second behavioral paradigm developed in flies is similar to conditioned place preference used with rodents, only that in the case of flies, the stimulus reinforced with ethanol vapor is an innocuous odor (Kaun et al. 2011). After conditioning, flies prefer the ethanol-paired odor, and they are willing to cross an aversive foot-shock grid to approach that odor. Interestingly, ethanol seems to be a stronger reinforcer than sucrose, which is generally used in appetitive conditioning (Kaun et al. 2011). Our novel FRAPPÉ assay combines the experience-dependent aspect of the odor-conditioning paradigm with the voluntary ethanol consumption of the CAFÉ, and allows for the high-throughput measure of ethanol preference in individual flies.
What kind of ethanol-experiences cause consumption preference in the FRAPPÉ? We show that pre-exposures to ethanol vapor that induces consumption preference include hyperactivating doses, as well as sedating doses (see Fig. 4a). The dose response of the consumption PI as a function of the pre-exposure dose shows a steady increase at low to medium doses, but then reaches a plateau at high doses (Fig. 1c). This plateau contrasts with ethanol-conditioned odor preference, where only hyperactivating, but not sedating doses of ethanol vapor induced subsequent odor preference (Kaun et al., 2011). This difference could have numerous causes: First, it could reflect a mechanistic difference between the FRAPPÉ and conditioned odor preference. Second, neuronal cell death in the antenna (French & Heberlein 2009) – the major olfactory sensory organ in flies – caused by the sedating conditioning dose of ethanol vapor could confound subsequent odor choices. Third, some of our pre-exposure regimens also might lead to ethanol aversion in the short term, followed by long-term ethanol preference. We have not tested flies in the FRAPPÉ assays within a few hours after the pre-exposure, but Kaun and colleagues (2011) found that even for reinforcing ethanol exposures, flies initially developed odor avoidance, which only later, after 12–15 hours changed into odor preference. Our finding that even a sedating dose of ethanol can increase subsequent ethanol preference and consumption is not unprecedented though. In rats, a single motor impairing dose of i.p. ethanol injection increased their subsequent ethanol intake compared to saline-injected rats (Tampier & Quintanilla 2002).
Ethanol-induced sedation causes stress, and induces expression of numerous stress-related genes (Kong et al., 2010). It seems unlikely that a generalized stress response to the pre-exposure is causing subsequent ethanol-preference in our FRAPPÉ assay. First, flies are food deprived for 18 hours prior to consumption choice, and this stress was insufficient to induce preference in naïve flies (Figures 1c, 2a). Second, two other stressors, 6-hour dehydration, and repeated mechanical stress by vortexing 24 hours before the choice assay, also did not lead to consumption preference (data not shown). Lastly, we found that ethanol consumption itself in a CAFÉ-like choice setting for 3 days was capable of causing subsequent ethanol consumption preference in the FRAPPÉ. Different ways of ethanol pre-exposure can thus lead to consumption preference, including voluntary consumption, which is unlikely to be stressful.
Our 3-day pre-feeding data, together with the gradual development of ethanol preference in the CAFÉ assay (see above), would indicate that ethanol-experience causes a gradual shift from slight aversion/indifference, towards preference. While nontasty, but caloric foods can act as reinforcers within minutes (via unknown sensory mechanisms; Burke & Waddell 2011; Fujita & Tanimura 2011), the 15 minute acute ethanol preference choice in the FRAPPÉ assay does not appear to be long enough for flies to “learn” to prefer ethanol. Since ethanol is not of caloric value to flies (see below), it presumably acts via a different reinforcing pathway that is yet to be determined. In line with this gradual learning of the reinforcing aspects of ethanol are the findings by Kaun and colleagues (2011) who showed that ethanol-conditioned odor preference only developed 12–15 hours after the ethanol conditioning. The kinetics of ethanol-mediated odor conditioning, and preference development thus seem slower than those observed in classical appetitive conditioning (Burke & Waddell 2011; Fujita & Tanimura 2011). Despite these differences in the kinetics of behavioral changes, it is noteworthy that the two mutations affecting ethanol consumption in the CAFÉ assay, kra (Devineni & Heberlein 2009) and rut (Xu et al. 2012), were both initially isolated and described as associative learning and memory mutants in Drosophila. Development of ethanol preference in flies may thus share molecular mechanisms that are also utilized for long-term learning and memory formation (Rothenfluh & Cowan 2013), in line with current thinking of addiction as long-lasting, maladaptive reinforcement learning (Grueter et al. 2012). Both experience-dependent consumption preference in the FRAPPÉ, as well as ethanol-conditioned odor preference are long-lasting and are still present even a week after the last ethanol experience (Fig. 3; Kaun et al., 2011). Further support for shared mechanisms between these ethanol-induced behavioral changes and other associative learning and memory processes comes from a survey of over 60 Drosophila learning and memory mutants. The results showed a striking overrepresentation of phenotypes in ethanol-induced behaviors, including tolerance (Berger et al., 2008).
In another small survey of behavioral ethanol mutants, Devineni and colleagues (2011) found that consumption preference phenotypes in the CAFÉ assay correlated with rapid tolerance phenotypes, but not with ethanol-induced sedation, or hyperactivity phenotypes. Our results (Fig. 4,5) indicate that sedation during the pre-exposure is neither necessary for, nor detrimental to preference induction. In addition, flies need not be tolerant to ethanol-induced sedation at the time of consumption choice. Furthermore, while flies can become hyperactive after ethanol ingestion, they do not routinely seem to in the CAFÉ (Devineni & Heberlein, 2009), or CAFÉ-like pre-feeding assay (Figure 5). This raises the question what ethanol does to flies to induce preference, and why flies consume alcohol in the first place. Two obvious answers spring to mind. First, they “like” the pharmacodynamic effects that ethanol has on the brain. This is what causes people to drink and abuse alcoholic beverages. Second, flies might prefer ethanol-containing food for the considerable calories that are provided by ethanol. Indeed, in our experiments, the difference between the food with and without ethanol was 907 vs 81 mcal/μL. One report altered this imbalance by varying sucrose, but not ethanol concentration in the CAFÉ and found no change in PI for ethanol (Devineni & Heberlein 2009), arguing against caloric imbalance being the driving force for preference. Xu and colleagues (2012) altered sucrose concentration and found a resulting change in volume (but not calories) consumed. Changes in ethanol concentration led to no change in volume, but in total calories consumed (including ethanol’s). This argues that sucrose, but not ethanol consumption is under homeostatic caloric control. In contrast, a recent paper investigated preference in the CAFÉ after counterbalancing ethanol’s calories with the same amount of calories in the other capillary. The complex sugar maltodextrin, and both sucrose and glucose, but not mannose, abrogated preference for the ethanol-containing capillary (Pohl et al. 2012), suggesting that it is the caloric content of ethanol that drives the preference. Alternatively though, preference for glucose and sucrose could be due to their highly appetitive sweet taste (Pohl et al. 2012), since taste is a major driving force in food choice (Stafford et al. 2012). Ethanol, in contrast, does not have any taste at the doses offered (Devineni & Heberlein 2009; Pohl et al. 2012). The remaining results with tasteless, but caloric maltodextrin and mannose were equivocal (Pohl et al. 2012). We decided to steer clear of maltodexrin, as it can be contaminated by simple, and tasty sugars (Burke & Waddell 2011), and instead used sorbitol to counterbalance ethanol’s calories. Sorbitol is a tasteless, caloric sugar (Burke & Waddell 2011; Fujita & Tanimura 2011; Stafford et al. 2012). Our results show that ethanol-exposed flies preferred to consume ethanol-containing food over isocaloric sorbitol-containing food, while we observed no preference in unexposed flies. When offered sucrose vs sucrose with sorbitol, flies strongly preferred the sorbitol-containing solution, irrespective of prior ethanol experience. These experiments argue that 1) ethanol provides an appetitive force that cannot solely be accounted for by the calories it contains, 2) even in our relatively acute FRAPPÉ paradigm, where flies choose for only 15 min, flies can detect, and prefer the caloric content of a taste-neutral sorbitol solution, and 3) ethanol pre-exposure does not affect the perception of tasteless calories. Taken together, the above experiments, with the one exception of the maltodextrin experiments, argue that ethanol does not provide significant calories as a driving force for preference. Indeed, when we tested survival of flies on 15% ethanol compared to “iso-caloric” sucrose or sorbitol, flies survived significantly longer on the sugars compared to ethanol (Fig. 6c,d), arguing that the theoretical calories provided by ethanol are not efficiently utilized by flies’ metabolism, and that flies prefer to consume ethanol for its pharmacodynamic effects.
One confound of our FRAPPÉ assay is the need for food-deprivation, in order to ensure significant amounts of consumption. While starvation in and of itself did not cause ethanol consumption preference, it is possible that it is a necessary gating mechanism for preference to be expressed. The similarities we have observed between the CAFÉ and the FRAPPÉ assays would argue against that, given that flies are not food-deprived in the CAFÉ assay. We are currently working on FRAPPÉ approaches that do not rely on food deprivation, to address this issue. Nevertheless, we have developed a novel experience-dependent ethanol consumption assay, which induces long-lasting ethanol consumption preference. Since this assay is quick, but allows for precise measurement of an individual preference index, it can be used in large-scale genetic screens to investigate the molecular mechanisms that are involved in the development of experience-dependent drinking, and should further our understanding of the processes leading to alcohol addiction.
Supplementary Material
Acknowledgements
We thank Angela Ozburn for valuable discussion. Supported by the NIH (T32 DA7290 to RLP and SAO, F31 AA021340 to SAO, and 5R01AA019526 to AR). AR is the Effie Marie Cain Scholar in Biomedical Research at UT Southwestern.
Footnotes
Author Contributions
RLP, SAO, PSP, MJN, SFA, ARR and AR designed experiments, analyzed and interpreted data. RLP, SAO, PSP, MJN, and TL performed experiments. SAO, RJD, PSP, SFA, ARR and AR wrote the paper, with input from all the authors.
References
- Bellen HJ, Tong C, Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci. 2010;11:514–522. doi: 10.1038/nrn2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KH, Kong EC, Dubnau J, Tully T, Moore MS, Heberlein U. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin Exp Res. 2008;32:895–908. doi: 10.1111/j.1530-0277.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CJ, Waddell S. Remembering Nutrient Quality of Sugar in Drosophila. Curr Biol. 2011;21:746–750. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. Preferential Ethanol Consumption in Drosophila Models Features of Addiction. Curr Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, McClure KD, Guarnieri DJ, Corl AB, Wolf FW, Eddison M, Heberlein U. The genetic relationships between ethanol preference, acute ethanol sensitivity, and ethanol tolerance in Drosophila melanogaster. Fly. 2011;5 doi: 10.4161/fly.5.3.16987. 191-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French RL, Heberlein U. Glycogen synthase kinase-3/Shaggy mediates ethanol-induced excitotoxic cell death of Drosophila olfactory neurons. Proc Natl Acad Sci USA. 2009;106:20924–20929. doi: 10.1073/pnas.0910813106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Curr Biol. 2011;21:751–755. doi: 10.1016/j.cub.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR. Genetics of alcohol dependence. Hum Genet. 2009;126:91–99. doi: 10.1007/s00439-009-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2012;22:545–551. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A Drosophila model for alcohol reward. Nat Neurosci. 2011;14:612–619. doi: 10.1038/nn.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun KR, Devineni AV, Heberlein U. Drosophila melanogaster as a model to study drug addiction. Hum Genet. 2012;131:959–975. doi: 10.1007/s00439-012-1146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong EC, Allouche L, Chapot PA, Vranizan K, Moore MS, Heberlein U, Wolf FW. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcohol Clin Exp Res. 2010;34:302–316. doi: 10.1111/j.1530-0277.2009.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-G, Kim Y-C, Dunning JS, Han K-A. Recurring ethanol exposure induces disinhibited courtship in Drosophila. PLoS ONE. 2008;3:e1391. doi: 10.1371/journal.pone.0001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl JB, Baldwin BA, Dinh BL, Rahman P, Smerek D, Prado FJ, Sherazee N, Atkinson NS. Ethanol Preference in Drosophila melanogaster is Driven by Its Caloric Value. Alcohol Clin Exp Res. 2012;36:1903–1912. doi: 10.1111/j.1530-0277.2012.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacol. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rodan AR, Rothenfluh A. The Genetics of Behavioral Alcohol Responses in Drosophila. Int Rev Neurobiol. 2010;91:25–51. doi: 10.1016/S0074-7742(10)91002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenfluh A, Cowan CW. Emerging roles of actin cytoskeleton regulating enzymes in drug addiction: Actin or reactin’? Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.01.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenfluh A, Threlkeld R, Bainton RJ, Tsai LT-Y, Lasek A, Heberlein U. Distinct Behavioral Responses to Ethanol Are Regulated by Alternate RhoGAP18B Isoforms. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28:261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- Stafford JW, Lynd KM, Jung AY, Gordon MD. Integration of taste and calorie sensing in Drosophila. J Neurosci. 2012;32:14767–14774. doi: 10.1523/JNEUROSCI.1887-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampier L, Quintanilla ME. Effect of a dose of ethanol on acute tolerance and ethanol consumption in alcohol drinkeRUChB) and non-drinker (UChA) rats. Addict Biol. 2002;7:279–284. doi: 10.1080/13556210220139488. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wolf FW, Rodan AR, Tsai LT-Y, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global Status Report on Alcohol. Geneva: 2004. Economic and social costs of alcohol use; pp. 65–66. [Google Scholar]
- Xu S, Chan T, Shah V, Zhang S, Pletcher SD, Roman G. The propensity for consuming ethanol in Drosophila requires rutabaga adenylyl cyclase expression within mushroom body neurons. Genes Brain Behav. 2012;11:727–739. doi: 10.1111/j.1601-183X.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.