Abstract
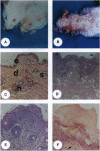
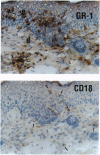
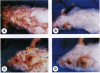
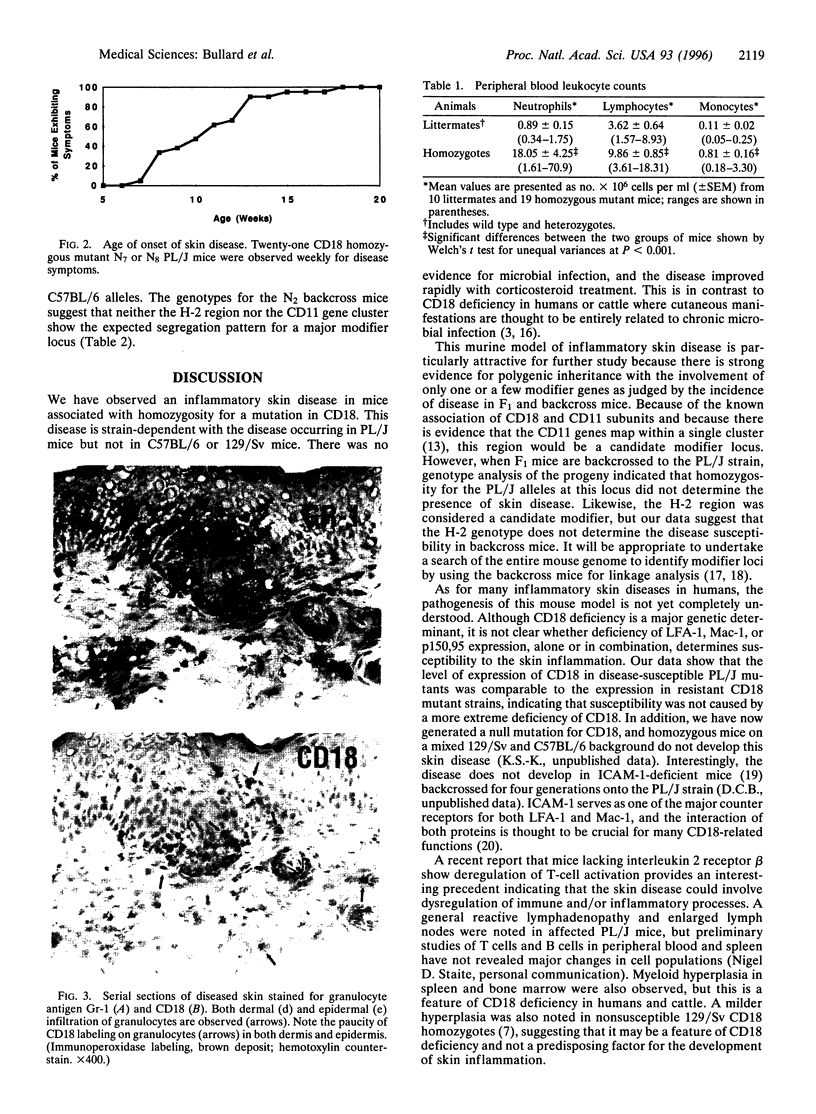
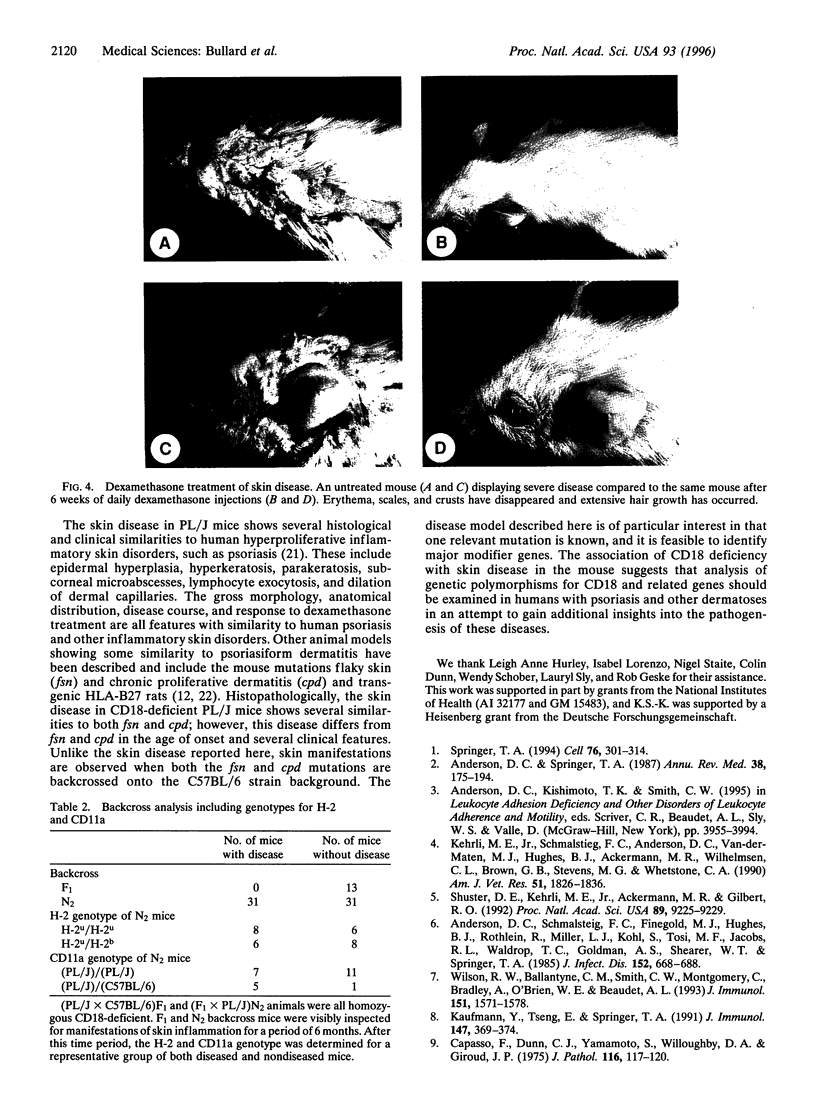
Previously, a hypomorphic mutation in CD18 was generated by gene targeting, with homozygous mice displaying increased circulating neutrophil counts, defects in the response to chemically induced peritonitis, and delays in transplantation rejection. When this mutation was backcrossed onto the PL/J inbred strain, virtually all homozygous mice developed a chronic inflammatory skin disease with a mean age of onset of 11 weeks after birth. The disease was characterized by erythema, hair loss, and the development of scales and crusts. The histopathology revealed hyperplasia of the epidermis, subcorneal microabscesses, orthohyperkeratosis, parakeratosis, and lymphocyte exocytosis, which are features in common with human psoriasis and other hyperproliferative inflammatory skin disorders. Repetitive cultures failed to demonstrate bacterial or fungal organisms potentially involved in the pathogenesis of this disease, and the dermatitis resolved rapidly after subcutaneous administration of dexamethasone. Homozygous mutant mice on a (PL/J x C57BL/6J)F1 background did not develop the disease and backcross experiments suggest that a small number of genes (perhaps as few as one), in addition to CD18, determine susceptibility to the disorder. This phenotype provides a model for inflammatory skin disorders, may have general relevance to polygenic human inflammatory diseases, and should help to identify genes that interact with the beta2 integrins in inflammatory processes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M. R., Kehrli M. E., Jr, Morfitt D. C. Ventral dermatitis and vasculitis in a calf with bovine leukocyte adhesion deficiency. J Am Vet Med Assoc. 1993 Feb 1;202(3):413–415. [PubMed] [Google Scholar]
- Aitman T. J., Hearne C. M., McAleer M. A., Todd J. A. Mononucleotide repeats are an abundant source of length variants in mouse genomic DNA. Mamm Genome. 1991;1(4):206–210. doi: 10.1007/BF00352326. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Capasso F., Dunn C. J., Yamamoto S., Willoughby D. A., Giroud J. P. Further studies on carrageenan-induced pleurisy in rats. J Pathol. 1975 Jun;116(2):117–124. doi: 10.1002/path.1711160208. [DOI] [PubMed] [Google Scholar]
- Corbi A. L., Larson R. S., Kishimoto T. K., Springer T. A., Morton C. C. Chromosomal location of the genes encoding the leukocyte adhesion receptors LFA-1, Mac-1 and p150,95. Identification of a gene cluster involved in cell adhesion. J Exp Med. 1988 May 1;167(5):1597–1607. doi: 10.1084/jem.167.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer R. E., Maika S. D., Richardson J. A., Tang J. P., Taurog J. D. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990 Nov 30;63(5):1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- Hogarth P. M., Eicher E. M., McKenzie I. F. Mapping of the murine Ly-15 (LFA-1) locus to chromosome 7. Immunogenetics. 1986;23(5):348–349. doi: 10.1007/BF00398800. [DOI] [PubMed] [Google Scholar]
- Kaufmann Y., Tseng E., Springer T. A. Cloning of the murine lymphocyte function-associated molecule-1 alpha-subunit and its expression in COS cells. J Immunol. 1991 Jul 1;147(1):369–374. [PubMed] [Google Scholar]
- Kehrli M. E., Jr, Schmalstieg F. C., Anderson D. C., Van der Maaten M. J., Hughes B. J., Ackermann M. R., Wilhelmsen C. L., Brown G. B., Stevens M. G., Whetstone C. A. Molecular definition of the bovine granulocytopathy syndrome: identification of deficiency of the Mac-1 (CD11b/CD18) glycoprotein. Am J Vet Res. 1990 Nov;51(11):1826–1836. [PubMed] [Google Scholar]
- Nepom G. T., Erlich H. MHC class-II molecules and autoimmunity. Annu Rev Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- Ord D. C., Edelhoff S., Dushkin H., Zhou L. J., Beier D. R., Disteche C., Tedder T. F. CD19 maps to a region of conservation between human chromosome 16 and mouse chromosome 7. Immunogenetics. 1994;39(5):322–328. doi: 10.1007/BF00189228. [DOI] [PubMed] [Google Scholar]
- Shuster D. E., Kehrli M. E., Jr, Ackermann M. R., Gilbert R. O. Identification and prevalence of a genetic defect that causes leukocyte adhesion deficiency in Holstein cattle. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9225–9229. doi: 10.1073/pnas.89.19.9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligh J. E., Jr, Ballantyne C. M., Rich S. S., Hawkins H. K., Smith C. W., Bradley A., Beaudet A. L. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Ballantyne C. M., Smith C. W., Montgomery C., Bradley A., O'Brien W. E., Beaudet A. L. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol. 1993 Aug 1;151(3):1571–1578. [PubMed] [Google Scholar]
- Wooley P. H., Luthra H. S., Stuart J. M., David C. S. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med. 1981 Sep 1;154(3):688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]