Abstract
Melanoma stem cells, also known as malignant melanoma-initiating cells, are identifiable through expression of specific biomarkers such as ABCB5 (ATP-binding cassette, sub-family B (MDR/TAP), member 5), NGFR (nerve growth factor receptor, CD271) and ALDH (aldehyde dehydrogenase), and drive melanoma initiation and progression based on prolonged self-renewal capacity, vasculogenic differentiation and immune evasion. As we will review here, specific roles of these aggressive subpopulations have been documented in tumorigenic growth, metastatic dissemination, therapeutic resistance, and malignant recurrence. Moreover, recent findings have provided pre-clinical proof-of-concept for the potential therapeutic utility of the melanoma stem cell concept. Therefore, melanoma stem cell-directed therapeutic approaches represent promising novel strategies to improve therapy of this arguably most virulent human cancer.
Keywords: Melanoma, cancer stem cell, ABCB5, vasculogenic mimicry, immune evasion, drug resistance, therapy
1. Introduction
Melanoma, perhaps even more than many other cancers, is an ideal clinicopathological example of a malignancy that fits the cancer stem cell model. In early evolutionary phases, melanoma grows as a flat, non-tumorigenic lesion (radial growth phase) that is virtually incapable of establishing a durable, tumorigenic metastasis. Accordingly, the vast majority of these early, flat melanomas are curable simply by surgical excision. However, once tumorigenic growth of the invasive component develops within the dermal microenvironment, even at a microscopic level when tumor volume is in the range of a cubic millimeter, potentially lethal metastases may ensue that themselves may grow expansively at distant sites. The cancer stem cell (CSC) model involves different subpopulations of malignant cells (as opposed to a homogeneous ‘clone’), and for many years it has been well-recognized that the tumorigenic component of primary melanoma is not homogeneous, as might be anticipated in a stochastic model of clonal tumorigenesis. Rather, melanomas exhibit ‘polyclonism’ (Laga and Murphy, 2010), a feature often characterized by a multiplicity of architectural, cytologic, and immunohistochemical compartments within a single tumor nodule. This patterning and apparent compartmentalization is consistent with hierarchically ordered subpopulations of tumor cells that may differ in their respective capacities to form and drive tumorigenesis through self-renewal, and engage in various pathways of cellular differentiation. Indeed, well before the discovery and experimental authentication of melanoma stem cells, Hendrix and co-workers noted differentiation heterogeneity in melanomas in the form of endothelial gene expression by tumor cells (Maniotis et al., 1999). This phenomenon, known as vasculogenic mimicry, was posited to be the consequence of differentiation plasticity in primitive, stem-like melanoma cells (Hendrix et al., 2003b), a prediction that more recently has been validated using melanoma stem cell biomarkers and experimental systems and clinical tissues (Boiko et al., 2010; Civenni et al., 2011; Fang et al., 2005; Klein et al., 2007; Monzani et al., 2007; Rappa et al., 2008; Schatton et al., 2008; Sharma et al., 2010; Vasquez-Moctezuma et al., 2010).
Primary melanomas are also highly immunogenic among human cancers, but seldom are they completely so. Two types of immune responses are involved in the primary evolution of melanoma, one directed against the more superficial component of the lesions, termed regression, and the other against the deeper invasive portion, referred to as the tumor infiltrating lymphocyte (TIL) response. Together, such immune responses may be remarkably successful at ‘melting away’ a substantial portion of the primary tumor, literally before the eyes of the afflicted patient. But success is generally only partial, and a portion of the tumor usually persists as cells that seem to be impervious to an otherwise impressive host immune response. This clinical phenomenon has suggested the possibility that subpopulations of virulent melanoma cells may preferentially express molecules that shield them from cytotoxic T cells, or alternatively fail to express melanoma-associated differentiation antigens. Accordingly, recent findings that cells expressing stem-like markers in human melanomas also selectively display co-stimulatory molecules capable of subverting host immune responses (Schatton and Frank, 2009; Schatton et al., 2010) now appear particularly relevant to melanoma as an informative paradigm for cancer stem cell behavior.
Once melanomas have spread beyond the primary site, they are extremely difficult to treat. In human cancer, this feature is known to be in part the result of resistance to drugs that effectively eradicate bulk tumor populations, but not melanoma stem cells. This protective attribute of stem cells may reside in expression of drug efflux transporters at the cell membrane capable of escorting cytotoxic agents to the extracellular space upon attempted entry (Aleman et al., 2003; Frank et al., 2005). Melanoma has turned out to be a paradigm for such stem-like drug resistance when it was shown to convert from a doxorubicin-resistant to a doxorubicin-sensitive phenotype upon experimental blockade of a drug efflux transporter later shown to be exclusively expressed by self-renewing, tumorigenic subpopulations (Frank et al., 2005). Thus, even on the surface, there exist a number of attributes to melanoma that suggest that it may be a neoplasm in which cancer stem cells play an important role. The review to follow unfortunately cannot possibly be inclusive and comprehensive, given the rapid proliferation of data supporting the existence of biomarker-identifiable melanoma cell subpopulations with self-renewing and tumorigenic properties. However, it is hoped that it will serve as an update regarding the translationally important new field of melanoma stem cell (MSC) biology, and will thus assist in driving forward the necessary research and clinical testing required to eradicate this most deadly of human cancers.
2. Conceptual Definition of Melanoma Stem Cells
The ability to recognize and study stem cells in any cancer depends on a rigorous and generally accepted definition. Failure to adhere to such criteria may result in overly restrictive definitions that contribute to unwarranted skepticism. The cancer stem cell concept recognizes that malignant tumors, like most healthy tissues, are hierarchically organized at a cellular level, with specific subpopulations that are primarily responsible for tumor initiation and propagation. According to this model, CSCs possesses 1) the capacity for prolonged and sustainable self-renewal that inexorably drives tumor growth; and 2) a capacity for differentiation to produce heterogeneous lineages of bulk tumor cells that form the bulk tumor mass but are themselves dispensable for tumor propagation. Implicit to this concept is that the CSC is capable of self-renewal through cell division that is asymmetrical, a process whereby two daughters are produced, one with potential to differentiate, and the second with capacity to continue to function as a CSC.
It is important to emphasize from the outset that it is critical for experimental models to recognize and adhere to such definitions. Over the years, a multiplicity of features have been ascribed to CSCs. Accordingly, researchers may emphasize certain characteristics to describe CSCs in the context of their hypotheses and related findings, producing the potential for bias and confusion. For example, if one regards rarity or a permanently fixed hierarchy as defining characteristics for CSCs, deviation from these features may confound data interpretation and resultant conclusions. Of particular relevance to this potential pitfall in scientific method and inquiry, the American Association for Cancer Research (AACR) in 2006 developed a working definition of a CSC, identifying it as “a cell within a tumor that possesses the capacity to self-renew and to cause heterogeneous lineages of cancer stem cells that comprise a tumor” (Clarke et al., 2006). The hallmark features of a CSC therefore are self-renewal (that drives inexorable and thus prolonged and sustained tumorigenesis), and differentiation. As will be seen in the pages to follow, melanoma is no exception to this definition.
3. Operational Definition of Melanoma Stem Cells (MSCs)
MSCs, like other CSCs, may be experimentally defined according to their ability to recapitulate the generation and perpetuation of a continuously-growing tumor. The gold standard assay for this attribute is the transplantation of patient-derived, purified MSC subpopulations into immunodeficient recipient mice capable of accepting human tumor grafts due to inability to mount an anti-tumor immune response. Because MSCs in vivo are defined as being capable of prolonged self-renewal that drives tumorigenesis, it is incumbent on such models to conduct experiments for sufficiently long periods in order to minimize the possibility that non-stem cells may deceptively appear to be stem-like only because they form tumors that enlarge over non-physiologically short durations. Unlike many other forms of human tumors, melanoma is also a special situation in that human melanomas tend to be highly immunogenic, and thus the more immunosuppressed the murine model employed for tumor graft formation, the potentially more non-physiologic becomes the tumor microenvironment.
The ability to segregate MSCs and controls (tumor bulk populations or non-MSCs) clearly is critically dependent upon the use of biomarkers for MSC identification and separation. Like physiologic stem cells, MSCs are relatively undifferentiated with respect to biomarkers, and identification of reliable markers has been the subject of intense investigation. Once separated and engrafted into immunosuppressed animals, however, rates of tumorigencity are determined and candidate marker-defined MSC subpopulations (or marker-negative bulk populations) are re-isolated from primary heterogeneous primary tumors and re-grafted to secondary, and sometimes again to tertiary experimental hosts. Such serial xenotransplantation assays are required to establish the tumorigenic capacity of MSC populations, and thus validating the necessary CSC requirement of prolonged and sustained self-renewal capacity. Serial xenotransplants also must produce tumors that upon immunohistochemical evaluation retain the phenocopy of cellular heterogeneity displayed in the original patient tumor, the result of differentiation capacity as well as self-renewal, an additional cardinal feature of the CSC. In addition, rigorous operational approaches to defining MSCs employ marker-specific genetic lineage tracing approaches that track individual cancer cell fates upon concurrent xenotransplantation of MSCs and bulk tumor populations. This provides rigorous confirmatory evidence for hierarchical tumor organization and permits further documentation of MSC phenotype and function. An added benefit of this type of experimental rigor is the opportunity to observe potentially novel interactions between MSCs and bulk tumor cell populations, such as MSC fusion with more differentiated tumor cells as a possible mechanism of resistance-associated gene transfer, or MSC secretion of extracellular matrix and growth factors required for efficient tumor initiation and expansion. Indeed, such cellular interactions that may be operative in naturally-occurring cancers, may escape detection when only purified subpopulations of cancer cells are studied (Frank et al., 2010).
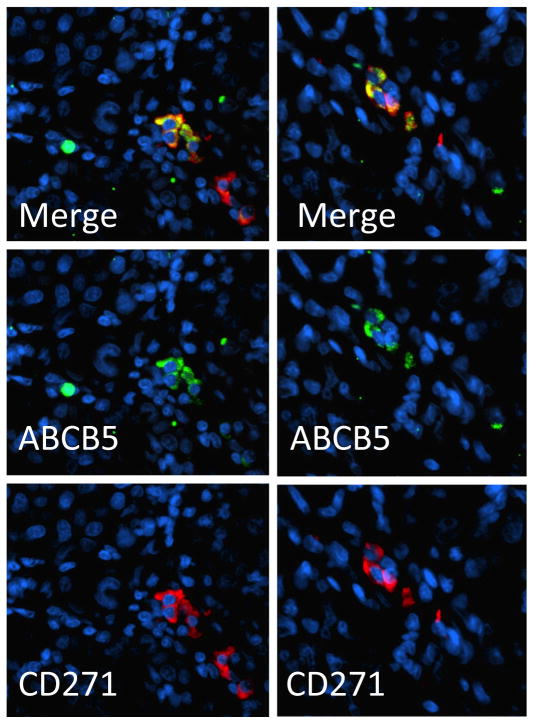
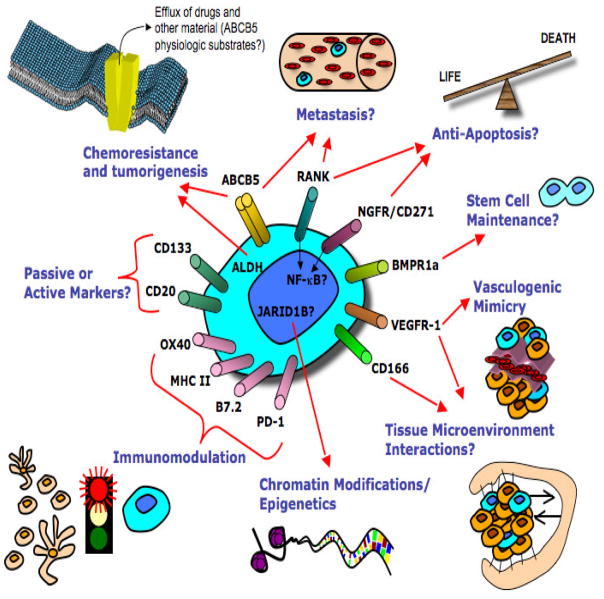
Today, MSCs are identified by biomarkers that either correlate with functional characteristics in keeping with formal definitions for CSCs, or that label associated antigens indicative of various functions and cellular behaviors that typify the MSC phenotype. Figure 1 shows two such markers (ABCB5 and CD271) in the context of a patient melanoma, and Figure 2 is a schematic that summarizes multiple markers in the context of their proposed MSC functions. These figures in themselves, and in conjunction with the descriptions that are to follow, should be of assistance in obtaining an overview of the rapidly evolving MSC field.
Figure 1. Dual Labeling for ABCB5 (green) and CD271 (red) melanoma stem cell markers in patient melanoma.
Note the restriction of reactivity to small clusters of tumor cells (two independent sites represented in left and right columns), and the co-expression of the two markers on the same cells, as evidenced by fluorochrome mixing to produce yellow-orange in the merged image (methods as per reference (Frank et al., 2011)).
Figure 2. MSC phenotypes and inferred roles of putative markers.
Clockwise from top left: Chemoresistance and tumorigenesis, metastasis, stem cell maintenance, vasculogenic mimicry, tumor microenvironment interactions, chromatin modifications/epigenetics, immunomodulation, and passive or active markers.
4. Discovery of Melanoma Stem Cells
Now that the conceptual basis and operational rigor required for the definition of CSCs, and more specifically MSCs, has been briefly addressed, we can focus on what is currently known about the identification and function of stem-like cells in human malignant melanoma (Figure 2). In 2005 (Fang et al., 2005), the Herlyn lab at the Wistar Institute examined melanoma cells isolated from patient metastases as well as from established cell lines and identified a subpopulation of melanoma cells that could be defined experimentally by their ability to proliferate as non-adherent spheres when cultured in medium suitable for human embryonic stem cells (hESCs). They found these “spheroid-forming” melanoma cells to be enriched in the CD20 surface marker, a hematopoietic phenotype that is normally associated with mature B lymphocytes. The CD20+ melanoma cells were capable of self-renewal and differentiation into adipocytic, osteocytic, and chondrocytic, as well as melanocytic lineages. These cells also exhibited increased tumorigenicity when compared to adherent cells upon xenografting into SCID (severe combined immunodeficiency) mice. However, the difference in tumor forming capacity between the non-adherent cells, spheroid-forming cells, and non spheroid-forming adherent cells was only modest. Regardless, the properties that were documented, namely of a) self-renewal, b) differentiation plasticity, and c) tumorigenesis provided important evidence that a subset of cancer stem cells might exist in human melanoma, as they do in many other forms of human cancer.
Additional evidence in support of the existence of MSCs emerged when detection of the cell surface antigen, CD133, a CSC marker previously applied to tumors of the brain (Singh et al., 2004) and colon (O’Brien et al., 2007; Ricci-Vitiani et al., 2007), was used to isolate a subset of stem-like melanoma cells from patient biopsies. These CD133+ cells exhibited enhanced tumorigenic potential when transplanted to immunodeficient mice, and accordingly were referred to as melanoma stem/initiating cells. In vitro, it was shown that certain long-term melanoma cell lines also highly expressed CD133, and that the CD133+ subset, as with patient melanoma cells, displayed increased tumorigenicity upon xenotransplantation. In addition, CD133+ melanoma cells were capable in vitro of differentiation into mesenchymal as well as neurogenic lineages and to grow as spheres under serum-free culture conditions. They also expressed angiogenic and lymphoangiogenic markers thought to contribute to tumorigenicity and later found to be relevant to differentiation plasticity akin to the phenomenon of vasculogenic mimicry. It remained unclear, however, whether CD133+ cells were truly capable of self-renewal, a defining quality of CSCs, and differentiation plasticity was demonstrated only for CD133+ melanoma cells from cell lines. Of interest, however, CD133 was highly co-expressed with ABCG2, a member of the ATP-binding cassette (ABC) transporter family associated with dye efflux capabilities characteristic of many stem cells (Monzani et al., 2007). In addition, a positive correlation between CD133 expression and melanoma progression was established by immunohistochemical staining of tissue microarrays (Klein et al., 2007). Furthermore, it was subsequently determined that down-regulation of CD133 in vitro and in vivo resulted in slower melanoma cell growth and decreased ability to metastasize (Rappa et al., 2008), further indicating a potential role for this biomarker in melanoma progression. Thus, the findings of several laboratories were converging to indicate that subpopulations of human melanoma cells did indeed show stem-like characteristics. However, a definitive demonstration correlating a melanoma stem cell marker with all of the necessary attributes of an authentic melanoma stem cell, as defined by the AACR for other CSCs, remained to be established.
An important insight into the existence of melanoma stem cells came in the form of a study concentrating on cell fusion (Frank et al., 2003). It was known that co-culture of pluripotent embryonic and mesenchymal stem cells with lineage-committed cell types resulted in hybrids as a result of cell fusion. Moreover, these hybrids could generate differentiated progeny in vitro and in vivo (Spees et al., 2003; Terada et al., 2002; Vassilopoulos et al., 2003; Wang et al., 2003; Ying et al., 2002). Although such fusion events were regarded to represent a model whereby progenitor cells express plasticity and renewal, the underlying mechanism for this phenomenon was unknown. One candidate for this type of cell fusion was the ABC superfamily of active membrane transporters known to be expressed on stem/progenitor cells (Chaudhary and Roninson, 1991; Zhou et al., 2001) and to mediate dye efflux capacity and multidrug resistance (Allikmets et al., 1998; Cole et al., 1992; Doyle et al., 1998; Goodell et al., 1996; Goodell et al., 1997; Gros et al., 1986; Leemhuis et al., 1996; Riordan et al., 1985; Roninson et al., 1986; Spangrude et al., 1995; Spangrude and Johnson, 1990; Ueda et al., 1986; Van der Bliek et al., 1987; Wolf et al., 1993; Zijlmans et al., 1995), as well as influence membrane fluidity and potential (Aleman et al., 2003). Indeed, one newly cloned ABC transporter, ABCB5 (ATP-binding cassette, subfamily B, member 5), was found to mark CD133+ melanocyte progenitor cells and also to determine the propensity for cell fusion (Frank et al., 2003). ABCB5 expression could also be detected in cells from an established human melanoma cell line. Thus, by defining a molecular mechanism for stem cell fusion that resulted in cell growth and differentiation, the potential relevance of this marker to human melanoma was an obvious next step. Because ABC transporter proteins also were known to confer drug resistance, it was speculated that ABCB5 may confer chemoresistance to primitive, stem-like melanoma cells. Indeed, ABCB5 was found to serve as a drug transporter and chemoresistance mediator in human melanoma. It also provided a molecular marker for a subpopulation of chemoresistant tumor cells with stem cell phenotypic markers among melanoma bulk populations. In melanoma cells, blockade of ABCB5 reversed resistance to doxorubicin, an agent that is ineffective in clinical melanoma therapy, to enhance cytotoxic efficacy (Frank et al., 2005). The question then emerged as to whether ABCB5, which defined melanoma cells with a progenitor, fusion-oriented phenotype, might be a marker for cells that fit a rigorous operational definition for the CSC phenotype.
In 2008, ABCB5 was indeed shown to be functional biomarker of melanoma stem cells (Schatton et al., 2008). In contrast to previous studies, the evidence that was assembled to demonstrate the existence of MSCs involved serial xenotransplantation of prospectively isolated subpopulations of melanoma cells, in vivo genetic lineage tracing, and proof-of-principle targeting of melanoma cells utilizing a monoclonal antibody to ABCB5. Specifically, when primary patient-derived tumor cells were serially transplanted to immunodeficient mice, only ABCB5+ melanoma cells proved capable of tumorigenesis, self-renewal, and differentiation into a heterogeneous population. By genetic lineage tracing, xenotransplantation revealed a tumor hierarchy in which ABCB5− cells displayed no differentiation capacity and gave rise only to ABCB5− cells, while ABCB5+ cells restored both subpopulations. This rigorously indicated that two fundamentally different phenotypes of cells existed within melanomas under these experimental conditions, with only one capable of self-renewal and differentiation. If this were true in vivo in humans, one might anticipate that with tumor progression there might be a positive correlation between ABCB5 expression and the natural evolution of the disease, from primary tumor to metastasis, and indeed this was identified using tissue microarrays. This correlation has been confirmed further by Sharma and co-workers (Sharma et al., 2010) who found increasing expression of ABCB5 with progression from nevi to primary melanoma to metastases.
Implicit to the CSC model is the notion that eradication of the stem cell component of the tumor, along with non-stem cancer cells, would be critical to effective cancer therapy. In this regard, it has been found that administration of anti-ABCB5 monoclonal antibodies to nude mice bearing melanoma xenografts impairs tumor initiation and growth and slows progression of established tumors via elicitation of antibody-dependent cell-mediated cytotoxicity directed against the ABCB5+ melanoma population (Schatton et al., 2008). This type of translational approach to MSC research provides important “proof-of-principle” with therapeutic implications. Further corroborative support for the stem cell properties of ABCB5+ melanoma cells has also come from in vitro studies of patient melanoma samples (Keshet et al., 2008). When melanoma cells were sorted by flow cytometry according to expression of multidrug-resistance gene product 1 (MDR1), which is co-expressed with ABCB5 and ABCC2, limiting dilution assays revealed that the MDR1+ fraction contains more clonogenic cells than MDR- cells, to be preferentially enriched with self-renewing cells, and to show higher anchorage independence than MDR1− cells when grown on ultra-low attachment plates. In addition, Kupas et al (Kupas et al., 2011) have shown that RANK (tumor necrosis factor receptor superfamily, member 11a, NFKB activator), a receptor activator of NF-κB (nuclear factor of kappa light polypeptide gene enhancer in B-cells 1), is expressed by melanoma cells in advanced disease, and that these cells co-express ABCB5 and CD133, cycle slowly, and exclusively initiate tumor growth in immunodeficient mice. The role of ABCB5+ cells in driving melanoma progression has been additionally fortified by Herlyn and co-workers, who demonstrated that certain mediators of MSC activity, such as tenascin-C, promote spherogenic growth characteristics and melanoma progression through maintenance of the ABCB5+ side population that displays stem cell characteristics (Fukunaga-Kalabis et al., 2010). Thus, the evolving weight of evidence supports the existence of MSCs, although the precise role of the systemic and local microenvironmental niche in determining their behavioral characteristics remains an area of active investigation.
Since these seminal early studies, a number of biomarkers for MSCs, as well as their potentially related implications in retaining the MSC phenotype and function, have been elucidated (summarized in Figure 2). In addition to ABCB5, a mediator of chemoresistance and tumorigenesis, the Aldefluor™ assay has been employed for detection of ALDH activity of this family of detoxifying enzymes to identify MSC-like cells, and ALDH1A shRNA knockdown was shown to sensitize melanoma cells to chemotherapy and to impact melanoma tumor growth (Luo et al., 2012). Regarding metastatic potential, ABCB5 has been observed to be abundantly expressed in extravasated circulating melanoma cells relative to primary tumor (Ma et al., 2012), which as already mentioned, was confirmed in a melanoma patient study where ABCB5 was co-expressed with Receptor Activator of NF-κB (RANK; (Kupas et al., 2011)) and a larger clinical study using circulating ABCB5+ MSC detection at the mRNA level (Reid et al., 2013). Whether ABCB5 or RANK are actively involved in metastasis remains to be investigated. With respect to anti-apoptosis mediators, RANK is a known activator of NF-κB, an anti-apoptotic transcription factor. Isoforms of the NGFR/CD271 protein (also expressed by MSCs (Boiko et al., 2010; Civenni et al., 2011); see Figure 1 and below) are also observed to activate NF-κB and can have anti-apoptotic roles (Colombo et al., 2012; Fiorentini et al., 2002). In the context of stem cell maintenance, BMPR1A (bone morphogenetic protein receptor, type IA) has been shown to be co-expressed with ABCB5 on MSCs (Schatton et al., 2008), and in glioblastoma, BMP4 (bone morphogenetic protein 4) signaling through BMPR1A regulates the available pool of cancer stem cells (Piccirillo et al., 2006), and thus may act similarly in melanoma. In terms of stem cell plasticity, the VEGFR1 (Flt-1) receptor has been observed to co-express with a sub-population of ABCB5+ melanoma cells, and been shown to be functionally required for tumorigenesis and the virulence-related phenomenon of vasculogenic mimicry (Frank et al., 2011). With respect to tumor microenvironment interactions, in addition to VEGF (vascular endothelial growth factor) interacting with the aforementioned FLT1/VEGFR1 (fms-related tyrosine kinase 1), the CD166 protein has been shown to be co-expressed with ABCB5 in melanoma (Frank et al., 2005). CD166 can affect cellular adhesion and primary tumor growth in melanoma (van Kempen et al., 2004) and play a role in the activation of matrix metalloproteinases (Lunter et al., 2005). In the context of chromatin modifications/epigenetic modifications factors, although JARID1B lysine histone demethylase has never been described or tested in the context of MSCs (there is currently no assay available to sort live cells based on its activity, as for ALDH), results using a single cell-line indicate that its activity might be important in regulating different phenotypes of melanoma cells that may possibly include MSCs or MSC-like cells, although this is currently speculative and requires experimental validation (Roesch et al., 2010). Concerning immunomodulation, co-expression with ABCB5 of OX40/TNFRSF4 (tumor necrosis factor receptor superfamily, member 4), OX40L (OX40 ligand), MHCII (major histocompatibility complex class II), B7-2 (CD86 molecule) and PD1 (programmed cell death 1) (among others) has been shown to be involved in the regulation of immune responses to MSC via activation of regulatory T-cells and downregulation of killer T-cells, potentially protecting MSCs from immune-mediated destruction (Schatton et al., 2010). Additional biomarkers include CD20, shown to be a possible MSC marker by multiple groups (Fang et al., 2005; Schlaak et al., 2012; Schmidt et al., 2011), which is active in the immune system on B lymphocytes, although a putative functional role has not been investigated in melanomas, indicating it could be a passive marker. The CD133 (Prom1) glycoprotein, shown to be co-expressed with ABCB5 in melanoma (Frank et al., 2005), has been claimed by various groups to be active or passive for cancers, including melanoma, and requires further investigation.
5. Relevance of Animal Models to Melanoma Stem Cells
Despite the compelling evidence in support of melanoma stem cells and the use of biomarkers like ABCB5 in their detection, there has existed a debate as to whether these cells behaved according to the CSC model. There is growing evidence that the confusion regarding the validity of the MSC concept resides in experimental differences among models and approaches that have resulted in failure in some laboratories to demonstrate that self-renewal, tumorigenic growth, and differentiation capacity resides exclusively in a stem cell subpopulation (Table 1). This issue has been recently reviewed by Shakhova and Sommer (Shakhova and Sommer, 2013), who point out that experimental differences among laboratories are likely to account for differences in the ability to detect stem cell properties.
Table 1.
Different experimental techniques associated with controversy regarding existence of MSCs*
| Studies Identifying MSCs | Authors Questioning The MSC Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Schatton et al. Nature 2008 | Kupas et al. J Invest Dermatol. 2011 | Boiko et al. Nature 2010 | Civenni et al. Cancer Res. 2011 | Boonyaratanakornkit et al. J Invest Dermatol. 2010 | Luo et al. Stem Cells 2012 | Quintana et al. Nature 2008 | Quintana et al. Cancer Cell 2010 |
| Marker | ABCB5 | RANK (ABCB5+) | CD271 | CD271 | ALDH | ALDH | Multiple | CD271, ABCB5 |
| Trypsin Dissociation | No | No | No | No | No | No | Yes | Yes |
| Sorting Technique | Magnetic Beads | FACS | FACS | FACS | FACS | FACS | FACS | FACS |
| Matrix | None | None reported | Matrigel | Matrigel | None reported | Matrigel | Matrigel | Matrigel |
| Host Strain | NOD/SCID | NSG | Rag2−/−gc−/− | NOD/SCID NSG | NOD/SCID NSG | NOD/SCID NSG | NSG | NSG |
| Transplant Rounds | 2 | 1 | 2 | 3 | 2–3 | 3 | 1–2 | 2 |
| Marker(+)MSC Enrichment | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| Genetic Lineage Tracing | Yes | No | No | No | No | No | No | No |
Noteworthy differences in protocols include the use of trypsin, the degree of immunosuppression in animal xenograft carriers, and use of the melanoma cell stimulant, laminin, in the form of Matrigel. In the absence of trypsin to harvest cells (trypsin cleaves ABCB5 and CD271 from cells, as shown by Civenni et al. (Civenni et al., 2011)), multiple independent researchers have observed the existence of cells that comply with the definition of MSCs (there is a potential for false negatives due to trypsin proteolytic cleavage of cell-surface markers). Comparison of highly immunocompromised NSG mice to NK-depleted nude or NOD/SCID mice has also revealed that in the former (1) the parental melanoma heterogeneity could not be recapitulated, and (2) CD271− cells may form tumors in the first round but may not be serially passaged, as would be anticipated for MSCs.
One point of contention has been whether MSCs are in fact ‘rare’. While the existing formal definition of CSCs does not have a specific requirement or threshold for rarity of CSCs among the bulk tumor population, it is often the case that the majority of cells within a tumor has a limited lifespan and qualifies as non-stem cells. Schatton et al. in 2008 (Schatton et al., 2008) indicated that in their hands, tumor initiation could be achieved with far fewer cells than would be required with bulk populations. Data from Quintana et al. (2008) indicated that the incidence of melanoma initiating cells was as high as 1 in 4 melanoma cells (Quintana et al., 2008). Although we do not currently know what the incidence of CSCs will be among all existing malignancies, Quintana et al. (2008) averred that their findings showed that melanoma does not follow the conventional cancer stem cell model. This assertion was soon challenged by Boiko et al. (2010), who discovered a CD271+ (low affinity nerve growth factor receptor-positive) melanoma subpopulation that was responsible for stem cell behavior in mice (Boiko et al., 2010). This work was supported further by Civenni et al. (2011) (Civenni et al., 2011) and Boonyaratanakornkit et al. (2010)(Boonyaratanakornkit et al., 2010), who employed markers for CD271 and ALDH (Aldehyde Dehydrogenase), respectively.
The reason why some investigators have failed to detect MSCs in the same manner of many other laboratories most likely relates to differences in cell isolation procedures and the animal models employed. In this regard, it is important to realize that human melanoma cells grow in a precisely-defined microenvironment that undoubtedly influences their behavior. Thus, stem cell-related tumorigenicity is not only the product of the stem cell itself, but also the result of the molecular cues to which it is exposed in the immediacy and dynamism of the extracellular matrix. These cues may become corrupted early in the experimental approach to MSC assays. For example, when patient melanoma tissue is dissociated by enzymatic digestion with collagenase or a combination of dispase and collagenase, MSC stem cell surface markers critical for assignment of the stem cell phenotype such as ABCB5 and CD271 are better preserved than upon treatment with trypsin (Civenni et al., 2011; Shakhova and Sommer, 2013). Trypsin thus depletes cells of MSC markers needed for their identification by FACS (fluorescence activated cell sorting), producing ‘false-negative’ stem cells capable of initiating tumorigenesis, resulting in the mistaken impression that non-stem cells behave like stem cells and thus potentially altering estimates of cell rarity or hierarchical organization among tumor cells (Table 1).
Another experimental pitfall relates to the fact that melanoma in patients is often highly immunogenic among human cancers. MSCs, however, have been shown to selectively express co-stimulatory pathways that may shield them from host immune responses (Schatton and Frank, 2009; Schatton et al., 2010), and proof-of-principle for the necessity of MSC immune targeting for inhibition of melanoma tumor formation has already been demonstrated (Schatton et al., 2008). Indeed, the dependency on the immune system of CD271+ MSCs to give rise to tumors upon xenotransplantation has been established by the observation that when CD271− cells are transplanted into partially immunocompromised mice, there is no melanoma formation, whereas NSG mice fully deficient in immunity provide, temporarily, a permissive growth environment for CD271− cells (Civenni et al., 2011). Importantly, tumors derived from such CD271− cells have a different cellular composition than those derived from CD271+ cells in that the former cannot be propagated extensively, and thus do not fulfill criteria for derivation from an authentic MSC (Civenni et al., 2011). Moreover, when MSC tumor initiation capability is also tested in the human-murine chimeric model that permits melanoma growth in the setting of intact human skin (not mouse subcutis), tumor initiation was restricted to the CD271+ subpopulation, consistent with the MSC model.
Finally, some investigators employed significant exposure to Matrigel, which contains laminin, in their experimental procedures. Laminin is a melanoma mitogen and stimulant of angiogenesis, and thus is theoretically capable of altering growth characteristics of melanoma cell subpopulations. The growth characteristics that are measured, as emphasized previously, ought not to be simply the ability to form a tumor over a relatively short interval (human melanomas grow over many months to several years), but rather the ability to sustain tumor growth over multiple rounds of xenotransplantation. In conclusion, if one carefully interrogates the relatively few studies that purport to show that melanoma does not conform to the CSC model, one will identify concerns regarding a) cell isolation procedures that may deplete markers from stem cells or abnormally stimulate non-stem cells, both potentially responsible for tumorigenic proliferation subject to possible misinterpretation; b) use of models devoid of any immune response that is a key component of the in vivo stem cell microenvironment; and c) assays for tumor formation that fall short of demonstrating sustained growth and retention of stem cell phenocopy over multiple rounds of tumorigenesis. Table 1 is offered to assist in comparing the experimental approaches utilized by several of the key studies where apparent disparities existed (Boiko et al., 2010; Boonyaratanakornkit et al., 2010; Civenni et al., 2011; Kupas et al., 2011; Luo et al., 2012; Quintana et al., 2010; Quintana et al., 2008; Schatton et al., 2008) with regard to use of potentially harsh enzymes (e.g. trypsin), variably immunocompromised mice, and Matrigel.
Since the inception of the debate regarding the existence of MSCs in 2008, the overwhelming majority of peer-reviewed publications elicited by the search term ‘melanoma stem cell’ provide positive data in support of this concept. Melanoma research in 2013 and beyond will be driven by a scientific community that must be fully cognizant of the weight of confirmatory evidence that has accrued regarding the existence and potential biological importance of melanoma stem cells. Accordingly, full appreciation of the basis for and deficiencies in the early debate regarding the existence of melanoma stem cells is key to informing targeted approaches designed to effectively eliminate these cells that are likely to be important contributors to the virulence in this most deadly form of human cancer.
6. Melanoma and the Immune Response
In the preceding section, the relevance of immunity to the study of MSCs was emphasized, specifically in the context of experimental modeling. While the ideal animal model consists of a fully humanized immune system in an immunocompromised mouse also xenografted with syngeneic patient melanoma cells (studies in progress in our laboratories), it is critical to understand the basis for how MSCs interact with host immunity, and how this may affect therapeutic maneuvers designed to eradicate them. Clinicians have recognized that immunocompromised patients have a heightened risk of developing cancer, including melanoma. As described above, a negative correlation has been observed between host immunocompetence and tumor initiation in animal models of human melanoma, as fewer melanoma cells were required to initiate tumor growth in more severely immunocompromised recipients (Quintana et al., 2008). However, when highly immunogenic cancers like melanoma are studied in immunocompromised animal hosts, such as NSG (IL-2Rγ (interleukin-2 receptor gamma)−/− NOD/SCID (non-obese diabetic/severe combined immunodeficiency)) mice, the frequency of tumor initiating cells may be overestimated because aspects of anti-tumor immunity have been unphysiologically eliminated (Frank et al., 2010; Schatton et al., 2010). While a relatively large number of melanoma cells can initiate tumors in severely immunodeficient mice, only a limited number are able to evade host anti-tumor immune responses and initiate melanoma in less immunocompromised NOD/SCID mice. This suggests that a subpopulation of cells may exist that is capable of immune evasion and modulation (Frank et al., 2010). Indeed, several of the mechanisms by which melanoma stem cells evade antitumor immunity have recently been identified, with one involving decreased expression of melanoma-associated antigens, such as MART-1 (melanoma antigen recognized by T cells-1) associated with melanocyte differentiation and T-cell recognition. T cells specific for and reactive against MART-1 have been identified in melanoma patients (Lee et al., 1999; Stockert et al., 1998) and thus by downregulating antigens such as MART-1, tumors are believed to escape anti-cancer immune responses (Khong et al., 2004). Melanoma stem cells that express ABCB5 (Schatton et al., 2008) have been found to have decreased expression of MART-1 in cell lines (Schatton and Frank, 2009) and patient samples (Schatton et al., 2010) as well as decreased expression of other tumor associated-antigens, including BIRC7/ML-IAP (baculoviral IAP repeat containing 7), CTAG1B/NY-ESO-1 (cancer/testis antigen 1B), and MAGE-A (melanoma antigen family A) (Schatton et al., 2010). Through downregulation of these antigens, MSCs may evade antitumor immune responses directed at non-stem cells expressing tumor antigens associated with a more differentiated malignant phenotype, thus achieving a state of immune privilege (Schatton and Frank, 2009). This could explain the relative ineffectiveness of CD8 tumor-reactive T cells in achieving a sustained and complete antitumor response (Lee et al., 1999).
MSCs also may evade the immune system via altered expression of major histocompatibility complex (MHC), induction of immunologic tolerance, and inhibition of IL-2 (interleukin-2) production. MHC class I molecules normally found on all nucleated cells classically present antigenic peptides to CD8+ T lymphocytes to activate the adaptive immune response. ABCB5+ melanoma cells have been demonstrated to have significantly reduced to absent expression of MHC class I molecules (Schatton et al., 2010). Without MHC class I expression, melanoma cells cannot be effectively recognized by CD8+ T cell responses, and antitumor immunity is thwarted. Overall, reduced MHC class I expression represents one of the principal mechanisms used by tumor cells to evade host antitumor immunity (Aptsiauri et al., 2007; Khong et al., 2004) and it is thus not surprising that decreased MHC class I expression is associated with melanoma progression, therapeutic failure, and poor clinical outcome (Cabrera et al., 2007; Carretero et al., 2008; van Houdt et al., 2008).
ABCB5+ MSCs may also resist immune-mediated rejection by inducing tolerance of melanoma-specific T cells. B7-2 and PD1 both represent negative costimulatory molecules, which downregulate immune responses by inducing T cell anergy and activating regulatory T cells (Treg cells) (Greenwald et al., 2005; Rothstein and Sayegh, 2003). Of relevance, B7-2 and PD1 are overexpressed on ABCB5+ melanoma cells in established xenografts and clinical tumor specimens (Schatton et al., 2010). This finding suggests that MSCs may evade antitumor immunity via downregulation of tumor-specific T cells through signals provided by negative costimulatory molecules. In this regard, B7-2 expressed by ABCB5+ MSCs induces CD4+CD25+FoxP3+ Treg cells and regulates their production and secretion of the immunosuppressive cytokine, interleukin (IL)-10 (Roncarolo et al., 2006; Schatton et al., 2010).
An additional mechanism by which melanoma stem cells may evade antitumor immunity is via inhibition of IL-2, an immunomodulatory cytokine that activates T cells. In vitro, ABCB5+ MSCs have been found to inhibit human peripheral blood mononuclear cell proliferation and IL-2 production more efficiently than ABCB5− melanoma cell populations (Schatton et al., 2010). Of interest to the discussion regarding animal modeling above, this may at least in part explain observed differences in frequency of tumorigenic melanoma cells in IL-2Rγ−/− compared with IL-2RγWT NOD/SCID murine hosts (Quintana et al., 2008; Schatton et al., 2008; Schatton et al., 2010). Moreover, tumorigenicity assays in the absence of IL-2 signaling may potentially overestimate the frequency of tumor-initiating cells due to an impaired antitumor host immune environment and thus may permit tumor bulk populations that ordinarily do not initiate tumors in immunocompetent hosts to generate tumors (Frank et al., 2010; Schatton and Frank, 2009). Indeed, if evasion of anti-tumor immunity by inhibition of IL-2 production is a feature of MSCs, then xenotransplantation into a murine model that is IL-2 receptor null is not a relevant environment for determination of frequency of tumor initiating cells in melanoma. In aggregate, there are a growing number of mechanisms by which MSCs evade the host immune system to promote tumor growth, including downregulation of melanoma-associated antigens, decreased expression of MHCI (major histocompatibility complex class I) molecules, induction of tolerance in T cells, and inhibition of IL-2 production. Given the association of multiple and discrete immunomodulatory characteristics with cells that express biomarkers operationally indicative of CSC function, these findings add further credence to the importance of MSCs as a well-defined subpopulation that requires serious recognition in translational approaches to immunotherapy.
7. Origin of Melanoma Stem Cells: Relationship to Physiologic Stem Cells
Cells presumed to represent normal melanocytic stem cells have been identified in the bulge region of the hair follicle where they exist as a reservoir for replenishing the epidermis upon demand (Nishimura, 2011; Nishimura et al., 2005). Dermal cells with potential for melanocytic differentiation also exist (Li et al., 2012). Although rigorous proof that these cells represent primary physiologic stem cells is accruing, whether melanomas arise from altered physiologic stem cells, or transform to a more stem-like phenotype as a consequence of mutations affecting a more differentiated melanocyte (or both), remain open questions. Nonetheless, as an extension of the CSC model, it is tempting to speculate that melanoma may arise from more primitive melanocytes rather than from their more differentiated counterparts. However, histopathological evidence supports the notion that most melanomas actually arise in the basal cell layer of the epidermis, not in the dermis or hair follicles where putative melanocyte stem cells are most concentrated. We have found that during embryogenesis, significant migration of dermal cells potentially representing melanocyte stem cells into the epidermal layer occurs (Gleason et al., 2008) and this has been substantiated experimentally (Li et al., 2012), raising the possibility that rare melanocyte stem cells may persist in the epidermis as potential targets for oncogenic mutation relevant to the genesis of early melanoma. Indeed, increasing attention is now being given to the possibility that melanoma arises in association with an extrafollicular melanocyte stem cell (Hoerter et al., 2012). Because different types of melanoma exist that display radically different architectural, cytologic, and antigenic characteristics, it is possible that MSCs arise from both melanocyte stem cells as well as from various stages in the differentiation pathway, thus potentially accounting for phenotypic heterogeneity among melanomas in a manner akin to that of different forms of leukemia within specific cell lineages.
It is of potential interest that after wounding or ultraviolet B irradiation, a known melanoma carcinogen, melanocyte stem cells in the hair follicle exit from their niche before their initial cell division, and migrate to the epidermis in a melanocortin 1 receptor (Mc1r)-dependent manner. Here they differentiate into functional epidermal melanocytes that provide melanin pigment synthesis critical to photoprotection against further damaging effects of ultraviolet irradiation (Chou et al., 2013). In addition to the possibility that this may promote accumulation of melanocyte stem cells in the epidermal layer, the involvement of the Mc1r receptor in this process is of interest in that patients expressing MC1R mutations are known to be susceptible to the development of melanoma through the PI3K (phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha)/AKT (v-akt murine thymoma viral oncogene homolog 1) signaling pathway (Cao et al., 2013). Accordingly, additional investigations concerning convergence of molecular pathways that affect melanocyte stem cell biology and melanoma evolution are clearly indicated (Lin and Fisher, 2007).
Might MSC markers themselves play a role in melanomagenesis? In a recent report, Lin and colleagues (Lin et al., 2013) genotyped over 1000 melanomas and matched controls for 44 ABCB5-tagging single nucleotide polymorphisms (SNPs) to span a region covering 108.2kb of the gene on the 7p21.1 locus. Three SNPs associated with decreased melanoma risk were identified, one associated with non-red, as compared to red hair. Functional studies in melanoma lines of this SNP correlated significantly with decreased ABCB5 transport capacity and increased melanin production, consistent with a novel ABCB5 polymorphism associated with human pigmentation phenotype and melanoma risk. Thus, functional variation in a prospective MSC marker may be associated with melanoma disease risk, providing provocative insight into future studies examining potential linkages between physiologic ABCB5 status in melanocyte precursors, and the role of ABCB5 once malignant transformation has occurred.
One promising model for discovery of potential links between melanocyte stem cells and melanoma is the Zebrafish (Hoerter et al., 2012). In this model, more primitive melanocytes (melanoblasts) have been shown to be responsible for continued regeneration of the melanocyte pigment pattern in the fish following multiple rounds of mature melanocyte ablation or fin amputation (Azevedo et al., 2011; Kelsh et al., 2000; O’Reilly-Pol and Johnson, 2008, 2009). Moreover, the cloning and expression of an early melanocyte stem cell biomarker, dopachrome tautomerase, allows for detection of melanoblasts at early developmental stages. Thus, the model is well-suited for studies that will mine the effects of known melanoma mutagens (e.g. ultraviolet light), or experimentally-induced gene mutations, on melanocyte stem cell populations.
8. Melanoma Stem Cells and Multistep Tumor Progression
Melanoma has long been regarded as a paradigm for cancer progression. Clark and Mihm originally advanced the concept (Clark et al., 1969) based on the evolution from a) melanocytic dysplasia to in situ/radial growth phase melanoma that is essentially incapable of metastasis, to b) vertical growth phase melanoma with metastatic capacity related to level and depth of invasion, to c) metastatic disease. An enormous number of studies have validated this concept at both genomic and proteomic levels. In tissue microarrays that involve high throughput analysis of biospecimens from each of these stages of progression, MSC markers have shown a consistent trend toward enhanced expression that correlates with the progression of disease (Klein et al., 2007; Schatton et al., 2008; Sharma et al., 2010). How, however, the complexities of MSC phenotype and function may govern melanoma progression, remains to be determined.
As has been emphasized in the preceding pages, primary tumor formation is driven by subpopulations of MSCs that express specific biomarkers, two notably being ABCB5 and CD271 (Boiko et al., 2010; Schatton et al., 2008). Circulating tumor cells during tumor progression to metastasis would therefore be anticipated to express MSC markers if they are to be capable of establishing durable tumorigenic metastases that are progressive and clinically detectable. Ma and colleagues (Ma et al., 2012) have shown in xenograft models that in animals bearing human melanomas, circulating tumor cells express MSC markers such as ABCB5, and the expression of such markers in the circulation correlates significantly with the development of lung metastases. In addition, prospectively isolated circulating tumor cells were found to be capable of primary tumor initiation and development of subsequent metastases upon xenotransplantation to secondary immunodeficient mice. As previously indicated, Kupas and coworkers (Kupas et al., 2011) have addressed this issue by examining in melanoma the expression of the Receptor activator of NF-κB (RANK)-RANKL(RANK ligand) pathways that are involved in migration and metastasis of epithelial tumor cells. They found RANK to be significantly increased in primary, circulating, and metastatic melanoma cells from patients with advanced (stage IV) disease, as opposed to tumor cells from earlier (stage I) disease. The RANK+ cells co-expressed the MSC markers ABCB5 and CD133, and these cells showed preferential tumorigenicity in xenograft assays (see also Table 1). Moreover, Reid and co-workers demonstrated circulating ABCB5+ MSCs to be a significant prognostic marker for disease recurrence in human melanoma patients (Reid et al., 2013). Thus, MSCs appear to acquire additional molecules of known importance in systemic dissemination during tumor evolution.
It is important to consider that aspects of the MSC model may also apply to other forms of cancer that exhibit similar modes of tumor progression. In this regard, it is of interest that the MSC marker, ABCB5, also has been shown in the setting of hepatocellular carcinoma, where it correlates with tumorigenic subpopulations and expression of the pluripotent growth factor, granulin-epithelin precursor (GEP) (Cheung et al., 2011), as well as in colorectal cancer, where it mediates 5-FU (fluoruracil) resistance in CD133+ colorectal cancer stem cells (Wilson et al., 2011).
9. Melanoma Stem Cells and “Epithelial-Mesenchymal Transition”
Epithelial-mesenchymal transition is a term that defines the ability of epithelial cells in cancers to transition to a more mesenchymal phenotype. This phenomenon is accompanied by a more invasive and metastatic phenotype, and with the expression of gene programs more typical of mesenchymal than of epithelial cells. Melanoma is capable of transitions from tumorigenic growth, where rounded epithelioid cells proliferate in a cohesive manner to form three-dimensional expansive nodules, to a more infiltrative phenotype where slender, fusiform cells infiltrate the extracellular matrix singly or in small, streamlined fascicles. Although both growth phases often co-exist in a given lesion of invasive primary melanoma, extremes exist where some melanomas may grow as primarily nodular lesions, while others may be exclusively infiltrative (for example, so called desmoplastic melanoma). Because a balance and ability to switch bidirectionally between tumorigenic growth and invasion would appear necessary for full tumor virulence, and because the invasive phenotype is regarded as a pre-requisite to effective metastasis, melanoma can certainly be regarded as having some EMT (epithelial-mesenchymal transition)-like features, leading to the question as to whether such features involve the expression of mesenchymal genes, and whether such behavior involves MSCs.
It has been known for some time that primitive melanoma cells may promote tumor growth via vasculogenic mimicry (VM), a phenomenon first described by Hendrix in 1999 as the ability of aggressive melanoma cells to form extracellular matrix (ECM)-rich, extravascular, patterned networks in three-dimensional cultures (Hendrix et al., 2003b). Vasculogenic mimicry involves the production of multiple, laminin-rich networks that are periodic acid-Schiff (PAS) positive and surround clusters of tumor cells (Seftor et al., 2001). VM does not form channels that are lined by authentic, CD31-positive endothelial cells, as in the case of conventional tumor angiogenesis, although mosaic areas where endothelium and tumor cells are intimately associated have been described (Zhang et al., 2006). Rather, VM networks are associated with expression within adjacent melanoma cells of the endothelium-associated gene CD144, also known as vascular-endothelial (VE) cadherin. Tubular structures formed in VM resemble primary sinusoidal networks formed during embryonic development, and these networks in tumors are hypothesized to serve as sites of nutritional exchange and dissemination routes for metastasis, which may be independent of or function in concert with angiogenesis (Hendrix et al., 2003a). While the full functional implications of VM remain to be elucidated, VM is clearly associated with tumor aggressiveness, poor clinical outcome, and high risk of recurrence (Seftor et al., 2001; Thies et al., 2001; Warso et al., 2001). Unfortunately, conventional anti-angiogenic therapies, such as endostatin, have been ineffective at inhibiting VM (van der Schaft et al., 2004).
Are the melanoma cells that result in VM related to MSCs? It is known that VM-producing tumor cells are highly invasive, multipotent cells that express primitive genes (Hendrix et al., 2003b) associated with multiple cell lineages, including those of endothelial, epithelial, pericyte, fibroblastic, hematopoietic, kidney, neuronal, muscle, and several other cell types (Bittner et al., 2000; Seftor et al., 2002), suggesting they are capable of differentiating into a variety of cellular phenotypes. Molecular analyses have revealed that these aggressive tumor cells also express several genes associated with embryonic stem cells, such as Nodal, a potent embryonic morphogen from the transforming growth factor (TGF)-β family. Nodal functions to maintain a stem cell-like phenotype in melanoma cells (Topczewska et al., 2006) and is required for VM and tumor growth. Moreover, Nodal-expressing melanoma cells are spatially associated with formation of the channel-like networks that characterize VM (McAllister et al., 2010), suggesting a possible key role for primitive, stem cell-like melanoma cells in the genesis of VM. Experimentally, inhibition of Nodal signaling impairs the capacity of aggressive melanoma cells to form VM on a three dimensional collagen network, reduces melanoma cell invasivenessi, and promotes transition of melanoma cells toward a more differentiated, melanocytic phenotype (Topczewska et al., 2006). Nodal also positively correlates with melanoma tumor progression (Topczewska et al., 2006), as is the case for several other MSC markers (discussed above).
Based on their gene and protein expression profiles, aggressive melanoma cells that are involved in VM most resemble undifferentiated, primitive, embryonic-like stem cells (Hendrix et al., 2003a). This suggests that MSCs may give rise to the patterned networks that typify VM. Indeed, ABCB5+ melanoma cells demonstrate preferential expression of the vasculogenic differentiation markers tyrosine kinase with Ig-like and EGF-like domains 1 (TIE-1), CD144, and bone morphogenetic protein receptor type 1A (BMPR1A) (Schatton et al., 2008). Thus, in the phenomenon of VM, MSCs appear to transform from a multipotent, stem-like phenotype into a more endothelial phenotype that produces vasculogenic-like networks (Frank et al., 2011).
In addition to forming laminin-rich scaffolds for possible support and stimulation of three-dimensional tumor growth and providing sinusoidal conduits that collaborate with leaky authentic tumor vessels to bathe metabolically active tumor cells with nutrients, VM may also promote metastases by exposing the most virulent tumor cells to the peripheral circulation. Because of this, metastasis of melanoma has been likened to the process of epithelial-mesenchymal transition (EMT), which confers metastatic and invasive properties to carcinomas (Thiery, 2002) and is a major determinant of melanoma progression (Alonso et al., 2007; Na et al., 2009; Yang et al., 2009). Classical EMT is a process whereby polarized, immotile epithelial cells lose cell-cell and cell-matrix connections and acquire the motile, migratory properties of mesenchymal cells. EMT often involves changes in cell adhesion molecules, including loss of E-cadherin expression and upregulation of N-cadherin (Bonitsis et al., 2006), and environmental influences, such as the fibroblast-secreted growth factor, TGF-β, may encourage this cadherin switch (Hsu et al., 2002; Lee and Herlyn, 2007). Thus, while VM involves transformation of primitive melanoma stem cells to those with a more endothelial-like phenotype, epithelial-like cells transform into a spindle-like, mesenchymal phenotype in EMT. Similar to VM, EMT occurs during embryogenesis, involves cell-matrix interactions, and is associated with disease progression. Accordingly, given the plasticity of MSCs, there is likely a link between primitive melanoma cells and EMT, though the precise relationship requires further investigation. Breast cancer trials recently demonstrated that EMT enriches the cancer stem cell population (Mani et al., 2008; Morel et al., 2008). However, whether this is the result of de-differentiation of mature cells, or accelerated division and increased proportion of self-renewing, pre-existing cancer stem cells remains to be elucidated (Turner and Kohandel, 2010).
It should be recognized that different MSC-like markers may indicate different pathways of virulence and cellular behavior. It has been shown, for example, that a subpopulation of melanoma cells express the embryonic neural crest stem cell transcription factor, SOX2 (Laga et al., 2010). SOX2+ melanoma cells show a more fusiform cytology, a more peripheral distribution of the stem cell marker, Nestin, and are more invasive in vitro and in xenograft models (Girouard et al., 2012; Laga et al., 2011). While this may be considered to link a stem cell marker, SOX2, with EMT-like behavior, it remains unclear as to how such markers will relate to other MSC markers, and whether the MSC phenotype will ultimately show complexity with regard to co-expression patterns of different, functionally significant markers of various components of tumor virulence and melanoma “stemness”.
10. Treatment Efficacy and Melanoma Stem Cells
MSCs are distinctive in a number of ways in addition to their ability to self-renew, form and perpetuate tumors, and differentiate; they also express a number of pathways that thwart both endogenous and iatrogenic strategies designed to effect their elimination. One such strategy is the expression of multidrug resistance (MDR) genes and related functional proteins. MDR describes the ability to inhibit cancer chemotherapeutic efficacy by mounting resistance to multiple, structurally unrelated therapeutic drugs with different mechanisms of action (Gottesman et al., 2002). An MDR pathway of special interest in melanoma is decreased intracellular drug accumulation accomplished by energy dependent efflux pumps. These pumps are known as ABC transporters that translocate solutes across the cellular membrane (Higgins, 1992). ABCB5 is a functional transporter that mediates melanoma resistance to multiple chemotherapeutic agents, such as doxorubicin (Elliott and Al-Hajj, 2009; Frank et al., 2005; Huang et al., 2004), and is also a molecular biomarker of melanoma stem cells (Schatton et al., 2008), as already discussed. ABCB5 blockade experimentally enhances intracellular drug accumulation and serves to render melanoma cells sensitive to the therapeutic effects of doxorubicin (Frank et al., 2005). Moreover, Chartrain et al. (2012) recently showed that ABCB5-expressing cells also selectively survive when exposed to dacarbazine, the reference treatment of metastatic melanoma, and vemurafenib, a new inhibitor of the mutated kinase V600E BRAF (v-raf murine sarcoma viral oncogene homolog B) (Chartrain et al., 2012). Importantly, Chartrain et al. (2012) also observed that ABCB5-expressing cells were enriched in melanoma patients in vivo following clinical temozolomide therapy (Chartrain et al., 2012). The multidrug-resistant gene product 1 (MDR1), another ABC transporter, is also preferentially expressed in human melanoma cells with stem cell properties (Keshet et al., 2008) and thus the expression of ABC transporter proteins in melanoma cells capable of self-renewal and differentiation identifies MDR as another mechanism by which melanoma stem cells promote tumor growth.
11. Targeted Approaches to Melanoma Stem Cells
Advanced, metastatic melanoma is an extraordinarily challenging cancer to treat due to its resistance to conventional therapies. Conventional anticancer therapeutics primarily eradicate the bulk tumor population, which have different properties than the MSC subpopulation. It has been hypothesized that cancer stem cells represent a pool of resistant cells in cancer patients (Frank et al., 2010), and as has been discussed in this review, MSCs are known to be chemoresistant and immunoevasive, and primitive melanoma cells are capable of the virulence-associated phenomenon of vasculogenic mimicry and possibly other EMT-like attributes. It follows that therapeutic targeting of the survival mechanisms employed by MSCs, in combination with eradication of bulk tumor populations, may increase the efficacy of anticancer therapies and decrease the risk of relapse and progression. Thus, targeting the very protective pathways and their markers that permit MSCs to evade detection and destruction may prove to be the most efficacious way to ultimately thwart this deadly disease. In order to muster all of the necessary resources to this end, it is also important that the scientific community fully appreciates the rapidly growing data concerning MSCs, and understands that methodological pitfalls and variations ought not to jeopardize understanding of the critical biological importance of this virulent subpopulation of cells. Cells shielded from detection and elimination that possess the selective capacity for sustained self-renewal and tumorigenesis must not be overlooked in translational research designed to eradicate melanoma, regardless of whether hierarchies are fixed or environmentally sensitive, or whether under some circumstances such cells are less rare than in others.
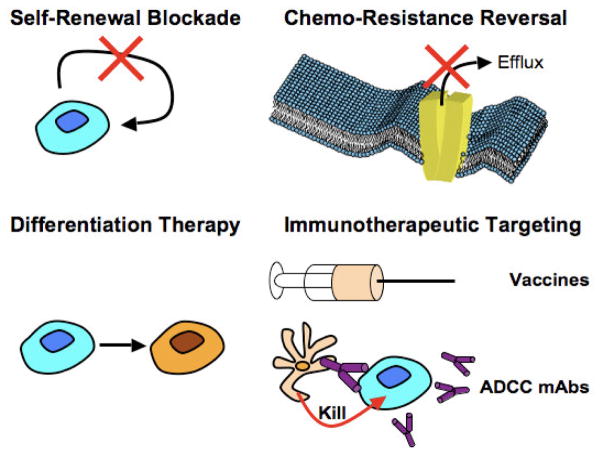
Targeted approaches eliminating MSCs, either used singly or combinatorially, must consider first those directed against surface antigens that MSCs uniquely express. Such “direct” approaches where MSC epitopes are targeted may take several forms in terms of the net cellular effect (Figure 3), including a) self-renewal blockade; b) induction of anti-MSC immune responses; c) reversal of drug resistance; and d) differentiation therapy. One key molecular marker to target is ABCB5, which is not only a biomarker of melanoma stem cells, but also provides a mechanism for chemoresistance. Potential therapies against ABCB5 already have been explored; these include use of monoclonal antibodies (Schatton et al., 2008) and short hairpin (sh) RNA-mediated knockdown (Elliott and Al-Hajj, 2009; Huang et al., 2004; Wilson et al., 2011). As proof-of-principle, selective killing of ABCB5-expressing MSCs via antibody-dependent cell mediated cytotoxicity has been shown to inhibit experimental melanoma growth in xenografts (Schatton et al., 2008). Notably, both anti-ABCB5 antibody (Frank et al., 2005)and siRNA gene silencing (Elliott and Al-Hajj, 2009) reversed melanoma resistance to doxorubicin, and gene silencing increased the sensitivity of melanoma cells to 5-fluorouracil and camptothecin (Huang et al., 2004), thus demonstrating that targeting of ABCB5 may have additive and potentially even synergistic effects by combining immune detection and induction of chemotherapeutic sensitization. Further confirmation of MSC targeting approaches is found in studies involving shRNA-mediated knockdown of MSC-associated CD133 (Frank et al., 2005), a maneuver that decreased the growth rate and impaired metastatic capacity of melanoma cells (Rappa et al., 2008). Other direct targeting approaches have involved the CD20 MSC marker, as described by the Herlyn laboratory (Fang et al., 2005). Using engineered T cells that recognize and destroy CD20+ cells, xenografted human melanomas were eradicated in immunodeficient mice (Schmidt et al., 2011), and clinical application of rituximab (an anti-CD20 antibody) to a limited number of patients with advanced metastatic melanoma has had promising results (Schlaak et al., 2012), although the precise mechanism of the therapeutic benefit (MSC elimination, anti-proliferative effects on melanoma cells, elimination of B lymphocytes) remains unclear.
Figure 3. Potential strategies to target MSCs.
Top left: Targeting of the self-renewal pathway of MSCs disabling their ability to replicate. Top right: Blocking chemotherapy resistance mediators (such as ABCB5 or ALDH) combined with standard chemotherapy to sensitize MSCs to drug-based killing. Bottom left: Modification of MSCs with drugs to initiate differentiation that would then undergo apoptosis, or be sensitized to chemotherapies. Bottom right: Utilization of MSC-specific, or MSC/bulk tumor-shared antigens to develop patient vaccines to enhance endogenous immune responses to destroy MSCs. Similarly, eliciting antibody-dependent cell cytotoxicity (ADCC) via administration of monoclonal antibodies to MSC-restricted antigens represents an additional means of for patient immune cells to specifically target the melanoma stem cell component of the tumor.
Another potential strategy directed at eradicating melanoma stem cells is the induction of a more differentiated phenotype. Differentiation of primitive tumor cells within a malignancy may result in tumor deterioration (Pierce, 1983), and differentiation therapy has proved useful in human glioblastoma where targeting of morphogen-driven signaling pathways by bone morphogenetic protein BMP4 inhibited tumor growth (Piccirillo et al., 2006). BMP4-dependent differentiation strategies may also be an effective method of targeting MSCs, as BMPR1A is known to be preferentially expressed on ABCB5+ cells (Schatton et al., 2008). The embryonic morphogen, Nodal, represents another potential target in melanoma given its role in maintenance of a stem cell-like phenotype in melanoma cells (Topczewska et al., 2006). Both BMP4 and Nodal are also potential targets in anti-VM therapy (see below), as downregulation of both proteins is known to impair VM and reduce expression of VM-related genes such as VE-cadherin (Rothhammer et al., 2007; Topczewska et al., 2006). It may also be possible to induce MSCs to differentiate by altering gene expression profiles using small non-coding microRNA (Yu et al., 2007) or epigenetic differentiation therapy via inhibition of DNA methyltransferases and/or histone deacetylases (Arce et al., 2006), both of which have been shown to induce differentiation of breast CSCs.
Inhibition of molecular pathways that confer “stemness” represents still another approach, although this remains in its infancy (see Shakhova and Sommer for recent review (Shakhova and Sommer, 2013)). More indirect therapies focused at VM and immune evasion and modulation are also potential strategies MSCs. Immunotherapeutic strategies of relevance to this discussion include cytokines IL-2 and IFNα-2b, as well as newer therapeutic developments, such as tumor vaccines and specific targeting of the molecules that induce immune tolerance to melanoma. IL-2 therapy is of interest given that melanoma stem cells suppress IL-2 production by T cells as a mechanism of immune evasion (Schatton et al., 2010), and thus by overcoming melanoma suppression of this cytokine, the anticancer immune response may be reawakened. Another immunotherapy, tumor vaccination, has been less efficacious thus far(Rosenberg et al., 2004). Specific molecular targeting of regulatory elements involved in immune tolerance should focus on negative regulators of the adaptive immune response, such as B7-2 and PD1, and inhibitory cytokines, such as IL-10, as all of these appear to be employed by MSCs to downregulate T cells (Schatton et al., 2010) and thus represent potential molecular targets for immunotherapy. CLTA4 (cytotoxic T-lymphocyte antigen 4) serves as a ligand receptor associated with B7-2, and its effect is negative regulation of T cell activation. Two anti-CTLA4 monoclonal antibodies, tremelimumab and ipilimumab, have already shown promise in initial clinical trials (Camacho et al., 2009; Ribas et al., 2005; Weber et al., 2009; Wolchok et al., 2010), and a randomized phase III trial has demonstrated marginally increased survival in patients with metastatic melanoma who received ipilimumab versus glycoprotein 100 peptide vaccine (Hodi et al., 2010).
Anti-VM therapy is another indirect strategy to target primitive melanoma cells, and this approach may be a particularly promising therapy against melanoma, because there is no known physiologic analog of VM in children or adults. Therefore, anti-VM therapies should have minimal effects on normal physiological processes (Folberg et al., 2000). Inhibitors of angiogenesis are ineffective against the tubular networks seen in VM, and it has been suggested that an efficient anti-VM therapy focus on three aspects: remodeling of the ECM and tumor microenvironment, blocking biochemical and molecular signaling pathways of VM, and inhibiting plasticity of tumor cells (Fan and Sun, 2010). Genes involved in the molecular signaling pathways of VM (e.g. MMP (matrix metalloproteinase), CD144/VE-cadherin (vascular endothelial cadherin), EPHA2 (EPH receptor A2), laminin 5γ2) can potentially be downregulated with therapeutic agents; indeed, in in vivo models of murine melanoma, thalidomide inhibited MMP-2 and MMP-9 expression and VM channel formation and promoted melanoma cell necrosis (Zhang et al., 2008), and many other such examples exist. Additional methods of impairing VM may employ monoclonal antibodies, antisense olignucleotides, or gene knockout of key proteins in signaling pathways. The potential reliance of VM on the microenvironment, including that immediately surrounding the genome, stresses the importance of the MSC niche in mediation of all such targetable attributes of cancer stem cells. Recently, experimental reversal of loss of 5-hydroxymethyl cytosine (5-hmC), a novel epigenetic marker for melanoma, was shown to inhibit melanomagenesis in several in vivo models (Lian et al., 2012). Given the importance of epigenetic events in stem cell behavior during embryogenesis, and considering the reversible and thus therapeutically amenable nature of epigenetic modifications, this new insight may provide an exciting platform for additional indirect strategies for manipulation of MSCs.
12. Conclusions and Future Directions
Malignant melanoma is arguably the most virulent cancer to which human flesh is heir. Because it is visible to inspection as it evolves, and in the context of the vast array of morphological, molecular, and genomic approaches that have been directed toward its understanding, melanoma has historically served as a model for how other cancers evolve and progress. Indeed, our concepts of pre-malignant dysplasia, in situ carcinoma, and how progressive stages of invasion correlate with systemic dissemination and development of durable metastases are largely derived from the study of melanoma. It is not surprising, therefore, that melanoma is also becoming a widely accepted model for the study of cancer stem cells. We already have learned from melanoma that stem cells employ extraordinary stealth in thwarting host defenses and therapies - fending off therapeutic drugs with efflux pumps; discouraging attacking T cells with co-stimulatory mechanisms that dampen immunity; deploying pathways for plasticity that enhance virulence, access to nutrition, and possibly invasion/metastasis; and engaging molecular mechanisms that protect against cell death by apoptosis. To accomplish these goals, however, melanoma stem cells express biomarkers that are both detectable and targetable.
The future of melanoma therapy must take these issues into serious consideration. Melanoma cells are heterogeneous, and it will be increasingly important to target all of the cells within a tumor, particularly the more virulent ones most capable of self-renewal and tumorigenicity. It is likely that combinatorial approaches will be needed to eliminate a tumor as deceptive and treacherous as melanoma in order to catch melanoma stem cells in a therapeutic “cross-fire” that will recognize all of the different, complementary ways to synergistically target stem cell-associated markers. It will now be possible to assess efficacy of therapy early on by defining and profiling the immunopathology of precisely which subpopulations within a given tumor are actually being eliminated. Just as individual melanomas are heterogeneous with respect to virulence-conferring cells, so too are melanomas likely to differ from patient to patient, further emphasizing the importance of personalized biomarker profiling for targeting of melanoma stem cells.
Despite the growing pains related to the need for standardized definitions and experimental approaches regarding melanoma stem cells, the recognition that melanoma harbors cancer stem cells represents a major advance in our thinking about this potentially deadly neoplasm. There is thus growing momentum to utilize this understanding toward therapeutic benefit, and the near future will hopefully fulfill the clinical promise so tightly linked to these fundamental biological insights.
Acknowledgments
This work was supported by the NIH/NCI grants R01CA113796, R01CA158467, R01CA138231 and P50CA093683, and by the Department of Veterans Affairs VA Merit Review Awards VA BLR&D 1I01BX000516 and VA RR&D 1I01RX000989.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman C, Annereau JP, Liang XJ, Cardarelli CO, Taylor B, Yin JJ, Aszalos A, Gottesman MM. P-glycoprotein, expressed in multidrug resistant cells, is not responsible for alterations in membrane fluidity or membrane potential. Cancer Res. 2003;63 (12):3084–3091. [PubMed] [Google Scholar]
- Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58 (23):5337–5339. [PubMed] [Google Scholar]
- Alonso SR, Tracey L, Ortiz P, Perez-Gomez B, Palacios J, Pollan M, Linares J, Serrano S, Saez-Castillo AI, Sanchez L, Pajares R, Sanchez-Aguilera A, Artiga MJ, Piris MA, Rodriguez-Peralto JL. A high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasis. Cancer Res. 2007;67 (7):3450–3460. doi: 10.1158/0008-5472.CAN-06-3481. [DOI] [PubMed] [Google Scholar]
- Aptsiauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol. 2007;601:123–131. doi: 10.1007/978-0-387-72005-0_13. [DOI] [PubMed] [Google Scholar]
- Arce C, Perez-Plasencia C, Gonzalez-Fierro A, de la Cruz-Hernandez E, Revilla-Vazquez A, Chavez-Blanco A, Trejo-Becerril C, Perez-Cardenas E, Taja-Chayeb L, Bargallo E, Villarreal P, Ramirez T, Vela T, Candelaria M, Camargo MF, Robles E, Duenas-Gonzalez A. A proof-of-principle study of epigenetic therapy added to neoadjuvant doxorubicin cyclophosphamide for locally advanced breast cancer. PLoS One. 2006;1:e98. doi: 10.1371/journal.pone.0000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo AS, Grotek B, Jacinto A, Weidinger G, Saude L. The regenerative capacity of the zebrafish caudal fin is not affected by repeated amputations. PLoS One. 2011;6 (7):e22820. doi: 10.1371/journal.pone.0022820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406 (6795):536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, Longaker MT, Weissman IL. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466 (7302):133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitsis N, Batistatou A, Karantima S, Charalabopoulos K. The role of cadherin/catenin complex in malignant melanoma. Exp Oncol. 2006;28 (3):187–193. [PubMed] [Google Scholar]
- Boonyaratanakornkit JB, Yue L, Strachan LR, Scalapino KJ, LeBoit PE, Lu Y, Leong SP, Smith JE, Ghadially R. Selection of tumorigenic melanoma cells using ALDH. J Invest Dermatol. 2010;130 (12):2799–2808. doi: 10.1038/jid.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera T, Lara E, Romero JM, Maleno I, Real LM, Ruiz-Cabello F, Valero P, Camacho FM, Garrido F. HLA class I expression in metastatic melanoma correlates with tumor development during autologous vaccination. Cancer Immunol Immunother. 2007;56 (5):709–717. doi: 10.1007/s00262-006-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho LH, Antonia S, Sosman J, Kirkwood JM, Gajewski TF, Redman B, Pavlov D, Bulanhagui C, Bozon VA, Gomez-Navarro J, Ribas A. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27 (7):1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- Cao J, Wan L, Hacker E, Dai X, Lenna S, Jimenez-Cervantes C, Wang Y, Leslie NR, Xu GX, Widlund HR, Ryu B, Alani RM, Dutton-Regester K, Goding CR, Hayward NK, Wei W, Cui R. MC1R Is a Potent Regulator of PTEN after UV Exposure in Melanocytes. Mol Cell. 2013;51 (4):409–422. doi: 10.1016/j.molcel.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero R, Romero JM, Ruiz-Cabello F, Maleno I, Rodriguez F, Camacho FM, Real LM, Garrido F, Cabrera T. Analysis of HLA class I expression in progressing and regressing metastatic melanoma lesions after immunotherapy. Immunogenetics. 2008;60 (8):439–447. doi: 10.1007/s00251-008-0303-5. [DOI] [PubMed] [Google Scholar]
- Chartrain M, Riond J, Stennevin A, Vandenberghe I, Gomes B, Lamant L, Meyer N, Gairin JE, Guilbaud N, Annereau JP. Melanoma chemotherapy leads to the selection of ABCB5-expressing cells. PLoS One. 2012;7 (5):e36762. doi: 10.1371/journal.pone.0036762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;66 (1):85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Cheung PF, Cheng CK, Wong NC, Ho JC, Yip CW, Lui VC, Cheung AN, Fan ST, Cheung ST. Granulin-epithelin precursor is an oncofetal protein defining hepatic cancer stem cells. PLoS One. 2011;6 (12):e28246. doi: 10.1371/journal.pone.0028246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WC, Takeo M, Rabbani P, Hu H, Lee W, Chung YR, Carucci J, Overbeek P, Ito M. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med. 2013;19 (7):924–929. doi: 10.1038/nm.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, Seifert B, Moch H, Dummer R, van den Broek M, Sommer L. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71 (8):3098–3109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- Clark WH, Jr, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29 (3):705–727. [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66 (19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258 (5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Colombo E, Romaggi S, Blasevich F, Mora M, Falcone C, Lochmuller H, Morandi L, Farina C. The neurotrophin receptor p75NTR is induced on mature myofibres in inflammatory myopathies and promotes myotube survival to inflammatory stress. Neuropathol Appl Neurobiol. 2012;38 (4):367–378. doi: 10.1111/j.1365-2990.2011.01212.x. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95 (26):15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AM, Al-Hajj MA. ABCB8 mediates doxorubicin resistance in melanoma cells by protecting the mitochondrial genome. Mol Cancer Res. 2009;7 (1):79–87. doi: 10.1158/1541-7786.MCR-08-0235. [DOI] [PubMed] [Google Scholar]
- Fan YZ, Sun W. Molecular regulation of vasculogenic mimicry in tumors and potential tumor-target therapy. World J Gastrointest Surg. 2010;2 (4):117–127. doi: 10.4240/wjgs.v2.i4.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65 (20):9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Guerra N, Facchetti M, Finardi A, Tiberio L, Schiaffonati L, Spano P, Missale C. Nerve growth factor regulates dopamine D(2) receptor expression in prolactinoma cell lines via p75(NGFR)-mediated activation of nuclear factor-kappaB. Mol Endocrinol. 2002;16 (2):353–366. doi: 10.1210/mend.16.2.0773. [DOI] [PubMed] [Google Scholar]
- Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156 (2):361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65 (10):4320–4333. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, Sayegh MH, Frank MH. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003;278 (47):47156–47165. doi: 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120 (1):41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank NY, Schatton T, Kim S, Zhan Q, Wilson BJ, Ma J, Saab KR, Osherov V, Widlund HR, Gasser M, Waaga-Gasser AM, Kupper TS, Murphy GF, Frank MH. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer Res. 2011;71 (4):1474–1485. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga-Kalabis M, Martinez G, Nguyen TK, Kim D, Santiago-Walker A, Roesch A, Herlyn M. Tenascin-C promotes melanoma progression by maintaining the ABCB5-positive side population. Oncogene. 2010;29 (46):6115–6124. doi: 10.1038/onc.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard SD, Laga AC, Mihm MC, Scolyer RA, Thompson JF, Zhan Q, Widlund HR, Lee CW, Murphy GF. SOX2 contributes to melanoma cell invasion. Lab Invest. 2012;92 (3):362–370. doi: 10.1038/labinvest.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason BC, Crum CP, Murphy GF. Expression patterns of MITF during human cutaneous embryogenesis: evidence for bulge epithelial expression and persistence of dermal melanoblasts. J Cutan Pathol. 2008;35 (7):615–622. doi: 10.1111/j.1600-0560.2007.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183 (4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3 (12):1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2 (1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Gros P, Ben Neriah YB, Croop JM, Housman DE. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986;323 (6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Molecular plasticity of human melanoma cells. Oncogene. 2003a;22 (20):3070–3075. doi: 10.1038/sj.onc.1206447. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003b;3 (6):411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363 (8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerter JD, Bradley P, Casillas A, Chambers D, Weiswasser B, Clements L, Gilbert S, Jiao A. Does melanoma begin in a melanocyte stem cell? J Skin Cancer. 2012;2012:571087. doi: 10.1155/2012/571087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu MY, Meier F, Herlyn M. Melanoma development and progression: a conspiracy between tumor and host. Differentiation. 2002;70 (9–10):522–536. doi: 10.1046/j.1432-0436.2002.700906.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Anderle P, Bussey KJ, Barbacioru C, Shankavaram U, Dai Z, Reinhold WC, Papp A, Weinstein JN, Sadee W. Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res. 2004;64 (12):4294–4301. doi: 10.1158/0008-5472.CAN-03-3884. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Schmid B, Eisen JS. Genetic analysis of melanophore development in zebrafish embryos. Dev Biol. 2000;225 (2):277–293. doi: 10.1006/dbio.2000.9840. [DOI] [PubMed] [Google Scholar]
- Keshet GI, Goldstein I, Itzhaki O, Cesarkas K, Shenhav L, Yakirevitch A, Treves AJ, Schachter J, Amariglio N, Rechavi G. MDR1 expression identifies human melanoma stem cells. Biochem Biophys Res Commun. 2008;368 (4):930–936. doi: 10.1016/j.bbrc.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Khong HT, Wang QJ, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J Immunother. 2004;27 (3):184–190. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20 (1):102–107. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- Kupas V, Weishaupt C, Siepmann D, Kaserer ML, Eickelmann M, Metze D, Luger TA, Beissert S, Loser K. RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells. J Invest Dermatol. 2011;131 (4):944–955. doi: 10.1038/jid.2010.377. [DOI] [PubMed] [Google Scholar]
- Laga AC, Lai CY, Zhan Q, Huang SJ, Velazquez EF, Yang Q, Hsu MY, Murphy GF. Expression of the embryonic stem cell transcription factor SOX2 in human skin: relevance to melanocyte and merkel cell biology. Am J Pathol. 2010;176 (2):903–913. doi: 10.2353/ajpath.2010.090495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laga AC, Murphy GF. Cellular heterogeneity in vertical growth phase melanoma. Arch Pathol Lab Med. 2010;134 (12):1750–1757. doi: 10.5858/2009-0394-RAR.1. [DOI] [PubMed] [Google Scholar]
- Laga AC, Zhan Q, Weishaupt C, Ma J, Frank MH, Murphy GF. SOX2 and nestin expression in human melanoma: an immunohistochemical and experimental study. Exp Dermatol. 2011;20 (4):339–345. doi: 10.1111/j.1600-0625.2011.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Herlyn M. Microenvironmental influences in melanoma progression. J Cell Biochem. 2007;101 (4):862–872. doi: 10.1002/jcb.21204. [DOI] [PubMed] [Google Scholar]
- Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5 (6):677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- Leemhuis T, Yoder MC, Grigsby S, Aguero B, Eder P, Srour EF. Isolation of primitive human bone marrow hematopoietic progenitor cells using Hoechst 33342 and Rhodamine 123. Exp Hematol. 1996;24 (10):1215–1224. [PubMed] [Google Scholar]
- Li L, Fukunaga-Kalabis M, Herlyn M. Isolation and cultivation of dermal stem cells that differentiate into functional epidermal melanocytes. Methods Mol Biol. 2012;806:15–29. doi: 10.1007/978-1-61779-367-7_2. [DOI] [PubMed] [Google Scholar]
- Lian CG, Xu Y, Ceol C, Wu F, Larson A, Dresser K, Xu W, Tan L, Hu Y, Zhan Q, Lee CW, Hu D, Lian BQ, Kleffel S, Yang Y, Neiswender J, Khorasani AJ, Fang R, Lezcano C, Duncan LM, Scolyer RA, Thompson JF, Kakavand H, Houvras Y, Zon LI, Mihm MC, Jr, Kaiser UB, Schatton T, Woda BA, Murphy GF, Shi YG. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150 (6):1135–1146. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445 (7130):843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- Lin JY, Zhang M, Schatton T, Wilson BJ, Alloo A, Ma J, Qureshi AA, Frank NY, Han J, Frank MH. Genetically determined ABCB5 functionality correlates with pigmentation phenotype and melanoma risk. Biochem Biophys Res Commun. 2013;436 (3):536–542. doi: 10.1016/j.bbrc.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunter PC, van Kilsdonk JW, van Beek H, Cornelissen IM, Bergers M, Willems PH, van Muijen GN, Swart GW. Activated leukocyte cell adhesion molecule (ALCAM/CD166/MEMD), a novel actor in invasive growth, controls matrix metalloproteinase activity. Cancer Res. 2005;65 (19):8801–8808. doi: 10.1158/0008-5472.CAN-05-0378. [DOI] [PubMed] [Google Scholar]
- Luo Y, Dallaglio K, Chen Y, Robinson WA, Robinson SE, McCarter MD, Wang J, Gonzalez R, Thompson DC, Norris DA, Roop DR, Vasiliou V, Fujita M. ALDH1A isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells. 2012;30 (10):2100–2113. doi: 10.1002/stem.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Lin JY, Alloo A, Wilson BJ, Schatton T, Zhan Q, Murphy GF, Waaga-Gasser AM, Gasser M, Stephen Hodi F, Frank NY, Frank MH. Isolation of tumorigenic circulating melanoma cells. Biochem Biophys Res Commun. 2012;402 (4):711–717. doi: 10.1016/j.bbrc.2010.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133 (4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155 (3):739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister JC, Zhan Q, Weishaupt C, Hsu MY, Murphy GF. The embryonic morphogen, Nodal, is associated with channel-like structures in human malignant melanoma xenografts. J Cutan Pathol. 2010;37(Suppl 1):19–25. doi: 10.1111/j.1600-0560.2010.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, Invernici G, Parati E, Alessandri G, La Porta CA. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43 (5):935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3 (8):e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na YR, Seok SH, Kim DJ, Han JH, Kim TH, Jung H, Lee BH, Park JH. Bone morphogenetic protein 7 induces mesenchymal-to-epithelial transition in melanoma cells, leading to inhibition of metastasis. Cancer Sci. 2009;100 (11):2218–2225. doi: 10.1111/j.1349-7006.2009.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24 (3):401–410. doi: 10.1111/j.1755-148X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307 (5710):720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445 (7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- O’Reilly-Pol T, Johnson SL. Neocuproine ablates melanocytes in adult zebrafish. Zebrafish. 2008;5 (4):257–264. doi: 10.1089/zeb.2008.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly-Pol T, Johnson SL. Melanocyte regeneration reveals mechanisms of adult stem cell regulation. Semin Cell Dev Biol. 2009;20 (1):117–124. doi: 10.1016/j.semcdb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444 (7120):761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- Pierce GB. The cancer cell and its control by the embryo. Rous-Whipple Award lecture. Am J Pathol. 1983;113 (1):117–124. [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, Morrison SJ. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18 (5):510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456 (7222):593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappa G, Fodstad O, Lorico A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008;26 (12):3008–3017. doi: 10.1634/stemcells.2008-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AL, Millward M, Pearce R, Lee M, Frank MH, Ireland A, Monshizadeh L, Rai T, Heenan P, Medic S, Kumarasinghe P, Ziman M. Markers of circulating tumour cells in the peripheral blood of patients with melanoma correlate with disease recurrence and progression. Br J Dermatol. 2013;168 (1):85–92. doi: 10.1111/bjd.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23 (35):8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445 (7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Deuchars K, Kartner N, Alon N, Trent J, Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature. 1985;316 (6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, Herlyn M. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141 (4):583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Roninson IB, Chin JE, Choi KG, Gros P, Housman DE, Fojo A, Shen DW, Gottesman MM, Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A. 1986;83 (12):4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10 (9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK. Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene. 2007;26 (28):4158–4170. doi: 10.1038/sj.onc.1210182. [DOI] [PubMed] [Google Scholar]
- Rothstein DM, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance. Immunol Rev. 2003;196:85–108. doi: 10.1046/j.1600-065x.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- Schatton T, Frank MH. Antitumor immunity and cancer stem cells. Ann N Y Acad Sci. 2009;1176:154–169. doi: 10.1111/j.1749-6632.2009.04568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451 (7176):345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, Zhou J, Hodi FS, Spagnoli GC, Murphy GF, Frank MH. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70 (2):697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaak M, Schmidt P, Bangard C, Kurschat P, Mauch C, Abken H. Regression of metastatic melanoma in a patient by antibody targeting of cancer stem cells. Oncotarget. 2012;3 (1):22–30. doi: 10.18632/oncotarget.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C, Abken H. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc Natl Acad Sci U S A. 2011;108 (6):2474–2479. doi: 10.1073/pnas.1009069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor EA, Meltzer PS, Schatteman GC, Gruman LM, Hess AR, Kirschmann DA, Seftor RE, Hendrix MJ. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Crit Rev Oncol Hematol. 2002;44 (1):17–27. doi: 10.1016/s1040-8428(01)00199-8. [DOI] [PubMed] [Google Scholar]
- Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V, Hendrix MJ. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61 (17):6322–6327. [PubMed] [Google Scholar]
- Shakhova O, Sommer L. Testing the cancer stem cell hypothesis in melanoma: The clinics will tell. Cancer Lett. 2013;338 (1):74–81. doi: 10.1016/j.canlet.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Sharma BK, Manglik V, Elias EG. Immuno-expression of human melanoma stem cell markers in tissues at different stages of the disease. J Surg Res. 2010;163 (1):e11–15. doi: 10.1016/j.jss.2010.03.043. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432 (7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Brooks DM, Tumas DB. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: in vivo expansion of stem cell phenotype but not function. Blood. 1995;85 (4):1006–1016. [PubMed] [Google Scholar]
- Spangrude GJ, Johnson GR. Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990;87 (19):7433–7437. doi: 10.1073/pnas.87.19.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003;100 (5):2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187 (8):1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416 (6880):542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2 (6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Thies A, Mangold U, Moll I, Schumacher U. PAS-positive loops and networks as a prognostic indicator in cutaneous malignant melanoma. J Pathol. 2001;195 (5):537–542. doi: 10.1002/path.988. [DOI] [PubMed] [Google Scholar]
- Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12 (8):925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- Turner C, Kohandel M. Investigating the link between epithelial-mesenchymal transition and the cancer stem cell phenotype: A mathematical approach. J Theor Biol. 2010;265 (3):329–335. doi: 10.1016/j.jtbi.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Ueda K, Cornwell MM, Gottesman MM, Pastan I, Roninson IB, Ling V, Riordan JR. The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem Biophys Res Commun. 1986;141 (3):956–962. doi: 10.1016/s0006-291x(86)80136-x. [DOI] [PubMed] [Google Scholar]
- Van der Bliek AM, Baas F, Ten Houte de Lange T, Kooiman PM, Van der Velde-Koerts T, Borst P. The human mdr3 gene encodes a novel P-glycoprotein homologue and gives rise to alternatively spliced mRNAs in liver. EMBO J. 1987;6 (11):3325–3331. doi: 10.1002/j.1460-2075.1987.tb02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaft DW, Seftor RE, Seftor EA, Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Griffioen AW, Hendrix MJ. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J Natl Cancer Inst. 2004;96 (19):1473–1477. doi: 10.1093/jnci/djh267. [DOI] [PubMed] [Google Scholar]
- van Houdt IS, Sluijter BJ, Moesbergen LM, Vos WM, de Gruijl TD, Molenkamp BG, van den Eertwegh AJ, Hooijberg E, van Leeuwen PA, Meijer CJ, Oudejans JJ. Favorable outcome in clinically stage II melanoma patients is associated with the presence of activated tumor infiltrating T-lymphocytes and preserved MHC class I antigen expression. Int J Cancer. 2008;123 (3):609–615. doi: 10.1002/ijc.23543. [DOI] [PubMed] [Google Scholar]
- van Kempen LC, Meier F, Egeblad M, Kersten-Niessen MJ, Garbe C, Weidle UH, Van Muijen GN, Herlyn M, Bloemers HP, Swart GW. Truncation of activated leukocyte cell adhesion molecule: a gateway to melanoma metastasis. J Invest Dermatol. 2004;122 (5):1293–1301. doi: 10.1111/j.0022-202X.2004.22531.x. [DOI] [PubMed] [Google Scholar]
- Vasquez-Moctezuma I, Meraz-Rios MA, Villanueva-Lopez CG, Magana M, Martinez-Macias R, Sanchez-Gonzalez DJ, Garcia-Sierra F, Herrera-Gonzalez NE. ATP-binding cassette transporter ABCB5 gene is expressed with variability in malignant melanoma. Actas Dermosifiliogr. 2010;101 (4):341–348. doi: 10.1016/j.ad.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422 (6934):901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422 (6934):897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Warso MA, Maniotis AJ, Chen X, Majumdar D, Patel MK, Shilkaitis A, Gupta TK, Folberg R. Prognostic significance of periodic acid-Schiff-positive patterns in primary cutaneous melanoma. Clin Cancer Res. 2001;7 (3):473–477. [PubMed] [Google Scholar]
- Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D, Siegel J, O’Day SJ. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15 (17):5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- Wilson BJ, Schatton T, Zhan Q, Gasser M, Ma J, Saab KR, Schanche R, Waaga-Gasser AM, Gold JS, Huang Q, Murphy GF, Frank MH, Frank NY. ABCB5 identifies a therapy-refractory tumor cell population in colorectal cancer patients. Cancer Res. 2011;71 (15):5307–5316. doi: 10.1158/0008-5472.CAN-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O’Day SJ, Lebbe C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11 (2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- Wolf NS, Kone A, Priestley GV, Bartelmez SH. In vivo and in vitro characterization of long-term repopulating primitive hematopoietic cells isolated by sequential Hoechst 33342-rhodamine 123 FACS selection. Exp Hematol. 1993;21 (5):614–622. [PubMed] [Google Scholar]
- Yang J, Price MA, Li GY, Bar-Eli M, Salgia R, Jagedeeswaran R, Carlson JH, Ferrone S, Turley EA, McCarthy JB. Melanoma proteoglycan modifies gene expression to stimulate tumor cell motility, growth, and epithelial-to-mesenchymal transition. Cancer Res. 2009;69 (19):7538–7547. doi: 10.1158/0008-5472.CAN-08-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416 (6880):545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131 (6):1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Zhang S, Guo H, Zhang D, Zhang W, Zhao X, Ren Z, Sun B. Microcirculation patterns in different stages of melanoma growth. Oncol Rep. 2006;15 (1):15–20. [PubMed] [Google Scholar]
- Zhang S, Li M, Gu Y, Liu Z, Xu S, Cui Y, Sun B. Thalidomide influences growth and vasculogenic mimicry channel formation in melanoma. J Exp Clin Cancer Res. 2008;27:60. doi: 10.1186/1756-9966-27-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7 (9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- Zijlmans JM, Visser JW, Kleiverda K, Kluin PM, Willemze R, Fibbe WE. Modification of rhodamine staining allows identification of hematopoietic stem cells with preferential short-term or long-term bone marrow-repopulating ability. Proc Natl Acad Sci U S A. 1995;92 (19):8901–8905. doi: 10.1073/pnas.92.19.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]