Significance
All cells age and do so in relation to how many times a cell divides (replicative aging) and how long a nondividing cell can live (chronological aging). Bakers’ yeast has been used to study both, but because yeast divides when nutrient levels permit, the genetics of its chronological lifespan has only been studied under calorie restriction, mimicked by starvation. Because many terminally differentiated animal cells are long-lived and rarely starve, we developed a model of cell lifespan under calorie-unrestricted conditions. When encapsulated and fed ad libitum, yeast goes into cell cycle arrest, continues to be metabolically active, and remains viable for weeks, offering a new experimental paradigm to study chronological lifespan in the absence of calorie restriction.
Keywords: cell longevity, Ca-alginate encapsulation
Abstract
Studies of replicative and chronological lifespan in Saccharomyces cerevisiae have advanced understanding of longevity in all eukaryotes. Chronological lifespan in this species is defined as the age-dependent viability of nondividing cells. To date this parameter has only been estimated under calorie restriction, mimicked by starvation. Because postmitotic cells in higher eukaryotes often do not starve, we developed a model yeast system to study cells as they age in the absence of calorie restriction. Yeast cells were encapsulated in a matrix consisting of calcium alginate to form ∼3 mm beads that were packed into bioreactors and fed ad libitum. Under these conditions cells ceased to divide, became heat shock and zymolyase resistant, yet retained high fermentative capacity. Over the course of 17 d, immobilized yeast cells maintained >95% viability, whereas the viability of starving, freely suspended (planktonic) cells decreased to <10%. Immobilized cells exhibited a stable pattern of gene expression that differed markedly from growing or starving planktonic cells, highly expressing genes in glycolysis, cell wall remodeling, and stress resistance, but decreasing transcription of genes in the tricarboxylic acid cycle, and genes that regulate the cell cycle, including master cyclins CDC28 and CLN1. Stress resistance transcription factor MSN4 and its upstream effector RIM15 are conspicuously up-regulated in the immobilized state, and an immobilized rim15 knockout strain fails to exhibit the long-lived, growth-arrested phenotype, suggesting that altered regulation of the Rim15-mediated nutrient-sensing pathway plays an important role in extending yeast chronological lifespan under calorie-unrestricted conditions.
Unicellular microbes senesce and die (for reviews, see refs. 1, 2) and can therefore be used as models to study the genetic control of lifespan in their multicellular relatives. Bakers’ yeast cells have a replicative lifespan (RLS), the number of times a mother cell undergoes mitosis (3), and a chronological lifespan (CLS), the length of time a nondividing cell remains alive (4). Under standard conditions, wild and laboratory yeast exhibit characteristic and highly variable RLS (5) and CLS (6), indicating that each trait has a genetic component. Indeed, we now know that genes such as SIR2, CYR1, and the protein kinase SCH9 influence yeast RLS and that each has a functional homolog in higher eukaryotes (7). Although yeast CLS has been reported to vary over a fourfold range among wild and laboratory strains (6), to date estimates of this parameter have been based on starvation as a means to keep cells from dividing. Perhaps not surprisingly, this approach has identified genes acting in stress-response pathways as regulators of cell lifespan, notably TPK and BCY1, which together encode cAMP-dependent protein kinase TOR, which encodes target-of-rapamycin protein kinase and SCH9 (4, 8–10). One virtue of the apparent need for this approach is that calorie restriction (CR) promotes longevity in all species studied (11).
However, many terminally differentiated metazoan cell types rarely starve and some, such as neurons, can consume a disproportionate share of an animal’s lifetime energy budget (12). Therefore, it is imperative to develop a different yeast model to study CLS, one in which nutrients are plentiful, but cell division ceases and metabolism remains highly active. Such a model would enable yeast to better serve as a surrogate for postmitotic cells in higher eukaryotes, including humans. To model CLS under nutrient-replete conditions, we eschewed the traditional approach of culturing yeast as free-floating, planktonic cells in liquid media, in favor of encapsulating cells in a matrix made of calcium alginate. We reasoned that such a matrix would more nearly approximate the physical milieu typically experienced by postmitotic metazoan cells. Previous studies have shown that relative to planktonic cells, encapsulated (i.e., immobilized) yeast exhibits increased glycolytic flux but greatly diminished production of biomass (13). These observations led us to investigate the possibility that encapsulated yeast could be used as a tool to study CLS in the absence of CR.
Immobilized yeast has been used for decades in brewing and bioethanol production (14, 15), and indeed virtually all studies of immobilized yeast have been aimed at optimizing its use in industrial processes. However, to the best of our knowledge, no study to date has brought the tools of functional genomics to bear on understanding the mechanism(s) by which metabolism might be uncoupled from reproduction in such cells, much less the consequences of this uncoupling for yeast’s life history. Here, for the first time according to the authors, we compare global gene expression of immobilized yeast to that of planktonic cells, from the perspective of yeast chronological aging. We find that nondividing yeast encapsulated in a permeable matrix and fed ad libitum resembles in key respects postmitotic metazoan cells (16, 17), offering an alternative paradigm for studying mechanisms that control CLS in all eukaryotes.
Results
To provide context for transcriptional profiling, we first compared the population structure and physiology of well-fed immobilized yeast to that of planktonic yeast growing at different rates and nutrient levels. In batch culture, log-phase planktonic cells experience excess nutrients and divide rapidly, whereas stationary-phase cells experience progressive nutrient depletion and ultimately undergo cell cycle arrest; in a chemostat, planktonic cells are nutrient-limited and divide at a rate fixed by the dilution rate (18). Yeast was cultured in Synthetic Minimal (SM) medium (19) using glucose as the sole carbon source in batch, in chemostats, and in immobilized cell reactors (ICRs) (for reactor schematic and photo, see Fig. S1 A and B). Because ICR yeast produces copious CO2, purging the bioreactor of oxygen, planktonic cells were also cultured under anoxic conditions, unless otherwise indicated. Unlike planktonic cells in batch, immobilized yeast cells were fed continuously: every 48 h as glucose fell below 5 g⋅L−1, the ICR feed was replaced with fresh media containing 100 g⋅L−1 glucose and an excess of micronutrients (Fig. S2).
Encapsulated Yeast Stops Dividing Under Nutrient-Sufficient Conditions.
Compared with batch- and chemostat-grown cells, the density of immobilized yeast cultures plateaued after 6–7 cell divisions. Planktonic batch cultures were inoculated at a starting density of ∼106 cells⋅mL−1, attained midlog phase 12 h postinoculation, then entered the stationary phase (∼108 cells⋅mL−1) 24 h later. Cells in chemostats reached the steady state (∼108 cells⋅mL−1) 24 h postinoculation, then continued to divide at the set dilution rate. Yeast encapsulated in ∼3 mm calcium alginate beads (∼2.3 × 106 cells⋅bead−1) and packed into ICRs underwent multiple cell divisions in the first 72 h of culture, forming microcolonies (Fig. S1D). Thereafter, mean cell density remained constant at ∼108 cells⋅bead−1. To discern replicative age structure in planktonic and immobilized populations, budding and bud scar indices were estimated. After 5 d, immobilized yeast populations had fourfold fewer budded cells than planktonic yeast in batch culture (4.3% vs. 17.2%) (Fig. S3A). At that time, a majority (∼70%) of the immobilized populations consisted of virgin, unbudded cells, and even older mothers having >2 bud scars were unbudded (Fig. S3B), further indicating that immobilized yeast proliferates only during the first few days of culture, then ceases to divide.
Nondividing, Well-Fed Yeast Shows No Loss in Viability During Prolonged ICR Culture.
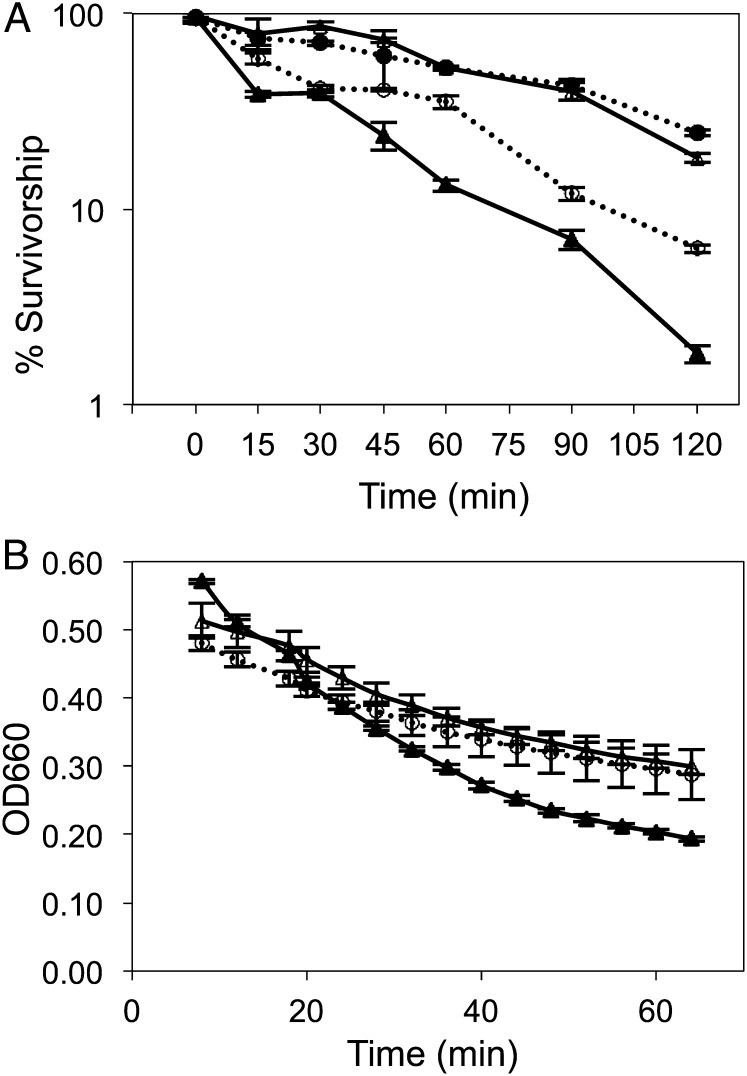
Yeast CLS is conventionally reported as the survivorship of nondividing cells in extended batch culture, estimated by their reproductive potential—that is, their ability to form colonies when transferred to solid medium (8). Planktonic cells in glucose-limited chemostats divide as a function of dilution rate (18), and under these conditions yeast showed >95% survivorship. In discontinuous batch culture, yeast quickly lost viability (Fig. 1A): after 5 d of anaerobic batch culture in SM medium, only 27% of cells could form colonies, and by day 10 and day 17, only 16% and ∼0.1% could do so, respectively. When yeast was batch-cultured under aerobic conditions, the decline in survivorship began ∼5 d later because these yeast could respire fermentation products following exhaustion of glucose. After 17 d, however, only ∼5% of cells in aerobic SM cultures could still produce colonies on solid medium, a value consistent with previous reports (20–22). By contrast, >95% of immobilized yeast remained reproductively competent over a 17-d experimental time course (Fig. 1A), indicating that division-arrested, metabolically active yeast cells are chronologically long-lived.
Fig. 1.
(A) Survivorship of immobilized yeast, anaerobic planktonic (freely suspended) yeast, and aerobic planktonic yeast over 17 d culture. Survivorship was estimated as the ratio of CFUs on rich solid medium relative to total cell number estimated by hemocytometry. Each experiment was repeated twice; error bars represent 1 SD from the mean value. (B) Glycogen content of immobilized and planktonic yeast (milligram of glucose equivalents per gram of dry weight biomass). Each experiment was repeated twice; error bars represent 1 SD (closed circle, immobilized, anaerobic; open triangle, planktonic, aerobic; closed triangle, planktonic, anaerobic).
To compare cell cycle status and DNA integrity in immobilized and planktonic yeast, we stained cells with SYTOX Green and performed flow cytometry (23). SYTOX fluorescence intensity is a direct measure of cellular DNA content and thus can be used to track progress through the cell cycle. Consistent with budding index data, some planktonic yeast in anaerobic batch culture were still dividing at day 5, as is evident from the second histogram peak corresponding to gap 2 (G2) in the cell cycle (Fig. 2A). The overall leftward shift in fluorescence intensity in starving planktonic populations (e.g., Fig. 2A, 17-d) may be evidence of DNA degradation (24); indeed, it has been argued that such cells become apoptotic (25). By contrast, flow cytometry confirmed that immobilized yeast fed ad libitum ceased replicating by day 5, was in G1-like arrest, and was not apoptotic (Fig. 2B).
Fig. 2.
Cell cycle status of (A) planktonic and (B) immobilized yeast through 17 d cultures. DNA content of planktonic batch cultured yeast and immobilized yeast released from its alginate matrix was assayed by flow cytometry of ethanol-fixed, SYTOX Green-stained cells. SYTOX Green is measured in mean fluorescence intensity. Red and black lines indicate fluorescence profiles of independent biological replicates. Insert displays fluorescence profiles of actively dividing planktonic haploid and diploid yeast, which display prominent G1 and G2 peaks indicative of mitotic activity.
Nondividing Immobilized Yeast Exhibits High Fermentative Capacity and Hyperaccumulates Glycogen.
Even though ICR yeast ceased dividing, extracellular glucose and ethanol concentrations continuously changed throughout each experiment (Fig. S2B), indicating that these cells continued to be metabolically highly active. As the glucose concentration fell only briefly below 5 g⋅L−1 and the ethanol concentration never exceeded 50 g⋅L−1, immobilized yeast experienced nutrient-sufficient conditions with little or no ethanol inhibition. In triplicate short-term (72 h) experiments, ethanol yield on glucose by immobilized cells (0.414 ± 0.012 g⋅g−1) was greater than that of log-phase planktonic cells (0.396 ± 0.007 g⋅g−1) (ANOVA, P < 0.05), whereas biomass yield on glucose was much less (0.006 ± 0.005 g dry wt ⋅g−1 vs. 0.027 ± 0.001 g dry wt ⋅g−1). Ethanol productivity per gram of cell biomass was therefore nearly fivefold greater in immobilized than in planktonic cells, consistent with previous reports of very high glycolytic flux in immobilized yeast (26). Thus, although immobilized well-fed yeast ceases to divide, it retains high fermentative capacity.
Plentiful glycogen provides yeast with survival and reproductive advantages (27). Indeed, CLS has been dramatically extended in starving planktonic cells by periodically exposing them to glucose concentrations that are sufficient to replenish glycogen reserves, but insufficient to support reproduction (28). Glycogen content in immobilized yeast (Fig. 1B) greatly exceeded that observed in either starving planktonic cells or in planktonic cells grown anaerobically in glucose-limited chemostats. Glycogen levels rose from 0.7 mg of glucose equivalents per gram of dry wt at day 1 to 4 mg of glucose equivalents at day 17, a >fivefold change. The steep increase in cells’ glycogen content commenced after day 3, the point at which immobilized yeast ceased to divide (Fig. 2B), suggesting that these cells reallocate resources to storage that would otherwise be directed toward growth and reproduction. No comparable increase was detected in yeast’s other major storage carbohydrate, trehalose, whose increase under aerobic conditions reportedly protects calorie-restricted planktonic cells from oxidative damage (29).
In summary, population, physiological, and cytometric data demonstrate that metabolism and reproduction are uncoupled in encapsulated, continuously fed yeast and that such cells remain viable for extended periods of time, features reminiscent of many types of postmitotic metazoan cells.
Patterns of Gene Expression in Immobilized Yeast Markedly Differ from Those in Planktonic Yeast.
To better understand how ICR yeast uncouples metabolism from reproduction and remains viable in prolonged culture, we performed microarray analysis using the Affymetrix platform. GeneSpring was first used to analyze transcript levels in immobilized cells and planktonic cells grown either in chemostat or in batch culture, the latter harvested at midlog and stationary phases. Overall, 4,308 of 5,804 genes assayed were found to be differentially expressed (Fig. S4A). Considering only immobilized yeast, 2,375 transcripts were differentially expressed, although most of these differences could be attributed to using stationary-phase planktonic cells for encapsulation. Immobilized yeast at day 0 was indistinguishable from stationary-phase yeast for >90% of transcripts assayed. Day 10 immobilized cells were harvested just before the medium was replenished, which did not change the sign of but rather exaggerated expression changes seen after day 3. Pairwise volcano plot comparisons showed that only 79 genes varied by more than twofold over the course of 2 wk of continuous immobilized culture (Fig. S4B). Over this interval, immobilized yeast consumed ∼1.2 kg of glucose with little or no cell division.
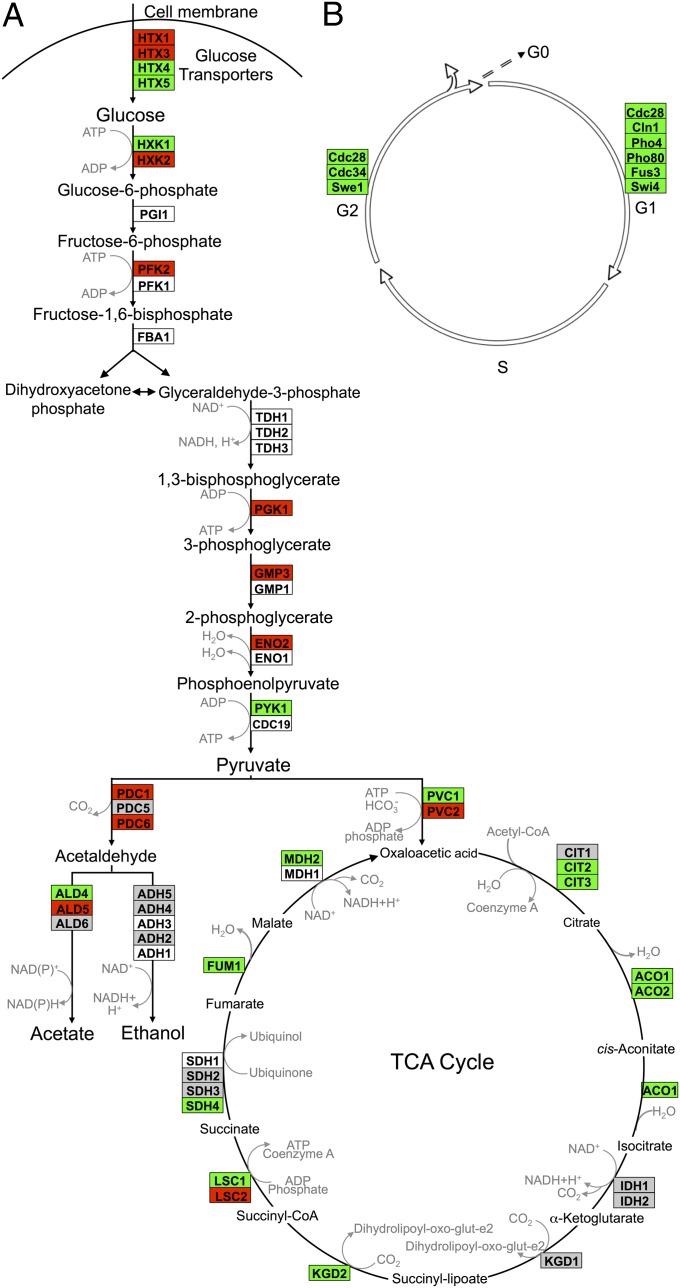
Next, the GeneSpring dataset was imported into GenMapp and criteria set to uncover genes differentially expressed by twofold or greater between immobilized cells and any of the planktonic states. Day 0 immobilized cells were excluded from GenMapp analyses, as their profile was essentially identical to that of planktonic stationary-phase yeast, as were day 1 immobilized cells, which were still dividing. GenMapp data were overlayed onto Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway maps for glycolysis, the tricarboxylic acid (TCA) cycle, and the yeast cell cycle. Consistent with observations that continuously fed immobilized yeast is highly fermentative, anaerobic, and not dividing (30), we found that many glycolytic genes, such as HXK2, PFK2, and PGK, were expressed highly, whereas TCA cycle genes, such as CIT1 and ACO1, were expressed at low levels (Fig. 3A). Strikingly, multiple genes whose products help drive transitions between G1, S, and G2 phases of the cell cycle were down-regulated relative to every planktonic state assayed (Fig. 3B and the full KEGG cell cycle pathway in Fig. S5). Low transcript levels of cyclin CLN1 and master cyclin-dependent kinase CDC28 were consistent with flow cytometric and demographic analyses indicating that immobilized yeast is in a state of G1-like arrest.
Fig. 3.
Comparison of gene expression in immobilized relative to planktonic yeast: (A) intermediary metabolism and (B) cell cycle. Differentially expressed transcripts identified using GeneSpring were overlayed onto the S. cerevisiae KEGG biological pathway maps using GenMapp software. Detailed KEGG pathway cell cycle results are presented in Fig. S5. Transcript levels in immobilized well-fed yeast for days 3, 5, 10, and 17 were averaged across replicate experiments. Red indicates instances where those values were at least twofold greater in immobilized cells than in planktonic cells. Green indicates instances where those values were at least twofold less in immobilized cells than in planktonic cells. White indicates that the gene was not found among the significant gene list identified earlier using GeneSpring. Gray denotes instances where transcript levels differed by less than twofold.
Two-class SAM reveals immobilization-specific changes in gene expression.
A two-class Significance Analysis of Microarrays (SAM) (31) was undertaken by grouping batch-log, batch-stationary, and chemostat expression data into a single planktonic group, then comparing their expression profiles to that of an immobilized group consisting of days 1 through 17. Although planktonic cells in chemostat, batch-log, and batch-stationary cultures obviously differ in their nutritional states, growth rates, and gene expression profiles (Fig. S4A), comparing their aggregated expression patterns to those of immobilized cells uncovered classes of genes whose altered regulation could be attributed to immobilization per se. SAM identified 379 significantly up-regulated and 204 down-regulated genes in immobilized relative to planktonic yeast (Table S1). To discern its dominant features, the two-class SAM dataset was further interrogated by K-means clustering and by Gene Ontology (GO) analysis using Database for Annotation, Visualization, and Integrated Discovery (DAVID).
K-means clustering indicates that immobilizing yeast favors cell wall remodeling, anaerobic fermentation, and cell cycle arrest.
Among genes up-regulated in immobilized but not in planktonic cells, K-means clustering associated genes whose products act in spore wall assembly (Fig. S6A), whose products localize to the cell membrane (Fig S6B), and that lie near telomeres (Fig. S6C). Conspicuously up-regulated are subtelomeric loci in the PAU seripauperin gene family, whose functions are unknown, and subtelomeric loci in the TIR cell wall mannoprotein gene family; both families have been described as being activated during anaerobic alcoholic fermentation (32, 33). Down-regulated clusters illuminate how immobilization uncouples reproduction from metabolism. One cluster (Fig. S6D) consisted of genes in nucleotide and amino acid synthesis, and the cell cycle, notably SWI5, a transcription factor that controls genes expressed at the M/G1 boundary and in late G1 (34). Another (Fig. S6E) included genes that act in DNA replication, chromosome segregation, and the spindle checkpoint, among them the forkhead family transcription factor, FKH1, which plays a key role in cell cycle progression at G2/M (35). We also observed down-regulation of RAS1 (Fig. S6E), a G protein essential for cell proliferation linked to the PKA-activating adenylate cyclase pathway (36). A third cluster of down-regulated transcripts (Fig. S6F) included those whose products play essential roles in iron assimilation and metabolism and in cytokinesis (e.g., HOF1, UTR2) (37, 38).
GO analysis further confirms that immobilized yeast remodels its cell wall, up-regulates glycolysis, and ceases to divide.
The two-class SAM dataset was further analyzed using DAVID, which clusters functionally similar GO terms into groups ranked by enrichment scores, with a score >1.3 being considered statistically significant (39). This procedure uncovered seven gene clusters that were significantly up-regulated in immobilized but not in planktonic yeast—cell wall (enrichment score, 6.3), cell membrane (3.5), spore wall (3.4), sporulation (2.5), zinc-finger proteins (2.0), GPI anchor (1.8), and thiamine biosynthesis (1.8)—as well as three clusters that were significantly down-regulated—cell cycle (2.0), chromosome segregation (1.6), and ribosome biogenesis (1.3) (Table S2).
Of 50 genes up-regulated in the cell wall (Fig. 4A) and spore wall (Fig. S7) clusters, 24 are involved in cell wall biogenesis. Altered expression of so many cell wall remodeling transcripts suggests that immobilization induces the Cell Wall Integrity (CWI) pathway. In this regard, RPI1 up-regulation in immobilized but not in planktonic cells is especially noteworthy (Fig. S7C). Rpi1 acts as an antagonist to the RAS–cAMP pathway and prepares yeast for entry into the stationary phase by inducing transcription of genes whose products fortify the cell wall (40). The TIR and DAN gene families, which encode cell wall mannoproteins and are induced under anaerobic conditions (41), were conspicuously up-regulated in ICRs. However, as planktonic yeast was also cultured under anaerobic conditions, and TIR and DAN genes were only up-regulated in immobilized cells, these genes must be specifically induced in response to being encapsulated and maintained in ICRs.
Fig. 4.
Functional annotation clustering using DAVID of gene expression differences in immobilized relative to planktonic yeast. Groups with significant (>1.3) enrichment scores include (A) cell wall (score, 6.3) and (B) sporulation (2.5).
Dramatically increased expression of essential genes in ascospore biogenesis was observed in immobilized, but not in planktonic, yeast (Fig. 4A). These included SPR1 and SPS22, whose products are required to synthesize the spore wall’s β-glucan layer (42); SPR3 and SPO19, which respectively encode meiosis-specific septin and prospore proteins (43); and CRR1, a glucoside hydrolase essential for spore wall assembly. Induction of these genes indicated overlap between the transcriptional programs of well-fed, nondividing vegetative cells and spores. Indeed, most genes belonging to the sporulation cluster (Fig. 4B) encode spore membrane and spore wall proteins—for example, OSW1—which is required for construction of the outer spore wall. The overexpression in immobilized haploid yeast of genes involved in spore formation was unexpected, as our strain carries mutations in HO and MAT that prevent mating-type switching. Moreover, flow cytometry showed that ICR yeast remained haploid; thus, mating, diploidization, and meiosis did not occur en masse in ICRs. However, the sporulation cluster also showed that immobilized yeast had elevated transcript levels for MSN4 and RIM15 that encode, respectively, a stress-responsive transcription factor and a glucose-repressible protein kinase required for nutrient signaling, especially during the onset of the stationary phase. RIM15 was originally identified in a genetic screen for mutants having a reduced ability to undergo meiosis (44), and was later shown to transiently induce early meiotic genes (45). Thus, RIM15 up-regulation may help explain induction of certain meiotic genes in well-fed, nondividing haploid yeast.
Zinc-finger proteins formed another GO cluster (Fig. S7B), members of which include MSN4, which acts in concert with MSN2 to activate transcription of stress-resistance genes (46). Significantly, the cytoplasmic-to-nuclear translocation of MSN2/MSN4 gene products depends not only on stress and on PKA (47) but also on Rim15 (48). Although TPK/BCY1 was not up-regulated in immobilized yeast, both RIM15 and MSN4 were (Fig. 4B), showing that immobilization per se activates these key stress response regulators (46, 49). Two other zinc-finger proteins in this cluster, NRG2 and MIG2, are transcription factors that negatively regulate expression of glucose-repressible genes. Up-regulation of NRG2, MIG2, and MIG3 in immobilized cells (Fig. S7B) likely reflects high glucose levels in their external milieu. Immobilization was also correlated with increased expression of transcription factor TYE7 (Table S1). Although not a zinc-finger protein, TYE7 binds E-boxes of glycolytic genes and contributes to their activation (50). Thus, its differential expression helps to explain elevated levels of ENO2, PGK1, and GPM1 (Fig. 3A), whose products help support immobilized yeast’s high rate of fermentation. Another gene of interest in the zinc-finger cluster is YPR015C, which encodes a protein of unknown function whose overexpression leads to cell cycle delay or arrest (51). Increased expression of YPR015C may be yet another factor contributing to cell cycle arrest in highly fermentative ICR yeast.
Consistent with other analyses, cell cycle (Fig. S8A) and chromosome segregation (Fig. S8B) clusters were both down-regulated in immobilized yeast, including genes such as HOF1 and BUD4, which encode structural proteins required for axial budding (52), and ACE2, which encodes a transcription factor required for cytokinesis (53). Expression of 22 genes related to ribosome biogenesis (Fig. S8C) was decreased in immobilized relative to planktonic yeast. As ribosomal gene expression is directly proportional to growth rate (54), low transcript levels here may result from G1-like arrest (54).
To validate our microarray results, we performed quantitative reverse-transcription PCR (qRT-PCR) to assay transcript levels for three key regulatory genes (RIM15, MSN4, and TYE7) and one constitutively expressed gene, ACT1, which encodes the structural protein actin. qRT-PCR results for all three regulatory genes, normalized to ACT1 expression, were consistent with array results, with correlation coefficients ranging from 0.68 to 0.93. If anything, microarray results tended to underestimate the magnitude of change in expression (Fig. S9).
Immobilized Yeast Is Thermotolerant and Zymolyase Resistant.
To test whether up-regulation of cell wall remodeling genes (e.g., CRR1 and RPI1) and stress response regulators (e.g., RIM15 and MSN4) made immobilized yeast more stress resistant than planktonic yeast, we tested its susceptibility to heat shock and zymolyase digestion. Thermal tolerance is often used as a proxy for stress resistance (55), which in yeast has been associated with CLS extension (56). Rapidly proliferating log-phase yeast is known to be more heat-shock sensitive than either growth-arrested (57) or slowly dividing yeast (58). We therefore compared the thermotolerance of cells either confined to or released from their calcium–alginate matrix to planktonic cells in the log or stationary phase by exposing each to 48 °C for 2 h (Fig. 5A). Immobilized and stationary-phase planktonic yeasts were significantly more heat-shock tolerant than planktonic yeast in the log phase (P = 0.00964 and P = 0.00637, respectively, by two-tailed t test). Thermal tolerance in immobilized cells was due not only to their physiological status, but also to the presence of the alginate matrix, as evidenced by the intermediate survivorship of cells released from beads.
Fig. 5.
(A) Heat shock tolerance and (B) zymolyase resistance in immobilized versus planktonic yeast. Survivorship following exposure to 48 °C was estimated as the ratio of CFUs to total cell number enumerated by hemocytometry. Digestion of the cell wall was followed by a change in optical density at 660 nm. Open triangle, planktonic stationary phase; closed triangle, planktonic log phase; closed circle, immobilized cells in calcium–alginate matrix; open circle, immobilized cells released from the matrix.
Yeast thermal tolerance is strongly correlated with changes in cell wall structure that occur in planktonic cells following sporulation or entry into the stationary phase (57). To test whether immobilized yeast underwent similar changes in cell wall structure, as suggested by its increased transcription of cell wall and sporulation genes, we assayed its resistance to zymolyase (Fig. 5B), a glucanase–protease suspension that enables the state and integrity of the yeast cell wall to be assessed qualitatively. As expected (59), midlog-phase planktonic cells were most susceptible, lysing at a higher rate (Vmax = –11.7) and to a greater extent over the experimental time course. Late stationary planktonic and day 5 immobilized yeast lysed at similar rates (Vmax = –5.49 and –5.81, respectively) and to the same extent. Immobilized cells were much more susceptible to zymolyase digestion on days 1 and 3, when they were still dividing, than on days 5, 10, and 17, when they were not.
Rim15 Helps Mediate Cell Cycle Arrest in Immobilized Yeast.
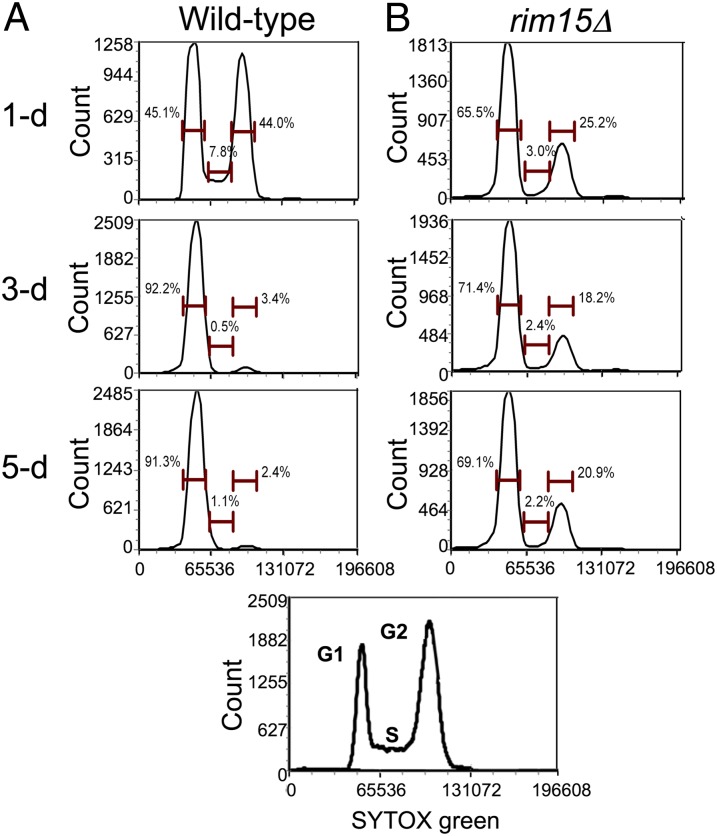
Immobilized yeast undergoes cell cycle arrest in the presence of excess nutrients. Because RIM15 was up-regulated in immobilized but not in planktonic cells, because RIM15 can play a role in cell cycle arrest, because multiple nutrient-sensing pathways converge on Rim15p (48), and because it promotes chronological longevity in calorie-restricted yeast (8, 10), we hypothesized that this master regulator played a key role in uncoupling metabolism from reproduction. To test this hypothesis, we immobilized a rim15Δ strain and cultured it in ICRs for 5 d under the same conditions as wild type. Flow cytometry of DNA content revealed that unlike wild type, rim15Δ cells exhibit a pronounced G2 peak at later time points, indicating that they continued to divide (Fig. 6). Moreover, at day 5, only ∼25% of the rim15Δ cells were viable, compared with >90% of immobilized wild-type cells. We therefore conclude that RIM15 helps mediate cell cycle arrest and stress resistance in well-fed immobilized yeast, attributes that contribute to its extraordinary chronological longevity.
Fig. 6.
Flow cytometry of immobilized wild-type and rim15Δ yeast over 5 d of continuous culture: (A) wild type and (B) rim15Δ. Immobilized wild-type and rim15Δ cells were released from their calcium–alginate matrix, stained with SYTOX Green, and analyzed by flow cytometry. SYTOX Green is measured in mean fluorescence intensity. Insert displays fluorescence profiles of actively dividing haploid and diploid yeast, which display prominent G1 and G2 peaks indicative of mitotic activity.
Discussion
CLS in yeast is measured as the survivorship of nondividing cells over time. Because yeast usually grows and divides when nutrients permit, to date CLS has only been estimated under starvation or severely nutrient-limited conditions (9, 22, 60). Thus, the CLS of division-arrested yeast under nutrient-replete conditions is completely unknown. Here, we describe a model system where calcium alginate-encapsulated yeast are placed in bioreactors and fed ad libitum. Under these calorie-unrestricted conditions, yeast ceases to divide, remains metabolically active, and exhibits no decline in viability over the course of 2 wk of continuous culture. Our findings are consistent with earlier studies suggesting that immobilized cells might be stress-resistant (61, 62) and long-lived (63). Furthermore, survivorship of nondividing immobilized yeast exceeds that reported for calorie-restricted cells, even that of several mutants known to prolong CLS (10, 64).
Growth-Arrested Immobilized Yeast Is Highly Fermentative.
Because immobilized yeast vigorously ferments glucose during prolonged culture, it has been used for ethanol production, reportedly attaining, relative to planktonic cells, >10-fold increases in volumetric ethanol productivity at high substrate concentrations (65). However, whereas high glycolytic flux typically triggers expression of genes required to pass through G1 (66), immobilized fermentative yeast does not respond to this signal. Indeed, RAS1, which encodes a component of the RAS/cAMP/PKA glucose-signaling pathway, is down-regulated in immobilized relative to planktonic cells, and the gene encoding the transcriptional regulator Rpi1, which acts antagonistically to RAS/cAMP/PKA, is up-regulated (Fig. S6E and Fig. S7C). Because RPI1 also regulates the CWI pathway, helping to prepare cells for entry into the stationary phase (40), elevated expression of this gene in highly fermentative ICR yeast may contribute to its heat-shock- and zymolyase-resistant phenotype.
In starved batch cultures, yeast CLS depends on respiration, not fermentation (67), as long-lived quiescent cells respire nonfermentable carbon that arises from autophagous nonquiescent cells (68). Immobilized yeast under calorie-unrestricted conditions exhibits long CLS in the absence of respiration, which is precluded in ICRs by glucose repression and low O2 tension. Interestingly, long-lived, planktonic tor, sch9, and ras mutants exhibit a metabolic shift away from respiration toward fermentation and increase glycerol production (69). Although encapsulated well-fed yeast is also fermentative and produces glycerol, our experiments were performed using wild-type cells under calorie-unrestricted conditions, not planktonic mutants under severe CR. Finally, immobilized fermentative yeast also produces acetate, which has been implicated in causing planktonic yeast to senesce under standard conditions (70). However, in ICR experiments, the “acetate effect” does not come into play, as the medium is exchanged frequently and its pH is controlled at 5.5.
Why Does Immobilized Yeast Cease Reproducing in the Presence of Excess Nutrients?
Possible causes for cell cycle arrest in ICR yeast include the following: copious CO2 production (71), ethanol toxicity (72), substrate and product mass transfer limitations, contact inhibition within cell aggregates, and/or hyperbaric stress arising from expansion of microcolonies into an imperfectly elastic bead matrix. We tested the possibility that copious CO2 production caused cell cycle arrest by culturing planktonic cells in medium sparged with CO2; these cells exhibited no evidence of growth arrest. Concerning ethanol, this metabolite varies cyclically in ICRs, but because the feed is replaced at least every 48 h, its concentration never exceeds 50 g⋅L−1, well below the threshold reported to cause growth arrest in fed-batch systems (80–100 g⋅L−1) (72). Mass transfer limitations are unlikely to cause growth arrest given the bead composition and geometry, as well as the high rate of ICR void volume exchange. Calculations using diffusivity values for glucose and ethanol through alginate indicate there should be no mass transfer limitations in beads <3.5 mm in diameter (73). In this regard, it is noteworthy that the biomass-specific rate of ethanol production by immobilized cells is several-fold greater than that of log-phase planktonic cells, and this high fermentation rate is maintained for the duration of the experiments. Concerning possible contact inhibition, immobilized cells highly express genes in the FLO family of cell wall glycoproteins (Fig. 4A and Table S1). Because FLO adhesion proteins are involved in cell aggregation and stress resistance (74), their increased expression may facilitate microcolony formation in the alginate matrix, a feature that may favor increased glycolytic flux (75). Indeed, scanning electron microscopy provides evidence for the existence of such structures (Fig. S1D), wherein yet-to-be discovered quorum sensing pathway(s) may help transduce information about local cell density into signals that mediate growth arrest.
Growth Arrest and Longevity in Immobilized Yeast May Be Related to Differential Expression of RIM15.
Even though the ultimate cause for cell cycle arrest in encapsulated cells remains elusive, our data indicate that key signal(s) may be transduced via Rim15. Relative to planktonic cells, immobilized wild-type yeast highly express this gene and its downstream target MSN4. Unlike wild type, immobilized rim15Δ cells continue to divide and exhibit much lower viability under nutrient-sufficient conditions. RIM15 encodes a glucose-repressible serine–threonine protein kinase that acts in signal transduction pathways responding to depletion of extracellular carbon, nitrogen, and phosphorus. Originally identified as a regulator of IME2, which stimulates early meiotic gene expression (and which is also up-regulated in ICRs) (45), RIM15 is crucial to the establishment of the stationary phase, especially the quiescent G0 state (76). Multiple nutrient-sensing pathways (TOR, SCH9, RAS/PKA) converge on Rim15, which in turn positively regulates stress-resistance transcription factors Msn2/Msn4 and Gis1 (48). Loss of RIM15 results in a pleiotropic phenotype that fails to enter the stationary phase; poorly accumulates trehalose and glycogen; derepresses HSP12, HSP26, and SSA3; and cannot induce either thermotolerance or starvation resistance (77).
Whereas previous work has shown how RIM15 is induced when nutrients are lacking, in ICR yeast we see RIM15 and its downstream target MSN4 induced when nutrients are abundant. If, as some suggest, Rim15 is activated by glucose uptake kinetics rather than by absolute glucose levels (76), then periodic variation in extracellular glucose in ICRs may help explain RIM15’s unexpected pattern of expression. Alternatively, Rim15 up-regulation under nutrient-sufficient conditions may result from signal decoupling in one or more of the nutrient sensor kinase pathways, a hypothesis that can be tested by assaying the phosphorylation status of kinases such as PKA, Sch9, the Pho80–Pho85 complex, as well as that of Rim15 itself. RIM15 confers thermal and oxidative stress resistance on calorie-restricted, chronologically long-lived planktonic yeast (10, 77). And indeed, the beneficial effects of CR on CLS can be negated either by deletion of RIM15 or by deletion of MSN2/MSN4 (10). Thus, experimental conditions that promote RIM15 overexpression could be expected to increase stress resistance and extend CLS. Well-fed encapsulated rim15Δ cells exhibit greatly reduced survivorship relative to that of encapsulated wild-type cells (25% vs. >90%). In fact, survivorship of encapsulated rim15Δ mutants is comparable to that of starved planktonic rim15Δ mutants in extended batch culture (20%) (77). Reduced viability of the deletion strain may be attributed to its inability to completely arrest, which leads to replicative stress (24, 78), and/or to diminished stress resistance, which is essential for stationary-phase survival. Given that RIM15 is highly expressed in encapsulated but not in planktonic yeast, and given that encapsulated wild-type but not rim15Δ cells cease to reproduce and show extended CLS, we conclude that differential expression of RIM15 facilitates cell cycle arrest and increases stress resistance in immobilized yeast.
Glycogen Accumulation May Help Extend CLS in Immobilized Yeast.
In high-glucose medium, respiratory-deficient strains accumulate glycogen and mobilize it upon glucose depletion (79). Although ICR cells are not glucose-limited, they are effectively respiratory-deficient due to anoxic reactor conditions, which helps to explain why they hyperaccumulate glycogen. Also, because loss of RIM15 prevents stationary-phase cells from accumulating glycogen (77), overexpression of RIM15 in immobilized cells may favor glycogen accumulation. The role that reserve storage carbohydrates play in chronological cell aging remains controversial. Although some (80) view their accumulation as a hallmark feature of senescence, others (28) have argued that periodic replenishment of glycogen reserves can dramatically extend yeast CLS. Our findings are consistent with the latter hypothesis.
Other Conditions Where Reproduction Is Uncoupled from Metabolism in Yeast.
Uncoupling of reproduction from metabolism has been observed in planktonic yeast during high-glucose, aerobic, fed-batch bioethanol fermentation (72). Here, uncoupling is attributed to ethanol (above an 80–100 g⋅L−1 threshold), which impairs ethanol-sensitive nutrient transporters, including those for glucose and ammonium. In effect, transporter inhibition prevents nutrient acquisition. While encapsulated, continuously fed yeast also uncouples reproduction from metabolism, the underlying cause is unlikely related to ethanol stress, as its medium is frequently exchanged so that ethanol never exceeds 50 g⋅L−1. Further, only 45% of “ethanol-uncoupled” planktonic cells remain viable compared with immobilized cells, which are essentially all viable even after being “uncoupled” for ∼2 wk (81). Expression profiling reveals that ethanol-uncoupled yeast up-regulates SNF1, ADR1, ASP3-1, and ASP2-1, indicating that, unlike the yeast in our study, these cells are sugar- and nitrogen-limited (81). Finally, in ethanol-uncoupled cells, genes involved in ribosomal biogenesis are up-regulated, whereas genes involved in cell wall remodeling are down-regulated. We observe exactly the opposite in immobilized cells; thus, the mechanisms that cause uncoupling in these two systems must be fundamentally different.
After planktonic cells exhaust glucose in the medium, they differentiate into several subtypes, one of which is quiescent. Quiescent cells consist primarily of daughter cells that have never budded, possess high stress tolerance, and retain high viability during prolonged periods of starvation (82). These cells seem poised to resume growth when resupplied with nutrients. Thus, quiescent cells in certain respects resemble encapsulated cells. However, whereas quiescent yeast meets minimum energy requirements via respiration, encapsulated yeast is highly fermentative. Both quiescent and encapsulated cell types possess certain characteristics of metazoan cells, with quiescent cells being similar to stem cells (82) and well-fed encapsulated cells being similar to postmitotic cells that are terminally differentiated.
Metabolic uncoupling occurs in yeast colonies growing on solid media, and does so in a manner that leaves older colonies stratified with respect to cell types that differ in activity and longevity (83). Similar to immobilized yeast, upper colony or “U-cells” are long-lived, resist stress, and accumulate glycogen. Also, like immobilized cells, but unlike quiescent cells, U-cell respiratory metabolism is down-regulated. U-cells up-regulate not only glycolysis but also pathways related to amino acid metabolism that enable them to survive on resources released by the lysis of lower “L-cells.” TOR pathway up-regulation in U-cells, but not in well-fed immobilized cells, indicates that the former are nutrient-limited whereas the latter are not.
Finally, yeast uncouples metabolism from reproduction when cultured in retentostats. In this system, planktonic cells are physically confined to a bioreactor operated under continuous glucose limitation at near-zero dilution rates (60, 84); such cells show exceptional longevity (∼80% viability after 2 wk). Significantly, although ICR yeast is metabolically very active and retentostat yeast is not, and although the expression profiles underlying these two states differ in certain respects, both hyperaccumulate glycogen but not trehalose and both highly express RIM15. Thus, these two systems could provide complementary approaches to study division-arrested yeast CLS in the presence and in the absence of CR.
An Alternative Paradigm for the Study of Aging in Postmitotic Cells.
To date, all studies of yeast CLS have focused on postmitotic viability under starvation (22) or near-starvation conditions (60). Although yeast CLS is held to mimic that of postmitotic metazoan cells (85), such cells are rarely calorie-restricted and many remain metabolically active throughout an animal’s lifetime. Terminally differentiated cells such as neurons and muscle arrest in G1 and uncouple metabolism from reproduction in the presence of ample nutrients. Differentiated myotubes show dramatic loss of core factors in their transcriptional machinery, which results in large-scale silencing of genes that control cell proliferation and cell cycle progression (86). Ideally, to model the lifespan of such cells, yeast should be studied under calorie-unrestricted conditions. In practice, this has been difficult to achieve as yeast divides when nutrient levels permit. Here we have shown that well-fed yeast confined within a matrix shuts down transcription of genes that drive the cell cycle, even as it continues to perform glycolysis at near maximal rates. Because reproduction is uncoupled from metabolism, resources that would otherwise be allocated to growth and cell division are redirected to storage and maintenance, a shift in yeast’s life history that greatly extends its CLS. In fact, how long a nondividing yeast cell can live under nutrient-replete conditions remains an open question. ICRs therefore offer an alternative experimental paradigm for investigating yeast CLS in the absence of CR, one that will yield fresh insights into the mechanisms that control cell lifespan in higher eukaryotes, including humans.
Materials and Methods
Strains and Culture Media.
Saccharomyces cerevisiae BY4722 (MATα leu2Δ ura3Δ0) was obtained from the American Type Culture Collection (ATCC No. 200884); a MAT-a leu2Δ0 ura3Δ0 rim15∆ derivative congenic to BY4730 was obtained from Open Biosystems. Culture media used in ICR, batch, and chemostat studies was the SM medium described by Verduyn et al. (19), modified for anaerobic culture. Because previous studies (21, 87) had shown that starvation for auxotrophic requirements accelerates cell aging, these were provided in fivefold excess to planktonic cells. As the medium feeding ICRs was regularly replenished, auxotrophic requirements were provided there at recommended concentrations (88). Details of routine strain storage and maintenance are described in SI Materials and Methods.
Preparation of Alginate Encapsulated Cells.
Early stationary yeast was encapsulated in high-viscosity sodium alginate to form ∼3 mm beads, as described in SI Materials and Methods. Beads were hardened by incubation in fresh, sterile 0.5 M CaCl2, then packed to a volume of 100 mL in a custom-built ICR.
Structural and Operational Features of the ICR.
A schematic of the ICR used in this study is shown in Fig. S1A. The Pyrex glass reactor column had a radius of 2.7 cm and a working length of 40 cm. Overall, the apparatus was similar to that described by ref. 89, modified by placing an in-line cell trap to remove planktonic cells arising from ruptured beads, and a stainless-steel piston at the base to permit bed recompression following sampling. Operational features are described in detail in SI Materials and Methods.
Planktonic Cell Studies.
Planktonic cells were cultured in either batch or chemostat mode using an ATR SixFors fermentation apparatus (ATR Biotechnologies) with the reactor working volume set at 300 mL Anaerobic conditions were maintained by sparging cultures with sterile-filtered, humidified N2. Batch cultures were initiated with 2 g⋅L−1 glucose; chemostat cultivation was carried out at 9 g⋅L−1 glucose with the dilution rate, D, fixed at 0.15 h−1. Temperature and pH were maintained at 32.5 °C and 5.5, respectively.
Sampling Procedures.
Detailed procedures for sampling planktonic and immobilized cell cultures are described in SI Materials and Methods.
Demographic Parameters: Population Density, Viability, Budding Index, and Replicative Age Structure.
Planktonic cells were enumerated either by hemocytometry or spectrophotometry at A600. Viability was determined by plating known dilutions onto yeast extract, peptone, dextrose (YEPD) agar, then counting colony-forming units (CFU) following 72 h of incubation at 30 °C. Following Calcofluor staining (90), budding index and bud scar analyses were performed by differential interference contrast and epifluorescence microscopy. For immobilized cells, demographic parameters were estimated on ICR yeast following their release from the calcium–alginate matrix by using sodium metaphosphate to chelate Ca2+, the cross-linking agent. Additional details are provided in SI Materials and Methods.
Assay of CLS.
CLS was evaluated as the time-dependent survivorship of nondividing cells, as in ref. 22. Survivorship was estimated in starving planktonic batch cultures and in ICRs as the ratio of CFUs to total cell count obtained by hemocytometry.
Metabolite Determinations.
Glucose and ethanol were determined either using a YSI 2700 analyzer (YSI Corp.) or, spectrophotometrically, using R-BIOPHARM enzyme kits (no. 716251 and no. 176290). Glycogen and trehalose were determined as described in ref. 91.
Resistance to Heat Shock and Zymolyase Digestion.
Thermotolerance was assayed as the change in cell survivorship over the course of a 2 h incubation at 48 °C, estimated as CFUs on YEPD agar. Susceptibility of the yeast cell wall to zymolyase digestion was assayed by the method of Ovalle et al. (92), but scaled for a 96-well format, as described in SI Materials and Methods.
Flow Cytometric Analysis of DNA Content and Integrity.
Cell cycle status and DNA integrity were assessed in planktonic yeast and immobilized yeast released from calcium alginate by staining ethanol-fixed cells with SYTOX Green and performing flow cytometry as previously described (23). Flow cytometric data were processed using FSC Express software (De Novo Software).
RNA Isolation.
Total RNA was isolated from planktonic cells using the hot acid phenol method (93) and from immobilized cells using a protocol developed in our laboratory and described in ref. 94, then analyzed by UV spectroscopy and Bioanalyser 2100 (Agilent Technologies).
Microarray Hybridization and Scanning.
Microarray analysis was performed using Yeast GenomeChip 2.0 arrays (AFFYMETRIX Inc.), using standard methods described in SI Materials and Methods. Microarray data have been deposited in the Gene Expression Omnibus repository under accession no. GSE21187.
GeneSpring Analysis.
Microarray data were first analyzed using GeneSpring software version 7.3, as described in SI Materials and Methods. A scatterplot of robust microarray-normalized (95) expression data was generated from biological replicates sampled on days 1, 3, 5, and 10 from ICRs. Analysis of this dataset showed that replicates from the same time point had Pearson correlation coefficients ranging from 0.85 to 0.98 (Mean = 0.92, SE = 0.0085). Minimal deviation was observed from the diagonal identity line among replicates, further indicating that our array results are highly reproducible (94).
GenMapp Analysis.
The set of statistically significant genes identified using GeneSpring 7.3 was imported into GenMapp software (GenMapp Version 2.1, Gladstone Institute, University of California, San Francisco) and criteria fixed to identify genes differentially expressed by a twofold-change threshold between immobilized cells and any planktonic state. Expression data for ICR yeast on day 0 and day 1 were excluded from this analysis, as day 0 transcript levels were indistinguishable from those of planktonic stationary-phase cells used for encapsulation and day 1 cells were still dividing. GenMapp data were overlayed onto KEGG pathway maps (96) for glycolysis, the TCA cycle, and the yeast cell cycle (97).
SAM was performed using TIGR (MEV) TM4 software based on ref. 31. An unpaired two-class SAM analysis was adopted to identify genes differentially expressed between the planktonic and immobilized states. Additional details are provided in SI Materials and Methods.
Functional Enrichment.
Significant genes were subjected to GO term analysis and functional enrichment clustering using the DAVID software, available as a web application (http://david.abcc.ncifcrf.gov/) (39, 98). As DAVID does not support yeast systematic names found at the Saccharomyces Genome Database (www.yeastgenome.org), Entrez GeneIDs were retrieved for significant genes and the analysis carried out using Entrez GeneIDs. qRT-PCR was performed on RIM15, MSN4, TYE7, and ACT1 using the BioRad MyiQ Real-Time PCR System (Biorad), using primers and conditions described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Matt Herron, Margie Kinnersley, Paul Levine, Gavin Sherlock, and Art Woods for useful discussions; Gretchen McAffrey for editorial comments; Pam Shaw for assistance in flow cytometry; and Jim Driver for electron microscopy. This work was supported by grants from the National Institutes of Health (NIH) (GM079762-01 and R15 GM79762-01A1) (to F.R.), the University of Montana’s Office of Sponsored Research, the Montana NIH–Biomedical Research Infrastructure Network program, and the University of Montana Fluorescence Cytometry Core Facility, which is in part supported by NIH Grant P20RR017670.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE21187).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323918111/-/DCSupplemental.
References
- 1.Fredriksson A, Nyström T. Conditional and replicative senescence in Escherichia coli. Curr Opin Microbiol. 2006;9(6):612–618. doi: 10.1016/j.mib.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Stephens C. Senescence: Even bacteria get old. Curr Biol. 2005;15(8):R308–R310. doi: 10.1016/j.cub.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Kaeberlein M, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310(5751):1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 4.Kaeberlein M. Lessons on longevity from budding yeast. Nature. 2010;464(7288):513–519. doi: 10.1038/nature08981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stumpferl SW, et al. Natural genetic variation in yeast longevity. Genome Res. 2012;22(10):1963–1973. doi: 10.1101/gr.136549.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin H, Lu M. Natural variation in replicative and chronological life spans of Saccharomyces cerevisiae. Exp Gerontol. 2006;41(4):448–456. doi: 10.1016/j.exger.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Smith ED, et al. Quantitative evidence for conserved longevity pathways between divergent eukaryotic species. Genome Res. 2008;18(4):564–570. doi: 10.1101/gr.074724.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 9.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20(2):174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei M, et al. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4(1):e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana L, Partridge L, Longo VD. Extending healthy life span—From yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: Update on dietary requirements for brain. Part 2: Macronutrients. J Nutr Health Aging. 2006;10(5):386–399. [PubMed] [Google Scholar]
- 13.Doran PM, Bailey JE. Effects of immobilization on growth, fermentation properties, and macromolecular composition of Saccharomyces cerevisiae attached to gelatin. Biotechnol Bioeng. 1986;28(1):73–87. doi: 10.1002/bit.260280111. [DOI] [PubMed] [Google Scholar]
- 14.Pacheco AM, Gondim DR, Goncalves LR. Ethanol production by fermentation using immobilized cells of Saccharomyces cerevisiae in cashew apple bagasse. Appl Biochem Biotechnol. 2010;161(1-8):209–217. doi: 10.1007/s12010-009-8781-y. [DOI] [PubMed] [Google Scholar]
- 15.Verbelen PJ, De Schutter DP, Delvaux F, Verstrepen KJ, Delvaux FR. Immobilized yeast cell systems for continuous fermentation applications. Biotechnol Lett. 2006;28(19):1515–1525. doi: 10.1007/s10529-006-9132-5. [DOI] [PubMed] [Google Scholar]
- 16.Blagosklonny MV. Cell senescence and hypermitogenic arrest. EMBO Rep. 2003;4(4):358–362. doi: 10.1038/sj.embor.embor806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flodin NW. The senescence of postmitotic mammalian cells: A cell-clock hypothesis. Mech Ageing Dev. 1984;27(1):15–27. doi: 10.1016/0047-6374(84)90079-4. [DOI] [PubMed] [Google Scholar]
- 18.Kubitschek HE, editor. Introduction to Research With Continuous Cultures. Englewood Cliffs, NJ: Prentice Hall; 1970. p. 195. [Google Scholar]
- 19.Verduyn C, Postma E, Scheffers WA, Van Dijken JP. Effect of benzoic acid on metabolic fluxes in yeasts: A continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8(7):501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- 20.Alvers AL, et al. Autophagy and amino acid homeostasis are required for chronological longevity in Saccharomyces cerevisiae. Aging Cell. 2009;8(4):353–369. doi: 10.1111/j.1474-9726.2009.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes P, Sampaio-Marques B, Ludovico P, Rodrigues F, Leão C. Low auxotrophy-complementing amino acid concentrations reduce yeast chronological life span. Mech Ageing Dev. 2007;128(5-6):383–391. doi: 10.1016/j.mad.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2003;2(2):73–81. doi: 10.1046/j.1474-9728.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 23.Haase SB, Reed SI. Improved flow cytometric analysis of the budding yeast cell cycle. Cell Cycle. 2002;1(2):132–136. [PubMed] [Google Scholar]
- 24.Weinberger M, et al. DNA replication stress is a determinant of chronological lifespan in budding yeast. PLoS ONE. 2007;2(8):e748. doi: 10.1371/journal.pone.0000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herker E, et al. Chronological aging leads to apoptosis in yeast. J Cell Biol. 2004;164(4):501–507. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galazzo JL, Bailey JE. Growing Saccharomyces cerevisiae in calcium-alginate beads induces cell alterations which accelerate glucose conversion to ethanol. Biotechnol Bioeng. 1990;36(4):417–426. doi: 10.1002/bit.260360413. [DOI] [PubMed] [Google Scholar]
- 27.François J, Parrou JL. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 2001;25(1):125–145. doi: 10.1111/j.1574-6976.2001.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 28.Samokhvalov V, Ignatov V, Kondrashova M. Reserve carbohydrates maintain the viability of Saccharomyces cerevisiae cells during chronological aging. Mech Ageing Dev. 2004;125(3):229–235. doi: 10.1016/j.mad.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Kyryakov P, et al. Caloric restriction extends yeast chronological lifespan by altering a pattern of age-related changes in trehalose concentration. Front Physiol. 2012;3:256. doi: 10.3389/fphys.2012.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulery TL, Jang SH, Jaehning JA. Glucose repression of yeast mitochondrial transcription: Kinetics of derepression and role of nuclear genes. Mol Cell Biol. 1994;14(2):1160–1170. doi: 10.1128/mcb.14.2.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98(9):5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abramova N, Sertil O, Mehta S, Lowry CV. Reciprocal regulation of anaerobic and aerobic cell wall mannoprotein gene expression in Saccharomyces cerevisiae. J Bacteriol. 2001;183(9):2881–2887. doi: 10.1128/JB.183.9.2881-2887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo Z, van Vuuren HJ. Functional analyses of PAU genes in Saccharomyces cerevisiae. Microbiology. 2009;155(Pt 12):4036–4049. doi: 10.1099/mic.0.030726-0. [DOI] [PubMed] [Google Scholar]
- 34.Moll T, Tebb G, Surana U, Robitsch H, Nasmyth K. The role of phosphorylation and the CDC28 protein kinase in cell cycle-regulated nuclear import of the S. cerevisiae transcription factor SWI5. Cell. 1991;66(4):743–758. doi: 10.1016/0092-8674(91)90118-i. [DOI] [PubMed] [Google Scholar]
- 35.Zhu G, et al. Two yeast forkhead genes regulate the cell cycle and pseudohyphal growth. Nature. 2000;406(6791):90–94. doi: 10.1038/35017581. [DOI] [PubMed] [Google Scholar]
- 36.Thevelein JM. The RAS-adenylate cyclase pathway and cell cycle control in Saccharomyces cerevisiae. Antonie van Leeuwenhoek. 1992;62(1-2):109–130. doi: 10.1007/BF00584466. [DOI] [PubMed] [Google Scholar]
- 37.Kamei T, et al. Interaction of Bnr1p with a novel Src homology 3 domain-containing Hof1p. Implication in cytokinesis in Saccharomyces cerevisiae. J Biol Chem. 1998;273(43):28341–28345. doi: 10.1074/jbc.273.43.28341. [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez-Peña JM, Cid VJ, Arroyo J, Nombela C. A novel family of cell wall-related proteins regulated differently during the yeast life cycle. Mol Cell Biol. 2000;20(9):3245–3255. doi: 10.1128/mcb.20.9.3245-3255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 40.Sobering AK, Jung US, Lee KS, Levin DE. Yeast Rpi1 is a putative transcriptional regulator that contributes to preparation for stationary phase. Eukaryot Cell. 2002;1(1):56–65. doi: 10.1128/EC.1.1.56-65.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sertil O, Cohen BD, Davies KJ, Lowry CV. The DAN1 gene of S. cerevisiae is regulated in parallel with the hypoxic genes, but by a different mechanism. Gene. 1997;192(2):199–205. doi: 10.1016/s0378-1119(97)00028-0. [DOI] [PubMed] [Google Scholar]
- 42.Coluccio A, et al. Morphogenetic pathway of spore wall assembly in Saccharomyces cerevisiae. Eukaryot Cell. 2004;3(6):1464–1475. doi: 10.1128/EC.3.6.1464-1475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Virgilio C, DeMarini DJ, Pringle JR. SPR28, a sixth member of the septin gene family in Saccharomyces cerevisiae that is expressed specifically in sporulating cells. Microbiology. 1996;142(Pt 10):2897–2905. doi: 10.1099/13500872-142-10-2897. [DOI] [PubMed] [Google Scholar]
- 44.Su SS, Mitchell AP. Identification of functionally related genes that stimulate early meiotic gene expression in yeast. Genetics. 1993;133(1):67–77. doi: 10.1093/genetics/133.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vidan S, Mitchell AP. Stimulation of yeast meiotic gene expression by the glucose-repressible protein kinase Rim15p. Mol Cell Biol. 1997;17(5):2688–2697. doi: 10.1128/mcb.17.5.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martínez-Pastor MT, et al. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE) EMBO J. 1996;15(9):2227–2235. [PMC free article] [PubMed] [Google Scholar]
- 47.Görner W, et al. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12(4):586–597. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swinnen E, et al. Rim15 and the crossroads of nutrient signalling pathways in Saccharomyces cerevisiae. Cell Div. 2006;1:3. doi: 10.1186/1747-1028-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cameroni E, Hulo N, Roosen J, Winderickx J, De Virgilio C. The novel yeast PAS kinase Rim 15 orchestrates G0-associated antioxidant defense mechanisms. Cell Cycle. 2004;3(4):462–468. [PubMed] [Google Scholar]
- 50.Sato T, et al. The E-box DNA binding protein Sgc1p suppresses the gcr2 mutation, which is involved in transcriptional activation of glycolytic genes in Saccharomyces cerevisiae. FEBS Lett. 1999;463(3):307–311. doi: 10.1016/s0014-5793(99)01654-3. [DOI] [PubMed] [Google Scholar]
- 51.Niu W, Li Z, Zhan W, Iyer VR, Marcotte EM. Mechanisms of cell cycle control revealed by a systematic and quantitative overexpression screen in S. cerevisiae. PLoS Genet. 2008;4(7):e1000120. doi: 10.1371/journal.pgen.1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanders SL, Herskowitz I. The BUD4 protein of yeast, required for axial budding, is localized to the mother/BUD neck in a cell cycle-dependent manner. J Cell Biol. 1996;134(2):413–427. doi: 10.1083/jcb.134.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laabs TL, et al. ACE2 is required for daughter cell-specific G1 delay in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2003;100(18):10275–10280. doi: 10.1073/pnas.1833999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jorgensen P, et al. A dynamic transcriptional network communicates growth potential to ribosome synthesis and critical cell size. Genes Dev. 2004;18(20):2491–2505. doi: 10.1101/gad.1228804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson TE, et al. Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans. Exp Gerontol. 2001;36(10):1609–1617. doi: 10.1016/s0531-5565(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 56.Harris N, MacLean M, Hatzianthis K, Panaretou B, Piper PW. Increasing Saccharomyces cerevisiae stress resistance, through the overactivation of the heat shock response resulting from defects in the Hsp90 chaperone, does not extend replicative life span but can be associated with slower chronological ageing of nondividing cells. Mol Genet Genomics. 2001;265(2):258–263. doi: 10.1007/s004380000409. [DOI] [PubMed] [Google Scholar]
- 57.Werner-Washburne M, Braun E, Johnston GC, Singer RA. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1993;57(2):383–401. doi: 10.1128/mr.57.2.383-401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu C, Brauer MJ, Botstein D. Slow growth induces heat-shock resistance in normal and respiratory-deficient yeast. Mol Biol Cell. 2009;20(3):891–903. doi: 10.1091/mbc.E08-08-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plesset J, Ludwig JR, Cox BS, McLaughlin CS. Effect of cell cycle position on thermotolerance in Saccharomyces cerevisiae. J Bacteriol. 1987;169(2):779–784. doi: 10.1128/jb.169.2.779-784.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boender LG, et al. Cellular responses of Saccharomyces cerevisiae at near-zero growth rates: Transcriptome analysis of anaerobic retentostat cultures. FEMS Yeast Res. 2011;11(8):603–620. doi: 10.1111/j.1567-1364.2011.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun ZJ, et al. Differential role of microenvironment in microencapsulation for improved cell tolerance to stress. Appl Microbiol Biotechnol. 2007;75(6):1419–1427. doi: 10.1007/s00253-007-0960-6. [DOI] [PubMed] [Google Scholar]
- 62.Norton S, Watson K, D’Amore T. Ethanol tolerance of immobilized brewers’ yeast cells. Appl Microbiol Biotechnol. 1995;43(1):18–24. doi: 10.1007/BF00170616. [DOI] [PubMed] [Google Scholar]
- 63.McGhee JE, St Julian G, Detroy RW. Continuous and static fermentation of glucose to ethanol by immobilized Saccharomyces cerevisiae cells of different ages. Appl Environ Microbiol. 1982;44(1):19–22. doi: 10.1128/aem.44.1.19-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matecic M, et al. A microarray-based genetic screen for yeast chronological aging factors. PLoS Genet. 2010;6(4):e1000921. doi: 10.1371/journal.pgen.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Najafpour G, Younesi H, Syahidah Ku Ismail K. Ethanol fermentation in an immobilized cell reactor using Saccharomyces cerevisiae. Bioresour Technol. 2004;92(3):251–260. doi: 10.1016/j.biortech.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 66.Newcomb LL, Diderich JA, Slattery MG, Heideman W. Glucose regulation of Saccharomyces cerevisiae cell cycle genes. Eukaryot Cell. 2003;2(1):143–149. doi: 10.1128/EC.2.1.143-149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5(4):265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davidson GS, et al. The proteomics of quiescent and nonquiescent cell differentiation in yeast stationary-phase cultures. Mol Biol Cell. 2011;22(7):988–998. doi: 10.1091/mbc.E10-06-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei M, et al. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet. 2009;5(5):e1000467. doi: 10.1371/journal.pgen.1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle. 2009;8(8):1256–1270. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Norton JS, Krauss RW. The inhibition of cell division in Sachharomyces cerevisiae (Meyen) by carbon dioxide. Plant Cell Physiol. 1972;13(1):139–149. [Google Scholar]
- 72.Cot M, Loret MO, François J, Benbadis L. Physiological behaviour of Saccharomyces cerevisiae in aerated fed-batch fermentation for high level production of bioethanol. FEMS Yeast Res. 2007;7(1):22–32. doi: 10.1111/j.1567-1364.2006.00152.x. [DOI] [PubMed] [Google Scholar]
- 73. Myers B (2005) Effects of cell immobilization on ethanol yield and cell growth rates with Saccharomyces cervisiae. Master’s thesis (Utah State Univ, Logan, UT)
- 74.Smukalla S, et al. FLO1 is a variable green beard gene that drives biofilm-like cooperation in budding yeast. Cell. 2008;135(4):726–737. doi: 10.1016/j.cell.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koschwanez JH, Foster KR, Murray AW. Sucrose utilization in budding yeast as a model for the origin of undifferentiated multicellularity. PLoS Biol. 2011;9(8):e1001122. doi: 10.1371/journal.pbio.1001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pedruzzi I, et al. TOR and PKA signaling pathways converge on the protein kinase Rim15 to control entry into G0. Mol Cell. 2003;12(6):1607–1613. doi: 10.1016/s1097-2765(03)00485-4. [DOI] [PubMed] [Google Scholar]
- 77.Reinders A, Bürckert N, Boller T, Wiemken A, De Virgilio C. Saccharomyces cerevisiae cAMP-dependent protein kinase controls entry into stationary phase through the Rim15p protein kinase. Genes Dev. 1998;12(18):2943–2955. doi: 10.1101/gad.12.18.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alcasabas AA, et al. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat Cell Biol. 2001;3(11):958–965. doi: 10.1038/ncb1101-958. [DOI] [PubMed] [Google Scholar]
- 79.Enjalbert B, Parrou JL, Vincent O, François J. Mitochondrial respiratory mutants of Saccharomyces cerevisiae accumulate glycogen and readily mobilize it in a glucose-depleted medium. Microbiology. 2000;146(Pt 10):2685–2694. doi: 10.1099/00221287-146-10-2685. [DOI] [PubMed] [Google Scholar]
- 80.Lin SS, Manchester JK, Gordon JI. Enhanced gluconeogenesis and increased energy storage as hallmarks of aging in Saccharomyces cerevisiae. J Biol Chem. 2001;276(38):36000–36007. doi: 10.1074/jbc.M103509200. [DOI] [PubMed] [Google Scholar]
- 81.Benbadis L, Cot M, Rigoulet M, Francois J. Isolation of two cell populations from yeast during high-level alcoholic fermentation that resemble quiescent and nonquiescent cells from the stationary phase on glucose. FEMS Yeast Res. 2009;9(8):1172–1186. doi: 10.1111/j.1567-1364.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 82.Werner-Washburne M, Roy S, Davidson GS. Aging and the survival of quiescent and non-quiescent cells in yeast stationary-phase cultures. Subcell Biochem. 2012;57:123–143. doi: 10.1007/978-94-007-2561-4_6. [DOI] [PubMed] [Google Scholar]
- 83.Váchová L, Cáp M, Palková Z. Yeast colonies: A model for studies of aging, environmental adaptation, and longevity. Oxid Med Cell Longev. 2012;2012:601836. doi: 10.1155/2012/601836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boender LG, de Hulster EA, van Maris AJ, Daran-Lapujade PA, Pronk JT. Quantitative physiology of Saccharomyces cerevisiae at near-zero specific growth rates. Appl Environ Microbiol. 2009;75(17):5607–5614. doi: 10.1128/AEM.00429-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MacLean M, Harris N, Piper PW. Chronological lifespan of stationary phase yeast cells: A model for investigating the factors that might influence the ageing of postmitotic tissues in higher organisms. Yeast. 2001;18(6):499–509. doi: 10.1002/yea.701. [DOI] [PubMed] [Google Scholar]
- 86.Deato MD, Tjian R. Switching of the core transcription machinery during myogenesis. Genes Dev. 2007;21(17):2137–2149. doi: 10.1101/gad.1583407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boer VM, Amini S, Botstein D. Influence of genotype and nutrition on survival and metabolism of starving yeast. Proc Natl Acad Sci USA. 2008;105(19):6930–6935. doi: 10.1073/pnas.0802601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pronk JT. Auxotrophic yeast strains in fundamental and applied research. Appl Environ Microbiol. 2002;68(5):2095–2100. doi: 10.1128/AEM.68.5.2095-2100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yadav BS, Rani U, Dhamija SS, Nigam P, Singh D. Process optimization for continuous ethanol fermentation by alginate-immobilized cells of Saccharomyces cerevisiae HAU-1. J Basic Microbiol. 1996;36(3):205–210. doi: 10.1002/jobm.3620360307. [DOI] [PubMed] [Google Scholar]
- 90.Pringle JR. Staining of bud scars and other cell wall chitin with calcofluor. Methods Enzymol. 1991;194:732–735. doi: 10.1016/0076-6879(91)94055-h. [DOI] [PubMed] [Google Scholar]
- 91.Parrou JL, François J. A simplified procedure for a rapid and reliable assay of both glycogen and trehalose in whole yeast cells. Anal Biochem. 1997;248(1):186–188. doi: 10.1006/abio.1997.2138. [DOI] [PubMed] [Google Scholar]
- 92.Ovalle R, et al. A spheroplast rate assay for determination of cell wall integrity in yeast. Yeast. 1998;14(13):1159–1166. doi: 10.1002/(SICI)1097-0061(19980930)14:13<1159::AID-YEA317>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 93.Schmitt ME, Brown TA, Trumpower BL. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990;18(10):3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kruckeberg AL, Nagarajan S, McInnerney K, Rosenzweig F. Extraction of RNA from Ca-alginate-encapsulated yeast for transcriptional profiling. Anal Biochem. 2009;391(2):160–162. doi: 10.1016/j.ab.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 96.Ogata H, et al. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999;27(1):29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet. 2002;31(1):19–20. doi: 10.1038/ng0502-19. [DOI] [PubMed] [Google Scholar]
- 98.Dennis G, Jr, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):3. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.