Abstract
The lymphatic system is important for body fluid balance as well as immunological surveillance. Due to the identification of new molecular markers during the last decade, there has been a recent dramatic increase in our knowledge on the molecular mechanisms involved in lymphatic vessel growth (lymphangiogenesis) and lymphatic function. Here we review data showing that although it is often overlooked, the extracellular matrix plays an important role in the generation of new lymphatic vessels as a response to physiological and pathological stimuli. Extracellular matrix-lymphatic interactions as well as biophysical characteristics of the stroma have consequences for tumor formation, growth and metastasis. During the recent years, anti-lymphangiogenesis has emerged as an additional therapeutic modality to the clinically applied anti-angiogenesis strategy. Oppositely, enhancement of lymphangiogenesis in situations of lymph accumulation is seen as a promising strategy to a set of conditions where few therapeutic avenues are available. Knowledge on the interaction between the extracellular matrix and the lymphatics may enhance our understanding of the underlying mechanisms and may ultimately lead to better therapies for conditions where reduced or increased lymphatic function is the therapeutic target
Keywords: tumor stroma, metastasis, integrins, microenvironment, lymphedema
Introduction
In this review the focus will be on the interstitial space, which is the physical and biochemical microenvironment of the cells in the body, and its interaction with the lymphatics. Essentially the interstitial space can be divided into two phases: the interstitial fluid that is synonymous to initial or afferent lymph and the structural molecules of the interstitial or the extracellular matrix (ECM).
We will briefly review recent data regarding the structure of the extracellular matrix (ECM) and factors in the ECM that affect formation of new lymphatics (lymphangiogenesis). Whereas it is often appreciated that the matrix per se, as well as neoplasms and inflammatory processes, will affect lymph vessel formation, the opposite effect, i.e. that the lymphatics will affect ECM structure and composition, has received less attention. We will provide a general description of the ECM, lymphatics and lymphangiogenic mediators before we discuss ECM factors that affect lymphangiogenesis and the consequences for inflammation as well as tumor formation, growth and metastasis. In the final part of the review we will discuss the potential clinical and therapeutical implications of recent data on interaction between ECM and lymphatics.
Structure of the ECM
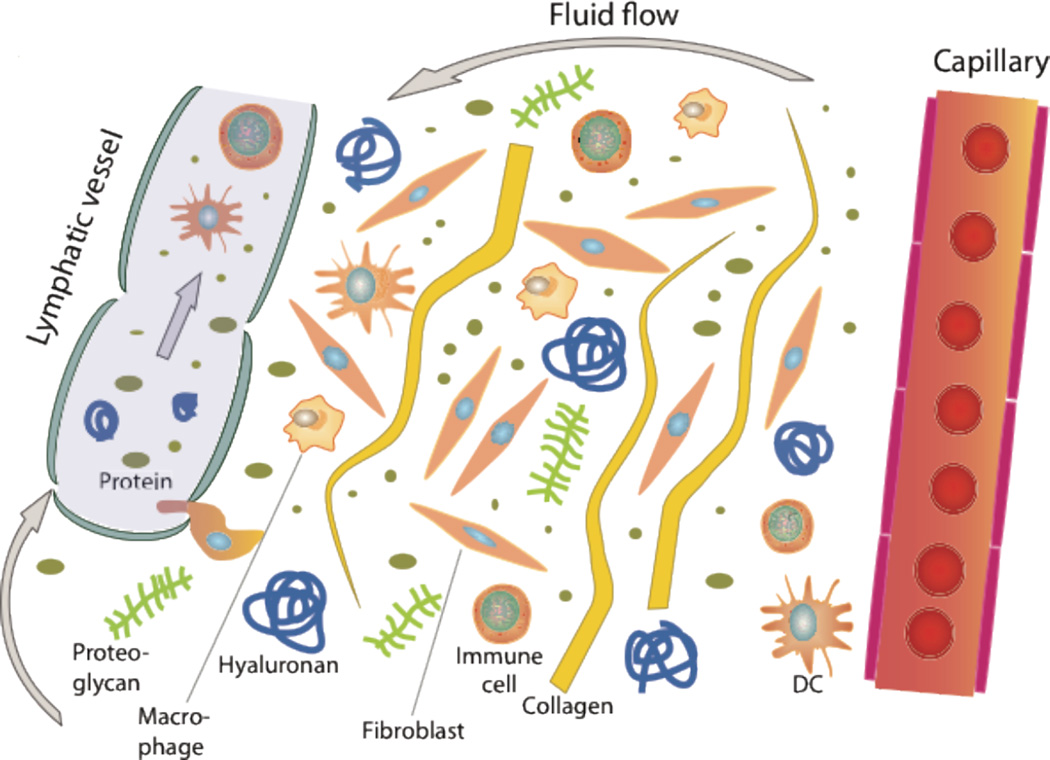
The extracellular or interstitial matrix constitutes the framework for the individual cells of an organ as well as for the body as a whole, and the extracellular or interstitial fluid constitutes the microenvironment of the connective tissue and parenchymal cells. All hormones, nutrients and waste products going to and from the cells must pass the interstitial or extracellular matrix. The lymphatic vasculature forms a vessel network in the interstitium of most tissues that has an important homeostatic role in the body. As schematized in Fig 1, this network drains filtered fluid and proteins and returns it to the blood. In addition, it serves an important role in the body’s immune defence since extravasated activated antigen presenting cells that enter the blind-ended lymphatic capillaries are transported to lymph nodes and may here initiate key immune responses (Alitalo et al., 2005; Tammela and Alitalo, 2010).
Fig. 1.
Schematic overview of the interstitium with some of its major its extracellular matrix components. Fluid containing plasma proteins and other solutes is filtered from the capillary percolates through the interstitium and is absorbed and thus returned to the circulation by lymph. In addition to proteins and solutes, immune cells migrate into lymphatic vessels and are transported to lymph nodes where they may initiate an immune response.
The composition and structure of the ECM has been the topic of several extensive recent reviews (Gelse et al., 2003; Heino, 2007; Kjaer, 2004; Raman et al., 2005; Sasisekharan et al., 2006), and thus only a brief description is given here. Whereas most reviews address the role of the ECM in the control of proliferation, differentiation and migration by mediating cell adhesion and communication, in the present context the focus is the major structural components collagen and glycosaminoglycans (GAGs; i.e. hyaluronan and different types of proteoglycans (PGs) because of their role in ECM-lymphatics interaction (Fig 1). Other major components of the ECM include elastin as well as the cell adhesion proteins, fibronectin, vitronectin, thrombospondins, and laminins (Miner and Yurchenco, 2004). Proteoglycans can be classified according to their core-proteins, their distribution (ECM, cell-surface, intracellular) or according to their GAG content (heparan sulfate PG, chondoitin sulfate PG or dermatan sulfate PG) (Raman et al., 2005). The matrix components interact through specific recognition sites contained in their protein and oligosaccharide sequences. Cells interact with the matrix via cell surface receptors including CD44 (hyaluronan and others), discoidin domain receptors (collagens), cell surface proteoglycans (fibronectin and others) and integrins (major ECM ligands including, fibronectin, vitronectin, fibrinogen, collagens, laminins). In some instances the recognition motifs in the ligands have been mapped which can be exemplified by the integrin motifs RGD in fibronectin, vitronectin, fibrinogen, LDV in fibronectin and GFOGER in collagens. In a previous review we have addressed RGD interactions because of its role in fluid volume regulation and thus lymph formation (Wiig et al., 2008; Wiig et al., 2003). A more extensive review of the elements constituting the ECM is therefore not given.
As pointed out by Aszodi and coworkers (Aszodi et al., 2006), the structural integrity of extracellular matrices is defined by (a) their macromolecular composition, (b) the complex interactions among the resident proteins, and (c) the capability of these ECM molecules to assemble a highly organized, three-dimensional architecture. In most tissues the primary scaffold is made of collagen fibrils or networks. These entrap various PGs and glycoproteins that assemble in specific supramolecular structures to fulfill the various mechanical requirements of connective tissues and basal membranes.
Structure and function of lymphatics
The lymphatic system consists of lymphatic vessels and lymphoid organs. One of the main functions of lymphatic vessels is to return extravasated fluid, proteins and cells back into the circulation (Alitalo et al., 2005; Mumprecht and Detmar, 2009; Schmid-Schonbein, 1990; Swartz, 2001) (Fig 1). Except for the avascular tissues such as epidermis, cartilage and cornea and some vascularized organs like brain and the retina, all organs have blind ended lymphatic capillaries, also known as initial lymphatics, that transport lymph to larger collecting lymphatic vessels that again return lymph to the general circulation in lymphatic-vascular junctions in the cervical area (Alitalo et al., 2005; Mumprecht and Detmar, 2009; Schmid-Schonbein, 1990).
From the tissue until entering into the blood stream, the lymph passes through the following conduits with increasing size, lymph capillaries (also called initial lymphatics), collecting vessels, lymph nodes, trunks and ducts (Swartz, 2001). The initial lymphatics are thin-walled, relatively large vessels compared to blood capillaries. They are composed by a single layer of endothelial cells, are not ensheathed by pericytes and smooth muscle cells, and have little or no basement membrane. The nonmuscular, so-called initial, lymphatics are the site of interstitial fluid absorption that requires only small and transient pressure gradients from the interstitium to enter the initial lymphatics. A fundamental question concerns the mechanism that causes expansion and compression of the initial lymphatics. It is assumed that the so-called anchoring filaments, connecting elastic fibers in the ECM and the abluminal part initial lymphatics (Leak, 1976) as well as tissue stress like contraction of surrounding muscles and arterial pulsations contribute in the initial lymph formation (Gerli et al., 2000; Schmid-Schonbein, 1990). The initial lymphatics have overlapping structures that may act as flaps and thus as primary valves in these vessels (Azzali, 2003; Baluk et al., 2007).
In addition to the propagation of initial lymph by tissue stress, lymph is moved centrally by spontaneous contractions of the smooth muscle cells lining collecting lymphatics. Such transport in unidirectional due to the presence of one-way valves. Larger collecting vessels are innervated and in addition are supposed to have pacemaker units (McGeown et al., 1987; McHale and Roddie, 1976; Muthuchamy and Zawieja, 2008; von der Weid et al., 1996). The functional units within the muscular lymphatics, lymphangions, are arranged in series and are separated by the lymphatic valves (Muthuchamy and Zawieja, 2008). As a part of the central movement, lymph in collecting vessels pass through lymph nodes, and as a consequence these vessels are classified as prenodal or postnodal (or afferent or efferent) thus saying that lymph is carried to or from the nodes, respectively. This is an important distinction, since the lymph composition is changed during its passage through the nodes (Adair et al., 1982; Schmid-Schonbein, 1990), and furthermore since important immune cell modifications occur in lymph nodes (Randolph et al., 2005) as will be discussed below.
The lymphatics are also an important element of the immune system, its vessels draining cells and fluid from practically all tissues into lymph nodes and other lymphoid tissues (von Andrian and Mempel, 2003), making lymphatics ideally suited for immune surveillance (Swartz et al., 2008) (Fig 1). This function applies to soluble antigens filtered by antigen presenting cells residing in the lymph node sinuses (Junt et al., 2007) as well as particulate antigens that are presented by dendritic cells traveling to lymph node after phagocytosis (Randolph et al., 2005; Randolph et al., 2008). These functions, that are fundamental for initiation and propagation of an immune response, are incompletely understood, but seem to involve chemokines CCL 21, CCL 19 and CCL 12 released by lymphatic endothelial cells that bind to their cognate receptors CCR7 and CXCR4 on mature dendritic cells to induce mobilization, vessel entry and trafficking (reviewed in (Jackson, 2009; Randolph, 2001)).
Lymphatic vessel markers and lymphangiogenic factors
Whereas lymph vessels were described at the beginning of the seventeenth century, growth factors and molecular markers for these vessels have been known for fifteen years only. During this last period, lymphatic vascular biology has advanced rapidly through the discovery of lymphangiogenic factors, identification of lymphatic vascular markers, isolation of lymphatic endothelial cells and the development of animal models to study lymphangiogenesis (Alitalo et al., 2005).
The discovery of several lymphatic vessel markers that can be used to distinguish lymphatic from blood vessels have been important for recent progress in the entire lymphatic biology field. The first of these were the homeobox transcription factor Prox1 (Wigle et al., 2002; Wigle and Oliver, 1999), the lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) (Banerji et al., 1999; Jackson, 2009), podoplanin (Breiteneder-Geleff et al., 1999), VEGFR- 3 (Kaipainen et al., 1995). Although these are the most commonly used, there are several other markers that can be used for differentiation of lymph and blood vessels (recently reviewed in e.g. (Jurisic and Detmar, 2009; Lohela et al., 2009)) and thus to isolate lymphatic endothelial cells for further studies in vitro.
There is a plethora of growth factors that induce growth of lymph vessels. The first to be identified were VEGF-C (Joukov et al., 1996) and VEGF-D (Achen et al., 1998), which both bind to VEGFR-3 (flt-4) on lymphatic endothelial cells and result in downstream signaling. Later, several other lymphatic growth factors have been identified, including hepatocyte growth factor (Kajiya et al., 2005), angiopoietin-1 (Morisada et al., 2005; Tammela et al., 2005), fibroblast growth factor-1 (Shin et al., 2006), insulin-like growth factor 1 and 2 (Bjorndahl et al., 2005), platelet derived growth factors (Cao et al., 2004), adrenomedullin (Fritz-Six et al., 2008), growth hormone (Banziger-Tobler et al., 2008) and endothelin (Spinella et al., 2009) as discussed in more detail in recent reviews (Jurisic and Detmar, 2009; Mumprecht and Detmar, 2009), but as recently pointed out these latter factors may have an indirect lymphangiogenic effect through their induction of VEGF-C and VEGF-D (Tammela and Alitalo, 2010). Many of the lymphangiogenetic factors are similar to the angiogenic factors, due to the common embryonic origins of lymphatic vessels and blood vessels (Karpanen and Alitalo, 2008). Whereas it is well established that integrin-ECM interactions are important in regulating angiogenesis (Cheresh and Stupack, 2008; Davis and Senger, 2005; Hynes, 2007), the corresponding role in lymphangiogenesis has not been addressed to a similar extent. Accumulating evidence is indicating a potential significance for ECM in regulating lymphangiogenesis, through integrin or non-integrin mediated interactions as will be discussed below.
ECM-factors that affect lymphangiogenesis
1. Integrins
Whereas integrins for several years has been appreciated as having a role in angiogenesis, more recently evidence have accumulated suggesting that integrins and ECM also influence normal lymphatic function and lymphangiogenesis (Avraamides et al., 2008) (Fig 2). The integrins are a superfamily of cell adhesion receptors important for cell-cell and cell-ECM connections that bind to extracellular matrix, cell-surface, and soluble ligands. They are transmembrane alphabeta heterodimers and at least 18 alpha and eight beta subunits are known in humans, generating 24 heterodimers (Avraamides et al., 2008; Hynes, 2002; Takada et al., 2007). Because integrins serve as transmembrane linkers between their extracellular ligands and the cytoskeleton, they have the capacity to influence cell migration during embryogenesis, angiogenesis, wound healing, immune and nonimmune defense mechanisms, hemostasis, and oncogenic transformation. As discussed above, in contrast to blood vessels, lymphatic microvessels lack a contiguous basement membrane, and this may be the reason why the role of integrin-matrix interactions in the lymphatic vessels have received less attention than in blood vessels.
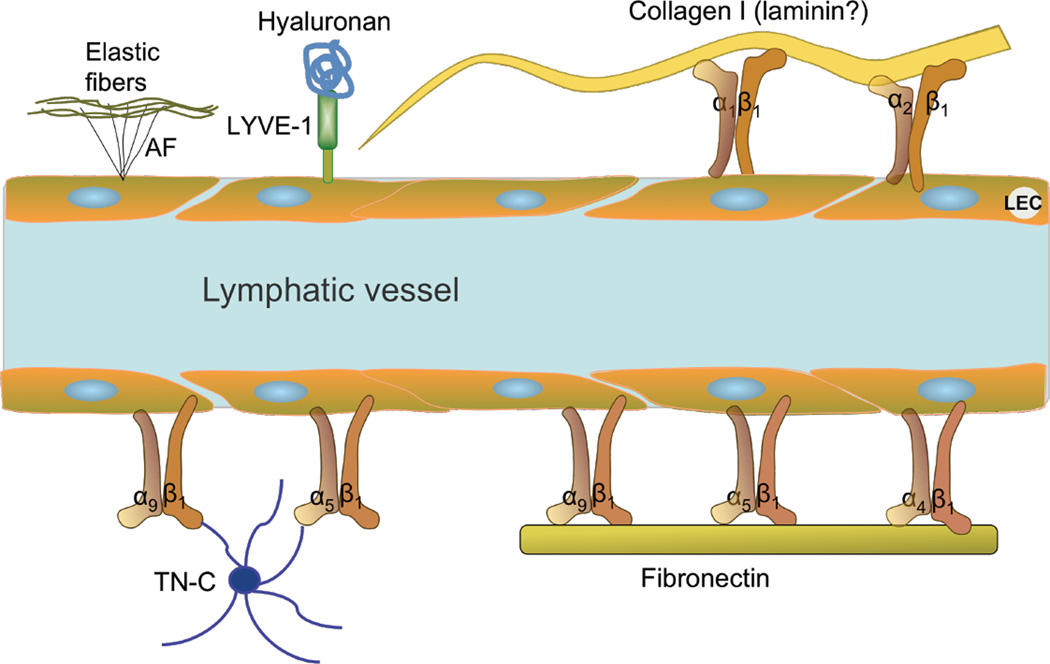
Fig. 2.
Schematic overview of known receptors on lymphatic vessels and binding of their extracellular matrix ligands. The anchoring filaments (AF) are adhered to the outside of the lymphatic endothelial cell (LEC) and connect to elastic fibers in the extracellular matrix. These filaments are important for lymphatic vessel function, allowing for fluid and cells to enter the initial lymphatic. TN-C: Tenascin C
One of the indications of an effect of integrins on lymphangiogenesis came from studies in mice lacking α9β1 integrin. These mice die perinatally due to respiratory failure caused by chylothorax (Huang et al., 2000). Since lymphatics are important for lipid absorption and transport, the chylothorax in these knockout mice suggested that there may be defects in the development of their lymphatic vessels (Huang et al., 2000), again suggesting that integrin α9β1 may be important for lymphangiogenesis. This assumption was supported by studies showing that integrin α9β1 is preferentially expressed on lymphatic endothelial cells, rather than blood endothelial cells (Kajiya et al., 2005; Petrova et al., 2002). Further support for a role of α9 came from experiments showing that Prox1, a lymphatic endothelial-cell selective transcription factor, regulated chemotaxis and migration of endothelial cells toward VEGF-C by regulating the expression of α9. These experiments also identified integrin α9 as a target gene for Prox1, thus participating in the acquisition of a lymphatic endothelial phenotype (Mishima et al., 2007). Recently it was shown that this integrin promotes VEGF-C and VEGF-D stimulated cell migration by direct binding of these growth factors, and that migration was abrogated by α9 function blocking antibodies (Vlahakis et al., 2005). In addition to its potential role in lymphangiogenesis, integrin α9β1 is important for formation of lymph valves. In a recent study it was shown that endothelial cell specific α9 deletion in mouse embryos resulted in the development of rudimentary valve leaflets again leading to retrograde lymphatic flow (Bazigou et al., 2009). This phenomenon may be the cause for the defective lymphatic function and formation of chylothorax in the systemic integrin α9 knockout mice. That this integrin also has a similar role in humans is supported by one recent study indicating a causal association between integrin α9 mutations and chylothorax in human fetuses (Ma et al., 2008).
The mechanism of action of α9β1 integrin has not fully established. Some studies have suggested that integrin α9β1 may bind to V-CAM1, ADAM12, ADAM15, VEGF-C, VEGF-D, fibronectin EIIIA, osteopontin (OPN) and tenascin-C (TN-C), but solid studies are lacking (Marcinkiewicz et al., 2000; Takada et al., 2007). The ECM ligands OPN and TN-C bind integrin α9β1 through non-RGD interactions (e.g. (Shinde et al., 2008)). In this regard, attachment of carcinoma cells to TN-C through integrin α9β1 induces proliferation via FAK-ERK signaling pathway (Yokosaki et al., 1996) and both OPN and TN-C are associated with increased lymphatic metastasis but the associated molecular mechanisms are unknown (Allan et al., 2006; Orend and Chiquet-Ehrismann, 2006). The best suggestion for a mechanism of action comes from the study of Bazigou and coworkers (Bazigou et al., 2009), showing that α9 directly regulates fibronectin fibril assembly that is essential for the formation of the extracellular matrix core of valve leaflets. Although TN-C, as well as its specific α9 binding ligand fibronectin EIIIA (Liao et al., 2002), is present in the vicinity of the integrin α9+ lymph valves, the functional effect of integrin α9β1 is specifically based on its interaction with fibronectin EIIIA. Notwithstanding the fact that integrin α9β1-fibronectin EIIIA interaction is crucial for the formation of the lymph valves during development, integrin α9β1 is not required for the maintenance of lymph valves and the surrounding matrix at later stages. Collectively, the studies discussed above suggest that integrin α9β1 has an important and crucial role in lymphangiogenesis and lymphatic function.
Recently, a role for integrin α4β1 was suggested in normal as well as tumor lymphangiogenesis (Garmy-Susini et al., 2010). Varner and collaborators found that the fibronectin-binding integrin α4β1 promoted the adhesion and migration of LECs during growth factor as well as tumor-induced lymphangiogenesis, suggesting that α4β1 expression is required for lymph vessel formation in vitro as well as in vivo.
One study has suggested that integrin α1β1 and α2β1 participate in lymphangiogenesis associated with wound healing. Using a model overexpressing VEGF-A in skin, these integrins were induced, and a combined blockade of the α1 and α2 integrins inhibited VEGF-A-driven lymphangiogenesis in vivo (Hong et al., 2004). In the same study it was speculated that their interaction with collagen type I lead to lymphangiogenesis in wound healing. While such studies are interesting and shed light on a possible role of integrins on lymphangiogenesis, the data remains inconclusive since they cannot distinguish the contribution of the individual α1 and α2 integrins and its impact on non-wounding lymphangiogenesis.
Integrin α5β1 has been shown to induce lymphatic endothelial cell (LEC) proliferation (Dietrich et al., 2007) in response to fibronectin attachment, but its role and mechanism of action is not understood. Integrin α5β1 is mainly implicated in the context of inflammatory lymphangiogenesis, in both corneal and airway inflammation models (Dietrich et al., 2007; Okazaki et al., 2009). Blockade of integrin α5β1 inhibits lymphangiogenesis (as well as angiogenesis) in these contexts, whereas αv integrin blockade inhibits angiogenesis, but not lymphangiogenesis, indicating some specificity for certain β1 containing integrins in the function in the lymphatic system.
2. Hyaluronan and LYVE-1
Hyaluronan (HA) (also known as hyaluronic acid and hyaluronate) is a glycosaminoglycan and an important component of the extracellular matrix within all tissues that also has a connection to the lymphatic system (Fig 2). The discovery of LYVE-1, the lymphatic vascular endothelial hyaluronan receptor, which expression is restricted almost entirely to lymph vessels and lymph node endothelia (Banerji et al., 1999), has been very important for the entire field of lymphatic research. Its lymphatic specific expression might indicate that lymphatic endothelial cells should be binding hyaluronan, but unexpectedly this was not the case (Banerji et al., 1999). The functional significance of the LYVE-1-hyaluronan interaction is not known in detail, although lymphatics have an important role in turnover of HA, and interaction of LECs with HA is occurring in e.g. inflammation (Jackson, 2009). Whereas previous studies suggested that HA was almost completely degraded in the lymph nodes, recent data indicate that this process occurs in lymphatic endothelial cells, indicating that the lymphatics may have a more important role in HA turnover than previously anticipated (reviewed in (Jackson, 2009)). It is speculated that through interaction between HA-receptors on lymphatics and cells having hyaluronan receptors (e.g. CD44 on leukocytes) could contribute to cell attachment and uptake in initial lymphatics (Jackson, 2009).
Hyaluronan could also be providing a pro-lymphanigogenic signal. Recent studies have associated the overproduction of HA in idiopathic pulmonary fibrosis with lymphangiogenesis, since HA was localizing around lymphatic vessels and that short fragment HA induced lymphangiogenesis (El-Chemaly et al., 2009). Although the studies discussed above indicate a lymphatic-HA interaction, LYVE-1−/− mice had no clear phenotype, and specifically no phenotype related to lymphatic function, leading to the conclusion that LYVE-1 may be partially redundant, at least in unchallenged conditions (Gale et al., 2007).
3. Collagens
Whereas collagen has an important role in supporting and maintaining the tissue structure, it also has an important role in regulating critical cellular functions like migration, survival, proliferation and differentiation (Sternlicht and Werb, 2001) (Fig 2). Several recent studies point to a role of this insoluble structure molecule in regulating lymphangiogenesis. One such indication is the influence through collagen-binding integrins α1β1 and α2β1 (Hong et al., 2004) discussed above. Another indication is recent genetic screen study in zebrafish showing that ccbe1 (collagen and calcium-binding EGF domain-1) is indispensible for embryonic lymphangiogenesis (Hogan et al., 2009). In an in vitro study exposing LEC and blood endothelial cells (BECs) to collagen type I, the LECs showed increased survival and an increased ability for form tubes without exogenously added growth factors (Podgrabinska et al., 2002) and thus a differential response to the matrix. A direct in vivo effect has also been shown for collagen type I. In secondary lymphedema induced by skin excision and lymph vessel ligation in the mouse tail, wound repair with collagen type I gel significantly reduced the expression of TGF-β1, decreased scarring/fibrosis, and significantly accelerated lymphatic regeneration (Clavin et al., 2008). Collagen also affected function of the regenerated lymph vessels as shown by washout of radioactive tracer. Although indirect, the finding that the collagenase MMP-2 is of importance for the growth of lymphatic cells in a lymphoma model and thus possibly lymphagiogenesis (Bruyere et al., 2008) is another indication for the role of collagen in this process. Interestingly though, an array comparing lymphangiogenic lymphatic endothelial cells isolated from VEGF-C overexpressing tumors with resident dermal lymphatic endothelial cells identified that various collagen genes are highly downregulated in the proliferating lymphatic endothelial cells (Clasper et al., 2008). As an additional proof of collagen involvement is the inhibitory effect of collagen fragments. Endostatin and Neostatin 7, both fragments of the vascular and epithelial collagen type XVIII, were both shown to inhibit lymphangiogenesis (Bruyere et al., 2008; Kojima et al., 2008), suggesting that the effect of collagen on lymphangiogenesis can be a target for therapy.
4. Laminin
The laminins are major components of the basement membranes that are heterotrimers consisting of one α, one β, and one γ chain and named according to their chain composition (Durbeej, 2009). Usually laminin is used as a blood vessel marker and thus to differentiate between blood vessels and lymphatics (e.g. (Hong et al., 2002)), whereas laminins have also been localized to the lymphatic endothelium in normal skin by immunohistochemistry (Vainionpaa et al., 2007). An indication of a possible effect on lymphangiogenesis comes from the fact that various laminins are shown to bind to α1β1, α2β1 and in particular α9β1 integrins (reviewed in (Durbeej, 2009)), molecules that have been discussed above and shown to have a role in lymphangiogenesis. Actually, in a recent paper it was shown that LECs produce 421-laminin that induces chemotaxis of melanoma cells (Saito et al., 2009), and thus that laminin can contribute to lymphangiogenesis and metastasis. Interestingly, reelin, a serine protease of the extracellular matrix that rapidly degrades laminin and fibronectin, is expressed in LEC but not on BEC (reviewed in (Ji, 2006a)) and may therefore influence the development and phenotype of lymphatics.
5. Fibronectin
Fibronectin is a cell adhesion protein found in the matrix surrounding normal cells, and mediates cell adhesion and anchorage through a number of fibronectin-binding integrins (Ruoslahti, 1999) (Fig 2). Of particular importance in this context is α5β1, which binds avidly with fibronectin. From the studies discussed above an important role in lymphangiogenesis may be inferred for the ligand for fibronectin, α5β1. Studies addressing this topic are, however, sparse. LECs have been found to express fibronectin to a similar extent as blood endothelial cells (Podgrabinska et al., 2002). Despite lacking a continuous basal lamina, lymphatic vessels are identified to have patches of fibronectin matrix around them (Oh et al., 1997). In an in vitro study, collagen type I was found to stimulate LEC tube formation in culture, whereas fibronectin had no effect (Leak and Jones, 1994). On the other hand, other studies indicated that lymphatic endothelial cells adhere and proliferate better when cultured on fibronectin-coated plates (Makinen et al., 2001b). Additionally, fibronectin enhances VEGF-C mediated proliferation of human microvascular endothelial cells in vitro, through VEGFR3 (Zhang et al., 2005). Blockade of integrin α5β1 inhibits VEGFR3 phosphorylation. Fibronectin attachment leads to PI3K induction through VEGFR3, which induces endothelial cell survival. Furthermore, fibronectin EDA domain facilitates tube formation of lymphatic endothelial cells in vitro, likely through its receptor integrin α9β1 (Ou et al.). Its interaction with integrin α9β1 additionally has a role in lymph valve development, as discussed previously. Collectively, these findings suggest that fibronectin likely has a role in lymphangiogenesis and lymphatic function, though different splice variants of fibronectin might have different levels of contributions to lymphangiogenesis, possibly depending on their respective receptors.
6. Tenascin-C
Tenascin-C (TN-C) is likely to affect lymphangiogenesis due to its interaction with integrins of potential importance for lymphangiogenesis discussed above (Fig 2). TN-C to is thought to orchestrate tissue morphogenesis by determining cell-cell and cell-matrix interactions, and by functioning as a provisional matrix providing cues for migration, proliferation, or apoptosis (Fiorilli et al., 2008; Hsia and Schwarzbauer, 2005; Jones and Jones, 2000). TN-C is transiently expressed during embryonic development and is greatly reduced in most adult tissues but increases markedly in pathological conditions (Hsia and Schwarzbauer, 2005; Jones and Jones, 2000). Tenascin-C knockout (TN-C-KO) mice are phenotypically normal and viable (Mackie and Tucker, 1999), whereas various phenotypes emerge when the mouse is challenged with pathological insults (Mackie and Tucker, 1999). TN-C can bind to various integrins, including α2β1 and ανβ3, which are expressed on endothelial cells (Joshi et al., 1993; Sriramarao et al., 1993), but TN-C-integrin α9β1 interaction is considered to be of higher avidity (Yokosaki et al., 1996). TN-C interacts with integrin α9β1 through a non-RGD interaction, while its interactions with other integrins are RGD dependent (Schneider et al., 1998; Yokosaki et al., 1998). TN-C expression in tumors correlates with lymphatic metastasis (Orend and Chiquet- Ehrismann, 2006) and it has been identified that proliferating integrin α9β1 expressing vessels have TN-C presence around them during wound healing (Hakkinen et al., 2000). Furthermore, an array has indicated that TN-C expression, as the only ECM molecule, is upregulated in lymphatic endothelial cells during lymphangiogenesis (Clasper et al., 2008). However, a direct link to lymphangiogenesis has not been identified. Indeed, integrin α9β1’s role in lymph valve formation is independent of TN-C (Bazigou et al., 2009).
7. Emilin-1
Emilin-1 is an ECM glycoprotein associated with elastic fibers (Bressan et al., 1993), and although this molecule is most abundant in large blood vessels and is involved in elastogenesis and blood vessel maintenance (Zanetti et al., 2004), it also has been shown to be of importance for lymphangiogenesis (Danussi et al., 2008). Emilin-1 was found to be highly expressed on LECs, it colocalized with lymphatic vessels, and emilin-1−/− mice had a reduction in anchoring filaments, hyperplastic and dysfunctional lymphatic vessels that may explain the observed mild lymphedema. Induction of lymphangioma resulted in larger lymphatics in emilin-1−/− than in wt mice, all of the above showing that this ECM component has a role in modulating lymphangiogenesis (Danussi et al., 2008).
8. Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are not components of the matrix per se but substances that degrade the matrix. MMPs, also called matrixins, are a family of over 20 zinc-containing endopeptidases that are capable of degrading various components of the ECM. All are produced as latent, pro-enzymes, which must be proteolytically processed to be activated (Nagase and Woessner, 1999; Rundhaug, 2005). They are essential for embryonic development, morphogenesis, reproduction, tissue resorption and remodeling, and in this context they may also influence lymphangiogenesis. Whereas an important role has been established for MMPs in angiogenesis (e.g. (Rundhaug, 2005)), the data regarding their role in lymphangiogenesis are more divergent. LECs do not express MMPs (Podgrabinska et al., 2002), and Ets 1, a transcription factor for several matrix-degrading proteases involved in angiogenesis, is not upregulated in lymphatic endothelium (Wernert et al., 2003). Furthermore, MMP-9, shown to be of importance for tumor angiogenesis (reviewed in (Rundhaug, 2005)), does not affect lymphangiogenesis in skin (Rutkowski et al., 2006). All of the above suggest a minor role for MMPs in the lymphangiogenesis process. In contrast, MMP-2 was found to be involved in tube formation of immortalized rat LECs (Matsuo et al., 2007). Moreover, using an in vitro lymphatic ring assay involving thoracic duct, a role for MMP-2 was also suggested in mice (Bruyere et al., 2008). In support of these data, Nakamura and collaborators were able to show that MMP-2 and MMP-9 are expressed in LECs isolated from lymphangiomas, and that the LEC’s tube formation as well as metastatic ability could be inhibited by a synthetic MMP inhibitor (Nakamura et al., 2004). To summarize, the data on MMPs in lymphangiogenesis are diverging. Part of the explanation is probably the variation in origin of the LECs and the experimental models used, a conclusion that calls for additional experiments in this area.
Effect of the biophysical microenvironment
Above we have discussed various growth factors and extracellular matrix elements that may affect lymphangiogenesis, but during the last years it has become increasingly clear that the biophysical microenvironment may also influence the growth of lymph vessels. As noted, one of the functions of the lymphatic system is to maintain tissue fluid balance and to clear proteins that are filtered across the capillaries. Fluid homeostasis is maintained through balancing hydrostatic and colloid osmotic pressures in the capillaries and the interstitium (Aukland and Reed, 1993; Wiig et al., 2003). During homeostasis, there is a net fluid filtration that is removed by lymph, and accordingly a steady flow of fluid towards the lymphatics. Whereas it is well known that mechanical stimuli in the form of shear stress is important for blood vessel growth and remodeling (recently reviewed in e.g. (Hahn and Schwartz, 2009; Shyy and Chien, 2002)) and that mechanical stimuli induce signaling and remodeling of the extracellular matrix (recently reviewed in (Chiquet et al., 2009; Wang et al., 2009)), the fact that interstitial fluid (i.e. initial lymph) per se induces ECM remodeling and lymphangiogenesis has received less attention. Recent in vitro as well as in vivo data, however, point to a role of a slow flow of interstitial fluid through the interstitium as an important morphoregulator and lymphangiogenic factor (Rutkowski and Swartz, 2007; Swartz and Fleury, 2007). In an in vitro model system, interstitial flow was found to induce lymphatic capillary formation (Ng et al., 2004), and the response to flow differed between LECs and BECs. In a skin regeneration model in mouse tail where interstitial flow and generation of initial lymphatics could be followed, flow occurring through interstitial channels were preceding and directing the formation of lymphatics (Boardman and Swartz, 2003). Moreover, the generation of VEGF-C in the tissue appears to be regulated by interstitial fluid flow (Goldman et al., 2007), suggesting that interstitial fluid flow is an important morphogenic cue. Another factor of importance is the driver of interstitial flow, namely the interstitial fluid pressure (Pif). Pif is regulated by the influx and outflux of fluid from the interstitium, but also by fibroblast-induced contraction of the collagen-network in the ECM through its α2β1 and α11β1 integrin receptors (Svendsen et al., 2009; Wiig et al., 2003).
The fact that there is a net fluid filtration in most, if not all, tissues suggests that such flow is of general importance to maintain lymphatic vessel integrity. As will be discussed, recent data suggest that this phenomenon also has implications for lymphatic vessel pathophysiology in relation to lymphedema and cancer metastasis.
Consequences of ECM-lymphatic interactions for tumor formation, growth and metastasis
The initial lymph vessels, apparently having no barrier in their basement membrane and no pericytes restricting cell uptake, may seem ideally suited for uptake of tumor cells. It may therefore not come as a surprise that the recipients of afferent lymphatics, the lymph nodes, are the most common sites for metastasis of many cancers (Sleeman and Thiele, 2009). With the discovery of new molecular markers and the discovery of lymphatic signaling substances, the area of tumor lymphangiogenesis and its effect on tumor growth and metastasis has become an area of intense research. Most of these studies have focused on the influence of the lymphangiogenic factors, especially VEGF-C/VEGF-D and VEGFR-3 signaling, and the reader is referred to several recent excellent reviews on this topic (e.g. (Achen et al., 2005; Achen and Stacker, 2006; Alitalo et al., 2005; Cao, 2005; Ji, 2006b; Lohela et al., 2009; Sleeman and Thiele, 2009)). Since the focus of this review is the ECM, we will consider tumor lymphangiogenesis in this perspective, i.e. the tumor microenvironment or stroma with its ECM elements and mixture of cells associated with the lymphatic endothelium.
Tumor stroma, consisting of fibroblasts, ECM, blood vessels, pericytes, lymphatic vessels, and immune cells, is known to contribute to the development and progression of cancer (Kalluri and Zeisberg, 2006; Nyberg et al., 2008) (Fig 3). As pointed out by Wong and Hynes (Wong and Hynes, 2007), the stroma cells can probably communicate with each other and with the tumor cells through secretion of various signals like growth factors, matrix molecules, chemokines and proteinases that influence the tumor’s ability to metastasize via lymphatics. Compared to the blood vasculature, little is known about the biology of the lymphatic vessels in tumors, the regulation of tumor lymphangiogenesis or the mechanisms that determine the interactions of tumor cells with the lymphatic vessels. Experiments in different tumor models and samples obtained from patients suggest that tumors can induce lymphangiogenesis to a varied extent (Achen and Stacker, 2006). Although many studies indicate that lymphangiogenesis correlates with lymphatic metastasis (Brakenhielm et al., 2007; Burton et al., 2008), there are others suggesting that lymphatic metastasis primarily occurs through the preexisting lymphatic vessels (Padera et al., 2002; Wong et al., 2005). Although peritumoural lymphatic vessels contribute to tumour metastasis, opposite views exist as to whether intratumoural lymphatics have any role in this context (for review see (Ji, 2006b; Saharinen et al., 2004)).
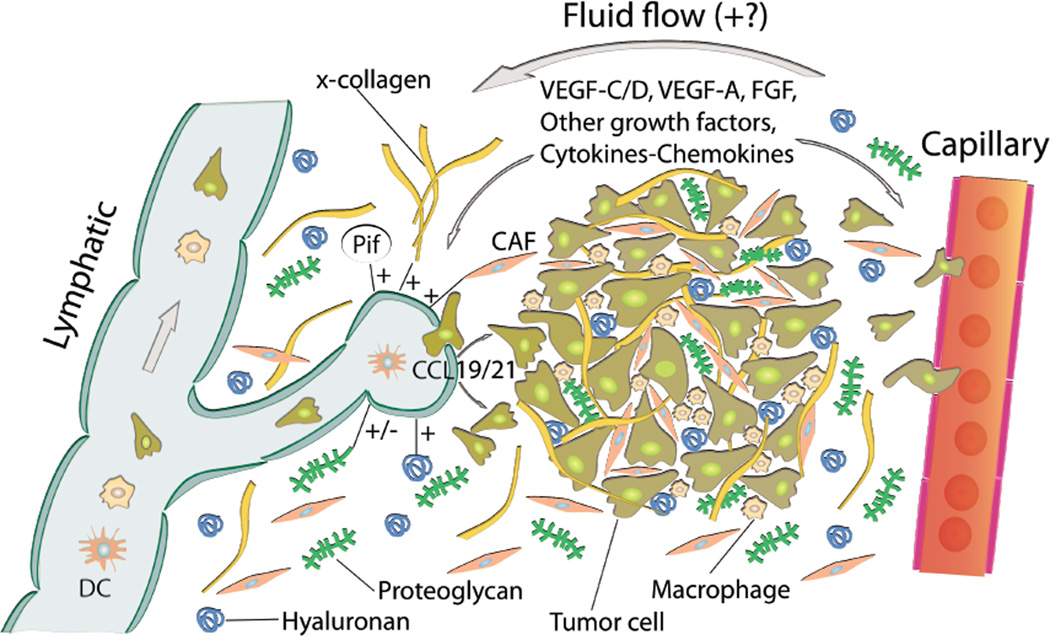
Fig. 3.
Role of the extracellular matrix and microenvironment in lymphangiogensis in tumors. Growth factors and cytokines produced by tumor cells and stroma are transported by fluid flow and down a diffusion gradient to lymphatics and blood capillaries. Tumor and immune cells (expressing CCR7) are chemoattracted to and enter peritumoral initial lymphatics expressing CCL19/21. + (plus) and − (minus) denotes stimulating and inhibiting lymphangiogenesis, respectively. x-collagen: crosslinked collagen, Pif: interstitial fluid pressure, CAF: cancer associated fibroblast.
As pointed out in several reviews, it is highly likely that the tumor cells themselves secrete lymphangiogenic factors and thus contribute to new vessels (e.g. (Alitalo et al., 2005; Mumprecht and Detmar, 2009; Sleeman and Thiele, 2009)). Overexpression studies using VEGF-C (Skobe et al., 2001) and VEGF-D (Stacker et al., 2001) both gave increased metastasis, although as pointed out by Wong and Hynes (Wong and Hynes, 2007) direct signalling between tumor cells and lymphatic endothelial cells has not been conclusively demonstrated in vivo.
The changes known to occur in the tumor stroma and microenvironment (Kalluri and Zeisberg, 2006; Nyberg et al., 2008) will significantly affect lymphangiogenesis. During malignant transformation, there is an influx of fibroblasts that acquire a cancer-activated phenotype named cancer-associated fibroblast (CAF). As a consequence, there is increased ECM deposition, increased inflammation and angiogenesis, most likely induced by a complicated exchange of paracrine signals (Wong and Hynes, 2007). Whereas it is well established that stroma cells and fibroblasts are central in secreting angiogenetic factors (Fukumura et al., 1998), less is known on lymphangiogenic factors in this setting. It is, however, likely that such secretion occurs since inflammation is a central element in tumor progression (Coussens and Werb, 2002; Mantovani et al., 2008), and immune cells are important sources for lymphangiogenetic factors. Macrophages, being capable of secreting lymphagiogenic factors and inducing lymphangiogenesis, are probably of special importance here (Schoppmann et al., 2002). Such an effect was shown in a recent study where infiltrating macrophages were judged to be responsible for lymphangiogenesis in an ovarian cancer model (Jeon et al., 2008). Of interest though, these new lymph vessels were shown to be dysfunctional, showing that a functional evaluation may be crucial to reveal the potential clinical importance of lymphangiogenesis.
Although a role for macrophages in lymphangiogenesis is established (reviewed in (Coffelt et al., 2009)), less is known on the role of the other cells types in stroma in this respect. Recently, however, it was shown that CAFs also contribute to lymphangiogenesis. Liao et al (Liao et al., 2009) eliminated CAFs an in vivo mouse model of breast carcinoma by a vaccine targeting a fibroblast activation protein. One of the resulting effects was reduced lymphangiogenesis, suggesting a role for fibroblasts in lymph vessel generation.
The fact that fibroblasts are activated thus inducing changes in the production of stroma factors and a more abundant ECM may per se affect the growth of lymphatics. In overexpression studies, such a role has been shown for HA in a transgenic mouse model of breast cancer (Koyama et al., 2008). These mice actively produce HA in Neu-initiated mammary tumors, resulting in a 16 fold greater density of intratumoral (defined as vessels located in the central part of the tumor) and 1.5 fold increase only in peritumoral (defined as vessels in the peripheral part of tumor tissues) lymphatic vessels. Importantly, in separate in vivo experiments it was shown that acceleration of stroma formation by HA-producing tumor activated fibroblasts co-implanted with tumor cells resulted in increased tumor growth and lymphangiogenesis. Although these experiments show a significant role of the stroma on lymphangiogenesis, the differential effect on tumoral and peritumoral lymphatics is puzzling. Considering that intratumoral lymphatics may not be functional (Padera et al., 2002), these newly generated lymphatics may not drain cells and fluid and calls for experiments to assess lymph drainage in this model. That CAFs may influence lymphangiogenesis was also shown in a study on human colorectal cancer demonstrating correlation between proliferating CAFs and peritumoral lymphagiogenesis, lymph vessel invasion and lymph node metastasis (Liang et al., 2005). Whether other glycosaminoglycans affect lymphangiogenesis is not known. Actually, data showing that heparanase, an enzyme degrading the heparan sulphate and thus the matrix, points in the opposite direction of what has been shown for HA (Cohen-Kaplan et al., 2008). Heparanase was associated with increased lymphatic density in human tumors, and induced lymphangiogenesis and VEGF-C production in vivo. These effects may be due to enzymatic activity and sprouting of endothelial cells as well as release of heparan sulphate bound growth factors and not to the amount of heparan sulphate per se.
Influence of the biophysical characteristics of stroma
The physico-chemical properties may also influence generation of new lymphatic vessels and lymphatic function. It is established that tumor solid stress resulting from increased interstitial fluid pressure may induce angiogenesis (Butcher et al., 2009), less is known about a corresponding effect in lymphangiogenesis. Recently, however, it was shown that an osteosarcoma cell line upregulated VEGF-C when exposed to an increased pressure mimicking that of a solid tumor (Nathan et al., 2008), suggesting that the high interstitial fluid pressure prevailing in a tumor may indeed affect lymphangiogenesis. Here it is interesting to note that tumor growth and invasion is affected by collagen crosslinking and tissue fibrosis, thereby involving integrins, notably α4β1 (Levental et al., 2009), which has been shown to have a central role in tumor lymphangiogenenesis (Garmy-Susini et al., 2010; Garmy-Susini et al., 2007). Another integrin, α11β1, also contributing in Pif regulation (Svendsen et al., 2009), has been found to strongly influence tumor growth when expressed on fibroblasts and co-implanted with several tumor cell lines (Zhu et al., 2007). It is likely that such crosslinking also affects Pif. As discussed above, integrins contribute in Pif regulation (Wiig et al., 2003), and since they may be targeted this may represent a way to reduce Pif and thereby reduce tumor lymphangiogenesis, growth and invasiveness.
Although likely to occur, the lymphangiogenic effect of interstitial fluid flow found in skin (Boardman and Swartz, 2003) has not been demonstrated in tumors. It is likely that such fluid flow directs tumor cells to lymphatics through a process called autologous chemotaxis and thus to promote tumor metastasis (Shields et al., 2007). Taken together, these phenomena shows that tumor lymphangiogenesis may be strongly affected by physico-chemical factors prevailing in the tumor microenvironment.
Effect of malfunctioning or missing lymphatics on ECM
Above we have discussed ECM-factors that may affect lymphangiogenesis. Oppositely, there can also be an effect on ECM by lymph vessels, most evident in situations where lymphatics are malfunctioning or missing, leading to fluid accumulation in the tissue called lymphedema. Lymphoedema can be primary if the cause is congenital or unknown or secondary if the condition results from an obstruction of lymphatics due to e.g. surgery or infection. Classically, the edema fluid in lymphedema is supposed to have a high protein concentration as a consequence of the edema’s lymphatic origin (Knight et al., 1987; Taylor et al., 1958), again supposedly resulting in an inflammatory reaction (Casley-Smith and Gaffney, 1981). As reviewed by Rockson (Rockson, 2001), chronic lymph stasis in humans often produces an increase in the number of fibroblasts, adipocytes, macrophages and keratinocytes in the edematous tissues. In most patients, there is an increase in collagen, fat and ECM deposition in the edematous skin and subcutaneous tissues. Ultimately, these processes lead to progressive subcutaneous fibrosis.
Although it is a widely held view that high interstitial protein concentration is an important pathogenetic factor in lymphedema (e.g. (Alitalo et al., 2005; Rockson, 2001)), more recent studies, however, have reported decreased (Bates et al., 1993) as well as unaltered (Olszewski, 2003) protein concentration in interstitial fluid and lymph from humans subject to secondary lymphedema, questioning this tenet. Another controversial issue that has not been resolved is whether an inflammatory reaction is an important part of the pathogenesis. In support of such a view, Olszewski (Olszewski, 2003) reported increased levels of inflammatory cytokines in lymph from secondary lymphedema patients, suggesting a role for inflammation in the pathogenesis of this condition, a conclusion that is also supported by animal studies (Tabibiazar et al., 2006). Recently, a new therapeutic modality working through the ECM was proposed for secondary lymphedema and wound healing. Mesenchymal stem cells were capable of expressing a lymphatic phenotype when stimulated with lymph-inductive media and purified VEGF-C, and to restore lymph flow when injected into a lymphedematous area (Conrad et al., 2009).
The pathogenetic characteristics and mechanisms behind lymphedema have also been addressed in animal models of primary lymphedema. In Chy-mice, having a mutation in the Vegfr-3-gene mimicking the human condition Milroy’s disease (Karkkainen et al., 2001), Karlsen et al demonstrated that these mice had high-protein edema of the hindlimbs (Karlsen et al., 2006) but no increase in inflammatory cytokines in their interstitial fluid that might be expected if an inflammatory reaction was prevalent. This observation is supported by experiments in the K14-VEGFR-3-Ig mouse model of primary lymphedema expressing soluble VEGFR-3 (Makinen et al., 2001a) also having a high relative concentration of interstitial fluid proteins and actually showing a reduced level of proinflammatory cytokines and macrophage infiltration in edematous skin (Markhus et el, unpublished). These observations indicate that a high tissue fluid protein concentration per se is not sufficient to induce the phenotypical tissue change associated with lymphedema. The discrepancy between primary and secondary lymphedema may be a result of the post-surgical and post-inflammatory condition that preceded the lymphedema and/or, in the case of patients, that they were studied at a more developed stage of the condition.
Another question is whether missing or dysfunctional lymphatics resulting in lymphedema induce fat accumulation, as suggested human studies and experimental models of acquired lymphedema (Rockson, 2001). In support of such a notion, increased lipid accumulation, especially in the intestine and mesentery, was found in Prox1+/−-mice having leaky and dysfunctional lymph vessels (Harvey et al., 2005). That lymphedema per se is not sufficient to induce fat accumulation was shown in a recent study by Rutkowski et al (Rutkowski et al., 2010). In the Chy and K14-VEGFR-3-Ig mice, both exhibiting lymphedema in the skin resulting from lack of dermal lymphatic capillaries, they demonstrated a markedly different tissue adaptation. Whereas lipid (and collagen) deposition was increased in the Chy model, thus mimicking the human condition, the K14-VEGFR-3-Ig mice actually had lower lipid content (and unaltered collagen) in lymphedematous tissue when compared to wild type mice, again impacting the hydraulic conductivities of the skin. Clearly, the difference between strains demonstrates the potential for lymphedema to be phenotypically similar, yet pathologically and functionally quite different on a case-to-case basis. There must be additional factors modulating the tissue response, calling for more mechanistic studies in this field.
Potential clinical implications
The emerging knowledge on the lymphatic system may have implications for therapy. This applies to conditions where it may be beneficial that lymphangiogenesis is reduced, e.g. in tumors and inflammatory conditions of the cornea as well as the opposite situation where dysfunction or obstruction results in lymph accumulation and accordingly lymphangiogenesis should be increased.
Due probably to the serious consequences, most focus has been given to the possibility of reducing tumor growth and metastasis by reducing lymphangiogenesis. When considering the molecular mechanisms, not unexpectedly the VEGF-C/VEGFD/VEGFR-3 signaling system is currently the most attractive target for antilymphangiogenic therapeutics designed to restrict cancer metastasis as covered in several excellent recent reviews, e.g. (Alitalo et al., 2005; Karpanen and Alitalo, 2008; Lohela et al., 2009; Mumprecht and Detmar, 2009; Sleeman and Thiele, 2009; Sundar and Ganesan, 2007; Zwaans and Bielenberg, 2007). From the ECM perspective used here, targeting the integrins expressed on tumors might seem a reasonable strategy. As reviewed by Varner and collaborators (Avraamides et al., 2008), several integrin antagonists are in phase I/II clinical studies. These antagonists, however, target integrins mainly expressed on blood and not lymphatic vessels, and whether they will affect lymphangiogenesis is unknown. One of these substances is volociximab, blocking α5β1-integrin, which although expressed in lymphatics in inflamed cornea do not appear to play a role in tumor lymphangiogenesis (Avraamides et al., 2008). Preliminary data, however, suggest that blocking α4β1- integrin expressed on lymphatics reduce tumor growth as well as metastasis (Garmy-Susini et al., 2007), but a more thorough evaluation is needed. Although not studied in tumors, other possible candidate molecules might be the collagen – binding α1β1, α2β1 and α11β1 integrins (Svendsen et al., 2009; Wiig et al., 2003). A possible outcome might be to reduce interstitial fluid pressure and thereby uptake of therapeutics. Additionally, it may also result in reduction of lymph flow and thus metastasis. Other possible targets might be the laminin-binding integrins α3β1 and α6β1, shown to mediate chemotaxis in melanoma cells and potential lymphatic metastasis (Saito et al., 2009), and α9β1 integrin shown to have a crucial role in formation of lymphatic vessels (Bazigou et al., 2009).
Various matrix derived antiangiogenic molecules, such as endostatin, tumstatin, canstatin and arresten have been identified (Clamp and Jayson, 2005; Nyberg et al., 2008). These substances are released as fragments of ECM molecules through degradation by MMPs during the process of formation of new blood vessels. Considering that MMPs are secreted by lymphatic endothelial cells as well during lymphangiogenesis, it is possible that these matrix derived endogeneous inhibitors might play a role in regulating lymphangiogenesis as well. Although lymphatics lack basement membranes, endostatin, a fragment of the basement membrane protein collagen type XVIII usually considered as an angiogenesis inhibitor, has been shown to inhibit lymphangiogenesis and lymphatic metastasis in two models of squamous cell carcinoma (Brideau et al., 2007; Fukumoto et al., 2005). A proposed mechanism of action was a reduction in inflammation and differentiation, as suggested by a reduced VEGF-C production through reduced mast cell infiltration of the tumor ECM in endostatin-treated animals. Whether endostatin has a direct effect on lymphatics, through its interaction with integrin α5β1 as in the context of angiogenesis is not known. Additionally, a study indicates that tumstatin inhibits lymphatic metastasis (Chung et al., 2008), although it is not yet clear whether this happens through inhibition of lymphangiogenesis.
Another potential target would be MMPs, where inhibitors are undergoing clinical testing due to antiangiogenesis effect (Roy et al., 2009). Whereas MMPs are likely to be less important in relation to lymphangiogenesis as discussed above and MMPs may have tumor promoting as well as repressing effects (e.g. reviewed in (Roy et al., 2009)), the fact that LEC tube formation as well as metastatic ability could be inhibited by a synthetic MMP inhibitor (Nakamura et al., 2004) should spur further investigation. Clearly, more knowledge on the phenotypical consequences of derangements in ECM genotype may shed new light on the role of these factors in tumor development. We must, however, also consider microenvironmental and ECM factors, the reason being these in a wider sense influence lymphangiogenesis and tumor metastasis, and that ECM-factors may even be targeted.
Angiogenesis inhibitors are in clinical use and several new ones are undergoing clinical trials (reviewed in (Crawford and Ferrara, 2009)). It is highly likely that part of their effect regarding growth and survival is related to reduction in lymphangiogenesis (and lymphatic metastasis) as observed in inflamed cornea (Bock et al., 2007). This assumption is supported by recent data showing that a monoclonal antibody, αPlGF, targeting placenta growth factor resulting in reduced angiogenesis and tumor growth, also reduced lymphangiogenesis and lymph node metastasis (Fischer et al., 2007). At least partially mediated through a reduced mobilization of macrophages, these results suggest that the tumor stroma can be targeted. It is furthermore possible that part of the effect ascribed to angiogenesis may be due to reduced lymphangiogenesis.
In contrast, in the lymphedema situation where enhanced lymphatic function is the desired therapeutic effect, promising results have been obtained in preclinical models using viral gene transfer vectors encoding for lymphangiogenic growth factors (reviewed in (Alitalo et al., 2005)), e.g. VEGF-C (Karkkainen et al., 2001). Other factors like inhibitors of transforming growth factor β, a factor shown to inhibit lymphangiogenesis (Oka et al., 2008), should also be considered. Still, our study in two lymphedema mouse strains, demonstrating pathophysiological difference in phenotypically similar mice, shows that extracellular matrix and tissue remodeling need to be taken into consideration during therapy (Rutkowski et al., 2010). These results indicate that therapies for lymphedema targeting the growth of new lymphatic vessels only or surgical correction of lymphatic ligations may face limited success. Rather, comprehensive therapeutic considerations should also integrate preventing or counteracting collagen and lipid accumulation to restore normal matrix composition and a reduction in adiposity to improve interstitial transport, remodel the tissue, and remediate fluid balance.
Summary
The lymphatic system is important for body fluid balance as well as immunological surveillance. Whereas often overlooked, the extracellular matrix is of central importance for the generation of new lymphatic vessels as a response to physiological and pathological stimuli. During the recent years, anti-lymphangiogenesis has emerged as an additional therapeutic modality to the clinically applied anti-angiogenesis strategy. Oppositely, enhancement of lymphangiogenesis in situations of lymph accumulation is seen as a promising strategy to a set of conditions where few therapeutic avenues are available. Knowledge on the interaction between the extracellular matrix and the lymphatics may enhance our understanding of the underlying mechanisms and may ultimately lead to better therapies for conditions where reduced or increased lymphatic function is the therapeutic target.
Acknowledgements
The work in the author’s laboratory is supported by the NIH grants CA125550 and DK 55001, the Champalimaud Metastasis Program and the research funds of the Division of Matrix Biology at the Beth Israel Deaconess Medical Center. HW is a recipient of grants from The Research Council of Norway (project 186128/V40) and the Faculty of Medicine and Dentistry, University of Bergen, Norway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell. 2005;7:121–127. doi: 10.1016/j.ccr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Achen MG, Stacker SA. Tumor lymphangiogenesis and metastatic spread-new players begin to emerge. Int J Cancer. 2006;119:1755–1760. doi: 10.1002/ijc.21899. [DOI] [PubMed] [Google Scholar]
- Adair TH, Moffatt DS, Paulsen AW, Guyton AC. Quantitation of changes in lymph protein concentration during lymph node transit. Am J Physiol. 1982;243:H351–H359. doi: 10.1152/ajpheart.1982.243.3.H351. [DOI] [PubMed] [Google Scholar]
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- Allan AL, George R, Vantyghem SA, Lee MW, Hodgson NC, Engel CJ, Holliday RL, Girvan DP, Scott LA, Postenka CO, et al. Role of the integrin-binding protein osteopontin in lymphatic metastasis of breast cancer. Am J Pathol. 2006;169:233–246. doi: 10.2353/ajpath.2006.051152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszodi A, Legate KR, Nakchbandi I, Fassler R. What mouse mutants teach us about extracellular matrix function. Annu Rev Cell Dev Biol. 2006;22:591–621. doi: 10.1146/annurev.cellbio.22.010305.104258. [DOI] [PubMed] [Google Scholar]
- Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzali G. Transendothelial transport and migration in vessels of the apparatus lymphaticus periphericus absorbens (ALPA) Int Rev Cytol. 2003;230:41–87. doi: 10.1016/s0074-7696(03)30002-6. [DOI] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger-Tobler NE, Halin C, Kajiya K, Detmar M. Growth hormone promotes lymphangiogenesis. Am J Pathol. 2008;173:586–597. doi: 10.2353/ajpath.2008.080060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO, Levick JR, Mortimer PS. Change in macromolecular composition of interstitial fluid from swollen arms after breast cancer treatment, and its implications. Clin Sci (Lond) 1993;85:737–746. doi: 10.1042/cs0850737. [DOI] [PubMed] [Google Scholar]
- Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Makinen T. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman KC, Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circ Res. 2003;92:801–808. doi: 10.1161/01.RES.0000065621.69843.49. [DOI] [PubMed] [Google Scholar]
- Bock F, Onderka J, Dietrich T, Bachmann B, Kruse FE, Paschke M, Zahn G, Cursiefen C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2545–2552. doi: 10.1167/iovs.06-0570. [DOI] [PubMed] [Google Scholar]
- Brakenhielm E, Burton JB, Johnson M, Chavarria N, Morizono K, Chen I, Alitalo K, Wu L. Modulating metastasis by a lymphangiogenic switch in prostate cancer. Int J Cancer. 2007;121:2153–2161. doi: 10.1002/ijc.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan GM, Daga-Gordini D, Colombatti A, Castellani I, Marigo V, Volpin D. Emilin, a component of elastic fibers preferentially located at the elastin-microfibrils interface. J Cell Biol. 1993;121:201–212. doi: 10.1083/jcb.121.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau G, Makinen MJ, Elamaa H, Tu H, Nilsson G, Alitalo K, Pihlajaniemi T, Heljasvaara R. Endostatin overexpression inhibits lymphangiogenesis and lymph node metastasis in mice. Cancer Res. 2007;67:11528–11535. doi: 10.1158/0008-5472.CAN-07-1458. [DOI] [PubMed] [Google Scholar]
- Bruyere F, Melen-Lamalle L, Blacher S, Roland G, Thiry M, Moons L, Frankenne F, Carmeliet P, Alitalo K, Libert C, et al. Modeling lymphangiogenesis in a three-dimensional culture system. Nat Methods. 2008;5:431–437. doi: 10.1038/nmeth.1205. [DOI] [PubMed] [Google Scholar]
- Burton JB, Priceman SJ, Sung JL, Brakenhielm E, An DS, Pytowski B, Alitalo K, Wu L. Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res. 2008;68:7828–7837. doi: 10.1158/0008-5472.CAN-08-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer. 2005;5:735–743. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- Casley-Smith JR, Gaffney RM. Excess plasma proteins as a cause of chronic inflammation and lymphodema: quantitative electron microscopy. J Pathol. 1981;133:243–272. doi: 10.1002/path.1711330307. [DOI] [PubMed] [Google Scholar]
- Cheresh DA, Stupack DG. Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene. 2008;27:6285–6298. doi: 10.1038/onc.2008.304. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 2009;1793:911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Chung IS, Son YI, Ko YJ, Baek CH, Cho JK, Jeong HS, et al. Peritumor injections of purified tumstatin delay tumor growth and lymphatic metastasis in an orthotopic oral squamous cell carcinoma model. Oral Oncol. 2008;44:1118–1126. doi: 10.1016/j.oraloncology.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Clamp AR, Jayson GC. The clinical potential of antiangiogenic fragments of extracellular matrix proteins. Br J Cancer. 2005;93:967–972. doi: 10.1038/sj.bjc.6602820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasper S, Royston D, Baban D, Cao Y, Ewers S, Butz S, Vestweber D, Jackson DG. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res. 2008;68:7293–7303. doi: 10.1158/0008-5472.CAN-07-6506. [DOI] [PubMed] [Google Scholar]
- Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A, Mehrara BJ. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol. 2008;295:H2113–H2127. doi: 10.1152/ajpheart.00879.2008. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796:11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Cohen-Kaplan V, Naroditsky I, Zetser A, Ilan N, Vlodavsky I, Doweck I. Heparanase induces VEGF C and facilitates tumor lymphangiogenesis. Int J Cancer. 2008;123:2566–2573. doi: 10.1002/ijc.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C, Niess H, Huss R, Huber S, von Luettichau I, Nelson PJ, Ott HC, Jauch KW, Bruns CJ. Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation. 2009;119:281–289. doi: 10.1161/CIRCULATIONAHA.108.793208. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford Y, Ferrara N. VEGF inhibition: insights from preclinical and clinical studies. Cell Tissue Res. 2009;335:261–269. doi: 10.1007/s00441-008-0675-8. [DOI] [PubMed] [Google Scholar]
- Danussi C, Spessotto P, Petrucco A, Wassermann B, Sabatelli P, Montesi M, Doliana R, Bressan GM, Colombatti A. Emilin1 deficiency causes structural and functional defects of lymphatic vasculature. Mol Cell Biol. 2008;28:4026–4039. doi: 10.1128/MCB.02062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Onderka J, Bock F, Kruse FE, Vossmeyer D, Stragies R, Zahn G, Cursiefen C. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol. 2007;171:361–372. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej M. Laminins. Cell Tissue Res. 2009 doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- El-Chemaly S, Malide D, Zudaire E, Ikeda Y, Weinberg BA, Pacheco-Rodriguez G, Rosas IO, Aparicio M, Ren P, MacDonald SD, et al. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci U S A. 2009;106:3958–3963. doi: 10.1073/pnas.0813368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorilli P, Partridge D, Staniszewska I, Wang JY, Grabacka M, So K, Marcinkiewicz C, Reiss K, Khalili K, Croul SE. Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation. Lab Invest. 2008;88:1143–1156. doi: 10.1038/labinvest.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118:40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Morifuji M, Katakura Y, Ohishi M, Nakamura S. Endostatin inhibits lymph node metastasis by a down-regulation of the vascular endothelial growth factor C expression in tumor cells. Clin Exp Metastasis. 2005;22:31–38. doi: 10.1007/s10585-005-3973-5. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, Thurston G, Jackson DG. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmy-Susini B, Avraamides CJ, Schmid MC, Foubert P, Ellies LG, Barnes L, Feral C, Papayannopoulou T, Lowy A, Blair SL. Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 2010;70:3042–3051. doi: 10.1158/0008-5472.CAN-09-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmy-Susini B, Makale M, Fuster M, Varner JA. Methods to study lymphatic vessel integrins. Methods Enzymol. 2007;426:415–438. doi: 10.1016/S0076-6879(07)26018-5. [DOI] [PubMed] [Google Scholar]
- Gelse K, Poschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gerli R, Solito R, Weber E, Agliano M. Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology. 2000;33:148–157. [PubMed] [Google Scholar]
- Goldman J, Conley KA, Raehl A, Bondy DM, Pytowski B, Swartz MA, Rutkowski JM, Jaroch DB, Ongstad EL. Regulation of lymphatic capillary regeneration by interstitial flow in skin. Am J Physiol Heart Circ Physiol. 2007;292:H2176–H2183. doi: 10.1152/ajpheart.01011.2006. [DOI] [PubMed] [Google Scholar]
- Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkinen L, Hildebrand HC, Berndt A, Kosmehl H, Larjava H. Immunolocalization of tenascin-C, alpha9 integrin subunit, and alphavbeta6 integrin during wound healing in human oral mucosa. J Histochem Cytochem. 2000;48:985–998. doi: 10.1177/002215540004800712. [DOI] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- Heino J. The collagen family members as cell adhesion proteins. Bioessays. 2007;29:1001–1010. doi: 10.1002/bies.20636. [DOI] [PubMed] [Google Scholar]
- Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, Schulte-Merker S. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet. 2009;41:396–398. doi: 10.1038/ng.321. [DOI] [PubMed] [Google Scholar]
- Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, Detmar M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- Hsia HC, Schwarzbauer JE. Meet the tenascins: multifunctional and mysterious. J Biol Chem. 2005;280:26641–26644. doi: 10.1074/jbc.R500005200. [DOI] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost. 2007;5(Suppl 1):32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol Rev. 2009;230:216–231. doi: 10.1111/j.1600-065X.2009.00803.x. [DOI] [PubMed] [Google Scholar]
- Jeon BH, Jang C, Han J, Kataru RP, Piao L, Jung K, Cha HJ, Schwendener RA, Jang KY, Kim KS, et al. Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res. 2008;68:1100–1109. doi: 10.1158/0008-5472.CAN-07-2572. [DOI] [PubMed] [Google Scholar]
- Ji RC. Lymphatic endothelial cells, lymphangiogenesis, and extracellular matrix. Lymphat Res Biol. 2006a;4:83–100. doi: 10.1089/lrb.2006.4.83. [DOI] [PubMed] [Google Scholar]
- Ji RC. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: New insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 2006b;25:677–694. doi: 10.1007/s10555-006-9026-y. [DOI] [PubMed] [Google Scholar]
- Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Joshi P, Chung CY, Aukhil I, Erickson HP. Endothelial cells adhere to the RGD domain and the fibrinogen-like terminal knob of tenascin. J Cell Sci. 1993;106(Pt 1):389–400. doi: 10.1242/jcs.106.1.389. [DOI] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Jurisic G, Detmar M. Lymphatic endothelium in health and disease. Cell Tissue Res. 2009;335:97–108. doi: 10.1007/s00441-008-0644-2. [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen TV, Karkkainen MJ, Alitalo K, Wiig H. Transcapillary fluid balance consequences of missing initial lymphatics studied in a mouse model of primary lymphoedema. J Physiol. 2006;574:583–596. doi: 10.1113/jphysiol.2006.108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Knight KR, Collopy PA, McCann JJ, Vanderkolk CA, Coe SA, Barton RM, Chen HC, O'Brien BM. Protein metabolism and fibrosis in experimental canine obstructive lymphedema. J Lab Clin Med. 1987;110:558–566. [PubMed] [Google Scholar]
- Kojima T, Azar DT, Chang JH. Neostatin-7 regulates bFGF-induced corneal lymphangiogenesis. FEBS Lett. 2008;582:2515–2520. doi: 10.1016/j.febslet.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Kobayashi N, Harada M, Takeoka M, Kawai Y, Sano K, Fujimori M, Amano J, Ohhashi T, Kannagi R, et al. Significance of tumor-associated stroma in promotion of intratumoral lymphangiogenesis: pivotal role of a hyaluronan-rich tumor microenvironment. Am J Pathol. 2008;172:179–193. doi: 10.2353/ajpath.2008.070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak LV. The structure of lymphatic capillaries in lymph formation. Fed Proc. 1976;35:1863–1871. [PubMed] [Google Scholar]
- Leak LV, Jones M. Lymphangiogenesis in vitro: formation of lymphatic capillary-like channels from confluent monolayers of lymphatic endothelial cells. In Vitro Cell Dev Biol Anim. 1994;30A:512–518. [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Hong JW, Ubukata H, Liu G, Katano M, Motohashi G, Kasuga T, Watanabe Y, Nakada I, Tabuchi T. Myofibroblasts correlate with lymphatic microvessel density and lymph node metastasis in early-stage invasive colorectal carcinoma. Anticancer Res. 2005;25:2705–2712. [PubMed] [Google Scholar]
- Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4:e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Ma GC, Liu CS, Chang SP, Yeh KT, Ke YY, Chen TH, Wang BB, Kuo SJ, Shih JC, Chen M. A recurrent ITGA9 missense mutation in human fetuses with severe chylothorax: possible correlation with poor response to fetal therapy. Prenat Diagn. 2008;28:1057–1063. doi: 10.1002/pd.2130. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Tucker RP. The tenascin-C knockout revisited. J Cell Sci. 1999;112(Pt 22):3847–3853. doi: 10.1242/jcs.112.22.3847. [DOI] [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001a;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001b;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz C, Taooka Y, Yokosaki Y, Calvete JJ, Marcinkiewicz MM, Lobb RR, Niewiarowski S, Sheppard D. Inhibitory effects of MLDG-containing heterodimeric disintegrins reveal distinct structural requirements for interaction of the integrin alpha 9beta 1 with VCAM-1, tenascin-C, and osteopontin. J Biol Chem. 2000;275:31930–31937. doi: 10.1074/jbc.M003209200. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Sakurai H, Koizumi K, Saiki I. Curcumin inhibits the formation of capillary-like tubes by rat lymphatic endothelial cells. Cancer Lett. 2007;251:288–295. doi: 10.1016/j.canlet.2006.11.027. [DOI] [PubMed] [Google Scholar]
- McGeown JG, McHale NG, Thornbury KD. The effect of electrical stimulation of the sympathetic chain on peripheral lymph flow in the anaesthetized sheep. J Physiol. 1987;393:123–133. doi: 10.1113/jphysiol.1987.sp016814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol. 1976;261:255–269. doi: 10.1113/jphysiol.1976.sp011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Mishima K, Watabe T, Saito A, Yoshimatsu Y, Imaizumi N, Masui S, Hirashima M, Morisada T, Oike Y, Araie M, et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol Cell. 2007;18:1421–1429. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, Maekawa H, Kimura Y, Ohmura M, Miyamoto T, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. J Cell Mol Med. 2009;13:1405–1416. doi: 10.1111/j.1582-4934.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuchamy M, Zawieja D. Molecular regulation of lymphatic contractility. Ann N Y Acad Sci. 2008;1131:89–99. doi: 10.1196/annals.1413.008. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Nakamura ES, Koizumi K, Kobayashi M, Saiki I. Inhibition of lymphangiogenesis-related properties of murine lymphatic endothelial cells and lymph node metastasis of lung cancer by the matrix metalloproteinase inhibitor MMI270. Cancer Sci. 2004;95:25–31. doi: 10.1111/j.1349-7006.2004.tb03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan SS, Huvos AG, Casas-Ganem JE, Yang R, Linkov I, Sowers R, DiResta GR, Gorlick R, Healey JH. Tumor interstitial fluid pressure may regulate angiogenic factors in osteosarcoma. J Orthop Res. 2008;26:1520–1525. doi: 10.1002/jor.20633. [DOI] [PubMed] [Google Scholar]
- Ng CP, Helm CL, Swartz MA. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc Res. 2004;68:258–264. doi: 10.1016/j.mvr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Salo T, Kalluri R. Tumor microenvironment and angiogenesis. Front Biosci. 2008;13:6537–6553. doi: 10.2741/3173. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Jeltsch MM, Birkenhager R, McCarthy JE, Weich HA, Christ B, Alitalo K, Wilting J. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR, Miyazono K. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood. 2008;111:4571–4579. doi: 10.1182/blood-2007-10-120337. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Ni A, Ayeni OA, Baluk P, Yao LC, Vossmeyer D, Zischinsky G, Zahn G, Knolle J, Christner C, et al. alpha5beta1 Integrin blockade inhibits lymphangiogenesis in airway inflammation. Am J Pathol. 2009;174:2378–2387. doi: 10.2353/ajpath.2009.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski WL. Pathophysiological aspects of lymphedema of human limbs: I. Lymph protein composition. Lymphat Res Biol. 2003;1:235–243. doi: 10.1089/153968503768330265. [DOI] [PubMed] [Google Scholar]
- Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett. 2006;244:143–163. doi: 10.1016/j.canlet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Ou JJ, Wu F, Liang HJ. Colorectal tumor derived fibronectin alternatively spliced EDA domain exserts lymphangiogenic effect on human lymphatic endothelial cells. Cancer Biol Ther. 2010;9 doi: 10.4161/cbt.9.3.10651. [DOI] [PubMed] [Google Scholar]
- Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A. 2002;99:16069–16074. doi: 10.1073/pnas.242401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol. 2005;12:267–277. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Randolph GJ. Dendritic cell migration to lymph nodes: cytokines, chemokines, and lipid mediators. Semin Immunol. 2001;13:267–274. doi: 10.1006/smim.2001.0322. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- Rockson SG. Lymphedema. Am J Med. 2001;110:288–295. doi: 10.1016/s0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- Rutkowski JM, Boardman KC, Swartz MA. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am J Physiol Heart Circ Physiol. 2006;291:H1402–H1410. doi: 10.1152/ajpheart.00038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski JM, Markhus CE, Gyenge CC, Alitalo K, Wiig H, Swartz MA. Dermal Collagen and Lipid Deposition Correlate with Tissue Swelling and Hydraulic Conductivity in Murine Primary Lymphedema. Am J Pathol. 2010 doi: 10.2353/ajpath.2010.090733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski JM, Swartz MA. A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol. 2007;17:44–50. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25:387–395. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Saito N, Hamada J, Furukawa H, Tsutsumida A, Oyama A, Funayama E, Saito A, Tsuji T, Tada M, Moriuchi T, et al. Laminin-421 produced by lymphatic endothelial cells induces chemotaxis for human melanoma cells. Pigment Cell Melanoma Res. 2009;22:601–610. doi: 10.1111/j.1755-148X.2009.00590.x. [DOI] [PubMed] [Google Scholar]
- Sasisekharan R, Raman R, Prabhakar V. Glycomics approach to structure-function relationships of glycosaminoglycans. Annu Rev Biomed Eng. 2006;8:181–231. doi: 10.1146/annurev.bioeng.8.061505.095745. [DOI] [PubMed] [Google Scholar]
- Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Schneider H, Harbottle RP, Yokosaki Y, Kunde J, Sheppard D, Coutelle C. A novel peptide, PLAEIDGIELTY, for the targeting of alpha9beta1-integrins. FEBS Lett. 1998;429:269–273. doi: 10.1016/s0014-5793(98)00612-7. [DOI] [PubMed] [Google Scholar]
- Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Shin JW, Min M, Larrieu-Lahargue F, Canron X, Kunstfeld R, Nguyen L, Henderson JE, Bikfalvi A, Detmar M, Hong YK. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol Biol Cell. 2006;17:576–584. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde AV, Bystroff C, Wang C, Vogelezang MG, Vincent PA, Hynes RO, Van De Water L. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities. J Biol Chem. 2008;283:2858–2870. doi: 10.1074/jbc.M708306200. [DOI] [PubMed] [Google Scholar]
- Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Sleeman JP, Thiele W. Tumor metastasis and the lymphatic vasculature. Int J Cancer. 2009;125:2747–2756. doi: 10.1002/ijc.24702. [DOI] [PubMed] [Google Scholar]
- Spinella F, Garrafa E, Di Castro V, Rosano L, Nicotra MR, Caruso A, Natali PG, Bagnato A. Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Res. 2009;69:2669–2676. doi: 10.1158/0008-5472.CAN-08-1879. [DOI] [PubMed] [Google Scholar]
- Sriramarao P, Mendler M, Bourdon MA. Endothelial cell attachment and spreading on human tenascin is mediated by alpha 2 beta 1 and alpha v beta 3 integrins. J Cell Sci. 1993;105(Pt 4):1001–1012. doi: 10.1242/jcs.105.4.1001. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar SS, Ganesan TS. Role of lymphangiogenesis in cancer. J Clin Oncol. 2007;25:4298–4307. doi: 10.1200/JCO.2006.07.1092. [DOI] [PubMed] [Google Scholar]
- Svendsen OS, Barczyk MM, Popova SN, Liden A, Gullberg D, Wiig H. The alpha11beta1 integrin has a mechanistic role in control of interstitial fluid pressure and edema formation in inflammation. Arterioscler Thromb Vasc Biol. 2009;29:1864–1870. doi: 10.1161/ATVBAHA.109.194308. [DOI] [PubMed] [Google Scholar]
- Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng. 2007;9:229–256. doi: 10.1146/annurev.bioeng.9.060906.151850. [DOI] [PubMed] [Google Scholar]
- Swartz MA, Hubbell JA, Reddy ST. Lymphatic drainage function and its immunological implications: from dendritic cell homing to vaccine design. Semin Immunol. 2008;20:147–156. doi: 10.1016/j.smim.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Tabibiazar R, Cheung L, Han J, Swanson J, Beilhack A, An A, Dadras SS, Rockson N, Joshi S, Wagner R, et al. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006;3:e254. doi: 10.1371/journal.pmed.0030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular Mechanisms and Future Promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Kinmonth JB, Dangerfield WG. Protein content of oedema fluid in lymphoedema. Br Med J. 1958;1:1159–1160. doi: 10.1136/bmj.1.5080.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainionpaa N, Butzow R, Hukkanen M, Jackson DG, Pihlajaniemi T, Sakai LY, Virtanen I. Basement membrane protein distribution in LYVE-1-immunoreactive lymphatic vessels of normal tissues and ovarian carcinomas. Cell Tissue Res. 2007;328:317–328. doi: 10.1007/s00441-006-0366-2. [DOI] [PubMed] [Google Scholar]
- Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, Crowe MJ, Van Helden DF. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J Physiol. 1996;493(Pt 2):563–575. doi: 10.1113/jphysiol.1996.sp021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Wernert N, Okuducu AF, Pepper MS. Ets 1 is expressed in capillary blood vessels but not in lymphatics. J Pathol. 2003;200:561–567. doi: 10.1002/path.1380. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Wiig H, Gyenge C, Iversen PO, Gullberg D, Tenstad O. The role of the extracellular matrix in tissue distribution of macromolecules in normal and pathological tissues: potential therapeutic consequences. Microcirculation. 2008;15:283–296. doi: 10.1080/10739680701671105. [DOI] [PubMed] [Google Scholar]
- Wiig H, Rubin K, Reed RK. New and active role of the interstitium in control of interstitial fluid pressure: potential therapeutic consequences. Acta Anaesthesiol Scand. 2003;47:111–121. doi: 10.1034/j.1399-6576.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res. 2005;65:9789–9798. doi: 10.1158/0008-5472.CAN-05-0901. [DOI] [PubMed] [Google Scholar]
- Wong SY, Hynes RO. Tumor-lymphatic interactions in an activated stromal microenvironment. J Cell Biochem. 2007;101:840–850. doi: 10.1002/jcb.21146. [DOI] [PubMed] [Google Scholar]
- Yokosaki Y, Matsuura N, Higashiyama S, Murakami I, Obara M, Yamakido M, Shigeto N, Chen J, Sheppard D. Identification of the ligand binding site for the integrin alpha9 beta1 in the third fibronectin type III repeat of tenascin-C. J Biol Chem. 1998;273:11423–11428. doi: 10.1074/jbc.273.19.11423. [DOI] [PubMed] [Google Scholar]
- Yokosaki Y, Monis H, Chen J, Sheppard D. Differential effects of the integrins alpha9beta1, alphavbeta3, and alphavbeta6 on cell proliferative responses to tenascin. Roles of the beta subunit extracellular and cytoplasmic domains. J Biol Chem. 1996;271:24144–24150. doi: 10.1074/jbc.271.39.24144. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Braghetta P, Sabatelli P, Mura I, Doliana R, Colombatti A, Volpin D, Bonaldo P, Bressan GM. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol Cell Biol. 2004;24:638–650. doi: 10.1128/MCB.24.2.638-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Groopman JE, Wang JF. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha5beta1. J Cell Physiol. 2005;202:205–214. doi: 10.1002/jcp.20106. [DOI] [PubMed] [Google Scholar]
- Zhu CQ, Popova SN, Brown ER, Barsyte-Lovejoy D, Navab R, Shih W, Li M, Lu M, Jurisica I, Penn LZ, et al. Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc Natl Acad Sci U S A. 2007;104:11754–11759. doi: 10.1073/pnas.0703040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaans BM, Bielenberg DR. Potential therapeutic strategies for lymphatic metastasis. Microvasc Res. 2007;74:145–158. doi: 10.1016/j.mvr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]