Abstract
Relative to childhood, peer relationships take on a heightened importance during adolescence. Might adolescents be highly attuned to information that concerns when and how they are being evaluated, and what their peers think of them? This review evaluates how continuing brain development - which influences brain function - partially explains or reflects adolescents’ attunement to social evaluation. Though preliminary, evidence is mounting to suggest that while processing information relevant to social evaluation and the internal states of other people, adolescents respond with greater emotional intensity and corresponding nonlinear recruitment of socioaffective brain circuitry. This review highlights research findings that relate trajectories of brain development and social behavior, and discusses promising avenues of future research that will inform how brain development might lead adolescents sensitized to social evaluation.
Keywords: Adolescence, social, brain, rejection, development, evaluation
When I was 14 years old and in eighth grade, I received an award at the end-of-year school assembly. Walking across the stage, I lost my footing and stumbled in front of the entire student body. To be clear, this was not falling flat on one’s face, spraining an ankle, or knocking over the school principal - it was a small misstep only noticeable to those in the audience who were paying close attention. Rushing off the stage, my heart pounded with embarrassment and self-consciousness, and weeks of speculation about the consequences of this missed step were set into motion. There were tears and loss of sleep. Did my friends notice? Will they stop wanting to hang out with me? Would a reputation for clumsiness follow me to high school?
Although tripping in public could be embarrassing at any age, the anecdote above illustrates one example of how events that entail social evaluation can be experienced as highly intense, salient, persistent, and emotionally evocative during the adolescent years – perhaps more so than other phases of the lifespan. Indeed, a defining feature of adolescence is a newfound importance of peer and romantic relationships. A shifting motivation toward social relatedness is thought to intensify the attention, salience and emotion relegated to processing information concerning social evaluations and social standing, referred to herein as social sensitivity. Understanding the mechanisms and consequences of adolescent social sensitivity, and the relationship between these behaviors and brain development, has received a surge of scientific interest.
Social sensitivity could ‘dial up’ socioemotional processes at numerous levels of complexity. If adolescents have high social sensitivity, they might be more emotionally reactive to explicit cues indicative of social inclusion or exclusion. They might also be more attuned to instances of real or perceived social evaluation, where individuals are led to believe that they are under evaluative scrutiny within certain contexts (for me, being onstage and aware of the hundreds of pairs of eyes in the audience). Adolescents might also consider with greater elaboration and emotional import what others are thinking and feeling, supported by mentalizing, or theory of mind processes, which enables speculation as to what others’ impressions of them might be. Although these are not the only possible manifestations of social sensitivity, they have been subject to experimental inquiry in neurodevelopmental research. As such, these components of adolescent social sensitivity, and what is known about their neural bases during adolescence, are explored here. This article highlights especially novel and informative advances focused on cross-age comparisons in healthy individuals, in the hope of motivating a more complete understanding of adolescent social sensitivity.
What is unique about the adolescent social life?
The term adolescence encapsulates a phase of life thought to begin around the onset of puberty and end when the individual achieves adult-like levels of independence. The social life of adolescents takes a central role in daily activities and thoughts. Relative to children, adolescents tend to spend less and less time with their families, and more time with peers (Barnes, Hoffman, Welte, Farrell, & Dintcheff, 2007; Larson, 2001), facilitated in part by greater freedom experienced with age. Digital peer communication also peaks, with a slightly higher frequency of internet, text messaging, and social media usage in 14–17 year olds than by adults aged 18–30 years (Lenhart, Ling, Campbell, & Purcell, 2010). This rise in peer interaction is not unique to humans. For example, “adolescent” rats spend more time engaged in social play than adult rats (Douglas, Varlinskaya, & Spear, 2004; Primus & Kellogg, 1989).
Not only do adolescents engage in more frequent contact with peers, the quality of peer interactions also changes. The function of social relationships tends to shift away from friends as activity partners, and toward intimate platonic and romantic relations. Finally, although adolescent social experiences are mostly positive, they also tend to be in a state of flux (Cairns, Leung, Buchanan, & Cairns, 1995). There is a tendency for adolescent relationships to wax and wane, and thus peer rejection becomes common during this time of life (Wang, Iannotti, & Nansel, 2009).
Developmental properties of socioaffective circuitry
Brain imaging complements behavioral science to inform the components and mechanisms of adolescent social sensitivity. Imaging methodology can be used to characterize the magnitude, eliciting conditions, and circuit-based coordination of neural responses to social and emotional cues. A powerful approach to reveal brain-behavior relationships that change across development is to relate shifts in structural, functional, or connectivity based measures with developmentally-mediated differences in laboratory-based or everyday behavior. Such age shifts could manifest along simple or complex trajectories (Somerville et al., under review). For instance, it is possible to detect adolescent-specific effects that are maximally engaged during adolescence compared to both older and younger individuals, which could parallel a temporary influx of hormones or other shifts that are unique to the adolescent years. Age-linear patterns would describe developmental effects that increase or decrease with age at a consistent pace, which could result from developmental progressions or regressions. A third pattern of interest could be termed adolescent-emergent, representing a nonlinear asymptotic pattern characterized by a rapid rate of change (and possible peak engagement during adolescence) that maintains or partially resolves into adulthood. Effects of this type imply developmentally-mediated shifts that are constrained by maturational processes that solidify in early adulthood. Examining linear and nonlinear patterns across transition into and out of adolescence can inform neurodevelopmental theory.
By drawing on a broad understanding of the functioning of neural systems, neuroimaging data can provide clues as to the putative mechanisms of adolescent emotional behavior, and serve as an indirect ‘readout’ of emotional and regulatory processes. Of particular relevance are the functional properties of what is termed here socioaffective circuitry, including the amygdala, striatum, the medial prefrontal cortex (MPFC), relative to regions within the ventromedial and lateral prefrontal cortices thought to flexibly regulate these responses (Blakemore, 2008; Nelson & Guyer, 2011). Broadly speaking, coordinated activity of these regions is critical for a) detection of salient information, b) assignment of hedonic, aversive, or emotional value to that information, c) social cognition, and d) utilization of that information to guide learning and behavior.
Theoretical and empirical accounts have proposed that socioaffective function might take a nonlinear trajectory during adolescence for at least two reasons. For one, regions of the prefrontal cortex continue to reach structural and functional maturity throughout the adolescent years (Shaw et al., 2008; Somerville, Hare, & Casey, 2011), and the connections between subcortical and cortical structures continue to strengthen (e.g., (Asato, Terwilliger, Woo, & Luna, 2010; Liston et al., 2006). Given the role of the prefrontal cortex in the regulation of behavior, still-maturing connections between it and subcortical structures might reduce the capacity to exert cognitive or emotional regulation particularly in emotionally salient contexts (Casey, Jones, & Hare, 2008; Steinberg, 2008). In addition, the influx of hormones during puberty are thought to sensitize functional properties of certain brain circuits due, in part, to the influence of hormones on neurotransmitter systems (see (Ernst, Romeo, & Andersen, 2009; Nelson, Leibenluft, McClure, & Pine, 2005; Sisk & Zehr, 2005), potentially resulting in adolescent-specific engagement patterns. Interestingly, the brain regions that are strongly affected by pubertal hormones share a high degree of overlap with socioaffective circuitry described above. Together, these observations motivate the prediction that sensitized socioaffective circuits of the brain, perhaps less efficiently regulated, could sharpen the detection of, and response to, salient social cues during adolescence.
Emotional processing in the social context
Information about our social standing is inherently laden with emotion. Finding out that a classmate called me “awkward” after tripping onstage is an example of information that is both negative in its valence and relevant to social standing (e.g., ‘she does not view me favorably’). Do adolescents react with greater emotional intensity when they find out whether others view them in a positive or negative light?
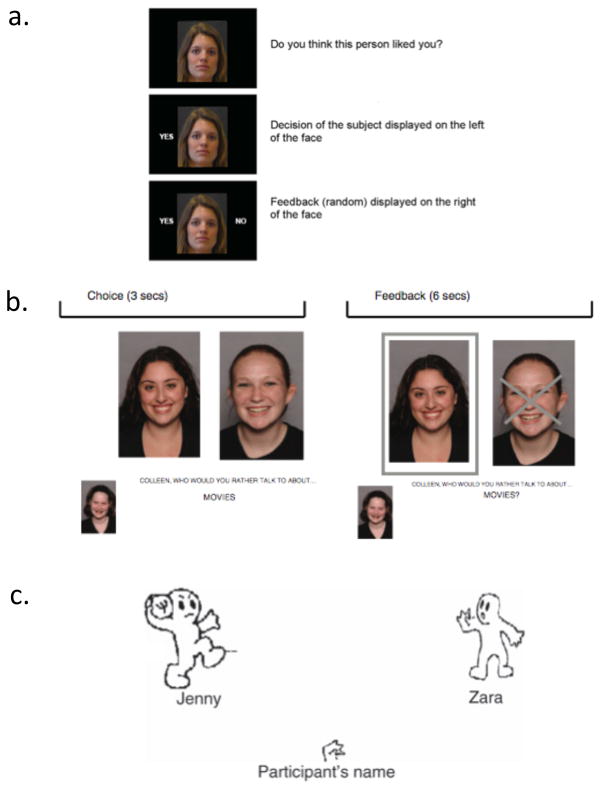
To address this question, researchers have been challenged to develop tasks that deliver self-relevant, salient, and believable social feedback to participants within the confines of the laboratory. Three such paradigms are highlighted in Figure 1. Figure 1A depicts a social feedback task (adapted from (Somerville, Heatherton, & Kelley, 2006) in which participants believe their photograph was rated by unfamiliar, peer-aged individuals based on how likeable the participant looked. Trial by trial, raters provide feedback to the participant indicating whether they did or did not like the participant’s photo. The ‘Chatroom Interact’ ((Silk et al., 2012); Figure 1B) and related ‘Chatroom’ tasks (Guyer, Choate, Pine, & Nelson, 2012; Guyer, McClure-Tone, Shiffrin, Pine, & Nelson, 2009) indicate whether the participant, or somebody else, was selected to chat online with a peer about a topic of mutual interest (in this case, the movies) over a series of trials. In the ‘Cyberball’ task adapted from (Williams, Cheung, & Choi, 2000) and used developmentally (Figure 1C; (Masten et al., 2009; Sebastian et al., 2011)), participants believe they are engaging in an online ball-tossing game, and the ball-tossing partners stop passing the participant the ball after a few mutual throws.
Figure 1.
Tasks developed to assess adolescent social sensitivity. 1A depicts a social feedback task for which participants receive feedback that another peer did, or did not, like the participant’s picture (Gunther Moor et al., 2010). 1B depicts an adaptation of the Chatroom task (from (Silk et al., 2012). Participants initially decide whether they would like to chat online with a peer about a topic of mutual interest, and then subsequently find out whether that individual chose to chat with them. 1C depicts a developmental adaptation of the Cyberball task (from (Sebastian et al., 2010)) which socially includes and excludes participants from a virtual ball-tossing game.
Though not always observed, adolescents have shown evidence of heightened positive and negative emotional responses in these experimental contexts. When experiencing negative social feedback, adolescents endorse a greater drop in mood and increase in anxiety relative to adults when excluded from the virtual ball-tossing game (Sebastian, Viding, Williams, & Blakemore, 2010), and expect less favorable positive feedback when their picture is supposedly judged by unfamiliar peers (Gunther Moor, van Leijenhorst, Rombouts, Crone, & Van der Molen, 2010). Silk and colleagues (2012) used eye tracking, an indirect measure of salience processing, to target implicit emotional and motivational responses to social feedback in the “Chatroom Interact” task depicted in Figure 1B. Whereas participants (9–17 years) showed a pupil difference to rejecting compared to accepting trials, this response was exaggerated in older adolescents. Further, heightened pupillary responses to rejecting social feedback predicted less connectedness in participants’ real-life social relationships. Interestingly, adolescent emotional reactivity is not limited to instances of social rejection. Adolescent participants report a boost in positive affect when experiencing social acceptance from a desirable peer (Guyer et al., 2012), though it is not yet known whether the magnitude of this effect differs relative to older or younger ages. Taken together, the findings suggest that these tasks are effective at indexing adolescent social sensitivity, emotional responses in adolescents are particularly robust, and heightened social sensitivity in the lab predicts less satisfaction in real-life social relationships.
Do adolescents show distinct recruitment of socioaffective circuitry when processing explicit positive and negative social feedback? Using the task depicted in Figure 1A, Gunther Moor and colleagues (2010) found that adolescents engaged similar regions of the brain as children and adults when processing positive and negative social feedback, but activity increased with age within the striatum and subgenual anterior cingulate cortex – regions thought to support emotional valuation - while anticipating positive feedback. Sebastian and colleagues (2011) observed that adolescents recruited the medial prefrontal cortex more strongly relative to adults and recruited ‘regulatory regions’ of the lateral prefrontal cortex less strongly while being actively excluded from the ball-tossing game. The latter study suggests that adolescents robustly engage socioaffective processes and reduce recruitment of regulatory circuitry while processing emotional qualities of social feedback compared to other ages. More research will be required to specify the implications of these activity patterns to brain maturation and to adolescent social sensitivity.
Vigilance to social evaluation
As I walked across the stage at the school assembly, I was painfully aware that others were watching and forming impressions of me. Yet, I was not privy to what they were actually thinking. Might the real or perceived experience of being evaluated contribute to adolescent social sensitivity? Historical accounts have proposed that adolescents are hyperaware of others’ evaluations and feel under constant scrutiny by an imaginary audience (Elkind & Bowen, 1979). Though this characterization has been challenged (Vartanian, 2000), adolescents do report a greater day-to-day tendency to feel self-conscious (Westenberg, Drewes, Goedhart, Siebelink, & Treffers, 2004) and in laboratory-based social stressor tasks, adolescents respond with greater release of cortisol (a stress hormone) when under social scrutiny compared to children (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Stroud et al., 2009). Thus, social evaluative situations – even those devoid of feedback - induce self-conscious emotion and engage stress systems of the body in adolescents.
Following these observations, we sought to assess whether adolescents demonstrate heightened self-consciousness and exaggerated engagement of socioaffective neural circuitry under minimal conditions– just knowing that someone is looking at them. We instructed participants that at times, they would be watched by a peer via live video feed during portions of a brain imaging scan (Somerville et al., under revision). Relative to both children and adults, adolescents experienced greater self-reported embarrassment (Figure 2A) evoked by ostensible video monitoring which partially subsided into adulthood, and uniquely heightened responding of the autonomic nervous system (indexed by skin conductance). The medial prefrontal cortex (MPFC; a key structure for integrating emotional and social information; see Figure 2B) showed robust age differences, with activity that drastically increased during adolescence and partially subsided into adulthood. Though preliminary, these findings suggest that adolescents’ social sensitivity extends to subtle evaluative contexts. Adolescent-emergent engagement of the MPFC could reflect, or perhaps result in, social evaluative situations being assigned a high degree of salience, emotional arousal, and self-relevance.
Figure 2.
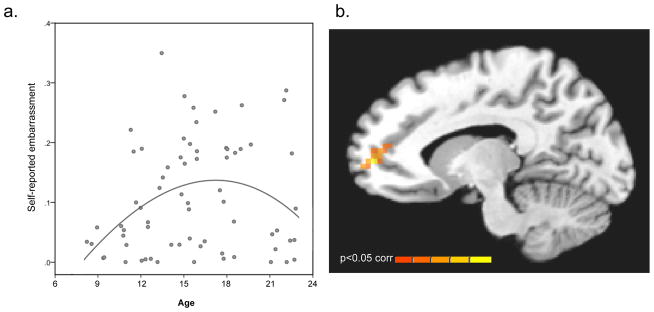
While ostensibly being viewed by a peer in a live video-feed, self-reported embarrassment rises rapidly during adolescence (A), mimicked by emergent recruitment of the medial prefrontal cortex (B).
Thinking about the thoughts of others
The night after I tripped onstage, I couldn’t sleep because I was thinking about my friends and classmates, predicting who would sympathize with me in my state of embarrassment and who would poke fun at me for my clumsiness. A third feature of adolescent social sensitivity is a tendency to speculate about the thoughts and feelings of peers, a cognitive ability referred to as mentalizing or theory of mind. Although adolescents excel at simple mentalizing tasks, they perform significantly worse than adults when the theory of mind tasks are made highly complex (Dumontheil, Apperly, & Blakemore, 2010), which suggests that mentalizing abilities continue to mature through adolescence.
A subset of the socioaffective circuitry described earlier is thought to support theory of mind processes. These regions, sometimes termed the ‘social brain’ (Blakemore, 2008) include the MPFC, temporoparietal junction, superior temporal sulcus and other regions that are consistently engaged across a wide variety of tasks that carry mentalizing demands. Such tasks include those in which participants are asked to reason about moral scenarios (Blakemore, Den Ouden, Choudhury, & Frith, 2007) and about social emotions like guilt and embarrassment relative to less socially-guided emotions like disgust and fear (e.g., (Burnett, Bird, Moll, Frith, & Blakemore, 2009)). Developmental investigations have demonstrated that MPFC recruitment is more robust in adolescents than in adults during tasks that share the common process of considering the thoughts and intentions of others (reviewed by (Burnett, Sebastian, Kadosh, & Blakemore, 2011). Burnett and Blakemore (2009) also observed heightened task-based functional connectivity between brain regions important for social cognition in adolescents compared to adults. Given that the prefrontal cortex continues to undergo changes in structural maturation throughout adolescence, heightened activity during adolescence has been proposed to reflect the MPFC’s continuing developmental course (Blakemore, 2008). It has also been proposed that adolescents utilize strategies for theory of mind that more strongly engage the MPFC. Alternatively, adolescents might consider thoughts and feelings of others to be more important or salient than adults do, which might strongly engage the processing resources of socioaffective neural circuitry through heightened motivation to understand others (and thus, greater effort allocated to mentalizing).
Conclusions
Convergent evidence suggests that adolescents display heightened sensitivity to social evaluation at various levels of complexity, and continue to refine their capacity to represent the thoughts and feelings of others. These features of adolescent social sensitivity appear to be instantiated by robust response properties within circuitries of the brain important to assigning value to social-affective information compared to other times of life. Though the present report focused on brain function, it should be acknowledged that adolescent social sensitivity is a product of multidirectional interactions between brain development, experience, and sociocultural factors (Crone & Dahl, 2012). Indeed, the greater independence afforded by adolescence poses a unique set of challenges that require adolescents to navigate their social worlds in ways that are new and challenging to them (Pfeifer & Peake, 2012), thus shaping brain development (Crone & Dahl, 2012; Pfeifer & Peake, 2012). Though speculative, it is possible that sensitized responding in socioaffective brain circuitry enables a heightened capacity to detect, learn from, and adapt to the myriad social challenges characteristic of adolescence, ultimately facilitating mature social competence.
It is important to consider that the studies presented above represent findings that have emerged within just the past few years. As a result, models pinpointing the neural mechanisms that give rise to adolescent-specific social sensitivity remain tentative. Studies that sample a large range of ages, including both pre- and post-adolescents, or track individuals longitudinally over time, provide the most compelling means to ask whether socioaffective sensitivity is specific to, emerges during, or is comparable to, older or younger ages. With a greater corpus of data, we will also be capable of refining the putative functional roles for subcomponents of socioaffective circuitry, which are much more complex than sketched here. Future research will also address the role of factors that likely influence adolescent social sensitivity in complex ways, including pubertal hormones, cultural norms, gender, social status, and self esteem, among many others (Crone & Dahl, 2012).
The goal of understanding the neural mechanisms of adolescent social sensitivity is not just compelling from a basic science standpoint. Social concerns are a primary source of anxiety for adolescents, and social exclusion during this time of life predicts risk for experiencing mood and anxiety disorders throughout the lifespan (Lev-Wiesel, Nuttman-Shwartz, & Sternberg, 2006). Initial clinical studies of social sensitivity have implicated biased responses in socioaffective circuitry in clinical and at-risk adolescent samples (Guyer et al., 2008; Masten et al., 2011). Thus, exaggerated responding in socioaffective brain circuitry might represent a common feature of adolescent-specific social sensitivity and emergent mood and anxiety dysregulation during adolescence, a connection that will be further specified with the study of clinical and at-risk samples.
When reflecting on formative events of one’s own adolescent years, I’ll bet they involve friends, love interests, or events experienced in social groups (in my case, embarrassing ones). The field of adolescent science is just beginning to uncover some of the causes and consequences of adolescent social sensitivity. Ultimately, it is hoped that this work informs the biological underpinnings of this core feature of the adolescent experience.
Acknowledgments
Thanks to Alea Skwara for assistance with manuscript preparation, and to BJ Casey and Jason Mitchell for providing comments on a draft of this manuscript. This work was supported by The National Institute of Mental Health NIMH R00MH087813.
References
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: A DTI study. Cerebral Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GM, Hoffman JH, Welte JW, Farrell MP, Dintcheff BA. Adolescents’ time use: Effects of substance use, delinquency and sexual activity. Journal of Youth and Adolescence. 2007;36:697–710. [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Social, Cognitive, and Affective Neuroscience. 2007;2(2):130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Bird G, Moll J, Frith C, Blakemore SJ. Development during adolescence of the neural processing of social emotion. Journal of Cognitive Neuroscience. 2009;21(9):1736–1750. doi: 10.1162/jocn.2009.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Blakemore SJ. Functional connectivity during a social emotion task in adolescents and in adults. European Journal of Neuroscience. 2009;29(6):1294–1301. doi: 10.1111/j.1460-9568.2009.06674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Kadosh KC, Blakemore SJ. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioral studies. Neuroscience and Biobehavioral Reviews. 2011;35:1654–1664. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RB, Leung MC, Buchanan L, Cairns BD. Friendships and social networks in childhood and adolescence: Fluidity, reliability, and interrelations. Child Development. 1995;66:1330–1345. [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare T. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Apperly IA, Blakemore SJ. Online usage of theory of mind continues to develop in late adolescence. Developmental Science. 2010;13(2):331–338. doi: 10.1111/j.1467-7687.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- Elkind D, Bowen R. Imaginary audience behavior in children and adolescents. Developmental Psychology. 1979;15(1):38–44. [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: A window into a neural systems model. Pharmacology Biochemistry and Behavior. 2009;93(3):199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther Moor B, van Leijenhorst L, Rombouts SA, Crone EA, Van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience. 2010;5(5–6):461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective responses to peer feedback in adolescence. Social, Cognitive, and Affective Neuroscience. 2012;7(1):82–91. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Nelson EE. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry. 2008;65(11):1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RW. How U.S. children and adolescents spend time: What it does (and doesn’t) tell us about their development. Current Directions in Psychological Science. 2001;10(4):160–164. [Google Scholar]
- Lenhart A, Ling R, Campbell SB, Purcell K. Teens and mobile phones. Pew Internet & American Life Project; 2010. http://pewinternet.org/Reports/2010/Teens-and-Mobile-Phones.aspx. [Google Scholar]
- Lev-Wiesel R, Nuttman-Shwartz O, Sternberg R. Peer rejection during adolescents: Psychological and long-term effects- A brief report. Journal of Loss and Trauma: International Perspectives on Stress and Coping. 2006;11(2):131–142. [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: A marker for adolescents’ risk for depression. Development and Psychopathology. 2011;23(1):283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Social, Cognitive, and Affective Neuroscience. 2009;4(2):143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Guyer AE. The development of ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience. 2011;1(3):233–245. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Peake SJ. Self-development: integrating cognitive, socioemotional, and neuroimaging perspectives. Developmental Cognitive Neuroscience. 2012;2(1):55–69. doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Developmental Psychobiology. 1989;22(6):633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Tan GCY, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: Implications of social neuroscience for education. Neuroimage. 2011;57:686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition. 2010;72:134–135. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani N, Lerch JP, Eckstrand K, Lenroot R, Gotay N, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Stroud LR, Siegle GJ, Dahl RE, Lee KH, Nelson EE. Peer acceptance and rejection through the eyes of youth: Pupillary, eyetracking and ecological data from the Chatroom Interact task. Social, Cognitive, and Affective Neuroscience. 2012;7:93–105. doi: 10.1093/scan/nsr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9(8):1007– 1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ. Medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. (under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian LR. Revisiting the Imaginary Audience and Personal Fable constructs of adolescent egocentrism: A conceptual review. Adolescence. 2000;35:639–661. [PubMed] [Google Scholar]
- Wang J, Iannotti RJ, Nansel TR. School bullying among US adolescents: Physical, verbal, relational, and cyber. Journal of Adolescent Health. 2009;45(4):368–375. doi: 10.1016/j.jadohealth.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenberg PM, Drewes MJ, Goedhart AW, Siebelink BM, Treffers PDA. A developmental analysis of self-reported fears in late childhood through mid-adolescence: social-evaluative fears on the rise? Journal of Child Psychology and Psychiatry. 2004;45(3):481–495. doi: 10.1111/j.1469-7610.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CKT, Choi W. CyberOstracism: Effects of being ignored over the Internet. Journal of Personality and Social Psychology. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
Recommended Readings
- •.Burnett S, Sebastian C, Kadosh KC, Blakemore SJ. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioral studies. Neuroscience and Biobehavioral Reviews. 2011;35:1654–1664. doi: 10.1016/j.neubiorev.2010.10.011. In-depth focus on the development of theory of mind and its neural bases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–650. doi: 10.1038/nrn3313. Rich assessment of the neurodevelopment of cognitive flexibility and social reorientation processes during adolescence. [DOI] [PubMed] [Google Scholar]
- •.Nelson EE, Liebenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. This early synthesis draws key linkages between adolescent social sensitivity, brain development, and risk for psychiatric illness. [DOI] [PubMed] [Google Scholar]
- •.Pfeifer JH, Peake SJ. Self-development: Integrating cognitive, socioemotional, and neuroimaging perspectives. Developmental Cognitive Neuroscience. 2012;2(1):55–69. doi: 10.1016/j.dcn.2011.07.012. Pfeifer and Peake review evidence informing the development of the self-concept from childhood to adulthood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. Presents a hypothesis of how subcortical-cortical interactions in the brain might function uniquely during adolescence, resulting in heightened sensitivity to emotional cues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. A ‘modern classic’ in the field, this article provides a comprehensive account of brain development during adolescence from a cross-species perspective, and considers its relation to unique features of adolescent behavior. [DOI] [PubMed] [Google Scholar]