Abstract
Contaminated soil from a former manufactured-gas plant site was treated in a laboratory-scale bioreactor. Desorbability and biodegradability of 14 polycyclic aromatic hydrocarbons (PAHs) and 4 oxygenated PAHs (oxy-PAHs) were investigated throughout a treatment cycle. Desorbability was determined using a mixed-function sorbent (Oasis® HLB) or a hydrophobic sorbent (Tenax®) in dialysis tubing suspended in the soil slurry. Toxicity and genotoxicity of the whole soil and the desorbable fractions were determined by DNA damage response analysis with the chicken DT40 B-lymphocyte isogenic cell line and its DNA repair-deficient mutant Rad54−/−. Biological treatment significantly removed both PAHs and oxy-PAHs, and their desorbability decreased throughout the bioreactor treatment cycle. Collectively, oxy-PAHs were more desorbable and biodegradable than the corresponding PAHs; for example, the oxy-PAH present at the highest concentration, 9,10-anthraquinone, was more desorbable and biodegradable than anthracene. For both PAHs and oxy-PAHs, the percentage removed in the bioreactor significantly exceeded the percentage desorbed from untreated soil, indicating that desorption did not control the extent of biodegradation. Consistent with previous results on the same soil, genotoxicity of the whole soil slightly increased after biological treatment. However, both toxicity and genotoxicity of the desorbable constituents in the soil decreased after treatment, suggesting that any genotoxic constituents that may have formed during treatment were primarily associated with less accessible domains in the soil.
Key words: : bioavailability, bioremediation, genotoxicity, MGP, quinones, soil
Introduction
Historically, the potential effects of contaminated sites on human or ecological health have been assessed based on the total contaminant concentrations as determined by vigorous chemical extraction techniques (Alexander, 2000). However, because of their association with different soil components, hydrophobic organic pollutants such as polycyclic aromatic hydrocarbons (PAHs) are only partly available for biodegradation by microorganisms or for exerting toxic effects (Alexander, 2000; Reid et al., 2000; Lei et al., 2004; Kreitinger et al., 2007; Vasseur et al., 2008). Poor correlation has been observed between vigorous solvent extraction of a given compound and its bioavailability to bacteria and earthworms in soil (Kelsey and Alexander, 1997; Kelsey et al., 1997). Recently, the U.S. Interstate Technology and Regulatory Council has advised incorporating bioavailability considerations into the evaluation of contaminated sediment sites to reduce the extent of cleanup required to be protective of the environment (Interstate Technology & Regulatory Council Contaminated Sediments Team, 2011).
A variety of biological and chemical techniques have been developed to estimate and predict bioavailability. Biological techniques involve measuring substrate uptake, mineralization, or toxicity (Kelsey et al., 1997; Stroo et al., 2000; Braida et al., 2004). Chemical techniques involve nonexhaustive extraction with “mild” solvents (Hatzinger and Alexander, 1995; Kelsey et al., 1997; Breedveld and Karlsen, 2000; Reid et al., 2000; Liste and Alexander, 2002) and solid-phase extraction (ten Hulscher et al., 2003; Lei et al., 2004; Gomez-Eyles et al., 2011). Solid-phase extraction is common for estimating bioavailability in contaminated soils and sediments, with polymeric adsorbent resins such as Tenax® serving as an infinite sink (Loehr et al., 2003). These sorbents work well with nonpolar compounds such as PAHs.
In a complex system such as soil, it is likely that some transformations of organic pollutants by microorganisms do not lead to complete metabolism of the parent compounds. In PAH-contaminated soil undergoing bioremediation, hundreds of hazardous compounds have been identified, covering a wide range of physicochemical properties. For example, log Kow values can range from 2 to 8 (Lundstedt et al., 2003), including polar and semi-polar metabolites of PAHs. Compounds other than parent PAHs are responsible at least in part for the toxicity and genotoxicity observed in studies on bioremediation of field-contaminated soil (Hu et al., 2012).
The objective of this study was to investigate the potential bioavailability of PAHs, selected oxygenated PAH (oxy-PAHs) metabolites, and other constituents in contaminated soil that collectively lead to toxicity or genotoxicity during biological treatment in a lab-scale, slurry-phase, sequencing batch bioreactor. Potential bioavailability was evaluated by desorption to both hydrophobic (Tenax) and universal (Oasis® HLB) sorptive resins. The DNA damage response assay using the chicken DT40 B-lymphocyte isogenic cell line and its DNA repair-deficient mutant Rad54−/− was applied to evaluate the toxicity and genotoxicity of whole soil and desorbed constituents throughout a treatment cycle in the bioreactor.
Materials and Methods
Materials
PAH standards (EPA 610 PAH Mixture), 9,10-phenanthrenequinone (PQ), and 9,10-anthraquinone (AQ) were purchased from Sigma-Aldrich (St. Louis, MO). 9-Fluorenone (FLO) and benz[a]anthracene-7,12-quinone (BAQ) were purchased from Acros Organics (Morris Plains, NJ). All solvents were high-pressure liquid chromatography (HPLC) grade and were purchased from Fisher Scientific (Pittsburgh, PA).
Tenax TA beads (60/80 mesh) and the universal (mixed-function) Oasis HLB resin (particle size 60 μm) were obtained from Alltech (Deerfield, IL) and Waters (Milford, MA), respectively. They were cleaned by Soxhlet extraction in acetone:hexane (50:50, v/v) and methanol:dichloromethane (50:50, v/v), respectively, overnight and air-dried before use. SnakeSkin® dialysis tubing (10,000 MWCO, 22 mm diameter, 10.5 m) was obtained from Thermo Scientific (Rockford, IL).
Contaminated soil (feed soil) used in this study was collected from a former manufactured-gas plant (MGP) site in Salisbury, North Carolina, processed, and characterized as described elsewhere (Richardson and Aitken, 2011; Hu et al., 2012). The total concentration of target PAHs (14 of the 16 USEPA priority PAHs, excluding acenaphthylene and indeno[1,2,3-cd]pyrene) was 362±23 μg/g and the total concentration of target oxy-PAHs (FLO, PQ, AQ, and BAQ) was 22.3±0.5 μg/g (dry mass basis, w/w). Among the oxy-PAHs, AQ was at the highest concentration (18.3 μg/g). Concentrations of individual PAHs and the other oxy-PAHs in the feed soil are shown in Supplementary Table S1. The feed soil was treated in a continuously stirred, semi-continuous (draw-and-fill or sequencing batch), laboratory-scale aerobic bioreactor with a working volume of ∼2 L, a solids concentration of 14% (w/w), and solids retention time of 35 days (Hu et al., 2012); treatment in the reactor relied on indigenous microorganisms present in the contaminated soil (i.e., no exogenous inoculum was necessary). Every week, 20% of the treated slurry was removed from the bioreactor and replaced with feed soil in a pH 7.5 buffer containing 5 mM phosphate and 5 mM ammonium nitrate.
Treated slurry was sampled at three time intervals during a given 7-days feeding cycle: immediately after feeding (d0), 1 day (d1) and 7 days (d7) after feeding. Concentrations of constituents in the d0 sample represent a weighted average of the concentration remaining at the end of the previous 7-day treatment cycle (80% of the reactor contents immediately after feeding) and the concentration in the added feed soil (20% of the reactor contents immediately after feeding); accordingly, concentrations in the d0 sample are substantially lower than in the feed soil. Samples of the slurry were centrifuged for 20 min to remove excess water prior to use for desorption experiments and toxicity assays. PAH and oxy-PAH concentrations of treated soil samples are shown in Supplementary Table S1.
Desorption experiments
The infinite-sink desorption method (Loehr et al., 2003) was employed to investigate desorption of PAHs and oxy-PAHs from feed soil or treated soil to the sorbents (Tenax beads or HLB resin). Because of the small particle size of HLB resin and difficulty separating it from the soil slurry, the conventional method (adding the sorbent directly to soil slurry) was adapted by placing the sorbent into dialysis tubing suspended in the soil slurry. Briefly, an aliquot of 0.1 g sorbent was weighed and transferred into 5 cm of dialysis tubing closed with knots on both ends. Approximately 4 g (wet wt.) of centrifuged soil sampled from the bioreactor and one dialysis tubing containing the sorbent were suspended in 50 mL phosphate buffer (pH 7.5) amended with 4.15 g/L NaN3 in a 60-mL centrifuge tube with a PTFE-lined septum and screw cap.
To evaluate the effects of incubation time on the extent of desorption, six centrifuge tubes were prepared for each soil sample for each desired time interval (desorption for 1, 2, 3, 7, 10, or 15 days): three tubes with dialysis tubing containing Tenax beads and three tubes with dialysis tubing containing HLB resin. The tubes were placed on a wrist-action shaker in the dark. After each desired time interval, the six centrifuge tubes for each soil sample were sacrificed. The dialysis tubing was removed from the centrifuge tube and rinsed with deionized water to detach soil particles from the exterior of the tubing. The dialysis tubing was then unknotted and the sorbents were rinsed with deionized water into a vacuum filtration apparatus to separate water from the resin. The soil slurry left in the centrifuge tube was centrifuged for 20 min and the supernatant was discarded. The sorbents and the soil after desorption were then extracted. These time-course measurements demonstrated that desorption of total PAHs (sum of 14 compounds) and total oxy-PAHs (sum of 4 compounds) from both feed soil and treated soil increased with time and approached apparent equilibrium in 7 days, as shown for the HLB resin in Supplementary Fig. S1. Therefore, all subsequent desorption experiments were conducted for 7 days. Recoveries of PAHs and oxy-PAHs were calculated by comparing the initial mass in the soil with the mass desorbed to Tenax beads or HLB resin plus the mass remaining in the soil after 7 days of desorption. Individual PAH and oxy-PAH recoveries are shown in Supplementary Table S2. The dialysis tubing did not sorb PAHs or oxy-PAHs (data not shown).
The conventional Tenax-bead desorption method was carried out as described elsewhere (Hu and Aitken, 2012). Briefly, ∼4 g of wet feed soil and 0.1 g Tenax beads were suspended in 50 mL phosphate buffer (pH 7.5) amended with 4.15 g/L NaN3 in each of triplicate 60-mL centrifuge tubes with a PTFE-lined septum and screw cap. The tubes were placed on a wrist-action shaker in the dark. After 7 days, the tubes were centrifuged for 20 min, and Tenax beads were removed from the tubes for subsequent extraction.
Sample extraction, chemical analysis, and DT40 bioassay
Tenax beads and HLB resin were extracted overnight with 10 mL methanol or 10 mL methanol:dichloromethane mixture (50:50, v/v), respectively, in a 20-mL glass vial with a PTFE-lined septum and screw cap. The extracts were filtered through a 0.2-μm pore-size nylon filter and brought to 25 mL with acetone. The soil samples were extracted overnight twice each with a mixture of 10 mL acetone and 10 mL dichloromethane as described elsewhere (Richardson et al., 2011). The extracts were filtered through a 0.2-μm pore-size nylon filter and brought to 50 mL with acetonitrile.
The filtered extracts were analyzed by HPLC for PAHs as described elsewhere (Richardson et al., 2011) and by liquid chromatography-tandem mass spectrometry (LC-MS/MS) for oxy-PAHs as described in Supplementary Data. Toxicity and genotoxicity of the filtered extracts were determined by DNA damage response analysis using a DT40 isogenic cell line and its mutant Rad54−/− knocked out in homologous recombination DNA repair pathway as described elsewhere (Ridpath et al., 2011; Hu et al., 2012). For the bioassays, extracts were first dried and the residue reconstituted in dimethyl sulfoxide as the delivery solvent (Hu et al., 2012).
Data analysis
SPSS® (v16.0; SPSS, Inc., Chicago, IL) was applied for statistical analysis. Student's t-test and one-way analysis of variance (ANOVA) followed by Tukey's test were employed to test for differences between two groups and among multiple groups, respectively. The LD50 of a sample for the parental DT40 cells and for the Rad54−/− mutant was calculated based on the dose–response relation and converted to an equivalent mass of soil as described elsewhere (Hu et al., 2012).
Results
Desorption from feed soil
Dialysis tubing was used to contain Tenax beads or HLB resin to investigate desorption of PAHs and oxy-PAHs from soil. Total PAH recovery in the Tenax-bead system was 95.7%±0.7% and in the HLB-resin system was 93.6%±1.1%; total oxy-PAH recovery in the Tenax-bead system was 101%±8% and in the HLB-resin system was 110%±5% (Supplementary Table S2). There was no significant difference in desorption from feed soil to Tenax beads versus HLB resin for any analyte (Fig. 1). To validate the dialysis tubing desorption approach, we compared it to the conventional method of suspending Tenax beads in the soil slurry. There was no difference between desorption to Tenax using the conventional approach versus the dialysis tubing method (Fig. 1).
FIG. 1.
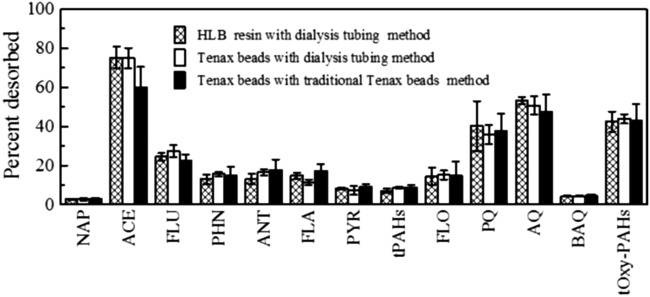
Comparison of desorption of polycyclic aromatic hydrocarbons (PAHs) and oxygenated PAHs (oxy-PAHs) from feed soil (FS) to HLB resin and Tenax beads in the dialysis-tubing desorption method and in the conventional Tenax-bead desorption method. Desorption was less than 2% for benz[a]anthracene (BaA), chrysene (CHR), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), dibenz[a,h]anthracene (DBA), and benzo[g,h,i]perylene (BgP) under all methods. Desorption time was 7 days. Values represent mean and standard deviation of triplicates. There was no significant difference (p>0.05) between methods for any of the analytes. NAP, naphthalene; ACE, acenaphthene; FLU, fluorene; PHN, phenanthrene; ANT, anthracene; FLA, fluoranthene; PYR, pyrene; tPAHs, total PAHs; FLO, 9-fluorene; PQ, 9,10-phenanthrene quinone; AQ, 9,10-anthraquinone; BAQ, BaA-7,12-quinone; tOxy-PAHs, total oxy-PAHs.
Biodegradability and desorbability of PAHs and oxy-PAHs
Treatment in the bioreactor significantly removed PAHs and oxy-PAHs from the contaminated soil, and the removal increased over the duration of the 7-day treatment cycle (Fig. 2). Note that removal is reported relative to concentrations in the feed soil; because the d0 sample represents dilution of the feed soil with slurry remaining in the reactor from the previous 7-day cycle (see Materials and Methods), the indicated removal is an apparent removal. The overall removal in the bioreactor is represented by the d7 sample, which is the effluent from the bioreactor at the end of a cycle. After 7 days, removal of total PAHs and total oxy-PAHs was 50% and 72%, respectively. Removal of each individual oxy-PAH and the corresponding parent PAH in the contaminated soil also increased with time. Compared to the respective parent PAH, there was greater removal of AQ and PQ but less removal of BAQ and FLO throughout the 7-day treatment cycle.
FIG. 2.
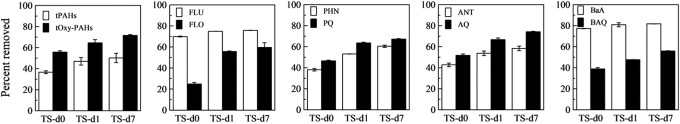
Removal of PAHs and oxy-PAHs in treated soils (TS) sampled at three time intervals during the 7-day treatment cycle: immediately after feeding (TS-d0), 1 day (TS-d1), and 7 days (TS-d7) after feeding. In all cases, there was a significant difference (p<0.05) between removal of an oxy-PAH and the corresponding parent PAH for a given sample. Removal is relative to the initial concentration of the respective analyte in the FS. Values represent mean and standard deviation of triplicates.
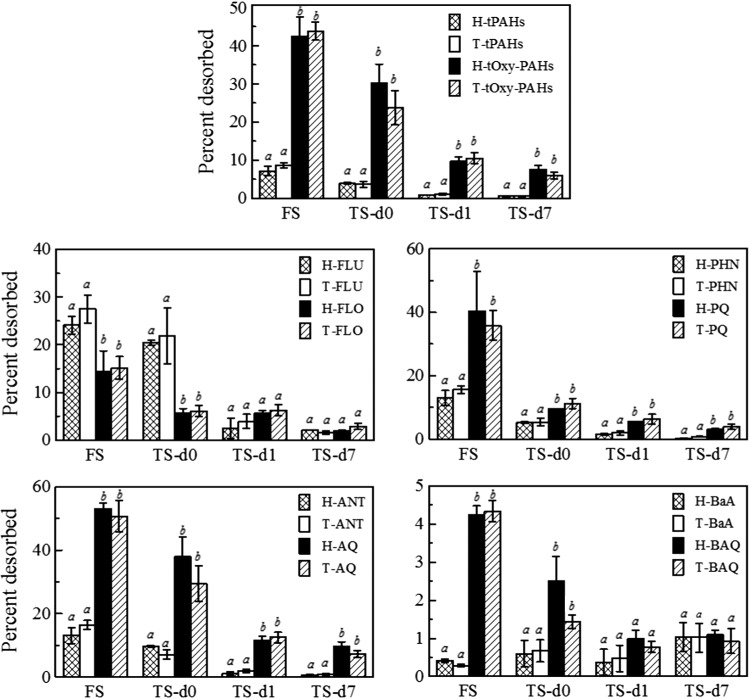
Desorption of PAHs and oxy-PAHs to HLB resin and Tenax beads from soils decreased with longer treatment time, except for benz[a]anthracene (BaA; Fig. 3); desorption of BaA in feed soil was slightly less than that in treated soil sampled at the end of the 7-day treatment cycle, although the desorbable fraction of BaA was negligible (2% or less in all samples). In both feed soil and treated soils, the percent desorption of total oxy-PAHs was significantly greater than that of total PAHs. At the end of the 7-day treatment cycle, 7.6%±1.1% and 6.0%±0.9% of total oxy-PAHs were still desorbable to HLB resin and Tenax beads, respectively, while only 0.5%±0.1% and 0.6%±0.1% of total PAHs were desorbable to HLB resin and Tenax beads, respectively. However, when comparing each individual oxy-PAH with its corresponding parent compound, desorption of only PQ and AQ were significantly greater than that of phenanthrene (PHN) and anthracene (ANT) throughout the bioreactor treatment cycle. Desorption of BAQ was significantly greater than that of BaA only in feed soil and in treated soil sampled immediately after feeding, while desorption of FLO was significantly less than that of FLU in those two samples. No differences between desorption of FLO or BAQ and that of their corresponding parent compounds were observed in treated soils sampled 1 and 7 days after feeding.
FIG. 3.
Desorption of PAHs and oxy-PAHs to HLB resin (H) and Tenax beads (T) from FS and TS sampled at three time intervals during the 7-day treatment cycle (abbreviations as in Fig. 2). a,bDifferent letters are assigned to conditions for which there was a significant difference (p<0.05) among analytes in a given sample. Values represent mean and standard deviation of triplicates.
The percentage of PAH and oxy-PAH desorbed from the feed soil was compared to the percentage of PAH and oxy-PAH removed in the bioreactor at the end of the bioreactor treatment cycle (Fig. 4). For most compounds, the removal in the bioreactor was greater than its desorption.
FIG. 4.
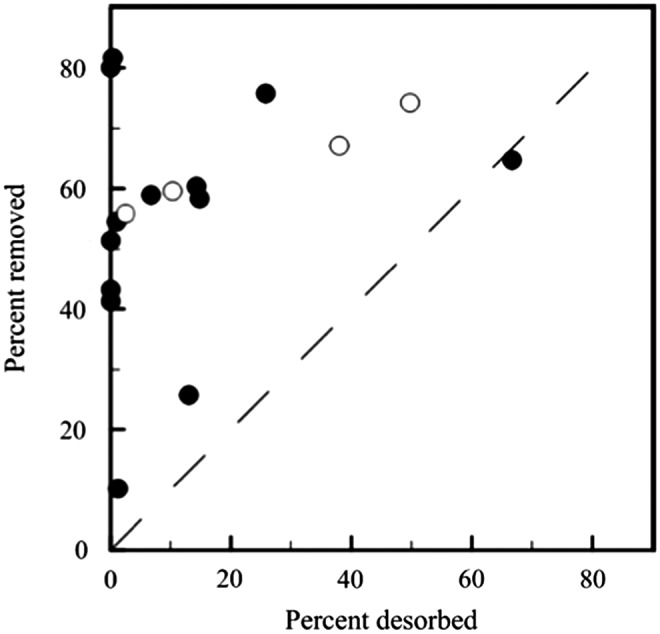
Percentage of PAH and oxy-PAH removed at the end of the 7-day treatment cycle versus percentage of PAH and oxy-PAH desorbed from the FS. Each point represents the mean value for an individual PAH or oxy-PAH. The dashed line represents a 1:1 correlation. Closed and open circles represent PAHs and oxy-PAHs, respectively. NAP is not included because no removal was observed.
Toxicity and genotoxicity
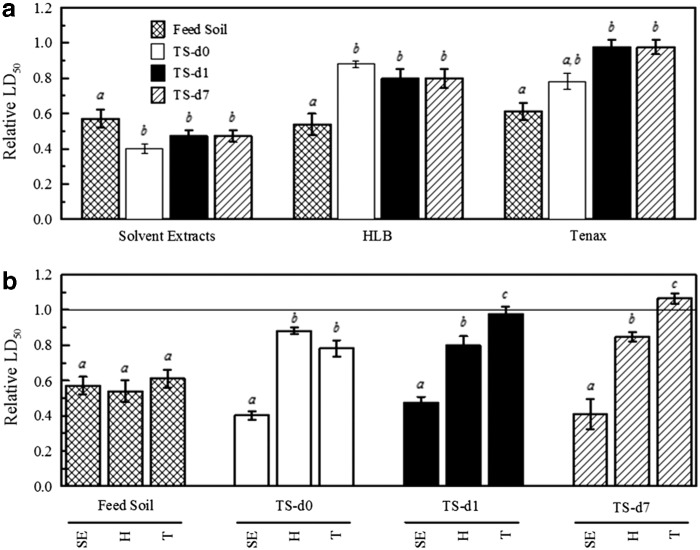
Toxicity and genotoxicity of whole-soil solvent extracts and of the desorbable constituents of soil samples were examined using the DT40 bioassay. For both the DT40 parental cell line and its Rad54−/− mutant, the LD50 of the solvent extracts increased with time during biological treatment (Fig. 5). The same trend was also observed for the constituents desorbed to HLB or Tenax. The LD50 of constituents desorbed to HLB or Tenax for both DT40 and Rad54−/− was significantly higher than that of the solvent extracts for all samples. No significant differences of LD50 were observed among the solvent extracts of each sample before or after desorption to HLB or to Tenax (Supplementary Fig. S2). The LD50 of constituents desorbed to HLB was significantly lower than that of constituents desorbed to Tenax in feed soil and treated soil sampled immediately after feeding (d0) for both DT40 and Rad54−/−. In contrast, the LD50 of constituents desorbed to HLB was significantly higher (p<0.05) than that of constituents desorbed to Tenax as treatment progressed during the 7-day treatment cycle (d1 and d7 samples) for DT40, while no significant differences were observed among LD50 values for Rad54−/− in these samples.
FIG. 5.
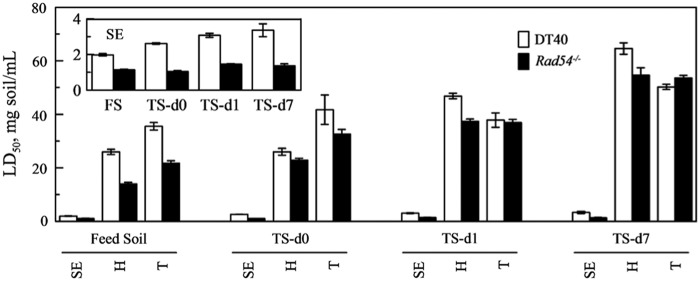
LD50 of solvent extracts (SE) and constituents desorbed to HLB (H) and Tenax (T) of soils for the parental DT40 cell line and its Rad54−/− mutant. The inset enlarges the results for LD50 of solvent extracts. Values represent mean and standard deviation of three separate experiments to quantify LD50 of a given sample.
For a quantitative comparison of genotoxicity, we also calculated the relative LD50 (LD50 of Rad54−/− divided by the LD50 of the parental DT40 cell line; Fig. 6). If the relative LD50 of a sample is significantly <1.0, that sample is defined as genotoxic; the lower the relative LD50, the more genotoxic the sample. The relative LD50 of solvent extracts decreased after biological treatment, while the relative LD50 of constituents desorbed to either HLB or Tenax increased as a result of biological treatment (Fig. 6a). The relative LD50 of constituents desorbed to HLB or Tenax was significantly higher than that of the solvent extracts for all samples of the treated soil (Fig. 6b). No significant differences of relative LD50 were observed among the solvent extracts of each sample before or after desorption to HLB or to Tenax (Supplementary Fig. S3). The relative LD50 of constituents desorbed to the HLB resin was not significantly different from that of constituents desorbed to Tenax in the feed soil and treated soil sampled immediately after feeding (d0), but was significantly lower in treated soil sampled at d1 and d7 during the 7-day treatment cycle (Fig. 6b). The relative LD50 of the constituents desorbed to Tenax in treated soil sampled at d1 and d7 was not significantly different from 1.0 (Fig. 6b).
FIG. 6.
Relative LD50 of solvent extracts (SE) and constituents desorbed to HLB (H) and Tenax (T) of soils. Data are organized by (a) extract type and (b) sample type. a,b,cDifferent letters are assigned to conditions for which there was a significant difference (p<0.05) among the samples for a given extract type (a) or among the extract types for a given sample (b).
Discussion
In the previous work (Hu et al., 2012), we reported that treatment of contaminated soil from an MGP site in a lab-scale bioreactor led to an increase in genotoxicity of the soil. In this study, we used solid-phase extraction to evaluate the potential bioavailability of the genotoxic constituents. Extraction with Tenax beads is commonly used to estimate and predict bioavailability of organic pollutants in contaminated soils and sediments (ten Hulscher et al., 2003; Gomez-Eyles et al., 2011). However, because oxy-PAHs and other possible products of aerobic bacterial metabolism can be more polar than the parent compounds, we decided to evaluate both hydrophobic (Tenax) and mixed-function (Oasis HLB) resins; the HLB resin is a hydrophilic-lipophilic balanced copolymer sorbent (Buchberger, 2007). We suspended the resins in dialysis tubing because the HLB resin particles could not be separated from the soil for quantitative analysis using the conventional method of mixing the sorbent with soil slurry directly. The method was validated by demonstrating that there was no significant difference between extraction with Tenax using the dialysis-tubing method and the conventional slurry-based desorption method (Fig. 1).
Bioreactor treatment significantly removed PAHs and oxy-PAHs from the contaminated soil (Fig. 2). In soils and sediments, the desorbability of PAHs is believed to be one of the major factors influencing the extent of biodegradation (Luthy et al., 1997; Lei et al., 2004). Some studies have suggested that fast-desorbing or total desorbing fractions of a hydrophobic contaminant can be used to predict bioavailability and, correspondingly, the achievable endpoint of bioremediation (Cornelissen et al., 1998; Hawthorne et al., 2001; Braida et al., 2004; Lei et al., 2004). However, we found that the percentage of most PAHs and oxy-PAHs removed at the end of the bioreactor treatment cycle exceeded the percentage desorbed from the feed soil (Fig. 4). This phenomenon was also observed for PAHs in a long-term study on the same soil using laboratory columns to simulate in situ bioremediation (Richardson and Aitken, 2011). The oxy-PAHs PQ and AQ were more biodegradable and desorbable than their parent compounds PHN and ANT, but FLO was less biodegradable and desorbable than the parent compound fluorene. The differences in desorbability could at least partially explain the differences in biodegradability between these oxy-PAHs and their respective parent compounds. However, we also observed that BAQ was more desorbable but less biodegradable than the parent compound BaA, which suggests that factors other than bioavailability limited the biodegradation of BAQ when compared to BaA.
Biological treatment decreased the toxicity of both desorbable and nondesorbable compounds in the soil; comparing LD50 values, the toxicity mainly came from the nondesorbable constituents (Fig. 5). For the desorbable compounds, the constituents that desorbed to HLB resin were more toxic than the constituents that desorbed to Tenax beads in the feed soil and in the treated soil immediately after feeding (d0), but less toxic in the treated soil sampled at d1 and d7 (Fig. 5). These results suggest that HLB resin and Tenax beads sorb different categories of compounds, although their ability to sorb 14 PAHs and 4 oxy-PAHs analyzed in this study was similar (Fig. 1). Moreover, the as-yet-unidentified toxic compounds sorbed by HLB resin were removed to a greater extent during the bioreactor treatment cycle than the toxic compounds sorbed by Tenax beads (Fig. 5).
Biological treatment slightly increased the genotoxicity of the whole soil, but it significantly reduced the genotoxicity of the desorbable constituents, especially the constituents desorbed to Tenax (which were not genotoxic at all after 1 day treatment during the 7-day treatment cycle; Fig. 6). Consistent with our previous work (Hu et al., 2012), these results indicate that bioremediation generated genotoxins, but it appears that the genotoxins were not desorbable. It is interesting that the desorbability of putative PAH metabolites such as oxy-PAHs also decreased during the bioreactor treatment cycle (Fig. 3); however, the increased genotoxicity resulting from treatment cannot be attributed to the four oxy-PAHs we analyzed because there was a net removal of these compounds during treatment (Fig. 2).
Findings from this work have potential implications for the influence of bioremediation on overall risk to human health and to ecological receptors. Bioremediation can clearly lead to the formation of products that are more genotoxic than the original constituents in a contaminated soil, which presumably can lead to increased risk upon exposure to the soil. However, if the genotoxic constituents in treated soil are less desorbable than those in untreated soil, they may be less bioavailable, with a corresponding decrease in overall risk. Further work is necessary to elucidate the nature of genotoxic constituents resulting from bioremediation and their bioavailability to relevant receptors.
Supplementary Material
Acknowledgments
We thank Bo Pan (Kunming University of Science and Technology, Kunming, China) for advice on dialysis tubing selection. We also thank Xin-Rui Xia and Xiang Q. Kong (both of North Carolina State University) for assistance with HLB resin application. This work was supported by the U.S. National Institute of Environmental Health Sciences' Superfund Research Program (grant 5 P42ES005948).
Author Disclosure Statement
All authors confirm that no competing financial interests exist.
References
- Alexander M. (2000). Aging, bioavailability, and overestimation of risk from environmental pollutants. Environ. Sci. Technol. 34, 4259 [Google Scholar]
- Braida W.J., White J.C., and Pignatello J.J. (2004). Indices for bioavailability and biotransformation potential of contaminants in soils. Environ. Toxicol. Chem. 23, 1585. [DOI] [PubMed] [Google Scholar]
- Breedveld G.D., and Karlsen D.A. (2000). Estimating the availability of polycyclic aromatic hydrocarbons for bioremediation of creosote contaminated soils. Appl. Microbiol. Biotechnol. 54, 255. [DOI] [PubMed] [Google Scholar]
- Buchberger W.W. (2007). Novel analytical procedures for screening of drug residues in water, waste water, sediment and sludge. Anal. Chim. Acta 593, 129. [DOI] [PubMed] [Google Scholar]
- Cornelissen G., Rigterink H., Ferdinandy M.M.A., and Van Noort P.C.M. (1998). Rapidly desorbing fractions of PAHs in contaminated sediments as a predictor of the extent of bioremediation. Environ. Sci. Technol. 32, 966 [Google Scholar]
- Gomez-Eyles J.L., Jonker M.T.O., Hodson M.E., and Collins C.D. (2011). Passive samplers provide a better prediction of PAH bioaccumulation in earthworms and plant roots than exhaustive, mild solvent, and cyclodextrin extractions. Environ. Sci. Technol. 46, 962. [DOI] [PubMed] [Google Scholar]
- Hatzinger P.B., and Alexander M. (1995). Effect of aging of chemicals in soil on their biodegradability and extractability. Environ. Sci. Technol. 29, 537. [DOI] [PubMed] [Google Scholar]
- Hawthorne S.B., Poppendieck D.G., Grabanski C.B., and Loehr R.C. (2001). PAH release during water desorption, supercritical carbon dioxide extraction, and field bioremediation. Environ. Sci. Technol. 35, 4577. [DOI] [PubMed] [Google Scholar]
- Hu J., and Aitken M.D. (2012). Desorption of polycyclic aromatic hydrocarbons from field-contaminated soil to a two-dimensional hydrophobic surface before and after bioremediation. Chemosphere 89, 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Nakamura J., Richardson S.D., and Aitken M.D. (2012). Evaluating the effects of bioremediation on genotoxicity of polycyclic aromatic hydrocarbon-contaminated soil using genetically engineered, higher eukaryotic cell lines. Environ. Sci. Technol. 46, 4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interstate Technology & Regulatory Council Contaminated Sediments Team. (2011). Incorporating Bioavailiability Considerations into the Evaluation of Contaminated Sediment Sites. Washington, DC: Interstate Technology & Regulatory Council [Google Scholar]
- Kelsey J.W., and Alexander M. (1997). Declining bioavailability and inappropriate estimation of risk of persistent compounds. Environ. Toxicol. Chem. 16, 582 [Google Scholar]
- Kelsey J.W., Kottler B.D., and Alexander M. (1997). Selective chemical extractants to predict bioavailability of soil-aged organic chemicals. Environ. Sci. Technol. 31, 214 [Google Scholar]
- Kreitinger J.P., Neuhauser E.F., Doherty F.G., and Hawthorne S.B. (2007). Greatly reduced bioavailability and toxicity of polycyclic aromatic hydrocarbons to Hyalella azteca in sediments from manufactured-gas plant sites. Environ. Toxicol. Chem. 26, 1146. [DOI] [PubMed] [Google Scholar]
- Lei L., Suidan M.T., Khodadoust A.P., and Tabak H.H. (2004). Assessing the bioavailability of PAHs in field-contaminated sediment using XAD-2 assisted desorption. Environ. Sci. Technol. 38, 1786. [DOI] [PubMed] [Google Scholar]
- Liste H.H., and Alexander M. (2002). Butanol extraction to predict bioavailability of PAHs in soil. Chemosphere 46, 1011. [DOI] [PubMed] [Google Scholar]
- Loehr R.C., Lamar M.R., and Poppendieck D.G. (2003). A protocol to estimate the release of anthropogenic hydrocarbons from contaminated soils. Environ. Toxicol. Chem. 22, 2202. [DOI] [PubMed] [Google Scholar]
- Lundstedt S., Haglund P., and Öberg L. (2003). Degradation and formation of polycyclic aromatic compounds during bioslurry treatment of an aged gasworks soil. Environ. Toxicol. Chem. 22, 1413. [PubMed] [Google Scholar]
- Luthy R.G., Aiken G.R., Brusseau M.L., Cunningham S.D., Gschwend P.M., Pignatello J.J., Reinhard M., Traina S.J., Weber W.J., Jr., and Westall J.C. (1997). Sequestration of hydrophobic organic contaminants by geosorbents. Environ. Sci. Technol. 31, 3341 [Google Scholar]
- Reid B.J., Jones K.C., and Semple K.T. (2000). Bioavailability of persistent organic pollutants in soils and sediments—A perspective on mechanisms, consequences and assessment. Environ. Pollut. 108, 103. [DOI] [PubMed] [Google Scholar]
- Richardson S.D., and Aitken M.D. (2011). Desorption and bioavailability of PAHs in contaminated soil subjected to long-term in situ biostimulation. Environ. Toxicol. Chem. 30, 2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S.D., Lebron B.L., Miller C.T., and Aitken M.D. (2011). Recovery of phenanthrene-degrading bacteria after simulated in situ persulfate oxidation in contaminated soil. Environ. Sci. Technol. 45, 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridpath J.R., Takeda S., Swenberg J.A., and Nakamura J. (2011). Convenient, multi-well plate-based DNA damage response analysis using DT40 mutants is applicable to a high-throughput genotoxicity assay with characterization of modes of action. Environ. Mol. Mutag. 52, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroo H.F., Jensen R., Loehr R.C., Nakles D.V., Fairbrother A., and Liban C.B. (2000). Environmentally acceptable endpoints for PAHs at a manufactured gas plant site. Environ. Sci. Technol. 34, 3831 [Google Scholar]
- ten Hulscher T.E.M., Postma J., den Besten P.J., Stroomberg G.J., Belfroid A., Wegener J.W., Faber J.H., van der Pol J.J.C., Hendriks A.J., and van Noort P.C.M. (2003). Tenax extraction mimics benthic and terrestrial bioavailability of organic compounds. Environ. Toxicol. Chem. 22, 2258. [DOI] [PubMed] [Google Scholar]
- Vasseur P., Bonnard M., Palais F., Eom I.C., and Morel J.L. (2008). Bioavailability of chemical pollutants in contaminated soils and pitfalls of chemical analyses in hazard assessment. Environ. Toxicol. 23, 652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.