Abstract
Tularemia, a highly infectious zoonotic disease caused by Francisella tularensis, occurs sporadically in Japan. However, little is known about the prevalence of the disease in wild animals. A total of 632 samples obtained from 150 Japanese black bears, 142 Japanese hares, 120 small rodents, 97 rats, 53 raptors, 26 Japanese monkeys, 21 Japanese raccoon dogs, 20 masked palm civets, and three Japanese red foxes between 2002 and 2010 were investigated for the presence of antibodies to F. tularensis by competitive enzyme-linked immunosorbent assay (cELISA) and the commonly used microagglutination (MA) test. Seropositive cELISA and MA results were obtained in 23 and 18 Japanese black bears, three and two Japanese raccoon dogs, and two and one small rodents, respectively. All MA-positive samples (n=21) were also positive by cELISA. Six of seven samples that were only positive by cELISA were confirmed to be antibody-positive by western blot analysis. These findings suggest that cELISA is a highly sensitive and useful test for serosurveillance of tularemia among various species of wild animals. Because this is the first study to detect F. tularensis–seropositive Japanese raccoon dogs, these could join Japanese black bears as sentinel animals for tularemia in the wild in Japan. Further continuous serosurveillance for F. tularensis in various species of wild animals using appropriate methods such as cELISA is important to assess the risks of human exposure and to improve our understanding of the ecology of F. tularensis in the wild.
Key Words: : Francisella tularensis, Tularemia, Serosurveillance, Wild animals, Japan
Introduction
Tularemia is a highly infectious zoonotic disease caused by an intracellular Gram-negative bacterium, Francisella tularensis, which is considered a viable biological warfare agent (Dennis et al. 2001). It is highly virulent in humans and animals such as rodents and lagomorphs. It is transmitted to humans mainly through handling of infected animals, arthropod bites, ingestion of contaminated water or food, and inhalation of infectious aerosols (Ellis et al. 2002). In humans, the clinical manifestation of the disease varies depending on the route of infection and virulence of the bacterial strain. The symptoms may range from skin ulcers to life-threatening pneumonia (Dennis et al. 2001). In animals, the severity of the disease varies among species. Highly susceptible animals such as rodents and lagomorphs die soon after infection. Other animal species, such as cats, dogs, and cattle, are relatively resistant to infection (Hopla 1974).
Tularemia surveillance data in wild animals are indispensable for understanding the host species responsible for maintenance of the pathogen in the wild and for assessing the risk of human exposure. Over the past few decades, the seroprevalence of tularemia has been assessed in North American and European countries using serum samples obtained from wild animals, such as bears (Chomel et al. 1998), hares, rabbits (Shoemaker et al. 1997), and wild boars (Al Dahouk et al. 2005). A serosurveillance study in Germany recently identified raccoon dogs and foxes as biological indicators of tularemia (Kuehn et al. 2013). However, in Asia, the ecology of tularemia is not well understood because of a paucity of surveillance studies. In Japan, Japanese black bears have been reported as a candidate sentinel species for tularemia (Hotta et al. 2012); however, there have been no other studies aiming to elucidate the distribution of F. tularensis among various wild animals.
Most serosurveillance studies that have been conducted to determine the distribution of F. tularensis in the wild have been based on the detection of serum antibodies (Al Dahouk et al. 2005, Hotta et al. 2012, Kuehn et al. 2013). The microagglutination (MA) test is the most commonly used method for tularemia screening across multiple animal species. However, the MA test is not very sensitive, requires large sample volumes, and cannot be used with hemolyzed sera. The indirect enzyme-linked immunosorbent assay (iELISA) is frequently used for serological surveys of tularemia and has high sensitivity (Al Dahouk et al. 2005); however, its usefulness in seroepidemiological studies of various wild animals is limited because of the unavailability of species-specific secondary antibodies. We recently developed a highly sensitive and specific monoclonal antibody (mAb)-based competitive ELISA (cELISA) for use in tularemia patients (Sharma et al. 2013). In the present study, we used this novel cELISA to examine the seroprevalence of tularemia among wild animals in Japan. We tested not only wild hares and bears (Hotta et al. 2012) but also rodents, birds, raccoon dogs, monkeys, foxes, and masked palm civets located in an area in which human tularemia is known to be endemic.
Materials and Methods
Blood samples from wild animals
A total of 632 blood samples obtained from nine different wild animal species between 2002 and 2010 were used in this study (Table 1). The blood samples from the Japanese black bears (Ursus thibetanus japonicus) and Japanese hares (Lepus brachyurus angustidens) were the same as those used in our previous study (Hotta et al. 2012). Sera or plasma samples from 150 Japanese black bears were obtained from six prefectures (Iwate, Fukushima, Ibaraki, Nagano, Gifu, and Hyogo) between 2002 and 2007, and 21 of these were identified as MA-positive (≥1:10) in our previous study (Hotta et al. 2012). A total of 142 samples from Japanese hares were collected from six prefectures (Aomori, Iwate, Akita, Yamagata, Fukushima, and Niigata) between 2006 and 2009. It appears that the healthy wild hares were shot by licensed hunters. Of the 142 samples, 97 blood samples from these hares were collected on filter paper (Toyo-roshi Ltd. Tokyo, Japan) and extracted from the paper in phosphate-buffered saline (PBS) containing 0.5% (vol/vol) Tween 20. Because the extracts from the filter paper were regarded as 1:50 dilutions of sera, the MA test was not applicable. The remaining 45 samples were collected in tubes and processed using standard protocols to obtain sera. These 45 sera samples did not show positive agglutination in the MA test (Hotta et al. 2012).
Table 1.
Seroprevalence of F. tularensis among Various Wild Animals in Japan Based on a Novel Competitive Enzyme-Linked Immunosorbent and the Microagglutination Test
| No. of positive | |||
|---|---|---|---|
| Animal species | No. of samples | cELISA | MA |
| Japanese black bear (Ursus thibetanus japonicus) | 150 | 23 | 18 |
| Japanese hare (Lepus brachyurus angustidens) | 142 | 0 | 0a |
| Small rodents (Apodemus argenteus, A. speciosus, Microtus montebelli) | 120 | 2 | 1 |
| Rats (Rattus norvegicus, R. rattus) | 97 | 0 | 0 |
| Raptorsb | 53 | 0 | 0 |
| Japanese monkey (Macaca fuscata) | 26 | 0 | 0 |
| Japanese raccoon dog (Nyctereutes procyonoides viverrinus) | 21 | 3 | 2 |
| Masked palm civet (Paguma larvata) | 20 | 0 | 0 |
| Japanese red fox (Vulpes vulpes japonica) | 3 | 0 | 0 |
| Total | 632 | 28 | 21 |
Only 45 of the 142 Japanese hare samples were tested using the MA test. All samples were obtained from animals captured in northeastern Japan, an area in which tularemia is endemic, and all were diluted 100 times.
Raptors include Accipiter gentilis, Buteo buteo, Falco peregrinus, F. tinnunculus, Haliaeetus albicilla, Milvus migrans, and Strix uralensis.
cELISA, competitive enzyme-linked immunosorbent assay; MA test, microagglutination test.
A total of 120 sera samples from three species of small rodents, namely the small Japanese field mouse (Apodemus argenteus), the large Japanese field mouse (A. speciosus), and the Japanese grass vole (Microtus montebelli), were collected between 2008 and 2010 and tested. The remaining samples included 97 sera samples from rats (Rattus norvegicus and Rattus rattus), 53 sera samples from different species of raptors (Accipiter gentilis, Buteo buteo, Falco peregrinus, F. tinnunculus, Haliaeetus albicilla, Milvus migrans, and Strix uralensis), 21 sera samples from Japanese raccoon dogs (Nyctereutes procyonoides viverrinus), 20 sera samples from masked palm civets (Paguma larvata), and three sera samples from Japanese red foxes (Vulpes vulpes japonica) collected between 2003 and 2008. All the above-mentioned samples were collected from wild animals captured from the Aomori, Fukushima, and Hokkaido prefectures, which are part of an area in northeastern Japan where tularemia is known to be endemic (Ohara et al. 1996).
cELISA
cELISA was performed to detect antibodies to F. tularensis in the blood samples of the wild animals, using previously described protocols with some modifications (Sharma et al. 2013). In brief, 96-well microtiter plates (Greiner Bio-One, Frickenhausen, Germany) were coated with purified lipopolysaccharide (LPS) from F. tularensis holarctica (strain NVF1, a Japanese isolate from a wild hare in 2009) in carbonate–bicarbonate buffer (pH 9.6) (2.5 μg/50 μL per well) at 37°C overnight. Thereafter, unbound antigens were removed and blocking was performed with 3% (wt/vol) skim milk in PBS containing 0.1% (vol/vol) Tween 20 (PBST) (150 μL/well). Duplicate 50-μL volumes of 1:100 dilutions of each sample in PBST containing 1% (wt/vol) skim milk were then added to the wells, and the plates were incubated at 37°C for 90 min. After the wells were washed three times with PBST, a biotin-labeled anti-LPS mAb (clone M14B11 recognizing F. tularensis LPS, 50 μL/well, 1:5000 dilution) was added to each well, and the plates were then incubated at 37°C for another 60 min. The bound biotin-labeled anti-LPS mAb was detected by subsequent reactions with streptavidin–peroxidase (Thermo Scientific, Rockford, IL) (50 μL/well, 1:5000 dilution) and 100 μL of 3,3′,5,5′-tetramethylbenzidine (TMB) enzyme substrate (SureBlue Reserve, TMB Microwell Peroxidase Substrate, KPL, Gaithersburg, MD). Finally, 100 μL of stop solution (1 N HCl) was added, and optical density (OD) was measured at 450 nm using a plate reader (Bio-Rad, iMark Microplate Reader) (BioRad, Hercules, CA). The cELISA percent inhibition (PI) values were calculated using the following formula: [1 − (ODsample − ODbackground)/(ODMAb − ODbackground)]×100, where ODsample and ODMAb were the absorbances observed in the presence and absence of samples, respectively, and ODbackground was obtained in the absence of a sample or labeled mAb. The cutoff value for cELISA was determined by calculating the mean PI+3 standard deviations (SDs) of all MA-negative (n=514) blood samples.
MA test
The MA test was performed as described previously (Hotta et al. 2012). In brief, 25 μL of two-fold serial dilutions of serum were mixed with an equal volume of formalin-inactivated F. tularensis holarctica whole-cell suspension (referred to as whole-cell antigen) (OD560=1.0) in a 96-well round-bottomed microtiter plate (IWAKI, Tokyo, Japan). Agglutination reactions in the plates were observed at 18 h after incubation at 37°C. Agglutination titers were expressed as reciprocals of the highest serum dilution showing agglutination with the antigen. A sample was considered seropositive for F. tularensis if the agglutination titer was ≥10.
Western blot analysis
To confirm the presence of antibodies to F. tularensis in blood samples showing positive results in cELISA but not in the MA test, western blot analysis was performed using purified LPS of the F. tularensis NVF1 strain. The LPS antigens were initially subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 12.5% gels and were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Immobilon; Millipore Corporation, Bedford, MA). After blocking the membrane with 3% skim milk in PBST at room temperature for 1 h and five washes with PBST, the membrane was incubated with the samples at 1:1000 dilution. After a further five times washing of membrane with PBST, horseradish peroxidase (HRP)-conjugated recombinant protein A/G (ICN Pharmaceuticals, Cappel) was applied at 1:50,000 dilution. The reactions were detected with an Amersham ECL Prime Western Blotting Detection Reagent kit (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) using a VersaDoc Imaging System (Bio-Rad Laboratories, Hercules, CA). The typical LPS ladder-like banding pattern was considered to indicate presence of specific antibodies in the samples.
Results
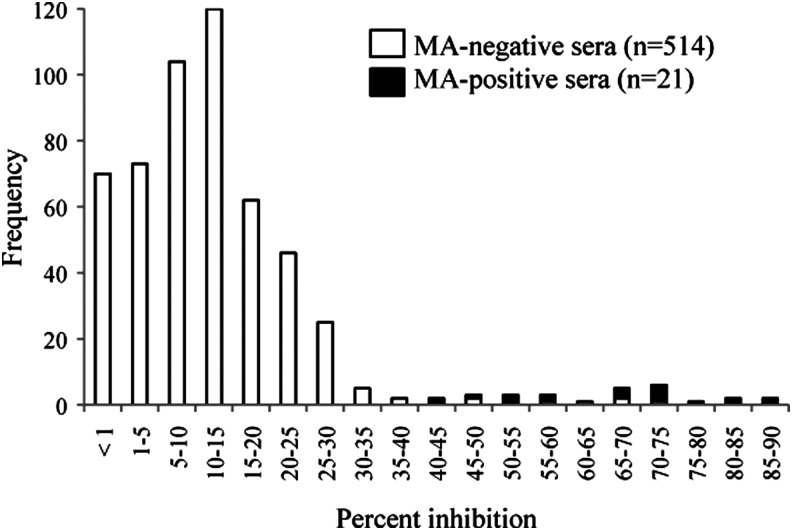
The presence of antibodies to F. tularensis in 632 blood samples collected from various wild animals was assessed by cELISA and the MA test (Table 1). Of the 535 samples subjected to the MA test, 21 showed agglutination of F. tularensis whole-cell antigen and were thus considered MA positive. Distribution of the cELISA PI values of the 514 MA-negative and 21 MA-positive blood samples is shown in Figure 1. The cutoff value for cELISA was determined based on the mean PI and SD of the 514 MA-negative samples. The MA-negative samples had a mean PI of 11.1% with an SD of 10.4. The cutoff value of cELISA was therefore set as 40% (approximate mean+3 SD), and samples showing more than 40% inhibition were considered to be positive in cELISA. A total of 28 (4.4%) of the 632 samples assessed by cELISA and 21 (3.9%) of the 535 samples assessed by MA were seropositive.
FIG. 1.
Inhibition of the binding of the F. tularensis lipopolysaccharide (LPS) monoclonal antibody (mAb) in the 514 microagglutination (MA)-negative and 21 MA test-positive serum samples in competitive enzyme-linked immunosorbent assay (cELISA). The percent inhibition at a serum dilution of 1:100 is plotted. On the basis of the distribution of these sera samples, the cELISA cutoff value was set at 40% (mean+3 standard deviations [SD]).
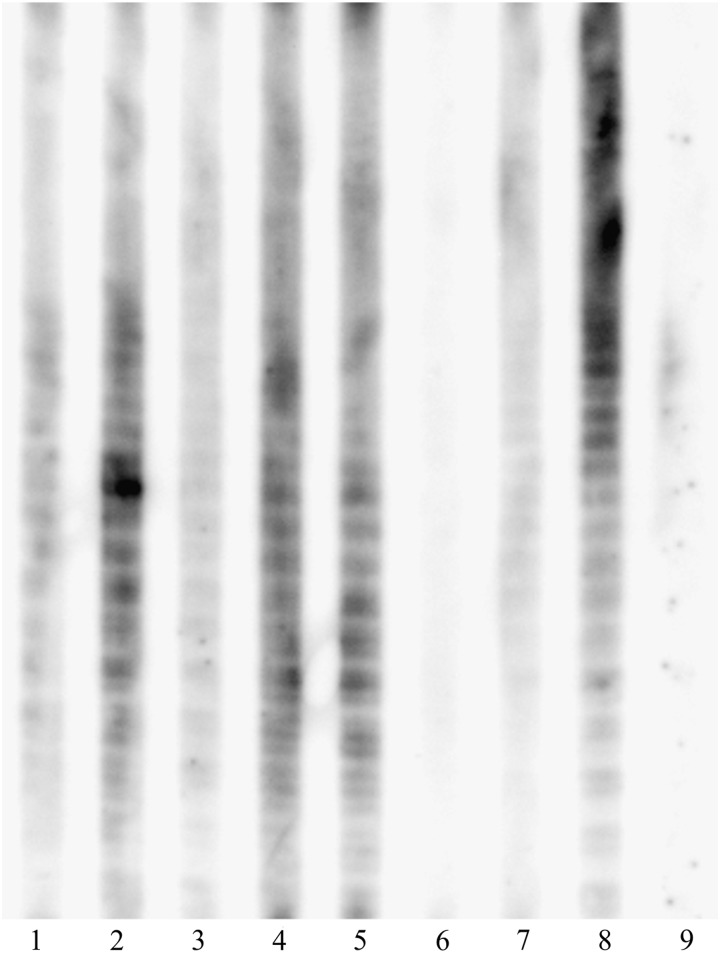
Eighteen samples from Japanese black bears (U. thibetanus japonicus), two samples from Japanese raccoon dogs (N. procyonoides viverrinus), and one sample from a small rodent (M. montebelli) agglutinated the whole-cell antigen in the MA test with titers ranging from 10 to 80. On the other hand, 20 black bears, three raccoon dogs, and two small rodents showed a positive reaction in cELISA, with PIs ranging from 41.0% to 88.5%. All MA-positive (n=21) samples from the bears, raccoon dogs, and small rodents were also positive in cELISA. Seven samples (five bears, one raccoon dog, and one mouse) were positive in cELISA but negative in the MA test. Further testing of these seven samples by WB analysis showed typical ladder-like bands using LPS antigen, except for the mouse sample (Fig. 2, lane 6). No antibodies were detected in the samples from the Japanese hares, Japanese monkeys, rats, raptors, foxes, or masked palm civets by either cELISA or the MA test.
FIG. 2.
Western blot for confirmation of samples that tested positive in competitive enzyme-linked immunosorbent assays (cELISA) but not in the microagglutination (MA) tests (n=7). Each well contains F. tularensis lipopolysaccharide (LPS) as an antigen. Typical LPS ladder-like bands were present in six of the seven samples positive in cELISA but not in the MA test. The serum sample from a mouse (lane 6) was negative in western blot. Lanes 1–5, 6, and 7 show the western blot patterns of the blood samples obtained from Japanese black bears, a small rodent and a Japanese raccoon dog. Lanes 8 and 9 show the western blot pattern of hyperimmune rabbit (positive control), and a normal rabbit (negative control) serum samples, respectively.
Discussion
Tularemia is an important zoonotic disease with complex transmission cycles including mammals and vectors as well as aerosolized air-borne transmission and water-borne transmission (Ellis et al. 2002). Although human cases of tularemia have occurred sporadically during recent years in Japan, the status of tularemia infection in various wild animals is not clearly understood. Therefore, to better understand the presence of tularemia in the wild, we attempted to study various wild animal populations with the aim of detecting F. tularensis antibodies using a novel cELISA and the traditional MA test.
Although the MA test is widely used for screening the sera of many animal species, its low sensitivity and cross-reaction with antibodies to bacteria such as Brucella spp. and Yersinia spp. make this assay less reliable (Behan and Klein 1982, Bevanger et al. 1988). We therefore used cELISA, which is well suited to samples from multiple animal species. Determination of the optimal cutoff value for cELISA was difficult because no standard positive and negative wild animal samples were available. Instead, we calculated the cutoff value from the mean PI and SD of 514 MA-negative blood samples (Fig. 1). With a cutoff value of 40% (mean+3 SD), all of the samples that showed positive agglutination in the MA test (n=21) clearly inhibited MAb binding to LPS in cELISA (Fig. 1). Furthermore, six of seven samples that were seropositive in cELISA but not in the MA test were confirmed to be seropositive by reacting with the LPS in the WB analysis (Fig. 2). These findings indicate that cELISA is a useful and sensitive test for tularemia screening in various species of wild animals.
In our previous study, Francisella novicida immunized sera exhibited slight inhibition (11%) in the cELISA, and no cross-reaction to antibodies to other possibly related bacterial species, such as Brucella spp. and Yersinia spp., was observed (Sharma et al. 2013). In the present study, the cutoff value in the cELISA for animal specimens was set as 40%, thus cross-reaction of antibodies to F. novicida was negligible. This might be supported by the previous report that showed no cross-reaction between F. tularensis and F. novicida (Grunow et al. 2000). Antibodies detected in the wild animals by the cELISA are therefore considered to be specific to F. tularensis LPS. However, it is important to note that we did not test this assay broadly against other Francisella spp. except F. novicida.
In Japan, a total of 1372 human tularemia cases were reported between 1924 and 1994 (Ohara et al. 1996). Most of these cases occurred in the northeastern part of Japan. The number of human tularemia cases declined from the 1960s (Ohara et al. 1996), but over the last 10 years, five human cases of tularemia have been reported (Infectious Diseases Weekly Report Japan, January 19, 2009). The only surveillance studies of tularemia conducted among wild animals in Japan have studied feral raccoons (Inoue et al. 2011) and wild hares and bears (Hotta et al. 2012). In the present study, we tested samples from wild hares and bears, which were similar to those tested in our previous study. These samples were subjected to the novel cELISA, and we found an additional five seropositive bear samples. This indicates that cELISA is more sensitive for detecting antibodies to F. tularensis in wild bears than the MA test. Although no clinical signs of tularemia have been observed in bears, the presence of antibodies to F. tularensis indicates that bears may be capable of surviving F. tularensis infection. They might be infected either through contact with the infected carcasses of hares or rodents or by bites from infected ticks. Bears may be a candidate sentinel species for F. tularensis infection in Japan, although they are not a major source of transmission of tularemia to humans (Hotta et al. 2012). A similar proportion of bears have been reported to be infected with the bacterium in Idaho in the United States (Binninger et al. 1980).
F. tularensis is known to infect various wild animals (Hopla 1974). Therefore, we tested samples from various wild animal species using cELISA and the MA test as well as western blot analysis. Three of 21 raccoon dogs were found to be seropositive by cELISA, and this is the first time the species has been shown to be seropositive for F. tularensis in Japan (Table 1). Raccoon dogs might be a sensitive indicator of tularemia in the wild, and our findings support previous reports from Germany (Kuehn et al. 2013). Like raccoons, raccoon dogs are scavengers (Berrada et al. 2006) that might become infected by preying on sick or dying tularemia-infected animals. The serum sample from a small rodent (M. montebelli) showing positive reaction in cELISA only and not in the MA test did not show positive LPS ladder like bands in the WB analysis (Table 1 and Fig. 2). The reason for these different reactions is not clear. However, they might be because of differences in the sensitivity of the assays or differences in the ability of the immunoglobulins in wild mice sera to recognize the epitopes of the LPS used in the assays. The isolation of the bacteria or positive PCR results from the specimens of the cELISA-positive animals would have confirmed the F. tularensis infection. However, we could not demonstrate the bacteria in the tissue specimens because they were not available. Protocols involving bacterial isolation or genome detection rather than serosurveillance are therefore preferable for surveillance of small rodents, although a few seropositive small rodent species have been reported in Europe (Omland et al. 1977).
Although most cases of human tularemia in Japan have been associated with direct contact with wild hares (Ohara et al. 1991), we did not find any seropositive Japanese hares through cELISA and the MA test, which confirms the findings of a previous report in Japan (Hotta et al. 2012). It is likely that the majority of infected hares die before raising an immune response because they are highly susceptible to F. tularensis infections (Mörner and Addison 2001). In contrast, in European brown hares, 5.1% seropositivity has been reported (Gyuranecz et al. 2011). The different seroprevalence rates might be because of the different susceptibilities of the Japanese and European brown hares to F. tularensis infection.
In the tularemia-endemic regions of the United States and Europe, antibodies to F. tularensis are frequently detected in sera samples from wild animals such as beavers, coyotes, wild boars, and foxes (Morner and Sandstedt 1983, Gese et al. 1997, Al Dahouk et al. 2005). However, except for wild bears and raccoon dogs, the wild animals examined in this study were all seronegative (Table 1). Testing of a large number of samples is necessary because animal species, such as the red fox, masked palm civet, and raptors, are known to prey on mice and hares, which might be a source of F. tularensis infection. A study in Bulgaria reported that raptors were infected with F. tularensis and that the bacteria could be transmitted from birds to humans (Padeshki et al. 2010). However, 53 samples from seven different species of raptors tested in our study were all seronegative. More careful surveillance of raptors may be necessary.
In this study, raccoon dogs and Japanese black bears were found to be seropositive for F. tularensis antibodies. Therefore, it is important that continuous serological surveillance of wild animals be conducted using suitable methods such as cELISA to assess the risks of F. tularemia infection in humans and to further understand the ecology of F. tularensis in the wild.
Acknowledgments
We are grateful to Toshio Tsubota (Hokkaido University, Hokkaido) and Mikiko Aoki (Iwate University, Morioka) for providing the samples from the Japanese black bears. We also thank Hiroaki Kariwa (Hokkaido University) for providing the samples from the rats. This study was supported in part by Health and Labor Science Research Grants for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor and Welfare in Japan (H22-Shinkou-Ippan-010). N.S. was supported by the Honjo International Scholarship Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- Al Dahouk S, Nockler K, Tomaso H, Splettstoesser WD, et al. . Seroprevalence of brucellosis, tularemia, and yersiniosis in wild boars (Sus scrofa) from north-eastern Germany. J Vet Med B Infect Dis Vet Public Health 2005; 52:444–455 [DOI] [PubMed] [Google Scholar]
- Behan KA, Klein GC. Reduction of Brucella species and Francisella tularensis cross-reacting agglutinins by dithiothreitol. J Clin Microbiol 1982; 16:756–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrada ZL, Goethert HK, Telford SR., 3rd.Raccoons and skunks as sentinels for enzootic tularemia. Emerg Infect Dis 2006; 12:1019–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevanger L, Maeland JA, Naess AI. Agglutinins and antibodies to Francisella tularensis outer membrane antigens in the early diagnosis of disease during an outbreak of tularemia. J Clin Microbiol 1988; 26:433–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binninger CE, Beecham JJ, Thomas LA, Winward LD. A serologic survey for selected infectious diseases of black bears in Idaho. J Wildl Dis 1980; 16:423–430 [DOI] [PubMed] [Google Scholar]
- Chomel BB, Kasten RW, Chappuis G, Soulier M, et al. . Serological survey of selected canine viral pathogens and zoonoses in grizzly bears (Ursus arctos horribilis) and black bears (Ursus americanus) from Alaska. Rev Sci Tech 1998; 17:756–766 [DOI] [PubMed] [Google Scholar]
- Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, et al. . Tularemia as a biological weapon: Medical and public health management. JAMA 2001; 285:2763–2773 [DOI] [PubMed] [Google Scholar]
- Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev 2002; 15:631–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gese EM, Schultz RD, Johnson MR, Williams ES, et al. . Serological survey for diseases in free-ranging coyotes (Canis latrans) in Yellowstone National Park, Wyoming. J Wildl Dis 1997; 33:47–56 [DOI] [PubMed] [Google Scholar]
- Grunow R, Splettstoesser W, McDonald S, Otterbein C, et al. . Detection of Francisella tularensis in biological specimens using a capture enzyme-linked immunosorbent assay, an immunochromatographic handheld assay, and a PCR. Clin Diagn Lab Immunol 2000; 7:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyuranecz M, Rigo K, Dan A, Foldvari G, et al. . Investigation of the ecology of Francisella tularensis during an inter-epizootic period. Vector Borne Zoonotic Dis 2011; 11:1031–1035 [DOI] [PubMed] [Google Scholar]
- Hopla CE. The ecology of tularemia. Adv Vet Sci Comp Med 1974; 18:25–53 [PubMed] [Google Scholar]
- Hotta A, Tanabayashi K, Yamamoto Y, Fujita O, et al. . Seroprevalence of tularemia in wild bears and hares in Japan. Zoonoses Public Health 2012; 59:89–95 [DOI] [PubMed] [Google Scholar]
- Inoue K, Kabeya H, Fujita H, Makino T, et al. . Serological survey of five zoonoses, scrub typhus, Japanese spotted fever, tularemia, Lyme disease, and Q fever, in feral raccoons (Procyon lotor) in Japan. Vector Borne Zoonotic Dis 2011; 11:15–19 [DOI] [PubMed] [Google Scholar]
- Kuehn A, Schulze C, Kutzer P, Probst C, et al. . Tularaemia seroprevalence of captured and wild animals in Germany: The fox (Vulpes vulpes) as a biological indicator. Epidemiol Infect 2013; 1:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörner T, Addison E. Tularemia. Ames, IA: Iowa State University Press, 2001:303–312 [Google Scholar]
- Morner T, Sandstedt K. A serological survey of antibodies against Francisella tularensis in some Swedish mammals. Nord Vet Med 1983; 35:82–85 [PubMed] [Google Scholar]
- Ohara Y, Sato T, Fujita H, Ueno T, et al. . Clinical manifestations of tularemia in Japan—analysis of 1,355 cases observed between 1924 and 1987. Infection 1991; 19:14–17 [DOI] [PubMed] [Google Scholar]
- Ohara Y, Sato T, Homma M. Epidemiological analysis of tularemia in Japan (yato-byo). FEMS Immunol Med Microbiol 1996; 13:185–189 [DOI] [PubMed] [Google Scholar]
- Omland T, Christiansen E, Jonsson B, Kapperud G, et al. . A survey of tularemia in wild mammals from Fennoscandia. J Wildlife Dis 1977; 13:393–399 [DOI] [PubMed] [Google Scholar]
- Padeshki PI, Ivanov IN, Popov B, Kantardjiev TV. The role of birds in dissemination of Francisella tularensis: First direct molecular evidence for bird-to-human transmission. Epidemiol Infect 2010; 138:376–379 [DOI] [PubMed] [Google Scholar]
- Sharma N, Hotta A, Yamamoto Y, Fujita O, et al. . Detection of Francisella tularensis-specific antibodies in patients with tularemia by a novel competitive enzyme-linked immunosorbent assay. Clin Vaccine Immunol 2013; 20:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker D, Woolf A, Kirkpatrick R, Cooper M. Humoral immune response of cottontail rabbits naturally infected with Francisella tularensis in southern Illinois. J Wildl Dis 1997; 33:733–737 [DOI] [PubMed] [Google Scholar]