Abstract
Metal homeostasis plays a critical role in antioxidative stress. Streptococcus oligofermentans, an oral commensal facultative anaerobe lacking catalase activity, produces and tolerates abundant H2O2, whereas Dpr (an Fe2+-chelating protein)-dependent H2O2 protection does not confer such high tolerance. Here, we report that inactivation of perR, a peroxide-responsive repressor that regulates zinc and iron homeostasis in Gram-positive bacteria, increased the survival of H2O2-pulsed S. oligofermentans 32-fold and elevated cellular manganese 4.5-fold. perR complementation recovered the wild-type phenotype. When grown in 0.1 to 0.25 mM MnCl2, S. oligofermentans increased survival after H2O2 stress 2.5- to 23-fold, and even greater survival was found for the perR mutant, indicating that PerR is involved in Mn2+-mediated H2O2 resistance in S. oligofermentans. Mutation of mntA could not be obtained in brain heart infusion (BHI) broth (containing ∼0.4 μM Mn2+) unless it was supplemented with ≥2.5 μM MnCl2 and caused 82 to 95% reduction of the cellular Mn2+ level, while mntABC overexpression increased cellular Mn2+ 2.1- to 4.5-fold. Thus, MntABC was identified as a high-affinity Mn2+ transporter in S. oligofermentans. mntA mutation reduced the survival of H2O2-pulsed S. oligofermentans 5.7-fold, while mntABC overexpression enhanced H2O2-challenged survival 12-fold, indicating that MntABC-mediated Mn2+ uptake is pivotal to antioxidative stress in S. oligofermentans. perR mutation or H2O2 pulsing upregulated mntABC, while H2O2-induced upregulation diminished in the perR mutant. This suggests that perR represses mntABC expression but H2O2 can release the suppression. In conclusion, this work demonstrates that PerR regulates manganese homeostasis in S. oligofermentans, which is critical to H2O2 stress defenses and may be distributed across all oral streptococci lacking catalase.
INTRODUCTION
Oxidative stress is encountered by all organisms on earth (1, 2), as deleterious reactive oxygen species (ROS), including superoxide ion (O2−), hydroxyl radical (HO·), and hydrogen peroxide (H2O2), are generated when molecular oxygen participates in electron transfer reactions or is auto-oxidized by reduced enzyme cofactors (2–4). Therefore, organisms have developed various mechanisms to protect against ROS, such as detoxifying enzymes (5, 6). Aerobes generally employ the superoxide dismutase (SOD)-catalase cascade to eliminate cellular ROS (4–7). Anaerobes use the superoxide reductase (SOR)-dependent oxide decomposition pathway to reduce O2− to H2O (8). Intact H2O2 is not particularly deleterious to cells, but it can react with cellular Fe2+ via Fenton chemistry to form highly toxic HO· (2). Ferritin and miniferritin (Dps family) proteins chelate Fe2+ to prevent the deleterious Fenton reaction (9). Moreover, not only is manganese ion (Mn2+) a cofactor of SOD (7, 10), but it does not undergo Fenton chemistry (11). Rather, it acts as an inorganic enzyme to dismute O2− by complexing with small molecules in bacterial cells (11–13). Hence, ROS damage to cells is intimately related to metal ion homeostasis (6).
Expression of antioxidant genes is controlled by redox-sensing transcriptional factors. A Fur family regulator, peroxide-responsive regulator (PerR), is the prototype of the redox-sensing transcriptional repressor found mainly in Gram-positive bacteria (6, 14, 15). Upon sensing cellular H2O2, PerR::Fe derepresses the transcription of antioxidant genes, including catalase, alkylhydroperoxide reductase (AhpC/AhpF), heme biosynthesis enzyme, and ferrous and zinc ion homeostatic genes (6). For example, the PerR protein of Bacillus subtilis regulates oxidant-detoxifying enzymes and metal ion homeostatic genes, namely, the Fe2+ binding protein MrgA (a Dps family homolog) and the Zn2+ transporter ZosA (6, 16, 17). PerR is also involved in Fe2+ and Zn2+ homeostasis and the oxidative-stress response in Streptococcus pyogenes (18, 19).
Streptococci are facultative anaerobes performing fermentative metabolism. During aerobic growth, they not only convert O2− to H2O2 via SOD, but produce H2O2 catalyzed by various oxidases (20–24). However, they do not possess the main H2O2-degrading enzyme catalase, and the identified peroxidases, such as AhpC/AhpF and glutathione peroxidase, also contribute little to H2O2 resistance in streptococci (20, 25). Thus, streptococci must employ unique mechanisms for H2O2 stress defense. Recent studies demonstrate that metal ion homeostasis is pivotal for H2O2 defense in streptococci (6). A dps-like nonspecific DNA-binding protein (Dpr), which chelates Fe2+, and the Zn2+ pump protein PmtA contribute to oxidative-stress resistance in streptococci (18, 26, 27). In addition, inactivation of Mn2+ transporter genes in some streptococci diminishes H2O2 and O2− resistance (28–30). Both dpr and pmtA are reported to be regulated by PerR (18, 26); however, how Mn2+ uptake is responsive to oxidative stress remains unclear.
Oral streptococci inhabit dental plaque biofilms, where dynamic interspecies interaction occurs and the outcome of competition determines oral health. The oral cavity is an environment under fluctuating oxidative stress. The ability of oral streptococci to defend against oxidants determines whether they can win the interspecies competition and therefore determines oral health. Streptococcus oligofermentans is isolated from noncariogenic dental plaque in humans (31). It generates copious H2O2 via multiple pathways (22–24) and inhibits the growth of the dental caries pathogen Streptococcus mutans. Compared to other streptococci, S. oligofermentans has the greatest H2O2 tolerance (32), making it a model organism in studying bacterial anti-oxidative-stress mechanisms. Although the Dpr protein plays a role in H2O2 resistance in S. oligofermentans (32), the dpr mutant still retains partial H2O2 tolerance, indicating that other mechanisms may confer oxidant resistance. Here, by using physiological, biochemical, and genetic approaches, we demonstrate that manganese is important for S. oligofermentans H2O2 tolerance and that, in the presence of H2O2, PerR releases repression of the manganese transporter mntABC and the Fe2+-chelating protein dpr genes.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. All Streptococcus strains were routinely incubated in brain heart infusion (BHI) broth (Difco, Detroit, MI) at 37°C as a static culture under low oxygen levels or anaerobically under 100% N2. BHI agar (1.5% [wt/vol]) plates were used to select mutant strains and count colonies. Antibiotics (1 mg ml−1 kanamycin and 1 mg ml−1 spectinomycin) were added to the BHI medium when necessary. The Escherichia coli strains (33) were grown in Luria-Bertani (LB) medium at 37°C with shaking and were used for plasmid amplification. When needed, kanamycin (50 μg ml−1) and spectinomycin (250 μg ml−1) were added for recombinant selection.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristics and descriptiona | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | supE44 lacU169 (ϕ80d lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 luxS | 33 |
| S. oligofermentans | ||
| Wild type | AS 1.3089; Kans Sps | 31 |
| ΔperR | AS 1.3089 perR::Kan; Kanr; AS 1.3089 with perR deletion | This study |
| ΔmntA | AS 1.3089 mntA::Kan; Kanr; AS 1.3089 with mntA deletion | This study |
| perR-com | AS 1.3089 perR::Kan pDL278-perR; Kanr Spr ΔperR with perR complement | This study |
| mntABC-exp | AS 1.3089 pDL278-mntABC; Spr; AS 1.3089 with mntABC ectopic expression | This study |
| PmntABC::luc | AS 1.3089 pFW5-PmntABC-luc; Spr; AS 1.3089 with PmntABC::luc fusion | This study |
| ΔperR PmntABC::luc | AS 1.3089 perR::Kan pFW5-PmntABC-luc; Kanr Spr ΔperR with PmntABC::luc fusion | This study |
| Plasmids | ||
| pALH124 | Kanr | 38 |
| pDL278 | Spr | 40 |
| pFW5-luc | Spr | 41 |
| pDL278-perR | Spr; pDL278 with AS 1.3089 perR gene under its inherent promoter | This study |
| pDL278-mntABC | Spr; pDL278 with AS 1.3089 mntABC gene under its inherent promoter | This study |
| pFW5-PmntABC-luc | Spr; pFW5-luc with AS 1.3089 mntABC promoter | This study |
Kanr, kanamycin resistant; Spr, spectinomycin resistant.
DNA manipulation.
Standard recombinant DNA techniques were used for plasmid construction and PCR product ligation. All restriction and ligation enzymes were purchased from New England BioLabs (Beverly, MA). S. oligofermentans genomic DNA was extracted and purified using the method of Marmur (34) with slight modifications (35). All primers (see Table S1 in the supplemental material) were designed according to the complete genome sequence of S. oligofermentans (36) and synthesized by Sangon Company (Shanghai, China). PCR amplifications were performed with KOD-Plus-Neo (Toyobo, Japan), and purification of PCR products was carried out with a Qiagen (Valencia, CA) QIAquick PCR Purification Kit. DNA extracted from agarose gels was purified with a Tiangen (Beijing, China) Tiangel Midi Purification Kit, and plasmids were extracted and purified with a Tiangen (Beijing, China) Tianprep Mini Plasmid Kit.
Construction of mutant strains.
Peroxide-responsive repressor (perR) and metal ABC transporter substrate-binding lipoprotein (mntA) gene deletion mutants were constructed by the PCR ligation method (37). Briefly, two ∼550-bp fragments of the upstream and downstream sequences of the perR and mntA genes, respectively, were amplified by PCR using S. oligofermentans genomic DNA as a template. The purified PCR products were digested with BamHI. The nonpolar kanamycin resistance gene cassette was cut from plasmid pALH124 (38) by digestion with BamHI. All three fragments were purified and mixed at a 1:1:1 molar ratio. A fused fragment was formed by T4 DNA ligase treatment and transformed into the S. oligofermentans wild-type strain using a published method (39). Transformants were selected on BHI (for the perR mutant) or BHI supplemented with 0.1 mM MnCl2 (for the mntA mutant) agar plates containing 1 mg ml−1 kanamycin. The corresponding gene deletion was confirmed with PCR and sequencing.
perR-complemented and mntABC overexpression strains were constructed as described below. The entire gene fragments, including the coding region and the promoter sequence of perR and mntABC, were amplified from chromosomal DNA of S. oligofermentans by PCR with the primers listed in Table S1 in the supplemental material. Then, 762- and 2,660-bp PCR products were purified and double digested with EcoRI and SalI. After gel purification, the PCR products were inserted into E. coli-Streptococcus shuttle plasmid pDL278 (40), which was cut with the same enzymes. Positive transformants were selected on LB agar plates containing 250 μg ml−1 spectinomycin and identified by PCR and sequencing. The recombinant plasmids pDL278-perR and pDL278-mntABC were then transformed into the S. oligofermentans perR mutant and wild-type strains, respectively. Transformants were selected on BHI agar plates containing 1 mg ml−1 kanamycin and 1 mg ml−1 spectinomycin or 1 mg ml−1 spectinomycin and identified by PCR and sequencing.
Construction of luciferase reporter strains.
For construction of the PmntABC-luc reporter strain, DNA fragments corresponding to a 430-bp sequence upstream of the start codon of the mntABC gene were amplified from chromosomal DNA using PCR with primers listed in Table S1 in the supplemental material. The purified PCR product was double digested with BamHI and NheI, gel purified, and ligated to plasmid pFW5-luc (41), which was digested with the same enzyme. The ligation mixtures were transformed into E. coli DH5α. Positive transformants were identified by PCR and sequencing. The recombinant plasmid pFW5-PmntABC-luc was then transformed into the S. oligofermentans wild-type strain and the perR mutant, and positive transformants were identified by PCR, sequencing, and luciferase activity.
Assay of hydrogen peroxide sensitivity.
S. oligofermentans strains were grown in BHI broth under static or anaerobic conditions at 37°C overnight. The overnight cultures were diluted 1:100 into fresh BHI broth and incubated under static or anaerobic conditions. When the optical density at 600 nm (OD600) reached 0.6 to 0.7, cells (1 ml) were removed and harvested by centrifugation. After two washings with phosphate-buffered saline (PBS), the cells were resuspended in 1 ml of fresh BHI broth. Cell aliquots (200 μl) were distributed into 1.5-ml Eppendorf tubes. One aliquot was challenged with 20 mM H2O2, and an aliquot without H2O2 treatment was used as a control. After incubation at 37°C for 10 min, the cells were collected, washed twice with PBS buffer, and resuspended in 200 μl BHI broth. Cell chains were separated by sonication for 30 s with a UP 200S sonicator (Germany). The samples were then serially 10-fold diluted. Appropriate dilutions were plated on BHI agar, and CFU were counted after 24 h of incubation in a candle jar at 37°C. Survival (percent) was calculated as the ratio of CFU in the H2O2-challenged sample to those in controls. Experiments were executed in triplicate, and each was repeated at least three times independently.
Metal content measurement.
Concentrations of iron, manganese, and zinc in static and anaerobic cultures of various S. oligofermentans strains were measured using inductively coupled plasma mass spectrometry (ICP-MS). Overnight BHI cultures of tested strains were diluted 1:50 in fresh BHI broth and incubated at 37°C under static or anaerobic conditions. Mid-log-phase cells were harvested by centrifugation at 13,400 × g for 10 min. The cell pellets were washed twice in PBS with 1 mM EDTA and once in PBS without EDTA and then resuspended in 1 ml of PBS. One hundred microliters of suspension was used to measure the protein concentration with a bicinchoninic acid (BCA) protein analysis kit according to the manufacturer's recommendations. The remaining 900 μl of suspension was collected by centrifugation at 13,400 × g for 10 min. The pelleted bacterial cells were resuspended in 500 μl of nitric acid (ultrapure). After overnight incubation at room temperature, the cell suspension was brought to 1.5 ml with deionized distilled water. Then, metal ions were analyzed by ICP-MS (DRCII; PerkinElmer) at Beijing University Health Science Center. Beryllium, indium, and uranium standard solutions (PerkinElmer; National Institute of Standards and Technology [NIST] certified) were used to calibrate the ICP-MS. Experiments were conducted in triplicate, and each was repeated at least three times. The metal content was expressed in nmol per mg protein.
Luciferase activity measurement.
Twenty-five microliters of 1 mM d-luciferin (Sigma-Aldrich, St. Louis, MO) solution (suspended in 1 mM citrate buffer, pH 6.0) was added to 100-μl samples, and luciferase activity assays were performed as previously described (39) using a TD 20/20 luminometer (Turner Biosystems, Sunnyvale, CA). The sample optical density (OD600) was measured with a 2100 visible spectrophotometer (Unico, Shanghai, China) and used to normalize the luciferase activity. All measurements were performed in triplicate, and all experiments were repeated at least three times.
Hydrogen peroxide measurement.
H2O2 in liquid culture was measured as described previously (24). Briefly, 650 μl of culture supernatant was added to 600 μl of solution containing 2.5 mM 4-amino-antipyrine (4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one; Sigma) and 0.17 M phenol. The reaction proceeded for 4 min at room temperature; horseradish peroxidase (Sigma) was then added to a final concentration of 50 mU/ml in 0.2 M potassium phosphate buffer (pH 7.2). After 4 min of incubation at room temperature, the OD510 was measured with a Unico (Shanghai, China) 2100 visible-light spectrophotometer. A standard curve was generated with known concentrations of chemical H2O2.
Quantitative PCR.
Total RNA was extracted from mid-log-phase (OD600 = 0.4 to 0.5) cells using TRIzol reagent (Invitrogen, Carlsbad, CA) as recommended by the suppliers. After quality confirmation with a 1% agarose gel, the RNA was treated with RNase-free DNase (Promega, Madison, WI) and analyzed by PCR for possible chromosomal DNA contamination. cDNA was generated from 2 μg total RNA with random primers using Moloney murine leukemia virus reverse transcriptase (Promega) according to the supplier's instructions and used for quantitative-PCR (qPCR) amplification with the corresponding primers (see Table S1 in the supplemental material). Amplifications were performed with a Mastercycler ep realplex2 (Eppendorf, Germany). To estimate copy numbers for a given mRNA, a standard curve of the tested gene was generated by quantitative PCR using 10-fold serially diluted PCR product as the template. The 16S rRNA gene was used as the biomass reference. The copy number of each gene was normalized to the number of 16S rRNA copies. The number of copies of the transcript of each gene per 1,000 16S rRNA copies is shown.
RESULTS
PerR functions as a peroxide-responsive repressor in S. oligofermentans.
To identify perR homologs in S. oligofermentans, the perR gene from B. subtilis was used as a probe to query the complete genome. An open reading frame (I872_05555) annotated as “ferric transport regulator protein” with 38% amino acid identity was hit, and it was noted as PerR. Furthermore, PerR orthologs were found in almost all oral streptococci. PerR from S. oligofermentans had the highest identity (97%) with that of Streptococcus cristatus and had 58 to 87% identity with other oral streptococci. Amino acid sequence alignment of PerR proteins from representative oral streptococci and B. subtilis (Fig. 1) revealed that the essential amino acid residues for metal ion binding (H37, H91, D85, and D104 for manganese or ferric ions; C96, C99, C136, and C139 for zinc ions) in B. subtilis (15) were conserved in all oral streptococcal PerR proteins examined except H93, where an asparagine (N) is found in four streptococcal species, namely, S. oligofermentans, Streptococcus gordonii, Streptococcus sanguinis, and Streptococcus salivarius. Sequence conservation suggests that the oral streptococcal PerR proteins function similarly to that of B. subtilis.
FIG 1.

Sequence alignment of PerR proteins from S. oligofermentans, B. subtilis, and other representative species of oral streptococci. Amino acid sequences of PerR were retrieved from the protein database of NCBI. *, conserved amino acid residue essential for manganese or ferric ion binding in B. subtilis; #, conserved cysteine residue for zinc ion binding. BSU08730, B. subtilis; soi_I872_05555, S. oligofermentans; SGO_0703, S. gordonii; SSA_0686, S. sanguinis; Ssal_01385, S. salivarius; SMU_593, S. mutans. Black shading indicates the homology level is 100%; gray shading indicates the homology level is ≥75%.
To identify the function of PerR in S. oligofermentans, a perR deletion mutant was constructed and verified by PCR and sequencing. Both the perR mutant and the wild-type strain were cultured under anaerobic condition to exclude endogenous H2O2 production. Mid-log-phase cultures (OD600 = 0.6 to 0.7) were collected and subjected to H2O2 pulsing (20 mM) for 10 min. Then, the survival rate was quantified as described in Materials and Methods. As shown in Fig. 2, perR mutant survival (22.05%) was ∼32-fold higher than that of the wild-type strain (0.67%). Furthermore, a perR-complemented strain, perR-com, showed significantly reduced survival (0.34%) compared with the perR mutant and similar to that of the wild-type strain. Thus, PerR has been demonstrated to be a peroxide-responsive repressor in S. oligofermentans.
FIG 2.
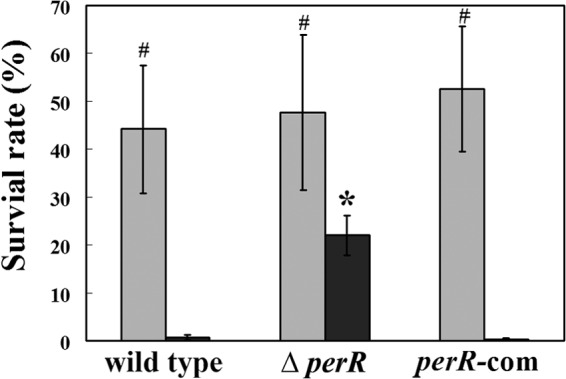
Survival of S. oligofermentans wild-type, perR mutant, and perR-complemented strains after H2O2 pulsing. The strains were incubated statically (gray bars) and anaerobically (black bars), and mid-log-phase cultures were collected and subjected to 20 mM H2O2 pulsing for 10 min. Viable cells were counted based on CFU on a BHI agar plate after serial dilution. Survival was calculated as the ratio of CFU in H2O2-pulsed samples to those without H2O2 treatment. The data are expressed as means ± standard deviations of three independent experiments. *, data are statistically significant in comparison to values of anaerobically cultured wild-type and perR-com strains as verified by Student's t test (P < 0.01); #, data are statistically significant compared to the values of the respective strains cultured anaerobically, as verified by Student's t test (P < 0.05).
perR deletion causes increased cellular manganese.
Because PerR regulates metal ion homeostasis in B. subtilis (6), its involvement in cellular metal ion balance in S. oligofermentans was examined. The wild-type, perR mutant, and perR-com strains were cultured anaerobically in BHI broth, and mid-log-phase cells (OD600 = 0.6 to 0.7) were collected to measure cellular metal by ICP-MS. Blank BHI broth was also included to determine the baseline metal content. As shown in Table 2, Mn2+ was 4.5-fold higher in the perR mutant, while its levels were similar in perR-com and the wild-type strain. However, iron and zinc were at similar levels in the three strains. This indicates that PerR specifically downregulates cellular manganese, likely by depressing the expression of Mn2+ transporters.
TABLE 2.
Cytoplasmic metal ion concentrations in various S. oligofermentans strains cultured statically or anaerobically
| Strain | Metal ion concna |
|||||
|---|---|---|---|---|---|---|
| Static culture |
Anaerobic culture |
|||||
| Mn | Fe | Zn | Mn | Fe | Zn | |
| Wild type | 4.13 ± 0.96d | 7.77 ± 1.42 | 2.37 ± 0.49 | 0.64 ± 0.15 | 6.33 ± 1.20 | 7.78 ± 1.50 |
| perR mutant | 4.59 ± 0.19 | 6.97 ± 0.56 | 2.12 ± 0.01 | 2.88 ± 0.29d | 7.17 ± 1.70 | 6.21 ± 0.70 |
| perR-com | ND | ND | ND | 0.58 ± 0.06 | 6.96 ± 0.27 | 7.30 ± 1.40 |
| mntA mutantb | 0.44 ± 0.04e | ND | ND | 0.33 ± 0.07e | ND | ND |
| mntABC-exp | 8.82 ± 0.21f | ND | ND | 2.86 ± 0.81d | ND | ND |
| Wild type-2.5Mnb | 9.02 ± 0.34 | ND | ND | 1.86 ± 0.33 | ND | ND |
| Wild type-Mnc | ND | ND | ND | 6.85 ± 0.09d | ND | ND |
| perR mutant-Mnc | ND | ND | ND | 9.80 ± 0.67g | ND | ND |
| mntABC-exp-Mnc | ND | ND | ND | 15.46 ± 3.6g | ND | ND |
Data are the means ± standard deviations of three independent cultures; metal content is expressed as nmol/mg protein. ND, not determined.
Strain grew in BHI broth with the addition of 2.5 μM MnCl2.
Strain grew in BHI broth with 0.1 mM MnCl2 added.
Value with significant difference from the anaerobically incubated wild-type strain (P < 0.05; Student's t test).
Value with significant difference from the wild-type strain growing in 2.5 μM MnCl2 (wild type-2.5Mn) (P < 0.01; Student's t test).
Value with significant difference from the statically incubated wild-type strain (P < 0.05; Student's t test).
Value with significant difference from the anaerobically incubated wild-type strain growing in 0.1 mM Mn2+ (wild type-Mn) (P < 0.01; Student's t test).
Mn2+ is important for H2O2 tolerance in S. oligofermentans.
To elucidate the role of cellular Mn2+ in H2O2 tolerance, the S. oligofermentans wild-type strain was cultured anaerobically in BHI broth supplemented with MnCl2 at different concentrations. Mid-log-phase cultures (OD600 = 0.6 to 0.7) were collected and subjected to H2O2 pulsing as described above. The survival of S. oligofermentans increased 2.5- and 23-fold upon addition of 0.1 and 0.25 mM MnCl2, respectively (Fig. 3). In addition, 10.7-fold more manganese was detected in cells grown with 0.1 mM MnCl2 than in cultures without MnCl2 supplementation (Table 2). These data suggest that Mn2+ plays a significant role in the H2O2 tolerance of S. oligofermentans. Furthermore, addition of 0.25 mM MnCl2 enhanced the shaking growth of S. oligofermentans by ∼35%, similar to that with catalase addition (200 U/ml); this confirms that Mn2+ can be an H2O2 scavenger in S. oligofermentans.
FIG 3.
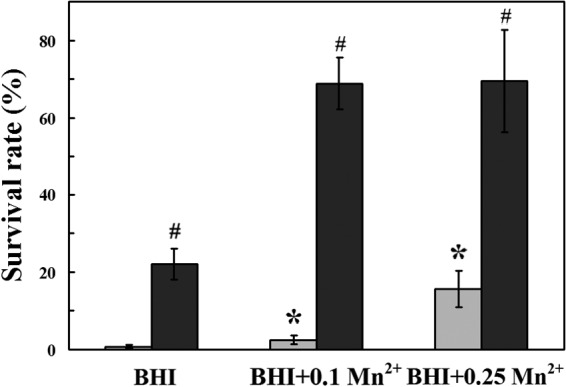
Impact of manganese ion on the H2O2-stressed survival of S. oligofermentans wild type and perR mutant. S. oligofermentans strains were anaerobically cultured in BHI broth with or without 0.1 and 0.25 mM MnCl2. After H2O2 pulsing, viable cells were counted, and survival was quantified as described for Fig. 2. The data are expressed as means ± standard deviations of three independent experiments. Gray bars, wild-type strain; black bars, perR mutant. *, data are statistically significant in comparison to the values of the wild-type strain growing in BHI broth, as verified by Student's t test (P < 0.05); #, data are statistically significant compared to the values of the wild-type strain growing in the same medium, as verified by Student's t test (P < 0.05).
perR inactivation increases Mn2+-assisted H2O2 survival of S. oligofermentans.
To find the possible linkage between PerR and Mn2+ in H2O2 resistance, an S. oligofermentans perR mutant was cultured anaerobically in BHI broth supplemented with 0.1 and 0.25 mM MnCl2 and then tested for H2O2 survival as described above. The results showed that perR inactivation increased H2O2 survival by 66% and 54% upon addition of 0.1 and 0.25 mM Mn2+, respectively, which was more than the increase in H2O2 survival (21%) in BHI broth (Fig. 3). Accordingly, addition of 0.1 mM MnCl2 increased cellular manganese more in the perR mutant than in the wild-type strain (Table 2). This indicates that PerR regulates manganese ion uptake, which contributes to H2O2 resistance of S. oligofermentans.
mntABC encodes a high-affinity Mn2+ transporter in S. oligofermentans.
To identify genes responsible for transporting Mn2+ in S. oligofermentans, two manganese transporter genes, scaCBA and mntH, which, respectively, belong to the manganese ABC transporter and the eukaryotic Nramp (natural resistance associated macrophage protein) families (42, 43), from S. gordonii were used as probes to query the complete genome of S. oligofermentans. Two targets were found: I872_09645-09655, a gene cluster encoding a putative manganese ABC transporter complex, designated mntABC (I872_09645, metal ABC transporter substrate-binding lipoprotein; I872_09650, ABC transporter membrane-spanning permease–manganese transport; I872_09655, ATP binding protein), and I872_08215, encoding a putative manganese transporter, Nramp, named mntH. The amino acid sequences of MntA, MntB, MntC, and MntH of S. oligofermentans shared 93%, 99%, 89%, and 89% identity with ScaA, ScaB, ScaC, and MntH from S. gordonii, respectively.
To measure the activity of the two manganese transport-related genes in S. oligofermentans, gene expression was measured in wild-type cells cultured statically or anaerobically in BHI broth. qPCR determined that in mid-log-phase cells (OD600 = 0.6 to 0.7), mntA expression was higher in all the cultures than mntH expression (Table 3), indicating that mntABC may be the main manganese ion transporter in S. oligofermentans. Both mntA and mntH increased (3.3- and 4.3-fold) their expression in the static culture, suggesting that the oxidative state induces the expression of the Mn2+ transporter genes mntABC and mntH.
TABLE 3.
Transcript levels of mntA, mntH, and dpr in the S. oligofermentans wild-type, perR mutant, and perR-complemented strains cultured statically or anaerobically
| Gene | Transcript levela |
|||||
|---|---|---|---|---|---|---|
| Wild type |
perR mutant |
perR-com |
||||
| Static | Anaerobic | Static | Anaerobic | Static | Anaerobic | |
| mntA | 14.86 ± 3.02 | 4.49 ± 1.37b | 11.88 ± 1.69 | 25.86 ± 8.13 | ND | 4.27 ± 0.74b |
| mntH | 0.26 ± 0.07 | 0.06 ± 0.01c | 0.30 ± 0.02 | 0.13 ± 0.06 | ND | ND |
| dpr | 6.17 ± 1.49 | 0.58 ± 0.15b | 15.90 ± 2.81 | 23.30 ± 8.81 | ND | 0.06 ± 0.01b,d |
Data are shown as means ± standard deviations of three independent experiments. Gene expression is shown as the means ± standard deviations of copy number/0.001 16S rRNA gene copy. ND, not determined.
Data are statistically significant compared to the corresponding genes in a statically cultured wild-type strain and a statically or anaerobically cultured perR mutant; Student's t test (P < 0.05).
Data are statistically significant compared to the corresponding genes in a statically cultured wild-type strain; Student's t test (P < 0.05).
Data are statistically significant compared to the corresponding genes in an anaerobically cultured wild-type strain; Student's t test (P < 0.05).
Inactivation of mntA could be achieved in BHI culture of S. oligofermentans only when supplemented with an additional ≥2.5 μM Mn2+. Since only 418 nM Mn2+ was detected in BHI broth, MntABC is predicted to be essential for S. oligofermentans in a low-Mn2+ environment. To identify the function of MntABC in the uptake of Mn2+, the wild-type strain and the mntA mutant were grown in BHI broth supplemented with 2.5 μM Mn2+, and mid-log-phase cells (OD600 = 0.6 to 0.7) were collected to measure cellular metal by ICP-MS. Compared to the wild-type strain, Mn2+ decreased by 95% and 82% in the statically and anaerobically cultured mntA mutant (Table 2), respectively, confirming that MntABC functions as a high-affinity Mn2+ transporter in S. oligofermentans.
Furthermore, 2.1- and 4.5-fold-greater manganese levels were found in a strain in which mntABC is ectopically expressed (mntABC-exp) when it was statically and anaerobically cultured, respectively (Table 2), verifying the metal-transporting function of MntABC in S. oligofermentans.
MntABC inactivation reduces the oxidative-stress tolerance of S. oligofermentans.
To investigate the role of the Mn2+ transporter MntABC in H2O2 tolerance in S. oligofermentans, both the wild-type strain and the mntA mutant were cultured statically in BHI broth supplemented with 2.5 μM Mn2+. Mid-log-phase cells (OD600 = 0.6 to 0.7) were collected and subjected to H2O2 pulsing as described above. Compared to the wild-type strain, mntA mutation reduced H2O2 survival 5.7-fold (Fig. 4), indicating that MntABC-mediated Mn2+ uptake plays a role in protecting S. oligofermentans from H2O2 attack.
FIG 4.
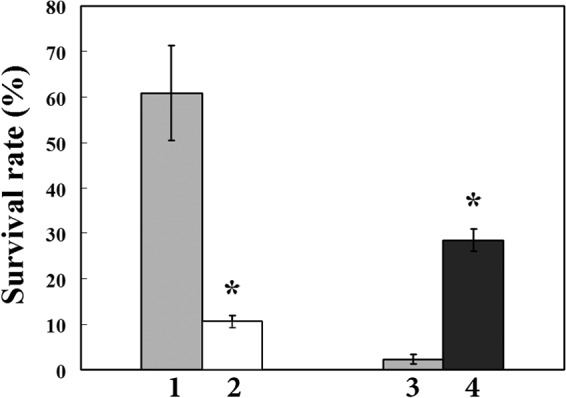
Role of MntABC-mediated manganese uptake in H2O2 stress tolerance in S. oligofermentans. The wild-type strain (1) and mntA mutant (2) were grown statically in BHI broth with addition of 2.5 μM MnCl2, and the wild-type strain (3) and mntABC-exp (4) were grown anaerobically in BHI broth with supplementation of 0.1 mM MnCl2. After H2O2 pulsing for 10 min, viable cells were counted, and survivors were quantified as described for Fig. 2. The data are expressed as means ± standard deviations of three independent experiments. *, data are statistically significant in comparison to the wild-type strain incubated under the same conditions, as verified by Student's t test (P < 0.01).
To further verify the role of MntABC in H2O2 resistance, the mntABC-exp strain was grown in BHI broth supplemented with 0.1 mM MnCl2 and anaerobically incubated. Cells at mid-log phase were treated with H2O2 (20 mM) for 10 min. About 12-fold-higher survival was determined for the mntABC-exp strain than for the wild-type strain, and cellular manganese was significantly increased in the mntABC-exp strain, as well (Table 2).
Inactivation of perR upregulates mntABC and dpr expression.
Increased Mn2+ was found in the perR mutant, so correlations among perR, mntABC, and mntH were tested in anaerobically cultured wild-type, perR mutant, and perR-com strains. Using qPCR, 5.8-fold upregulation of mntA was detected in the perR mutant, while similar expression levels were found in perR-com and the wild-type strain. Similarly, dpr, encoding an Fe2+-chelating protein, was upregulated 40-fold in the perR mutant, whereas perR complementation greatly reduced dpr expression to as much as ∼10-fold lower than that in the wild-type strain (Table 3). This shows that PerR represses expression of both mntABC and dpr. However, no significant change in mntH expression was detected in the perR mutant (Table 3), indicating that mntH is not subjected to PerR regulation.
Furthermore, a putative PerR-binding sequence “Per box” (AATTAGAAGCATTATAATT) was found in the promoter region of dpr but not in that of mntABC. Electrophoretic mobility shift assay (EMSA) detected PerR's binding only to the dpr promoter (see Fig. S1 in the supplemental material) but not to mntABC (data not shown). This suggests direct regulation of dpr by PerR but indirect regulation of mntABC in S. oligofermentans.
H2O2 releases PerR repression of mntABC.
Since an effect of perR mutation on cellular manganese, H2O2 survival, and mntABC expression was not observed in statically cultured S. oligofermentans (Tables 2 and 3 and Fig. 2) and abundant H2O2 was produced only in statically cultured cells (see Fig. S2 in the supplemental material) while none was detected in anaerobic cultures under the detection limit of 1.90 μM H2O2, H2O2 may be a substance that interferes with PerR regulation of mntABC. To confirm this, an mntABC luciferase reporter strain was constructed by introducing the integrative plasmid pFW5-PmntABC-luc into the S. oligofermentans wild-type strain and the perR mutant. The constructed S. oligofermentans PmntABC::luc and ΔperR PmntABC::luc strains were cultured anaerobically, and 20 μM H2O2, a concentration similar to that produced in statically cultured cells (see Fig. S2 in the supplemental material), was added to the early log-phase cells (OD600, ∼0.2). After cultivation for 20, 40, 60, and 80 min, the luciferase activity and OD600 were measured. mntABC expression was significantly upregulated in H2O2-pulsed cultures (Fig. 5A); however, no significant difference in mntABC expression was found between the H2O2-pulsed and nonpulsed perR mutant cells (Fig. 5B). This indicates that H2O2-induced mntABC expression depends on the presence of perR. Similar to the qPCR results (Table 3), 9- to 10-fold-elevated mntABC expression was detected in the non-H2O2-pulsed perR mutant compared to wild-type cells (Fig. 5), supporting the conclusion that PerR negatively regulates transcription of mntABC.
FIG 5.
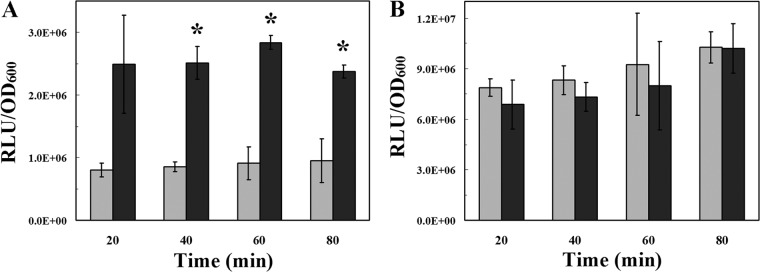
H2O2 induced mntABC expression in the wild-type strain (A) and perR mutant (B) of S. oligofermentans. Overnight BHI cultures of S. oligofermentans::PmntABC-luc and S. oligofermentans ΔperR::PmntABC-luc were diluted 1:30 in fresh BHI broth and incubated anaerobically. Then, 20 μM H2O2 was added to early-log-phase cells (OD600 = ∼0.2). Cells were collected at 20, 40, 60, and 80 min. The OD600 and luciferase activity (relative light units [RLU]) were measured as described in Materials and Methods. mntABC expression is expressed as RLU/OD600 unit. Gray bars, no H2O2 addition; black bars, 20 μM H2O2 addition. The experiments were repeated 3 times, and the data are expressed as means ± standard deviations of three reads of each independent experiment. *, data are statistically significant in comparison to the values of the wild-type strain growing in BHI broth without H2O2 addition at the respective time points, as verified by Student's t test (P < 0.01).
DISCUSSION
The oral commensal S. oligofermentans produces ample H2O2 (4.6 mM) via multiple pathways. In particular, lactate oxidase activity enables it to inhibit the caries pathogen S. mutans (22–24). S. oligofermentans is also resistant to higher H2O2 levels (5.5 mM) than other bacterial species, although it has no catalase (22). However, the activity of the ferric iron-chelating protein Dpr is insufficient for such higher H2O2 tolerance, as demonstrated by our previous work (32). Here, we report that S. oligofermentans contains extraordinarily high concentrations of manganese, which acts synergistically with Dpr to enable greater H2O2 tolerance than in other bacteria. Figure 6 depicts a model of anti-oxidative stress in S. oligofermentans. In the presence of endogenous or exogenous H2O2, PerR derepressed the expression of the manganese transporter mntABC and ferric iron-chelating protein dpr genes. MntABC facilitates Mn2+ internalization. Mn2+ can act as a cofactor of SOD or a catalase-like inorganic catalyzer; it can also substitute for Fe2+ in protein active sites to reduce protein oxidation. Dpr protein, by trapping Fenton reaction-stimulating Fe2+, prevents inert H2O2 from converting to highly reactive HO·.
FIG 6.
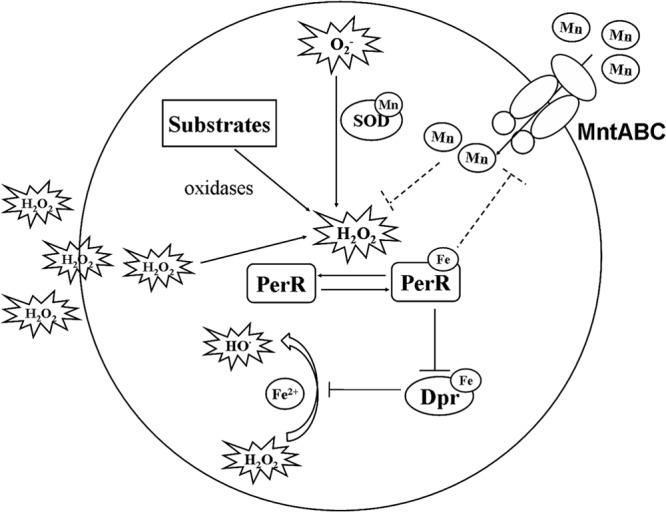
Diagram depicting the antioxidative mechanisms in S. oligofermentans. Endogenous H2O2 produced during aerobic growth or exogenous H2O2 releases PerR's inhibition of the Mn2+ transporter gene mntABC and the Fe2+-chelating protein gene dpr. Mn2+ might protect S. oligofermentans from O2− by activating SOD and from H2O2 by unknown mechanisms. Dpr prevents Fenton reactions by chelating Fe2+, avoiding the production of toxic HO·.
Fe and Mn are essential trace metal elements for bacteria, although the cellular Fe level is usually higher than that of Mn, e.g., the Mn/Fe ratio of 0.0072 (0.0197 nmol Mn and 2.72 nmol Fe/mg protein) found in E. coli (44). It has been reported that the poor Fenton reagent metal ions Mn2+ and Zn2+ prevent H2O2-derived HO· production (11), and a higher Mn/Fe ratio (0.24) contributes to oxidative-stress and radiation resistance in Deinococcus radiodurans (44). Moreover, the SOD-lacking Lactobacillus plantarum accumulates a high concentration of cellular Mn (20 to 35 mM) to defend against oxidative stress (10, 45). In this work, a high Mn/Fe ratio (0.48) was also detected in statically cultured S. oligofermentans (Table 2), but a high zinc level was not observed. Furthermore, a higher cellular Mn2+ level was found in S. oligofermentans (4.13 ± 0.96 nmol/mg protein) than in other streptococci (1.12 ± 0.23 and 1.57 ± 0.14 nmol/mg protein in Streptococcus pneumoniae and S. mutans) in the parallel measurements in this study. Supplementation experiments also confirmed the role of Mn2+ in the protection of S. oligofermentans against H2O2 (Fig. 3). How Mn2+ offers protection against H2O2 has been hypothesized. Stadtman et al. predicted a catalase-like activity of the Mn2+-bicarbonate complex in scavenging H2O2 (46), a finding consistent with the observation in S. oligofermentans that, although high H2O2 concentrations are found in the culture media, cellular H2O2 is undetectable. The H2O2-scavenging activity of Mn2+ must be confirmed, as only barely detectable H2O2 degradation was found by mixed incubation of MnCl2 with cell extracts of S. oligofermentans (data not shown).
Mn2+ is thought to reduce chemical oxidation of many proteins by substitution for the active Fenton-reactive Fe2+ at protein active sites (47). The growth of streptococci is reported to require iron (48), and high cellular iron levels (6 to 8 nmol/mg protein) are found in S. oligofermentans, as well, when cultured in BHI broth (Table 2). This suggests that Mn2+ substitution for Fe2+ can occur in the oral streptococcus under the current experimental conditions. Released Fe2+ might then be chelated by Dpr protein, avoiding Fenton chemistry.
Among the three types of manganese transporters, the manganese ABC transporter is key for bacterial uptake of Mn2+ (49). Manganese ABC transporter mutation affects biofilm formation and oxidative-stress resistance of S. gordonii and S. mutans (28, 49, 50), as well as the virulence of a number of bacterial pathogens, including S. pneumoniae, S. pyogenes, and Yersinia pestis (11, 29, 49). The mntA mutant can be obtained only in BHI medium (which contains trace amounts of Mn2+ [∼0.4 μM]) supplemented with ≥2.5 μM Mn2+. This suggests that MntABC acts as a main Mn2+ transporter at low Mn2+ levels in S. oligofermentans, while other manganese transporters, such as MntH, might function at relatively high extracellular Mn2+ levels. MntABC protects S. oligofermentans, not only from H2O2 pulsing (Fig. 4), but also from attack by paraquat, an O2− producer (data not shown), suggesting that MntABC-transported Mn2+ plays an important role in anti-oxidative stress in S. oligofermentans.
In response to H2O2 stress, E. coli employs OxyR, a transcriptional activator, to promote expression of the manganese transporter mntH and a Fur family regulator, which controls iron transporter genes (47, 51, 52). Gram-positive bacteria use PerR to repress zinc and iron transporters. In the absence of H2O2, PerR::Fe binds to the conservative DNA sequence (Per box) in the promoter regions, while H2O2 can oxidize and inactivate PerR::Fe protein, leading to derepression of zinc- and iron-transporting genes (6, 15, 53, 54). PerR in S. oligofermentans has been verified to be a peroxide-responsive repressor, as well (Fig. 2). However, unlike those of B. subtilis and S. pyogenes (6, 18), the PerR protein of S. oligofermentans represses the manganese transporter gene mntABC, in addition to dpr, so enhanced cytoplasmic Mn2+ was observed in the perR mutant (Tables 2 and 3 and Fig. 5), and this contributed to H2O2 resistance (Fig. 3). In addition, a physiological concentration of H2O2-induced mntABC transcription occurs only in the anaerobically cultured wild-type strain, but not in the perR mutant (Fig. 5). This indicates that the PerR protein of S. oligofermentans is involved in H2O2-induced mntABC gene expression. Collectively, PerR has been determined to play a pivotal role in sensing the cellular oxidative status and regulating Mn2+ and Fe2+ homeostasis in S. oligofermentans.
MntR, a member of the DtxR family, has been shown to regulate manganese transporter transcription in response to the cellular manganese concentration (55). This study also found that an MntR ortholog repressed the expression of mntABC in S. oligofermentans; however, mntR expression was not affected by perR mutation (data not shown). These data suggest that mntABC can independently respond to the cell redox status and manganese levels. Detailed regulation by PerR of mntABC and manganese homeostasis is under study in our laboratory through transcriptomics.
perR mutation does not significantly affect the antioxidant phenotype and mntABC expression when S. oligofermentans is statically cultured (Tables 2 and 3 and Fig. 2), suggesting that H2O2 produced under low oxygen levels inactivates perR. In accordance with this finding, PerR represses mntABC and dpr much more in anaerobic culture than in statically cultured cells (Table 3), and accordingly, more manganese is measured in the statically cultured wild-type strain (Table 2). More importantly, the survival of statically cultured S. oligofermentans is significantly higher than that in anaerobic culture after high-dose H2O2 pulsing (Fig. 2), indicating that antioxidant gene derepression promotes S. oligofermentans resistance to a high level of H2O2. Therefore, S. oligofermentans produces H2O2, not only as a chemical weapon in interspecies competition, but also as a strategy of “autoimmunity” to defend against more drastic oxidative stress. Orthologs of PerR and MntABC are found in almost all oral streptococci, suggesting that a PerR-regulated Mn2+-based antioxidative mechanism can be generally used by these streptococci devoid of catalase. The oral commensals, such as S. sanguinis and S. gordonii, also suppress the growth of S. mutans by producing H2O2 (21), and this plays a role in oral health.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by the National Natural Science Foundation of China, grant no. 31370098.
Footnotes
Published ahead of print 31 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00064-14.
REFERENCES
- 1.Lushchak VI. 2011. Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 153:175–190. 10.1016/j.cbpc.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 2.Imlay JA. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57:395–418. 10.1146/annurev.micro.57.030502.090938 [DOI] [PubMed] [Google Scholar]
- 3.Korshunov S, Imlay JA. 2010. Two sources of endogenous hydrogen peroxide in Escherichia coli. Mol. Microbiol. 75:1389–1401. 10.1111/j.1365-2958.2010.07059.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 11:443–454. 10.1038/nrmicro3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77:755–776. 10.1146/annurev.biochem.77.061606.161055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faulkner MJ, Helmann JD. 2011. Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid. Redox Signal. 15:175–189. 10.1089/ars.2010.3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yesilkaya H, Kadioglu A, Gingles N, Alexander JE, Mitchell TJ, Andrew PW. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 68:2819–2826. 10.1128/IAI.68.5.2819-2826.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenney FE, Jr, Verhagen MF, Cui X, Adams MW. 1999. Anaerobic microbes: oxygen detoxification without superoxide dismutase. Science 286:306–309. 10.1126/science.286.5438.306 [DOI] [PubMed] [Google Scholar]
- 9.Ilari A, Ceci P, Ferrari D, Rossi GL, Chiancone E. 2002. Iron incorporation into Escherichia coli Dps gives rise to a ferritin-like microcrystalline core. J. Biol. Chem. 277:37619–37623. 10.1074/jbc.M206186200 [DOI] [PubMed] [Google Scholar]
- 10.Archibald FS, Fridovich I. 1981. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J. Bacteriol. 146:928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culotta VC, Daly MJ. 2013. Manganese complexes: diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxid. Redox Signal. 19:933–944. 10.1089/ars.2012.5093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archibald FS, Fridovich I. 1981. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J. Bacteriol. 145:442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daly MJ. 2009. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat. Rev. Microbiol. 7:237–245. 10.1038/nrmicro2073 [DOI] [PubMed] [Google Scholar]
- 14.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann JD. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189–198. 10.1046/j.1365-2958.1998.00921.x [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Helmann JD. 2006. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature 440:363–367. 10.1038/nature04537 [DOI] [PubMed] [Google Scholar]
- 16.Helmann JD, Wu MFW, Gaballa A, Kobel PA, Morshedi MM, Fawcett P, Paddon C. 2003. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J. Bacteriol. 185:243–253. 10.1128/JB.185.1.243-253.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Z, Lee JW, Helmann JD. 2011. Identification of altered function alleles that affect Bacillus subtilis PerR metal ion selectivity. Nucleic Acids Res. 39:5036–5044. 10.1093/nar/gkr095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenot A, Weston BF, Caparon MG. 2007. A PerR-regulated metal transporter (PmtA) is an interface between oxidative stress and metal homeostasis in Streptococcus pyogenes. Mol. Microbiol. 63:1185–1196. 10.1111/j.1365-2958.2006.05577.x [DOI] [PubMed] [Google Scholar]
- 19.Grifantini R, Toukoki C, Colaprico A, Gryllos I. 2011. Peroxide stimulon and role of PerR in group A Streptococcus. J. Bacteriol. 193:6539–6551. 10.1128/JB.05924-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yesilkaya H, Andisi VF, Andrew PW, Bijlsma JJ. 2013. Streptococcus pneumoniae and reactive oxygen species: an unusual approach to living with radicals. Trends Microbiol. 21:187–195. 10.1016/j.tim.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 21.Zhu L, Kreth J. 2012. The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxid. Med. Cell. Longev. 2012:717843. 10.1155/2012/717843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong H, Chen W, Merritt J, Qi F, Shi W, Dong X. 2007. Streptococcus oligofermentans inhibits Streptococcus mutans through conversion of lactic acid into inhibitory H2O2: a possible counteroffensive strategy for interspecies competition. Mol. Microbiol. 63:872–880. 10.1111/j.1365-2958.2006.05546.x [DOI] [PubMed] [Google Scholar]
- 23.Tong H, Chen W, Shi W, Qi F, Dong X. 2008. SO-LAAO, a novel l-amino acid oxidase that enables Streptococcus oligofermentans to outcompete Streptococcus mutans by generating H2O2 from peptone. J. Bacteriol. 190:4716–4721. 10.1128/JB.00363-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Tong H, Dong X. 2012. Function of the pyruvate oxidase-lactate oxidase cascade in interspecies competition between Streptococcus oligofermentans and Streptococcus mutans. Appl. Environ. Microbiol. 78:2120–2127. 10.1128/AEM.07539-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King KY, Horenstein JA, Caparon MG. 2000. Aerotolerance and peroxide resistance in peroxidase and perR mutants of Streptococcus pyogenes. J. Bacteriol. 182:5290–5299. 10.1128/JB.182.19.5290-5299.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujishima K, Kawada-Matsuo M, Oogai Y, Tokuda M, Torii M, Komatsuzawa H. 2013. dpr and sod in Streptococcus mutans are involved in coexistence with S. sanguinis, and PerR is associated with resistance to H2O2. Appl. Environ. Microbiol. 79:1436–1443. 10.1128/AEM.03306-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsou CC, Chiang-Ni C, Lin YS, Chuang WJ, Lin MT, Liu CC, Wu JJ. 2010. Oxidative stress and metal ions regulate a ferritin-like gene, dpr, in Streptococcus pyogenes. Int. J. Med. Microbiol. 300:259–264. 10.1016/j.ijmm.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 28.Jakubovics NS, Smith AW, Jenkinson HF. 2002. Oxidative stress tolerance is manganese (Mn2+) regulated in Streptococcus gordonii. Microbiology 148:3255–3263 [DOI] [PubMed] [Google Scholar]
- 29.Janulczyk R, Ricci S, Bjorck L. 2003. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect. Immun. 71:2656–2664. 10.1128/IAI.71.5.2656-2664.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng HJ, McEwan AG, Paton JC, Jennings MP. 2002. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect. Immun. 70:1635–1639. 10.1128/IAI.70.3.1635-1639.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong H, Gao X, Dong X. 2003. Streptococcus oligofermentans sp. nov., a novel oral isolate from caries-free humans. Int. J. Syst. Evol. Microbiol. 53:1101–1104. 10.1099/ijs.0.02493-0 [DOI] [PubMed] [Google Scholar]
- 32.Zhu B, Tong H, Chen W, Dong X. 2009. Role of Dpr in hydrogen peroxide tolerance of Streptococcus oligofermentans. Wei Sheng Wu Xue Bao 49:1341–1346 [PubMed] [Google Scholar]
- 33.Surette MG, Miller MB, Bassler BL. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. U. S. A. 96:1639–1644. 10.1073/pnas.96.4.1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marmur J. 1961. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J. Mol. Biol. 3:208–218. 10.1016/S0022-2836(61)80047-8 [DOI] [Google Scholar]
- 35.Dong X, Xin Y, Jian W, Liu X, Ling D. 2000. Bifidobacterium thermacidophilum sp. nov., isolated from an anaerobic digester. Int. J. Syst. Evol. Microbiol. 1:119–125. 10.1099/00207713-50-1-119 [DOI] [PubMed] [Google Scholar]
- 36.Tong H, Shang N, Liu L, Wang X, Cai J, Dong X. 2013. Complete genome sequence of an oral commensal, Streptococcus oligofermentans strain AS 1.3089. Genome Announc. 1:pii: e00353-13. 10.1128/genomeA.00353-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193–205. 10.1016/S0167-7012(01)00369-4 [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Zeng L, Burne RA. 2009. AguR is required for induction of the Streptococcus mutans agmatine deiminase system by low pH and agmatine. Appl. Environ. Microbiol. 75:2629–2637. 10.1128/AEM.02145-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong H, Zhu B, Chen W, Qi F, Shi W, Dong X. 2006. Establishing a genetic system for ecological studies of Streptococcus oligofermentans. FEMS Microbiol. Lett. 264:213–219. 10.1111/j.1574-6968.2006.00453.x [DOI] [PubMed] [Google Scholar]
- 40.LeBlanc DJ, Lee LN, Abu-Al-Jaibat A. 1992. Molecular, genetic, and functional analysis of the basic replicon of pVA380-1, a plasmid of oral streptococcal origin. Plasmid 28:130–145. 10.1016/0147-619X(92)90044-B [DOI] [PubMed] [Google Scholar]
- 41.Podbielski A, Spellerberg B, Woischnik M, Pohl B, Lutticken R. 1996. Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS). Gene 177:137–147. 10.1016/0378-1119(96)84178-3 [DOI] [PubMed] [Google Scholar]
- 42.Kolenbrander PE, Andersen RN, Baker RA, Jenkinson HF. 1998. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J. Bacteriol. 180:290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kehres DG, Zaharik ML, Finlay BB, Maguire ME. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 36:1085–1100. 10.1046/j.1365-2958.2000.01922.x [DOI] [PubMed] [Google Scholar]
- 44.Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D. 2004. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025–1028. 10.1126/science.1103185 [DOI] [PubMed] [Google Scholar]
- 45.Archibald F. 1986. Manganese: its acquisition by and function in the lactic acid bacteria. Crit. Rev. Microbiol. 13:63–109. 10.3109/10408418609108735 [DOI] [PubMed] [Google Scholar]
- 46.Stadtman ER, Berlett BS, Chock PB. 1990. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc. Natl. Acad. Sci. U. S. A. 87:384–388. 10.1073/pnas.87.1.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anjem A, Varghese S, Imlay JA. 2009. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 72:844–858. 10.1111/j.1365-2958.2009.06699.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown JS, Holden DW. 2002. Iron acquisition by Gram-positive bacterial pathogens. Microbes Infect. 4:1149–1156. 10.1016/S1286-4579(02)01640-4 [DOI] [PubMed] [Google Scholar]
- 49.Jakubovics NS, Jenkinson HF. 2001. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147:1709–1718 [DOI] [PubMed] [Google Scholar]
- 50.Arirachakaran P, Luengpailin S, Banas JA, Mazurkiewicz JE, Benjavongkulchai E. 2007. Effects of manganese on Streptococcus mutans planktonic and biofilm growth. Caries Res. 41:497–502. 10.1159/000110882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antelmann H, Helmann JD. 2011. Thiol-based redox switches and gene regulation. Antioxid. Redox Signal. 14:1049–1063. 10.1089/ars.2010.3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng M, Storz G. 2000. Redox sensing by prokaryotic transcription factors. Biochem. Pharmacol. 59:1–6. 10.1016/S0006-2952(99)00289-0 [DOI] [PubMed] [Google Scholar]
- 53.Mongkolsuk S, Helmann JD. 2002. Regulation of inducible peroxide stress responses. Mol. Microbiol. 45:9–15. 10.1046/j.1365-2958.2002.03015.x [DOI] [PubMed] [Google Scholar]
- 54.Herbig AF, Helmann JD. 2001. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol. Microbiol. 41:849–859. 10.1046/j.1365-2958.2001.02543.x [DOI] [PubMed] [Google Scholar]
- 55.Que Q, Helmann JD. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 35:1454–1468. 10.1046/j.1365-2958.2000.01811.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


