Abstract
Forty-nine clinical isolates of multidrug-resistant Acinetobacter baumannii were obtained from 12 hospitals in 7 prefectures throughout Japan. Molecular phylogenetic analysis revealed the clonal spread of A. baumannii sequence type 208 (ST208) and ST455 isolates harboring the armA gene and ST512 harboring the armA and blaOXA-72 genes. These findings show that A. baumannii isolates harboring armA are disseminated throughout Japan, and this is the first report to show that A. baumannii strains harboring blaOXA-72 and armA are emerging in hospitals in Japan.
TEXT
Multidrug-resistant Acinetobacter baumannii has become a threatening nosocomial pathogen worldwide (1). Most strains of this species develop resistance to carbapenems by mechanisms associated with carbapenem-hydrolyzing class D OXA-type β-lactamases (CHDLs) (2). The overproduction of intrinsic chromosomal OXA-51-like enzymes and the production of acquired OXA-23-, OXA-24/40-, OXA-58-, OXA-143-, and OXA-235-like enzymes have been associated with carbapenem-resistant A. baumannii isolates (3–5). The gene blaOXA-72, one of the blaOXA-40-like genes, was first identified in an A. baumannii strain isolated in 2004 in Thailand (GenBank accession no. AY739646). Since then, Acinetobacter spp. harboring blaOXA-72 have been reported in Brazil (6), China (7), Colombia (8), Croatia (9), France (10), Italy (11), Lithuania (12), South Korea (13), Spain (14), Taiwan (15), and the United States (16).
The armA gene, encoding a 16S rRNA methylase that confers aminoglycoside resistance, was initially identified in Citrobacter freundii in 2002 in Poland (17), and it was later detected in several Gram-negative bacterial spp., including A. baumannii, in Africa, Asia, Europe, and North America (18).
From July to December 2012, the BML Biomedical Laboratories R&D Center (Kawagoe, Saitama, Japan) acquired 16,343 isolates of Acinetobacter spp. from 3,015 medical settings located in 47 prefectures throughout Japan. These included 49 isolates of multidrug-resistant A. baumannii obtained from 49 patients in 12 hospitals located in 7 prefectures in Japan (see Fig. S1 in the supplemental material). Of these 49 isolates, 41 were from respiratory tract, 7 from urinary tract, and 1 from blood samples. The multidrug-resistant A. baumannii strains were defined as having MICs of ≥16 μg/ml to imipenem/meropenem, ≥32 μg/ml to amikacin, and ≥8 μg/ml to levofloxacin/gatifloxacin or ≥4 μg/ml to ciprofloxacin, according to the criteria of the Japanese Nosocomial Infection Surveillance System (JANIS) of the Japanese Ministry of Health, Labor and Welfare (MHLW). The species were determined by the Vitek system (bioMérieux SA, Marcy l'Etoile, France) and by the sequences of the 16S rRNA, gyrB, and blaOXA-51-like genes.
The MICs were determined using the microdilution method, as described in the guidelines of the Clinical and Laboratory Standards Institute (19).
Whole genomes of the 49 multidrug-resistant isolates were extracted by DNeasy blood and tissue kits (Qiagen, Tokyo, Japan) and sequenced by MiSeq (Illumina, San Diego, CA). More-than-10-fold coverage was archived for each isolate. To identify single-nucleotide polymorphisms (SNPs) in these genomes, the sequence of A. baumannii strain MDR-TJ (GenBank accession no. CP003500) (20) was used as a control, with all reads of each isolate aligned against the MDR-TJ sequence using CLC Genomics Workbench version 5.5 (CLC bio, Tokyo, Japan). SNP concatenated sequences were aligned using MAFFT (http://mafft.cbrc.jp/alignment/server/). A maximum likelihood phylogenetic tree was constructed from the SNP alignment using PhyML 3.0 (21). The probability of node branching was evaluated with 100 bootstrappings. The multilocus sequence types (MLST) were deduced as described in the protocols of the Institut Pasteur MLST (IP-MLST) (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Abaumannii.html) and PubMLST (http://pubmlst.org/abaumannii/) databases. Clonal complexes were determined by eBURST version 3 (http://eburst.mlst.net). The sequences of 916 drug resistance genes, including β-lactamase-encoding genes (www.lahey.org/studies), aminoglycoside resistance genes (22, 23), and quinolone resistance genes (24), were determined using CLC Genomics Workbench version 5.5. Pulsed-field gel electrophoresis (PFGE) analysis was performed as described previously (25).
The genome of A. baumannii strain NCGM237, one of the 49 multidrug-resistant isolates, was sequenced using a PacBio RSII platform (Pacific Biosciences of California, Inc., Menlo Park, CA).
All 49 isolates were resistant to most of the antibiotics tested (Table 1), including to imipenem and meropenem, with MICs of ≥16 μg/ml. Five isolates showed higher MICs to imipenem and meropenem of 64 and 128 μg/ml, respectively, than the other 44 isolates. All 49 isolates were resistant to amikacin, arbekacin, and gentamicin, with MICs of ≥512 μg/ml, and to ciprofloxacin, with MICs of 32 to 1,024 μg/ml. Of the 49 isolates, 45 were susceptible to colistin.
TABLE 1.
MIC50 and MIC90 values and percent antimicrobial resistance of A. baumannii clinical isolates (n = 49)
| Antimicrobial agent | Breakpoint for resistance (μg/ml)a | % resistance | MIC data (μg/ml) |
||
|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | |||
| Amikacin | ≥64 | 100 | >1,024 | >1,024 | >1,024 |
| Arbekacin | 1,024 to >1,024 | >1,024 | >1,024 | ||
| Colistin | ≥4 | 8 | ≤0.25–4 | 2 | 2 |
| Ciprofloxacin | ≥4 | 100 | 32–1,024 | 256 | 512 |
| Gentamicin | ≥16 | 100 | 512 to >1,024 | >1,024 | >1,024 |
| Imipenem | ≥16 | 100 | 16–64 | 16 | 64 |
| Meropenem | ≥16 | 100 | 16–128 | 16 | 128 |
| Tigecyclineb | ≤0.25–4 | 1 | 4 | ||
Breakpoints for antimicrobial resistance were determined according to guidelines of the Clinical and Laboratory Standards Institute (document M07-A9 [19]).
The MICs to tigecycline were 4 μg/ml for 6 isolates, 2 μg/ml for 7 isolates, 1 μg/ml for 18 isolates, 0.5 μg/ml for 12 isolates, and <0.25 μg/ml for 6 isolates.
IP-MLST showed that all isolates belonged to sequence type 2 (ST2). According to the MLST scheme from www.pasteur.fr, all isolates belonging to ST2 belong to the worldwide clonal lineage II (European clone II) (26), indicating that A. baumannii European clone II isolates have been spreading in Japan. PubMLST showed that 23, 21, and 5 isolates belonged to ST455, ST208 (clonal complex 92 [CC92]), and ST512 (CC92), respectively. CC92 is the most widely disseminated clonal complex worldwide (27). ST455 does not belong to CC92. Molecular phylogenetic analysis based on SNP concatenation showed that the 49 isolates could be clustered into 3 clades (Fig. 1). The PFGE pattern analysis showed 2 clusters (clusters I and II) (see Fig. S2 in the supplemental material). Cluster I had the isolates belonging to ST455, and cluster II had the isolates belonging to ST208 and ST512.
FIG 1.
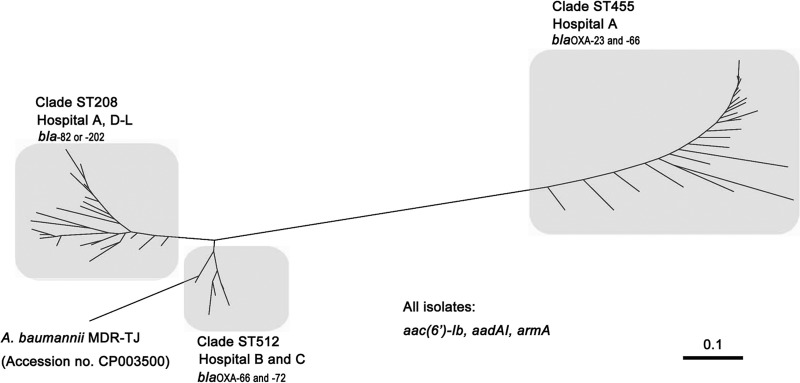
Molecular phylogeny of the 49 A. baumannii strains. Molecular phylogenetic analysis based on SNP concatenation revealed that the 49 isolates were clustered into 3 clades, with the ST208 clade composed of 2 subclades. The genes harbored by each ST are listed with the clades, and all isolates had aac(6′)-Ib, aadAI, and armA.
The 23 isolates belonging to the ST455 clade had both blaOXA-23 and blaOXA-66 genes (Table 2). Of the 21 isolates belonging to the ST208 clade, 17 had blaOXA-82 genes and 4 had blaOXA-202 genes (Table 2). The five isolates belonging to the ST512 clade had both blaOXA-66 and blaOXA-72 genes (Table 2). The blaOXA-66, blaOXA-82, and blaOXA-202 genes are blaOXA-51-like variants. Among these blaOXA genes, blaOXA-23, blaOXA-82, and blaOXA-202 were flanked by ISAba1, whereas blaOXA-66 and blaOXA-72 were not. Sixteen isolates had blaTEM-1, and all 49 had the AmpC-encoding gene, blaADC-30, flanked by ISAbaI. None of the isolates had any other β-lactamase-encoding genes registered at http://www.lahey.org/studies/. It has been reported that A. baumannii isolates producing OXA-66, OXA-82, or OXA-202 belong to European clone II (28). There were no differences in the drug susceptibility profiles of the isolates belonging to the three clades, except for their susceptibility to carbapenems. Five isolates belonging to the ST512 clade were more resistant to imipenem and meropenem, with MICs of 64 μg/ml and 128 μg/ml, respectively. These isolates harbored blaOXA-72.
TABLE 2.
MLST and drug resistance genes in A. baumannii isolates
| MLST | No. of isolates in ST | β-Lactamase-encoding genesa | Aminoglycoside resistance genesa |
|---|---|---|---|
| ST208 | 21 | blaOXA-82 (17/21), blaOXA-202 (4/21), blaADC-30, blaTEM-1 (10/21) | armA, aac(6′)-Ib, aac(3)-Ia (16/21), aadA1, aph(3′)-Ib (14/21) |
| ST455 | 23 | blaOXA-23, blaOXA-66, blaADC-30, blaTEM-1 (1/23) | armA, aac(6′)-Ib, aadA1, aph(3′)-Ib (1/23) |
| ST512 | 5 | blaOXA-66, blaOXA-72, blaADC-30, blaTEM-1 | armA, aac(6′)-Ib, aadA1, aph(3′)-Ib |
| ST369 (MDR-TJ)b | 1 | blaOXA-66, blaADC-30 | armA, aac(6′)-Ib, aadA1, aph(3′)-Ib |
Shown in parentheses are the numbers of isolates with the respective genes out of the total number of isolates for that ST, if not all isolates in that ST had those particular genes.
MDT-TJ belonging to ST369 strain was cited to compare drug resistance genes (20).
In the 5 isolates harboring blaOXA-72, this gene was located on pAB-NCGM253 (GenBank accession no. AB823544). The genetic organization of pAB-NCGM253 was similar to that of pABVA01 harboring blaOXA-24 (29) and p2ABAYE harboring no β-lactamase gene (30), which were obtained from clinical isolates in Italy and France, respectively. The entire sequences of pAB-NCGM253 (8,970 bp) had >96% identity with those of pABVA01, and most of pAB-NCGM253 (86.0%; nucleotide [nt] 1 to nt 5194 and nt 6098 to nt 8619) had >99% identity with those of p2ABAYE. The blaOXA-72 gene was flanked by XerC/XerD recombination sites, which were identical to those of pABVA01 (29), indicating mobilization by the site-specific recombination mechanism. The sequence analysis using MiSeq revealed that all 5 isolates had the same sequence of pAB-NCGM253. Isolates other than these 5 that harbored blaOXA-72 had no plasmid. The genetic environments surrounding blaOXA-23 (from nt 3159090 to nt 3162816 in the entire genome sequence [GenBank accession no. AP013357]) were ISAbaI-blaOXA-23-ISAbaI, which was identical to A. baumannii transposon Tn2006 (GenBank accession no. GQ861439) (26). All isolates had armA, aac(6′)-Ib, and aadA1, but none had the genes encoding the other 16S rRNA methylases, 6′-N-aminoglycoside acetyltransferases and aminoglycoside adenylyltransferases. The genetic environments surrounding armA (from nt 1398519 to nt 1416800 in the entire genome sequence [GenBank accession no. AP013357]) were identical to those of A. baumannii MDR-TJ isolated in China (31) and TYTH-1 isolated in Taiwan (32). The sequences from nt 1405067 to nt 1409153 in the entire genome sequence (GenBank accession no. AP013357) were identical to A. baumannii transposon Tn1548 (GenBank accession no. EU014811) (33), which included the ISCR1 insertion sequence. tnpU, a putative transposase gene, was located upstream of armA, which was followed downstream by another putative transposase gene, tnpD. A class I integron, including intI1-aac(6)-Ib-catB8-aadA1-qacEdeltaI-sulI, was located upstream of the ISCR1 insertion sequence.
A complete genome sequence of A. baumannii strain NCGM237, determined using a combination of PacBio and MiSeq platforms, revealed that the armA and blaOXA-23 were located on the chromosome (GenBank accession no. AP013357). The genome consists of a single circular chromosome of 4,021,920 bp, with an average G+C content of 39.1%. The details of the A. baumannii NCGM237 genome and its comparative analysis will be reported elsewhere. The armA and blaOXA-23 genes will be located on the chromosome in other strains belonging to ST455. The whole-genome sequences using MiSeq revealed that the genomic environments surrounding the armA and blaOXA-23 genes (18.3 kbp and 17.9 kbp, respectively) were identical to each other, and NCGM237 and PFGE analyses showed that no plasmid was found in all the isolates belonging to ST455.
All 49 isolates tested had point mutations in the quinolone resistance-determining regions of gyrA and parC, with amino acid substitutions of S83L in GyrA and S80L in ParC. The amino acid substitutions in GyrA and ParC were reported to be associated with the ciprofloxacin resistance in A. baumannii (24).
This is the first report of A. baumannii ST455 and ST512 isolates in Japan. A. baumannii ST455 isolates were originally identified as a causative agent of nosocomial infections in Taiwan (C.-H. Chiu, Chang Gung Memorial Hospital and Chang Gung University College of Medicine, personal communication), and were registered in 2012 in the A. baumannii MLST database website (http://pubmlst.org/abaumannii/). ST455 isolates have not been reported elsewhere. The A. baumannii ST512 isolates were relatively similar to A. baumannii MDR-TJ (Fig. 1), which had been isolated in China (20). Both the ST512 isolates and MDR-TJ had drug resistance genes, including blaOXA-66, armA, aac(6′)-Ib, and aadA1; however, not all isolates had blaOXA-72 (31), i.e., the ST512 isolates, but not MDR-TJ, had blaOXA-72 (Table 2). ST208 isolates were found in various regions in Japan (Fig. 1; see also Fig. S2 in the supplemental material), being first identified in 2012 in the Kanto and Kyushu areas (34), although it was not reported whether these isolates were resistant to aminoglycosides and possessed the armA gene.
To our knowledge, this is the first report showing that highly carbapenem-resistant A. baumannii strains harboring blaOXA-72 are emerging in Japan. OXA-72 was primarily responsible for carbapenem resistance in A. baumannii clinical isolates from a Taiwan hospital (15), although OXA-23 is much more prevalent worldwide (3). The expression of OXA-72 in Escherichia coli resulted in 6.0-, 2.7-, and 3.9-fold increases in the MICs to imipenem, meropenem, and doripenem, respectively, compared with that of the control (8). It is necessary to monitor highly carbapenem-resistant A. baumannii producing OXA-72 in Japan, because outbreaks due to metallo-β-lactamase producers with high MICs to carbapenems have caused serious health problems throughout Japan (35). Some A. baumannii isolates producing CHDLs show lower MICs to carbapenems (36), but a part of this population may have been undetected in this study.
The present study strongly suggests that A. baumannii isolates producing a 16S rRNA methylase, ArmA, have emerged and disseminated in medical settings throughout Japan. Bacteria producing 16S rRNA methylases are resistant to clinically important aminoglycosides (37, 38). In Japan, clinical isolates of aminoglycoside-resistant Gram-negative bacteria producing 16S rRNA methylases were first reported in 2003 (39); since then, 38 of these strains have been reported throughout Japan (38–43). A nationwide surveillance of 16S rRNA methylase-producing Gram-negative pathogens in 2004 in Japan (38) revealed that only 26 of 87,626 isolates (0.03%) produced 16S rRNA methylases. Recently, there was an outbreak of A. baumannii harboring armA at a university hospital in Japan (43). In this study, we focused on multidrug-resistant A. baumannii isolates but not aminoglycoside-resistant isolates. Therefore, a subset of aminoglycoside-resistant A. baumannii isolates, such as carbapenem-sensitive ArmA-producers, might have been missed. It is necessary to continue surveying aminoglycoside-resistant A. baumannii isolates in Japan.
Supplementary Material
ACKNOWLEDGMENTS
We thank Takashi Hirano, Kazuhito Satou, and Kuniko Teruya (Okinawa Institute of Advanced Sciences) for the complete genome sequence of A. baumannii NCGM237 using PacBio.
This study was supported by grants from International Health Cooperation Research (24-S-5), the Ministry of Health, Labor, and Welfare of Japan (H24-Shinko-Ippan-010), and JSPS KAKENHI (grant 24790432).
Footnotes
Published ahead of print 18 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01212-13.
REFERENCES
- 1.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582. 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz-Price LS, Weinstein RA. 2008. Acinetobacter infection. N. Engl. J. Med. 358:1271–1281. 10.1056/NEJMra070741 [DOI] [PubMed] [Google Scholar]
- 3.Poirel L, Naas T, Nordmann P. 2010. Diversity, epidemiology, and genetics of class D beta-lactamases. Antimicrob. Agents Chemother. 54:24–38. 10.1128/AAC.01512-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins PG, Poirel L, Lehmann M, Nordmann P, Seifert H. 2009. OXA-143, a novel carbapenem-hydrolyzing class D β-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:5035–5038. 10.1128/AAC.00856-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins PG, Pérez-Llarena FJ, Zander E, Fernández A, Bou G, Seifert H. 2013. OXA-235, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 57:2121–2126. 10.1128/AAC.02413-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werneck JS, Picão RC, Carvalhaes CG, Cardoso JP, Gales AC. 2011. OXA-72-producing Acinetobacter baumannii in Brazil: a case report. J. Antimicrob. Chemother. 66:452–454. 10.1093/jac/dkq462 [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Guo P, Sun H, Wang H, Yang Q, Chen M, Xu Y, Zhu Y. 2007. Molecular epidemiology of clinical isolates of carbapenem-resistant Acinetobacter spp. from Chinese hospitals. Antimicrob. Agents Chemother. 51:4022–4028. 10.1128/AAC.01259-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montealegre MC, Maya JJ, Correa A, Espinal P, Mojica MF, Ruiz SJ, Rosso F, Vila J, Quinn JP, Villegas MV. 2012. First identification of OXA-72 carbapenemase from Acinetobacter pittii in Colombia. Antimicrob. Agents Chemother. 56:3996–3998. 10.1128/AAC.05628-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goic-Barisic I, Towner KJ, Kovacic A, Sisko-Kraljevic K, Tonkic M, Novak A, Punda-Polic V. 2011. Outbreak in Croatia caused by a new carbapenem-resistant clone of Acinetobacter baumannii producing OXA-72 carbapenemase. J. Hosp. Infect. 77:368–369. 10.1016/j.jhin.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 10.Barnaud G, Zihoune N, Ricard JD, Hippeaux MC, Eveillard M, Dreyfuss D, Branger C. 2010. Two sequential outbreaks caused by multidrug-resistant Acinetobacter baumannii isolates producing OXA-58 or OXA-72 oxacillinase in an intensive care unit in France. J. Hosp. Infect. 76:358–360. 10.1016/j.jhin.2010.05.026 [DOI] [PubMed] [Google Scholar]
- 11.Di Popolo A, Giannouli M, Triassi M, Brisse S, Zarrilli R. 2011. Molecular epidemiological investigation of multidrug-resistant Acinetobacter baumannii strains in four Mediterranean countries with a multilocus sequence typing scheme. Clin. Microbiol. Infect. 17:197–201. 10.1111/j.1469-0691.2010.03254.x [DOI] [PubMed] [Google Scholar]
- 12.Povilonis J, Seputiene V, Krasauskas R, Juskaite R, Miskinyte M, Suziedelis K, Suziedeliene E. 2012. Spread of carbapenem-resistant Acinetobacter baumannii carrying a plasmid with two genes encoding OXA-72 carbapenemase in Lithuanian hospitals. J. Antimicrob. Chemother. 68:1000–1006. 10.1093/jac/dks499 [DOI] [PubMed] [Google Scholar]
- 13.Lee K, Kim MN, Choi TY, Cho SE, Lee S, Whang DH, Yong D, Chong Y, Woodford N, Livermore DM, KONSAR Group 2009. Wide dissemination of OXA-type carbapenemases in clinical Acinetobacter spp. isolates from South Korea. Int. J. Antimicrob. Agents 33:520–524. 10.1016/j.ijantimicag.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 14.Candel FJ, Calvo N, Head J, Sánchez A, Matesanz M, Culebras E, Barrientos A, Picazo J. 2010. A combination of tigecycline, colistin, and meropenem against multidrug-resistant Acinetobacter baumannii bacteremia in a renal transplant recipient: pharmacodynamic and microbiological aspects. Rev. Esp. Quimioter. 23:103–108 [PubMed] [Google Scholar]
- 15.Lu PL, Doumith M, Livermore DM, Chen TP, Woodford N. 2009. Diversity of carbapenem resistance mechanisms in Acinetobacter baumannii from a Taiwan hospital: spread of plasmid-borne OXA-72 carbapenemase. J. Antimicrob. Chemother. 63:641–647. 10.1093/jac/dkn553 [DOI] [PubMed] [Google Scholar]
- 16.Tian GB, Adams-Haduch JM, Bogdanovich T, Pasculle AW, Quinn JP, Wang HN, Doi Y. 2011. Identification of diverse OXA-40 group carbapenemases, including a novel variant, OXA-160, from Acinetobacter baumannii in Pennsylvania. Antimicrob. Agents Chemother. 55:429-432. 10.1128/AAC.01155-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golebiewski M, Kern-Zdanowicz I, Zienkiewicz M, Adamczyk M, Zylinska J, Baraniak A, Gniadkowski M, Bardowski J, Ceglowski P. 2007. Complete nucleotide sequence of the pCTX-M3 plasmid and its involvement in spread of the extended-spectrum beta-lactamase gene blaCTX-M-3. Antimicrob. Agents Chemother. 51:3789–3795. 10.1128/AAC.00457-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wachino J, Arakawa Y. 2012. Exogenously acquired 16S rRNA methyltransferases found in aminoglycoside-resistant pathogenic Gram-negative bacteria: an update. Drug Resist. Updat. 15:133–148. 10.1016/j.drup.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 9th ed. Approved standard M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 20.Gao F, Wang Y, Liu YJ, Wu XM, Lv X, Gan YR, Song SD, Huang H. 2011. Genome sequence of Acinetobacter baumannii MDR-TJ. J. Bacteriol. 193:2365–2366. 10.1128/JB.00226-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59:307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 22.Ramirez MS, Tolmasky ME. 2010. Aminoglycoside modifying enzymes. Drug Resist. Updat. 13:151–171. 10.1016/j.drup.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tada T, Miyoshi-Akiyama T, Shimada K, Shimojima M, Kirikae T. 2013. Novel 6′-n-aminoglycoside acetyltransferase AAC(6′)-Iaj from a clinical isolate of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 57:96–100. 10.1128/AAC.01105-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopes BS, Amyes SG. 2013. Insertion sequence disruption of adeR and ciprofloxacin resistance caused by efflux pumps and gyrA and parC mutations in Acinetobacter baumannii. Int. J. Antimicrob. Agents 41:117–121. 10.1016/j.ijantimicag.2012.08.012 [DOI] [PubMed] [Google Scholar]
- 25.Seifert H, Dolzani L, Bressan R, van der Reijden T, van Strijen B, Stefanik D, Heersma H, Dijkshoorn L. 2005. Standardization and interlaboratory reproducibility assessment of pulsed-field gel electrophoresis-generated fingerprints of Acinetobacter baumannii. J. Clin. Microbiol. 43:4328–4335. 10.1128/JCM.43.9.4328-4335.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mugnier PD, Poirel L, Naas T, Nordmann P. 2010. Worldwide dissemination of the blaOXA-23 carbapenemase gene of Acinetobacter baumannii. Emerg. Infect. Dis. 16:35–40. 10.3201/eid1601.090852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamouda A, Evans BA, Towner KJ, Amyes SG. 2010. Characterization of epidemiologically unrelated Acinetobacter baumannii isolates from four continents by use of multilocus sequence typing, pulsed-field gel electrophoresis, and sequence-based typing of blaOXA-51-like genes. J. Clin. Microbiol. 48:2476–2483. 10.1128/JCM.02431-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zander E, Nemec A, Seifert H, Higgins PG. 2012. Association between β-lactamase-encoding bla(OXA-51) variants and DiversiLab rep-PCR-based typing of Acinetobacter baumannii isolates. J. Clin. Microbiol. 50:1900–1904. 10.1128/JCM.06462-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Andrea MM, Giani T, D'Arezzo S, Capone A, Petrosillo N, Visca P, Luzzaro F, Rossolini GM. 2009. Characterization of pABVA01, a plasmid encoding the OXA-24 carbapenemase from Italian isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:3528–3533. 10.1128/AAC.00178-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallenet D, Nordmann P, Barbe V, Poirel L, Mangenot S, Bataille E, Dossat C, Gas S, Kreimeyer A, Lenoble P, Oztas S, Poulain J, Segurens B, Robert C, Abergel C, Claverie JM, Raoult D, Médigue C, Weissenbach J, Cruveiller S. 2008. Comparative analysis of Acinetobacters: three genomes for three lifestyles. PLoS One 3:e1805. 10.1371/journal.pone.0001805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H, Yang ZL, Wu XM, Wang Y, Liu YJ, Luo H, Lv X, Gan YR, Song SD, Gao F. 2012. Complete genome sequence of Acinetobacter baumannii MDR-TJ and insights into its mechanism of antibiotic resistance. J. Antimicrob. Chemother. 67:2825–2832. 10.1093/jac/dks327 [DOI] [PubMed] [Google Scholar]
- 32.Liou ML, Liu CC, Lu CW, Hsieh MF, Chang KC, Kuo HY, Lee CC, Chang CT, Yang CY, Tang CY. 2012. Genome sequence of Acinetobacter baumannii TYTH-1. J. Bacteriol. 194:6974. 10.1128/JB.01860-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doi Y, Adams JM, Yamane K, Paterson DL. 2007. Identification of 16S rRNA methylase-producing Acinetobacter baumannii clinical strains in North America. Antimicrob. Agents Chemother. 51:4209-4210. 10.1128/AAC.00560-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kouyama Y, Harada S, Ishii Y, Saga T, Yoshizumi A, Tateda K, Yamaguchi K. 2012. Molecular characterization of carbapenem-non-susceptible Acinetobacter spp. in Japan: predominance of multidrug-resistant Acinetobacter baumannii clonal complex 92 and IMP-type metallo-β-lactamase-producing non-baumannii Acinetobacter species. J. Infect. Chemother. 18:522–528. 10.1007/s10156-012-0374-y [DOI] [PubMed] [Google Scholar]
- 35.Sekiguchi J, Asagi T, Miyoshi-Akiyama T, Kasai A, Mizuguchi Y, Araake M, Fujino T, Kikuchi H, Sasaki S, Watari H, Kojima T, Miki H, Kanemitsu K, Kunishima H, Kikuchi Y, Kaku M, Yoshikura H, Kuratsuji T, Kirikae T. 2007. Outbreaks of multidrug-resistant Pseudomonas aeruginosa in community hospitals in Japan. J. Clin. Microbiol. 45:979–989. 10.1128/JCM.01772-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endo S, Yano H, Hirakata Y, Arai K, Kanamori H, Ogawa M, Shimojima M, Ishibashi N, Aoyagi T, Hatta M, Yamada M, Tokuda K, Kitagawa M, Kunishima H, Kaku M. 2012. Molecular epidemiology of carbapenem-non-susceptible Acinetobacter baumannii in Japan. J. Antimicrob. Chemother. 67:1623–1626. 10.1093/jac/dks094 [DOI] [PubMed] [Google Scholar]
- 37.Doi Y, Arakawa Y. 2007. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 45:88-94. 10.1086/518605 [DOI] [PubMed] [Google Scholar]
- 38.Yamane K, Wachino J, Suzuki S, Shibata N, Kato H, Shibayama K, Kimura K, Kai K, Ishikawa S, Ozawa Y, Konda T, Arakawa Y. 2007. 16S rRNA methylase-producing, Gram-negative pathogens, Japan. Emerg. Infect. Dis. 13:642–646. 10.3201/eid1304.060501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokoyama K, Doi Y, Yamane K, Kurokawa H, Shibata N, Shibayama K, Yagi T, Kato H, Arakawa Y. 2003. Acquisition of 16S rRNA methylase gene in Pseudomonas aeruginosa. Lancet 362:1888-1893. 10.1016/S0140-6736(03)14959-8 [DOI] [PubMed] [Google Scholar]
- 40.Doi Y, Yokoyama K, Yamane K, Wachino J, Shibata N, Yagi T, Shibayama K, Kato H, Arakawa Y. 2004. Plasmid-mediated 16S rRNA methylase in Serratia marcescens conferring high-level resistance to aminoglycosides. Antimicrob. Agents Chemother. 48:491-496. 10.1128/AAC.48.2.491-496.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wachino J, Yamane K, Shibayama K, Kurokawa H, Shibata N, Suzuki S, Doi Y, Kimura K, Ike Y, Arakawa Y. 2006. Novel plasmid-mediated 16S rRNA methylase, RmtC, found in a Proteus mirabilis isolate demonstrating extraordinary high-level resistance against various aminoglycosides. Antimicrob. Agents Chemother. 50:178-184. 10.1128/AAC.50.1.178-184.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wachino J, Shibayama K, Kurokawa H, Kimura K, Yamane K, Suzuki S, Shibata N, Ike Y, Arakawa Y. 2007. Novel plasmid-mediated 16S rRNA m1A1408 methyltransferase, NpmA, found in a clinically isolated Escherichia coli strain resistant to structurally diverse aminoglycosides. Antimicrob. Agents Chemother. 51:4401–4409. 10.1128/AAC.00926-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada Y, Suwabe A. 2012. Diverse carbapenem-resistance mechanisms in 16S rRNA methylase-producing Acinetobacter baumannii. J. Med. Microbiol. 62(Pt 4):618–622. 10.1099/jmm.0.048991-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


