Abstract
Burkholderia thailandensis contains three acyl-homoserine lactone quorum sensing circuits and has two additional LuxR homologs. To identify B. thailandensis quorum sensing-controlled genes, we carried out transcriptome sequencing (RNA-seq) analyses of quorum sensing mutants and their parent. The analyses were grounded in the fact that we identified genes coding for factors shown previously to be regulated by quorum sensing among a larger set of quorum-controlled genes. We also found that genes coding for contact-dependent inhibition were induced by quorum sensing and confirmed that specific quorum sensing mutants had a contact-dependent inhibition defect. Additional quorum-controlled genes included those for the production of numerous secondary metabolites, an uncharacterized exopolysaccharide, and a predicted chitin-binding protein. This study provides insights into the roles of the three quorum sensing circuits in the saprophytic lifestyle of B. thailandensis, and it provides a foundation on which to build an understanding of the roles of quorum sensing in the biology of B. thailandensis and the closely related pathogenic Burkholderia pseudomallei and Burkholderia mallei.
INTRODUCTION
We have a general interest in acyl-homoserine lactone (AHL) quorum sensing (QS) and the benefit this type of cell-to-cell communication can provide to bacteria living in different environments. AHL QS, which has been identified in dozens of species of Proteobacteria, usually involves gene pairs coding for LuxI-family AHL signal synthases and LuxR-family AHL signal receptors, which function as transcription factors. The AHL signals are diffusible, and when they reach a critical environmental concentration, they can interact with their cognate LuxR homolog to alter global patterns of gene expression (see reference 1 for review).
AHL QS is common to many Burkholderia species, including Burkholderia thailandensis, a nonpathogenic tropical soil saprophyte. B. thailandensis is closely related to two pathogenic species, Burkholderia pseudomallei and Burkholderia mallei (2–4). We call these three related species the Bptm group (5). Members of the Btpm group have homologous QS systems. B. thailandensis and B. pseudomallei contain three complete QS circuits, quorum sensing circuit 1 (QS-1), QS-2, and QS-3. B. mallei has retained QS-1 and QS-3, but not QS-2. The B. thailandensis QS-1 circuit consists of the BtaI1-BtaR1 pair and the signal N-octanoyl homoserine lactone (C8-HSL) (6, 7), QS-2 consists of BtaI2-BtaR2 and N-3-hydroxy-decanoyl homoserine lactone (3OHC10-HSL) (6, 8), and QS-3 consists of BtaI3-BtaR3 and N-3-hydroxy-octanoyl homoserine lactone (3OHC8-HSL) (6, 7). Additionally, each member of the Bptm group contains two orphan LuxR homologs (LuxR homologs without a cognate LuxI homolog). The B. thailandensis orphans are called BtaR4 and BtaR5 (6). The B. thailandensis QS-1 system controls aggregation, motility, and oxalic acid production, QS-2 controls synthesis of the broad-spectrum bactobolin antibiotics, and we do not know what functions are controlled by QS-3 (6–9).
We believe that B. thailandensis QS research will advance our understanding of several aspects of AHL signaling. First, there is not a deep understanding of why certain bacteria like B. thailandensis possess multiple quorum sensing systems. B. thailandensis can serve as a model to study what advantages multiple systems provide. Second, B. thailandensis, B. pseudomallei, and B. mallei are closely related species with generally conserved QS systems. B. mallei is a host-restricted pathogen, and B. pseudomallei is a soil bacterium as well as a highly infectious opportunistic pathogen. Work with either B. pseudomallei or B. mallei requires elaborate biosafety containment. Because B. thailandensis is not a human pathogen, it serves as a convenient model to study QS and other conserved aspects of the biology of the Bptm group using less-stringent non-select agent biocontainment conditions (10–13). Ultimately, we hope that comparisons of the QS regulons in B. thailandensis, B. pseudomallei, and B. mallei will provide insight about the evolution of AHL QS.
Little is known about the networks of genes controlled by QS in the Bptm group or how the multiple QS circuits might intersect. Here we describe results of a transcriptome sequencing (RNA-seq) study in which numerous QS-controlled genes are identified. This is a first step toward understanding the value of gene regulation by multiple QS circuits; it is a step toward understanding how QS benefits a saprophytic species and toward understanding how quorum sensing might benefit a saprophyte versus an opportunistic pathogen versus a host-adapted pathogen.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were grown in Luria-Bertani (LB) broth (10 g tryptone per liter, 5 g yeast extract, 5 g NaCl per liter) supplemented with 50 mM morpholinepropanesulfonic acid (MOPS) buffer (pH 7.0) when indicated. Antibiotics were added to bacteria at the following concentrations as appropriate: for Escherichia coli, 100 μg/ml trimethoprim (Tp), 25 μg/ml zeocin (Zeo), 100 μg/ml ampicillin (Ap), and 15 μg/ml gentamicin (Gm); for Burkholderia thailandensis, Tp, 100 μg/ml; and Zeo, 2 mg/ml. Where indicated, 3OHC10-HSL (2 μM), 3OHC8-HSL (4 μM), and C8-HSL (2 μM) were added. Except where indicated, bacteria were grown at 37°C with shaking.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Description or relevant genotypea | Source or reference |
|---|---|---|
| Bacterial strains | ||
| DH10B | E. coli cloning vehicle | Invitrogen |
| E264 | Wild-type B. thailandensis | 3 |
| JBT112 | E264 ΔbtaI1 ΔbtaI2 ΔbtaI3 | 7 |
| JBT107 | E264 ΔbtaR1 | 7 |
| JBT108 | E264 ΔbtaR2 | 7 |
| JBT109 | E264 ΔbtaR3 | 7 |
| JBT110 | E264 ΔbtaR4 | 7 |
| JBT111 | E264 ΔbtaR5 | 7 |
| CM183 | E264 ΔcdiAIB::tmp; Tpr | This study |
| CM219 | E264 glmS1 attn7::tmp; Tpr | This study |
| JBT101 | E264 ΔbtaI1 | 7 |
| JBT102 | E264 ΔbtaI2 | 7 |
| JBT103 | E264 ΔbtaI3 | 7 |
| Plasmids | ||
| pTNS2 | R6K replicon TnsABC+D vector | 68 |
| pUC18T-mini-Tn7T-tmp | Cloning vector | 69 |
| pBD4 | pJN105 with bmaR1; Gmr | 16 |
| pBD5 | pQF50 with the bmaI1 promoter; Apr | 16 |
| pJNR2 | pJN105 with btaR2; Gmr | 8 |
| pI2P50 | pQF50 with the btaI2 promoter; Apr | 8 |
Tpr, trimethoprim resistant; Zeor, zeocin resistant; Gmr, gentamicin resistant; Apr, ampicillin resistant.
For RNA isolation, inocula were from 5-ml overnight LB-MOPS B. thailandensis cultures grown in 16-mm tubes. Fresh LB-MOPS with or without AHLs (15 ml in 125-ml flasks) was inoculated to a starting optical density at 600 nm (OD600) of 0.05. Biological replicates were from different days.
Mutant construction.
The contact-dependent inhibition (CDI) mutant CM183 was constructed by first generating a deletion fragment with PCR and then introducing the DNA fragment into B. thailandensis via natural transformation as described previously (14). To create the ΔcdiAIB::tmp deletion fragment, we first used PCR to generate three DNA molecules. The first consisted of approximately 1,000 bp of sequence upstream of the cdi genes and was generated with primers OCM83 and OCM85 (Table 2). This fragment contained a 3′ primer-encoded sequence complementary to the fragment carrying trimethoprim resistance. The second fragment contained the trimethoprim resistance cassette from pUC18T-mini-Tn7T-tmp and was made with primers OCM84 and OCM92 (Table 2). The third fragment contained approximately 1,000 bp of sequence downstream of the cdi genes and was made with primers OCM91 and OCM86 (Table 2). The 5′ end of this fragment contained primer-encoded sequence complementary to the 3′ end of the fragment carrying trimethoprim resistance. We next stitched the DNA molecules together by Gibson product ligation (New England BioLabs). The assembled fragment was then amplified in a final PCR with primers OCM83 and OCM86, purified, and used to transform B. thailandensis to yield the Tp-resistant (Tpr) mutant strain CM183.
TABLE 2.
Primers used in this study
| Primer | Sequencea | Description |
|---|---|---|
| OCM83 | ATGTCCCGGGCGAAAAATGGGGATAGTGGA | Upstream cdi primer |
| OCM84 | ACACTCTTTGACGCTGCCTTGGATCTTGAAGTACCTATTC | Upstream forward primer for cdi and tmp junction |
| OCM85 | GAATAGGTACTTCAAGATCCAAGGCAGCGTCAAAGAGTGT | Upstream reverse primer for cdi and tmp junction |
| OCM86 | TGAACCCGGGTTTCAGGGTTGGCAAGTAGC | Downstream cdi primer |
| OCM91 | GCGCTTTTGAAGCTAATTCGTTGCATATGCTCGTCTGGTC | Downstream forward primer for cdi and tmp junction |
| OCM92 | GACCAGACGAGCATATGCAACGAATTAGCTTCAAAAGCGC | Downstream reverse primer for cdi and tmp junction |
Restriction endonuclease sites are underlined.
To create the Tpr-marked B. thailandensis strain CM219, pUC18T-mini-Tn7T-tmp and pTNS2 were introduced into B. thailandensis strain E264 by electroporation. Briefly, the cells were grown to mid-exponential phase (OD600 of 0.5), pelleted by centrifugation, and washed. First the cells were washed with an equal volume of sterile water, then with 25 ml sterile water, and then with 1 ml of 10% sterile glycerol. The pellet was resuspended in 100 μl of 10% sterile glycerol, and 1 μg of each plasmid was used for electrotransformation. The electroporation mixture was allowed a 3- to 5-h outgrowth prior to plating on selective media. Tpr colonies were selected, and we used PCR to show that the marker was inserted specifically at the attn7 site near glmS1.
Measurement of C8-HSL, 3OHC8-HSL, and 3OHC10-HSL in B. thailandensis cultures.
To measure AHLs in B. thailandensis cultures, we twice extracted 5 ml of a culture grown to an OD600 of 4.0 with acidified ethyl acetate. The analysis was performed as described elsewhere (15), except that high-performance liquid chromatography (HPLC) fractions containing 3OHC10-HSL were also collected. We measured the AHLs in appropriate HPLC fractions by using bioassays as described elsewhere (16). Synthetic C8-l-HSL was purchased from Sigma Chemical Co. 3OHC10-l-HSL was purchased from the School of Molecular Medical Sciences at the University of Nottingham (http://www.nottingham.ac.uk/quorum/compounds.htm), and synthetic 3OHC8-l-HSL was synthesized as previously described (15).
RNA isolation.
About 1 × 109 bacteria from cultures at an OD600 of 0.5, 2.0, or 4.0 were suspended in RNAprotect bacteria reagent (Qiagen) and pelleted by centrifugation. The cell pellets were stored at −80°C. Thawed cells were suspended in 1 ml RLT buffer (Qiagen) containing 2-mercaptoethanol and lysed by bead beating. RNA was purified by using the miRNAeasy minikit (Qiagen). Contaminating DNA was removed with Turbo DNase (Ambion), and RNA was obtained by using RNeasy MinElute cleanup kit (Qiagen).
RNA-seq library construction.
Bptm-specific primers were based on the sequenced genomes of B. thailandensis E264, B. pseudomallei K96243, and B. mallei ATCC 23344 using methods similar to the “not-so-random” primer method previously described (17). Primers predicted to anneal to the 5S, 16S, and 23S ribosomal sequences of each species were removed from a random set of hexamers. Two test RNA-seq analyses were run on RNA isolated from wild-type B. thailandensis cells harvested at an OD600 of 2.0, and the remaining primers that annealed to rRNA were removed from a final B. thailandensis-specific hexamer pool of 949 primers. In silico analyses of the specific primers showed an average primer density (hits per gene) of 106 for B. thailandensis, 91 for B. mallei, and 87 for B. pseudomallei. The primer sequences are provided in Table S8 in the supplemental material.
We prepared cDNA libraries as described elsewhere (18) by using the 949-primer set and isolated RNA. Briefly, a cDNA strand was generated for the RNA. Then, the first DNA strand was converted to double-stranded DNA by RNase H-mediated nick translation. The double-stranded DNA was purified, and the ends were prepared for adapter ligation. Following adapter ligation (containing bar-coded DNA sequences), the products were PCR amplified, purified, and sequenced on an Illumina Genome Analyzer II.
RNA-seq mapping and analysis.
Raw sequencing reads were sorted by bar-coded adapters and then aligned to the B. thailandensis E264 genome (the GenBank accession no. for chromosome 1 is CP000086.1 and for chromosome 2, it is CP000085.1). Aligned reads were analyzed with the Avadis NGS software package version 1.4.5 (Strand Scientific Intelligence, CA). We removed remaining reads mapping to the ribosomal sequences, determined differentially regulated genes for biological replicates using the DESeq package (with a false discovery rate [FDR] cutoff of 0.05), and proceeded with genes showing 2-fold or more regulation relative to the reference condition. Reads that partially overlapped with a gene contributed to its raw count. The Avadis NGS software package was used to compare gene expression between conditions. The data have been deposited in the NCBI sequence read archive (SRA database) (BioProject identification [ID] PRJNA233628).
Operon, ortholog, and pseudogene analysis.
We used Regulatory Sequence Analysis Tools (RSAT) (19) to predict operons based on two criteria: genes within a predicted operon must be no more than 100 bp apart and must be transcribed in the same direction. To identify orthologs and paralogs among B. thailandensis, B. pseudomallei, and B. mallei, we employed a two-pronged approach. First, all genes showing QS control at an OD600 of 2.0 were imported into the Burkholderia Prokaryotic Genome Analysis Tool (PGAT) (20). Orthologs and paralogs in B. thailandensis E264, B. pseudomallei 1026b, and B. mallei ATCC 23344 were identified by using PGAT. We used the same QS-controlled gene list to query all open reading frames in a six-frame translation of each genome in order to identify any potentially nonannotated sequence or sequences that were annotated as pseudogenes. Gene matches with 80% or more identity using basic local alignment search tool (BLAST) (21) were identified as orthologs.
The B. thailandensis genome and each QS-controlled gene list was further analyzed using PGAT tools to identify genes containing signal peptides and transmembrane domains and to assign each gene a functional category of gene product (COG).
Competition experiments.
Overnight cultures of target or inhibitor B. thailandensis strains were back diluted into fresh LB-MOPS broth with or without AHLs to an OD600 of 0.05 and grown to logarithmic phase (OD600 of 0.2 to 0.6). Then, each culture was adjusted to a final OD600 of 0.2 in phosphate buffer (pH 7.0). The partners were mixed at a 1,000:1 or 1:1 ratio of inhibitor and target. Twenty microliters of the competition mixture was then spotted onto an LB-MOPS agar plate with or without AHLs, when indicated. The plates were incubated at 30°C for 24 h. The bacteria were scraped from the agar, suspended in phosphate buffer (pH 7.0), subjected to water bath sonication for 10 min to disrupt aggregates. The bacteria were enumerated by plate counting on selective media. A competitive index (CI) was generated for each competition by dividing the final ratio of target to inhibitor cells by the starting ratio of target to inhibitor cells.
RESULTS
AHL-regulated genes.
We used RNA-seq transcriptomic analysis to identify B. thailandensis QS-controlled genes. AHL-regulated genes were identified by comparing transcripts in an AHL-negative B. thailandensis strain (JRC112) grown with or without exogenously added synthetic AHLs. Because the AHL-negative strain possesses wild-type copies of all five of its luxR homologs, it can respond to added AHLs. We identified genes regulated by the addition of the QS-1 signal (C8-HSL), the QS-2 signal (3OHC10-HSL), or the QS-3 signal (3OHC8-HSL) during logarithmic growth (L growth) (OD600 of 0.5), the transition from logarithmic growth to stationary phase (T phase) (OD600 of 2.0), and stationary phase (S phase) (OD600 of 4.0).
As a prelude to the transcriptomic analysis, we measured wild-type S-phase culture levels of AHLs so that we could add reasonable concentrations of these signals to the AHL synthesis mutant. We found 370 ± 100 nM C8-HSL, 2.5 ± 0.5 μM 3OHC10-HSL, and 190 ± 50 nM 3OHC8-HSL in culture fluid. For transcriptome analysis, we used AHL concentrations similar to or exceeding the measured amounts in wild-type culture fluid (2 μM C8-HSL, 2 μM 3OHC8-HSL, or 4 μM 3OHC10-HSL). We note that the addition of AHLs to the growth medium results in artificially high AHL levels during early growth phases. Despite the continuous presence of AHLs throughout culturing, they did not influence gene expression during L growth. However, transcript levels of dozens to hundreds of genes were affected during the T and S phases (Table 3). The greatest influence of AHLs on the transcriptome was during the T phase. Furthermore, the majority of T-phase AHL-responsive genes showed activation (65%), whereas in S phase, the majority of AHL-responsive genes were repressed (64%).Tables S1 and S2 in the supplemental material show all AHL-regulated genes. The effects of AHLs at different phases of growth are consistent with studies of other bacteria where AHL signaling is required but not sufficient for transcriptional regulation (22).
TABLE 3.
Numbers of QS-regulated genes
| AHL or transcription factor | No. of genes regulated during the followinga: |
|||||
|---|---|---|---|---|---|---|
| T phase |
S phase |
|||||
| Activated | Repressed | Total | Activated | Repressed | Total | |
| C8-HSL | 24 | 11 | 35 | 29 | 68 | 97 |
| 3OHC10-HSL | 69 | 46 | 115 | 21 | 31 | 52 |
| 3OHC8-HSL | 125 | 62 | 187 | 35 | 54 | 89 |
| BtaR1 | 101 | 63 | 164 | NDb | ND | ND |
| BtaR2 | 20 | 0 | 20 | ND | ND | ND |
| BtaR3 | 12 | 3 | 15 | ND | ND | ND |
| BtaR4 | 0 | 0 | 0 | ND | ND | ND |
| BtaR5 | 0 | 1 | 1 | ND | ND | ND |
Number of genes regulated during the transition (T) and stationary (S) phase. Regulation was determined by comparing transcripts from the AHL synthesis mutant (strain JRC112) grown with AHLs to transcripts from the mutant grown without the indicated AHL or by comparing a specific BtaR transcription factor mutant to the wild type.
ND indicates that analyses were not done.
Thirty-five genes were regulated by C8-HSL in T phase, and 97 were regulated in S phase, but only 4 showed a C8-HSL response in both the T and S phases (see Table S3 in the supplemental material). Of the many 3OHC10-HSL-regulated genes, 32 showed a response in both T and S phases. Of the many 3OHC8-HSL-regulated genes, 27 showed a response in both T and S phases. Interestingly, there was considerable overlap among the QS-2 and QS-3 signal-controlled genes; 23 of the genes regulated in T and S phases by 3OHC10-HSL were also regulated by 3OHC8-HSL in T and S phases (Table S3).
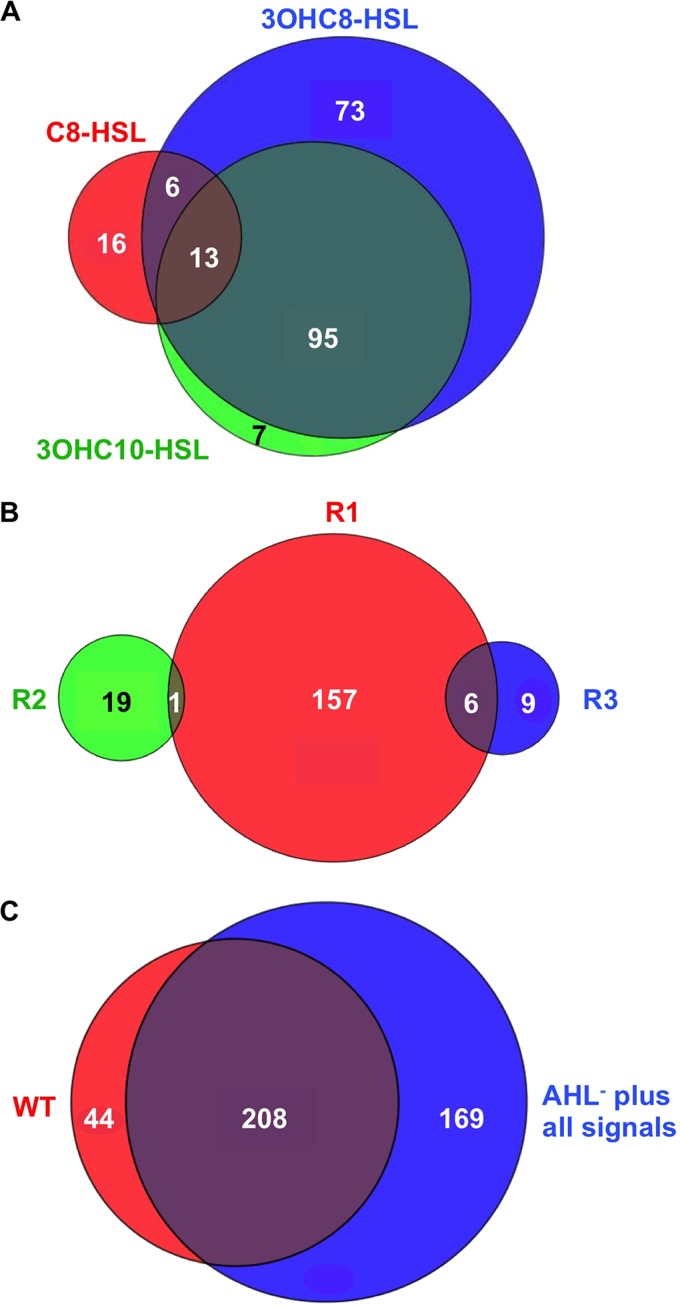
To gain additional insight, we compared the transcriptomes at T phase of the AHL-negative mutant and the wild type, and the AHL-negative mutant grown with and without all three of the AHL signals added together (Fig. 1; see Tables S1 and S4 in the supplemental material). These comparisons address two issues. The first comparison allows a normal accumulation of each of the three signals during growth and allows us to identify QS-controlled genes that might require multiple signals. The second comparison independently addresses the issue of whether multiple signals might be required for QS control of some genes. We interpret differences in the QS-regulated genes in these two comparisons to indicate that the artificial addition of relatively high AHL concentrations from the time of inoculation results in some experimental artifacts. Regardless, our results show a large overlap in the two comparisons with 208 genes showing differential regulation in either comparison (Fig. 1C and Table S4 in the supplemental material). There were, however, 169 genes showing differential expression only in the signal-add back comparison and 44 genes showing differential expression only in the comparison of the wild type to the AHL synthesis mutant. We cannot draw conclusions about these results, but they suggest that timing of expression can be affected by exogenous additions of AHLs for some, but not all, QS-regulated genes.
FIG 1.
Comparisons of T-phase QS-controlled genes. Venn diagrams show the relationships between QS-controlled genes in different RNA-seq experiments during T phase (the transition between exponential and stationary-phase growth). The circles show overlapping regulons under different conditions (the numbers of genes are also given). (A) The AHL synthesis mutant grown without any signals and grown with the indicated AHL. (B) The wild-type parent compared to the btaR1 (R1), btaR2 (R2), or btaR3 (R3) mutant (grown in medium with all three AHLs added at the time of inoculation). (C) The wild type (WT) or the AHL synthase mutant grown with all three AHLs (AHL− plus all signals) compared to the AHL mutant grown without added AHLs.
Validation of RNA-seq analysis.
Our RNA-seq analysis identified genes known to be controlled by QS or genes coding for functions known to be controlled by QS in B. thailandensis (see Tables S1, S2, S3, and S4 in the supplemental material). QS-1 promotes oxalate accumulation in T- and S-phase B. thailandensis cultures (23). RNA-seq showed that the QS-1 signal (C8-HSL) induced transcription of obc-1, which codes for the oxalate biosynthetic enzyme. Interestingly, the other two AHLs made by B. thailandensis, 3OHC8-HSL and 3OHC10-HSL, also activated obc-1. B. thailandensis QS mutants are hypermotile (6, 7), and RNA-seq showed that 17 flagellar genes and 4 methyl-accepting chemotaxis protein genes were repressed by QS. QS controls aggregation in B. thailandensis and activates transcription of the putative exopolysaccharide (EPS) genes bceABCDEFGHIJ and bceNOPRSTU (7, 24). RNA-seq confirmed this finding. RNA-seq showed that the regulation of genes for aerobic respiration is repressed by QS. This is consistent with the finding that QS mutants have increased rates of respiration compared to the wild type (7). Finally, we know that genes required for synthesis of bactobolin antibiotics are activated by QS (8, 25). RNA-seq showed strong induction of these genes by AHLs. Thus, RNA-seq reliably identified known QS-regulated genes among a much larger overall set.
Signal receptor specificity in the QS regulon.
The experiments described above provide information about the AHL-controlled regulons but do not provide direct information on which of the regulated genes respond to specific signal-responsive transcription factors. There are five LuxR homologs coded in the B. thailandensis genome, BtaR1 to BtaR5; BtaR1 to BtaR3 are cognate with BtaI1 to BtaI3, and BtaR4 and BtaR5 are orphan LuxR homologs. To gain insights into which signal receptors control what genes, we compared the transcriptome of T-phase wild-type cells to T-phase cells of strains with single null mutations in btaR1, btaR2, btaR3, btaR4, or btaR5. Because we were concerned that signal production by one system might depend on another system, we grew bacteria in the presence of added QS-1 to QS-3 AHLs.
BtaR1 affected transcript levels of 164 genes, BtaR2 regulated 20 genes, and BtaR3 controlled 15 genes (Table 3; see Table S5 in the supplemental material). Under the conditions of our analysis, BtaR4 did not significantly influence expression of any genes. The RNA-seq data indicated that btaR4 was expressed at very low levels. BtaR5 affected only one transcript, a region immediately upstream of the btaR5 open reading frame (ORF), which itself is deleted in the btaR5 mutant we used. We presume that the upstream sequence is an untranslated btaR5 leader. This result suggests that BtaR5 serves as a btaR5 repressor. However, we found that exogenous AHLs activated btaR5 transcription in the QS signal synthesis mutant. It is difficult to draw many conclusions about the orphan LuxR homologs other than to say that under the conditions of our experiments they had very little impact on the B. thailandensis transcriptome.
Although there was some overlap in the genes regulated by the three different B. thailandensis AHLs, particularly genes regulated by 3OHC8-HSL and 3OHC10-HSL, there was very little overlap in the genes regulated by BtaR1, BtaR2, and BtaR3 (Fig. 1). We presume that the AHL receptors regulate generally unique sets of genes and are most sensitive to their cognate AHL. However, the AHL receptors may show some response to the noncognate signals, which we added in relative abundance to the AHL synthesis mutant. Furthermore, there may be complex regulatory networks among the QS systems that we are just beginning to understand. It is of interest that BtaR1 activated transcription of btaR3 (see Table S5 in the supplemental material). Thus, we suggest that QS-1 induces QS-3 in a QS regulatory cascade.
We next identified genes with overlapping regulation by a signal receptor and the cognate signal. For example, we identified genes regulated by both BtaR1 and C8-HSL. We believe that these genes are regulated by QS-1 specifically. Fourteen genes were activated by BtaR1 and its cognate signal C8-HSL, 13 genes were coactivated by BtaR2 and 3OHC10-HSL, and 10 genes were activated and 2 were repressed by BtaR3 and 3OHC8-HSL (Table 4). All of the QS-2-specific genes code for bactobolin synthesis. Interestingly, almost all of the genes that appeared to be regulated by a specific signal-signal receptor system are activated rather than repressed.
TABLE 4.
QS-controlled genes regulated by an AHL and its cognate receptor during T phase
| AHL and its cognate receptor and locus taga | Gene | Operonb | Description | Fold change |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WTc | QS-1d | QS-2d | QS-3d | Alld | R1e | R2e | R3e | ||||
| BtaR1 and C8-HSL | |||||||||||
| BTH_I1956 | BTH_I1967-I1955 | Nonribosomal peptide synthetase, putative | 11.6+ | 3.5+ | 3.6+ | 13.8+ | 7.8+ | ||||
| BTH_I1957 | BTH_I1967-I1955 | Hypothetical protein | 14.3+ | 5.0+ | 4.7+ | 18.1+ | 19.9+ | ||||
| BTH_I1960 | BTH_I1967-I1955 | Hypothetical protein | 28.0+ | 6.1+ | 5.2+ | 42.3+ | 17.1+ | ||||
| BTH_I2813 | BTH_I2814-I2813 | Hypothetical protein | 3.2+ | 3.2+ | 3.9+ | ||||||
| BTH_II0204 | BTH_II0206-II0204 | Peptide synthetase, putative | 5.5+ | 8.0+ | 9.6+ | 7.7+ | |||||
| BTH_II0627 | BTH_II0627-II0626 | Hypothetical protein | 20.3+ | 8.2+ | 10.0+ | 17.4+ | 43.3+ | 26.3+ | |||
| BTH_II1071 | obc-1 | Oxalate biosynthesis enzyme | 11.7+ | 8.7+ | 10.0+ | 15.4+ | 31.7+ | 15.5+ | |||
| BTH_II1161 | LysR family transcriptional regulator | 5.2+ | 4.7+ | 4.3+ | 7.9+ | 4.4+ | |||||
| BTH_II1210 | BTH_II1219-II1209 | Hypothetical protein | 19.8+ | 6.9 | 4.7+ | 6.8+ | 32.8+ | 15.0+ | |||
| BTH_II1211 | BTH_II1219-II1209 | Polyketide synthase | 10.8+ | 4.5 | 4.2+ | 13.6+ | 12.7+ | ||||
| BTH_II1339 | Hypothetical protein | 10.8+ | 4.0+ | 15.6+ | 30.2+ | 32.1+ | 5.3+ | ||||
| BTH_II1925 | BTH_II1925-II1923 | Chitin-binding domain-containing protein | 218.9+ | 23.4+ | 54.6+ | 71.5+ | 242.7+ | 4.4+ | 91.7+ | ||
| BTH_II1997 | Hypothetical protein | 5.8+ | 3.8+ | ||||||||
| BTH_II2000 | BTH_II2001-II2000 | Hypothetical protein | 3.3+ | 6.1+ | 6.5+ | 5.5+ | |||||
| BtaR2 and 3OHC10-HSL | |||||||||||
| BTH_II1223 | BTH_II1224-II1223 | Hypothetical protein | 66.8+ | 19.8+ | 19.4+ | 46.0+ | 12.9+ | ||||
| BTH_II1224 | btaA | BTH_II1224-II1223 | CmaB | 236.4+ | 10.5 | 130.2+ | 146.5+ | 241.2+ | 17.2+ | ||
| BTH_II1225 | btaC | BTH_II1228-II1225 | Phosphopantetheine-containing protein | 414.6+ | 220.8+ | 278.0+ | 373.0+ | 17.5+ | |||
| BTH_II1226 | btaE | BTH_II1228-II1225 | Peptide synthetase, putative | 327.1+ | 11.5+ | 171.3+ | 206.8+ | 331.2+ | 16.6+ | ||
| BTH_II1233 | btaK | BTH_II1241-II1233 | Peptide synthetase, putative | 149.4+ | 24.4+ | 19.2+ | 151.1+ | 36.4+ | |||
| BTH_II1234 | btaL | BTH_II1241-II1233 | JamP | 158.7+ | 20.2+ | 16.7+ | 122.2+ | 48.0+ | |||
| BTH_II1235 | btaM | BTH_II1241-II1233 | JamP | 127.9+ | 23.2+ | 23.1+ | 138.1+ | 30.4+ | |||
| BTH_II1236 | btaN | BTH_II1241-II1233 | Nonribosomal peptide synthetase, putative | 111.9+ | 21.0+ | 21.5+ | 128.4+ | 45.5+ | |||
| BTH_II1237 | btaO | BTH_II1241-II1233 | Thiotemplate mechanism natural product synthetase | 79.8+ | 15.8+ | 14.9+ | 96.9+ | 33.8+ | |||
| BTH_II1238 | btaP | BTH_II1241-II1233 | Polyketide synthase | 46.6+ | 29.8+ | 28.1+ | 54.4+ | 27.0+ | |||
| BTH_II1240 | btaS | BTH_II1241-II1233 | Thioesterase II | 47.0+ | 14.0+ | 11.8+ | 70.6+ | 20.0+ | |||
| BTH_II1241 | btaT | BTH_II1241-II1233 | Drug resistance transporter, Bcr/CflA family protein, putative | 75.4+ | 15.9+ | 12.7+ | 88.2+ | 21.6+ | |||
| BTH_II1242 | btaU | TauD/TfdA family dioxygenase | 220.4+ | 6.6+ | 104.3+ | 118.8 | 390.2+ | 29.8+ | |||
| BtaR3 and 3OHC8-HSL | |||||||||||
| BTH_I0814 | cysI | BTH_I0819-I0814 | Sulfite reductase | 2.6+ | 4.5+ | 3.8+ | |||||
| BTH_I1020 | kdpF | BTH_I1023-I1020 | Potassium-transporting ATPase, KdpF subunit-related protein | 15.2+ | 23.2+ | 30.5+ | 37.0+ | 28.3+ | |||
| BTH_I1021 | kdpA | BTH_I1023-I1020 | Potassium-transporting ATPase subunit A | 6.1+ | 13.3+ | 15.1+ | 10.3+ | 7.5+ | |||
| BTH_I2299 | BTH_I2301-I2299 | LacI family transcription regulator | 4.2+ | 8.9+ | 11.5+ | 6.0+ | 3.4+ | 8.4+ | |||
| BTH_II0022 | Sperm-specific protein Phi-1 | 7.3+ | 9.2+ | 11.5+ | 7.3+ | 5.6+ | |||||
| BTH_II1170 | nirB | BTH_II1172-II1169 | Nitrite reductase [NAD(P)H], large subunit | 8.5+ | 6.8+ | 10.5+ | 8.3+ | 3.5+ | 4.3+ | ||
| BTH_II1172 | BTH_II1172-II1169 | Nitrate reductase | 9.2+ | 6.0+ | 7.0+ | 8.4+ | 3.9+ | 9.3+ | |||
| BTH_II1279 | BTH_II1281-II1276 | Glyoxalase family protein family | 11.5+ | 6.6+ | 8.6+ | 15.1+ | 6.5+ | ||||
| BTH_II1307* | Hypothetical protein | 22.2+ | 6.1+ | 7.4+ | 17.6+ | 4.5+ | |||||
| BTH_II1720 | Outer membrane porin OpcP | 2.7− | 3.9− | 7.8− | 7.9− | 4.2− | |||||
| BTH_II1925 | BTH_II1925-II1923 | Chitin-binding domain-containing protein | 218.9+ | 23.4+ | 54.6+ | 71.5+ | 242.7+ | 4.4+ | 91.7+ | ||
| BTH_II2089 | malB | BTH_II2089-II2088 | Hypothetical protein | 16.6− | 18.4− | 2.8− | |||||
Locus tags correspond to the B. thailandensis E264 genome. An asterisk after a locus tag indicates that it is a PGAT (20) predicted pseudogene.
When indicated, the loci in a predicted operon are given.
Fold change value and induction (+) or repression (−) in the wild-type (WT) strain compared to the AHL synthesis mutant strain JRC112 without added AHLs.
Fold change value and induction (+) or repression (−) by AHLs (QS-1 for C8-HSL, QS-2 for 3OHC10-HSL, QS-3 for 3OHC8-HSL, and All for all three AHLs) when added to strain JRC112.
Fold change value and induction (+) or repression (−) in the wild-type strain compared to the indicated btaR mutants (btaR1 [R1] to btaR3 [R3]).
Chromosomal distribution of QS-controlled genes.
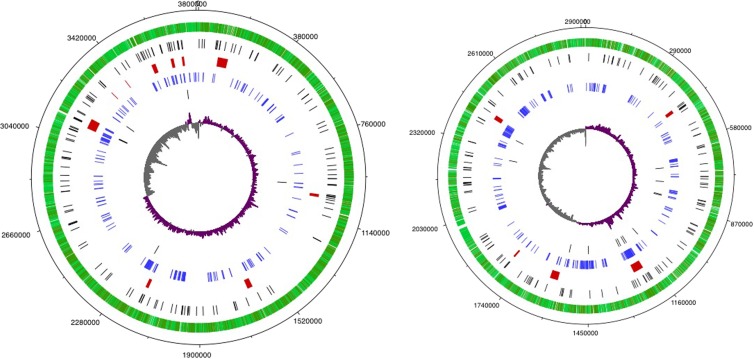
B. thailandensis contains two circular chromosomes (Fig. 2). Chromosome 1 is the larger of the two with 3,282 predicted ORFs, and chromosome 2 has 2,363 predicted ORFs (26). For many bacteria with two chromosomes, including members of the Bptm group, the larger chromosome is enriched in genes coding for essential functions, and the smaller chromosome is enriched in genes of unknown function and genes associated with adaptation (27). Interestingly, all three of the QS cognate pairs of genes (btaI1-btaR1, btaI2-btaR2, and btaI3-btaR3) and one of the two orphan receptor genes (btaR4) are found on chromosome 2. Of the 542 genes controlled by QS in the T phase, most (308) are on chromosome 2. The density of QS-controlled genes on chromosome 2 was nearly twice that of QS-controlled genes on chromosome 1 (13% of the chromosome 2 genes versus 7% of the chromosome 1 genes). Figure 2 shows the distribution of the QS-controlled genes across the B. thailandensis chromosomes and the pseudogene distribution. There was no enrichment for pseudogenes in the QS regulon compared to genome-wide distribution of pseudogenes.
FIG 2.
Diagrams of the large and small B. thailandensis chromosomes. Base numbers are shown in the outermost ring. The next ring shows annotated genes in light and dark green for strand orientation. The ring of black hash marks indicates pseudogenes. The ring showing red bars illustrates genomic islands. QS-controlled genes are shown in blue in the next ring followed by pseudogenes in QS regulon (black). The innermost ring shows GC skew (positive values in purple and negative values in gray). The images were generated with DNAplotter (67).
The B. thailandensis genome contains 15 genomic islands (GIs), which have atypical GC content or code for bacteriophage-related genes or phage-like elements (26). The content and location of the GIs differ among strains of B. thailandensis and also among other Burkholderia species (26, 28, 29). Eighty-four QS-controlled genes mapped to 7 of the 15 genomic islands (see Table S6 in the supplemental material). Fifty-five of the 84 genes on GIs were on GI 12, which lies between BTH_II1011 and BTH_II1070. GI 12 genes are predicted to code for a bacteriophage. B. thailandensis is able to produce functional phages; however, it is not known which genes are precisely responsible for phage production (30, 31).
Do orthologs of B. thailandensis QS-controlled genes occur in B. mallei and B. pseudomallei? We identified orthologs of the B. thailandensis QS-controlled genes in other members of the Bptm group (see Table S7 in the supplemental material). We sought to gain insight into which QS-regulated factors are associated with saprophytic survival. This idea was based on the fact that the genome of B. mallei has experienced reductive evolution, in which it lost over 20% of its ancestral genome. Presumably, B. mallei lost genes of specific value for saprophytic life. Thus, the absence of B. thailandensis QS-controlled orthologs in the B. mallei genome would suggest a role in saprophytic life for those genes. We found that 77 B. thailandensis QS-controlled genes are absent from both B. mallei and B. pseudomallei. Forty-two of the 77 are on GIs. We identified many more orthologs of QS-regulated genes, 142, in B. pseudomallei but not B. mallei. Perhaps these genes also code for functions involved in the free-living lifestyle. Most of the B. thailandensis QS-regulated genes, 323, have orthologs in both B. mallei and B. pseudomallei.
Predicted functions and locations of QS-controlled gene products.
Secreted products are overrepresented in the QS regulon of the well-studied bacterium Pseudomonas aeruginosa (32). An in silico analysis indicated that in B. thailandensis secreted polypeptides are not overrepresented in the large group of genes identified as QS. There was not an overrepresentation of polypeptides containing signal peptides or transmembrane domains. We also asked whether gene products predicted to code for production of secreted or excreted products might be enriched in the B. thailandensis QS regulon by an analysis of functional categories of gene products (COG) (see Fig. S1 in the supplemental material). Three COG groups appeared to be overrepresented among the QS-controlled regulon in comparison to genome-wide distributions: secondary metabolite biosynthesis, lipid metabolism, and those with unknown functions. The following COG groups were underrepresented: translation, transcription, intracellular trafficking and secretion, and ion transport and metabolism.
QS controls production of predicted and characterized secondary metabolites.
QS controlled a large number of genes associated with 11 predicted and characterized secondary metabolites (Table 5). It was previously reported that QS controls the production of bactobolin (8, 25). Indeed, we observed that the genes for bactobolin production were activated by QS. We also observed that QS controls genes associated with the production of burkholdac, malleobactin, terphenyl, thailandamide, 2-alkyl-4-quinolone, rhamnolipids, and malleilactone, and genes in 3 additional uncharacterized clusters that either contain polyketide synthase (PKS) or nonribosomal peptide synthase (NRPS) genes. Interestingly, some secondary metabolite genes are QS activated, and others are QS repressed. This suggests that QS does not act as a global activator of secondary metabolite production but specifically activates and represses certain products in different conditions. For example, genes for bactobolin, burkholdac, and genes in two uncharacterized clusters (designated unknown-1 and unknown-3) showed the strongest T-phase QS activation. Alternatively, genes for malleobactin showed QS repression only during S phase. Yet other genes associated with the production of malleilactone, 2-alkyl-4-quinolone, unknown product-2, terphenyl, and thailandamide showed complex regulation with activation or repression depending on the QS signal added and the growth phase.
TABLE 5.
QS control of genes for production of known or predicted secondary metabolitesa
| Secondary metabolite and locus tagb | Gene | Descriptionc | Fold change |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T phase |
S phase |
||||||||||||
| WTd | QS-1e | QS-2e | QS-3e | Alle | R1f | R2f | R3f | QS-1e | QS-2e | QS-3e | |||
| Unknown-1 | |||||||||||||
| BTH_I1950 | AcrB/AcrD/AcrF family protein | 6.9+ | 8.4+ | 5.4+ | |||||||||
| BTH_I1951 | mexE | Multidrug efflux RND membrane fusion protein MexE | 10.0+ | 8.5+ | |||||||||
| BTH_I1952 | Adenylylsulfate kinase | 11.4+ | 8.7+ | 5.4+ | |||||||||
| BTH_I1953 | Peptide synthetase domain-containing protein | 14.2+ | 15.6+ | 12.0+ | |||||||||
| BTH_I1954 | Hypothetical protein | 23.0+ | 28.4+ | 19.5+ | |||||||||
| BTH_I1955 | Hypothetical protein | 14.0+ | 7.3+ | ||||||||||
| BTH_I1956 | Nonribosomal peptide synthetase, putative | 11.6+ | 3.5+ | 3.6+ | 13.8+ | 7.8+ | |||||||
| BTH_I1957 | Hypothetical protein | 14.3+ | 5.0+ | 4.7+ | 18.1+ | 19.9+ | |||||||
| BTH_I1958 | Dioxygenase, TauD/TfdA | 7.1+ | 5.1+ | 8.2+ | 13.7+ | ||||||||
| BTH_I1960 | Hypothetical protein | 28.0+ | 6.1+ | 5.2+ | 42.3+ | 17.1+ | |||||||
| BTH_I1961* | Hypothetical protein | 20.6+ | 9.5+ | ||||||||||
| BTH_I1963 | Transketolase, C-terminal subunit | 10.0+ | |||||||||||
| BTH_I1965 | Acyltransferase family protein | 3.9+ | 5.9+ | 6.2+ | |||||||||
| BTH_I1966 | serC2 | Phosphoserine aminotransferase | 7.2+ | 9.9+ | |||||||||
| BTH_I1967 | Glycosyltransferase, group 2 family protein | 5.3+ | 8.5+ | 5.6+ | |||||||||
| BTH_I1969 | Kinase, putative | 5.8+ | 6.1+ | ||||||||||
| BTH_I1970 | Cysteine synthase/cystathionine beta-synthase family protein | 11.8+ | 27.0+ | ||||||||||
| BTH_I1971 | Argininosuccinate lyase | 3.5+ | 5.1+ | 8.0+ | |||||||||
| Burkholdac | |||||||||||||
| BTH_I2367 | Dihydroaeruginoic acid synthetase | 4.0+ | |||||||||||
| Malleobactin | |||||||||||||
| BTH_I2415 | TonB-dependent siderophore receptor | 3.2− | |||||||||||
| BTH_I2417 | Nonribosomal peptide synthetase, putative | 5.1− | |||||||||||
| BTH_I2418 | Peptide synthetase-like protein | 2.8− | |||||||||||
| Terphenyl | |||||||||||||
| BTH_II0204 | Peptide synthetase, putative | 5.5+ | 8.0+ | 9.6+ | 7.7+ | 6.4− | 4.8− | ||||||
| BTH_II0205 | Hypothetical protein | 11.4+ | 25.6+ | 12.9+ | |||||||||
| BTH_II0206 | Hypothetical protein | 15.3+ | |||||||||||
| BTH_II0207 | Hypothetical protein | 14.6+ | 13.7+ | 10.3+ | |||||||||
| Unknown-2 | |||||||||||||
| BTH_II0562 | BarD | 5.3− | 8.6− | 10.2− | |||||||||
| BTH_II0563 | Peptide synthetase, putative | 4.6− | 9.8− | 11.4− | |||||||||
| BTH_II0564 | BarB2 | 4.1+ | 11.5+ | ||||||||||
| BTH_II0566 | Demethylmenaquinone methyltransferase | 4.8− | 9.3− | 9.2− | |||||||||
| BTH_II0567 | Branched-chain amino acid aminotransferase | 3.8− | 7.2− | 6.3− | |||||||||
| BTH_II0569 | mhpF | Acetaldehyde dehydrogenase | 4.1− | 6.6− | 6.2− | ||||||||
| BTH_II0570 | mhpE | 4-Hydroxy-2-ketovalerate aldolase | 5.1− | 10.0− | 9.6− | ||||||||
| BTH_II0571 | Pectin degradation protein KdgF | 4.2− | 6.3− | ||||||||||
| Unknown-3 | |||||||||||||
| BTH_II1209 | Hypothetical protein | 18.6+ | 4.6+ | 8.3+ | 54.6+ | 32.8+ | |||||||
| BTH_II1210 | Hypothetical protein | 19.8+ | 6.9+ | 4.7+ | 6.8+ | 32.8+ | 15.0+ | ||||||
| BTH_II1211 | Polyketide synthase | 10.8+ | 4.5+ | 4.2+ | 13.6+ | 12.7+ | |||||||
| BTH_II1212 | Syringomycin biosynthesis enzyme, putative | 17.0+ | |||||||||||
| BTH_II1213 | Peptide synthetase-like protein | 10.7+ | 18.0+ | 8.2+ | |||||||||
| BTH_II1214 | Peptide synthetase, putative | 11.1+ | 4.7+ | 18.2+ | 6.6+ | ||||||||
| BTH_II1216 | d-Cysteine desulfhydrase, putative | 10.5+ | |||||||||||
| BTH_II1218* | AMP-binding domain-containing protein | 27.7+ | 5.3+ | 6.4+ | 28.4+ | 12.5+ | |||||||
| Bactobolin | |||||||||||||
| BTH_II1222 | 4-Hydroxyphenylpyruvate dioxygenase | 12.8+ | 11.5+ | 9.7+ | |||||||||
| BTH_II1223 | Hypothetical protein | 66.8+ | 19.8+ | 19.4+ | 46.0+ | 12.9+ | 13.5+ | ||||||
| BTH_II1224 | btaA | CmaB | 236.4+ | 10.5+ | 130.2+ | 146.5+ | 241.2+ | 17.2+ | 2.8+ | 35.0+ | 18.4+ | ||
| BTH_II1225 | btaC | Phosphopantetheine-containing protein | 414.6+ | 220.8+ | 278.0+ | 373.0+ | 17.5+ | ||||||
| BTH_II1226 | btaE | Peptide synthetase, putative | 327.1+ | 11.5+ | 171.3+ | 206.8+ | 331.2+ | 16.6+ | 69.9+ | ||||
| BTH_II1227 | btaI2 | N-acyl homoserine lactone synthase | 1563+ | 17.6+ | |||||||||
| BTH_II1228 | btaF | Hypothetical protein | 8.5+ | ||||||||||
| BTH_II1229 | btaG | Sodium/hydrogen exchanger | 24.3+ | 11.0+ | 23.4+ | 30.7+ | |||||||
| BTH_II1230 | btaH | Hypothetical protein | 5.2+ | 6.2+ | 4.9+ | ||||||||
| BTH_II1231 | btaR2 | ATP-dependent transcription regulator LuxR | 3.6+ | 26.2+ | |||||||||
| BTH_II1232 | btaJ | Oligopeptidase A | 11.3+ | 7.5+ | 8.3+ | 13.7+ | |||||||
| BTH_II1233 | btaK | Peptide synthetase, putative | 149.4+ | 24.4+ | 19.2+ | 151.1+ | 36.4+ | ||||||
| BTH_II1234 | btaL | JamP | 158.7+ | 20.2+ | 16.7+ | 122.2+ | 48.0+ | ||||||
| BTH_II1235 | btaM | JamP | 127.9+ | 23.2+ | 23.1+ | 138.1+ | 30.4+ | ||||||
| BTH_II1236 | btaN | Nonribosomal peptide synthetase, putative | 111.9+ | 21.0+ | 21.5+ | 128.4+ | 45.5+ | ||||||
| BTH_II1237 | btaO | Thiotemplate mechanism natural product synthetase | 79.8+ | 15.8+ | 14.9+ | 96.9+ | 33.8+ | ||||||
| BTH_II1238 | btaP | Polyketide synthase | 46.6+ | 29.8+ | 28.1+ | 54.4+ | 27.0+ | ||||||
| BTH_II1239 | btaQ | Acetyltransferase | 56.7+ | 75.5+ | 30.0+ | ||||||||
| BTH_II1240 | btaS | Thioesterase II | 47.0+ | 14.0+ | 11.8+ | 70.6+ | 20.0+ | ||||||
| BTH_II1241 | btaT | Drug resistance transporter, Bcr/CflA family protein, putative | 75.4+ | 15.9+ | 12.7+ | 88.2+ | 21.6+ | ||||||
| BTH_II1242 | btaU | TauD/TfdA family dioxygenase | 220.4+ | 6.6+ | 104.3+ | 118.8+ | 390.2+ | 29.8+ | 4.0+ | ||||
| Thailandamide | |||||||||||||
| BTH_II1674 | Polyketide synthase | 3.5+ | |||||||||||
| BTH_II1677 | Phenolpthiocerol synthesis type i polyketide synthase PpsA | 4.1− | 6.0− | ||||||||||
| Rhamnolipid | |||||||||||||
| BTH_II1075 | rhlA1 | Rhamnosyltransferase 1, subunit A | 3.0+ | ||||||||||
| BTH_II1076* | rhlB1 | Rhamnosyltransferase I, subunit B | 5.4+ | ||||||||||
| BTH_II1077 | EmrB/QacA family drug resistance transporter | 5.2+ | |||||||||||
| BTH_II1081 | Multidrug resistance protein | 3.5+ | |||||||||||
| BTH_II1879 | EmrB/QacA family drug resistance transporter | 3.7+ | |||||||||||
| BTH_II1880* | rhlB2 | Rhamnosyltransferase I, subunit B | 5.7+ | ||||||||||
| 2-Alkyl-4-quinolone | |||||||||||||
| BTH_II1929 | hmqG | Hypothetical protein | 3.7− | 3.1− | 6.3− | 5.9− | |||||||
| BTH_II1930 | hmqF | AMP-binding domain-containing protein | 10.8+ | 3.7− | 4.2− | 5.7− | 4.6− | ||||||
| BTH_II1931 | hhqE | Metallo-beta-lactamase domain-containing protein | 5.3− | ||||||||||
| BTH_II1932 | hhqD | 3-Oxoacyl-(acyl carrier protein) synthase III | 5.4− | 5.2− | 3.2− | 7.7− | 8.5− | ||||||
| BTH_II1933 | hhqC | Hypothetical protein | 5.6− | 4.3− | |||||||||
| BTH_II1935 | hhqA | Acetyl-CoA synthetase, putative | 5.2− | 4.4− | 4.9− | 4.3− | |||||||
| Malleilactone | |||||||||||||
| BTH_II2088 | malA | Thiotemplate mechanism natural product synthetase | 12.6− | 16.6− | 5.0− | 11.8− | 13.5− | ||||||
| BTH_II2089 | malB | Hypothetical protein | 16.6− | 18.4− | 2.8− | 4.4− | 10.9− | 15.7− | |||||
| BTH_II2090 | malC | Syringomycin synthesis regulator SyrP, putative | 10.1− | 10.8− | 5.3− | 9.5− | 10.6− | ||||||
| BTH_II2091 | malD | Hypothetical protein | 8.6+ | 9.9− | 9.6− | 8.0+ | 4.4− | 7.9− | 12.2− | ||||
| BTH_II2092 | malE | Gamma-aminobutyraldehyde dehydrogenase | 5.4- | 4.4- | 4.4- | 26.4- | 7.5- | ||||||
| BTH_II2093 | malF | Polyketide synthase, putative | 7.0− | 7.0− | 6.2+ | 3.8− | 8.1− | 8.8− | |||||
| BTH_II2094 | malG | Ketol-acid reductoisomerase | 3.5− | 6.2− | 6.6− | ||||||||
| BTH_II2095 | malI | Diaminopimelate decarboxylase, putative | 5.6+ | 5.7− | 6.7− | 4.1− | 8.5− | 8.5− | |||||
| BTH_II2096 | malJ | Long-chain-fatty-acid–CoA ligase, putative | 4.9+ | 5.3− | 5.6− | 3.6+ | 3.8− | 8.4− | 8.7− | ||||
| BTH_II2097 | malK | Putative lipoprotein | 4.9− | ||||||||||
| BTH_II2098 | malL | Malonyl CoA-acyl carrier protein transacylase | 4.5− | 7.3− | 9.3− | ||||||||
| BTH_II2099 | malM | AMP-binding domain-containing protein | 9.8+ | 5.6− | 5.1− | 11.0+ | 4.0+ | 4.1− | 7.9− | 8.5− | |||
QS-controlled genes during T or S phase predicted or shown to code for the production of secondary metabolites.
Locus tags correspond to the B. thailandensis E264 genome. An asterisk after a locus tag indicates that it is a pseudogene predicted by PGAT (20) analysis.
RND, resistance-nodulation-cell division; CoA, coenzyme A.
Fold change value and induction (+) or repression (−) in the wild-type strain compared to the AHL synthesis mutant strain JRC112 without added AHLs.
Fold change value and induction (+) or repression (−) by AHLs (QS-1 for C8-HSL, QS-2 for 3OHC10-HSL, QS-3 for 3OHC8-HSL, and All for all three AHLs) when added to strain JRC112.
Fold change value and induction (+) or repression (−) in the wild-type strain compared to the indicated btaR mutants (btaR1 [R1] to btaR3 [R3]).
BtaR2 was the sole activator of the bactobolin genes and did not activate other known or predicted PKS or NRP genes. BtaR1 activated genes in multiple clusters associated with the production of terphenyl, malleilactone, and unknown-1 and unknown-3. The BtaR1-controlled genes BTH_II1209 through BTH_II1218 (unknown-3 cluster) lie immediately upstream of the QS-2 controlled bactobolin genes. BtaR3 repressed a single gene in the malleilactone gene cluster. It seems possible that the QS systems contribute independent and even opposing regulation to some secondary metabolite genes.
We do not know why QS regulation of secondary metabolites is so complex. It is tempting to speculate that QS acts to shuttle energy and precursors toward synthesis of certain products while limiting the synthesis of others. The significance of this might become clear as we learn more about the functions of the B. thailandensis secondary metabolites.
QS controls contact-dependent growth inhibition.
Our analysis also revealed that 13 GI 5 genes were QS activated (see Table S6 in the supplemental material). GI 5 contains contact-dependent growth inhibition (CDI) genes. B. thailandensis and B. pseudomallei CDI systems are composed of the CdiA and CdiB two-partner secretion system and an immunity protein, CdiI. The CdiA and CdiB proteins inhibit growth of nonimmune Burkholderia cells (33, 34). The genes for CdiA, CdiI, CdiB, and several uncharacterized downstream genes (BTH_I2719-I2720 and BTH_I2713-I2716) were activated by the addition of the QS-2 signal (3OHC10-HSL), the QS-3 signal (3OHC8-HSL), or all three AHLs together. However, BtaR1 alone activated cdiI, cdiA, BTH_I2719, and BTH_I2716 (Table S6). These data suggest that BtaR1 activates the CDI genes but raises the possibility that BtaR1 regulation is indirect.
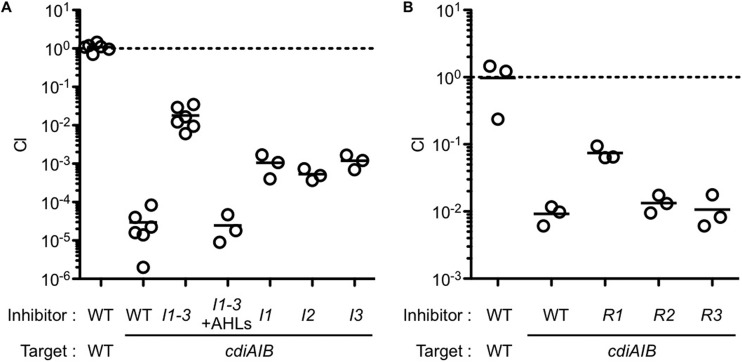
To test the hypothesis that QS activates CDI in B. thailandensis, we asked whether QS mutants were limited in their ability to inhibit growth of susceptible bacteria by using competition experiments. An AHL synthesis mutant was unable to confer wild-type levels of growth inhibition to the cdiAIB mutant (Fig. 3A). Additionally, wild-type levels of CDI were restored to the AHL synthesis mutant by exogenous addition of AHLs (Fig. 3A). Next, we asked which of the BtaI AHL synthases and which BtaR regulators contribute to CDI. The btaI1, btaI2, or btaI3 signal synthase mutants were unable to confer wild-type levels of CDI; however, they were not as CDI defective as the triple btaI123 mutant [Fig. 3A]). The btaR1 mutant was unable to confer wild-type levels of CDI. However, the btaR2 or btaR3 mutant showed near wild-type CDI levels (Fig. 3B). Thus, we believe that BtaR1 is the primary QS transcriptional regulator of CDI. However, there may be substantial complexity because the QS-2 and QS-3 AHLs activated the CDI genes, and each signal synthase appears to contribute to CDI activity. We note that the competition experiments serve as another type of RNA-seq validation result.
FIG 3.
QS controls contact-dependent growth inhibition. Unmarked inhibitor cells of either wild-type B. thailandensis E264 (WT), the btaI1-3 mutant (I1-3), or individual btaR (R1, R2, or R3) or btaI (I1, I2, or I3) mutants were cocultured with Tpr-marked target cells of either the WT (CM219) or a cdiAIB mutant (CM183) at a starting inhibitor-to-target ratio of 1,000:1 (A) or 1:1 (B). AHLs (C8-HSL, 3OHC8-HSL, and 3OHC10-HSL were added where indicated. The competitive index (CI) is the final ratio of target to inhibitor cells divided by the starting ratio of target to inhibitor cells. A CI of 1 (indicated by the broken line) shows equal fitness. Each symbol represents the value for a biological replicate, and the short black bars show the mean values.
QS activates CPS genes.
The B. thailandensis genome contains multiple gene clusters that code for the production of four polysaccharides that contribute to the capsule (capsular polysaccharide [CPS]) or to EPS production: CPS I, CPS II, CPS III, and CPS IV (26, 35). Our RNA-seq findings confirm a previous observation that B. thailandensis QS activates two clusters of genes (the bceI and bceII genes), which are similar to the Burkholderia cenocepacia cepacian biosynthetic genes (7, 24). In B. pseudomallei, bceI orthologs are required for the production of CPS III (35). In T phase, select bce genes were activated by BtaR1, the QS-2 AHL, and the QS-3 AHL (see Tables S1 and S5 in the supplemental material). In S phase, the bce genes showed continued activation by the QS-2 and QS-3 AHLs but also showed activation by the QS-1 AHL (Table S2). Additionally, genes in the CPS II gene cluster (BTH_II1972-BTH_II1994) were activated as much as 30-fold by AHL additions to the AHL synthesis mutant (Table S1). These genes showed strongest QS regulation when all AHLs were present and by BtaR1 (Tables S1 and S5). We also observed that three CPS I genes (BTH_I1330, BTH_I1331, and BTH_I1341) showed modest QS activation in T phase (Table S1).
DISCUSSION
We have generated a deep transcriptomics data set to begin to learn about the activities controlled by QS in B. thailandensis. The data set includes transcriptomes for an AHL synthesis mutant at three different points in growth with or without each of the three B. thailandensis AHLs added individually and at one point in growth with all three AHLs added together. We also generated transcriptome data for the wild type and for strains with single deletions of btaR1, btaR2, btaR3, btaR4, and btaR5. These data allow us to paint a general picture of genes controlled by QS, of which there are many. However, even with this large data set, we do not have a complete picture of the QS-controlled regulon. The data reveal that different genes are QS induced or repressed at different points during culture growth, that genes can be regulated by noncognate signals and receptors, and that some regulated genes likely respond to multiple signals and receptors. These features are not unexpected. Studies with P. aeruginosa have shown that many QS-controlled genes are coregulated by other factors (see reference 32 for an overview). Accumulation of AHL signals is necessary but not sufficient for activation of many genes (control is via AND logic gates). We also know that P. aeruginosa lasB transcription responds to both LasR and RhlR (36, 37). Our data show that based on transcriptomics alone, it is difficult to determine which genes are directly regulated by a specific QS transcription factor, which QS circuits might be controlled by others, and whether there is a hierarchy of QS circuits. Yet we can get a sense of the breadth of the QS-controlled regulon and although we cannot derive a comprehensive list of genes that are influenced by QS under any condition and at any stage of growth, we can obtain a picture of the types of activities influenced by QS. It is clear that B. thailandensis QS has a large global influence on gene expression.
Many bacteria contain multiple AHL QS systems, and numerous efforts have been directed toward understanding the independent and combined roles of each signaling circuit in a given species. For example, B. cenocepacia (38), P. aeruginosa (22, 32, 39), Pseudomonas chloroaphis (40), and several Yersinia species (41–43) each contain two or more complete QS systems, which act synergistically, independently, and even in opposition to regulate QS-controlled factors. By examining both the AHL signal- and AHL signal receptor-regulated transcripts in B. thailandensis, we learned that there is considerable overlap among the AHL-regulated factors and more divergence among the receptor-regulated genes. For example, there were 108 genes regulated by both the QS-2 or QS-3 signals (3OHC10-HSL and 3OHC8-HSL). However, there were no genes regulated by both BtaR2 and BtaR3. The overlapping regulation by the QS-2 and QS-3 AHLs can in part be explained by the fact that BtaR2 can respond to both 3OHC10-HSL and 3OHC8-HSL (8). We assume that BtaR2 and BtaR3 regulate unique gene sets but that particularly at high concentrations the QS-3 signal substitutes for the QS-2 signal and vice versa.
There was also overlap in the genes regulated by BtaR1 and BtaR3. Because our btaR1 null mutant showed reduced btaR3 expression compared to the wild type, we believe the overlap might result in part from a hierarchical QS network with BtaR1 controlling the BtaR3 system.
What types of B. thailandensis activities are regulated by QS? We found that many genes involved in production of secondary metabolites were QS controlled. Secondary metabolites are often excreted or secreted during stationary phase, and they are not catabolic end products. Microbial secondary metabolites can function as antibiotics, surfactants, siderophores, pigments, immunosuppressants, signaling molecules, and virulence factors. It is not uncommon for QS to control production of secondary metabolites. Examples include carbapenem production by Erwinia carotovora (44–46), phenazine production by Pseudomonas chloroaphis (47), violacein by Chromobacterium violaceum (48), bactobolin by B. thailandensis (8, 25), mupirocin by Pseudomonas fluorescens (49), and rhamnolipids, pyocyanin, hydrogen cyanide, and pyoverdin by P. aeruginosa (50–53). Control of secondary metabolite genes by QS in B. thailandensis is complex. Examples of B. thailandensis QS-controlled secondary metabolite genes include those coding for functions involved in the synthesis of malleobactin, malleilactone, terphenyl, thailandamide, quinolones, three compounds of unknown function, rhamnolipids, and bactobolin. Two gene clusters for metabolites of unknown function (designated unknown-1 and unknown-3) were strongly activated by multiple AHLs. They also showed regulation by BtaR1, but not BtaR2 or BtaR3. In contrast, bactobolin production was activated by BtaR2, but not BtaR1 or BtaR3.
The QS control of quinolone synthesis genes, malleilactone synthesis genes (malABCDEFGIJKLM), and unknown product-2 was particularly complex in that depending on conditions, these genes can either be induced or repressed by QS. For example, the addition of C8-HSL, 3OHC10-HSL, or 3OHC8-HSL individually to the AHL synthase mutant repressed many mal genes during both T and S phases. However, when all three AHLs were added together or when we compared the wild-type strain to the AHL− mutant, QS activated a subset of mal genes in T phase. Additionally, we observed that BtaR1 activated malM and BtaR3 repressed malB. Previous work showed that malleilactone had iron-binding activity and mild antibacterial activity against Gram-positive bacteria (54). It is of interest that the mal genes are adjacent to the orphan QS receptor gene, btaR4. There is some evidence that BtaR4 might activate the mal genes under certain conditions (54). Although little or no malleilactone was produced during laboratory growth, B. thailandensis malF and btaR4 mutants were attenuated for virulence of the worm Caenorhabditis elegans and the slime mold Dictyostelium discoideum (54). Presumably BtaR4 activates mal gene expression during infection (54). Our RNA-seq analysis of the btaR4 mutant is consistent with this idea. We did not find any BtaR4-dependent gene expression in laboratory-grown T-phase cells.
Our analysis revealed themes regarding roles of each QS system. QS-1 regulates factors that are likely involved in cell aggregation or biofilm formation. Specifically, BtaR1 or C8-HSL activates three separate gene clusters important for CPS or EPS production, the bceI and bceII genes for CPS III, and the genes for CPS II. The role of CPS III in B. pseudomallei is controversial; one publication reports that it is a virulence factor (55), and another indicates it is involved in the saprophytic lifestyle of this species and is not a virulence factor (35). The other QS-controlled CPS, CPS II, remains largely uncharacterized. Like CPS III, the genes for CPS II are present in B. thailandensis and B. pseudomallei, but not in B. mallei.
Additionally, we discovered that BtaR1 activates the CDI operon. CDI mediates intraspecies growth inhibition or killing and also promotes biofilm growth in B. thailandensis (33, 34, 56). Finally, QS-1 represses many motility genes. Repression of motility genes often correlates with biofilm formation. Thus, the coregulation genes for CPS or EPS, CDI, and motility by BtaR1 or C8-HSL suggest that QS-1 may promote an aggregate or biofilm lifestyle.
One additional B. thailandensis QS-1-controlled trait is oxalate production (23). QS-1-controlled oxalate production can spare B. thailandensis from catastrophic high-pH-induced stationary-phase cell death when growing on amino acids as the primary carbon source (23). The RNA-seq analysis showed that the oxalate biosynthetic gene, obc-1, is activated by QS. Furthermore, another gene important for oxalate production, qsmR, was controlled by QS. However, qsmR was repressed by QS in B. thailandensis (see Table S1 in the supplemental material) but is required for oxalate production in B. thailandensis (23). This may represent a situation where obc-1 activation leads to oxalate production, and this is followed by QsmR repression of oxalate synthesis such that an extracellular pH homeostasis is achieved.
What is the primary role of QS-2? The QS-2 and QS-3 signals are similar (3OHC10-HSL and 3OHC8-HSL), and there is overlap in the genes they regulate, possibly because BtaR2 and BtaR3 can respond to either signal. Therefore, to assess which genes are activated by QS-2, we can sort for those regulated by both 3OHC10-HSL in the signal synthase mutant and BtaR2 (wild type versus a btaR2 null mutant). This list encompasses 13 bactobolin synthesis genes. There were five additional bactobolin genes regulated by BtaR2 that did not appear in the list of genes regulated by 3OHC10-HSL in the AHL synthase mutant. Perhaps this is related to differences in timing of gene expression. This is consistent with previous investigations showing that some bactobolin genes are activated by QS-2 and that bactobolin production itself depends on QS-2 (8). The bactobolin genes are found in B. thailandensis and B pseudomallei, but not B. mallei (8). Our results suggest that the primary role of QS-2 is control of bactobolin synthesis. It appears that the bactobolin genes serve a role in saprophyte growth.
The QS-3 circuit strongly activates BTH_II1925, which codes for a predicted chitin-binding domain-containing protein (CBP). BMAA1785, the B. mallei ortholog of BTH_II1925 (>95% amino acid sequence identity) contributes to virulence in an insect infection model (57). This gene is also conserved in B. pseudomallei. Chitin-binding proteins, as well as chitinases, have been identified in the QS regulons of multiple species, including P. aeruginosa (18, 58), B. cenocepacia (38, 59), and C. violaceum (60). A gene annotated as kdpF is also strongly activated by BtaR3 (28-fold), and is adjacent to and presumably cotranscribed with kdpA (8-fold). These genes, along with three other genes (kdpB, kpdC, and kdpD), are predicted to code for a P-type ATPase high-affinity potassium ion transporter complex that functions in adaptation to osmotic stress in several bacteria (61). Interestingly, kdpB did not show BtaR3 activation, but it was activated by the addition of AHL to the AHL synthesis mutant.
Generally, AHL QS has been considered in the context of a transition between a free-living and host-associated lifestyle (5). B. thailandensis is considered to be a soil saprophyte. Our analysis indicates that genes coding for production of secondary metabolites are heavily represented in the QS regulon. A number of the secondary metabolites have antimicrobial properties and may provide groups of B. thailandensis a competitive advantage in multispecies soil habitats.
Of the B. thailandensis genes we identified as QS controlled, 40% are absent from the genome of the host-restricted pathogen B. mallei. The B. mallei genome has experienced massive reductive evolution, presumably losing genes for saprophytic survival and maintaining those for host colonization and persistence (62). Thus, we believe many of the 40% of B. thailandensis QS-controlled genes absent in the B. mallei genome are for adaptation to variations in saprophytic habitats. This includes many, but not all, of the genes for characterized and predicted antimicrobial factors. It also includes certain CPS and EPS genes. Might specific exopolysaccharides promote survival and group activities in the soil, while others play roles in host association?
This deep transcriptomics analysis of QS-controlled genes in B. thailandensis lays the groundwork for comparative transcriptome studies in B. pseudomallei and B. mallei. A global survey of QS-controlled factors in these pathogens has not yet been done despite the link between QS and virulence in both species (15, 63–66). Conserved elements may provide insights about the role of QS during infections and how QS can be adapted from a system providing benefit in a saprophyte to one providing benefit in a pathogen.
Supplementary Material
ACKNOWLEDGMENT
This research was supported by the Northwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases U54AI057141.
Footnotes
Published ahead of print 24 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01405-13.
REFERENCES
- 1.Fuqua C, Winans SC, Greenberg EP. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50:727–751. 10.1146/annurev.micro.50.1.727 [DOI] [PubMed] [Google Scholar]
- 2.Smith MD, Angus BJ, Wuthiekanun V, White NJ. 1997. Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect. Immun. 65:4319–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brett PJ, DeShazer D, Woods DE. 1998. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48(Part 1):317–320. 10.1099/00207713-48-1-317 [DOI] [PubMed] [Google Scholar]
- 4.Dance DA. 2000. Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human-animal hosts. Acta Trop. 74:159–168. 10.1016/S0001-706X(99)00066-2 [DOI] [PubMed] [Google Scholar]
- 5.Majerczyk C, Greenberg EP, Chandler JR. 2013. Quorum sensing in Burkholderia, p 40–57 In Vasil M, Darwin A. (ed), Regulation of bacterial virulence. ASM Press, Washington, DC [Google Scholar]
- 6.Ulrich RL, Hines HB, Parthasarathy N, Jeddeloh JA. 2004. Mutational analysis and biochemical characterization of the Burkholderia thailandensis DW503 quorum-sensing network. J. Bacteriol. 186:4350–4360. 10.1128/JB.186.13.4350-4360.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandler JR, Duerkop BA, Hinz A, West TE, Herman JP, Churchill MEA, Skerrett SJ, Greenberg EP. 2009. Mutational analysis of Burkholderia thailandensis quorum sensing and self-aggregation. J. Bacteriol. 191:5901–5909. 10.1128/JB.00591-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duerkop BA, Varga J, Chandler JR, Peterson SB, Herman JP, Churchill MEA, Parsek MR, Nierman WC, Greenberg EP. 2009. Quorum-sensing control of antibiotic synthesis in Burkholderia thailandensis. J. Bacteriol. 191:3909–3918. 10.1128/JB.00200-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulrich RL. 2004. Quorum quenching: enzymatic disruption of N-acylhomoserine lactone-mediated bacterial communication in Burkholderia thailandensis. Appl. Environ. Microbiol. 70:6173–6180. 10.1128/AEM.70.10.6173-6180.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haraga A, West TE, Brittnacher MJ, Skerrett SJ, Miller SI. 2008. Burkholderia thailandensis as a model system for the study of the virulence-associated type III secretion system of Burkholderia pseudomallei. Infect. Immun. 76:5402–5411. 10.1128/IAI.00626-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West TE, Frevert CW, Liggitt HD, Skerrett SJ. 2008. Inhalation of Burkholderia thailandensis results in lethal necrotizing pneumonia in mice: a surrogate model for pneumonic melioidosis. Trans. R. Soc. Trop. Med. Hyg. 102(Suppl 1):S119–S126. 10.1016/S0035-9203(08)70028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasselbring BM, Patel MK, Schell MA. 2011. Dictyostelium discoideum as a model system for identification of Burkholderia pseudomallei virulence factors. Infect. Immun. 79:2079–2088. 10.1128/IAI.01233-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilátová M, Dionne MS. 2012. Burkholderia thailandensis is virulent in Drosophila melanogaster. PLoS One 7:e49745. 10.1371/journal.pone.0049745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thongdee M, Gallagher LA, Schell M, Dharakul T, Songsivilai S, Manoil C. 2008. Targeted mutagenesis of Burkholderia thailandensis and Burkholderia pseudomallei through natural transformation of PCR fragments. Appl. Environ. Microbiol. 74:2985–2989. 10.1128/AEM.00030-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majerczyk C, Kinman L, Han T, Bunt R, Greenberg EP. 2013. Virulence of Burkholderia mallei quorum sensing mutants. Infect. Immun. 81:1471–1478. 10.1128/IAI.00048-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duerkop BA, Ulrich RL, Greenberg EP. 2007. Octanoyl-homoserine lactone is the cognate signal for Burkholderia mallei BmaR1-BmaI1 quorum sensing. J. Bacteriol. 189:5034–5040. 10.1128/JB.00317-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armour CD, Castle JC, Chen R, Babak T, Loerch P, Jackson S, Shah JK, Dey J, Rohl CA, Johnson JM, Raymond CK. 2009. Digital transcriptome profiling using selective hexamer priming for cDNA synthesis. Nat. Methods 6:647–649. 10.1038/nmeth.1360 [DOI] [PubMed] [Google Scholar]
- 18.Chugani S, Kim BS, Phattarasukol S, Brittnacher MJ, Choi SH, Harwood CS, Greenberg EP. 2012. Strain-dependent diversity in the Pseudomonas aeruginosa quorum-sensing regulon. Proc. Natl. Acad. Sci. U. S. A. 109:E2823–E2831. 10.1073/pnas.1214128109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Helden J. 2003. Regulatory sequence analysis tools. Nucleic Acids Res. 31:3593–3596. 10.1093/nar/gkg567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brittnacher MJ, Fong C, Hayden HS, Jacobs MA, Radey M, Rohmer L. 2011. PGAT: a multistrain analysis resource for microbial genomes. Bioinformatics 27:2429–2430. 10.1093/bioinformatics/btr418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 22.Schuster M, Lostroh CP, Ogi T, Greenberg EP. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066–2079. 10.1128/JB.185.7.2066-2079.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goo E, Majerczyk CD, An JH, Chandler JR, Seo Y-S, Ham H, Lim JY, Kim H, Lee B, Jang MS, Greenberg EP, Hwang I. 2012. Bacterial quorum sensing, cooperativity, and anticipation of stationary-phase stress. Proc. Natl. Acad. Sci. U. S. A. 109:19775–19780. 10.1073/pnas.1218092109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira AS, Leitão JH, Silva IN, Pinheiro PF, Sousa SA, Ramos CG, Moreira LM. 2010. Distribution of cepacian biosynthesis genes among environmental and clinical Burkholderia strains and role of cepacian exopolysaccharide in resistance to stress conditions. Appl. Environ. Microbiol. 76:441–450. 10.1128/AEM.01828-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seyedsayamdost MR, Chandler JR, Blodgett JAV, Lima PS, Duerkop BA, Oinuma K-I, Greenberg EP, Clardy J. 2010. Quorum-sensing-regulated bactobolin production by Burkholderia thailandensis E264. Org. Lett. 12:716–719. 10.1021/ol902751x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y, Kim HS, Chua HH, Lin CH, Sim SH, Lin D, Derr A, Engels R, Deshazer D, Birren B, Nierman WC, Tan P. 2006. Genomic patterns of pathogen evolution revealed by comparison of Burkholderia pseudomallei, the causative agent of melioidosis, to avirulent Burkholderia thailandensis. BMC Microbiol. 6:46. 10.1186/1471-2180-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper VS, Vohr SH, Wrocklage SC, Hatcher PJ. 2010. Why genes evolve faster on secondary chromosomes in bacteria. PLoS Comput. Biol. 6:e1000732. 10.1371/journal.pcbi.1000732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holden MTG, Titball RW, Peacock SJ, Cerdeño-Tárraga AM, Atkins T, Crossman LC, Pitt T, Churcher C, Mungall K, Bentley SD, Sebaihia M, Thomson NR, Bason N, Beacham IR, Brooks K, Brown KA, Brown NF, Challis GL, Cherevach I, Chillingworth T, Cronin A, Crossett B, Davis P, Deshazer D, Feltwell T, Fraser A, Hance Z, Hauser H, Holroyd S, Jagels K, Keith KE, Maddison M, Moule S, Price C, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Simmonds M, Songsivilai S, Stevens K, Tumapa S, Vesaratchavest M, Whitehead S, Yeats C, Barrell BG, Oyston PCF, Parkhill J. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. U. S. A. 101:14240–14245. 10.1073/pnas.0403302101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuanyok A, Leadem BR, Auerbach RK, Beckstrom-Sternberg SM, Beckstrom-Sternberg JS, Mayo M, Wuthiekanun V, Brettin TS, Nierman WC, Peacock SJ, Currie BJ, Wagner DM, Keim P. 2008. Genomic islands from five strains of Burkholderia pseudomallei. BMC Genomics 9:566. 10.1186/1471-2164-9-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronning CM, Losada L, Brinkac L, Inman J, Ulrich RL, Schell M, Nierman WC, Deshazer D. 2010. Genetic and phenotypic diversity in Burkholderia: contributions by prophage and phage-like elements. BMC Microbiol. 10:202. 10.1186/1471-2180-10-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woods DE, Jeddeloh JA, Fritz DL, Deshazer D. 2002. Burkholderia thailandensis E125 harbors a temperate bacteriophage specific for Burkholderia mallei. J. Bacteriol. 184:4003–4017. 10.1128/JB.184.14.4003-4017.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuster M, Greenberg EP. 2006. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 296:73–81. 10.1016/j.ijmm.2006.01.036 [DOI] [PubMed] [Google Scholar]
- 33.Nikolakakis K, Amber S, Wilbur JS, Diner EJ, Aoki SK, Poole SJ, Tuanyok A, Keim PS, Peacock S, Hayes CS, Low DA. 2012. The toxin/immunity network of Burkholderia pseudomallei contact-dependent growth inhibition (CDI) systems. Mol. Microbiol. 84:516–529. 10.1111/j.1365-2958.2012.08039.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson MS, Garcia EC, Cotter PA. 2012. The Burkholderia bcpAIOB genes define unique classes of two-partner secretion and contact dependent growth inhibition systems. PLoS Genet. 8:e1002877. 10.1371/journal.pgen.1002877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reckseidler-Zenteno SL, Viteri D-F, Moore R, Wong E, Tuanyok A, Woods DE. 2010. Characterization of the type III capsular polysaccharide produced by Burkholderia pseudomallei. J. Med. Microbiol. 59:1403–1414. 10.1099/jmm.0.022202-0 [DOI] [PubMed] [Google Scholar]
- 36.Toder DS, Gambello MJ, Iglewski BH. 1991. Pseudomonas aeruginosa LasA: a second elastase under the transcriptional control of lasR. Mol. Microbiol. 5:2003–2010. 10.1111/j.1365-2958.1991.tb00822.x [DOI] [PubMed] [Google Scholar]
- 37.Pearson JP, Pesci EC, Iglewski BH. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756–5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Grady EP, Viteri DF, Malott RJ, Sokol PA. 2009. Reciprocal regulation by the CepIR and CciIR quorum sensing systems in Burkholderia cenocepacia. BMC Genomics 10:441. 10.1186/1471-2164-10-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pesci EC, Iglewski BH. 1997. The chain of command in Pseudomonas quorum sensing. Trends Microbiol. 5:132–135. 10.1016/S0966-842X(97)01008-1 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Pierson LS. 2001. A second quorum-sensing system regulates cell surface properties but not phenazine antibiotic production in Pseudomonas aureofaciens. Appl. Environ. Microbiol. 67:4305–4315. 10.1128/AEM.67.9.4305-4315.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkinson S, Throup JP, Stewart GS, Williams P. 1999. A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol. Microbiol. 33:1267–1277 [DOI] [PubMed] [Google Scholar]
- 42.Kirwan JP, Gould TA, Schweizer HP, Bearden SW, Murphy RC, Churchill MEA. 2006. Quorum-sensing signal synthesis by the Yersinia pestis acyl-homoserine lactone synthase YspI. J. Bacteriol. 188:784–788. 10.1128/JB.188.2.784-788.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atkinson S, Chang C-Y, Patrick HL, Buckley CMF, Wang Y, Sockett RE, Cámara M, Williams P. 2008. Functional interplay between the Yersinia pseudotuberculosis YpsRI and YtbRI quorum sensing systems modulates swimming motility by controlling expression of flhDC and fliA. Mol. Microbiol. 69:137–151. 10.1111/j.1365-2958.2008.06268.x [DOI] [PubMed] [Google Scholar]
- 44.Bainton NJ, Stead P, Chhabra SR, Bycroft BW, Salmond GP, Stewart GS, Williams P. 1992. N-(3-oxohexanoyl)-L-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem. J. 288(Part 3):997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGowan S, Sebaihia M, Jones S, Yu B, Bainton N, Chan PF, Bycroft B, Stewart GS, Williams P, Salmond GP. 1995. Carbapenem antibiotic production in Erwinia carotovora is regulated by CarR, a homologue of the LuxR transcriptional activator. Microbiology (Reading, Engl.) 141(Part 3):541–550. 10.1099/13500872-141-3-541 [DOI] [PubMed] [Google Scholar]
- 46.McGowan SJ, Barnard AML, Bosgelmez G, Sebaihia M, Simpson NJL, Thomson NR, Todd DE, Welch M, Whitehead NA, Salmond GPC. 2005. Carbapenem antibiotic biosynthesis in Erwinia carotovora is regulated by physiological and genetic factors modulating the quorum sensing-dependent control pathway. Mol. Microbiol. 55:526–545 [DOI] [PubMed] [Google Scholar]
- 47.Pierson LS, Keppenne VD, Wood DW. 1994. Phenazine antibiotic biosynthesis in Pseudomonas aureofaciens 30-84 is regulated by PhzR in response to cell density. J. Bacteriol. 176:3966–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology (Reading, Engl.) 143:3703–3711. 10.1099/00221287-143-12-3703 [DOI] [PubMed] [Google Scholar]
- 49.El-Sayed AK, Hothersall J, Thomas CM. 2001. Quorum-sensing-dependent regulation of biosynthesis of the polyketide antibiotic mupirocin in Pseudomonas fluorescens NCIMB 10586. Microbiology (Reading, Engl.) 147:2127–2139 [DOI] [PubMed] [Google Scholar]
- 50.Ochsner UA, Reiser J. 1995. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 92:6424–6428. 10.1073/pnas.92.14.6424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochsner UA, Koch AK, Fiechter A, Reiser J. 1994. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J. Bacteriol. 176:2044–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latifi A, Winson MK, Foglino M, Bycroft BW, Stewart GS, Lazdunski A, Williams P. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333–343. 10.1111/j.1365-2958.1995.mmi_17020333.x [DOI] [PubMed] [Google Scholar]
- 53.Stintzi A, Evans K, Meyer JM, Poole K. 1998. Quorum-sensing and siderophore biosynthesis in Pseudomonas aeruginosa: lasR/lasI mutants exhibit reduced pyoverdine biosynthesis. FEMS Microbiol. Lett. 166:341–345. 10.1111/j.1574-6968.1998.tb13910.x [DOI] [PubMed] [Google Scholar]
- 54.Biggins JB, Ternei MA, Brady SF. 2012. Malleilactone, a polyketide synthase-derived virulence factor encoded by the cryptic secondary metabolome of Burkholderia pseudomallei group pathogens. J. Am. Chem. Soc. 134:13192–13195. 10.1021/ja3052156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarkar-Tyson M, Thwaite JE, Harding SV, Smither SJ, Oyston PCF, Atkins TP, Titball RW. 2007. Polysaccharides and virulence of Burkholderia pseudomallei. J. Med. Microbiol. 56:1005–1010. 10.1099/jmm.0.47043-0 [DOI] [PubMed] [Google Scholar]
- 56.Garcia EC, Anderson MS, Hagar JA, Cotter PA. 2013. Burkholderia BcpA mediates biofilm formation independently of interbacterial contact-dependent growth inhibition. Mol. Microbiol. 89:1213–1225. 10.1111/mmi.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schell MA, Lipscomb L, Deshazer D. 2008. Comparative genomics and an insect model rapidly identify novel virulence genes of Burkholderia mallei. J. Bacteriol. 190:2306–2313. 10.1128/JB.01735-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Folders J, Algra J, Roelofs MS, van Loon LC, Tommassen J, Bitter W. 2001. Characterization of Pseudomonas aeruginosa chitinase, a gradually secreted protein. J. Bacteriol. 183:7044–7052. 10.1128/JB.183.24.7044-7052.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huber B, Riedel K, Hentzer M, Heydorn A, Gotschlich A, Givskov M, Molin S, Eberl L. 2001. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology (Reading, Engl.) 147:2517–2528 [DOI] [PubMed] [Google Scholar]
- 60.Chernin LS, Winson MK, Thompson JM, Haran S, Bycroft BW, Chet I, Williams P, Stewart GS. 1998. Chitinolytic activity in Chromobacterium violaceum: substrate analysis and regulation by quorum sensing. J. Bacteriol. 180:4435–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bramkamp M, Altendorf K, Greie J-C. 2007. Common patterns and unique features of P-type ATPases: a comparative view on the KdpFABC complex from Escherichia coli. Mol. Membr. Biol. 24:375–386. 10.1080/09687680701418931 [DOI] [PubMed] [Google Scholar]
- 62.Nierman WC, Deshazer D, Kim HS, Tettelin H, Nelson KE, Feldblyum T, Ulrich RL, Ronning CM, Brinkac LM, Daugherty SC, Davidsen TD, Deboy RT, Dimitrov G, Dodson RJ, Durkin AS, Gwinn ML, Haft DH, Khouri H, Kolonay JF, Madupu R, Mohammoud Y, Nelson WC, Radune D, Romero CM, Sarria S, Selengut J, Shamblin C, Sullivan SA, White O, Yu Y, Zafar N, Zhou L, Fraser CM. 2004. Structural flexibility in the Burkholderia mallei genome. Proc. Natl. Acad. Sci. U. S. A. 101:14246–14251. 10.1073/pnas.0403306101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valade E, Thibault FM, Gauthier YP, Palencia M, Popoff MY, Vidal DR. 2004. The PmlI-PmlR quorum-sensing system in Burkholderia pseudomallei plays a key role in virulence and modulates production of the MprA protease. J. Bacteriol. 186:2288–2294. 10.1128/JB.186.8.2288-2294.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ulrich RL, Deshazer D, Brueggemann EE, Hines HB, Oyston PC, Jeddeloh JA. 2004. Role of quorum sensing in the pathogenicity of Burkholderia pseudomallei. J. Med. Microbiol. 53:1053–1064. 10.1099/jmm.0.45661-0 [DOI] [PubMed] [Google Scholar]
- 65.Song Y, Xie C, Ong Y-M, Gan Y-H, Chua KL. 2005. The BpsIR quorum-sensing system of Burkholderia pseudomallei. J. Bacteriol. 187:785–790. 10.1128/JB.187.2.785-790.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ulrich RL, Deshazer D, Hines HB, Jeddeloh JA. 2004. Quorum sensing: a transcriptional regulatory system involved in the pathogenicity of Burkholderia mallei. Infect. Immun. 72:6589–6596. 10.1128/IAI.72.11.6589-6596.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carver T, Thomson N, Bleasby A, Berriman M, Parkhill J. 2009. DNAPlotter: circular and linear interactive genome visualization. Bioinformatics 25:119–120. 10.1093/bioinformatics/btn578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi K-H, Gaynor JB, White KG, Lopez C, Bosio CM, Karkhoff-Schweizer RR, Schweizer HP. 2005. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2:443–448. 10.1038/nmeth765 [DOI] [PubMed] [Google Scholar]
- 69.Choi K-H, Schweizer HP. 2006. Mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat. Protoc. 1:153–161. 10.1038/nprot.2006.24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.