Abstract
O antigen (O polysaccharide) is an important and highly variable cell component present on the surface of cells which defines the serospecificity of Gram-negative bacteria. Most O antigens of Shigella flexneri, a cause of shigellosis, share a backbone composed of →2)-α-l-RhapIII-(1→2)-α-l-RhapII-(1→3)-α-l-RhapI-(1→3)-β-d-GlcpNAc-(1→ repeats, which can be modified by adding various substituents, giving rise to 19 serotypes. The known modifications include glucosylation on various sugar residues, O-acetylation on RhaI, and phosphorylation with phosphoethanolamine on RhaII or/and RhaIII. Recently, two new O-antigen modifications, namely, O-acetylation at position 3 or 4 of RhaIII and position 6 of GlcNAc, have been identified in several S. flexneri serotypes. In this work, the genetic basis for the 3/4-O-acetylation on RhaIII was elucidated. Bioinformatic analysis of the genome of S. flexneri serotype 2a strain Sf301, which carries 3/4-O-acetylation on RhaIII, revealed an O-acyltransferase gene designated oacB. Genetic studies combined with O-antigen structure analysis demonstrated that this gene is responsible for the 3/4-O-acetylation in serotypes 1a, 1b, 2a, 5a, and Y but not serotype 6, which has a different O-antigen backbone structure. The oacB gene is carried by a transposon-like structure located in the proA-adrA region on the chromosome, which represents a novel mechanism of mobilization of O-antigen modification factors in S. flexneri. These findings enhance our knowledge of S. flexneri O-antigen modifications and shed light on the origin of new O-antigen variants.
INTRODUCTION
Shigella flexneri is the major cause of shigellosis in developing countries. It is estimated that there are 125 million shigellosis cases annually in Asia, with 14,000 deaths, the majority of which are children under 5 years of age (1). The O-polysaccharide chain of the lipopolysaccharide, called O antigen, is an important and highly variable cell component present on the surface, and it provides the chemical basis for serotyping of S. flexneri. Except for serotype 6, all S. flexneri serotypes share a polysaccharide backbone composed of the tetrasaccharide repeats (O units) of three rhamnose (RhaI to RhaIII) and one 2-acetamido-2-deoxy-d-glucose (GlcNAc) [→2)-α-l-RhapIII-(1→2)-α-l-RhapII-(1→3)-α-l-RhapI-(1→3)-β-d-GlcpNAc-(1→] (2). The backbone can be modified by adding various chemical groups to different sugars, giving rise to diverse O-antigen structures and, correspondingly, to various serotypes (3).
The first two O-antigen modification types identified in S. flexneri were O-acetylation at position 2 of RhaI (2-O-acetylation) and glucosylation at different positions on each of the four sugar residues in the O-unit (3). 2-O-acetylation occurs in serotypes 1b, 3a, 3b, 4b, and 7b and confers on the host a group 6 antigenic determinant (O-factor 6) (4, 5). A single O-acetyltransferase-encoding gene (oac) carried by the temperate bacteriophage Sf6 mediates the 2-O-acetylation (6, 7). Glucosylation defines type I, IC, II, IV, and V antigenic determinants as well as the group 7,8 antigenic determinant in various serotypes (3, 8). Factors responsible for glucosylation are arranged in a single operon known as the gtr gene cluster, with 3 genes, including gtrA, gtrB (both conserved), and gtr (type specific), carried by bacteriophages SfI, SfIC, SfII, SfIV, SfV, and SfX (7–14). The Sf6 and SfIC genomes are integrated into the host chromosome at the tRNA-argW site adjacent to a conserved gene, yfdC, and at the site adjacent to the yejO locus, respectively (8, 15). The other serotype-converting phages map to tRNA-thrW, located between genes proA and adrA (3, 16).
A third known type of modification is the addition of phosphoethanolamine (PEtN) to position 3 of either RhaIII or RhaII or both (17–20). The PEtN group confers the host with monoclonal antibody MASF IV-1 reactivity and defines the variant (v) factor in newly named serotypes Xv, 4av, and Yv (17–20). A single gene, opt (O-antigen phosphoethanolamine transferase gene), which is carried on a 6.8-kb plasmid (pSFxv_2 or pSFyv_2), mediates the PEtN modification (17, 18, 20).
Recently, two new O-antigen modifications have been identified: (i) O-acetylation at either position 3 or position 4 of RhaIII (3/4-O-acetylation) in serotypes 1a, 1b, 2a, 5a, Y, and 6 and (ii) O-acetylation at position 6 of GlcNAc (6-O-acetylation) in serotypes 2a, 3a, Y, and Yv (2, 21–23). The degree of the 3/4-O-acetylation varies not only between serotypes but also between strains of the same serotype in the range of 30 to 70% at position 3 and 15 to 30% at position 4 (2, 24). The latter variation likely is due to storage and/or cultivation conditions. Further studies indicated that the 3/4-O-acetylation on RhaIII interfered with the PEtN phosphorylation at the same sugar residue and reduced the MASF IV-1 determinant manifestation (24). Until now, the genetic basis for the 3/4-O-acetylation on RhaIII and 6-O-acetylation on GlcNAc remained unknown.
O-antigen modifications are considered an important virulence factor. The host immune response has been shown to be serotype specific (3). Thus, antigenic diversity enhances the bacterium's survival, as it helps the bacterium escape the host defense (3). Furthermore, some modifications, such as glucosylation on GlcNAc, RhaI, and RhaII, have been demonstrated to promote bacterial invasion into host cells mediated by the type III secretion system (25). Therefore, elucidation of the 3/4-O-acetylation mechanism is important for understanding the S. flexneri antigenicity and pathogenicity. In this study, we identified a homolog of the oac gene for O-acyltransferase, designated oacB, and demonstrated that this gene is responsible for the 3/4-O-acetylation in S. flexneri serotypes 1a, 1b, 2a, 5a, and Y but not serotype 6. oacB was carried by a transposon-like structure located upstream of the adrA gene on the chromosome.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culturing conditions.
Strains and plasmids used in this study are listed in Table 1. S. flexneri strain Sf301 (serotype 2a) with 3/4-O-acetylated RhaIII in the O antigen (Fig. 1) was used as the reference strain for oacB gene amplification and deletion analysis. S. flexneri strains 51251 (serotype 2b) and 51580 (serotype X), lacking O-acetylation on RhaIII (Fig. 1), were employed as hosts for the oacB gene transformation analysis. Thirty-one S. flexneri strains with known O-antigen structures (see Table S1 in the supplemental material) were used for oacB gene PCR detection analysis. They were either clinical isolates from our collection, purchased from the National Collection of Type Cultures (NCTC), or kindly donated by B. Liu (Nankai University, Tianjin, China). Escherichia coli JM109 was used for plasmid propagation. pMD20T vector (TaKaRa, Japan) was used for DNA sequencing and oacB gene function analysis. Plasmid pRS551 was used for kanamycin resistance gene amplification. pKOBEG, encoding a homologous recombination system, was used in oacB gene deletion analysis. Strains were grown in a 37°C incubator or orbital shaker in Luria-Bertani (LB) broth supplemented with ampicillin (100 μg ml−1), kanamycin (40 μg ml−1), or chloramphenicol (50 μg ml−1) when appropriate.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristic(s) | Reference or source |
|---|---|---|
| S. flexneri strains | ||
| Sf301 | Serotype 2a reference strain used for oacB gene cloning and inactivation analysis, Aps Kms | NC_004337 |
| 301ΔoacB | Sf301 with the oacB gene replaced by the kanamycin resistance gene (kan) from pSR551, Kmr Aps | This work |
| 301ΔoacB_pSQZ4 | 301ΔoacB transformed by plasmid pSQZ4 | This work |
| 51521 | Serotype 2b strain used as host for the oacB gene function analysis, Aps | 24 |
| 51521_pSQZ4 | 51251 transformed by plasmid pSQZ4 | This work |
| 51580 | Serotype X strain used as host for the oacB gene function analysis, Aps | 24 |
| 51580_pSQZ4 | 51580 transformed by plasmid pSQZ4 | This work |
| E. coli DH5α | Used for plasmid propagation and gene cloning | TaKaRa |
| Plasmids | ||
| pMD20T | TA vector, Apr | TaKaRa |
| pSR551 | Kmr, used for kan gene cloning | 31 |
| pKOBEG | Thermosensitive replicon that carries the λ phage redγβα operon expressed under the control of the arabinose-inducible pBAD promoter | 32 |
| pSQZ4 | pMD20T carrying the whole sequence of the oacB gene from strain Sf301, Apr | This work |
FIG 1.

Structures of the O polysaccharides of S. flexneri strains studied. In addition to the major structure shown, a negligible amount (<5%) of glucosylated structure characteristic of serotype X was found in transformant 51580_pSQZ4, indicating incomplete blocking of glucosylation by O-acetylation on RhaIII.
Bioinformatics analysis.
The protein sequence of the O-acetyltransferase Oac (accession no. NP_958191.1) of bacteriophage Sf6 was compared to the genome of S. flexneri strain Sf301 using tBLASTn and BLASTP (http://www.ncbi.nlm.nih.gov/BLAST). Searches for homologs to the gene oacB in the NCBI database were carried out using the BLASTP search engine.
DNA techniques.
Chromosomal DNA was isolated from S. flexneri strains using a DNA extraction kit according to the manufacturer's instructions (Qiagen, Germany). Primers used in this study are listed in Table 2. Primer pair oacB-1 was used for oacB gene detection. Primer pair oacB-2 was used for oacB gene function analysis. Primer pair oac-adrA was used to amplify regions downstream of oacB in S. flexneri strains 019 (serotype 1a), G1662 (1b), G1036 (5a), 51581, and G1040 (both Y). Primers spr1, spr2, and spr3, which are complementary to the reverse sequence of oacB, were used in genomic walking PCR to analyze the regions upstream of oacB. Oligonucleotide primers were synthesized by Sangon Biotech (Shanghai). PCR amplifications were performed using a TaKaRa PCR amplification kit (TaKaRa, Japan) by following a standard protocol. PCR products using primer pair oacB-2 were purified and cloned into the pMD20T vector (TaKaRa, Japan) to generate the oacB expression plasmid pSQZ4. The recombinant plasmid was first transformed into commercial E. coli DH5α competent cells (TaKaRa, Japan) and then into S. flexneri strains 51251 and 51580 using a standard chemical protocol (26). The transformants were selected on LB plates supplemented with ampicillin (100 μg ml−1) and confirmed by PCR amplification of the oacB gene.
TABLE 2.
Primers used in this study
| Primer | Primer sequence (5′–3′) | Target gene(s) |
|---|---|---|
| oacB-1 | ||
| Forward | TCATCTGGAGTATGGGAAG | oacB |
| Reverse | CAAAGAATCAGTGGTAGCG | |
| oacB-2 | ||
| Forward | GGTGTGTCTCCGTTTTGTTTC | oacB |
| Reverse | CGACGTTGCTACTGGTGTTTC | |
| kan | ||
| Forward | GCAGGAATAATCAAATAGATGGAATGCGGGGGTTCTTAGCAATTTTCGTGCTTATTCATCACGCACGTTGTGTCTCAAAATCT | kan and oacB |
| Reverse | CACGTCATTAGGCAATAAAGGAATATCCCATGCAGAAGGTAAACGCTGTAGGTAGTTTCTCCTAGCTTTCTGCGTCCCGTCAAGTCAGCGTA | |
| oacB-adrA | ||
| Forward | ACCAGAAAGCTAGGAGAAACTAC | oacB, adrA |
| Reverse | GCAATCGGTAAGAACATGCCAG | |
| spr | ||
| spr1 | GCTAACAGATTTGATGAAGGTGCTTCCC | oacB |
| spr2 | GCTAAGAACCCCCGCATTCCATC | |
| spr3 | CCCCACAGCTAATAACGACATC |
To determine whether an oacB homolog is present in serotype 6 strains, Southern hybridization was carried out. Genomic DNA extracted from serotype 6 strains was digested with the restriction enzyme NotI and subjected to agarose gel electrophoresis. The separated DNA was transferred onto a nylon membrane (Amersham Biosciences, Sweden) using a vacuum blotter (Bio-Rad, Hercules, CA). Hybridization was performed using an ECLTM direct nucleic acid labeling and detection system (Amersham) as recommended by the manufacturer. DNA product amplified from strain Sf301 using primer pair oacB-1 was prepared for a biotin-labeling DNA probe. The hybridization temperature was 42°C under 50% formamide.
oacB gene functional deletion and complementation analysis.
Deletion of the oacB gene was performed on S. flexneri strain Sf301 using a one-step method as described previously (27). The Kmr gene was PCR amplified from plasmid pRS551 using the kan primer pair (Table 2). The PCR products were electroporated into strain Sf301 carrying plasmid pKOBEG and selected on an LB plate with chloramphenicol (50 μg ml−1) and kanamycin (40 μg ml−1). The oacB gene deletion mutant 301ΔoacB was confirmed by PCR amplification of oacB using primer pairs oacB-1 and oacB-2 (Fig. 2). Plasmid pSQZ4 was transformed into mutant strain 301ΔoacB, giving rise to oacB-complemented strain 301ΔoacB_pSQZ4.
FIG 2.
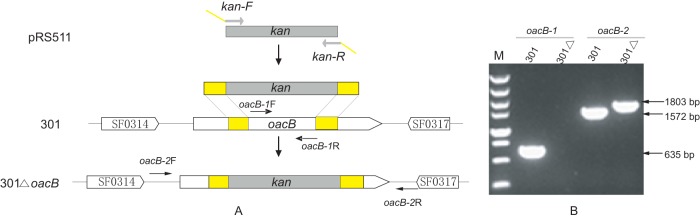
Schematic presentation of the oacB gene deletion inactivation using a one-step method and detection by PCR amplification. (A) Structural presentation of the oacB gene deletion by a one-step method. Open reading frames are shown as thick arrows, and primers used for detection are shown as thin arrows. The kanamycin resistance gene kan (gray) is amplified from plasmid pRS551 and flanked by DNA sequences complementary to the oacB gene (yellow). (B) PCR analysis of deletion mutants using primer pairs oacB-1 and oacB-2.
Serotyping.
Serological features of S. flexneri wild-type strains and transformants were determined by slide agglutination test using a commercially available monovalent antiserum kit (Denka Seiken, Japan) specific for all known type and group O factors of S. flexneri O antigens.
O-polysaccharide isolation and structure analysis.
Lipopolysaccharides of wild-type strains and transformants were isolated by the phenol-water extraction of dried bacterial cells (28). The crude extract without separation of the layers was dialyzed against tap water, nucleic acids and proteins were precipitated by adding aqueous 50% CCl3CO2H at 4°C to pH 2, the supernatant was dialyzed against distilled water, and the supernatant was freeze-dried. The purified lipopolysaccharide preparations were degraded with aqueous 2% acetic acid at 100°C, and the O polysaccharides were isolated in yields of 27 to 44% by gel permeation chromatography on Sephadex G-50 Superfine (Amersham Biosciences, Sweden) in 0.05 M pyridinium acetate buffer, pH 4.5, monitored with a differential refractometer (Knauer, Germany).
Structures of the O polysaccharides were elucidated using two-dimensional nuclear magnetic resonance (NMR) spectroscopy essentially as described previously (20). Assignment of the 1H and 13C NMR spectra (see Table S2 in the supplemental material) was performed using correlation spectroscopy (COSY), total correlation spectroscopy (TOCSY), and 1H,13C heteronuclear single-quantum coherence (HSQC) experiments. Positions of O-acetyl groups were determined by characteristic low-field displacements of the NMR signals for 1H and 13C atoms at the O-acetylation sites and by a comparison to the corresponding O-deacetylated polysaccharides studied earlier (2, 22). The degree of O-acetylation was determined by relative integral intensities of the 1H NMR signals of the O-acetylated and non-O-acetylated RhaIII and GlcNAc residues. The O-polysaccharide structure of the parental strain Sf301 (2a) was essentially identical to that of another serotype 2a strain, G1663, studied earlier (22).
RESULTS
Identification of an O-acyltransferase gene that is present only in S. flexneri strains with 3/4-O-acetylation on RhaIII.
2-O-Acetylation of RhaI in the O antigens of S. flexneri serotypes 1b, 3a, 3b, 4b, and 7b is mediated by Oac, an acyltransferase family protein encoded by the oac gene of bacteriophage Sf6 (6, 7). We hypothesized that the 3/4-O-acetylation of RhaIII in serotypes 1a, 1b, 2a, 5a, Y, and 6 is mediated by an Oac homolog. BLAST search using the Oac protein sequence of Sf6 against the genome of S. flexneri serotype 2a strain Sf301 that carries the 3/4-O-acetylation (Fig. 1) retrieved a hypothetical protein encoded by gene SF0315, which showed the highest homology to Oac with 26% identity, 51% coverage, and an E value of 3e−06 at the protein level. The protein contained 390 amino acids and possessed conserved domains of the acyltransferase family (COG1835 or acyl_trans_3).
BLAST search indicated that this hypothetical acetyltransferase is also present in completely or partially sequenced S. flexneri strains 2457T (WP_000570107), 4343-70 (WP_005094470), 2930-71 (WP_000570109), and 2747-71 (WP_005090992) with 99 to 100% identity at the protein or DNA level. It showed 72 to 74% identity to predicted acyltransferases of S. flexneri strains CDC 796-83 (WP_005054336) and CCH060 (WP_005114037) and 25 to 39% identity to predicted acyltransferases of Pseudomonas sp. strain S9 (WP_010486952.1), Pseudomonas sp. strain GM55 (WP_008016406.1), Dechloromonas aromatica (YP_285087.1), Flavobacterium columnare (YP_004943102.1), and some other species. We name the SF0315 protein 3/4-O-acyltransferase, or OacB, and the corresponding gene oacB, following the designation convention for oac of Sf6, which we suggest renaming as oacA. The function of oacB was confirmed as described below.
PCR screening of 31 strains with known O-antigen structures (see Table S1 in the supplemental material), using primer pair oacB-1 (Table 2), revealed a complete correlation between the 3/4-O-acetylation and the presence of the oacB gene. Except for three serotype 6 strains, the expected PCR product (653 bp) was amplified from all strains carrying 3/4-O-acetylation on RhaIII, including two strains each of serotypes 1a, 1b, 2a, 5a, and Y (Table 3). In contrast, strains lacking 3/4-O-acetylation did not carry the oacB gene (Table 3). The whole oacB gene sequence in the 11 oacB-positive strains was PCR amplified and sequenced using primer pair oacB-2. The oacB gene sequences in all of these strains were found to be identical to that of strain Sf301.
TABLE 3.
Correlation between the 3/4-O-acetylation on RhaIII and the presence of the oacB gene in S. flexneri strains
| S. flexneri strain | Serotype | 3/4-O-acetylation on RhaIII | oacB gene PCR result | O-antigen structure referencea |
|---|---|---|---|---|
| 51571 | 1a | + | + | 24 |
| G1661 | 1a | + | + | 22 |
| 51572 | 1b | + | + | 24 |
| G1662 | 1b | + | + | 22 |
| X6 | 1c | − | − | 24 |
| HN153 | 1d | − | − | 33 |
| 51250 | 2a | + | + | 24 |
| G1663 | 2a | + | + | 22 |
| 51251 | 2b | − | − | This work |
| 51575 | 3a | − | − | 24 |
| G1665 | 3a | − | − | 2 |
| G1666 | 3b | − | − | 2 |
| NCTC9725 | 4a | − | − | 17 |
| G1668 | 4av | − | − | 19 |
| 51577 | 4b | − | − | 24 |
| G1669 | 4b | − | − | 2 |
| 51247 | 5a | + | + | 24 |
| G1036 | 5a | + | + | 21 |
| 51580 | X | − | − | 17 |
| G1039 | X | − | − | 2 |
| 2002017 | Xv | − | − | 17 |
| 2003055 | Xv | − | − | 17 |
| 51581 | Y | + | + | 24 |
| G1040 | Y | + | + | 2 |
| 036 | Y | − | − | 20 |
| HN006 | Yv | − | − | 20 |
| AH012 | Yv | − | − | 20 |
| HN011 | Yv | − | − | 20 |
| 51579 | 6 | + | − | 24 |
| G1038 | 6 | + | − | 2 |
| G1671 | 6 | + | − | 2 |
The O-antigen structures are shown in Table S1 in the supplemental material.
Southern hybridization on the three serotype 6 strains 51579, G1038, and G1671 was performed using the oacB gene PCR product (653 bp) as the probe and showed no positive hybridization signal, indicating the absence of the oacB gene from these strains (data not shown).
Functional oacB gene mediates the 3/4-O-acetylation of RhaIII in S. flexneri O antigens.
The entire oacB gene of 1,173 bp, together with 399-bp sequences upstream and downstream to cover its promoter and terminator sequences, was cloned from S. flexneri strain Sf301 (serotype 2a) into expression plasmid pMD20T to construct plasmid pSQZ4, which was then transformed into S. flexneri serotype 2b strain 51251 and serotype X strain 51580. Serological analysis of the 51521_pSQZ4 and 51580_pSQZ4 transformants using commercial Shigella antisera (Denka Seiken, Japan) showed that, compared to the parental strains, both transformants lost reactivity with group 7,8 antiserum and gained reactivity with group 3,4 antiserum (Table 4). Therefore, according to the current serotyping scheme, the 51521_pSQZ4 and 51580_pSQZ4 transformants converted from serotypes 2b and X into serotypes 2a and Y, respectively (Table 4), indicating that oacB affected another modification, namely, glucosylation on RhaIII that defines group O-factor 7,8.
TABLE 4.
Serological features of S. flexneri strains studied
| Strain (serotype)a | Reactivity with type and group antisera |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | 3,4 | 6 | 7,8 | |
| 51251 (2b) | − | + | − | − | − | − | − | − | + |
| 51521_pSQZ4 (2a) | − | + | − | − | − | − | + | − | − |
| 51580 (X) | − | − | − | − | − | − | − | − | + |
| 51580_pSQZ4 (Y) | − | − | − | − | − | − | + | − | − |
| 301 (2a) | − | + | − | − | − | − | + | − | − |
| 301ΔoacB (2a) | − | + | − | − | − | − | + | − | − |
| 301ΔoacB_pSQZ4 (2a) | − | + | − | − | − | − | + | − | − |
Serotypes were determined using antisera of Denka Seiken, Japan, according to the current serotyping scheme of S. flexneri.
The O polysaccharides of the transformants were isolated and analyzed using NMR spectroscopy as described in Materials and Methods (for assigned 1H and 13C NMR chemical shifts, see Table S2 in the supplemental material). Compared to the parental strains 51251 and 51580, both transformants acquired an O-acetyl group at either position 3 or 4 of RhaIII and lost the original glucosylation at position 3 of the same sugar (Fig. 1). The degree of O-acetylation at positions 3 and 4 was ∼60% and 20% in 51521_pSQZ4 and ∼65% and 25% in 51580_pSQZ4, respectively. Like the parental serotype 2b and X strains, neither transformant contained the 6-O-acetyl group on the GlcNAc residue.
The function of the oacB gene was confirmed by deletion and complementation assay performed on strain Sf301 using the one-step method. A stretch of 740 bp of the oacB gene from bp 172 to 911 was replaced by the kanamycin resistance gene (Fig. 2). The deletion mutant was selected on a chloramphenicol and kanamycin resistance plate and confirmed by PCR amplification of the oacB gene using primer pairs oacB-1 and oacB-2. A PCR product of 1,803 bp was amplified from 301ΔoacB using primer pair oacB-2, which was the expected size of the product containing the kanamycin resistance gene (Fig. 2). No serological difference was observed between deletion mutant 301ΔoacB and parent strain Sf301 using Shigella antisera from Denka Seiken (Table 4).
Analysis of the O-polysaccharide structure by NMR spectroscopy (see Table S2 in the supplemental material) showed that the deletion mutant lost the 3/4-O-acetylation on RhaIII compared to its parental strain, and this modification was restored by transformation of the mutant with the oacB-carrying plasmid pSQZ4 (Fig. 1). The NMR data also indicated that the oacB gene deletion did not affect the 6-O-acetylation on GlcNAc in 301ΔoacB. Accordingly, the oacB gene could not be PCR amplified from strains 51575 and G1665 (both serotype 3a) or HN006, AH012, and HN011 (all serotype Yv), which carried 6-O-acetylation on GlcNAc but lacked 3/4-O-acetylation on RhaIII (see Table S1 in the supplemental material).
oacB gene is carried by a transposon-like structure located upstream of the adrA gene on the chromosome.
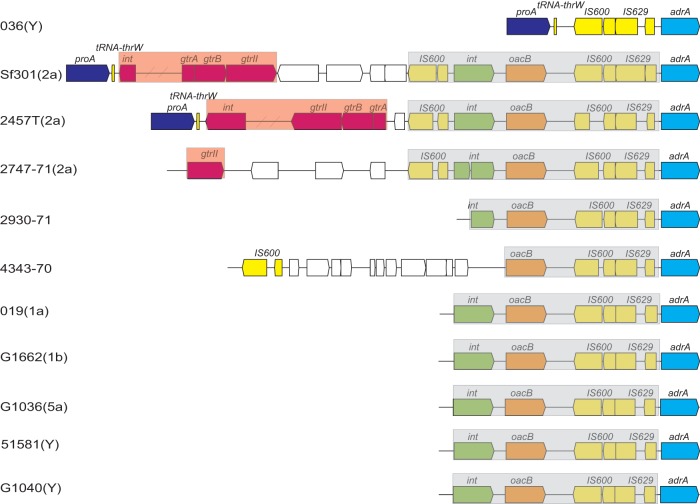
Analysis of the regions flanking the oacB gene in sequenced strains Sf301, 2457T, and 2747-71 (all 2a) as well as 4343-70 and 2930-71 (both of unknown serotype) showed that in all 5 strains, the oacB gene was located downstream of an integrase-encoding gene (int). Furthermore, in all strains studied except for strain 2930-71, whose DNA sequence upstream of int had not been sequenced, the int-oacB locus was flanked by insertion sequences (IS), with IS600 upstream and IS600 and IS629 downstream, giving rise to a transposon-like structure (Fig. 3). In serotype 2a strains Sf301, 2457T, and 2747-71, this structure was located immediately downstream of the SfII prophage genome sequence and was followed by the conserved adrA gene (Fig. 3). In strains 4343-70 and 2930-71 of unknown serotype, the oacB-carrying transposon was also found upstream of the adrA gene (Fig. 3). This observation was confirmed by PCR amplification and sequencing of the DNA regions upstream and downstream of the oacB gene in strains 019 (1a), G1662 (1b), G1036 (5a), 51581, and G1040 (both Y), which revealed the same structure in all five strains (Fig. 3).
FIG 3.
Genetic organizations of the genomic regions carrying the oacB gene in serotype 1a, 1b, 2a, 5a, and Y strains. Sequences of Sf301, 2457T, 2747-71, 2930-71, and 4343-70 were obtained from the NCBI database. The serotypes of 2930-71 and 4343-70 are unknown. Genomic sequences of 019 (1a), G1662 (1b), G1036 (5a), and 51581 and G1040 (both Y) were obtained by PCR amplification. The open reading frames are annotated as sequences in NCBI or predicted using ORF Finder and are shown as thick arrows. The conserved genes are shown in different colors: proA, dark blue; adrA, blue; IS600 and IS629, yellow; oacB, orange; integrase gene (int), green; prophage SfII genome genes, red. The serotype-converting prophage and oacB-carrying transposon are highlighted in red and gray, respectively.
DISCUSSION
The present data revealed a clear correlation between the presence of the oacB gene and 3/4-O-acetylation on RhaIII and demonstrated the functional oacB gene to be responsible for this O-antigen modification in S. flexneri serotypes 1a, 1b, 2a, 5a, and Y. This conclusion was supported by the following pieces of evidence: (i) the OacB protein encoded by the oacB gene showed significant similarity to acyltransferase family proteins; (ii) the cloned oacB gene mediated the 3/4-O-acetylation of RhaIII upon transformation; and (iii) deletion of the functional oacB gene resulted in the loss of 3/4-O-acetyltion. The fact that one gene mediates modification at both positions 3 and 4 of RhaIII suggests that OacB is a bifunctional enzyme with respect to the substrate O-acetylation site. Such bifunctional catalysis is also found on the PEtN transferase Opt, which mediates the addition of a PEtN residue(s) to position 3 of either RhaIII, RhaII, or both (17–20, 24).
Although serotype 6 strains also possess 3/4-O-acetylation on RhaIII (2) (see Table S1 in the supplemental material), they lack oacB. The serotype 6 O antigen has a linear tetrasaccharide repeat containing one residue each of N-acetylgalactosamine and galacturonic acid and two rhamnose residues (RhaII and RhaIII) (29), and the structural difference from the O antigens of the other S. flexneri serotypes (1 to 5, 7, X, and Y) might require another mechanism of 3/4-O-acetylation on RhaIII. Indeed, in serotype 6 strains, the 3/4-O-acetylation was found to be mediated by another acyltransferase encoded by a gene named oacC, which presents 57.1% similarity to oacB and maps in a phage-like region on the chromosome as well (unpublished data). It is also evident that yet another unidentified acyltransferase gene is responsible for the 6-O-acetylation on GlcNAc in serotypes 2a, 3a, Y, and Yv.
Two known mechanisms of mobilization of S. flexneri O-antigen modification factors involve temperate bacteriophages (for 2-O-acetylation of RhaI and glucosylation) or a plasmid (for PEtN phosphorylation). In this study, the oacB gene was found to be carried by a transposon-like structure, suggesting that it is transferred by a transposon mechanism among strains in nature. The serotype-converting bacteriophages for O-antigen glucosylation (SfI, SfII, SfIV, SfV, and SfX) are integrated into the host chromosome at the tRNA-thrW site located between genes proA and adrA (3, 8–14, 16). The transposon-like locus responsible for the 3/4-O-acetylation of RhaIII also maps upstream of the adrA gene. Therefore, one can speculate that the proA-adrA region is a conserved insertion site for both oacB-carrying transposons and serotype-converting bacteriophages in all serotypes involved.
The oacB-mediated addition of the O-acetyl group to positions 3 and 4 of RhaIII prevents this sugar from being glucosylated and results in serotype conversion of the host strains, as observed in the conversion of serotypes 2b and X into 2a and Y, respectively. Glucosylation at position 3 of RhaIII defines the group 7,8 antigenicity, and the loss of this modification upon transformation with an oacB-carrying plasmid pSQZ4 abolishes the reactivity with group 7,8 antiserum (Table 4). It is noteworthy that the 7,8 antiserum reactivity of transformants 51580_pSQZ4 and 51251_pSQZ4 could be recovered by curing the pSQZ4 plasmid (data not shown), suggesting that the potential glucosylation activity of the gtrX locus retained in the transformants and the loss of glucosylation upon transformation occurred by a competitive occupation with O-acetyl groups.
Group O-factor 3,4 is linked to the linear →3)-α-l-RhapI-(1→3)-β-d-GlcpNAc-(1→2)-α-l-RhapIII-(1→ trisaccharide fragment of the O-antigen backbone (30). This epitope is masked by glucosylation on RhaIII, whereas glucosylation on GlcNAc and 2-O-acetylation on RhaI abolish the 3,4 reactivity only when they occur simultaneously (30). Our data (Table 4) and published data (2) indicate that, in contrast to glucosylation, 3/4-O-acetylation on RhaIII does not affect O-factor 3,4. Finally, the 3/4-O-acetylation also interferes with PEtN modification of RhaIII mediated by the plasmid-borne opt gene, and PEtN phosphorylation occurs at RhaII only. As a result, the level of the reactivity with monoclonal antibody MASF IV-1 specific to PEtN-linked epitopes decreases significantly (24).
In conclusion, the genetic basis of the 3/4-O-acetylation of RhaIII was elucidated in the O antigens of S. flexneri serotypes 1a, 1b, 2a, 5a, and Y. A new O-acyltransferase responsible for this modification, OacB, is encoded by the oacB gene, which is carried by a transposon-like structure inserted into the proA-adrA region on the chromosome. The 3/4-O-acetylation of RhaIII interferes with glucosylation and PEtN phosphorylation at the same sugar residue. These findings enhance our understanding of the mechanisms of the O-antigen variation and enable further studies to understand the contribution of the 3/4-O-acetylation to the antigenicity and pathogenicity of S. flexneri.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (no. 81271788); the National Basic Research Priorities Program (2011CB504901); the Project of State Key Laboratory for Infectious Disease Prevention and Control (2011SKLID203); the National Key Program for Infectious Diseases of China (2013ZX10004221, 2013ZX10004216-001-002, 2012ZX10004215); and the Russian Foundation for Basic Research (12-04-00172).
Footnotes
Published ahead of print 7 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01393-13.
REFERENCES
- 1.Bardhan P, Faruque AS, Naheed A, Sack DA. 2010. Decrease in shigellosis-related deaths without Shigella spp.-specific interventions, Asia. Emerg. Infect. Dis. 16:1718–1723. 10.3201/eid1611.090934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perepelov AV, Shekht ME, Liu B, Shevelev SD, Ledov VA, Senchenkova SN, L'Vov VL, Shashkov AS, Feng L, Aparin PG, Wang L, Knirel YA. 2012. Shigella flexneri O-antigens revisited: final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity. FEMS Immunol. Med. Microbiol. 66:201–210. 10.1111/j.1574-695X.2012.01000.x [DOI] [PubMed] [Google Scholar]
- 3.Allison GE, Verma NK. 2000. Serotype-converting bacteriophages and O-antigen modification in Shigella flexneri. Trends Microbiol. 8:17–23. 10.1016/S0966-842X(99)01646-7 [DOI] [PubMed] [Google Scholar]
- 4.Foster RA, Carlin NI, Majcher M, Tabor H, Ng LK, Widmalm G. 2011. Structural elucidation of the O-antigen of the Shigella flexneri provisional serotype 88-893: structural and serological similarities with S. flexneri provisional serotype Y394 (1c). Carbohydr. Res. 346:872–876. 10.1016/j.carres.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 5.Kenne L, Lindberg B, Petersson K, Katzenellenbogen E, Romanowska E. 1978. Structural studies of Shigella flexneri O-antigens. Eur. J. Biochem. 91:279–284. 10.1111/j.1432-1033.1978.tb20963.x [DOI] [PubMed] [Google Scholar]
- 6.Verma NK, Brandt JM, Verma DJ, Lindberg AA. 1991. Molecular characterization of the O-acetyl transferase gene of converting bacteriophage SF6 that adds group antigen 6 to Shigella flexneri. Mol. Microbiol. 5:71–75. 10.1111/j.1365-2958.1991.tb01827.x [DOI] [PubMed] [Google Scholar]
- 7.Clark CA, Beltrame J, Manning PA. 1991. The oac gene encoding a lipopolysaccharide O-antigen acetylase maps adjacent to the integrase-encoding gene on the genome of Shigella flexneri bacteriophage Sf6. Gene 107:43–52. 10.1016/0378-1119(91)90295-M [DOI] [PubMed] [Google Scholar]
- 8.Stagg RM, Tang SS, Carlin NI, Talukder KA, Cam PD, Verma NK. 2009. A novel glucosyltransferase involved in O-antigen modification of Shigella flexneri serotype 1c. J. Bacteriol. 191:6612–6617. 10.1128/JB.00628-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Q, Lan R, Wang Y, Wang J, Li P, Du P, Xu J. 2013. Isolation and genomic characterization of SfI, a serotype-converting bacteriophage of Shigella flexneri. BMC Microbiol. 13:39. 10.1186/1471-2180-13-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mavris M, Manning PA, Morona R. 1997. Mechanism of bacteriophage SfII-mediated serotype conversion in Shigella flexneri. Mol. Microbiol. 26:939–950. 10.1046/j.1365-2958.1997.6301997.x [DOI] [PubMed] [Google Scholar]
- 11.Guan S, Bastin DA, Verma NK. 1999. Functional analysis of the O antigen glucosylation gene cluster of Shigella flexneri bacteriophage SfX. Microbiology 145:1263–1273. 10.1099/13500872-145-5-1263 [DOI] [PubMed] [Google Scholar]
- 12.Allison GE, Angeles D, Tran-Dinh N, Verma NK. 2002. Complete genomic sequence of SfV, a serotype-converting temperate bacteriophage of Shigella flexneri. J. Bacteriol. 184:1974–1987. 10.1128/JB.184.7.1974-1987.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adhikari P, Allison G, Whittle B, Verma NK. 1999. Serotype 1a O-antigen modification: molecular characterization of the genes involved and their novel organization in the Shigella flexneri chromosome. J. Bacteriol. 181:4711–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams MM, Allison GE, Verma NK. 2001. Type IV O antigen modification genes in the genome of Shigella flexneri NCTC 8296. Microbiology 147:851–860 http://mic.sgmjournals.org/content/147/4/851.long [DOI] [PubMed] [Google Scholar]
- 15.Casjens S, Winn-Stapley DA, Gilcrease EB, Morona R, Kuhlewein C, Chua JE, Manning PA, Inwood W, Clark AJ. 2004. The chromosome of Shigella flexneri bacteriophage Sf6: complete nucleotide sequence, genetic mosaicism, and DNA packaging. J. Mol. Biol. 339:379–394. 10.1016/j.jmb.2004.03.068 [DOI] [PubMed] [Google Scholar]
- 16.Sun Q, Lan R, Wang Y, Wang J, Luo X, Zhang S, Li P, Ye C, Jing H, Xu J. 2011. Genesis of a novel Shigella flexneri serotype by sequential infection of serotype-converting bacteriophages SfX and SfI. BMC Microbiol. 11:269–274. 10.1186/1471-2180-11-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Q, Knirel YA, Lan R, Wang J, Senchenkova SN, Jin D, Shashkov AS, Xia S, Perepelov AV, Chen Q, Wang Y, Wang H, Xu J. 2012. A novel plasmid-encoded serotype conversion mechanism through addition of phosphoethanolamine to the O-antigen of Shigella flexneri. PLoS One 7(9):e46095. 10.1371/journal.pone.0046095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Q, Lan R, Wang J, Xia S, Wang Y, Jin D, Yu B, Knirel YA, Xu J. 2013. Identification and characterization of a novel Shigella flexneri serotype Yv in China. PLoS One 8:e70238. 10.1371/journal.pone.0070238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perepelov AV, L'vov VL, Liu B, Senchenkova SN, Shekht ME, Shashkov AS, Feng L, Aparin PG, Wang L, Knirel YA. 2009. A new ethanolamine phosphate-containing variant of the O-antigen of Shigella flexneri type 4a. Carbohydr. Res. 344:1588–1591. 10.1016/j.carres.2009.03.022 [DOI] [PubMed] [Google Scholar]
- 20.Knirel YA, Lan R, Senchenkova SN, Wang J, Shashkov AS, Wang Y, Perepelov AV, Xiong Y, Xu J, Sun Q. 2013. O-antigen structure of Shigella flexneri serotype Yv and effect of the lpt-O gene variation on phosphoethanolamine modification of S. flexneri O-antigens. Glycobiology 23:475–485. 10.1093/glycob/cws222 [DOI] [PubMed] [Google Scholar]
- 21.Perepelov AV, Shevelev SD, Liu B, Senchenkova SN, Shashkov AS, Feng L, Knirel YA, Wang L. 2010. Structures of the O-antigens of Escherichia coli O13, O129, and O135 related to the O-antigens of Shigella flexneri. Carbohydr. Res. 345:1594–1599. 10.1016/j.carres.2010.04.023 [DOI] [PubMed] [Google Scholar]
- 22.Perepelov AV, L'Vov VL, Liu B, Senchenkova SN, Shekht ME, Shashkov AS, Feng L, Aparin PG, Wang L, Knirel YA. 2009. A similarity in the O-acetylation pattern of the O-antigens of Shigella flexneri types 1a, 1b, and 2a. Carbohydr. Res. 344:687–692. 10.1016/j.carres.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 23.Kubler-Kielb J, Vinogradov E, Chu C, Schneerson R. 2007. O-acetylation in the O-specific polysaccharide isolated from Shigella flexneri serotype 2a. Carbohydr. Res. 342:643–647. 10.1016/j.carres.2006.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Q, Knirel YA, Lan R, Wang J, Senchenkova SN, Shashkov AS, Wang Y, Luo X, Xu J. 2014. Dissemination and serotype modification potential of pSFxv_2, an O-antigen PEtN modification plasmid in Shigella flexneri. Glycobiology 24:305–313. 10.1093/glycob/cwt115 [DOI] [PubMed] [Google Scholar]
- 25.West NP, Sansonetti P, Mounier J, Exley RM, Parsot C, Guadagnini S, Prevost MC, Prochnicka-Chalufour A, Delepierre M, Tanguy M, Tang CM. 2005. Optimization of virulence functions through glucosylation of Shigella LPS. Science 307:1313–1317. 10.1126/science.1108472 [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 27.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westphal O, Jann K. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 5:83–90 [Google Scholar]
- 29.Dmitriev BA, Knirel YA, Sheremet OK, Shashkov AS, Kochetkov NK, Hofman IL. 1979. Somatic antigens of Shigella. The structure of the specific polysaccharide of Shigella newcastle (Sh. flexneri type 6) lipopolysaccharide. Eur. J. Biochem. 98:309–316 [DOI] [PubMed] [Google Scholar]
- 30.Carlin NI, Lindberg AA. 1987. Monoclonal antibodies specific for Shigella flexneri lipopolysaccharides: clones binding to type IV, V, and VI antigens, group 3,4 antigen, and an epitope common to all Shigella flexneri and Shigella dysenteriae type 1 stains. Infect. Immun. 55:1412–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simons RW, Houman F, Kleckner N. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85–96. 10.1016/0378-1119(87)90095-3 [DOI] [PubMed] [Google Scholar]
- 32.Pradel N, Ye C, Livrelli V, Xu J, Joly B, Wu LF. 2003. Contribution of the twin arginine translocation system to the virulence of enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 71:4908–4916. 10.1128/IAI.71.9.4908-4916.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shashkov AS, Senchenkova SN, Sun Q, Lan R, Wang J, Perepelov AV, Knirel YA, Xu J. 2013. Structure of the O-antigen of a novel Shigella flexneri serotype, 1d (I: 7,8). Carbohydr. Res. 373:93–96. 10.1016/j.carres.2013.03.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.