Abstract
Vibrio cholerae is the causative agent of the diarrheal disease cholera. The ability of V. cholerae to colonize and cause disease requires the intricately regulated expression of a number of virulence factors during infection. One of the signals sensed by V. cholerae is the presence of oxygen-limiting conditions in the gut. It has been shown that the virulence activator AphB plays a key role in sensing low oxygen concentrations and inducing the transcription of another key virulence activator, TcpP. In this study, we used a bacterial two-hybrid system to further examine the effect of oxygen on different virulence regulators. We found that anoxic conditions enhanced the interaction between TcpP and ToxR, identified as the first positive regulator of V. cholerae virulence genes. We further demonstrated that the TcpP-ToxR interaction was dependent on the primary periplasmic protein disulfide formation enzyme DsbA and cysteine residues in the periplasmic domains of both ToxR and TcpP. Furthermore, we showed that in V. cholerae, an interaction between TcpP and ToxR is important for virulence gene induction. Under anaerobic growth conditions, we detected ToxR-TcpP heterodimers, which were abolished in the presence of the reducing agent dithiothreitol. Our results suggest that V. cholerae may sense intestinal anoxic signals by multiple components to activate virulence.
INTRODUCTION
The Gram-negative bacterium Vibrio cholerae is responsible for the diarrheal disease cholera. It is a facultative pathogen that resides predominantly in a variety of aqueous environments (1), and human infection normally starts with the ingestion of food or water contaminated with V. cholerae. Subsequently, the bacteria enter the small intestine, where they must then penetrate the mucus lining to colonize the epithelial cells beneath and cause disease (2). As it colonizes, V. cholerae produces an array of virulence factors, including cholera toxin (CT) and toxin-coregulated pili (TCP) (3, 4). These virulence factors are transcriptionally regulated by multiple systems (5). The primary, direct transcriptional activator of the V. cholerae genes encoding CT and TCP is ToxT (6). Transcription of toxT is regulated, in turn, by the ToxR and TcpP proteins (7). The expression of tcpP is activated by two additional activators, AphA and AphB (8, 9), whereas the expression of toxR is thought to be constitutive and can be modulated by AphB (10). Both TcpP and ToxR are inner membrane proteins with N-terminal cytoplasmic domains homologous to winged helix-turn-helix transcriptional activators and C-terminal periplasmic domains (11). ToxR and TcpP can interact with each other (12, 13), but the exact mechanism by which ToxR facilitates TcpP-mediated activation of the toxT promoter is unclear. It has been hypothesized that ToxR binds to three helical turns upstream of TcpP in the toxT promoter (14) and recruits TcpP proteins to activate toxT transcription (13).
The environmental cues influencing the expression of virulence genes in vivo are not well characterized. It has been reported that certain environmental conditions such as temperature, osmolarity, pH, and iron availability influence the expression of virulence genes in vitro (15). For example, quorum-sensing systems negatively regulate virulence genes by repressing aphA expression through HapR (16–18). Fatty acids in bile and bicarbonate regulate ToxT activity (19, 20). We recently reported that a set of bile salts induce virulence genes by enhancing TcpP protein activity, possibly by promoting TcpP-TcpP intermolecular disulfide bond formation (21). Moreover, we found that under oxygen-limiting conditions in the gastrointestinal tract (22), V. cholerae virulence genes are highly expressed. We showed that oxygen-limiting conditions enhance the dimerization and activity of AphB, which leads to the production of virulence factors (23). To further examine whether, in addition to AphB, the activity of other virulence regulators may be affected by the oxygen concentration, we used a bacterial two-hybrid system to examine protein-protein interactions between subunits of virulence regulators. We found that while interactions between TcpP and TcpP, ToxR and ToxR, and ToxT and ToxT were insensitive to the environmental oxygen concentration, the interaction between ToxR and TcpP was increased under oxygen-limiting conditions. We further demonstrated that the periplasmic domains of both TcpP and ToxR are important for this interaction and cysteine residues in the periplasmic domains of both proteins were critical for the TcpP-ToxR interaction. We show that the anaerobiosis-enhanced TcpP-ToxR interaction contributes to V. cholerae virulence gene expression.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
For the strains, plasmids, and primers used in this study, see Tables S1 to S3 in the supplemental material. All of the V. cholerae strains used in this study were derived from E1 Tor C6706 (24) and propagated in Luria broth (LB) containing appropriate antibiotics at 37°C. Cultures were grown aerobically (with shaking at 250 rpm), microaerophilically (stationary growth), or anaerobically (Vinyl Anaerobic Chambers; Coy Laboratory Products) unless otherwise noted. Escherichia coli strains DH5α and DH5α λpir were used for cloning. E. coli XL1-Blue was used for the bacterial adenylate cyclase two-hybrid (BACTH) system (25) and recombinant fusion construct propagation. E. coli BTH101 (ΔcyaA) (25) was used for bacterial hybrid analysis. Full-length toxR or tcpP, truncated fragments thereof, and cysteine mutant derivatives were PCR amplified and cloned into the inducible expression vectors pUT18C and pKT25 (25), respectively. The arabinose-inducible PBAD-tcpPH plasmids were described in reference 21. The isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Plac-toxRS plasmid was constructed by PCR amplifying full-length toxRS genes and cloning them into the pSRKtc vector (26). The PctxA-luxCDABE transcriptional reporter plasmid was constructed by cloning the promoter of ctxA sequences into the pBBR-lux plasmid (27). The tcpA-sh ble construct was described previously (28). Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; streptomycin, 100 μg/ml; kanamycin, 50 μg/ml. For E. coli, we used the following: chloramphenicol, 30 μg/ml; tetracycline, 10 μg/ml. For V. cholerae, we used the following: chloramphenicol, 2 μg/ml; tetracycline, 2 μg/ml, streptomycin, 100 μg/ml.
Bacterial two-hybrid system for analysis of the ToxR-TcpP interaction.
Overnight cultures of E. coli BTH101 or BTH101ΔdsbA containing plasmid pKT25-ToxR (and its derivatives) and pUT-18C-TcpP (and its derivatives) were subcultured in LB containing 0.5 mM PTG and grown aerobically (with shaking at 250 rpm), microaerophilically (stationary growth), or anaerobically (anaerobic incubator) at 37°C until the optical density at 600 nm (OD600) reached approximately 0.3. β-Galactosidase activities were then measured as described previously (29). The toxR constructs contain full-length toxS in the same operon with toxR, and the tcpP constructs contain full-length tcpH in the same operon with tcpP.
Measurement of toxT transcription in V. cholerae.
The PtoxT-lacZ plasmid reporter was constructed by cloning toxT promoter DNA into LacZ transcriptional reporter plasmid pAH6 (30). Overnight cultures of the V. cholerae ΔtcpPH ΔtoxRS mutant containing PtoxT-lacZ reporters and various PBAD-tcpPH and Ptac-toxRS plasmids were diluted 1:50 (for microaerophilic growth) or 1:2,000 (for aerobic growth) into fresh AKI medium (1.5% Bacto peptone, 0.4% yeast extract, 0.5% sodium chloride, pH 7.5) supplemented with the appropriate antibiotics, 0.01% arabinose, and 1 μM IPTG and incubated at 37°C (stationary or shaking) until the OD600 reached 0.3. β-Galactosidase activities were then assayed as described previously (29).
Western blot assays and SDS-PAGE.
Overnight cultures of wild-type or ΔtoxRS ΔtcpPH mutant V. cholerae containing plasmids PBAD-tcpPH and Plac-toxRS were diluted into fresh AKI medium supplemented with appropriate antibiotics and the various concentrations of arabinose and IPTG indicated. The cultures were incubated at 37°C (stationary or shaking) until the OD600 reached 0.3. The cultures were then pelleted and resuspended in phosphate-buffered saline. Cell pellets normalized by the total protein amounts were then resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sampling buffer with or without dithiothreitol (DTT) and loaded at the same volumes for each sample onto a 15% polyacrylamide gel. Proteins were transferred to Immobilon-P SQ polyvinylidene difluoride transfer membrane for Western blot analysis with polyclonal rabbit anti-ToxR or anti-TcpP antibodies (10, 21), followed by enhanced-chemiluminescence anti-rabbit IgG secondary antibody (GE Healthcare). Proteins were detected by use of Thermo Scientific SuperSignal West Pico Chemiluminescent Substrate.
RESULTS AND DISCUSSION
Effect of oxygen concentration on protein-protein interactions of V. cholerae virulence regulators.
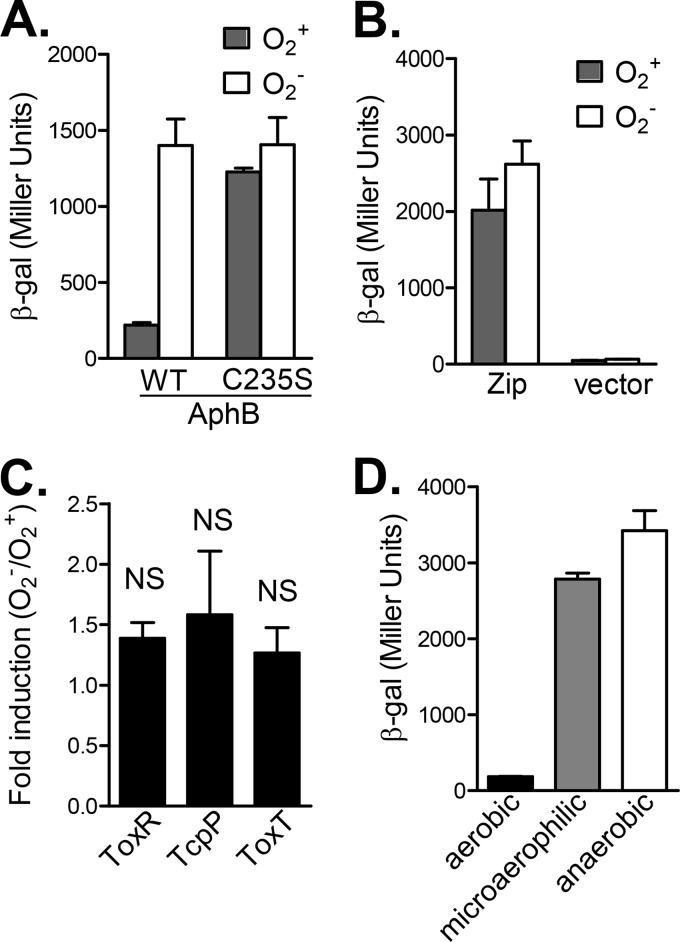
Protein-protein interactions have been shown to play critical roles in gene regulation in all living organisms. There are a number of genetic approaches to the analysis of protein-protein interactions. Among them, a BACTH system is a proven, simple, and fast approach to the characterization of these interactions in E. coli cells (25). This system has been used to examine V. cholerae TcpP-ToxR (12) and TcpP-TcpP interactions in response to bile salt signals (21). To test whether this method is suitable for elucidation of the effects of oxygen on protein-protein interactions, we first constructed fusions of T25 and T18, both of which are complementary fragments of the catalytic domain of adenylate cyclase (CyaA) from Bordetella pertussis (25), with wild-type AphB and with a cysteine 235-to-serine AphB mutant that is insensitive to oxygen (23). We then measured the β-galactosidase activity in an E. coli Δcya mutant strain grown aerobically or anaerobically. The AphB-AphB interaction brings the two Cya fragments together and leads to cyclic AMP (cAMP) generation and increased β-galactosidase production. Figure 1A shows that wild-type AphB proteins interacted significantly better under anaerobic growth than under aerobic growth, whereas the strength of interaction between AphBC235S mutant proteins was similar, regardless of the oxygen conditions during growth. These results are consistent with our previous findings (23) that oxygen-limiting conditions enhance AphB dimerization and AphBC235S mutant proteins form dimers even during aerobic growth. In addition, oxygen availability did not affect β-galactosidase production in the strains containing either leucine zipper of GCN4 T18/25 (25) fusions (positive controls) or empty vectors (negative controls) (Fig. 1B). These data suggest that the BACTH system is suitable for assaying the effects of growth conditions on protein-protein interactions. We then used this system to examine whether interactions of other V. cholerae virulence regulators such as ToxR, TcpP, and ToxT are affected by the oxygen concentration. We found that ToxR proteins could interact with each other to activate β-galactosidase production, but this induction was not oxygen concentration dependent (Fig. 1C). Similarly, TcpP-TcpP and ToxT-ToxT interactions were also O2 independent (Fig. 1C, second and third columns). Of note, we always included tcpH with tcpP and toxS with toxR on the T18/T25 fusion plasmids to avoid TcpP and ToxR protein instability (31, 32). Finally, we examined TcpP-ToxR interaction when bacteria were grown aerobically, microaerophilically, or anaerobically. Figure 1D shows that under oxygen-limiting conditions during either microaerophilic or anaerobic growth, the TcpP-ToxR interaction was greatly enhanced, suggesting that, in addition to modulating AphB dimerization, anaerobiosis also influences other important components of the virulence regulatory cascade.
FIG 1.
Effects of oxygen on protein interactions between V. cholerae virulence regulators in a bacterial two-hybrid system. The E. coli cAMP-based two-hybrid system was used in this study (25). See Materials and Methods for details. The BTH101 strains containing different combinations of T25-T18 fusion pairs were grown aerobically, microaerophilically, and anaerobically at 37°C until mid-log phase, and β-galactosidase activity was measured and recorded in Miller units (29). (A) Effects of oxygen on AphB-AphB and AphBC235S-AphBC235S interactions. (B) Effects of oxygen on positive controls (leucine zipper of GCN4) and negative controls (empty vectors). (C) ToxR-ToxR, TcpP-TcpP, and ToxT-ToxT interactions. The ratios of β-galactosidase units from cultures grown anaerobically and aerobically are presented. (D) ToxR-TcpP interactions. The results are the mean of three experiments ± the standard deviation. β-gal, β-galactosidase; WT, wild type; NS, not significantly different.
Periplasmic domains of ToxR and TcpP are critical for the anaerobiosis-dependent TcpP-ToxR interaction.
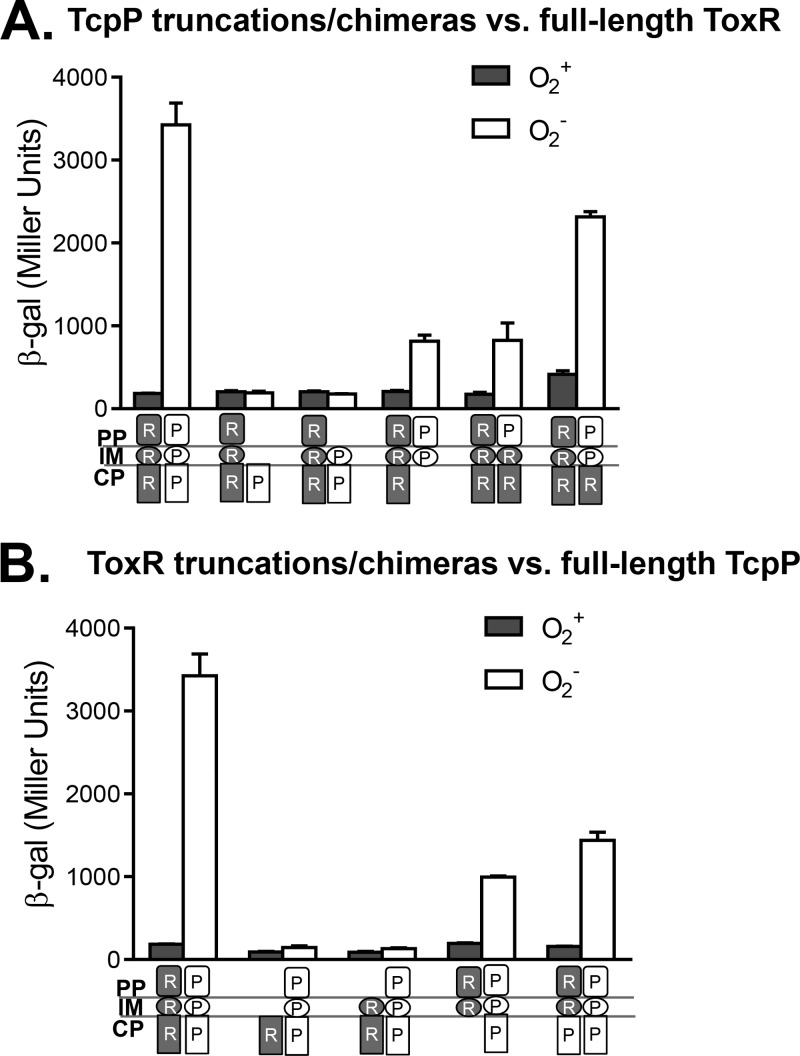
To investigate the mechanism of an anaerobiosis-enhanced ToxR-TcpP interaction, we first examined how truncated TcpP proteins affect this interaction. We introduced T18 fusions of full-length TcpP or TcpP truncations into E. coli cyaA mutants containing the T25 fusion of full-length ToxR and assayed for LacZ production during aerobic or anaerobic growth. Figure 2A shows that compared to that of wild-type TcpP, truncated TcpP containing only the cytoplasmic domain or the cytoplasmic and transmembrane (TM) domains could not interact with ToxR under anaerobic conditions. Truncated TcpP containing both the periplasmic and TM domains, as well as chimeric proteins consisting of the TcpP periplasmic domain and the TM domain of ToxR-TcpP and the ToxR cytoplasmic domain, however, could partially interact with ToxR in the absence of oxygen. We examined the levels and localization of the proteins produced by those constructs in E. coli and found that they produced similar amount of proteins and that they were localized at the predicted locations (data not shown; 21). These data suggest that the periplasmic domain of TcpP is critical for interaction with ToxR under oxygen-limiting conditions.
FIG 2.
Domain analysis of effects of oxygen on ToxR-TcpP interactions. (A) Full-length ToxR interacts with various TcpP truncations and chimeras. (B) Full-length TcpP interacts with various ToxR truncations and chimeras. The BTH101 strains containing those pairs were grown aerobically (gray bars) or anaerobically (white bars) at 37°C to an OD600 of ∼0.3, and β-galactosidase activity was measured and recorded in Miller units (29). β-gal, β-galactosidase; PP, periplasm; IM, inner membrane; CP, cytoplasm. White symbols, TcpP (P) domains; shaded symbols, ToxR (R) domains.
To examine the domain requirement of ToxR, we tested the interaction between a T18 fusion of full-length or truncated ToxR and a T25 fusion of full-length TcpP by assaying β-galactosidase production under aerobic or anaerobic conditions. We found that ToxR's periplasmic domain was also important for interaction with TcpP under anaerobic conditions, as LacZ activity was abolished in any constructs where the periplasmic domain of ToxR was deleted (Fig. 2B). These data indicate that the ToxR periplasmic domain contributes to the anaerobiosis-enhanced interaction with TcpP proteins, likely through the TcpP periplasmic domain. Intriguingly, Crawford et al. previously showed that the periplasmic domain of ToxR is not required for TcpP-dependent activation of virulence (33). In that study, a classical strain of V. cholerae was used and the cultures were grown aerobically at 30°C. It will be interesting to examine whether virulence genes are further induced under anaerobic conditions in classical strains.
Disulfide bond formation is required for the ToxR-TcpP interaction.
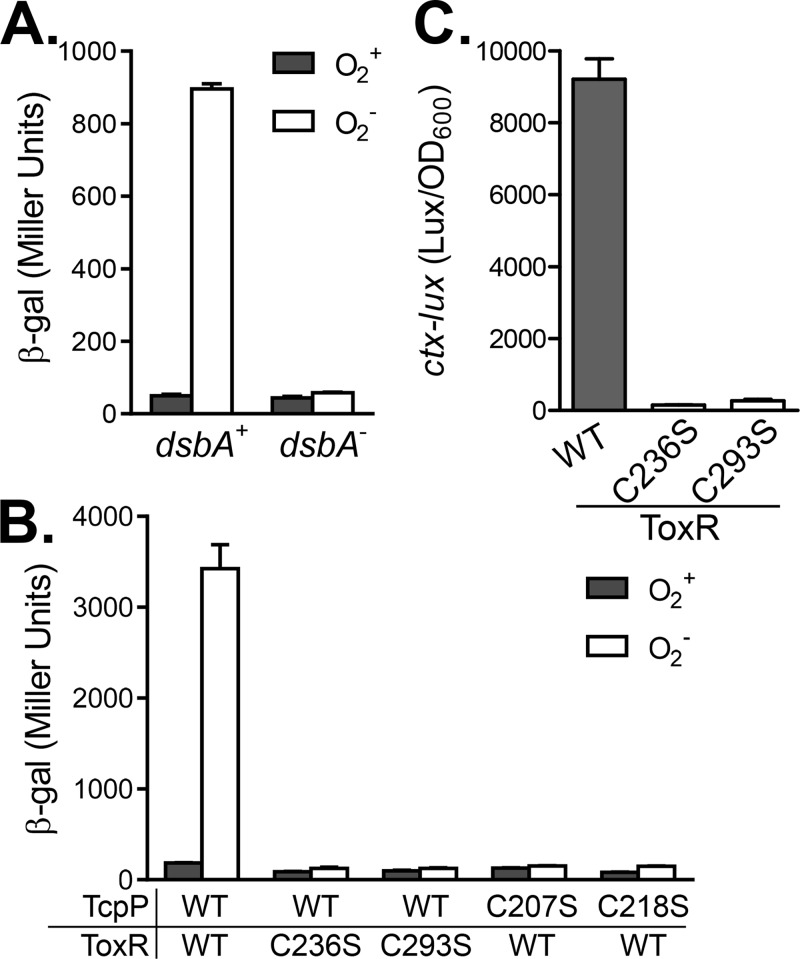
It has been shown that intermolecular disulfide bond formation plays an important role in bile salt-induced TcpP activity (21). To examine whether disulfide bond formation is involved in the ToxR-TcpP interaction, we first tested the bacterial two-hybrid system in a dsbA mutant, which lacks the ability to form disulfide bonds in periplasmic proteins (21). DsbA is a primary periplasmic protein thiol:disulfide oxidoreductase found in eukaryotic endoplasmic reticulum and in the bacterial periplasm. It oxidizes about 300 proteins exported to the periplasm in E. coli (34, 35). We found that compared to that of the wild type, the ToxR-TcpP interaction was significantly reduced in the dsbA mutant. The positive-control plasmids that express Zip-T18/T25 fusions still produced similar amount of LacZ in both the dsbA+ and dsbA mutant backgrounds (data not shown), indicating that the two-hybrid system, in general, is functional in dsbA mutants. There are two cysteine residues in the periplasmic region of ToxR (C236 and C293) and TcpP (C207 and C218). To test which cysteine residues are involved in the ToxR-TcpP interaction, we mutated each cysteine residue to a serine in the periplasmic domains of ToxR and TcpP and examined the effects of these changes on the ToxR-TcpP interaction with or without oxygen. Figure 3B shows that mutation of either C236 or C293 of ToxR abolished the ToxR-TcpP interaction independently of the oxygen concentration. Similarly, cysteine mutant TcpP also failed to interact with ToxR. These data suggest that these cysteine residues are critical for the ToxR-TcpP interaction. However, we could not rule out the possibility that mutations in cysteine residues may change the protein's conformation and therefore cause loss of stability. Previously, we showed that the C207S mutant of TcpP is inactive whereas the C218S mutant is active even in the absence of bile salts (21). The ToxR periplasmic domain has been proposed to act as a sensor of environmental stimuli and contains two cysteine residues that can form both intra- and intermolecular disulfide bonds (36). Interestingly, Fengler et al. have recently reported that the double cysteine-to-serine mutation of ToxR does not affect virulence gene activation but does abolish the ability of ToxR to activate ompU (37). The reason for this phenotype remains unclear. To examine whether ToxR periplasmic cysteine residues are important for its function in our systems, we compared the abilities of wild-type ToxR and single cysteine mutant ToxR to activate ctx expression in E. coli. We found that both cysteine residues in ToxR were important for ToxR activity, as both single cysteine mutations virtually abolished ToxR activity in E. coli (Fig. 3C). We do not know why our data are inconsistent with the previous report (37), and we cannot rule out the possibility that either ToxR or TcpP cysteine mutations interrupted interactions by changing their conformation, rather than the involvement of disulfide bond formation. Moreover, it has been reported that in the absence of ToxS, ToxR interchain disulfide bond formation is enhanced (37). It will be interesting to examine the TcpP-ToxR interaction in the absence of ToxS in the future.
FIG 3.
Effects of periplasmic disulfide bonds and cysteine residues on a ToxR-TcpP interaction. (A) ToxR-TcpP interaction in the dsbA mutant. BTH101 and BTH101 ΔdsbA containing pKT25-ToxRS and pUT18C-TcpPH were grown aerobically and anaerobically at 37°C to an OD600 of ∼0.3. β-Galactosidase (β-gal) activity was then measured and recorded in Miller units. (B) Effects of periplasmic cysteine mutations. BTH101 containing different combinations of ToxR and TcpP cysteine mutant proteins were grown aerobically and anaerobically at 37°C to an OD600 of ∼0.3. β-Galactosidase activity was then measured. (C) Effect of ToxR cysteine mutations on ctx expression in E. coli. E. coli strains containing PctxA-luxCDABE reporter and Plac-toxR (wild type and its cysteine mutant derivatives) plasmids were grown in LB containing 0.5 mM IPTG to an OD600 of ∼0.3. Luminescence was then measured and reported as light units/OD600 unit. Data represent the mean ± the standard deviation of three independent experiments. WT, wild type.
Oxygen limitation-dependent interaction of TcpP and ToxR is important for virulence induction in V. cholerae.
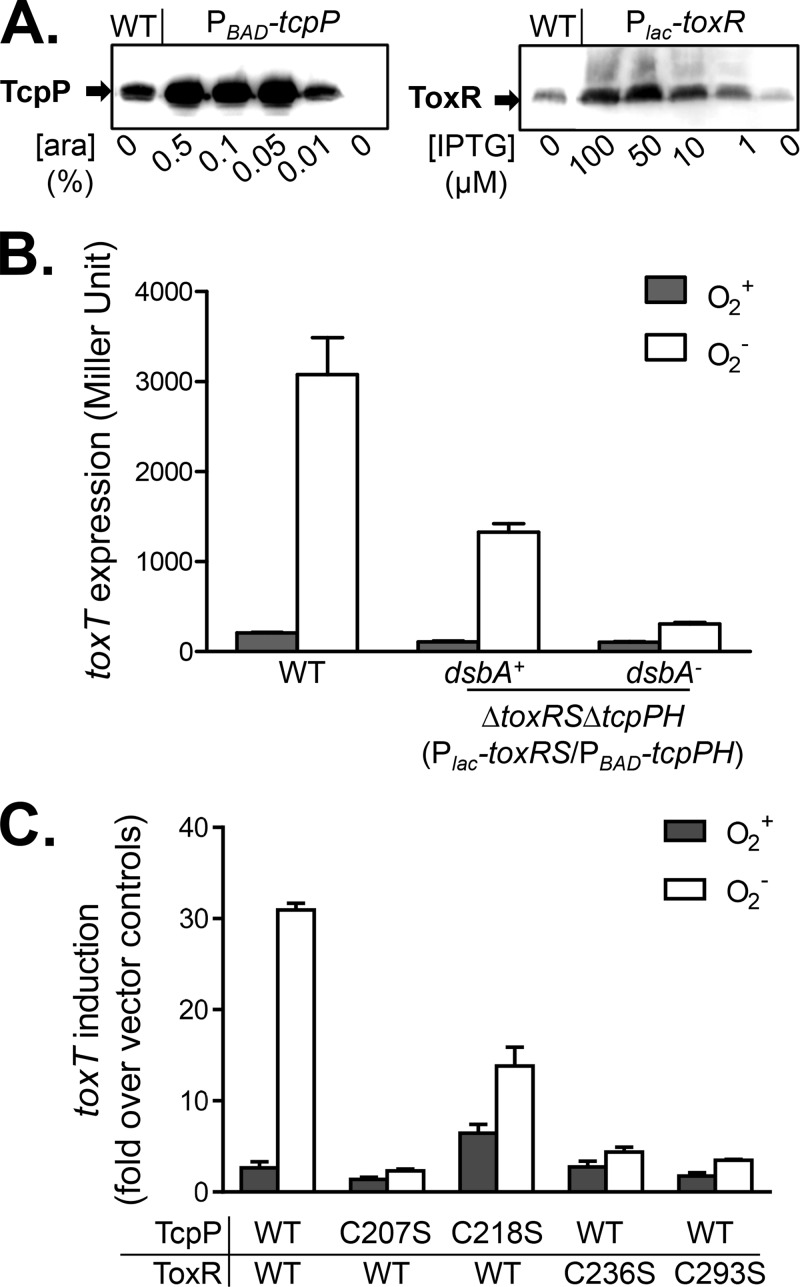
We have shown that the ToxR-TcpP interaction was enhanced under oxygen-limiting conditions and that this interaction was dependent on DsbA and cysteine residues in both ToxR and TcpP periplasmic domains. To investigate whether this interaction is also important for virulence activation in V. cholerae, we first decoupled the expression of tcpP from the oxygen concentration. It has been reported that tcpP expression was increased under oxygen-limiting conditions because of the thiol-based switch of AphB (23). We developed a controllable system to express tcpPH and toxRS independently of oxygen by placing their coding sequences under the control of the PBAD and Plac promoters, respectively. We introduced these two plasmids into a mutant strain of V. cholerae lacking both toxRS and tcpPH. We then performed Western blot analysis to determine the amounts of arabinose and IPTG inducers needed to express amounts of TcpP and ToxR similar to those of the wild type under anaerobic condition. We found that using 0.01% arabinose and 1 μM IPTG to induce tcpP and toxR, respectively, could achieve wild-type levels of production of these proteins (Fig. 4A). Therefore, we used these conditions to express tcpPH and toxRS in the ΔtoxRS ΔtcpPH mutant to examine the effects of oxygen on the TcpP-ToxR interaction by measuring toxT expression. We found that both ToxR and TcpP are required for toxT expression, as the addition of either arabinose or IPTG failed to induce toxT expression (see Fig. S1 in the supplemental material). With both inducers, this strain showed strong toxT expression when cultures were grown anaerobically but not when they were grown aerobically (Fig. 4B). Similarly, the expression of tcpA, the major virulence determinant in the same strain background and under the same inducing conditions was strongly enhanced under anaerobic conditions (see Fig. S2 in the supplemental material). Taken together, these data suggest that anaerobically enhanced TcpP-ToxR interaction contributes to virulence induction. In the dsbA mutant of V. cholerae, toxT was not expressed (Fig. 4B), suggesting that disulfide bond formation is involved in the ToxR-TcpP interaction in V. cholerae. Furthermore, we examined the role of cysteine residues in periplasmic domains of ToxR and TcpP in toxT activation. Figure 4C shows that toxT expression was low when the TcpPC207S, ToxRC236S, and ToxRC293S mutant proteins were present; however, a TcpPC218S mutant protein could induce toxT expression even under aerobic conditions. Previously, we have shown that a TcpPC218S mutant protein could activate virulence in the absence of virulence-inducing bile salts because of the intermolecular disulfide bond formation between the mutant proteins (21). This may explain why this mutant protein could activate toxT in the presence of oxygen. More importantly, under anaerobic conditions, toxT induction by the TcpPC218S mutant protein was lower than that by wild-type TcpP. As TcpPC218S could not interact with ToxR (Fig. 3B), these results again suggest that TcpP-ToxR interactions under anaerobic conditions may be important for virulence activation.
FIG 4.
Effects of oxygen on the ToxR-TcpP interaction and virulence expression in V. cholerae. (A) Concentrations of inducers needed to express TcpP and ToxR to wild-type (WT) levels. Cultures of wild-type or ΔtoxRS ΔtcpPH mutant V. cholerae containing PBAD-tcpPH and Plac-toxRS were grown in AKI medium in the presence of various amounts of arabinose (ara) or IPTG without shaking at 37°C to an OD600 of ∼0.3. The cell pellets were subjected to Western blot assays with anti-TcpP (left panel) and anti-ToxR (right panel) antibodies. (B) Analysis of ToxR-TcpP interactions and activation of toxT. Wild-type V. cholerae (PtoxT-lacZ), a ΔtoxRS ΔtcpPH mutant, and a ΔtoxRS ΔtcpPH ΔdsbA mutant containing PBAD-tcpPH, Plac-toxRS, and PtoxT-lacZ reporter plasmids were grown aerobically or anaerobically in AKI medium supplemented with 0.01% arabinose and 1 μM IPTG to an OD600 of ∼0.3. β-Galactosidase activity was then measured. Data represent the mean ± the standard deviation of three independent experiments. (C) Analysis of the effects of periplasmic cysteine mutations on ToxR-TcpP interactions and activation of toxT. The ΔtoxRS ΔtcpP mutant (21) containing a PBAD-tcpPH (or its cysteine→serine derivatives), Plac-toxRS (or its cysteine→serine derivative mutant forms), or PtoxT-lacZ reporter plasmid was grown aerobically or anaerobically in AKI medium supplemented with 0.01% arabinose and 1 μM IPTG to an OD600 of ∼0.3. β-Galactosidase activity was then measured. Data represent the mean ± the standard deviation of three independent experiments.
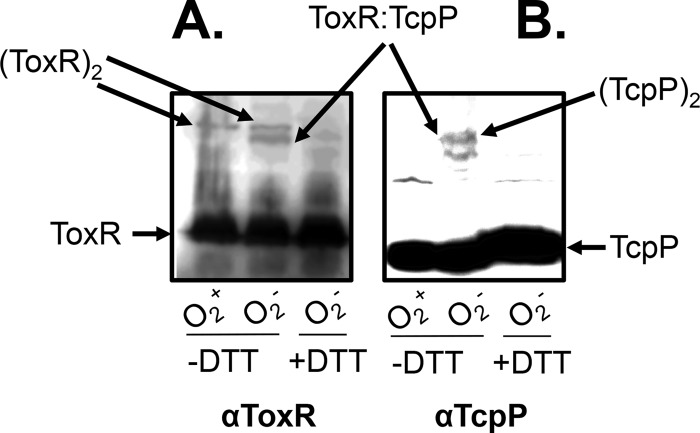
To further demonstrate that TcpP-ToxR interacts better under anaerobic conditions, we performed a Western blot analysis with cultures of the V. cholerae ΔtoxRS ΔtcpPH (PBAD-tcpPH) (Plac-toxRS) mutant strain grown in the presence of 0.01% arabinose and 1 μM IPTG aerobically and anaerobically. We used anti-ToxR (Fig. 5A) and anti-TcpP (Fig. 5B) antibodies. We found that in the absence of the reducing agent DTT, a band corresponding to a ToxR dimer (65 kDa) was present in the sample obtained from the aerobically grown culture, whereas an additional 58-kDa band that corresponds to the size of a ToxR-TcpP heterodimer was present in the sample from the anaerobically grown culture (Fig. 5A). This 58-kDa band was absent from the anaerobically grown culture when no TcpP was expressed (data not shown) and from the sample treated with DTT (Fig. 5, the last lane). Similarly, ToxR-TcpP heterodimers were detected only in anaerobically grown cultures without DTT treatment when anti-TcpP antibody was used (Fig. 5B). These data suggest that the ToxR-TcpP interaction is enhanced during anaerobic growth and that this interaction may be covalently linked through disulfide bond formation.
FIG 5.
ToxR-TcpP interactions in V. cholerae. The ΔtoxRS ΔtcpPH mutant harboring Plac-toxRS or PBAD-tcpPH was grown aerobically or anaerobically in AKI medium at 37°C and induced with 0.01% arabinose and 1 μM IPTG. Cell pellets were collected and resuspended in SDS-PAGE sampling buffer with or without DTT. Samples containing 0.1 μg total proteins were loaded onto a 15% polyacrylamide gel and subjected to Western blot analysis with anti-ToxR antibody (A) or anti-TcpP antibody (B).
V. cholerae depends on a complex transcriptional cascade to activate virulence and colonization genes to survive in the host. One of the environmental signals used by V. cholerae to regulate virulence is an anoxic signal (23, 38), as gastrointestinal tracts are generally considered to be oxygen deficient (39). Reduced forms of AphB have been shown to activate tcpP transcription under oxygen-limiting conditions (23). Here, we discovered that ToxR-TcpP interacted better under these conditions. This interaction also played an important role in activating virulence gene expression. We further demonstrated the importance of the cysteine residues in the periplasmic domains of ToxR and TcpP in ToxR-TcpP interactions. Intriguingly, we recently showed that a set of bile salts serve as virulence inducers by promoting TcpP intermolecular disulfide bond formation (21). Interestingly, the AKI medium used to culture V. cholerae in this study may contain sufficient bile salts. It has been reported that Bacto peptone, the major component of AKI medium, has approximately 10 mg/g of bile salts (40). Further investigation is required to better understand the relationships among periplasmic disulfide bond formation, ToxR-TcpP interaction, and virulence induction in host intestinal environments.
Supplementary Material
ACKNOWLEDGMENTS
We thank Stephen Farrand for providing the pSRKtc vector and Menghua Yang for various TcpP constructs.
This study was supported by Natural Science Foundation of China key project 30830008 (B.K.), NIH/NIAID R01 AI080654 and R56 AI072479 (J.Z.), and Fulbright Fellowship 68434010 (N.J.).
Footnotes
Published ahead of print 3 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01377-13.
REFERENCES
- 1.Colwell RR, Huq A. 1994. Environmental reservoir of Vibrio cholerae. The causative agent of cholera. Ann. N. Y. Acad. Sci. 740:44–54. 10.1111/j.1749-6632.1994.tb19852.x [DOI] [PubMed] [Google Scholar]
- 2.Butler SM, Camilli A. 2005. Going against the grain: chemotaxis and infection in Vibrio cholerae. Nat. Rev. Microbiol. 3:611–620. 10.1038/nrmicro1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller VL, Taylor RK, Mekalanos JJ. 1987. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell 48:271–279. 10.1016/0092-8674(87)90430-2 [DOI] [PubMed] [Google Scholar]
- 4.Herrington DA, Hall RH, Losonsky G, Mekalanos JJ, Taylor RK, Levine MM. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487–1492. 10.1084/jem.168.4.1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matson JS, Withey JH, DiRita VJ. 2007. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect. Immun. 75:5542–5549. 10.1128/IAI.01094-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiRita VJ, Parsot C, Jander G, Mekalanos JJ. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 88:5403–5407. 10.1073/pnas.88.12.5403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Häse CC, Mekalanos JJ. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 95:730–734. 10.1073/pnas.95.2.730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacikova G, Skorupski K. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skorupski K, Taylor RK. 1999. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol. Microbiol. 31:763–771. 10.1046/j.1365-2958.1999.01215.x [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Stern AM, Liu Z, Kan B, Zhu J. 2010. Virulence regulator AphB enhances toxR transcription in Vibrio cholerae. BMC Microbiol. 10:3. 10.1186/1471-2180-10-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krukonis ES, Yu RR, Dirita VJ. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol. Microbiol. 38:67–84. 10.1046/j.1365-2958.2000.02111.x [DOI] [PubMed] [Google Scholar]
- 12.Morgan SJ, Felek S, Gadwal S, Koropatkin NM, Perry JW, Bryson AB, Krukonis ES. 2011. The two faces of ToxR: activator of ompU, co-regulator of toxT in Vibrio cholerae. Mol. Microbiol. 81:113–128. 10.1111/j.1365-2958.2011.07681.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krukonis ES, DiRita VJ. 2003. DNA binding and ToxR responsiveness by the wing domain of TcpP, an activator of virulence gene expression in Vibrio cholerae. Mol. Cell 12:157–165. 10.1016/S1097-2765(03)00222-3 [DOI] [PubMed] [Google Scholar]
- 14.Goss TJ, Morgan SJ, French EL, Krukonis ES. 2013. ToxR recognizes a direct repeat element in the toxT, ompU, ompT, and ctxA promoters of Vibrio cholerae to regulate transcription. Infect. Immun. 81:884–895. 10.1128/IAI.00889-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krukonis ES, DiRita VJ. 2003. From motility to virulence: Sensing and responding to environmental signals in Vibrio cholerae. Curr. Opin. Microbiol. 6:186–190. 10.1016/S1369-5274(03)00032-8 [DOI] [PubMed] [Google Scholar]
- 16.Kovacikova G, Skorupski K. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46:1135–1147. 10.1046/j.1365-2958.2002.03229.x [DOI] [PubMed] [Google Scholar]
- 17.Rutherford ST, van Kessel JC, Shao Y, Bassler BL. 2011. AphA and LuxR/HapR reciprocally control quorum sensing in vibrios. Genes Dev. 25:397–408. 10.1101/gad.2015011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 99:3129–3134. 10.1073/pnas.052694299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowden MJ, Skorupski K, Pellegrini M, Chiorazzo MG, Taylor RK, Kull FJ. 2010. Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes. Proc. Natl. Acad. Sci. U. S. A. 107:2860–2865. 10.1073/pnas.0915021107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abuaita BH, Withey JH. 2009. Bicarbonate induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect. Immun. 77:4111–4120. 10.1128/IAI.00409-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang M, Liu Z, Hughes C, Stern AM, Wang H, Zhong Z, Kan B, Fenical W, Zhu J. 2013. Bile salt-induced intermolecular disulfide bond formation activates Vibrio cholerae virulence. Proc. Natl. Acad. Sci. U. S. A. 110:2348–2353. 10.1073/pnas.1218039110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson M. 2004. Microbial inhabitants of humans: their ecology and role in health and disease. Cambridge University Press, Cambridge, England [Google Scholar]
- 23.Liu Z, Yang M, Peterfreund GL, Tsou AM, Selamoglu N, Daldal F, Zhong Z, Kan B, Zhu J. 2011. Vibrio cholerae anaerobic induction of virulence gene expression is controlled by thiol-based switches of virulence regulator AphB. Proc. Natl. Acad. Sci. U. S. A. 108:810–815. 10.1073/pnas.1014640108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joelsson A, Liu Z, Zhu J. 2006. Genetic and phenotypic diversity of quorum sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect. Immun. 74:1141–1147. 10.1128/IAI.74.2.1141-1147.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karimova G, Pidoux J, Ullmann A, Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 95:5752–5756. 10.1073/pnas.95.10.5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan SR, Gaines J, Roop RM, II, Farrand SK. 2008. Broad-host-range expression vectors with tightly regulated promoters and their use to examine the influence of TraR and TraM expression on Ti plasmid quorum sensing. Appl. Environ. Microbiol. 74:5053–5062. 10.1128/AEM.01098-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammer BK, Bassler BL. 2007. Regulatory small RNAs circumvent the conventional quorum sensing pathway in pandemic Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 104:11145–11149. 10.1073/pnas.0703860104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Miyashiro T, Tsou A, Hsiao A, Goulian M, Zhu J. 2008. Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing. Proc. Natl. Acad. Sci. U. S. A. 105:9769–9774. 10.1073/pnas.0802241105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 30.Hsiao A, Xu X, Kan B, Kulkarni RV, Zhu J. 2009. Direct regulation by the Vibrio cholerae regulator ToxT to modulate colonization and anticolonization pilus expression. Infect. Immun. 77:1383–1388. 10.1128/IAI.01156-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller VL, DiRita VJ, Mekalanos JJ. 1989. Identification of toxS, a regulatory gene whose product enhances toxR-mediated activation of the cholera toxin promoter. J. Bacteriol. 171:1288–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matson JS, DiRita VJ. 2005. Degradation of the membrane-localized virulence activator TcpP by the YaeL protease in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 102:16403–16408. 10.1073/pnas.0505818102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crawford JA, Krukonis ES, DiRita VJ. 2003. Membrane localization of the ToxR winged-helix domain is required for TcpP-mediated virulence gene activation in Vibrio cholerae. Mol. Microbiol. 47:1459–1473. 10.1046/j.1365-2958.2003.03398.x [DOI] [PubMed] [Google Scholar]
- 34.Dutton RJ, Boyd D, Berkmen M, Beckwith J. 2008. Bacterial species exhibit diversity in their mechanisms and capacity for protein disulfide bond formation. Proc. Natl. Acad. Sci. U. S. A. 105:11933–11938. 10.1073/pnas.0804621105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiniker A, Bardwell JC. 2004. In vivo substrate specificity of periplasmic disulfide oxidoreductases. J. Biol. Chem. 279:12967–12973. 10.1074/jbc.M311391200 [DOI] [PubMed] [Google Scholar]
- 36.Ottemann KM, Mekalanos JJ. 1996. The ToxR protein of Vibrio cholerae forms homodimers and heterodimers. J. Bacteriol. 178:156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fengler VH, Boritsch EC, Tutz S, Seper A, Ebner H, Roier S, Schild S, Reidl J. 2012. Disulfide bond formation and ToxR activity in Vibrio cholerae. PLoS One 7:e47756. 10.1371/journal.pone.0047756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sengupta N, Paul K, Chowdhury R. 2003. The global regulator ArcA modulates expression of virulence factors in Vibrio cholerae. Infect. Immun. 71:5583–5589. 10.1128/IAI.71.10.5583-5589.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Contag CH, Bachmann MH. 2002. Advances in in vivo bioluminescence imaging of gene expression. Annu. Rev. Biomed. Eng. 4:235–260. 10.1146/annurev.bioeng.4.111901.093336 [DOI] [PubMed] [Google Scholar]
- 40.Kamekura M, Oesterhelt D, Wallace R, Anderson P, Kushner DJ. 1988. Lysis of halobacteria in Bacto-Peptone by bile acids. Appl. Environ. Microbiol. 54:990–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.