Abstract
One of the first issues that emerges when a prokaryotic organism of interest is encountered is the question of what it is—that is, which species it is. The 16S rRNA gene formed the basis of the first method for sequence-based taxonomy and has had a tremendous impact on the field of microbiology. Nevertheless, the method has been found to have a number of shortcomings. In the current study, we trained and benchmarked five methods for whole-genome sequence-based prokaryotic species identification on a common data set of complete genomes: (i) SpeciesFinder, which is based on the complete 16S rRNA gene; (ii) Reads2Type that searches for species-specific 50-mers in either the 16S rRNA gene or the gyrB gene (for the Enterobacteraceae family); (iii) the ribosomal multilocus sequence typing (rMLST) method that samples up to 53 ribosomal genes; (iv) TaxonomyFinder, which is based on species-specific functional protein domain profiles; and finally (v) KmerFinder, which examines the number of cooccurring k-mers (substrings of k nucleotides in DNA sequence data). The performances of the methods were subsequently evaluated on three data sets of short sequence reads or draft genomes from public databases. In total, the evaluation sets constituted sequence data from more than 11,000 isolates covering 159 genera and 243 species. Our results indicate that methods that sample only chromosomal, core genes have difficulties in distinguishing closely related species which only recently diverged. The KmerFinder method had the overall highest accuracy and correctly identified from 93% to 97% of the isolates in the evaluations sets.
INTRODUCTION
Rapid identification of the species of isolated bacteria is essential for surveillance for human and animal health and for choosing optimal treatment and control measures. Since the beginning of microbiology more than a century ago, this has to a large extent been based on morphology and biochemical testing. However, for more than 30 years, 16S rRNA sequence data have served as the backbone for the classification of prokaryotes (1), and tremendous amounts of 16S rRNA sequences are available in public repositories (2–4). However, due to the conserved nature of the 16S rRNA gene, the resolution is often too low to adequately resolve different species and sometimes is not even adequate for genus delineation (5, 6). Furthermore, many prokaryotic genomes contain several copies of the 16S rRNA gene with substantial intergene variation (7, 8). It is also considered problematic that this gene represents only a tiny fraction, roughly about 0.1% or less, of the coding part of a microbial genome (9).
Second- and third-generation sequencing techniques have the potential to revolutionize the classification and characterization of prokaryotes and is now being used routinely in some clinical microbiology labs. However, so far no consensus on how to utilize the vast amount of information in whole-genome sequence (WGS) data has emerged (10). Nevertheless, a number of different methods have been proposed. Roughly, they can be divided into those that require annotation of genes in the data and those that employ the nucleotide sequences directly (9).
One of the first attempts to employ WGS data for taxonomic purposes was carried out in 1999 (11). At the time, 13 completely sequenced genomes of unicellular organisms were available, and distance-based phylogeny was constructed on the basis of the presence and absence of suspected orthologous (direct common ancestry) gene pairs. Later, it was recognized that methods that take into account gene content can be greatly influenced by horizontal gene transfer (HGT), and alternative methods were developed that used homologous groups (gene family content) (12) or protein domains (13).
Functional protein domains also form the basis of a recent approach developed by our group (14). Here, the protein domains are combined into functional profiles of which some are species specific and can thus be used for inferring taxonomy.
As an extension of 16S rRNA analysis, which focuses on a single locus, super multilocus sequence typing (SuperMLST) has been proposed (15). It relies on the selection of a set of genes that are highly conserved and hence can be used with any organism. In a publication from 2012, Jolley et al. suggested that 53 genes encoding ribosomal proteins be used for bacterial classification in an approach called ribosomal MLST (rMLST) (16). Not all 53 genes were found in all bacterial genomes, but due to the relatively high number of sampled loci, this is not considered problematic. The rMLST method forms the basis of a proposed reclassification of Neisseria species (17) and has also been used for analyzing human Campylobacter isolates (18).
It is also possible to employ the sequence data directly without preannotation of genes. For instance, this can be done using BLAST (19). An alternative, faster approach would be to look at k-mers (substrings of k nucleotides in DNA sequence data) and use the number of cooccurring k-mers in two bacterial genomes as a measure of evolutionary relatedness. Using the k-mer-based approach, we have developed a method, KmerFinder, which examines all regions of the genomes, not only core genes (20). Furthermore, a gene segment will score highly despite the transposition of a gene segment within the genome since only the flanking regions will be mismatched.
In the current study, we have trained five different methods for species identification on a common data set of complete prokaryotic genomes: (i) the SpeciesFinder method, which serves as the baseline as it is based solely upon the 16S rRNA gene; (ii Reads2Type which is a variant that searched for species-specific 50-mers, predominantly within the 16S rRNA gene, with the help of non-species-specific 50-mers to quickly narrow the search; (iii) rMLST, which predicts species by examining 53 ribosomal genes; (iv) TaxonomyFinder, which is based on species-specific functional protein domain profiles; and finally (v) KmerFinder, which predicts species by examining the number of overlapping 16-mers.
The publicly available databases contain ample amounts of WGS data from prokaryotes, enabling us to conduct a large-scale benchmark study of the proposed methods. Hence, the process of reaching a consensus on how the WGS data should optimally be used for prokaryotic taxonomy is initiated.
MATERIALS AND METHODS
Data set. (i) Training data.
In August 2011 a total of 1,647 complete genomes originating from Bacteria (1,535) and Archaea (112) were downloaded from the National Center for Biotechnology Information (NCBI [http://www.ncbi.nlm.nih.gov/genome]). For each genome, the annotated taxonomy according to GenBank was compared to the taxonomy according to Entrez, which was retrieved using the taxonomy module of BioPerl. Discrepancies were checked and corrected manually. For each genome, it was also examined if the annotated name was in accordance to the List of Prokaryotic Names with Standing in Nomenclature (http://www.bacterio.cict.fr/allnames.html) (21). When possible, names that were not in accordance were corrected to valid ones. In this way, 1,426 genomes were assigned to 847 approved genus and species names. The remaining 221 genomes, which were either assigned only to a genus, e.g., Vibrio spp., or assigned to species with informal names, e.g., Synechococcus islandicus, were kept in the training data under the assumption that they would influence the different methods for species identification equally. An overview of the training data is available in Table S1 in the supplemental material.
(ii) Evaluation data.
Three data sets were generated for the purpose of evaluating the methods. The first consisted of assembled complete or draft genomes with assigned species which were downloaded from NCBI in September 2012 and were not already part of the training data. Only genomes assigned to species that were also present in the training data were included. The set was called NCBIdrafts and consisted of genomes from 695 isolates covering 81 genera and 149 species. The set includes three members of the Archaea, two Methanobrevibacter smithii isolates and one Sulfolobus solfataricus isolate. An overview of the data can be seen in Table S2 in the supplemental material.
Furthermore, in January 2012, 11,768 sets of Illumina raw reads with assigned species were downloaded from the NCBI Sequence Reads Archive (SRA [http://www.ncbi.nlm.nih.gov/sra]) (22). A total of 10,517 of these had been sequenced by the Illumina Genome Analyzer II sequencer, while the remaining 1,251 had been sequenced by the Illumina HiSeq 2000 sequencer. A total of 1,361 sets of reads originated from species that were not part of the training data and were removed. The final SRAreads data set consisted of 8,798 sets of paired-end reads and 1,609 sets of single reads, giving a total of 10,407 sets.
For the short reads of the SRAreads set, the optimal k-mer length was estimated and used for de novo assembly as described previously (23) using Velvet, version 1.1.04 (24). The resulting set of draft genomes constituted the SRAdrafts evaluation set. To measure the qualities of the draft assemblies, the N50 values were calculated (25). The draft assemblies had an average N50 of 77,018, with a range of 101 to 779,945 (see Fig. S1 in the supplemental material), an average number of scaffolds of 697, and an average size of 3,301 kb.
The SRAreads and SRAdrafts sets both cover 167 different species from 120 genera with more than 5,000 strains from the Streptococcus, Staphylococcus, and Salmonella genera. There are no species from Archaea. An overview of the SRAreads and SRAdrafts sets is available in Table S3 in the supplemental material.
Methods for species identification. (i) SpeciesFinder.
SpeciesFinder predicts the prokaryotic species based on the 16S rRNA gene. The concept of using the 16S rRNA gene for taxonomic purposes goes back to 1977 (1), but the implementation used in this study was developed by our group. A 16S database was built from the genomes of the common training data using RNAmmer (26). The species predictions were performed differently depending on the input type. If the input was short reads, the prediction was done in the following way. (a) The reads were mapped against the 16S database using the Smith-Waterman Burrows-Wheeler aligner (BWA) (27). (b) The mapped reads were assembled using Trinity (28) to obtain the 16S rRNA sequences. (c) The BLAST algorithm (19) was used to search the output from Trinity against the 16S database. (d) The best BLAST hit (see below) was chosen, and the species associated with the best hit was given as the final prediction.
When the input sequence was a draft or complete genome, the prediction was performed as follows. (a) The 16S rRNA gene was predicted from the input sequence using RNAmmer. (b) Using the BLAST algorithm, the predicted sequence was aligned against the 16S database. (c) The best BLAST hit (see below) was chosen, and the species associated with it given as the final prediction.
The best BLAST hit was chosen by ranking the output from the BLAST alignment by the best cumulative rank of coverage, percent identity, bit score, number of mismatches, and number of gaps. The highest ranked hit was chosen for the prediction.
SpeciesFinder is freely available at http://cge.cbs.dtu.dk/services/SpeciesFinder/.
(ii) rMLST.
The rMLST method predicts bacterial species based on 53 ribosomal genes originally defined by Jolley et al. (16). The set of genes can be used in an approach similar to multilocus sequence typing (MLST), where each locus in the query genome is considered identical or nonidentical to alleles of the corresponding locus in the reference database, and an allelic profile based on arbitrary numbers assigned to each of the alleles in the database is generated accordingly. Since the strains that we compare are more diverse than the ones compared in MLST, it is likely that many loci would have no identical matches in the database, making a simple cluster analysis based on allelic profiles problematic. To improve the resolution of the method, in our implementation of rMLST, the nucleotide sequence of each locus is aligned to the alleles in the reference database, and a measure of the similarity of the locus and the best matching allele is used subsequently, as described below.
Briefly, for each of the genomes in the training data, the 53 ribosomal genes were extracted by BLAST and provided to us by Keith Jolley, Department of Zoology, University of Oxford, United Kingdom. In this way, for each genome, a gene collection of up to 53 ribosomal genes was assigned. To predict the species of a query genome, the query genome was first aligned to each gene collection using Blat (29). Only hits with at least 95% identity and 95% coverage were considered potential matches. If there were several potential matches, the best match was selected based on the best cumulative rank of coverage, percent identity, bit score, number of mismatches, and number of gaps in the alignments. The final prediction was given as the organism with the highest number of best hits across all genes. Our implementation of rMLST performs predictions for draft or complete genomes but not short reads.
(iii) TaxonomyFinder.
The TaxonomyFinder method is based on taxonomy group-specific protein profiles developed by our group (14; Lukjancenko et al., submitted). It performs predictions for draft or complete genomes but not for short reads. The common training data were used to create the taxonomy-specific profile database. Briefly, for each genome, functional profiles were assigned based on three collections of hidden Markov model (HMM) databases: PfamA (30), TIGRFAM (31), and Superfamily (32). Genes that did not match any entry in the HMM databases were clustered using CD-HIT (33). Further, genomes were grouped according to the taxonomy level, either phylum or species, and profiles that were specific to each taxonomic group were extracted. Profiles were considered specific to a taxonomic group if they were conserved in 30 to 100% of the genomes within a phylum/species group and absent in all genomes outside the group. The actual threshold for conservation depended on the size of the group, with large groups having smaller thresholds for conservation. The workflow of the TaxonomyFinder method is a four-step process, as follows. (a) The open reading frame is predicted using Prodigal (34). (b) Functional profiles are constructed from protein coding sequences. (c) Functional profiles are assigned. (d) Functional profiles are compared to the taxonomy-specific profile database. The number of architectures, matched to each of the taxonomy groups, is recorded, and the fraction of taxon-specific genes (score) is calculated. The best-matching taxonomy group is selected based on a consensus of the best score and highest number of matched architectures.
TaxonomyFinder is freely available at http://cge.cbs.dtu.dk/services/TaxonomyFinder/.
(iv) KmerFinder.
The KmerFinder method was developed by our group and predicts prokaryotic species based on the number of overlapping (cooccurring) k-mers, i.e., 16-mers, between the query genome and genomes in a reference database (20). Initially, all genomes in the common training data were split into overlapping 16-mers with step size of one, meaning that if the first 16-mer is initiated at position N and ends at position N + 15, the next 16-mer is initiated at position N + 1 and ends at position N + 16, and so on. To reduce the size of the final 16-mer database, only 16-mers with the prefix ATGAC were kept. These 16-mers were stored in a hash table with links to the original genomes. The length of the k-mers was chosen to be 16 since a parallel study showed that this resulted in the highest performance of the method (results not shown). The prefix ATGAC was initially selected in an attempt to focus the 16-mers on coding regions (ATG is the start codon for protein coding sequences), while the A and C were chosen arbitrarily as the first two nucleotides when the four nucleotides are sorted alphabetically. Later studies have shown that the nucleotide sequence of the prefix has little influence on the performance of the method as long as strongly repetitive sequences, e.g., CCCCC or AAAAA, are omitted (data not shown). When the prediction is performed, the species of the query genome is predicted to be identical to the species of the genome in the training data with which it has the highest number of 16-mers in common, regardless of position. In the case of ties, the species were sorted alphabetically according to their name and the first species selected. The input for KmerFinder can be draft or complete genomes as well as short reads. KmerFinder is freely available at http://cge.cbs.dtu.dk/services/KmerFinder/.
(v) Reads2Type.
Reads2Type was developed by our group and identifies the prokaryotic species based on a database of 50-mer probes generated from chosen marker genes (D. Saputra, S. Rasmussen, M. V. Larsen, N. Haddad, F. M. Aarestrup, O. Lund O, and T. Sicheritz-Pontén, unpublished data). The version of Reads2Type evaluated in this study requires short reads as input. For bacterial species not belonging to the Enterobacteriaceae family, the 50-mer database relies on the 16S rRNA locus, while for Enterobacteriaceae the gyrB locus is used. Briefly, the following steps were applied for building the 50-mer probe database. (a) The 16S rRNA sequences of the complete bacterial genomes of the common training set were predicted using RNammer (26). (b) For species belonging to the Enterobacteriaceae family, the gyrB sequences were downloaded from NCBI. (c) The above sequences were pooled, and all possible 50-bp fragments were generated from that pool. (d) 16S rRNA probes unique for Enterobacteriaceae were removed from the pool of 50-mers. (e) All 50-mer duplicates associated to the conserved regions of different strains but the same species were removed. (f) To further reduce the size of the final 50-mers database, 25 consecutive 50-mers previously fragmented from one ≥50-bp stretch of 16S rRNA belonging to the same list of organisms were removed.
The resulting 50-mer probe database consists of a number of sequences found uniquely in one species, as well as other sequences shared between several species. Subsequently, each read was compressed into a suffix tree, which is a data structure for fast string matching. The compressed short reads were aligned to the 50-mer probe database using a hierarchical “narrow-down” strategy: when a compressed read matched a probe belonging to a group of species, a much smaller probe database excluding other species was created on the fly, causing the read progress to be faster and the species to be identified more quickly.
The Reads2Type method is freely available as a web server (http://cge.cbs.dtu.dk/services/Reads2Type/) and as a console. The web-based Reads2Type is unique in not requiring the short read file to be uploaded to the server. Instead, the 4.6-MB 50-mer probe database is automatically transferred into the client computer's memory before species identification is initiated. All computations needed for the species identification is fully performed on the client's computer, minimizing the data transfer and avoiding the network bottleneck on the server.
Testing the speed.
The speed of the methods was evaluated on nonpublished internal data from up to 450 strains covering eight species (Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Escherichia fergusonii, Klebsiella pneumoniae, Salmonella enterica, Staphylococcus aureus, and Vibrio cholerae) that had been sequenced by the Illumina sequencing method. Draft genomes were de novo assembled as described above for the SRAdrafts set. The speed was tested on a cluster with ×86_64 architecture, 128 nodes, 4 cores per node, and 30 GB or 7 GB RAM per node. SpeciesFinder used 4 cores per job, TaxonomyFinder used up to 10 cores per job, and the other methods used 1 core per job.
RESULTS
Five methods for species identification were trained on a common data set of completed prokaryotic genomes. The performances of the methods were subsequently evaluated on three data sets of draft genomes or short sequence reads.
Performances on NCBI draft genomes.
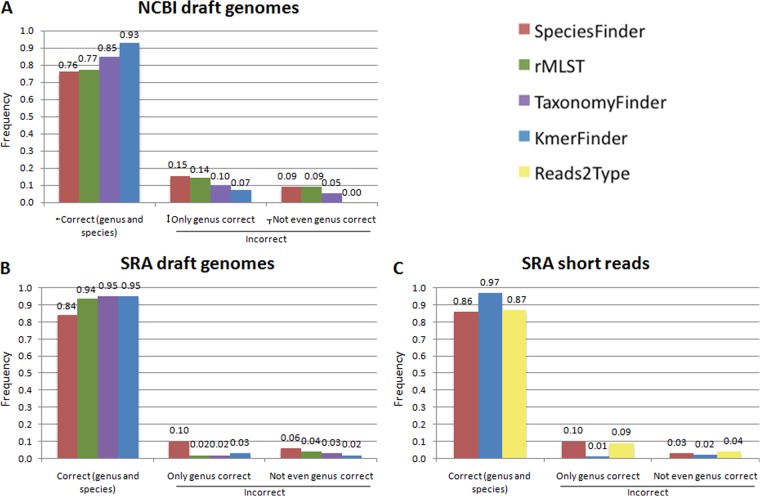
The SpeciesFinder, rMLST, TaxonomyFinder, and KmerFinder methods are able to perform species predictions on draft or completed prokaryotic genomes. Their performances were evaluated on the NCBIdrafts set of 695 draft genomes covering 149 species. File S1 in the supplemental material lists all predictions, while Fig. 1A summarizes the results. Overall, SpeciesFinder, which is based on the 16S rRNA gene, had the poorest performance, correctly identifying only 76% of the isolates down to species level. KmerFinder, which is based on cooccurring 16-mers, had the highest performance and correctly identified 93% of the isolates. For only three isolates (0.43%), KmerFinder did not get even the genus correct. These three isolates were two E. coli isolates predicted as Shigella sonnei and one Providencia alcalifaciens isolate predicted as Yersinia pestis.
FIG 1.
Performance of the five methods for species identification on the indicated data sets. The rMLST and TaxonomyFinder methods take only draft or complete genomes as input, while Reads2Type works only for short reads. Correct (genus and species), predicted genus and species are in accordance with the annotation; only genus correct, the predicted genus is in accordance with the annotation, but the species is not; not even genus correct, neither predicted genus nor species is in accordance with the annotation.
The NCBIdrafts set contained three archaeal isolates: two M. smithii isolates and one S. solfataricus isolate. SpeciesFinder, TaxonomyFinder, and KmerFinder predicted the species of all three isolates correctly, while rMLST, which was intended only for characterization of bacteria (16), predicted the M. smithii isolate correctly but was unable to make a prediction for the S. solfataricus.
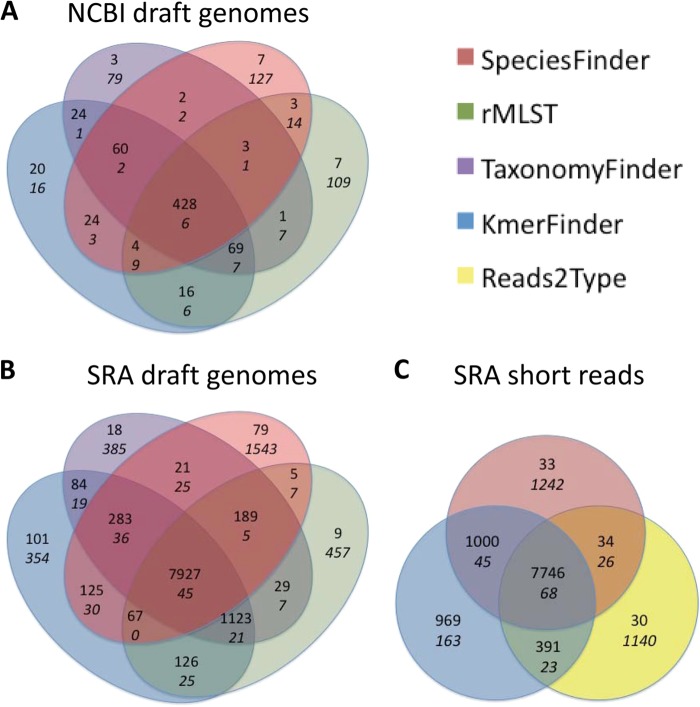
The overlap in predictions of SpeciesFinder, rMLST, TaxonomyFinder, and KmerFinder was examined and is illustrated in Fig. 2A. All four methods correctly identified 428 out of 695 isolates (62%), and all methods misidentified the same six isolates. These six isolates were also misidentified by the BLAST-based method. Table 1 lists these six isolates. Since all five methods agreed on these predictions, the isolates are possibly wrongly annotated. Alternatively, the annotations of the isolates in the training data that the predictions were based on are incorrect.
FIG 2.
Overlap in predictions by the five methods for species identification. Numbers written in regular font indicate the number of isolates for which the predicted species corresponds to the annotated species. Numbers written in italics indicate the number of isolates for which the predicted and annotated species differ. The methods used and data sets evaluated are indicated.
TABLE 1.
Isolates of the NCBIdrafts set for which all five methods predict the species to be different from its present annotation
| RefSeq accession no.a | Strain namec | Annotated species | Predicted species |
|---|---|---|---|
| NZ_ACLX00000000 | AH621 (uid55161) | Bacillus cereus | Bacillus weihenstephanensis |
| NZ_ACMD00000000 | BDRD ST196 (uid55169) | Bacillus cereus | Bacillus weihenstephanensis |
| NZ_ABDQ00000000 | C Eklund (uid54841) | Clostridium botulinum | Clostridium novyi |
| NZ_ABXZ00000000 | FTG (uid55313) | Francisella novicida | Francisella tularensis |
| NZ_AHIE00000000 | DC283 (uid86627) | Pantoea stewartii | Pantoea ananatis |
| NZ_AEPO00000000b | ATCC 49296 (uid61461) | Streptococcus sanguinis | Streptococcus oralis |
NCBI Reference Sequence (RefSeq) accession number from GenBank.
NZ_AEPO00000000 has been reannotated as Streptococcus oralis since we collected the data in 2011.
uid, unique identification number.
As seen in Fig. 2A, isolate predictions agreed upon by several methods are more accurate that predictions unique to a particular method. However, the KmerFinder method made unique predictions for 36 isolates, of which 20 were in concordance with the annotation.
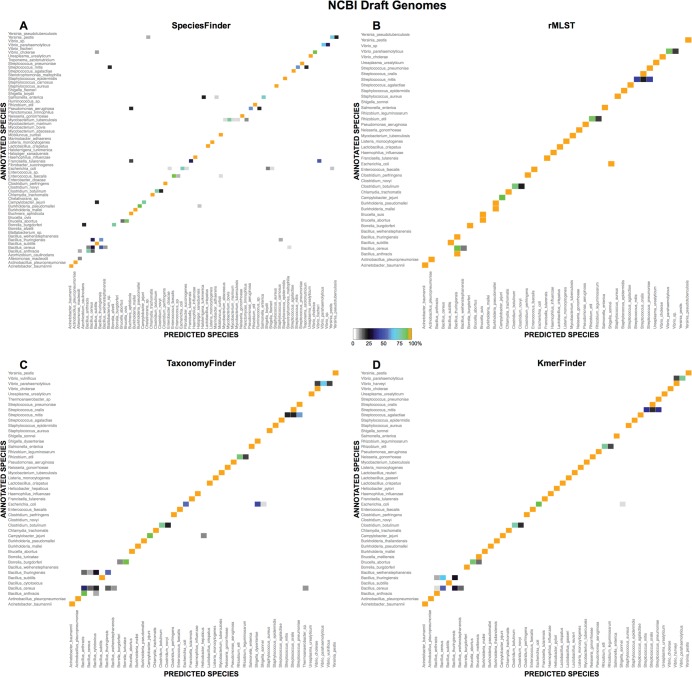
Predictions for the most common species in the NCBIdrafts data set were examined more closely and are illustrated in Fig. 3 and in File S2 in the supplemental material. In general, the “wrong” predictions by SpeciesFinder (that is, the ones that were in disagreement with the NCBI annotation) were typically scattered, often consisting of a few wrong predictions of each type. The rMLST method was, on the other hand, more consistent in its incorrect predictions. As an example, the rMLST method wrongly annotated all 14 Bacillus anthracis isolates as Bacillus thuringiensis, all 8 Brucella abortus isolates as Brucella suis, and all 6 Burkholderia mallei isolates as Burkholderia pseudomallei. In general, all four methods had difficulties identifying species within the Bacillus genus, such as isolates annotated as B. thuringiensis but predicted to be Bacillus cereus or vice versa. Another mistake common to all methods was Streptococcus mitis being predicted as Streptococcus oralis or Streptococcus pneumoniae. Also, none of the methods was able to correctly identify all annotated E. coli isolates but identified at least some of them as Shigella spp. Both SpeciesFinder and TaxonomyFinder had problems identifying the Borrelia burgdorferi isolates, while SpeciesFinder and rMLST had problems distinguishing Yersinia pestis from Yersinia pseudotuberculosis. SpeciesFinder was the only method that had difficulties identifying Mycobacterium tuberculosis isolates, often predicting them to be Mycobacterium bovis isolates.
FIG 3.
Predictions for the most common species of the NCBIdrafts set. For each method, indicated at the top of each panel, the results for a given species are only shown if the method made a prediction for five or more isolates annotated as this species (e.g., if there are five isolates annotated as species A in the data set, but the method was not able to make a prediction for one of the isolates, the species is not shown) or if two or more isolates are predicted as this species (e.g., if there are no isolates annotated as species B in the data set but two isolates annotated as species C are predicted to be species B, then species B is shown).
Performance rates on SRA draft genomes.
The SpeciesFinder, rMLST, TaxonomyFinder, and KmerFinder methods were next evaluated on the SRAdrafts set of 10,407 draft genomes covering 167 species. The performances on the draft genomes, for which the methods were able to make a prediction, are depicted in Fig. 1B, while the overlap in predictions is illustrated in Fig. 2B. Again, SpeciesFinder had the lowest performance, with only 84% correct predictions. The rMLST, TaxonomyFinder, and KmerFinder methods had almost equal performance rates of 94%, 95%, and 95%, respectively. There was, however, a difference in the percentage of draft genomes for which each of the methods failed to make any prediction. SpeciesFinder and KmerFinder were the most robust methods, failing to make predictions for only 0.2% and 0.4% of the draft genomes, respectively. TaxonomyFinder was not able to make a prediction for 1.8% of the draft genomes, and rMLST was not able to for 3.5%. That rMLST was the least robust method is at least partly due to our implementation of the method, where only hits with at least 95% identity and 95% coverage were considered potential matches. On the other hand, the N50 values for the draft genomes that SpeciesFinder and KmerFinder could not make a prediction for were approximately half the size of the corresponding values for rMLST and TaxonomyFinder (data not shown), meaning that the quality of the draft genomes has to be higher for rMLST and TaxonomyFinder to be able to make a prediction. This is in accordance with these methods relying on the presence of many complete genes.
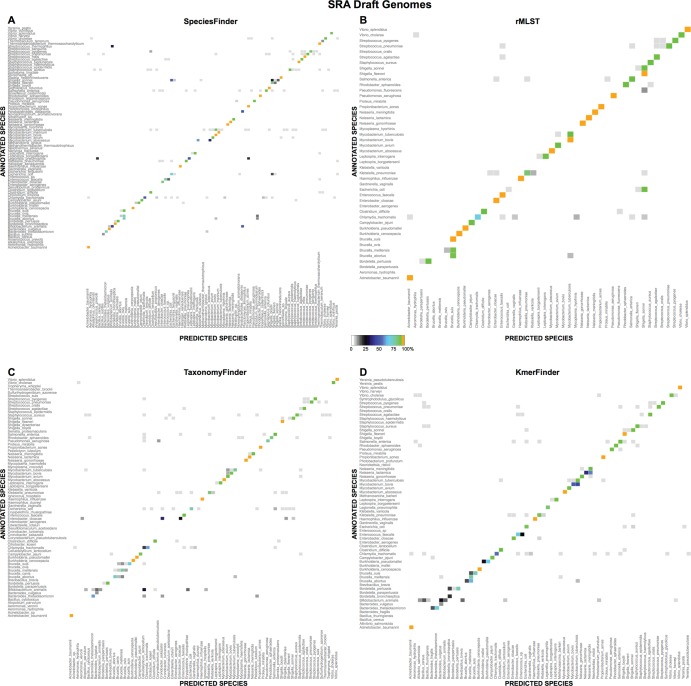
Predictions for the most common species in the SRAdrafts data set are shown in Fig. 4 and in File S2 in the supplemental material. As seen previously when the NCBIdrafts set was used for evaluations, the rMLST method was more consistent in its predictions for a given species than the other methods. For instance, rMLST predicted all 15 Mycobacterium bovis isolates to be M. tuberculosis. As also seen when the NCBIdrafts set was used for evaluations, it is evident that all methods had difficulties distinguishing E. coli from species within the Shigella genus. Furthermore, species within the Brucella genus were often wrongly identified. In particular, it was only TaxonomyFinder that was able to correctly identify most Brucella abortus isolates. Some of the common problems that were obvious when the NCBIdrafts set was used for evaluations were not obvious when the SRAdrafts set was used for evaluations since the problematic species were too scarcely represented here. For instance, there were only five species from the Bacillus genus and only one S. mitis isolate in the SRAdrafts data set. The difference in species distribution between the NCBIdrafts and SRAdrafts sets also explain why SpeciesFinder, TaxonomyFinder, and rMLST all have increased performance on the SRAdrafts set: while more than half of the isolates in the SRAdrafts set belong to the Salmonella, Staphylococcus, and Streptococcus genera, which none of the methods have particular problems identifying, these genera constitute less than 20% of the NCBIdrafts set. Conversely, the NCBIdrafts set contains a high proportion of the problematic species E. coli (8.8%) and the genus Bacillus (10%). The corresponding proportions for SRAdrafts are 3.5% E. coli isolates and 0.05% isolates of the Bacillus genus. Furthermore, the NCBIdrafts set is proportionally more diverse, consisting of 149 species, while the almost 15-times-larger SRAdrafts set consists of only 168 different species.
FIG 4.
Predictions for the most common species in the SRAdrafts data set. For each method, indicated at the top of each panel, the results for a given species is shown only if the method made a prediction for 10 or more isolates annotated as this species or if two or more isolates are predicted as this species.
Performances on short reads from SRA.
Only three of the methods were able to perform species predictions directly on short reads without first assembling the reads. These methods were SpeciesFinder, KmerFinder, and Reads2Type. Their performances on the SRAreads set of 10,407 sets of short reads representing 168 species are shown in Fig. 1C.
Again, the SpeciesFinder method had the poorest performance, with 86% of the isolates being correctly predicted. Reads2Type performed marginally better (87%), while KmerFinder achieved 97% correct reads.
Figure 2C illustrates the overlap in predictions between the three methods, while predictions for the most common species are shown in Fig. S2 in the supplemental material. In general, the results correspond to those observed for the SRAdrafts set.
Speed.
The speed of the methods was evaluated on a subset of draft genomes and short reads as described in Materials and Methods (Table 2). Since the actual speed experienced by the user will depend on a number of factors, for instance, the network bandwidth capacity of the client computer and the number of jobs queued at the server, the relative speed of the different methods in comparison to each other is more relevant than the absolute speed.
TABLE 2.
Speed of the tested methods
| Method | Speed (mm:ss) on:a |
|
|---|---|---|
| Draft genomes | Short reads | |
| SpeciesFinder | 00:13 | 3:14 |
| Reads2Type | NA | 1:20 |
| rMLST | 00:45 | NA |
| TaxonomyFinder | 11:33 | NA |
| KmerFinder | 00:09 | 03:10 |
NA, not applicable.
DISCUSSION
In the present study, we trained five different methods for prokaryotic species identification on a common data set and evaluated their performance on three data sets of draft genomes or short sequence reads.
The SpeciesFinder method is based on the 16S rRNA gene, which has served as the backbone of prokaryotic systematics since 1977 (1). Accordingly, sequencing of the 16S rRNA gene is a well-established method for identification of prokaryotes and has, in all likelihood, been used for annotating some of the isolates in the training and evaluation sets. In the light of this potential advantage of the SpeciesFinder method over the other methods, it is noteworthy that it had the lowest performance on all evaluation sets. Previous studies, however, have also pointed to the many limitations of the 16S rRNA gene for taxonomic purposes (5–9). Examples, which are also observed in this study, include its inadequacy for the delineation of species within the Borrelia burgdorferi sensu lato complex and the Mycobacterium tuberculosis complex (35). Similarly, in silico studies of the applicability of the 16S rRNA gene for the identification of medically important bacteria led to the authors concluding that although the method is useful for identification to the genus level, it is able to identify only 62% of anaerobic bacteria (36) and less than 30% of aerobic bacteria (37) confidently to the species level.
The performance of SpeciesFinder was surpassed only marginally by Reads2Type. This is not surprising since the two methods are conceptually very similar: SpeciesFinder utilizes the entire 16S rRNA gene of approximately 1,540 nucleotides, while for most species, Reads2Type searches for species-specific 50-mers in the same gene. In terms of its future usability, Reads2Type has, however, one advantage over the other methods: like most of the other methods it is available as a web server, but uniquely it does not require the read data to be uploaded to the server. Instead, a small 50-mer database is transferred to the user's computer, and all computations are performed there. As a result, bottleneck problems on the server are avoided, and the data transfer is minimized, which may be particularly advantageous for users with limited Internet access.
While SpeciesFinder and Reads2Type sample only one locus, the rMLST method samples up to 53 loci—all ribosomal genes located to the chromosome of the bacteria. Evaluating on the data set of SRA draft genomes, rMLST, TaxonomyFinder, and KmerFinder performed equally well. However, on the more diverse and difficult set of NCBI draft genomes, the rMLST method performed only marginally better than SpeciesFinder and significantly worse than TaxonomyFinder and KmerFinder. In particular, the rMLST method consistently made incorrect identifications of a number of closely related species, e.g., Y. pestis versus Y. pseudotuberculosis (38) and M. tuberculosis versus M. bovis (39). Also, rMLST consistently predicted the human pathogen B. anthracis to be B. thuringiensis. The latter is used extensively as a biological pesticide and is generally not considered harmful for humans. B. anthracis and B. thuringiensis are both members of the B. cereus group and genetically very similar, with most of the disease and host specificity being attributable to their plasmid content (40, 41). It has even been suggested that all members of the B. cereus group should be considered to be B. cereus and only subsequently be differentiated by their plasmids (42). Hence, in concordance with rMLST sampling only chromosomal, core genes, it is not surprising that the method fails to distinguish these isolates. A similar example is given by the rMLST method identifying all E. coli isolates as Shigella sonnei. Although Shigella sp. isolates have been rewarded their own genus, the separation of the genus from Escherichia spp. is mainly historical (43–45). To be sure, some of the mistakes commonly made by rMLST as well as the other methods highlight taxonomic taxa that are intrinsically difficult to distinguish due to a suboptimal initial classification. Although Shigella has for several years been considered a substrain of E. coli, the practical implications of renaming it are considered insurmountable. It should also be noted that the rMLST method was not developed for usage with a fixed training set but, rather, with all known alleles. Accordingly, the performance of the method is expected to improve with increased size of the reference rMLST database, which is currently expanding rapidly (Keith Jolley, Department of Zoology, University of Oxford, United Kingdom, personal communication).
The TaxonomyFinder method was the second most accurate method on the set of NCBI draft genomes and performed in the top for the SRA drafts set. In contrast to the other methods, it does not work directly on the nucleotide sequence of the isolates but, rather, on the proteome, utilizing functional protein domain profiles for the species prediction. It was the slowest of the tested methods, but in return for the extra time, the user is rewarded with an annotated genome.
The KmerFinder method performs its predictions on the basis of cooccurring k-mers, regardless of their location in the chromosome. It had the overall highest accuracy, worked on complete or draft genomes as well as short reads, and was found to be very robust as well as fast. Furthermore, the KmerFinder method holds promise for future improvements as the implementation used for this study was very simple. Only the raw number of cooccurring k-mers between the query and reference genome was considered although a parallel analysis indicated that the performance could be improved even further if more sophisticated measures were used, also taking into account the total number of k-mers in the query and reference genome. KmerFinder took approximately 9 s per query genome, which makes it the fastest of the tested methods. To test the general applicability of sampling the entire genome and not preselected genes or sets of genes for the species prediction, we also implemented a whole-genome BLAST-based method. The method used hit aggregation of significant matches between the query genome and all genomes in the common training set. As the final prediction, the species for which the query genome had the most bases matched was selected. The performance of this whole-genome BLAST-based method was tested on the NCBIdrafts and SRAdrafts evaluation sets and found to be very similar to that of KmerFinder (see File S2 in the supplemental material). The method was, however, almost 20 times slower than KmerFinder, taking approximately 3 min per genome.
It has previously been noted that some of the isolates present in public databases and, hence, used in this study, are wrongly annotated (17, 46, 47). Based on the current study, it is likely that at least the six isolates from the NCBIdrafts set that all methods identified as something other than the annotated species are wrongly annotated or, alternatively, most closely related to an isolate in the common training data that is wrongly annotated. In agreement with this, one of the isolates has indeed been reannotated since we initially downloaded the data. Of the remaining five isolates, two B. cereus isolates were found to be most closely related to the Bacillus weihenstephanensis strain KBAB4 of the common training set. This strain is the single representative of the species in the public database and not the type strain. Hence, there is no guarantee that the sequenced strain represents the named taxon (48). The same is the case for the Clostridium botulinum strain C Eklund, which is predicted to be a Clostridium novyi based on its close resemblance to C. novyi strain NT of the training set. Clostridium novyi strain NT is the only representative of this species in the database and not the type strain. Obviously, all the evaluated methods are highly dependent on the size and the accuracy of the set of genomes that they are trained on. Accordingly, all methods have the potential to improve their performance in the future when more genomes become available and when the present mistakes in the public databases are corrected. Another way to ensure future improvement is to combine the individual predictions of the methods and let the final predicted species of a query genome be decided by a majority vote. We are currently planning to implement such a system.
In the current study, we included only species in the evaluation sets which were also present in the training set. We have hence not tested how the methods would perform when presented with a species not included in the training set. SpeciesFinder searches for the closest match in the query genome to a database of 16S rRNA genes. If the species of the query genome is not represented in the database, the closest match is likely to be of a closely related species, but the method will also test if the percent identity and coverage of the 16S rRNA gene are above 98% and mark the prediction as “failed” if the match is below this threshold. The rMLST method searches for closest matches in a database of 53 different ribosomal genes. In our implementation, the method will not provide an output if the percent identity and coverage of the matches are below a threshold of 95%, and hence it will be able to select only a closely related species for species that are not represented in the training set. Other implementations of the rMLST method, however, would not necessarily have this limitation. The TaxonomyFinder method uses species- or phylum-specific protein profiles and would hence identify the correct phylum if the species of the query genome was not in the training set. Along with the predicted species, the KmerFinder outputs the number of cooccurring k-mers that the selection was based on. A high number of k-mers indicates that the identification is probable, while low numbers of k-mers indicate that the predicted species is likely to be a related species and that the actual species is not in the training data. Further investigations would be necessary to identify a threshold for the number of k-mers to make this distinction.
While some taxonomists consider the goal of bacterial taxonomy to “mirror the order of nature and describe the evolutionary order back to the origin of life” (6, 49), a more pragmatic and applied view is likely to be advantageous for epidemiological purposes, where most outbreaks last less than 6 months. The number of prokaryotic genomes in public databases is currently sufficiently high to replace theoretical views of which loci to sample for optimal species identification by actual testing of how different approaches perform. One locus (the 16S rRNA gene) was initially used for sequenced-based examination of relationships between bacteria, and when the approach was found to have limitations, more loci were added in MLST and multilocus sequence analysis (MLSA) (50, 51). The addition of still more loci has been suggested for improving MLSA even further (16, 35). This study suggests that an optimal approach should not be limited to a finite number of genes but, rather, look at the entire genome.
Conclusion.
The 16S rRNA gene has served prokaryotic taxonomy well for more than 30 years, but the emergence of second- and third-generation sequencing technologies enables the use of WGS data with the potential of higher resolution and more phylogenetically accurate classifications. Methods that sample the entire genome, not just core genes located to the chromosome, seem particularly well suited for taking up the baton.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Center for Genomic Epidemiology at the Technical University of Denmark and funded by grant 09-067103/DSF from the Danish Council for Strategic Research.
We are grateful to John Damm Sørensen for excellent technical assistance. We are grateful to Keith Jolley, Department of Zoology, University of Oxford, United Kingdom, for providing us with the rMLST genes for the genomes of the training data.
Footnotes
Published ahead of print 26 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02981-13.
REFERENCES
- 1.Fox GE, Peckman KJ, Woese CE. 1977. Comparative cataloging of 16S ribosomal ribonucleic acid: molecular approach to procaryotic systematics. Int. J. Syst. Evol. Bacteriol. 27:44–57. 10.1099/00207713-27-1-44 [DOI] [Google Scholar]
- 2.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72:5069–5072. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glockner FO. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35:7188–7196. 10.1093/nar/gkm864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363–1371. 10.1093/nar/gkh293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tindall BJ, Rossello-Mora R, Busse HJ, Ludwig W, Kampfer P. 2010. Notes on the characterization of prokaryote strains for taxonomic purposes. Int. J. Syst. Evol. Microbiol. 60:249–266. 10.1099/ijs.0.016949-0 [DOI] [PubMed] [Google Scholar]
- 6.Kampfer P. 2012. Systematics of prokaryotes: the state of the art. Antonie Van Leeuwenhoek 101:3–11. 10.1007/s10482-011-9660-4 [DOI] [PubMed] [Google Scholar]
- 7.Tindall BJ, Schneider S, Lapidus A, Copeland A, Glavina Del Rio T, Nolan M, Lucas S, Chen F, Tice H, Cheng JF, Saunders E, Bruce D, Goodwin L, Pitluck S, Mikhailova N, Pati A, Ivanova N, Mavrommatis K, Chen A, Palaniappan K, Chain P, Land M, Hauser L, Chang YJ, Jeffries CD, Brettin T, Han C, Rohde M, Goker M, Bristow J, Eisen JA, Markowitz V, Hugenholtz P, Klenk HP, Kyrpides NC, Detter JC. 2009. Complete genome sequence of Halomicrobium mukohataei type strain (arg-2). Stand. Genomic Sci. 1:270–277. 10.4056/sigs.42644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walcher M, Skvoretz R, Montgomery-Fullerton M, Jonas V, Brentano S. 2013. Description of an unusual Neisseria meningitidis isolate containing and expressing Neisseria gonorrhoeae-specific 16S rRNA gene sequences. J. Clin. Microbiol. 51:3199–3206. 10.1128/JCM.00309-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klenk HP, Goker M. 2010. En route to a genome-based classification of Archaea and Bacteria? Syst. Appl. Microbiol. 33:175–182. 10.1016/j.syapm.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 10.Koser CU, Ellington MJ, Cartwright EJ, Gillespie SH, Brown NM, Farrington M, Holden MT, Dougan G, Bentley SD, Parkhill J, Peacock SJ. 2012. Routine use of microbial whole genome sequencing in diagnostic and public health microbiology. PLoS Pathog. 8:e1002824. 10.1371/journal.ppat.1002824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snel B, Bork P, Huynen MA. 1999. Genome phylogeny based on gene content. Nat. Genet. 21:108–110. 10.1038/5052 [DOI] [PubMed] [Google Scholar]
- 12.House CH, Fitz-Gibbon ST. 2002. Using homolog groups to create a whole-genomic tree of free-living organisms: an update. J. Mol. Evol. 54:539–547. 10.1007/s00239-001-0054-5 [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Doolittle RF, Bourne PE. 2005. Phylogeny determined by protein domain content. Proc. Natl. Acad. Sci. U. S. A. 102:373–378. 10.1073/pnas.0408810102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukjancenko O, Thomsen MC, Larsen MV, Ussery DW. 2013. PanFunPro: PAN-genome analysis based on FUNctional PROfiles. F1000Research 2:265. 10.12688/f1000research.2-265.v1 [DOI] [Google Scholar]
- 15.Ciccarelli FD, Doerks T, von Mering C, Creevey CJ, Snel B, Bork P. 2006. Toward automatic reconstruction of a highly resolved tree of life. Science 311:1283–1287. 10.1126/science.1123061 [DOI] [PubMed] [Google Scholar]
- 16.Jolley KA, Bliss CM, Bennett JS, Bratcher HB, Brehony C, Colles FM, Wimalarathna H, Harrison OB, Sheppard SK, Cody AJ, Maiden MC. 2012. Ribosomal multilocus sequence typing: universal characterization of bacteria from domain to strain. Microbiology 158:1005–1015. 10.1099/mic.0.055459-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett JS, Jolley KA, Earle SG, Corton C, Bentley SD, Parkhill J, Maiden MC. 2012. A genomic approach to bacterial taxonomy: an examination and proposed reclassification of species within the genus Neisseria. Microbiology 158:1570–1580. 10.1099/mic.0.056077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cody AJ, McCarthy ND, Jansen van Rensburg M, Isinkaye T, Bentley SD, Parkhill J, Dingle KE, Bowler IC, Jolley KA, Maiden MC. 2013. Real-time genomic epidemiological evaluation of human campylobacter isolates by use of whole-genome multilocus sequence typing. J. Clin. Microbiol. 51:2526–2534. 10.1128/JCM.00066-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasman H, Saputra D, Sicheritz-Ponten T, Lund O, Svendsen CA, Frimodt-Moller N, Aarestrup FM. 2014. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples. J. Clin. Microbiol. 52:139–146. 10.1128/JCM.02452-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Euzeby JP. 1997. List of Bacterial Names with Standing in Nomenclature: a folder available on the Internet. Int. J. Syst. Bacteriol. 47:590–592. 10.1099/00207713-47-2-590 [DOI] [PubMed] [Google Scholar]
- 22.Kodama Y, Shumway M, Leinonen R. 2012. The Sequence Read Archive: explosive growth of sequencing data. Nucleic Acids Res. 40:D54–56. 10.1093/nar/gkr854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Ponten T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 50:1355–1361. 10.1128/JCM.06094-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829. 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JR, Koren S, Sutton G. 2010. Assembly algorithms for next-generation sequencing data. Genomics 95:315–327. 10.1016/j.ygeno.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. 2007. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35:3100–3108. 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Durbin R. 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26:589–595. 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29:644–652. 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kent WJ. 2002. BLAT—the BLAST-like alignment tool. Genome Res. 12:656–664. 10.1101/gr.229202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer EL, Eddy SR, Bateman A, Finn RD. 2012. The Pfam protein families database. Nucleic Acids Res. 40:D290–301. 10.1093/nar/gkr1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haft DH, Selengut JD, White O. 2003. The TIGRFAMs database of protein families. Nucleic Acids Res. 31:371–373. 10.1093/nar/gkg128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gough J, Karplus K, Hughey R, Chothia C. 2001. Assignment of homology to genome sequences using a library of hidden Markov models that represent all proteins of known structure. J. Mol. Biol. 313:903–919. 10.1006/jmbi.2001.5080 [DOI] [PubMed] [Google Scholar]
- 33.Li W, Godzik A. 2006. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22:1658–1659. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- 34.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almeida LA, Araujo R. 2013. Highlights on molecular identification of closely related species. Infect. Genet. Evol. 13:67–75. 10.1016/j.meegid.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 36.Woo PC, Chung LM, Teng JL, Tse H, Pang SS, Lau VY, Wong VW, Kam KL, Lau SK, Yuen KY. 2007. In silico analysis of 16S ribosomal RNA gene sequencing-based methods for identification of medically important anaerobic bacteria. J. Clin. Pathol. 60:576–579. 10.1136/jcp.2006.038653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teng JL, Yeung MY, Yue G, Au-Yeung RK, Yeung EY, Fung AM, Tse H, Yuen KY, Lau SK, Woo PC. 2011. In silico analysis of 16S rRNA gene sequencing based methods for identification of medically important aerobic Gram-negative bacteria. J. Med. Microbiol. 60:1281–1286. 10.1099/jmm.0.027805-0 [DOI] [PubMed] [Google Scholar]
- 38.Achtman M, Zurth K, Morelli G, Torrea G, Guiyoule A, Carniel E. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. U. S. A. 96:14043–14048. 10.1073/pnas.96.24.14043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sreevatsan S, Pan X, Stockbauer KE, Connell ND, Kreiswirth BN, Whittam TS, Musser JM. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. U. S. A. 94:9869–9874. 10.1073/pnas.94.18.9869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasko DA, Altherr MR, Han CS, Ravel J. 2005. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 29:303–329. 10.1016/j.fmrre.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 41.Jimenez G, Urdiain M, Cifuentes A, Lopez-Lopez A, Blanch AR, Tamames J, Kampfer P, Kolsto AB, Ramon D, Martinez JF, Codoner FM, Rossello-Mora R. 2013. Description of Bacillus toyonensis sp. nov., a novel species of the Bacillus cereus group, and pairwise genome comparisons of the species of the group by means of ANI calculations. Syst. Appl. Microbiol. 36:383–391. 10.1016/j.syapm.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 42.Helgason E, Okstad OA, Caugant DA, Johansen HA, Fouet A, Mock M, Hegna I, Kolsto AB. 2000. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—one species on the basis of genetic evidence. Appl. Environ. Microbiol. 66:2627–2630. 10.1128/AEM.66.6.2627-2630.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lan R, Reeves PR. 2002. Escherichia coli in disguise: molecular origins of Shigella. Microbes Infect. 4:1125–1132. 10.1016/S1286-4579(02)01637-4 [DOI] [PubMed] [Google Scholar]
- 44.Lukjancenko O, Wassenaar TM, Ussery DW. 2010. Comparison of 61 sequenced Escherichia coli genomes. Microb. Ecol. 60:708–720. 10.1007/s00248-010-9717-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karaolis DK, Lan R, Reeves PR. 1994. Sequence variation in Shigella sonnei (Sonnei), a pathogenic clone of Escherichia coli, over four continents and 41 years. J. Clin. Microbiol. 32:796–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. 2007. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 57:81–91. 10.1099/ijs.0.64483-0 [DOI] [PubMed] [Google Scholar]
- 47.Yarza P, Richter M, Peplies J, Euzeby J, Amann R, Schleifer KH, Ludwig W, Glockner FO, Rossello-Mora R. 2008. The All-Species Living Tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst. Appl. Microbiol. 31:241–250. 10.1016/j.syapm.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 48.Richter M, Rossello-Mora R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. U. S. A. 106:19126–19131. 10.1073/pnas.0906412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kampfer P, Glaeser SP. 2012. Prokaryotic taxonomy in the sequencing era—the polyphasic approach revisited. Environ. Microbiol. 14:291–317. 10.1111/j.1462-2920.2011.02615.x [DOI] [PubMed] [Google Scholar]
- 50.Gevers D, Cohan FM, Lawrence JG, Spratt BG, Coenye T, Feil EJ, Stackebrandt E, Van de Peer Y, Vandamme P, Thompson FL, Swings J. 2005. Opinion: re-evaluating prokaryotic species. Nat. Rev. Microbiol. 3:733–739. 10.1038/nrmicro1236 [DOI] [PubMed] [Google Scholar]
- 51.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140–3145. 10.1073/pnas.95.6.3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.