Abstract
The Candida parapsilosis complex is composed of Candida parapsilosis sensu stricto, Candida orthopsilosis, Candida metapsilosis, and the closely related species Lodderomyces elongisporus. An exon-primed intron-crossing PCR assay was developed here to distinguish the members of the species complex on the basis of the distinct sizes of amplicons, and Candida orthopsilosis and Candida metapsilosis were further discriminated by restriction enzyme analysis.
TEXT
The Candida parapsilosis complex has been reported as the second most common species isolated from patients with invasive candidiasis such as candidemia or superficial candidiasis in many studies (1–5). On the basis of genetic analysis, the C. parapsilosis complex consists of three genetically distinct species, namely, C. parapsilosis sensu stricto, C. orthopsilosis, and C. metapsilosis, which are phenotypically indistinguishable from each other (6). The three species exhibit differences in epidemiology, virulence, biofilm formation, and antifungal susceptibility (7–17). C. metapsilosis has been demonstrated to be the least virulent member of the complex (7, 8). Lodderomyces elongisporus, a closely related species, has also been verified as a cause of infection in case reports and epidemiologic studies (2, 18–20). Thus, a four-species complex has been suggested to replace the three-species complex (19). Thus, it is clinically important to distinguish among the members of this species complex.
So far, various molecular approaches, such as PCR-restriction fragment length polymorphism (RFLP), random amplified polymorphic DNA, real-time PCR, PCR with specific primers, matrix-assisted laser desorption ionization-time of flight mass spectrometry, sequencing analysis, and a simple PCR developed recently, were established to differentiate the former three-species complex (6, 21–27). Although turquoise blue colonies on BBL CHROMagar Candida medium (Becton, Dickinson and Company, Sparks, MD) or sequence analysis could be used to distinguish L. elongisporus from the three-species complex (19), no other molecular tool has been well established. PCR analyses based on intron size differences or intron loss have been used to easily differentiate closely related clinically important yeast species, and exon-primed intron-crossing (EPIC) PCR analyses of intron length polymorphisms were also widely used as a molecular typing tool for multiple eukaryotes (28–31). Herein, an EPIC PCR combined with restriction enzyme analysis was developed to differentiate the present four-species complex and verified to be a simple, inexpensive, and reliable method.
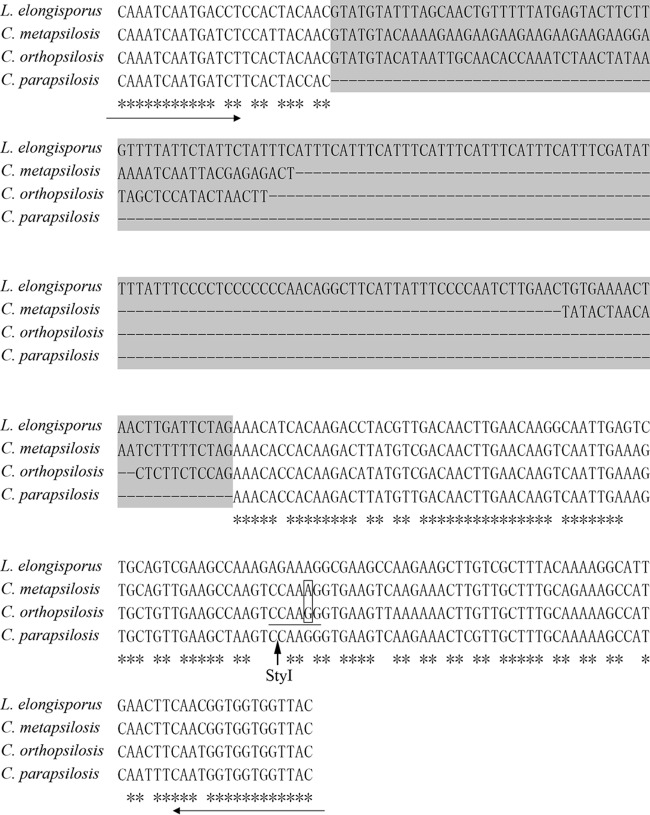
Genomic sequence data for the type strains C. parapsilosis CDC 317, C. orthopsilosis Co90-125, and L. elongisporus YB-4239 (available at http://www.ncbi.nlm.nih.gov/genome) were aligned and analyzed by LAGAN as described previously (28). C. metapsilosis was not included in the analysis because of unavailability of the genomic data online until now. The gene for manganese superoxide dismutase (MnSOD), a phylogenetic marker previously used to identify closely related fungal species (32), was chosen because of its marked difference in intron length among C. parapsilosis CDC 317, C. orthopsilosis Co90-125, and L. elongisporus YB-4239, and its homologous sequence in C. metapsilosis MCO448 was cloned and sequenced. A slight difference in intron length between C. metapsilosis and C. orthopsilosis was found, which is consistent with the fact that C. metapsilosis is more closely related to C. orthopsilosis than to the others (6). We calculated the intron length of one allele of the MnSOD gene as 169 bp for L. elongisporus YB-4239, 79 bp for C. metapsilosis MCO448, and 64 bp for C. orthopsilosis Co 90-125 and established intron loss by C. parapsilosis CDC 317 (Fig. 1).
FIG 1.
Multiple-sequence alignment of fragments of the MnSOD gene orthologs in type strains. The intron within the gene fragment is on a gray background. Intron loss and size differences are indicated by dashes. The positions of the primers used are indicated by the horizontal arrows. The cutting site of the StyI enzyme is indicated by the vertical arrow. The sequence recognized by StyI is underlined. The nucleotide difference between C. metapsilosis and C. orthopsilosis in the recognition site is boxed.
A degenerate primer pair, MnSODF (5′ GCTTTAGTGGACAAATCAATGAYCT 3′) and MnSODR (5′ AGTTGATGTAACCACCACCRTTG 3′), was designed on the basis of the exon region of the MnSOD gene. PCR was performed in a final volume of 50 μl containing 50 ng of DNA; 1× PCR buffer with 2 mM MgSO4; 0.2 mM each dATP, dCTP, dGTP, and dTTP; 0.2 μM each primer; and 2.5 U of Taq polymerase. PCR was performed in an Eppendorf Mastercycler at 94°C for 5 min for initial denaturation, followed by 30 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 20 s and a final extension step of 72°C for 6 min. PCR products were separated on a 2.0% (wt/vol) agarose gel at 90 V for 50 min. All PCRs were conducted in duplicate. PCR products with sizes similar to those of C. metapsilosis and C. orthopsilosis were further discriminated by restriction digestion with FastDigest enzyme StyI with the cutting site located in the exon region of the C. orthopsilosis gene but not C. metapsilosis (Fig. 1). Restriction digestions were performed according to the manufacturer's instructions (Fermentas, Vilnius, Lithuania), and the reaction mixture was incubated at 37°C for 20 min before separation of the product on a 2.0% (wt/vol) agarose gel at 90 V for 50 min.
A total of 210 strains belonging to the C. parapsilosis complex (112 C. parapsilosis isolates, 56 C. metapsilosis isolates, 30 C. orthopsilosis isolates, and 12 L. elongisporus isolates) were involved in the assay. Among them, nonreference strains were identified as belonging to one of the four species by sequencing of the internal transcribed spacer region as previously described (28). For detailed information regarding the strains tested, see Table S1 in the supplemental material. Genomic DNA was extracted with the MasterPure Yeast DNA Purification kit (Epicentre Biotechnologies, Madison, WI) according to the manufacturer's instructions. Additionally, reference strains of C. albicans, C. glabrata, C. tropicalis, C. krusei, C. guilliermondii, C. lusitaniae, and C. famata were chosen to verify the specificity of the primer pair.
As expected, electrophoretic analysis revealed an approximately 340-bp amplicon for L. elongisporus, a 250-bp amplicon for C. metapsilosis, a 235-bp amplicon for C. orthopsilosis, and a 171-bp amplicon for C. parapsilosis (Fig. 2). No StyI cutting site was found in any of the amplicons from C. metapsilosis isolates, but amplicons from all of the C. orthopsilosis isolates were cut into 165- and 70-bp fragments in restriction enzyme analyses (Fig. 2). All of the isolates belonging to the four-species complex involved were correctly identified, and none of the other Candida species tested produced an amplification product in this assay.
FIG 2.
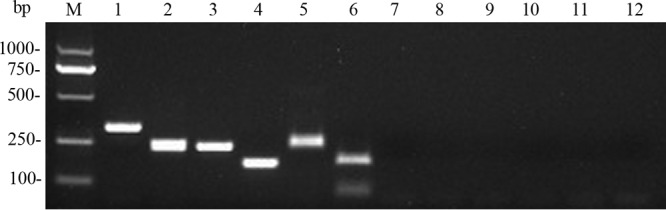
Agarose gel electrophoresis of PCR and enzyme digestion products. Lanes: M, DL2000 ladder; 1, L. elongisporus CBS10974; 2, C. metapsilosis MCO448; 3, C. orthopsilosis Y27733; 4, C. parapsilosis ATCC 22019; 5, C. metapsilosis MCO448 enzyme digestion product; 6, C. orthopsilosis Y27733 enzyme digestion product; 7, C. albicans ATCC 10231; 8, C. tropicalis ATCC 750; 9, C. guilliermondii CBS 2030; 10, C. glabrata ATCC 90030; 11, C. krusei ATCC 6258; 12, negative control.
It was reported that C. parapsilosis sensu stricto was the predominant species in the group, followed by C. orthopsilosis and C. metapsilosis, which together accounted for fewer than 10% of the C. parapsilosis complex isolates from infections (16). In addition, L. elongisporus was the least frequently encountered species in this group in clinical samples (2, 16). Unlike the PCR-RFLP analyses reported in other studies (6, 21, 23), only strains of C. orthopsilosis and C. metapsilosis that are difficult to discriminated by agarose gel electrophoresis are required to be further distinguished by rapid restriction enzyme analysis. Most of the strains can be identified without the further restriction enzyme analysis because C. orthopsilosis and C. metapsilosis are less commonly recovered from clinical specimens. The proportion of L. elongisporus isolates in clinical samples may be underestimated because of the unavailability of a suitable molecular tool, and our approach can solve this problem. This approach could be used to distinguish the members of the four-species complex among untyped Candida isolates or strains routinely identified as C. parapsilosis in a clinical laboratory. Finally, we report here a simple PCR assay with restriction enzyme analysis that performed well in discriminating among the members of the present four-species complex of C. parapsilosis sensu lato.
Nucleotide sequence accession number.
The sequence of the MnSOD gene fragment of reference strain MCO448 was deposited in GenBank under accession number KF974528.
Supplementary Material
ACKNOWLEDGMENTS
We thank Arianna Tavanti (University of Pisa, Pisa, Italy), Dominique Sanglard and Jamel Eddouzi (University Hospital Lausanne and University Hospital Center, Lausanne, Switzerland), James Swezey (ARS Culture Collection), Orazio Romeo (University of Messina, Messina, Italy), Donna MacCallum (University of Aberdeen, Aberdeen, United Kingdom), Oliver Bader (University Medical Center Göttingen, Göttingen, Germany), László Majoros (University of Debrecen, Debrecen, Hungary), Elisa Borghi (Università degli Studi di Milano, Milan, Italy), Attila Gacser (University of Szeged, Szeged, Hungary), and Jozef Nosek (Comenius University, Bratislava, Slovak Republic) for generously contributing strains for this study.
This work was supported by grants from the National Key Basic Research Program of China (2013CB531601, 2013531606), the Major Infectious Disease Fund (2013ZX1000 4612), the Shanghai Science and Technology Commission Fund (10dz2220100), and the Shanghai Key Laboratory of Molecular Medical Mycology Fund (20110001).
Footnotes
Published ahead of print 12 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00105-14.
REFERENCES
- 1.Won EJ, Shin JH, Lee K, Kim MN, Lee HS, Park YJ, Joo MY, Kim SH, Shin MG, Suh SP, Ryang DW. 2013. Accuracy of species-level identification of yeast isolates from blood cultures from 10 university hospitals in South Korea by use of the matrix-assisted laser desorption ionization-time of flight mass spectrometry-based Vitek MS system. J. Clin. Microbiol. 51:3063–3065. 10.1128/JCM.00945-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Xiao M, Chen SC, Kong F, Sun ZY, Liao K, Lu J, Shao HF, Yan Y, Fan H, Hu ZD, Chu YZ, Hu TS, Ni YX, Zou GL, Xu YC. 2012. In vitro susceptibilities of yeast species to fluconazole and voriconazole as determined by the 2010 National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study. J. Clin. Microbiol. 50:3952–3959. 10.1128/JCM.01130-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassetti M, Merelli M, Righi E, Diaz-Martin A, Rosello EM, Luzzati R, Parra A, Trecarichi EM, Sanguinetti M, Posteraro B, Garnacho-Montero J, Sartor A, Rello J, Tumbarello M. 2013. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J. Clin. Microbiol. 51:4167–4172. 10.1128/JCM.01998-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng X, Ling B, Yang G, Yu X, Ren D, Yao Z. 2012. Prevalence and distribution profiles of Candida parapsilosis, Candida orthopsilosis and Candida metapsilosis responsible for superficial candidiasis in a Chinese university hospital. Mycopathologia 173:229–234. 10.1007/s11046-011-9496-5 [DOI] [PubMed] [Google Scholar]
- 5.Cisterna R, Ezpeleta G, Telleria O. 2010. Nationwide sentinel surveillance of bloodstream Candida infections in 40 tertiary care hospitals in Spain. J. Clin. Microbiol. 48:4200–4206. 10.1128/JCM.00920-10 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Tavanti A, Davidson AD, Gow NA, Maiden MC, Odds FC. 2005. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43:284–292. 10.1128/JCM.43.1.284-292.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertini A, De Bernardis F, Hensgens LA, Sandini S, Senesi S, Tavanti A. 2013. Comparison of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis adhesive properties and pathogenicity. Int. J. Med. Microbiol. 303:98–103. 10.1016/j.ijmm.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 8.Németh T, Toth A, Szenzenstein J, Horvath P, Nosanchuk JD, Grozer Z, Toth R, Papp C, Hamari Z, Vagvolgyi C, Gacser A. 2013. Characterization of virulence properties in the C. parapsilosis sensu lato species. PLoS One 8:e68704. 10.1371/journal.pone.0068704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treviño-Rangel RJ, Gonzalez JG, Gonzalez GM. 2013. Aspartyl proteinase, phospholipase, esterase and hemolysin activities of clinical isolates of the Candida parapsilosis species complex. Med. Mycol. 51:331–335. 10.3109/13693786.2012.712724 [DOI] [PubMed] [Google Scholar]
- 10.de Toro M, Torres MJ, Maite R, Aznar J. 2011. Characterization of Candida parapsilosis complex isolates. Clin. Microbiol. Infect. 17:418–424. 10.1111/j.1469-0691.2010.03302.x [DOI] [PubMed] [Google Scholar]
- 11.Cantón E, Espinel-Ingroff A, Peman J, Del CL. 2010. In vitro fungicidal activities of echinocandins against Candida metapsilosis, C. orthopsilosis, and C. parapsilosis evaluated by time-kill studies. Antimicrob. Agents Chemother. 54:2194–2197. 10.1128/AAC.01538-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romeo O, Delfino D, Costanzo B, Cascio A, Criseo G. 2012. Molecular characterization of Italian Candida parapsilosis isolates reveals the cryptic presence of the newly described species Candida orthopsilosis in blood cultures from newborns. Diagn. Microbiol. Infect. Dis. 72:234–238. 10.1016/j.diagmicrobio.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Effron G, Cantón E, Peman J, Dilger A, Roma E, Perlin DS. 2012. Epidemiology and echinocandin susceptibility of Candida parapsilosis sensu lato species isolated from bloodstream infections at a Spanish university hospital. J. Antimicrob. Chemother. 67:2739–2748. 10.1093/jac/dks271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantón E, Peman J, Quindos G, Eraso E, Miranda-Zapico I, Alvarez M, Merino P, Campos-Herrero I, Marco F, de la Pedrosa EG, Yague G, Guna R, Rubio C, Miranda C, Pazos C, Velasco D. 2011. Prospective multicenter study of the epidemiology, molecular identification, and antifungal susceptibility of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis isolated from patients with candidemia. Antimicrob. Agents Chemother. 55:5590–5596. 10.1128/AAC.00466-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva AP, Miranda IM, Lisboa C, Pina-Vaz C, Rodrigues AG. 2009. Prevalence, distribution, and antifungal susceptibility profiles of Candida parapsilosis, C. orthopsilosis, and C. metapsilosis in a tertiary care hospital. J. Clin. Microbiol. 47:2392–2397. 10.1128/JCM.02379-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockhart SR, Messer SA, Pfaller MA, Diekema DJ. 2008. Geographic distribution and antifungal susceptibility of the newly described species Candida orthopsilosis and Candida metapsilosis in comparison to the closely related species Candida parapsilosis. J. Clin. Microbiol. 46:2659–2664. 10.1128/JCM.00803-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Lopez A, Alastruey-Izquierdo A, Rodriguez D, Almirante B, Pahissa A, Rodriguez-Tudela JL, Cuenca-Estrella M. 2008. Prevalence and susceptibility profile of Candida metapsilosis and Candida orthopsilosis: results from population-based surveillance of candidemia in Spain. Antimicrob. Agents Chemother. 52:1506–1509. 10.1128/AAC.01595-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daveson KL, Woods ML. 2012. Lodderomyces elongisporus endocarditis in an intravenous drug user: a new entity in fungal endocarditis. J. Med. Microbiol. 61:1338–1340. 10.1099/jmm.0.047548-0 [DOI] [PubMed] [Google Scholar]
- 19.Lockhart SR, Messer SA, Pfaller MA, Diekema DJ. 2008. Lodderomyces elongisporus masquerading as Candida parapsilosis as a cause of bloodstream infections. J. Clin. Microbiol. 46:374–376. 10.1128/JCM.01790-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad S, Khan ZU, Johny M, Ashour NM, Al-Tourah WH, Joseph L, Chandy R. 2013. Isolation of Lodderomyces elongisporus from the catheter tip of a fungemia patient in the Middle East. Case Rep. Med. 2013:560406. 10.1155/2013/560406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirhendi H, Bruun B, Schonheyder HC, Christensen JJ, Fuursted K, Gahrn-Hansen B, Johansen HK, Nielsen L, Knudsen JD, Arendrup MC. 2010. Molecular screening for Candida orthopsilosis and Candida metapsilosis among Danish Candida parapsilosis group blood culture isolates: proposal of a new RFLP profile for differentiation. J. Med. Microbiol. 59:414–420. 10.1099/jmm.0.017293-0 [DOI] [PubMed] [Google Scholar]
- 22.Souza AC, Ferreira RC, Goncalves SS, Quindos G, Eraso E, Bizerra FC, Briones MR, Colombo AL. 2012. Accurate identification of Candida parapsilosis (sensu lato) by use of mitochondrial DNA and real-time PCR. J. Clin. Microbiol. 50:2310–2314. 10.1128/JCM.00303-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Effron G, Cantón E, Peman J, Dilger A, Roma E, Perlin DS. 2011. Assessment of two new molecular methods for identification of Candida parapsilosis sensu lato species. J. Clin. Microbiol. 49:3257–3261. 10.1128/JCM.00508-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Pilar Vercher M, Garcia MJ, Cantón E, Peman J, Gomez GM, Gomez EV, Del CAL. 2011. Differentiation of Candida parapsilosis, C. orthopsilosis, and C. metapsilosis by specific PCR amplification of the RPS0 intron. Int. J. Med. Microbiol. 301:531–535. 10.1016/j.ijmm.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 25.Hays C, Duhamel C, Cattoir V, Bonhomme J. 2011. Rapid and accurate identification of species belonging to the Candida parapsilosis complex by real-time PCR and melting curve analysis. J. Med. Microbiol. 60:477–480. 10.1099/jmm.0.026633-0 [DOI] [PubMed] [Google Scholar]
- 26.Quiles-Melero I, Garcia-Rodriguez J, Gomez-Lopez A, Mingorance J. 2012. Evaluation of matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry for identification of Candida parapsilosis, C. orthopsilosis and C. metapsilosis. Eur. J. Clin. Microbiol. Infect. Dis. 31:67–71. 10.1007/s10096-011-1277-z [DOI] [PubMed] [Google Scholar]
- 27.Prandini TH, Theodoro RC, Bruder-Nascimento AC, Scheel CM, Bagagli E. 2013. Analysis of Inteins in the Candida parapsilosis complex for simple and accurate species identification. J. Clin. Microbiol. 51:2830–2836. 10.1128/JCM.00981-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng X, Fu X, Ling B, Wang L, Liao W, Yao Z. 2013. Development of a singleplex PCR assay for rapid identification and differentiation of Cryptococcus neoformans var. grubii, Cryptococcus neoformans var. neoformans, Cryptococcus gattii, and hybrids. J. Clin. Microbiol. 51:1920–1923. 10.1128/JCM.00064-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng X, Fu X, Ling B, Wang L, Liao W, Pan W, Yao Z. 2013. Rapid differentiation of cryptic species within Cryptococcus gattii by a duplex PCR assay. J. Clin. Microbiol. 51:3110–3112. 10.1128/JCM.01455-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enache-Angoulvant A, Guitard J, Grenouillet F, Martin T, Durrens P, Fairhead C, Hennequin C. 2011. Rapid discrimination between Candida glabrata, Candida nivariensis, and Candida bracarensis by use of a singleplex PCR. J. Clin. Microbiol. 49:3375–3379. 10.1128/JCM.00688-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Zhao X, Zhu J, Wu W. 2005. Genome-wide investigation of intron length polymorphisms and their potential as molecular markers in rice (Oryza sativa L.). DNA Res. 12:417–427. 10.1093/dnares/dsi019 [DOI] [PubMed] [Google Scholar]
- 32.Fréalle E, Noel C, Nolard N, Symoens F, Felipe MS, Dei-Cas E, Camus D, Viscogliosi E, Delhaes L. 2006. Manganese superoxide dismutase based phylogeny of pathogenic fungi. Mol. Phylogenet. Evol. 41:28–39. 10.1016/j.ympev.2006.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.