Abstract
Limited information is available about the effects of HIV and subsequent antiretroviral treatment on host-microbe interactions. This study aimed to determine the salivary microbial composition for 10 HIV-seropositive subjects, before and 6 months after highly active antiretroviral therapy (HAART), compared with that for 10 HIV-seronegative subjects. A conventional culture and two culture-independent analyses were used and consistently demonstrated differences in microbial composition among the three sets of samples. HIV-positive subjects had higher levels of total cultivable microbes, including oral streptococci, lactobacilli, Streptococcus mutans, and Candida, in saliva than did HIV-negative subjects. The total cultivable microbial levels were significantly correlated with CD4+ T cell counts. Denaturing gradient gel electrophoresis (DGGE), which compared the overall microbial profiles, showed distinct fingerprinting profiles for each group. The human oral microbe identification microarray (HOMIM) assay, which compared the 16S rRNA genes, showed clear separation among the three sample groups. Veillonella, Synergistetes, and Streptococcus were present in all 30 saliva samples. Only minor changes or no changes in the prevalence of Neisseria, Haemophilus, Gemella, Leptotrichia, Solobacterium, Parvimonas, and Rothia were observed. Seven genera, Capnocytophaga, Slackia, Porphyromonas, Kingella, Peptostreptococcaceae, Lactobacillus, and Atopobium, were detected only in HIV-negative samples. The prevalences of Fusobacterium, Campylobacter, Prevotella, Capnocytophaga, Selenomonas, Actinomyces, Granulicatella, and Atopobium were increased after HAART. In contrast, the prevalence of Aggregatibacter was significantly decreased after HAART. The findings of this study suggest that HIV infection and HAART can have significant effects on salivary microbial colonization and composition.
INTRODUCTION
In 2012, more than 35 million people were living with HIV, and more people than ever received life-saving antiretroviral therapy worldwide (1). The availability of antiretroviral therapy has significantly reduced the number of AIDS-related deaths. Concurrently, people living with HIV are continuously challenged by diseases associated with a compromised host immune system, including opportunistic infections (2). Oropharyngeal candidiasis is the most common oral infection (3), and it can be detected in the early stages of HIV infection (4). This opportunistic infection and others can be a consequence of immune impairment induced by HIV, changes in saliva composition and function (5, 6), the presence of advanced caries lesions (7), and/or progressive periodontal infections (8).
Despite the overall decline in HIV-related deaths, studies have suggested clinical associations between HIV infection and both caries and periodontal diseases; for example, immunocompromised individuals, especially children and young adults, have shown increased prevalence rates of dental caries (7, 9–11) and necrotizing periodontal diseases (12, 13). However, a few studies have shown no differences between HIV-infected and healthy subjects in caries severity, chronic periodontitis, or advanced periodontal diseases (14, 15). Previously, we reported salivary microbial changes in HIV-infected patients (16). Others have observed positive correlations between HIV infection and increased oral Candida colonization (6, 17–19). It has also been suggested that impairment of systemic defense mechanisms by reduction of CD4+ T cells below protective levels and impairment of local immunity by reduction of levels of salivary IgA, defensins, or epithelial cell-mediated cytokines in the saliva might lead to the conversion of commensal Candida to microorganisms with increased pathogenicity, causing an imbalance in the host oral microbial composition and hence increased risk for opportunistic infections (20, 21). Dramatic reductions in oral candidiasis after highly active antiretroviral therapy (HAART) have been observed consistently (22–25); however, the mechanisms underlying host-microbe interactions relative to HIV infection and subsequent HAART, all in the context of oral microbial composition, are not well understood. Currently, a wide range of molecular techniques are available to help identify and characterize microorganisms, including sequencing of 16S rRNA genes using DNA hybridization with custom-designed oligonucleotide probes, fingerprinting of the microbial flora with denaturing gradient gel electrophoresis (DGGE), and other PCR-based techniques.
The present study aimed to evaluate the microbial colonization and composition in samples collected from HIV-positive subjects and healthy controls. We hypothesized that individuals immunocompromised by HIV infection would be at greater risk for increased microbial colonization and diversity than healthy controls, leading to increased prevalence and severity of oral diseases. We also hypothesized that HAART might reverse the HIV-associated changes in the microbial composition in saliva, restoring balance in the oral microbiota and hence improving the oral health of individuals with HIV. A conventional culture method was used to evaluate total cultivable microbes in saliva, and two culture-independent methods based on 16S rRNA were used to determine the effects of HIV infection and HAART on changes in salivary microbial composition. The molecular fingerprints generated by DGGE provided a direct cross-sectional comparison of microbial compositions of the targeted bacterial 16S rRNA gene (26, 27). In addition, the human oral microbe identification microarray (HOMIM) assay enabled us to further distinguish the observed differences in microbial diversity at the microbial genus or species level.
(A preliminary report of this work was presented at the International Association for Dental Research/American Association for Dental Research/Canadian Association for Dental Research 91st General Session and Exhibition, Seattle, WA, 20 to 23 March 2013.)
MATERIALS AND METHODS
Ethics statement.
The study was conducted at two sites, Bellevue Hospital Center and New York University College of Dentistry, and was approved by the Institutional Review Board of the New York University School of Medicine (for the College of Dentistry) and the Institutional Review Board of the New York City Health and Hospital Corporation (for Bellevue Hospital Center). Written informed consent was obtained from all participants.
Study participants.
Twenty subjects were randomly selected from an HIV cohort study (16). Ten subjects who were seropositive for HIV and were HAART naive or off therapy for at least 6 months were recruited prior to the initiation of HAART, which was provided by the New York University Clinical Trial Unit, Bellevue Hospital Center (New York, NY). The other 10 subjects were seronegative for HIV and were recruited from the Bluestone Center for Clinical Research, New York University College of Dentistry. Demographic data (for all subjects) and medical data (for HIV-positive subjects), including age, sex, ethnicity, HIV load, CD4+ and CD8+ T cell counts, and type and date of initiation of antiretroviral medications, were obtained from the medical records; data were collected and evaluated before and 6 months after initiation of HAART. For HIV-negative subjects, seronegative status was confirmed using the OraQuick ADVANCE Rapid HIV-1/2 antibody test.
Oral examination and sample collection.
At the initial evaluation, each subject received a comprehensive oral examination performed by one of two standardized clinical examiners. Caries status was determined at the tooth surface level according to criteria modified from National Health and Nutrition Examination Survey III, as well as an index of decayed and filled teeth (DFT) and decayed and filled tooth surfaces (DFS) (28). Periodontal examinations were performed for the 6 Ramfjord index teeth (29), at 6 sites on each tooth (mesiobuccal, buccal, distobuccal, distolingual, lingual, and mesiolingual sites). Periodontal bleeding on probing (BOP) was recorded as a dichotomous outcome for each site and was deemed positive if bleeding occurred within 15 s after the assessment of probing depth.
Stimulated whole-saliva samples were collected for this study. In order to minimize potential variations, saliva sample collection was conducted in the morning, if possible. Each subject was asked to refrain from eating or drinking for at least 2 h prior to sample collection. After resting for 5 min with no talking, subjects were asked to rinse their mouths with sterile water, chew on a piece of paraffin wax for 30 s, and expectorate directly into a graduated 50-ml sample collection tube on ice. A portion of the saliva sample (2 ml) was transferred immediately, on ice, to a microbiology laboratory (New York University College of Dentistry) and was processed within 2 h. For HIV-seropositive subjects, a second saliva sample was collected 6 months after the initiation of HAART.
Quantitative evaluations of oral microbial colonization and microbial diversity were performed with 3 methods, namely, conventional culture, denaturing gradient gel electrophoresis (DGGE), and the human oral microbe identification microarray (HOMIM) assay.
Microbial cultivation.
Saliva samples were sonicated (30 s) and diluted (10−1 to 10−4). The diluted samples (50 μl) were plated on selective media for cultivation of Streptococcus mutans (mitis salivarius agar with potassium tellurite-bacitracin; Difco Laboratories Inc., Detroit, MI) (30), Lactobacillus species (Rogosa agar; Thermo Scientific, Lenexa, KS) (31), total oral streptococci (mitis salivarius agar; Anaerobe Systems, Morgan Hill, CA), and Candida species (CHROMagar Candida; CHROMagar, Paris, France) (32, 33). The saliva samples were also plated on nonselective enriched tryptic soy agar (ETSA) (Anaerobe Systems, Morgan Hill, CA) for cultivation of total cultivable oral bacteria. All bacterial samples were plated using an Autoplate 4000 system (Advanced Instruments, Norwood, MA) and incubated anaerobically (85% nitrogen, 10% carbon dioxide, and 5% hydrogen) or aerobically for 72 h, and colonies on each culture plate were counted manually. For statistical analysis, CFU values were transformed logarithmically to normalize the variation in distribution and to estimate the concentration of each targeted microorganism in saliva. The microbial CFU were analyzed for association with viral loads, CD4+ and CD8+ T cell counts, caries status (DFT/DFS scores), and periodontal health status (BOP scores).
Extraction of bacterial genomic DNA.
Whole-saliva samples (1 ml) were used for bacterial genomic DNA extraction, as described previously (34, 35). Briefly, each saliva sample was centrifuged (18,000 × g for 3 min), the supernatant was discarded, and total bacterial genomic DNA was extracted from the pellet using a DNA purification kit (MasterPure; Epicentre, Madison, WI). Samples were treated with a solution of phenol, chloroform, and isoamyl alcohol (25:24:1 [pH 8.0]) with mutanolysin (5,000 U/ml; 2 μl). DNA was precipitated from the aqueous phase with isopropanol and recovered by centrifugation. The pellet was resuspended in 20 μl of Tris-EDTA buffer. Final DNA quality and concentration were measured using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). The bacterial DNA was stored at −20°C.
DGGE assay.
The DGGE assay is commonly used in studies of microbial ecology, microbiology, and environmental microbiology (36–38). Previously, we showed that the assay can provide rapid assessment of the oral bacterial community without cultivation, can differentiate the major components of the microbial profile, and enabled longitudinal monitoring of changes in microbial flora in the same subjects (35, 39–42). In this study, we applied DGGE to all bacterial genomic DNA samples. The complete 16S rRNA gene locus (1,500 bp) was preamplified with universal 16S rRNA gene primers (8f to 1492r), as described previously (43). Nested amplification of the V4 to V5 hypervariable region of the 16S rRNA gene was performed using an internal set of primers (prbac1 and prbac2) (44) with a 40-nucleotide GC clamp added at the 5′ end of the forward primer (39, 45). After PCR amplification (GeneAmp PCR System 9700; PE Applied Biosystems, Foster City, CA), the PCR products (>300 bp) were separated on linear denaturing gradient polyacrylamide gels (40% to 60%, DCode System; Bio-Rad, Hercules, CA). Electrophoresis was performed (constant 60 V at 58°C for 16 h) in Tris-acetate-EDTA (TAE) buffer (pH 8.5). After electrophoresis, the gels were stained with ethidium bromide (0.5 mg/ml). The DGGE profile images were captured digitally using an AlphaImager 3300 system (Alpha Innotech, San Leandro, CA).
HOMIM assay.
The bacterial genomic DNA samples were analyzed for identification of specific microbial species using the human oral microbial identification microarray (HOMIM) assay (Forsyth Institute, Cambridge, MA). The HOMIM assay allowed simultaneous detection of >300 predominant oral bacterial species in a single hybridization experiment. The quality of the bacterial DNA was verified with PCR using a new set of universal 16S rRNA primers (forward, AACTGGAGGAAGGTGGGGAT; reverse, AGGAGGTGATCCAACCGCA). A nested PCR was performed to incorporate the fluorescent materials (Cy3-dCTP) into the targeted DNA (46). The labeled 16S rRNA amplicons were loaded on aldehyde-coated glass slides containing >430 unique 16S rRNA-based reverse-capture oligonucleotide probes targeting >300 bacterial taxa (Forsyth Institute, Cambridge, MA) and were hybridized overnight at 55°C (46). The slides were washed with buffer (2× saline sodium citrate, 10% sodium dodecyl sulfate) at 55°C, dried, and scanned (Axon GenePix 4000B microarray scanner; Molecular Devices, Sunnyvale, CA). The raw data were normalized by comparing individual signal intensities with the average signal from universal probes for 16S rRNA genes (GenePix Pro software; Molecular Devices, Sunnyvale, CA) (46). The final microarray data were graded (range, 0 to 5) based on the presence or absence of the hybridization signals and the degree of signal intensity and were imported to software for analysis (MeV v4.8.1 software; Dana-Farber Cancer Institute, Boston, MA) (47).
Statistical analysis.
Bacterial CFU for each targeted microorganism, viral loads, CD4+ and CD8+ T cell counts, dental caries, and periodontal status scores were evaluated with the nonparametric Mann-Whitney test for mean comparisons and the Spearman rank correlation test for correlation analyses between HIV-positive and HIV-negative groups, as well as within the HIV-positive group before and after HAART. The DGGE microbial flora profiles were analyzed with BioNumerics 6.0 software (Applied Maths, Austin, TX). Levels of similarity between the fingerprints were calculated based on the Dice coefficient for pairwise comparisons. Cluster analyses were performed and dendrograms were constructed based on the Ward method and algorithm (48). The numbers of detected DGGE bands and their frequency distribution patterns were determined and compared between groups. Shannon diversity index values were calculated on the basis of the relative abundance and evenness of the 16S rRNA gene fragments detected by DGGE.
HOMIM data were correlated to identify the relative abundance of oral microbial genes of interest and were compared among the three groups based on the presence or absence of genes and the HOMIM hybridization intensities. Hierarchical clustering analysis was performed based on the presence or absence of bands and the average linkage method (49). Dendrograms were constructed by clustering correlation matrices that included all elements of gene comparisons. An experiment-wise false discovery rate (FDR) of 5% was used. The final P values for the analysis were adjusted for multiple comparisons using Holm's method (50). Principal-component analysis was performed using MeV v4.8.1 software.
All data analyses were performed with statistical software (SAS/STAT software [SAS Institute, Cary, NC] or SPSS Statistics software [IBM Corp., Somers, NY]). The nonparametric Wilcoxon-Mann-Whitney test was used to compare the results for HIV-positive subjects and HIV-negative controls. To compare the data for HIV-positive subjects before and after HAART, Friedman's chi-square rank test, equivalent to the Cochran-Mantel-Haenszel chi-square test, was used.
RESULTS
HIV infection status.
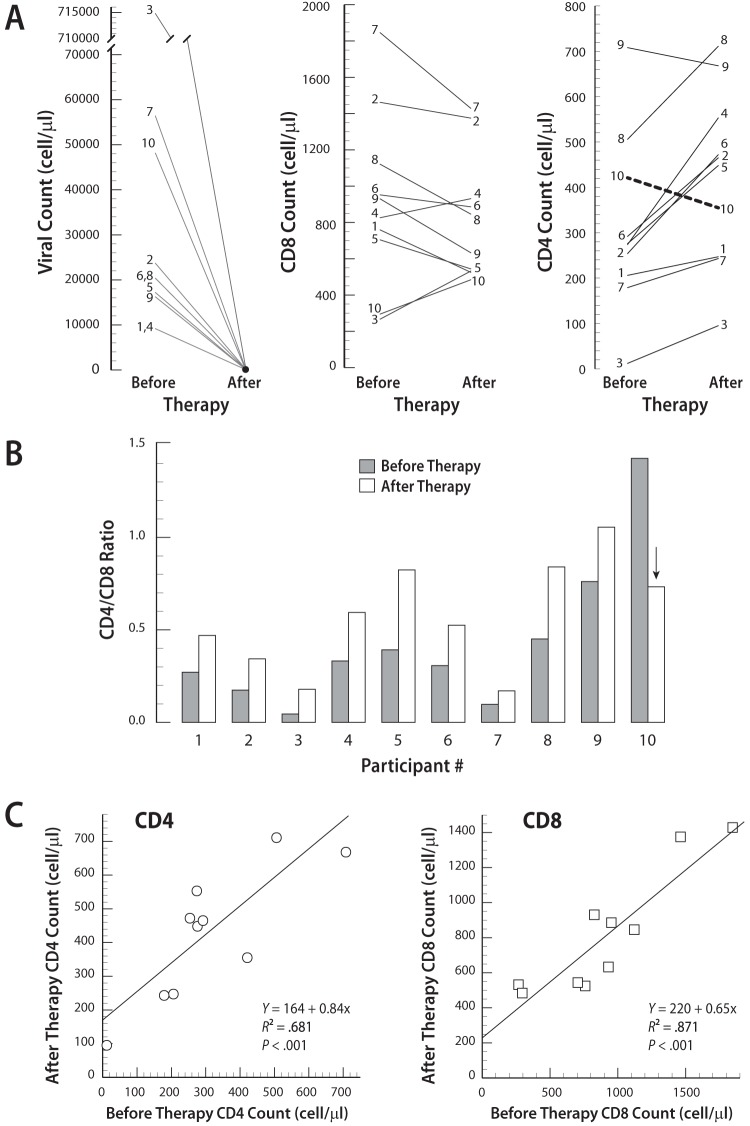
A schematic diagram of the study design is presented in Fig. 1. No significant difference in the mean age was observed between the two groups. HIV-seropositive subjects had more dental caries (mean DFS score) but similar levels of gingivitis and periodontitis (mean BOP score) (Table 1). Among the HIV-positive subjects, HIV loads ranged from 9.0 × 103 to 7.1 × 105 copies/ml, but these results were markedly reduced for each subject after HAART (P < 0.001, Mann-Whitney test) (Fig. 2A). At 6 months after HAART, the mean CD4+ T cell count was increased by 36% (before therapy, 313 ± 192 cells/μl; after therapy, 426 ± 194 cells/μl), and the mean ratio of CD4+ T cells to CD8+ T cells (CD4/CD8 ratio) was increased by 33% (before therapy, 0.43 ± 0.41; after therapy, 0.57 ± 0.29) (Fig. 2B). All HIV-positive subjects, with the exception of subject 10, experienced improved CD4+ T cell counts. The CD4+ and CD8+ T cell counts before HAART were highly correlated with the cell counts after HAART for each HIV-seropositive subject (Fig. 2C).
FIG 1.
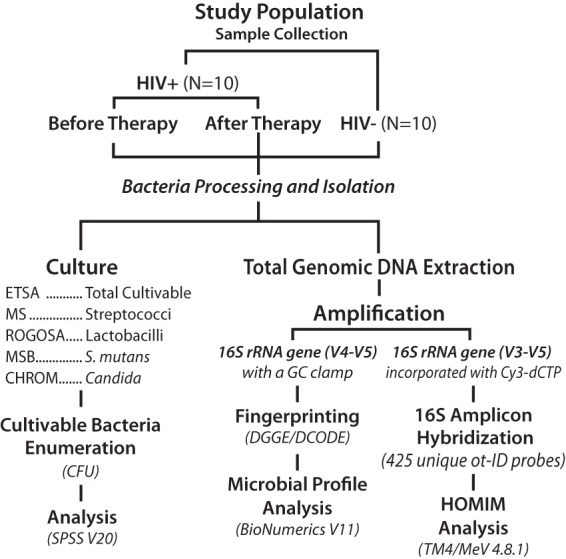
Schematic diagram of the study design. Three methods were used to evaluate the oral microbial composition in saliva, i.e., one method based on culture and two methods based on genomic DNA extraction. DGGE, denaturing gradient gel electrophoresis; HOMIM, human oral microbe identification microarray.
TABLE 1.
Demographic and clinical characteristics of HIV-seropositive and HIV-seronegative subjects
| Characteristic | Seronegative | Seropositive, before HAART |
|---|---|---|
| No. of patients | 10 | 10 |
| Age (mean ± SD) (yr) | 43 ± 13 | 39 ± 10 |
| Sex (no.) | ||
| Male | 5 | 7 |
| Female | 5 | 2 |
| Transgender | 0 | 1 |
| Ethnicity (no.) | ||
| Non-Hispanic | 9 | 9 |
| Hispanic | 1 | 1 |
| Race (no.) | ||
| Black/African American | 6 | 7 |
| White | 2 | 3 |
| Asian | 2 | 0 |
| Oral examination results | ||
| DFS score (mean ± SD)a | 18 ± 20 | 29 ± 26 |
| BOP score (mean ± SD)b | 0.4 ± 0.1 | 0.4 ± 0.4 |
DFS, decayed and filled tooth surfaces, for assessment of the dental caries status based on the total number of tooth surfaces examined in the oral cavity.
BOP, bleeding on probing, for assessment of the periodontal status of the oral cavity based on the 6 Ramfjord index teeth.
FIG 2.
HIV loads, CD4+ T cell counts, and CD8+ T cell counts before and after HAART in HIV-seropositive subjects. (A) After HAART, decreased viral loads and partial immune restoration for CD4 were observed. It is noteworthy that all of the HIV-infected subjects except subject 10 experienced improved CD4+ T cell counts. (B) After HAART, all of the HIV-infected subjects except subject 10 (arrow) experienced improved CD4/CD8 ratios; however, the difference was not statistically significant. (C) Nonparametric correlation analysis showed that the CD4+ T cell counts (ρ = 0.770, P < 0.001) and CD8+ T cell counts (ρ = 0.855, P < 0.001) before HAART were highly correlated with the cell counts after HAART.
Oral microbial colonization.
Of the 30 salivary samples, 24 were collected between 10 a.m. and 12 p.m.; only 6 samples were collected between 1 p.m. and 4 p.m. The salivary cultures showed that HIV-seropositive subjects had greater CFU counts for total cultivable microbes, total oral streptococci, Lactobacillus species, S. mutans, and Candida in saliva than did HIV-seronegative subjects. Significant correlations were noted between CD4+ T cell counts and both total cultivable microbial CFU counts (ρ = 0.608, P < 0.01) and total oral streptococcal CFU counts (ρ = 0.684, P < 0.01) (Table 2). BOP scores (indicators of tissue inflammatory responses to bacterial pathogens) were positively correlated with CD8+ T cell counts (ρ = 0.584, P ≤ 0.01). A bivariate linear relationship was also observed between Candida levels and DFS caries scores (ρ = 0.478, P < 0.01) (Table 2).
TABLE 2.
Correlations between microbial CFU values, DFS scores, BOP scores, and HIV infection
| Parameter | Correlation coefficient for: |
|||||||
|---|---|---|---|---|---|---|---|---|
| CD4+ cell count | CD8+ cell count | DFS score | BOP score | S. mutans | Oral streptococci | Lactobacilli | Candida | |
| CD8+ cell count | 0.032 | |||||||
| DFS score | −0.329 | 0.081 | ||||||
| BOP score | −0.160 | 0.584a | 0.207 | |||||
| S. mutans | −0.085 | 0.068 | 0.160 | 0.439b | ||||
| Oral streptococci | 0.684a | −0.172 | −0.137 | 0.121 | 0.249 | |||
| Lactobacilli | −0.101 | 0.222 | 0.176 | 0.436b | 0.590a | 0.325 | ||
| Candida | −0.163 | 0.103 | 0.478a | 0.173 | −0.008 | −0.218 | 0.127 | |
| Total cultivable microbes | 0.608a | −0.170 | −0.063 | 0.132 | 0.274 | 0.900a | 0.392b | −0.171 |
Spearman's correlation, P < 0.01 (2-tailed).
Spearman's correlation, P < 0.05 (2-tailed).
Denaturing gradient gel electrophoresis.
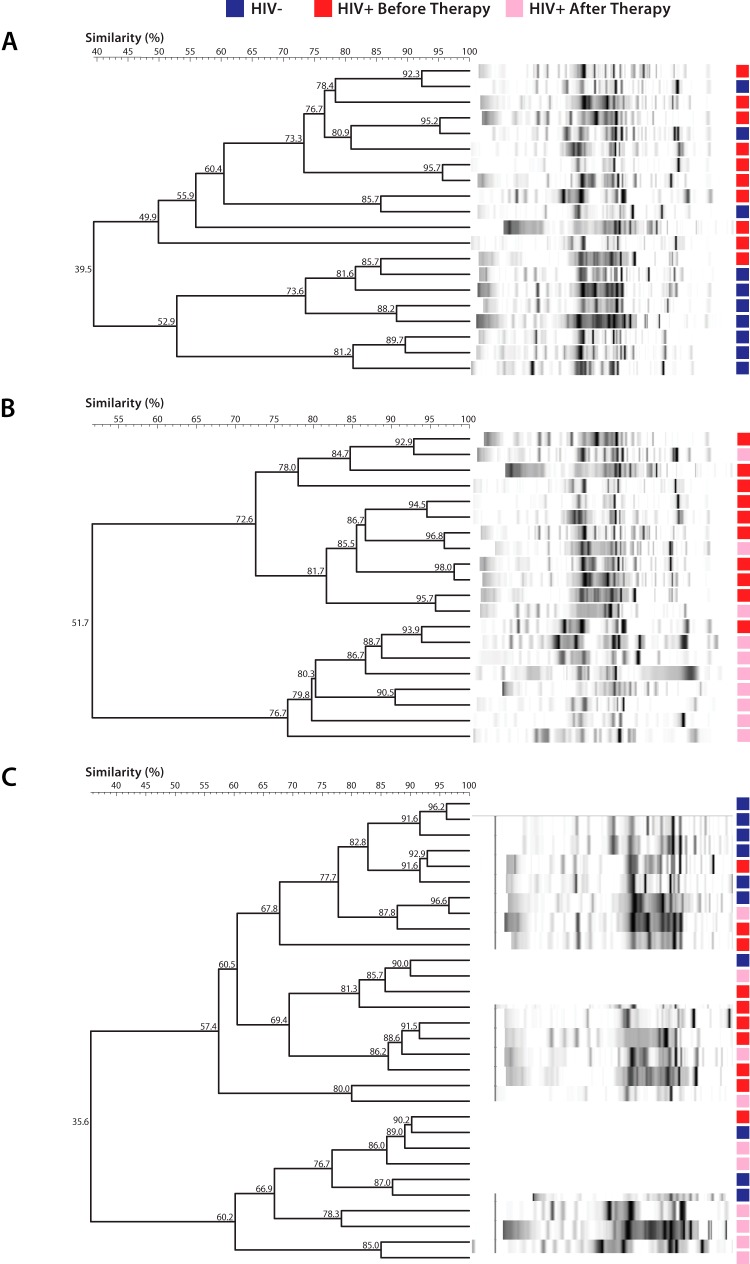
After PCR amplification, the 16S rRNA gene fragments (>300 bp) were separated by DGGE and stained, and DNA fingerprint profiles (banding patterns) were obtained. Based on the relative distribution and intensities of the banding patterns detected on the DGGE gels, the profile analysis showed that the samples from HIV-negative subjects clustered separately from the samples from HIV-positive subjects before HAART (Fig. 3A). In addition, the samples from HIV-positive subjects before HAART were clustered separately from the samples after therapy (Fig. 3B). Simultaneous comparison of samples from all 3 sample groups (HIV-negative group, HIV-positive group before therapy, and HIV-positive group after therapy) showed that 60% to 70% of samples from the same group were clustered in one branch (Fig. 3C). A total of 42 distinct amplicons (bands) were detected from all DGGE profiles. The mean numbers of bands were 33 ± 6 bands for the HIV-negative group, 31 ± 3 bands for the HIV-positive group before therapy, and 27 ± 5 bands for the HIV-positive group after therapy (Fig. 4A). Compared with the HIV-negative group, fewer bands were found in the HIV-positive group before therapy and the HIV-positive group after therapy (P = 0.05, Mann-Whitney U test). The differences between groups were confirmed by Shannon diversity index values; the HIV-positive group had significantly lower Shannon diversity scores than did the two other groups (P = 0.025, Mann-Whitney U test) (Fig. 4B).
FIG 3.
Cluster analyses of salivary microbial flora profiles determined from DGGE for subjects who were seropositive or seronegative for HIV. (A) Samples from 70% of the HIV-negative subjects clustered separately from samples from 90% of the HIV-positive subjects before HAART. (B) Samples from 90% of the HIV-positive subjects before HAART clustered separately from samples from 70% of the HIV-positive subjects after HAART. (C) Simultaneous comparison of samples from all 3 groups (HIV-negative group, HIV-positive group before therapy, and HIV-positive group after therapy) showed that 60% of samples within each group were clustered in the same branch.
FIG 4.
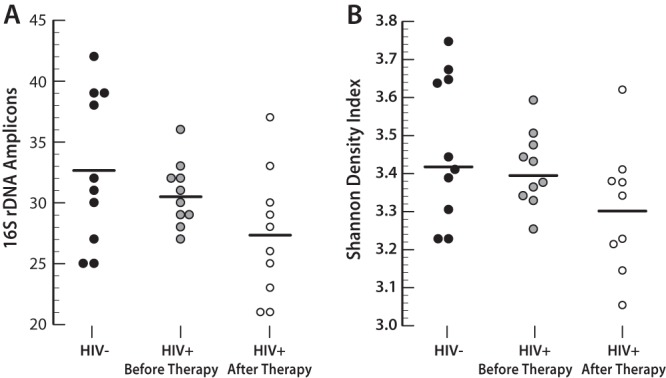
Microbial diversity analysis of salivary microbial flora. Profiles were determined through denaturing gradient gel electrophoresis (DGGE) for subjects who were HIV positive or HIV negative (horizontal lines indicate mean values). (A) Forty-two distinct amplicon fragments (bands) were detected from all DGGE profiles. Compared with the HIV-negative group, fewer bands were found in the HIV-positive group before therapy and the HIV-positive group after therapy (P = 0.05, Mann-Whitney U test). (B) The differences between groups were confirmed by Shannon diversity index values, which showed that HAART significantly decreased the overall microbial diversity (P = 0.025, Mann-Whitney U test).
Human oral microbe identification microarray assay.
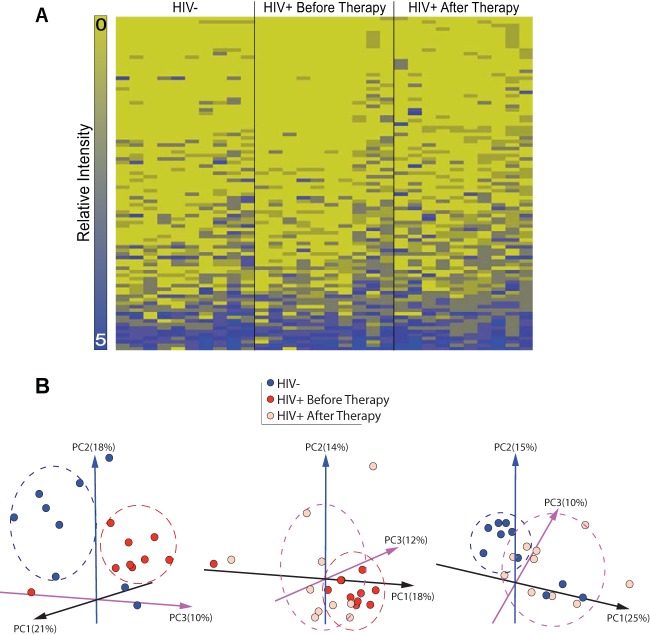
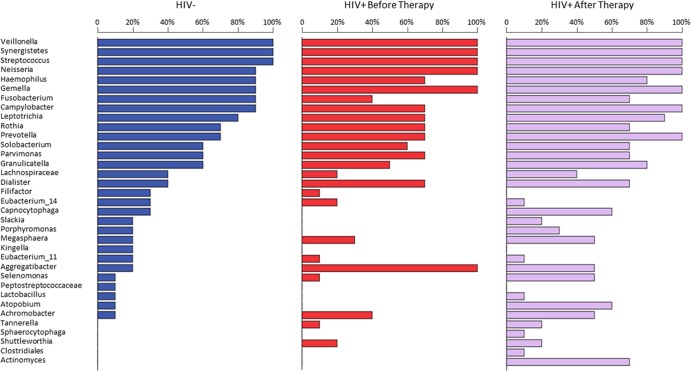
The HOMIM assay was performed to distinguish the observed differences in DGGE findings for the 30 DNA samples. Among the 423 bacterial probes included in the DNA microarray, 121 (29%) were detected with positive hybridization with scores of ≥1. They were categorized in 6 bacterial phyla (see Fig. S1A in the supplemental material), 11 classes (see Fig. S1B), 14 orders (see Fig. S1C), 23 families (see Fig. S1D), 34 genera (see Fig. S1E), and 90 species (see Fig. S1F). The heat map showed the top 94 positive probe reactions and their distributions among the 3 sample groups (Fig. 5A). The principal-component analysis of all 121 positive probes showed clear separation among the HIV-negative group, the HIV-positive group before therapy, and the HIV-positive group after therapy, as well as between the HIV-negative group and the HIV-positive group after therapy (Fig. 5B). The predominant phylogenetic characteristics included the phylum Firmicutes (65%), more specifically Streptococcus species (34%) and Veillonella species (11%) (Table 2). Although the prevalence and distribution of the bacterial genera differed among the three sample groups (Fig. 6), the difference in microbial composition (Table 3) was statistically significant only when the HIV-positive group before therapy was compared with the HIV-positive group after therapy (chi-square = 5.0, P = 0.025, nonparametric Friedman test). More specifically, 30 genera of bacteria, on average, were identified in the oral cavities of HIV-negative individuals. Three genera, Veillonella, Synergistetes, and Streptococcus, were present in all 30 saliva samples. Only minor or no changes in the prevalence of Neisseria, Haemophilus, Gemella, Leptotrichia, Solobacterium, Parvimonas, and Rothia were observed. Seven genera, including Capnocytophaga, Slackia, Porphyromonas, Kingella, Peptostreptococcaceae, Lactobacillus, and Atopobium, were present only in HIV-negative controls and not in the HIV-positive group. Additionally, several genera, such as Fusobacterium, Campylobacter, Prevotella, Capnocytophaga, Selenomonas, Granulicatella, Actinomyces, and Atopobium, were notably increased after HAART. In contrast, Aggregatibacter was significantly decreased in HIV-positive groups. Using a P value of <0.05 as a cutoff for selecting candidates of a specific phylum or genus for the comparison, we found that 2 phyla (Bacteroidetes and Actinobacteria) and 5 genera (Actinomyces, Atopobium, Aggregatibacter, Fusobacterium, and Prevotella) were significantly different (Table 3). When the analysis was adjusted for multiple comparisons, only 3 genera (Actinomyces, Atopobium, and Aggregatibacter) were significantly different from baseline values after HAART.
FIG 5.
Human oral microbe identification microarray (HOMIM) assay of salivary microbial profiles. The HOMIM assay showed that bacterial profiles differed among the 3 sample groups (HIV-negative group, HIV-positive group before therapy, and HIV-positive group after therapy). (A) The heat map showed the top 94 of 121 positive probe reactions and their distribution among the 3 sets of samples. Hybridization signals were read on a 6-level scale (range, 0 to 5). (B) The principal-component analysis showed clear separation between the HIV-negative samples (blue circles) and the HIV-positive samples before therapy (red circles), between the HIV-positive samples before and after therapy (pink circles), and between the HIV-negative samples and the HIV-positive samples after therapy, indicating that microbial compositions were different for the 3 sets of samples.
FIG 6.
Comparison of microbial distribution. Differences in the prevalence of oral bacteria at the genus level were observed among the 3 sample groups. About 30 genera of bacteria were identified in the oral cavities of HIV-negative individuals. HIV infection and HAART showed no effects on the prevalence of Veillonella, Synergistetes, and Streptococcus groups. Seven genera were present only in HIV-negative controls. The prevalence of Fusobacterium, Campylobacter, and Leptotrichia were decreased in the HIV-positive group but increased in the HIV-positive group after therapy. The differences in the overall prevalence and distribution of the bacteria before and after HAART were statistically significant (chi-square = 5.0, P = 0.025, nonparametric Friedman test).
TABLE 3.
Microbial profiles of HIV-seropositive and HIV-seronegative subjects
| Categorya | Prevalence (%) |
|||
|---|---|---|---|---|
| Total | Seronegative | Seropositive, before HAART | Seropositive, after HAART | |
| Phylum | ||||
| Actinobacteriab | 5.82 | 5.35 | 3.67 | 7.79 |
| Bacteroidetesb | 7.65 | 5.61 | 6.78 | 9.89 |
| Firmicutes | 65.0 | 64.97 | 68.64 | 62.32 |
| Fusobacteriac | 5.32 | 6.68 | 4.24 | 5.05 |
| Proteobacteria | 13.72 | 14.71 | 13.84 | 12.84 |
| Synergistetes | 2.49 | 2.67 | 2.82 | 2.11 |
| Genus | ||||
| Actinomyces | 0.67 | 0.0 | 0.0 | 1.69 |
| Aggregatibacter | 1.50 | 0.80 | 2.82 | 1.06 |
| Atopobium | 0.58 | 0.27 | 0.0 | 1.27 |
| Achromobacter | 0.83 | 0.27 | 1.13 | 1.06 |
| Campylobacter | 4.32 | 5.60 | 3.66 | 3.81 |
| Capnocytophaga | 0.75 | 0.80 | 0.0 | 1.27 |
| Clostridiales | 0.08 | 0.0 | 0.0 | 0.21 |
| Dialister | 2.16 | 1.07 | 2.82 | 2.54 |
| Eubacterium | 1.08 | 1.07 | 1.41 | 0.85 |
| Filifactor | 0.58 | 1.60 | 0.28 | 0.00 |
| Fusobacterium | 2.91 | 4.53 | 1.69 | 2.54 |
| Gemella | 4.32 | 4.53 | 4.23 | 4.23 |
| Granulicatella | 2.99 | 2.67 | 2.25 | 3.81 |
| Haemophilus | 3.41 | 4.0 | 3.10 | 3.17 |
| Kingella | 0.17 | 0.53 | 0.0 | 0.0 |
| Lachnospiraceae | 1.08 | 1.33 | 0.85 | 1.06 |
| Lactobacillus | 0.17 | 0.27 | 0.0 | 0.21 |
| Leptotrichia | 2.41 | 2.13 | 2.54 | 2.54 |
| Megasphaera | 1.25 | 0.53 | 1.41 | 1.69 |
| Neisseria | 3.49 | 3.47 | 3.10 | 3.81 |
| Parvimonas | 3.24 | 3.20 | 3.66 | 2.96 |
| Peptostreptococcaceae | 0.08 | 0.27 | 0.0 | 0.0 |
| Porphyromonas | 0.50 | 0.80 | 0.0 | 0.63 |
| Prevotella | 6.07 | 4.0 | 6.48 | 7.40 |
| Rothia | 4.24 | 4.80 | 3.94 | 4.02 |
| Selenomonas | 0.83 | 0.53 | 0.28 | 1.48 |
| Shuttleworthia | 0.33 | 0.0 | 0.56 | 0.42 |
| Slackia | 0.33 | 0.53 | 0.0 | 0.42 |
| Solobacterium | 2.24 | 1.87 | 2.25 | 2.54 |
| Sphaerocytophaga | 0.08 | 0.00 | 0.00 | 0.21 |
| Streptococcus | 33.50 | 35.47 | 36.34 | 29.81 |
| Synergistetes | 2.49 | 2.67 | 2.82 | 2.11 |
| Tannerella | 0.25 | 0.0 | 0.28 | 0.42 |
| Veillonella | 11.06 | 10.40 | 12.11 | 10.78 |
Microbial identification was performed with the human oral microbe identification microarray (HOMIM) assay.
P < 0.05, nonparametric Wilcoxon-Mann-Whitney test, for comparisons between HIV-positive and HIV-negative samples.
P < 0.05, nonparametric Friedman's chi-square rank test, for comparisons of HIV-positive samples before and after HAART.
DISCUSSION
Even though HIV infection has been associated with increased risks of opportunistic oral infections, dental caries, and aggressive periodontal diseases, reports of oral microbial characterization among immunocompromised individuals are limited. Since the immunodeficiency that results from HIV infection is characterized by the depletion of CD4+ lymphocytes, the main objective of HAART is to improve CD4+ T cell levels in order to decrease the HIV burden and to prevent opportunistic infections. In this study, we first demonstrated that the HIV-positive subjects, after 6 months of HAART, experienced significant decreases in viral loads and 0.4- to 3-fold increases in CD4+/CD8+ T cell ratios. These findings were expected and were consistent with previous studies that showed inverse correlation of CD4+/CD8+ T cell ratios with antiretroviral therapy (51–53). As the particular focus of the present study, the microbial analysis data showed differences in oral microbial colonization among HIV-seronegative subjects, HIV-seropositive subjects before HAART, and HIV-seropositive subjects 6 months after HAART. We found correlations between CD4+ T cell counts and levels of total cultivable bacteria or total oral streptococci at the early stages of HAART, supporting our initial hypothesis that HIV infection can affect salivary microbial colonization and hence contribute to increased risks of declining oral health in individuals living with HIV.
While evidence has indicated increased CD4+ T cell counts after HAART, it has also been reported that noncytotoxic CD8+ T cell anti-HIV responses may contribute significantly to immune system reconstitution by suppressing HIV replication (54). Clinical studies have demonstrated the use of the CD4/CD8 ratio as an indicator of immunocompromise in HIV-infected individuals, with high sensitivity (98%) and specificity (>98%), particularly for children <2 years of age (55, 56). However, a recent study by Leung et al. concluded that CD4/CD8 ratio normalization might not have additional short-term predictive value for clinical outcomes after accounting for other risk factors, such as age and HIV load (57). In our study, we found only 1 subject who did not experience an increased CD4/CD8 ratio after HAART, most likely as a result of antiretroviral resistance or treatment nonadherence (58), as evidenced by low CD4+ and CD8+ T cell counts during the 6 months after treatment. Our findings that correlated decreased viral loads with increased CD4/CD8 ratios support the notion that the CD4/CD8 ratio can be used as an additional marker to monitor treatment and prognosis.
HIV infection is also associated with changes in the diversity of genital flora, including increased diversity of the genital flora in HIV-infected women with bacterial vaginosis (59). In patients with cystic fibrosis, increased lung microbial diversity has been suggested to be beneficial for overall health status improvement (60). In the present study, we hypothesized that immunocompromise resulting from HIV infection can significantly disrupt the normally constituted commensal bacterial colonization in the oral microbiota. Such disruption might cause an increase in microbial diversity, but it has also been suggested that impairment of systemic defense mechanisms by reduction of CD4+ T cells below protective levels and impairment of local immunity by reduction of the levels of salivary IgA, defensins, or epithelial cell-mediated cytokines in the saliva might lead to the conversion of commensal microorganisms, e.g., Candida, to microorganisms with increased pathogenicity, causing an imbalance in the host oral microbial composition and hence increased risk for opportunistic infections (20, 21). On the other hand, HAART might reverse such HIV-associated microbial alterations in saliva and improve oral health. Unexpectedly, our data showed a decrease in microbial diversity, along with changes in the overall oral microbiota in HIV-positive samples. Since the levels of Candida species and a number of genera, including Dialister, Aggregatibacter, Atopobium, and Actinomyces, were increased in the HIV-positive samples, we postulate that decreased diversity may be attributed to increased proportions of opportunistic microbes as a result of immunocompromise. In our previous study of caries-associated microbial flora, it was demonstrated that microbial diversity was decreased in the dental plaque of children with severe caries, compared with caries-free children (35). We believe that the reduced diversity may be attributed to increased proportions of acidogenic and aciduric bacteria, which may become dominant in the oral cavity during the progression of caries lesions. The notion that microbial diversity is closely associated with an individual's health status was supported by a study showing that children with Crohn's disease were characterized by significant decreases in oral microbial diversity (61). The same study also suggested that specific phyla and oral microbial compositions might represent a specific disease status (61).
The present study used two 16S rRNA methods, i.e., DGGE and the HOMIM assay, to determine the effects of HIV infection and HAART on microbial diversity. Findings of the two approaches were consistent, suggesting complementarity. DGGE analysis of the numbers of bands, band intensities, and migration distributions of the 16S rRNA gene amplicons clearly showed that the microbial profiles were similar within each group but remarkably different among groups. These findings were similar to those of a periodontal microbial study in which the microbial profiles in subgingival plaque associated with putative pathogens were different in HIV-seropositive subjects versus HIV-seronegative subjects, even though different molecular approaches were used (62).
HOMIM analysis, based on 121 positive DNA hybridization reactions, showed that 30 genera of bacteria, on average, were identified in the oral cavities of HIV-negative healthy controls. However, changes in microbial composition did occur in the three sample groups. Specifically, among the 34 identified genera, Streptococcus (33.5%) and Veillonella (11.1%) were the predominant groups. Although both genera are commensal components of oral flora, they include numerous clinically important species that may cause infections in humans (63, 64). Interestingly, 7 genera were present only in HIV-negative controls and not in the HIV-positive group. HIV infection and HAART showed no effects on the prevalence of Streptococcus and Veillonella, along with Synergistetes and Rothia groups, which are commonly detected in patients with periodontitis (65–67). We also observed the increased prevalence of Aggregatibacter, Achromobacter, Dialister, Shuttleworthia, and Gemella in the HIV-positive group, compared with the HIV-negative control group. Several groups of bacteria, such as Fusobacterium, Campylobacter, Prevotella, Capnocytophaga, Actinomyces, and Atopobium, were increased 6 months after HAART. In contrast, the prevalence of Aggregatibacter was significantly decreased after HAART. We propose that these observed HIV- and HAART-associated microbial changes might have resulted from immunocompromise induced by HIV infection, immune system reversal/reconstitution by HAART, or direct effects of antimicrobial prophylaxis in HIV-positive individuals (68).
Of the 90 identified species, 7% were detected only in HIV-negative samples, while 24% were detected only in HIV-positive samples. The group-characteristic composition shown in our study also suggests that specific types of bacteria might be associated with HIV infection. Most of the species that showed significant differences among the three sample groups were common members of the human oral microbiota, as well as opportunistic pathogens such as Actinomyces gerencseriae, Atopobium spp., and Aggregatibacter segnis. Studies suggested that microorganisms that were previously considered nonpathogenic can become opportunistic pathogens in immunosuppressed patients receiving antibiotics (23, 69). HIV-associated changes in microbial composition may favor pathogen selection (70) and play a role in increased risks of opportunistic infections in HIV-infected patients. However, the mechanisms and clinical importance of such changes are not fully understood and must be confirmed with large sample sizes. It is also critical to define and to understand the “normal” oral microbiota, so that any changes in the abundance of commensal organisms that typically characterize the healthy state can serve as indicators of oral cavity dysfunction.
HIV-positive individuals are at greater risk of dental caries and periodontal diseases than are HIV-negative individuals (71–73). The increased risk may be caused by depletion of CD4+ and CD8+ lymphocytes and resultant decreased host immune responses (16, 74), increased S. mutans colonization (16), reduced salivary secretion (75, 76), and/or poor oral hygiene (77). In the present study, culture methods showed that HIV infection can increase salivary microbial colonization. The total cultivable microbial counts were positively correlated with the total oral streptococcal counts (data not shown). A correlation was noted between S. mutans and Lactobacillus, which have similar ecological niches and cariogenic abilities. However, the positive correlation of CD4+ T cell counts with total cultivable microbial counts was unexpected. CD4+ T cell counts are markedly reduced in acute HIV infection but rapidly increase after HAART; therefore, it is unknown whether the positive correlation between CD4+ T cell counts and bacterial levels reflects a true association between oral microbial colonization and immunosuppression or immunoreconstruction.
Furthermore, our study showed that the CD8+ T cell counts were significantly correlated with the periodontal inflammation scores (BOP scores) and that the levels of Candida in saliva were significantly correlated with the caries scores. Moreover, we found decreases in total Candida CFU counts in the saliva samples after HAART and an increased prevalence of periodontal pathogens, as well as various anaerobic groups of bacteria. These findings indicate continuous interactions between HIV infection status, host immunocompetence, microbial pathogens, and clinical outcomes. Thus, different members of the microbial community might respond differently to HAART. Previously, a significant correlation was noted between CD8+ T cell activation levels and total bacterial 16S rRNA gene levels in stool samples from HIV-infected subjects (78). We demonstrated a positive association between CD8+ T cell counts and the level of S. mutans colonization in HIV-infected individuals (16). In addition to known pathogens associated with aggressive periodontal disease, other microbial species, such as Gemella morbillorum, Saccharomyces cerevisiae, and Candida albicans, may contribute to periodontal disease in HIV-seropositive subjects (70). Longitudinal follow-up studies are needed to clarify these complex microbe-microbe and host-pathogen interactions and to determine factors associated with HIV that may mediate oral microbial colonization.
The present study showed that salivary microbial colonization and composition were changed in HIV-infected individuals, both before and after HAART, in comparison with HIV-negative controls. Although the sample size was limited, the effects of HAART on the molecular phylogenetic characteristics of the bacterial population were compelling. Since all of the HIV-positive subjects were carefully screened and enrolled before the initiation of HAART, the study was able to minimize potential treatment bias and presented exploratory evidence regarding the potential real associations between host immunological changes, microbiological interspecies interactions, and clinical outcome assessments. More importantly, our findings suggest that HAART may be able to directly or indirectly reverse salivary microbial alterations associated with HIV infection, allowing reconstitution of the oral microbiota. However, the polymicrobial characteristics of the oral microbiota and their association with HIV infection and HAART remain challenging. Elucidating the molecular mechanisms underlying the responses of the oral microbiota to changes in the host immune system will be useful for monitoring HIV infection and evaluating disease prognosis.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Institute of Dental and Craniofacial Research (grant U19 DE018385).
The funders had no role in the study design, data collection or analysis, the decision to publish, or preparation of the manuscript. We declare no potential conflicts of interest with respect to the authorship or publication of this article.
We thank Isaac R. Rodriguez-Chavez, David Wong, Louis Terracio, Fred Valentine, and Martin Blazer for their support and insightful advice. We also thank Maura Laverty, Patty Wang, and staff members of the Bluestone Center for Clinical Research at New York University for their assistance with subject recruitment and sample collection.
Footnotes
Published ahead of print 12 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02954-13.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS. 2013. Global report: UNAIDS report on the global AIDS epidemic 2013. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland [Google Scholar]
- 2.Aberg J, Powderly W. 2010. HIV: primary and secondary prophylaxis for opportunistic infections. Clin. Evid. 2010:0908 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3217757/pdf/2010-0908.pdf [PMC free article] [PubMed] [Google Scholar]
- 3.Greenspan D. 1994. Treatment of oral candidiasis in HIV infection. Oral Surg. Oral Med. Oral Pathol. 78:211–215. 10.1016/0030-4220(94)90149-X [DOI] [PubMed] [Google Scholar]
- 4.Owotade FJ, Shiboski CH, Poole L, Ramstead CA, Malvin K, Hecht FM, Greenspan JS. 2008. Prevalence of oral disease among adults with primary HIV infection. Oral Dis. 14:497–499. 10.1111/j.1601-0825.2007.01407.x [DOI] [PubMed] [Google Scholar]
- 5.Boackle RJ. 1991. The interaction of salivary secretions with the human complement system: a model for the study of host defense systems on inflamed mucosal surfaces. Crit. Rev. Oral Biol. Med. 2:355–367 [DOI] [PubMed] [Google Scholar]
- 6.Fidel PLJ. 2011. Candida-host interactions in HIV disease: implications for oropharyngeal candidiasis. Adv. Dent. Res. 23:45–49. 10.1177/0022034511399284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owotade FJ, Patel M, Ralephenya TR, Vergotine G. 2013. Oral Candida colonization in HIV-positive women: associated factors and changes following antiretroviral therapy. J. Med. Microbiol. 62:126–132. 10.1099/jmm.0.047522-0 [DOI] [PubMed] [Google Scholar]
- 8.Ryder MI, Nittayananta W, Coogan M, Greenspan D, Greenspan JS. 2012. Periodontal disease in HIV/AIDS. Periodontol 2000 60:78–97. 10.1111/j.1600-0757.2012.00445.x [DOI] [PubMed] [Google Scholar]
- 9.Sales-Peres SH, Mapengo MA, de Moura-Grec PG, Marsicano JA, Sales-Peres AC. 2012. Oral manifestations in HIV+ children in Mozambique. Cien. Saude Colet. 17:55–60. 10.1590/S1413-81232012000100008 [DOI] [PubMed] [Google Scholar]
- 10.Beena JP. 2011. Prevalence of dental caries and its correlation with the immunologic profile in HIV-infected children on antiretroviral therapy. Eur. J. Paediatr. Dent. 12:87–90 [PubMed] [Google Scholar]
- 11.Fine DH, Tofsky N, Nelson EM, Schoen D, Barasch A. 2003. Clinical implications of the oral manifestations of HIV infection in children. Dent. Clin. North Am. 47:159–174, xi-xii. 10.1016/S0011-8532(02)00057-5 [DOI] [PubMed] [Google Scholar]
- 12.Ramos MPdA, Ferreira SMS, Silva-Boghossian CM, Souto R, Colombo AP, Noce CW, Goncalves LdS. 2012. Necrotizing periodontal diseases in HIV-infected Brazilian patients: a clinical and microbiologic descriptive study. Quintessence Int. 43:71–82 [PubMed] [Google Scholar]
- 13.Umadevi M, Adeyemi O, Patel M, Reichart PA, Robinson PG. 2006. (B2) Periodontal diseases and other bacterial infections. Adv. Dent. Res. 19:139–145. 10.1177/154407370601900125 [DOI] [PubMed] [Google Scholar]
- 14.Fricke U, Geurtsen W, Staufenbiel I, Rahman A. 2012. Periodontal status of HIV-infected patients undergoing antiretroviral therapy compared to HIV-therapy naive patients: a case control study. Eur. J. Med. Res. 17:2. 10.1186/2047-783X-17-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khammissa R, Feller L, Altini M, Fatti P, Lemmer J. 2012. A comparison of chronic periodontitis in HIV-seropositive subjects and the general population in the Ga-Rankuwa Area, South Africa. AIDS Res. Treat. 2012:620962. 10.1155/2012/620962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G, Saxena D, Chen Z, Norman RG, Phelan JA, Laverty M, Fisch GS, Corby PM, Abrams W, Malamud D, Li Y. 2012. HIV infection affects Streptococcus mutans levels, but not genotypes. J. Dent. Res. 91:834–840. 10.1177/0022034512454298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassone A, Cauda R. 2012. Candida and candidiasis in HIV-infected subjects: where commensalism, opportunistic behavior and frank pathogenicity lose their borders. AIDS 26:1457–1472. 10.1097/QAD.0b013e3283536ba8 [DOI] [PubMed] [Google Scholar]
- 18.Leigh JE, Shetty K, Fidel PLJ. 2004. Oral opportunistic infections in HIV-positive individuals: review and role of mucosal immunity. AIDS Patient Care STDS 18:443–456. 10.1089/1087291041703665 [DOI] [PubMed] [Google Scholar]
- 19.Greenspan JS, Greenspan D. 2002. The epidemiology of the oral lesions of HIV infection in the developed world. Oral Dis. 8(Suppl 2):34–39. 10.1034/j.1601-0825.2002.00009.x [DOI] [PubMed] [Google Scholar]
- 20.Fidel PLJ. 2002. Immunity to Candida. Oral Dis. 8(Suppl 2):69–75. 10.1034/j.1601-0825.2002.00015.x [DOI] [PubMed] [Google Scholar]
- 21.Leigh JE, Steele C, Wormley FLJ, Luo W, Clark RA, Gallaher W, Fidel PLJ. 1998. Th1/Th2 cytokine expression in saliva of HIV-positive and HIV-negative individuals: a pilot study in HIV-positive individuals with oropharyngeal candidiasis. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:373–380. 10.1097/00042560-199812010-00008 [DOI] [PubMed] [Google Scholar]
- 22.Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, Holmberg S, Jones JL. 2000. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 30(Suppl 1):S5–S14. 10.1086/313843 [DOI] [PubMed] [Google Scholar]
- 23.Navazesh M, Mulligan R, Pogoda J, Greenspan D, Alves M, Phelan J, Greenspan J, Slots J. 2005. The effect of HAART on salivary microbiota in the Women's Interagency HIV Study (WIHS). Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 100:701–708. 10.1016/j.tripleo.2004.10.011 [DOI] [PubMed] [Google Scholar]
- 24.Nicolatou-Galitis O, Velegraki A, Paikos S, Economopoulou P, Stefaniotis T, Papanikolaou IS, Kordossis T. 2004. Effect of PI-HAART on the prevalence of oral lesions in HIV-1 infected patients: a Greek study. Oral Dis. 10:145–150. 10.1046/j.1601-0825.2003.00994.x [DOI] [PubMed] [Google Scholar]
- 25.Yang YL, Lo HJ, Hung CC, Li Y. 2006. Effect of prolonged HAART on oral colonization with Candida and candidiasis. BMC Infect. Dis. 6:8. 10.1186/1471-2334-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muyzer G. 1999. DGGE/TGGE a method for identifying genes from natural ecosystems. Curr. Opin. Microbiol. 2:317–322. 10.1016/S1369-5274(99)80055-1 [DOI] [PubMed] [Google Scholar]
- 27.Muyzer G, de Waal EC, Uitterlinden AG. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, Eke PI, Beltran-Aguilar ED, Horowitz AM, Li CH. 2007. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat. 11(248):1–92 http://www.cdc.gov/nchs/data/series/sr_11/sr11_248.pdf [PubMed] [Google Scholar]
- 29.Ramfjord SP. 1959. Indices for prevalence and incidence of periodontal disease. J. Periodontol. 30:51–59 [Google Scholar]
- 30.Gold OG, Jordan HV, Van Houte J. 1973. A selective medium for Streptococcus mutans. Arch. Oral Biol. 18:1357–1364. 10.1016/0003-9969(73)90109-X [DOI] [PubMed] [Google Scholar]
- 31.Rogosa M, Mitchell JA, Wiseman RF. 1951. A selective medium for the isolation and enumeration of oral lactobacilli. J. Dent. Res. 30:682–689. 10.1177/00220345510300051201 [DOI] [PubMed] [Google Scholar]
- 32.Beighton D, Ludford R, Clark DT, Brailsford SR, Pankhurst CL, Tinsley GF, Fiske J, Lewis D, Daly B, Khalifa N, Marren V, Lynch E. 1995. Use of CHROMagar Candida medium for isolation of yeasts from dental samples. J. Clin. Microbiol. 33:3025–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odds FC, Bernaerts R. 1994. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J. Clin. Microbiol. 32:1923–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu G, Saxena D, Deng H, Norman RG, Chen Z, Abrams WR, Malamud D, Li Y. 2010. Effect of protease inhibitors on the quantitative and qualitative assessment of oral microorganisms. FEMS Microbiol. Lett. 312:63–70. 10.1111/j.1574-6968.2010.02100.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Ge Y, Saxena D, Caufield PW. 2007. Genetic profiling of the oral microbiota associated with severe early-childhood caries. J. Clin. Microbiol. 45:81–87. 10.1128/JCM.01622-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muyzer G, Smalla K. 1998. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Van Leeuwenhoek 73:127–141. 10.1023/A:1000669317571 [DOI] [PubMed] [Google Scholar]
- 37.Knapp LA. 2005. Denaturing gradient gel electrophoresis and its use in the detection of major histocompatibility complex polymorphism. Tissue Antigens 65:211–219. 10.1111/j.1399-0039.2005.00368.x [DOI] [PubMed] [Google Scholar]
- 38.Ferguson AS, Huang WE, Lawson KA, Doherty R, Gibert O, Dickson KW, Whiteley AS, Kulakov LA, Thompson IP, Kalin RM, Larkin MJ. 2007. Microbial analysis of soil and groundwater from a gasworks site and comparison with a sequenced biological reactive barrier remediation process. J. Appl. Microbiol. 102:1227–1238. 10.1111/j.1365-2672.2007.03398.x [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Ku CY, Xu J, Saxena D, Caufield PW. 2005. Survey of oral microbial diversity using PCR-based denaturing gradient gel electrophoresis. J. Dent. Res. 84:559–564. 10.1177/154405910508400614 [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Saxena D, Barnes VM, Trivedi HM, Yao G, Xu T. 2006. Polymerase chain reaction-based denaturing gradient gel electrophoresis in the evaluation of oral microbiota. Oral Microbiol. Immunol. 21:333–339. 10.1111/j.1399-302X.2006.00301.x [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Ismail AI, Ge Y, Tellez M, Sohn W. 2007. Similarity of bacterial populations in saliva from African-American mother-child dyads. J. Clin. Microbiol. 45:3082–3085. 10.1128/JCM.00771-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji X, Pushalkar S, Li Y, Glickman R, Fleisher K, Saxena D. 2012. Antibiotic effects on bacterial profile in osteonecrosis of the jaw. Oral Dis. 18:85–95. 10.1111/j.1601-0825.2011.01848.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–147 In Stackebrandt E, Goodfellow M. (ed), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, New York, NY [Google Scholar]
- 44.Rupf S, Merte K, Eschrich K. 1999. Quantification of bacteria in oral samples by competitive polymerase chain reaction. J. Dent. Res. 78:850–856. 10.1177/00220345990780040501 [DOI] [PubMed] [Google Scholar]
- 45.Sheffield VC, Cox DR, Lerman LS, Myers RM. 1989. Attachment of a 40-base-pair G+C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc. Natl. Acad. Sci. U. S. A. 86:232–236. 10.1073/pnas.86.1.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst F, Paster BJ. 2009. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J. Periodontol. 80:1421–1432. 10.1902/jop.2009.090185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. 2003. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378 [DOI] [PubMed] [Google Scholar]
- 48.Ward JHJ. 1963. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 58:236–244. 10.1080/01621459.1963.10500845 [DOI] [Google Scholar]
- 49.Eisen MB, Spellman PT, Brown PO, Botstein D. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U. S. A. 95:14863–14868. 10.1073/pnas.95.25.14863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6:65–70 [Google Scholar]
- 51.Grelli S, d'Ettorre G, Lauria F, Montella F, Di Traglia L, Lichtner M, Vullo V, Favalli C, Vella S, Macchi B, Mastino A. 2004. Inverse correlation between CD8+ lymphocyte apoptosis and CD4+ cell counts during potent antiretroviral therapy in HIV patients. J. Antimicrob. Chemother. 53:494–500. 10.1093/jac/dkh105 [DOI] [PubMed] [Google Scholar]
- 52.Torti C, Prosperi M, Motta D, Digiambenedetto S, Maggiolo F, Paraninfo G, Ripamonti D, Cologni G, Fabbiani M, Caputo SL, Sighinolfi L, Ladisa N, El-Hamad I, Quiros-Roldan E, Frank I. 2012. Factors influencing the normalization of CD4+ T-cell count, percentage and CD4+/CD8+ T-cell ratio in HIV-infected patients on long-term suppressive antiretroviral therapy. Clin. Microbiol. Infect. 18:449–458. 10.1111/j.1469-0691.2011.03650.x [DOI] [PubMed] [Google Scholar]
- 53.Izadi N, Goetz MB, Graber CJ. 2012. Inverse correlation of initial CD8 lymphocyte count and CD4 lymphocyte response to combination antiretroviral therapy in treatment-naive HIV-infected patients. J. Acquir. Immune Defic. Syndr. 59:e1–e3. 10.1097/QAI.0b013e31823d3277 [DOI] [PubMed] [Google Scholar]
- 54.Killian MS, Roop J, Ng S, Hecht FM, Levy JA. 2009. CD8+ cell anti-HIV activity rapidly increases upon discontinuation of early antiretroviral therapy. J. Clin. Immunol. 29:311–318. 10.1007/s10875-009-9275-y [DOI] [PubMed] [Google Scholar]
- 55.Zijenah LS, Katzenstein DA, Nathoo KJ, Rusakaniko S, Tobaiwa O, Gwanzura C, Bikoue A, Nhembe M, Matibe P, Janossy G. 2005. T lymphocytes among HIV-infected and -uninfected infants: CD4/CD8 ratio as a potential tool in diagnosis of infection in infants under the age of 2 years. J. Transl. Med. 3:6. 10.1186/1479-5876-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunt PW, Cao HL, Muzoora C, Ssewanyana I, Bennett J, Emenyonu N, Kembabazi A, Neilands TB, Bangsberg DR, Deeks SG, Martin JN. 2011. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS 25:2123–2131. 10.1097/QAD.0b013e32834c4ac1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leung V, Gillis J, Raboud J, Cooper C, Hogg RS, Loutfy MR, Machouf N, Montaner JS, Rourke SB, Tsoukas C, Klein MB. 2013. Predictors of CD4:CD8 ratio normalization and its effect on health outcomes in the era of combination antiretroviral therapy. PLoS One 8:e77665. 10.1371/journal.pone.0077665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fellay J, Boubaker K, Ledergerber B, Bernasconi E, Furrer H, Battegay M, Hirschel B, Vernazza P, Francioli P, Greub G, Flepp M, Telenti A. 2001. Prevalence of adverse events associated with potent antiretroviral treatment: Swiss HIV Cohort Study. Lancet 358:1322–1327. 10.1016/S0140-6736(01)06413-3 [DOI] [PubMed] [Google Scholar]
- 59.Spear GT, Sikaroodi M, Zariffard MR, Landay AL, French AL, Gillevet PM. 2008. Comparison of the diversity of the vaginal microbiota in HIV-infected and HIV-uninfected women with or without bacterial vaginosis. J. Infect. Dis. 198:1131–1140. 10.1086/591942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Filkins LM, Hampton TH, Gifford AH, Gross MJ, Hogan DA, Sogin ML, Morrison HG, Paster BJ, O'Toole GA. 2012. Prevalence of streptococci and increased polymicrobial diversity associated with cystic fibrosis patient stability. J. Bacteriol. 194:4709–4717. 10.1128/JB.00566-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Docktor MJ, Paster BJ, Abramowicz S, Ingram J, Wang YE, Correll M, Jiang H, Cotton SL, Kokaras AS, Bousvaros A. 2012. Alterations in diversity of the oral microbiome in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 18:935–942. 10.1002/ibd.21874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paster BJ, Russell MK, Alpagot T, Lee AM, Boches SK, Galvin JL, Dewhirst FE. 2002. Bacterial diversity in necrotizing ulcerative periodontitis in HIV-positive subjects. Ann. Periodontol. 7:8–16. 10.1902/annals.2002.7.1.8 [DOI] [PubMed] [Google Scholar]
- 63.Marsh P, Martin M. 1999. Acquisition, adherence, distribution and metabolism of the oral microflora, p 34–57 In Marsh P, Martin M. (ed), Oral microbiology, 4th ed. Reed Educational and Professional Publishing Ltd., Bodmin, Cornwall, United Kingdom [Google Scholar]
- 64.Liljemark WF, Gibbons RJ. 1971. Ability of Veillonella and Neisseria species to attach to oral surfaces and their proportions present indigenously. Infect. Immun. 4:264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.You M, Mo S, Watt RM, Leung WK. 2013. Prevalence and diversity of Synergistetes taxa in periodontal health and disease. J. Periodontal Res. 48:159–168. 10.1111/j.1600-0765.2012.01516.x [DOI] [PubMed] [Google Scholar]
- 66.Belibasakis GN, Ozturk VO, Emingil G, Bostanci N. 2013. Synergistetes cluster A in saliva is associated with periodontitis. J. Periodontal Res. 48:727–732. 10.1111/jre.12061 [DOI] [PubMed] [Google Scholar]
- 67.Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, Podar M, Leys EJ. 2012. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 6:1176–1185. 10.1038/ismej.2011.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feikin DR, Feldman C, Schuchat A, Janoff EN. 2004. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect. Dis. 4:445–455. 10.1016/S1473-3099(04)01060-6 [DOI] [PubMed] [Google Scholar]
- 69.Dang AT, Cotton S, Sankaran-Walters S, Li CS, Lee CY, Dandekar S, Paster BJ, George MD. 2012. Evidence of an increased pathogenic footprint in the lingual microbiome of untreated HIV infected patients. BMC Microbiol. 12:153. 10.1186/1471-2180-12-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aas JA, Barbuto SM, Alpagot T, Olsen I, Dewhirst FE, Paster BJ. 2007. Subgingival plaque microbiota in HIV positive patients. J. Clin. Periodontol. 34:189–195. 10.1111/j.1600-051X.2006.01034.x [DOI] [PubMed] [Google Scholar]
- 71.Baqui A, Meiller T, Jabra-Rizk M, Zhang M, Kelley J, Falkler W. 1999. Association of HIV viral load with oral diseases. Oral Dis. 5:294–298 [DOI] [PubMed] [Google Scholar]
- 72.Patel M, Coogan M, Galpin JS. 2003. Periodontal pathogens in subgingival plaque of HIV-positive subjects with chronic periodontitis. Oral Microbiol. Immunol. 18:199–201. 10.1034/j.1399-302X.2003.00064.x [DOI] [PubMed] [Google Scholar]
- 73.Goncalves LS, Soares Ferreira SM, Souza CO, Souto R, Colombo AP. 2007. Clinical and microbiological profiles of human immunodeficiency virus (HIV)-seropositive Brazilians undergoing highly active antiretroviral therapy and HIV-seronegative Brazilians with chronic periodontitis. J. Periodontol. 78:87–96. 10.1902/jop.2007.060040 [DOI] [PubMed] [Google Scholar]
- 74.Johnson NW. 1997. Essential questions concerning periodontal diseases in HIV infection. Oral Dis. 3(Suppl 1):S138–S140. 10.1111/j.1601-0825.1997.tb00347.x [DOI] [PubMed] [Google Scholar]
- 75.Lin AL, Johnson DA, Sims CA, Stephan KT, Yeh CK. 2006. Salivary gland function in HIV-infected patients treated with highly active antiretroviral therapy (HAART). Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 102:318–324. 10.1016/j.tripleo.2005.07.021 [DOI] [PubMed] [Google Scholar]
- 76.Phelan JA, Mulligan R, Nelson E, Brunelle J, Alves ME, Navazesh M, Greenspan D. 2004. Dental caries in HIV-seropositive women. J. Dent. Res. 83:869–873. 10.1177/154405910408301109 [DOI] [PubMed] [Google Scholar]
- 77.John CN, Stephen XG, Africa CWJ. 2012. BANA-positive plaque samples are associated with oral hygiene practices and not CD4+ T cell counts in HIV-positive patients. Int. J. Dent. 2012:157641. 10.1155/2012/157641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ellis CL, Ma ZM, Mann SK, Li CS, Wu J, Knight TH, Yotter T, Hayes TL, Maniar AH, Troia-Cancio PV, Overman HA, Torok NJ, Albanese A, Rutledge JC, Miller CJ, Pollard RB, Asmuth DM. 2011. Molecular characterization of stool microbiota in HIV-infected subjects by panbacterial and order-level 16S ribosomal DNA (rDNA) quantification and correlations with immune activation. J. Acquir. Immune Defic. Syndr. 57:363–370. 10.1097/QAI.0b013e31821a603c [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.