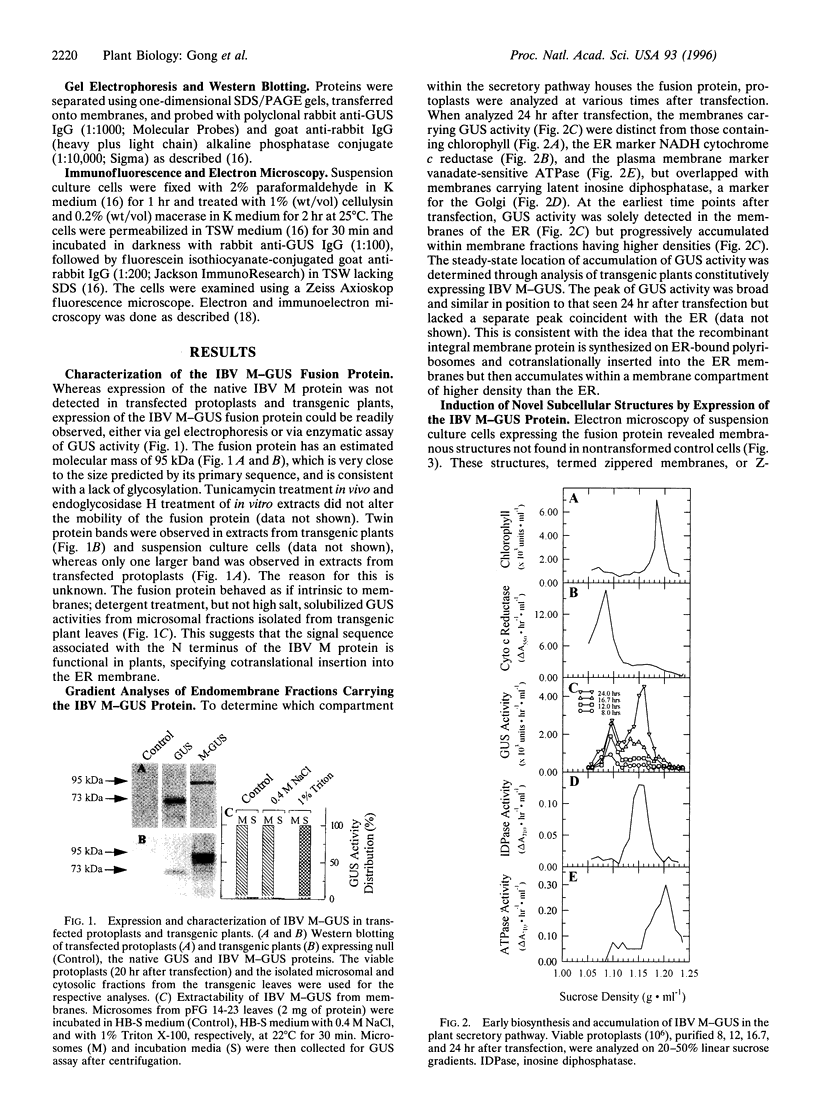
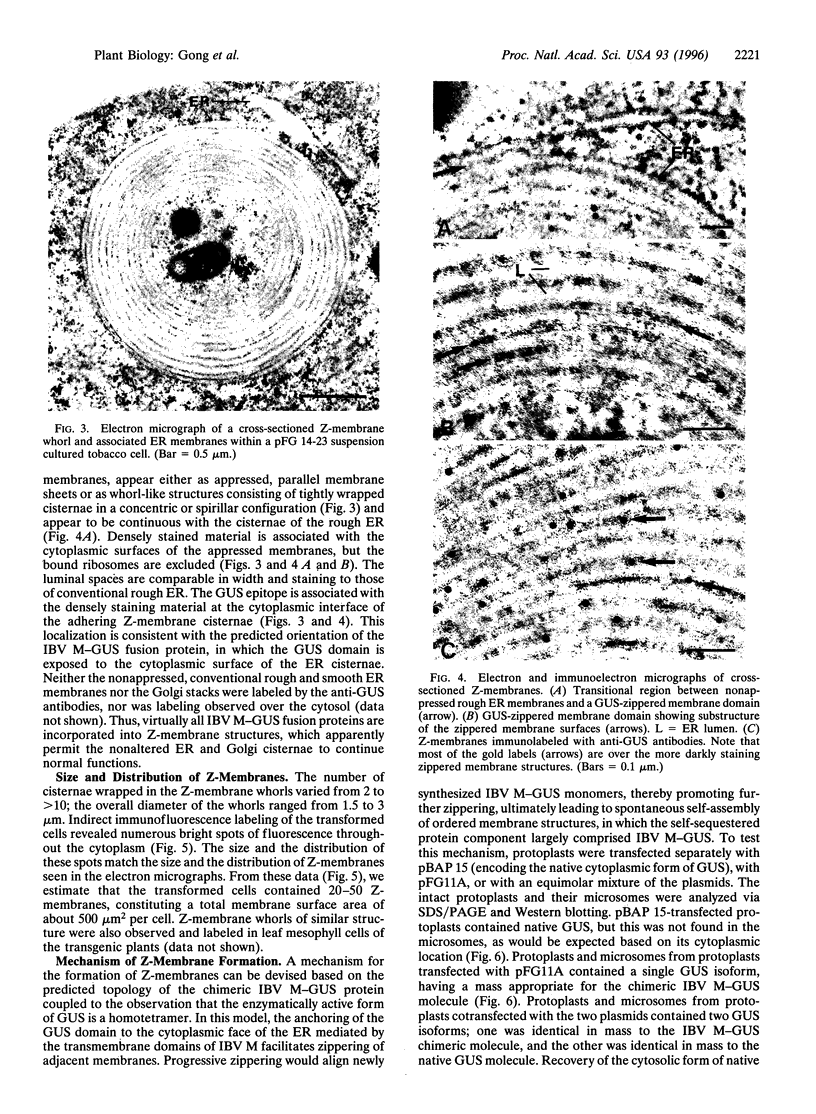
Abstract
We have expressed a fusion protein formed between the avian infectious bronchitis virus M protein and the bacterial enzyme beta-glucuronidase in transgenic tobacco cells. Electron microscope images of such cells demonstrate that overexpression of this fusion protein gives rise to a type of endoplasmic reticulum membrane domain in which adjacent membranes become zippered together apparently as a consequence of the oligomerizing action of beta-glucuronidase. These zippered (Z-) membranes lack markers of the endoplasmic reticulum (NADH cytochrome c reductase and ribosomes) and accumulate in the cells in the form of multilayered scroll-like structures (up to 2 micrometers in diameter; 20-50 per cell) without affecting plant growth. The discovery of Z-membranes has broad implications for biology and biotechnology in that they provide a means for accumulating large quantities of recombinant membrane proteins within discrete domains of native membranes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Niemann H., Smeekens S., Rottier P., Warren G. Sequence and topology of a model intracellular membrane protein, E1 glycoprotein, from a coronavirus. Nature. 1984 Apr 19;308(5961):751–752. doi: 10.1038/308751a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek S. Y., Raikhel N. V. Intracellular trafficking of secretory proteins. Plant Mol Biol. 1992 Oct;20(1):133–150. doi: 10.1007/BF00029156. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat J., Nijenhuis M., Janssen H., Tulp A., Dusseljee S., Wubbolts R., Neefjes J. Major histocompatibility complex class II molecules induce the formation of endocytic MIIC-like structures. J Cell Biol. 1994 Aug;126(4):967–977. doi: 10.1083/jcb.126.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Staehelin L. A. Budding, fission, transport, targeting, fusion--frontiers in secretion research. Plant Cell. 1992 Sep;4(9):1008–1016. doi: 10.1105/tpc.4.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driouich A., Faye L., Staehelin L. A. The plant Golgi apparatus: a factory for complex polysaccharides and glycoproteins. Trends Biochem Sci. 1993 Jun;18(6):210–214. doi: 10.1016/0968-0004(93)90191-o. [DOI] [PubMed] [Google Scholar]
- Garoff H. Using recombinant DNA techniques to study protein targeting in the eucaryotic cell. Annu Rev Cell Biol. 1985;1:403–445. doi: 10.1146/annurev.cb.01.110185.002155. [DOI] [PubMed] [Google Scholar]
- Guerineau F., Woolston S., Brooks L., Mullineaux P. An expression cassette for targeting foreign proteins into chloroplasts. Nucleic Acids Res. 1988 Dec 9;16(23):11380–11380. doi: 10.1093/nar/16.23.11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins K. R., Jefferson R. A., Kavanagh T. A., Bevan M. W., Galbraith D. W. Expression of photosynthesis-related gene fusions is restricted by cell type in transgenic plants and in transfected protoplasts. Proc Natl Acad Sci U S A. 1990 Jan;87(2):816–820. doi: 10.1073/pnas.87.2.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W., Tang B. L. Protein trafficking along the exocytotic pathway. Bioessays. 1993 Apr;15(4):231–238. doi: 10.1002/bies.950150403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch R. B., Klee H. J. Rapid assay of foreign gene expression in leaf discs transformed by Agrobacterium tumefaciens: Role of T-DNA borders in the transfer process. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4428–4432. doi: 10.1073/pnas.83.12.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Burgess S. M., Hirsh D. beta-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Mentone S. A., Rose J. K., Farquhar M. G. The E1 glycoprotein of an avian coronavirus is targeted to the cis Golgi complex. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6944–6948. doi: 10.1073/pnas.87.18.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. A specific transmembrane domain of a coronavirus E1 glycoprotein is required for its retention in the Golgi region. J Cell Biol. 1987 Sep;105(3):1205–1214. doi: 10.1083/jcb.105.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Lam D. M., Arntzen C. J. Expression of hepatitis B surface antigen in transgenic plants. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11745–11749. doi: 10.1073/pnas.89.24.11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Afonso C. L., Galbraith D. W. Isolation and characterization of monoclonal antibodies directed against plant plasma membrane and cell wall epitopes: identification of a monoclonal antibody that recognizes extensin and analysis of the process of epitope biosynthesis in plant tissues and cell cultures. J Cell Biol. 1988 Jul;107(1):163–175. doi: 10.1083/jcb.107.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Hoe M. H., Slusarewicz P., Rabouille C., Watson R., Hunte F., Watzele G., Berger E. G., Warren G. Kin recognition between medial Golgi enzymes in HeLa cells. EMBO J. 1994 Feb 1;13(3):562–574. doi: 10.1002/j.1460-2075.1994.tb06294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak R. K., Luskey K. L., Anderson R. G. Biogenesis of the crystalloid endoplasmic reticulum in UT-1 cells: evidence that newly formed endoplasmic reticulum emerges from the nuclear envelope. J Cell Biol. 1986 Jun;102(6):2158–2168. doi: 10.1083/jcb.102.6.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryer N. K., Wuestehube L. J., Schekman R. Vesicle-mediated protein sorting. Annu Rev Biochem. 1992;61:471–516. doi: 10.1146/annurev.bi.61.070192.002351. [DOI] [PubMed] [Google Scholar]
- Roe J. N. Biosensor development. Pharm Res. 1992 Jul;9(7):835–844. doi: 10.1023/a:1015828311073. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The structure and insertion of integral proteins in membranes. Annu Rev Cell Biol. 1990;6:247–296. doi: 10.1146/annurev.cb.06.110190.001335. [DOI] [PubMed] [Google Scholar]
- Vergères G., Yen T. S., Aggeler J., Lausier J., Waskell L. A model system for studying membrane biogenesis. Overexpression of cytochrome b5 in yeast results in marked proliferation of the intracellular membrane. J Cell Sci. 1993 Sep;106(Pt 1):249–259. doi: 10.1242/jcs.106.1.249. [DOI] [PubMed] [Google Scholar]
- Weisz O. A., Swift A. M., Machamer C. E. Oligomerization of a membrane protein correlates with its retention in the Golgi complex. J Cell Biol. 1993 Sep;122(6):1185–1196. doi: 10.1083/jcb.122.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesuriya D. C., Rechnitz G. A. Biosensors based on plant and animal tissues. Biosens Bioelectron. 1993;8(3-4):155–160. doi: 10.1016/0956-5663(93)85027-l. [DOI] [PubMed] [Google Scholar]
- Zhang G. F., Staehelin L. A. Functional compartmentation of the Golgi apparatus of plant cells : immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol. 1992 Jul;99(3):1070–1083. doi: 10.1104/pp.99.3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984 Aug;3(8):1791–1797. doi: 10.1002/j.1460-2075.1984.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]