ABSTRACT
Human immunodeficiency virus type 1 (HIV-1) replication in dendritic cells (DCs) is restricted by SAMHD1. This factor is counteracted by the viral protein Vpx; Vpx is found in HIV-2 and simian immunodeficiency virus (SIV) from sooty mangabeys (SIVsm) or from macaques (SIVmac) but is absent from HIV-1. We previously observed that HIV-1 replication in immature DCs is stimulated by cocultivation with primary T and B lymphocytes, suggesting that HIV-1 restriction in DCs may be overcome under coculture conditions. Here, we aimed to decipher the mechanism of SAMHD1-mediated restriction in DC-lymphocyte coculture. We found that coculture with lymphocytes downregulated SAMHD1 expression and was associated with increased HIV-1 replication in DCs. Moreover, in infected DC-T lymphocyte cocultures, DCs acquired maturation status and secreted type 1 interferon (alpha interferon [IFN-α]). The blockade of DC-lymphocyte cross talk by anti-ICAM-1 antibody markedly inhibited the stimulation of HIV-1 replication and prevented the downregulation of SAMHD1 expression in cocultured DCs. These results demonstrate that, in contrast to purified DCs, cross talk with lymphocytes downregulates SAMHD1 expression in DCs, triggering HIV-1 replication and an antiviral immune response. Therefore, HIV-1 replication and immune sensing by DCs should be investigated in more physiologically relevant models of DC/lymphocyte coculture.
IMPORTANCE SAMHD1 restricts HIV-1 replication in dendritic cells (DCs). Here, we demonstrate that, in a coculture model of DCs and lymphocytes mimicking early mucosal HIV-1 infection, stimulation of HIV-1 replication in DCs is associated with downregulation of SAMHD1 expression and activation of innate immune sensing by DCs. We propose that DC-lymphocyte cross talk occurring in vivo modulates host restriction factor SAMHD1, promoting HIV-1 replication in cellular reservoirs and stimulating immune sensing.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) replication has been proposed to be highly restricted in myeloid dendritic cells (DCs) (1, 2). The poor capacity of these cells to support replication was recently explained by the presence of the host restriction factor SAMHD1 (3–5). Restriction by SAMHD1 was observed in DCs infected with cell-free HIV-1 and in the presence of infected CD4 T cells (6). SAMHD1 diminishes intracellular pools of deoxynucleoside triphosphates (dNTPs), substrates necessary for the synthesis of viral DNA (7–9), and its antiviral activity is inhibited following phosphorylation (10–12). Of note, HIV-1 inhibition by SAMHD1 is counteracted by the viral protein Vpx found in HIV-2 and in simian immunodeficiency virus (SIV) from macaques (SIVmac) (3, 4); Vpx is absent from HIV-1 (13, 14). Vpx degrades SAMHD1 in numerous cell types (3, 4, 6, 9, 15–21), allowing efficient viral DNA synthesis and significantly enhanced HIV-1 replication in DCs (2, 22). The paralogous viral protein of Vpx is Vpr, which is encoded by all lineages of lentivirus (23). In lentiviral lineages, which do not encode SAMHD1 antagonist Vpx, the Vpr protein has also been found to degrade SAMHD1 (24, 25). These findings of viral adaptation to host restriction suggest that SAMHD1 antagonism is a component of viral fitness in the context of natural infections (26).
Monocyte-derived dendritic cells (MoDCs) have been used as model for myeloid DCs (27, 28). These cells do not undergo maturation following HIV-1 infection (29–33) and produce only small amounts of interferon (IFN) (6, 33). Intracellular delivery of Vpx to MoDCs induces sensing of HIV-1 with production of type 1 IFN, upregulation of the costimulatory molecule CD86, and triggering of DC maturation (21, 34, 35). Therefore, sensing of HIV-1 in DCs has been proposed to be limited by the presence of SAMHD1.
We have previously demonstrated that HIV-1 replication in primary HIV-1 isolate-loaded immature DCs is enhanced when they are cocultured with autologous primary CD4 T or B lymphocytes (29, 36). In this study, we investigated whether this enhanced HIV-1 replication in cocultured DCs was due to modulation of SAMHD1 expression. We found that the stimulation of HIV-1 replication in DCs during cross talk with primary lymphocytes was associated with the decreased expression of SAMHD1. In addition, IFN-α was secreted into the medium of infected DC-T lymphocyte cocultures, and DCs acquired the maturation status. These results demonstrate for the first time that coculture with lymphocytes downregulates the expression of the host restriction factor SAMHD1 in DCs, and this decreased expression is associated with both efficient HIV-1 replication in DCs and the triggering of an antiviral immune response.
MATERIALS AND METHODS
Antibodies.
Mouse anti-human CD3-VioBlue (BW264/56) and CD83-allophycocyanin (APC) (HB15) monoclonal antibodies (MAbs) were purchased from Miltenyi Biotec SAS (France). Peridinin chlorophyll protein (PerCP)-Cy5.5-conjugated mouse MAb against human CD209 (DC-SIGN; DCN46) was purchased from BD Pharmingen (San Diego, CA). HIV-1 antigen (Ag) p24 APCA700 and goat F(ab′)2 fragment anti-mouse IgG1-phycoerythrin (PE) Abs were purchased from Beckman-Coulter (Roissy, France). Anti-ICAM-1 antibody (clone 1H4 [azide free]) was purchased from Abcam (United Kingdom). Mouse monoclonal IgG1 directed against human SAMHD1 antibody (clone I19-18) was kindly provided by Olivier Schwartz and Françoise Porrot (Institut Pasteur, Unité Virus et Immunité, Paris, France): this antibody was selected for its ability to recognize SAMHD1 in flow cytometry assays (6, 17, 37).
Cell preparation.
All human blood samples were collected from healthy anonymized donors seronegative for HIV-1 and hepatitis C virus (HCV) (EFS, Strasbourg, France). The primary human immune cells, immature monocyte-derived dendritic cells (MoDCs), were obtained by purification of human blood CD14+ monocytes (with a high degree of purity, >99.5%) by immunomagnetic bead isolation after Ficoll-Hypaque sedimentation (AutoMACS; Miltenyi Biotec). Highly purified CD14+ monocytes were differentiated to MoDCs at 2 × 106 cells/ml in RPMI 1640 plus GlutaMAX (GIBCO) medium supplemented with 5% fetal calf serum (FCS), 10 ng/ml of granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems), and 20 ng/ml interleukin-4 (IL-4) (R&D Systems) for 6 days, as previously described (29, 30, 36). Autologous CD19+ B lymphocytes and CD4+ T lymphocytes were purified by positive selection (AutoMACS; Miltenyi Biotec) after CD14+ purification. All nonactivated CD19+ B lymphocytes and nonactivated CD4+ T lymphocytes were frozen immediately after purification. The resting primary purified CD4+ T lymphocytes were activated by incubation with phytohemagglutinin A (PHA) (2 μg/ml) for 3 days in RPMI 1640 plus GlutaMAX medium supplemented with 10% FCS. Cells were stored frozen and thawed 1 day before use.
Cells of the MT4 T cell line (NIH) was cultured in RPMI 1640 plus GlutaMAX medium supplemented with 10% FCS at 37°C under 5% CO2; all media used were supplemented with 100 U/ml of penicillin and 100 μg/ml of streptomycin (GIBCO).
Virus preparation.
The primary clinical HIV-1 isolates were amplified on human blood leukocytes, as previously described (30). Virus stocks collected at peak virus production were concentrated 70-fold with a 100-kDa-cutoff polyethersulfone filter (Centricon Plus-70 Biomax filter; Millipore, Molsheim, France). The primary clinical R5 isolate HIV-1BaL (subtype B) was provided by S. Gartner, M. Popovic, and R. Gallo from the National Institutes of Health (NIH). HIV-1SF162 (subtype B, R5 strain) was obtained from the AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH). HIV-1QH0 (subtype B, R5 strain) was obtained from the National Institute for Biological Standards and Control (NIBSC). An SIV type 3 (SIV-3)-positive wild-type strain containing vesicular stomatitis virus G glycoprotein (VSV-G)-pseudotyped virus and Vpx (SIV-Vpx-VSV-G) and named VLP-Vpx was kindly provided by Olivier Schwartz and Françoise Porrot (Institut Pasteur, Paris, France) (6).
HIV-1 transfer experiments.
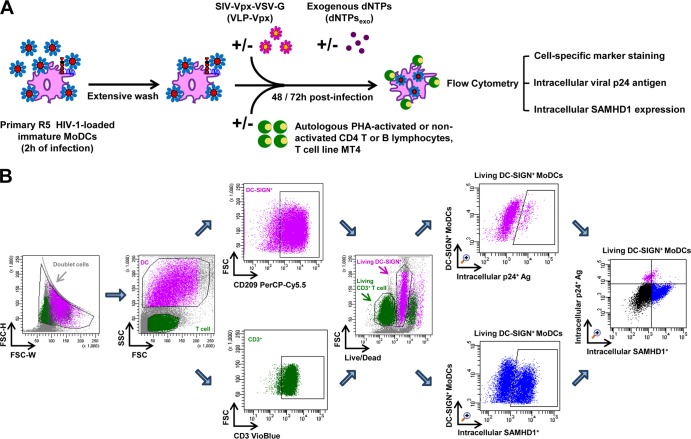
The conditions used for MoDC infection and coculture with PHA-activated autologous CD4 T lymphocytes, nonactivated CD4 T lymphocytes, and nonactivated B lymphocytes were similar to those previously described (29, 36) (Fig. 1A). Briefly, immature MoDCs were infected with primary R5 HIV-1 isolates at a concentration of 500 ng/ml viral p24 antigen such that >2% of the cells were infected. After 2 h of incubation, MoDCs were thoroughly washed three times to remove unbound free viral particles. Twenty-five microliters of HIV-1-loaded MoDCs (at 6 × 106 cells/ml) was incubated with or without 25 μl of autologous PHA-activated or nonactivated CD4 T and B lymphocytes (at 24 × 106 cells/ml, 1:4 ratio) or MT4 cells (at 6 × 106 cells/ml, 1:1 ratio) in 96-well plates. Where indicated, virus-like particles containing Vpx (VLP-Vpx) or exogenous deoxynucleotide triphosphates (dNTPs) (10 μM) (Invitrogen Life Science, Applied Biosystems Inc., CA) were added to cells as controls to decrease SAMHD1 levels and stimulate HIV-1 replication, respectively. For some experiments, the HIV-1 reverse transcriptase inhibitor azidothymidine (AZT [Sigma]) was added (5 μM) 2 h after MoDC infection, concomitantly with the addition of CD4 T lymphocytes to prevent productive HIV-1 replication. Viral p24 antigen detected in AZT-treated wells was therefore indicative of residual p24 detected in the absence of de novo virus synthesis. The HIV-1 protease inhibitor indinavir (IDV) (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH), added at 1 μM (38) to MoDCs at the same time as CD4 T lymphocytes, limits the infection to a single cycle of HIV-1 replication, therefore preventing DCs from de novo virus infection. Unless otherwise stated, productive infection was quantified by flow cytometry, based on the detection of intracellular viral p24 antigen in the MoDC population after 48 and 72 h of culture (Fig. 1A).
FIG 1.
Schematic representation of HIV-1 cell-to-cell transfer assay and the gating strategy for flow cytometry analysis. (A) HIV-1 cell-to-cell transfer assay. Immature MoDCs were pulsed with primary R5 HIV-1 isolates for 2 h and then thoroughly washed to remove unbound free viral particles. HIV-1-loaded MoDCs were incubated with or without autologous PHA-activated or nonactivated CD4 T or B lymphocytes or cells of the MT4 T cell line in 96-well plates. Where indicated, virus-like particles containing Vpx (VLP-Vpx) or exogenous dNTPs were added to infected MoDCs at the same time as lymphocytes as controls to decrease SAMHD1 levels and stimulate HIV-1 replication, respectively. After 48 and 72 h of culture, productive infection in MoDCs was quantified by detection of intracellular viral p24 antigen and intracellular SAMHD1 levels simultaneously by flow cytometry using cell-specific marker staining. (B) Quantification of productive infection and intracellular SAMHD1 levels by flow cytometry. Among all events, forward width and forward area were used to exclude doublet cells; forward angle and side scatter light gating were used to exclude cell debris. The DC population can easily be distinguished from lymphocytes according to size. Within the DC population, Ab directed against human CD209 (DC-SIGN, a DC-specific surface marker) was used to select DC-SIGN+ CD3− MoDCs; in the T cell population, Ab directed against human CD3 was used to select CD3+ CD4 T cells. Dead cells were then excluded with LIVE/DEAD fixable dead cell stain fluorescence kits. Percentages of living DC-SIGN+ MoDCs that are infected and contain intracellular SAMHD1 can be determined simultaneously. Double staining for HIV-1 infection and SAMHD1 expression in living DC-SIGN+ MoDCs is shown. The final analysis was performed with FACS Diva software, which generated a graphical output. FSC, forward scatter; SSC, side scatter.
Inhibition of dendritic cell-lymphocyte cross talk.
In order to prevent the cross talk between MoDCs and autologous lymphocytes, anti-ICAM-1 monoclonal antibody was added at 20 μg/ml to HIV-1-loaded MoDCs prior to the coculture with autologous lymphocytes for 48 h.
Cell staining and flow cytometry assay.
Briefly, cells were labeled with LIVE/DEAD fixable dead cell stain fluorescence kits (Invitrogen Life Science, Applied Biosystems, Inc., CA) for 10 min at room temperature. The cells were then washed, fixed and permeabilized with the Cytofix and Perm/Wash kit solutions (BD Biosciences), and subjected to intracellular SAMHD1 staining for 15 min at 4°C and then incubated for 15 min at 4°C with goat F(ab′)2 fragment anti-mouse IgG1-PE. The samples were then washed, a mixture of Abs directed against the human CD209, CD3, and intracellular p24 antigen was added, and the samples were incubated for 10 min at 4°C. The percentages of infection and SAMHD1 labeling were determined simultaneously by flow cytometry for each of DC-SIGN+ (CD209+) CD3− MoDCs, CD3+ DC-SIGN− CD4 T lymphocytes, and CD19+ DC-SIGN− B lymphocytes. For MoDC maturation kinetics studies, a mixture of Abs directed against human CD209, CD83, and CD3 was incubated with the samples for 10 min at 4°C, and the cells were then fixed and permeabilized. Multicolor samples were acquired with a LSRII SORP cytometer (BD Bioscience, San Jose, CA). Cytometer setup and tracking (CST) calibration particles were used to ensure that fluorescence intensity measurement was consistent in all experiments. Flow cytometry CompBeads kits (BD) were used for compensation. Forward angle and side scatter light gating were used to exclude cell debris from the analysis. Forward width and forward area were used to exclude doublet cells, and dead cells were excluded with the LIVE/DEAD kit solution. The final analysis was performed with FACS (i.e., fluorescence-activated cell sorter) Diva software version 6.1.2 (BD), which generated a graphical output. The strategies for the analysis of flow cytometry data are detailed in Fig. 1B.
ELISA.
The number of HIV-1 particles released into the supernatant of cell cultures was detected by dosage of HIV-1 p24 antigen by enzyme-linked immunosorbent assay (ELISA) (Innogenetics/Ingen) following the manufacturer's instruction. Supernatants from cell cultures treated with AZT were used as a negative control to determine residual p24 due to the virus inoculum.
Cytokine analysis.
Supernatants were collected at various time points during HIV-1 infection for analysis of cytokine production. IFN-α was assayed with the Verikine human IFN-α multisubtype ELISA kit (PBL Interferon Source, NJ). This kit is specifically formulated to detect 14 of the 15 identified human IFN-α subtypes using a VMax Kinetic ELISA microplate reader to determine the absorbance at 450 nm (Molecular Devices, California, USA).
Statistical analysis.
All data plotted with error bars are expressed as means ± standard deviations (SD). The P values were generated by analyzing data with a one-way analysis of variance (ANOVA) (Kruskal-Wallis test) for group comparisons or by two-tailed pairwise comparison based on Wilcoxon matched-pairs signed rank test. P values of <0.05 were considered statistically significant. Correlations were analyzed by calculating Pearson's correlation coefficient, with P values of <0.05 considered statistically significant. GraphPad Prism 5.04 software (GraphPad, San Diego, CA) was used for all statistical analyses.
RESULTS
The increased HIV-1 replication in immature MoDCs in the presence of activated CD4 T lymphocytes is inversely correlated with SAMHD1 expression.
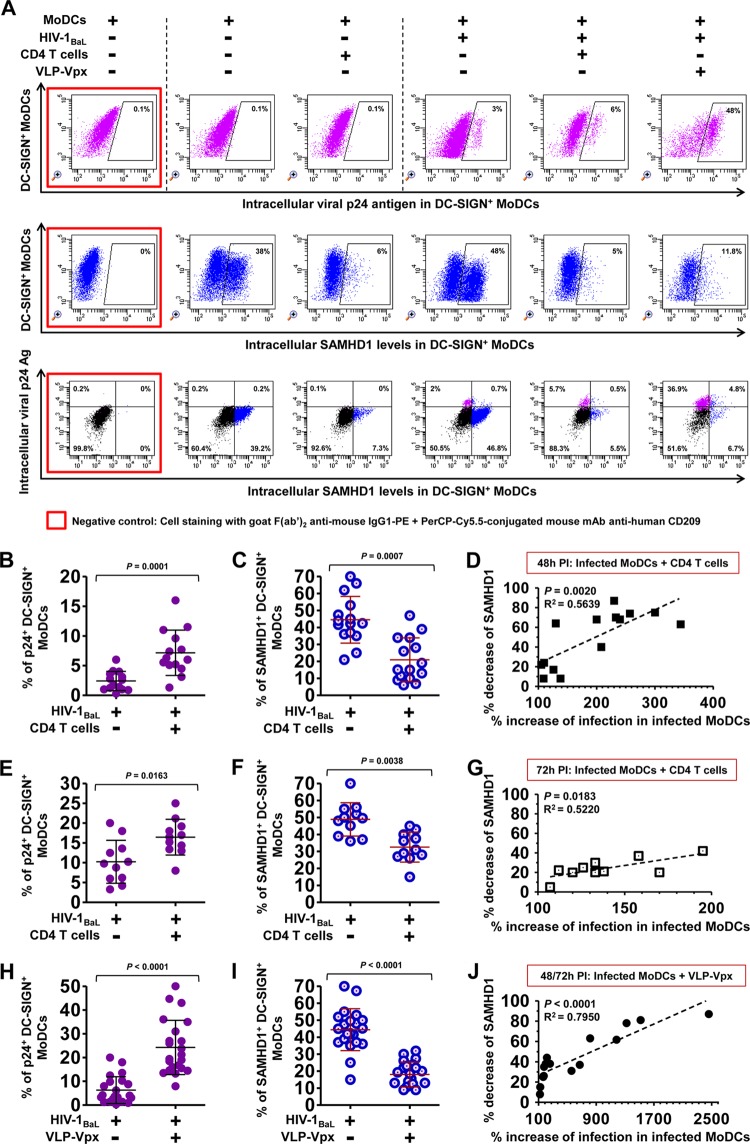
Using a coculture model (Fig. 1), we observed that coculture with autologous PHA-activated CD4 T lymphocytes significantly increased the percentage of HIV-1 infected MoDCs after 48 h (Fig. 2A and B) and 72 h (Fig. 2E) of culture. Enhancement of HIV-1 replication was also observed in DCs transduced with VLP-Vpx, which was added as control (Fig. 2A and H), suggesting that, like Vpx, the coculture with CD4 T lymphocytes may regulate HIV-1 replication in DCs by modulating SAMHD1 expression.
FIG 2.
Detection of intracellular HIV-1 p24 antigen and SAMHD1, and the correlation between the two markers in immature MoDCs. (A) Dot plot showing the percentages of HIV-1 intracellular p24 Ag (top), intracellular SAMHD1 expression (middle), and double intracellular p24 Ag and SAMHD1 staining (bottom) within DC-SIGN+ MoDCs at 48 h postinfection (PI) by primary HIV-1BaL in the presence or absence of activated CD4 T lymphocytes or VLP-Vpx. One representative experiment from 12 independent experiments performed with 12 different healthy blood donors is shown. Shown are percentages of infected MoDCs with primary HIV-1BaL (B and E) and SAMHD1 expression in DC-SIGN+ MoDCs (C and F) after 48 h (B and C) or 72 h (E and F) of infection as percentages of cells in the absence or presence of activated CD4 T cells. The percentages of infected MoDCs (H) and SAMHD1 expression (I) among DC-SIGN+ MoDCs were measured 48 and 72 h postinfection in the absence or presence of VLP-Vpx. Data are means ± standard deviations (SD) from at least 12 independent experiments performed with cells from 12 healthy blood donors in duplicate. (Each point represents the result from one donor.) Horizontal bars denote medians, and whiskers denote interquartile ranges. The significance of differences was determined with two-tailed Wilcoxon matched-pairs signed rank test, and P < 0.05 is considered significant. Plotted is the correlation between the percent decrease of SAMHD1 levels (with respect to control infected MoDCs alone) and the percent increase of infection in cocultured DC-SIGN+ MoDCs with activated CD4 T lymphocytes at 48 h (D) and 72 h (G) postinfection and infected DC-SIGN+ MoDCs alone in the presence of VLP-Vpx as a control (J). Repeated independent experiments were performed with cells from at least 12 healthy donors. Correlations were analyzed by calculating Pearson's correlation coefficient, with P < 0.05 considered significant.
Therefore, to investigate SAMHD1-mediated restriction in DC-lymphocyte coculture, we analyzed HIV-1 infection and SAMHD1 expression in DC-SIGN+ MoDCs at 48 and 72 h postinfection. Double staining of HIV-1 intracellular p24 antigen and SAMHD1 revealed that SAMHD1 expression was significantly lower in HIV-1-infected MoDCs when they were cultivated with activated CD4 T lymphocytes (Fig. 2A and C, 48 h postinfection; F, 72 h postinfection). This decreased SAMHD1 expression was also detected when MoDCs were treated with VLP-Vpx (Fig. 2A and I), indicating that the presence of autologous CD4 T lymphocytes downregulate SAMHD1 expression in DCs to a similar extent as Vpx. Most MoDCs positive for intracellular p24+ were SAMHD1 negative (Fig. 2A), consistent with a restriction of HIV-1 infection by SAMHD1 (3, 6). Moreover, the decrease of SAMHD1 expression was strongly associated with the increase of HIV-1 replication in cocultured MoDCs at 48 and 72 h postinfection (Fig. 2D and G). A similar association between decreased SAMHD1 expression and increased HIV-1 replication was observed in VLP-Vpx-transduced and HIV-1-infected MoDCs (Fig. 2J). These results suggest that the increased HIV-1 replication in DCs following DC/T coculture is due to the downregulation of SAMHD1 expression.
Stimulation of HIV-1 replication in cocultured MoDCs occurs before viral assembly.
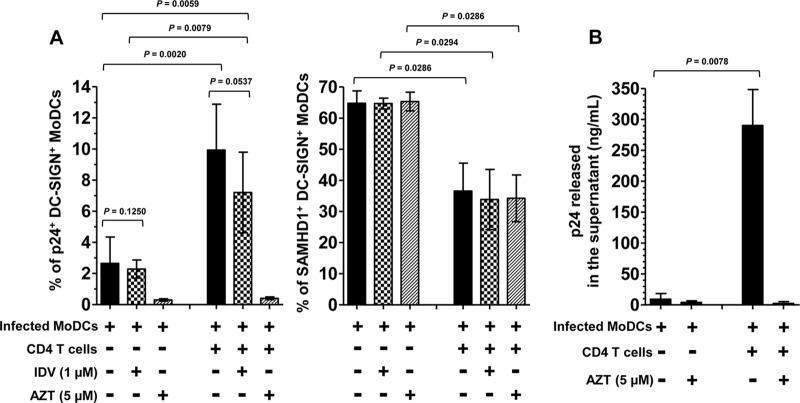
First, we confirmed that the percentage of p24+ cells detected by flow cytometry corresponds to newly synthesized HIV-1 by treatment of the cells with a reverse transcriptase inhibitor, AZT. We observed that treatment with AZT strongly inhibited the percentage of infected cells (Fig. 3A, left panel) (36, 38). In addition, significant viral p24 released was detected in the supernatants of coculture (Fig. 3B). These results demonstrate that the percentage of p24+ cells corresponds to infected cells producing de novo virus particles released in the supernatant of coculture. Next, we investigated whether the increased HIV-1 replication in cocultured MoDCs was due to the stimulation of HIV-1 replication in these cells or to an external refeeding with virions newly synthesized by PHA-activated CD4 T lymphocytes. We therefore added a protease inhibitor, indinavir (IDV), 2 h after the infection of MoDCs, at the same time as activated CD4 T lymphocytes. IDV prevents new cycles of infection by blocking final assembly and maturation of newly synthesized virions and thus restraining HIV-1 to a single cycle of replication. In the presence of IDV, the level of infected MoDCs in the coculture corresponds to 80% of the level of control infected MoDCs in the absence of IDV (Fig. 3A, left panel). The HIV-1 replication detected in MoDCs after 48 h was therefore mainly due to a single cycle of infection. This percentage of infected MoDCs treated by IDV was increased in coculture by 3.1-fold compared to treated MoDCs in the absence of CD4 T lymphocytes (Fig. 3A, left panel). This increased infection indicates that the stimulation of HIV-1 replication in cocultured MoDCs occurs before viral assembly and is not the consequence of refeeding of MoDCs with virions newly synthesized by PHA-activated CD4 T lymphocytes. Interestingly, the presence of inhibitors IDV and AZT did not modify the expression of SAMHD1 in MoDCs (Fig. 3A, right panel). The downregulation of SAMHD1 expression in MoDCs was related to the presence of CD4 T lymphocytes, suggesting that CD4 T lymphocytes and not HIV-1 replication trigger the decrease in SAMHD1 expression.
FIG 3.
Single cycle of HIV-1 infection in cocultured MoDCs. (A) Percentages of HIV-1BaL-infected DC-SIGN+ MoDCs (left panel) were measured at 48 h postinfection in the presence or absence of activated CD4 T lymphocytes treated with HIV-1 protease inhibitor indinavir (IDV) at 1 μM to prevent infection of new virus produced by MoDCs or T lymphocytes or treated with HIV-1 reverse transcriptase inhibitor azidothymidine (AZT) at 5 μM to prevent HIV-1 replication as negative controls. Data are expressed as means ± SD from 10 independent experiments performed with 10 healthy blood donors. Under the same conditions, we measured intracellular SAMHD1 levels in DC-SIGN+ MoDCs (right panel). Data are expressed as means ± SD from four healthy blood donors. (B) Quantification of the amounts of viral p24 released into the supernatant when HIV-1-infected immature MoDCs were cultured with or without activated CD4 T cells in the presence or absence of negative controls treated with AZT. The means ± SD from eight different donors after 48 h of infection are shown. The significance of differences was determined with two-tailed Wilcoxon matched-pairs signed rank test. P values of <0.05 were considered statistically significant.
Coculture with various primary lymphocyte subtypes downregulates SAMHD1 expression in MoDCs.
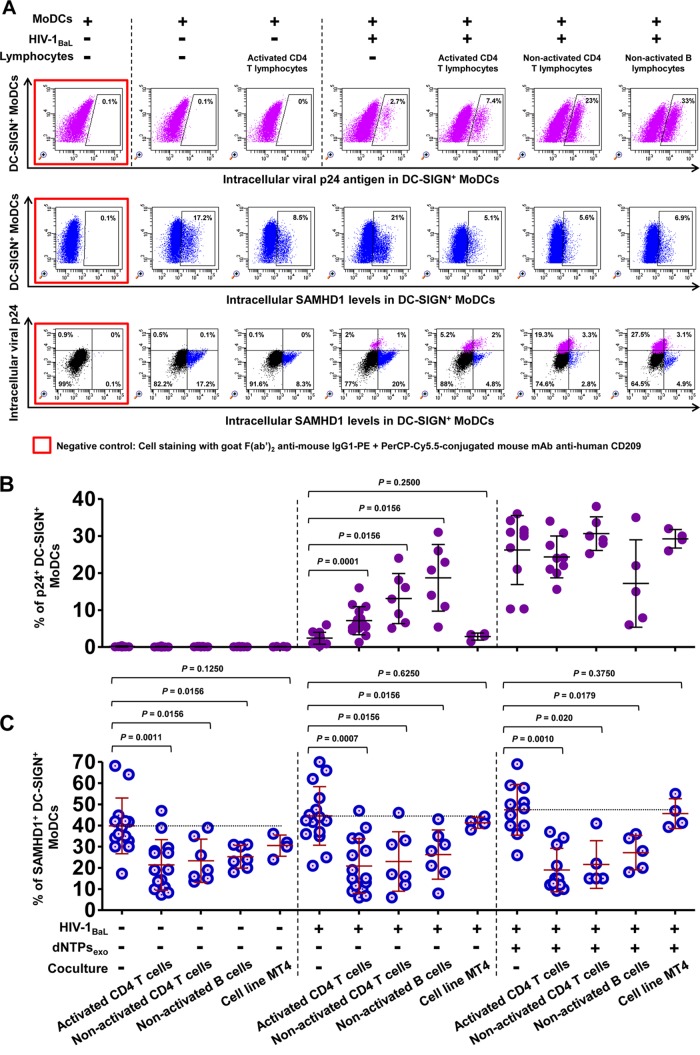
As PHA-activated primary CD4 T lymphocytes contain high concentrations of dNTPs (3 to 30 μM) (9, 39, 40), we first investigated whether dNTPs carried by activated CD4 T lymphocytes had any effect on HIV-1 replication and SAMHD1 restriction in DCs. Exogenous dNTPs were thus added to MoDCs (Fig. 4). The presence of exogenous dNTPs markedly enhanced HIV-1 replication in DCs without modifying SAMHD1 levels (Fig. 4). Thus, the downregulation of SAMHD1 expression in cocultured DCs does not appear to be due to the abundant dNTPs carried over from activated CD4 T lymphocytes.
FIG 4.
Measurement of HIV-1 infection and SAMHD1 expression in immature MoDCs cocultivated with various primary lymphocyte subtypes and MT4. (A) Dot plot showing the percentages of HIV-1 infection (top), intracellular SAMHD1 expression (middle), and double intracellular p24 Ag and SAMHD1 staining (bottom) among DC-SIGN+ MoDCs in the absence or presence of virus, PHA-activated CD4 T lymphocytes, or nonactivated CD4 T and B lymphocytes at 48 h postinfection. One representative experiment from seven independent experiments performed with seven different healthy blood donors is shown. HIV-1 infection (B) and SAMHD1 expression (C) were measured as percentages of DC-SIGN+ MoDCs at 48 h postinfection in the absence or presence of virus, autologous primary lymphocytes, T cell line MT4, or exogenous dNTPs. The data are the means ± SD from at least seven independent experiments performed with DCs from seven healthy blood donors in duplicate (except for coculture with MT4, where n = 4). Horizontal bars denote the medians, and error bars denote the interquartile ranges. The significance of differences was determined with two-tailed Wilcoxon matched-pairs signed rank test, with P < 0.05 considered significant.
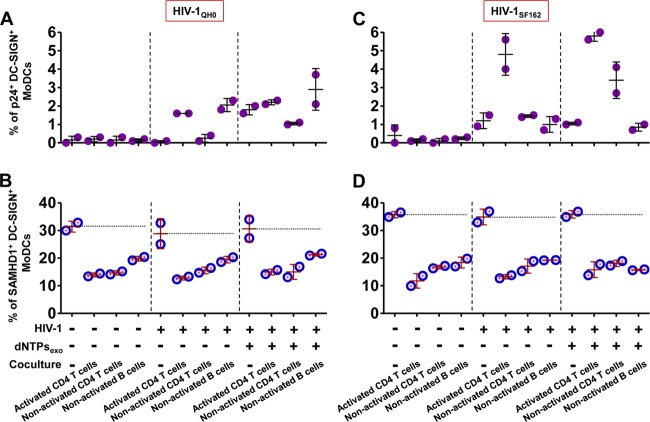
To further assess whether virions newly synthesized by activated CD4 T lymphocytes affect SAMHD1 expression, MoDCs were cocultivated with autologous nonactivated CD4 T or B lymphocytes, two cell types that are naturally resistant to HIV-1 replication. Indeed, nonactivated T/B lymphocytes did not replicate HIV-1 under these coculture conditions although they strongly stimulated HIV-1 replication in MoDCs (Fig. 4A and B). Interestingly, we found that the expression of SAMHD1 by MoDCs was significantly decreased by cocultivation with these various lymphocyte populations following infection by primary clinical isolate HIV-1BaL (Fig. 4A and C) or HIV-1QH0 and HIV-1SF162 (Fig. 5). Therefore, modulation of SAMHD1 expression in DCs is independent of HIV-1 replication and is observed in the presence of various types of autologous primary lymphocytes. These decreased SAMHD1 levels and increased HIV-1 replication in cocultured MoDCs were also observed in the presence of exogenous dNTPs (Fig. 4C and 5).
FIG 5.
Measurement of HIV-1 infection and SAMHD1 expression in immature MoDCs infected with primary HIV-1 isolates HIV-1QH0 and HIV-1SF162 when cocultured with autologous primary lymphocyte populations. Dot plots show the percentages of HIV-1 infection and SAMHD1 levels in DC-SIGN+ MoDCs in the absence or presence of PHA-activated CD4 T lymphocytes or nonactivated CD4 T or B lymphocytes at 48 h postinfection, infected with primary clinical isolate HIV-1QH0 (A and B) or HIV-1SF162 (C and D). The data are the means ± SD from one representative experiment.
Interestingly, in the absence of HIV-1 infection, SAMHD1 levels in MoDCs were also significantly decreased by cocultivation with autologous lymphocytes for 48 h (P < 0.05) (Fig. 4C and 5B and D). In contrast, the addition of cells of the T cell line MT4 to infected MoDCs had no effect on the percentage of p24+ DC-SIGN+ MoDCs (Fig. 4B) and also had no effect on the levels of SAMHD1 expression (Fig. 4C). These results suggest that decrease of SAMHD1 expression in immature MoDCs is associated with the formation of stable immunological synapses between DCs and autologous primary lymphocytes that may not occur with the T cell line MT4.
Dendritic cell-lymphocyte cross talk is required for the stimulation of HIV-1 production in MoDCs.
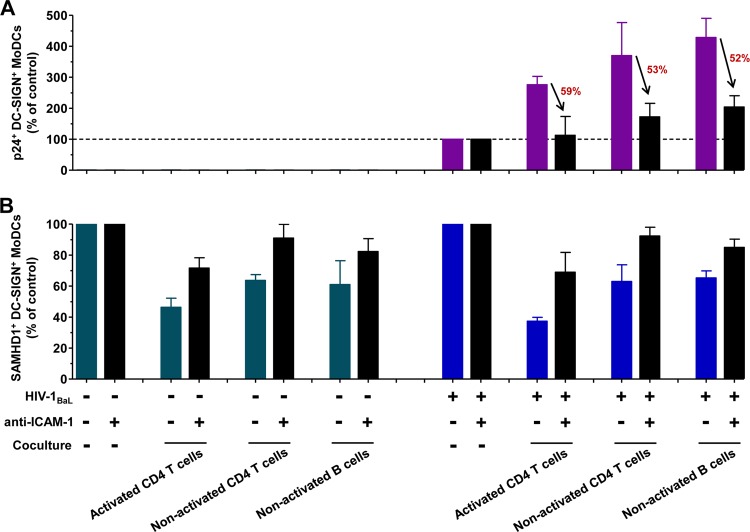
The interaction between adhesion molecule LFA-1 and its ligand ICAM-1 plays significant roles not only in the formation of immunological synapses and antigen-presenting cell-T cell interactions but also in cell-to-cell HIV-1 transmission (36, 41–44). To investigate the functional role of DC-lymphocyte cross talk, we used an anti-ICAM-1 monoclonal antibody to prevent the formation of immunological synapse between immature MoDCs and autologous lymphocytes. The addition of 20 μg/ml antibody directed against ICAM-1 to HIV-1-infected MoDCs prior to the coculture with autologous lymphocytes markedly inhibited by 50 to 60% the stimulation of HIV-1 replication in MoDCs (Fig. 6A). In parallel to this decreased HIV-1 replication, the presence of anti-ICAM-1 prevented SAMHD1 downregulation as 70 to 92% SAMHD1 expression was detected compared to that in control MoDCs cultured without lymphocytes (Fig. 6B). The blockade of immunological synapse formation by anti-ICAM-1 also preserved high levels of SAMHD1 expression in the absence of HIV-1 infection (Fig. 6B). Thus, the cellular interactions between MoDCs and autologous lymphocytes modulate the expression of SAMHD1 in MoDCs. Overall, the downregulation of SAMHD1 expression promotes HIV-1 replication in HIV-1-infected DCs.
FIG 6.
Blockade of dendritic cell-lymphocyte cross talk by anti-ICAM-1. Anti-ICAM-1 antibody (1H4; 20 μg/ml) was added to HIV-1-infected or uninfected MoDCs prior to coculture with autologous lymphocytes. At 48 h postinfection, intracellular HIV-1 infection (A) and SAMHD1 expression (B) were measured and expressed as a percentage of the p24+ or SAMHD1+ DC-SIGN+ MoDCs compared to control cells in the absence of coculture. The percent decrease of HIV-1 infection was calculated and is shown in red. The data are means ± SD from two independent healthy blood donors.
Coculture with CD4 T lymphocytes induces innate sensing of HIV-1-infected MoDCs.
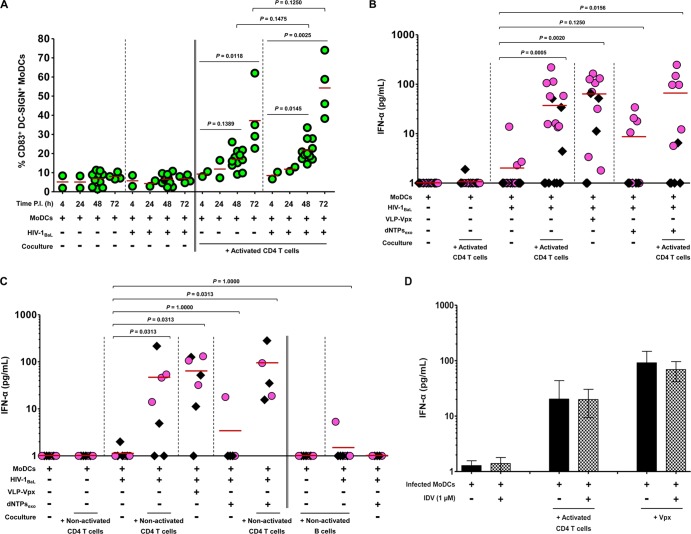
Impairment of SAMHD1 induces the triggering of the immune response (6). We therefore evaluated the kinetics of MoDC maturation by assaying expression of the DC maturation marker CD83 on the surface of DC-SIGN+ MoDCs (Fig. 7A). HIV-1-infected MoDCs matured efficiently in cocultures with CD4 T lymphocytes, and the extents of maturation were greater after 48 and 72 h of culture (P = 0.0145 and P = 0.0025, respectively). MoDC maturation was also greater in the presence of HIV-1 replication than that in uninfected cells, although the difference was not statistically significant (P = 0.1475 at 48 h postinfection and P = 0.1250 at 72 h postinfection) (Fig. 7A). There was no such maturation by HIV-1 in the absence of CD4 T lymphocytes (Fig. 7A).
FIG 7.
Coculture with CD4 T lymphocytes induces innate sensing of HIV-1-infected MoDCs. (A) The kinetics of MoDC maturation were assessed at various time points. The percentage of CD83+ DC-SIGN+ MoDCs was determined in the absence or presence of virus and/or activated CD4 T lymphocytes. Each point represents the result from one donor. (B) Detection of the production of IFN-α by MoDCs in the supernatants of uninfected or infected MoDCs cultured alone or cocultured with activated CD4 T lymphocytes, VLP-Vpx, or dNTPs or with combinations of these factors collected at 48 h (black diamonds) and 72 h (pink circles) postinfection (P.I.). At least 12 independent experiments were performed with DCs from 12 healthy blood donors. (C) IFN-α was assessed under same conditions as in panel B, cocultured with nonactivated CD4 T or B lymphocytes. Data represent the results from seven independent experiments performed with DCs from seven healthy blood donors. Each point represents the result for one donor, and horizontal red bars denote the medians in the plots. (D) IFN-α was measured under same conditions as in panel B and cocultured with PHA-activated CD4 T lymphocytes or VLP-Vpx-transduced MoDCs in the absence or presence of HIV-1 protease inhibitor IDV at 1 μM to prevent the final assembly and maturation of newly synthesized virions. Data are expressed as means ± SD from four healthy blood donors. Groups were compared by one-way ANOVA (Kruskal-Wallis test) and two-tailed pairwise comparison based on Wilcoxon matched-pairs signed rank test, with P < 0.05 considered significant.
IFN-α production was also evaluated. IFN-α was detected in the supernatant of HIV-1-infected MoDCs cocultivated with activated CD4 T lymphocytes at 48 and 72 h postinfection; the induction of this production was donor dependent (Fig. 7B). It is noteworthy that this increase was statistically significant at 72 h postinfection, indicating that the type I IFN is released at a later time point as a DC maturation marker (Fig. 7B). Interestingly, nonactivated CD4 T lymphocytes also induced IFN-α release from HIV-1-infected MoDCs, whereas nonactivated B lymphocytes did not (Fig. 7C), despite inducing increased HIV-1 replication and decreased SAMHD1 expression. Of note, the increased IFN-α release in the presence of CD4 T lymphocytes was similar to that observed in the presence of VLP-Vpx (Fig. 7B and C). Addition of exogenous dNTPs did not significantly modify IFN-α release by infected MoDCs alone (Fig. 7B and C). Furthermore, no IFN-α was detected in either uninfected or infected MoDCs in the absence of CD4 T lymphocytes or in uninfected or infected CD4 T lymphocytes, consistent with previous reports (35). Thus, coculture with autologous CD4 T lymphocytes is necessary for HIV-1-infected MoDCs to produce IFN-α. To determine if IFN-α production is due to the HIV-1 infection by newly synthesized virions, the protease inhibitor IDV was added to the coculture. We observed that IFN-α production by HIV-1-infected cocultured MoDCs or VLP-Vpx-transduced MoDCs was not significantly modified following IDV treatment (Fig. 7D). Therefore, the stimulation of IFN-α production by HIV-1 is due to a viral replication step that occurs before viral assembly and release (known as viral maturation) of newly synthesized virus.
Taken together, these findings indicate that coculture with CD4 T lymphocytes downregulates the expression of the host restriction factor SAMHD1, thereby promoting efficient HIV-1 replication and stimulating an antiviral immune response in DCs.
DISCUSSION
Restriction of HIV-1 infection in DCs in vitro has been observed at various steps of HIV-1 replication (3, 45–52; reviewed in reference 53). SAMHD1 was identified as one of several cellular restriction factors, the expression of which prevents HIV-1 reverse transcription in myeloid DCs (3, 4). It is generally admitted that DCs support only low levels of HIV-1 replication, and infection of DCs with cell-free virions does not induce DC maturation (29–33). These results have been obtained using purified DCs cultivated alone. Here, we used a coculture model mimicking early mucosal HIV-1 infection and dissemination, in which DCs bind and take up the virus, which is then transferred to CD4 T lymphocytes (44, 54–59). Under these conditions, a significant proportion of virus particles released in DC-lymphocyte coculture were produced by DCs carried the DC-specific marker CD1a (36). Moreover, these newly synthesized virions were infectious, as they productively infected MoDCs and peripheral blood mononuclear cells (PBMCs) (36). Here, we demonstrated that the high HIV-1 replication observed in cocultured DCs was associated with a decreased expression of SAMHD1. SAMHD1 expression by DCs was also downregulated in cocultures with autologous primary nonactivated CD4 T or B lymphocytes, which are naturally resistant to HIV-1 replication. However, the presence of the T cell line MT4 had no effect on HIV-1 replication or SAMHD1 expression by infected MoDCs. Of note, MT4 cells are highly permissive to cell-free HIV-1 but do not establish strong immunological/virological synapses (60). Indeed, HIV-1 can be transmitted through direct cell-to-cell contact via such synapses (56, 61–63; reviewed in references 64, 65, 66, and 67). The formation of an immunological/virological synapse contributes to DC-lymphocyte cross talk and involves the local reorganization of various molecules (including CD3, CD4, CD20, TCR, ICAM-1/LFA-1, DC-SIGN/gp120, CD40/CD40L, and the major histocompatibility complex [MHC]) important to viral pathogenesis, antigen presentation, and immune responses (68–71). The impairment of DC-lymphocyte cross talk by anti-CD3 or anti-ICAM-1/LFA-1 prevents the stimulation of HIV-1 replication in MoDCs cocultivated with primary CD4 T lymphocytes (36); this confirmed that the formation of an immunological synapse is required for efficient HIV-1 replication in MoDCs. In addition, we showed that the blockade of immunological synapse formation by anti-ICAM-1 monoclonal antibody prevented the downregulation of SAMHD1 expression, demonstrating that DC-lymphocyte cross talk modulates SAMHD1 expression and consequently inhibits the stimulation of HIV-1 replication in the presence of anti-ICAM-1. DC-lymphocyte cross talk may therefore induce signal transduction across cell membranes like gene transcription by unknown retrograde signals delivered to DCs. Recently, Puigdomenech et al. analyzed SAMHD1 expression by MoDCs in the presence of productively infected CD4 T cells or the T cell line MT4 and found that the presence of infected CD4 T cells did not modify SAMHD1 expression in DCs (6). Indeed, in HIV-1-infected T cells, cross talk with DCs may be limited because of the altered intracellular trafficking of T cell receptor (TCR), Lck, and CD3 in a Nef-dependent manner and thereby impair the formation of the immunological synapse (72, 73; reviewed in references 74 and 75). This would explain the lack of downregulation of SAMHD1 expression in this previous study involving HIV-1-infected CD4 T lymphocytes (6). Interestingly, we observed decreased expression of SAMHD1 in cocultured MoDCs in the absence of HIV-1 infection, indicating that downregulation of SAMHD1 expression in DCs is not the consequence of HIV-1 replication but requires coculture with autologous lymphocytes. These findings suggest that SAMHD1 not only plays a critical role in HIV-1 restriction but also interferes with other biological functions in many other cells or tissues during DC-lymphocyte contact that need to be identified. It will be worthwhile for further studies to determine which molecules are involved in signal transduction pathways that regulate SAMHD1 variation within the MoDCs during DC-lymphocyte cross talk.
In pure MoDC cultures, intracellular HIV-1 capsid, provided by incoming viral particles, failed to induce expression of the costimulatory molecule CD86 (35), and IFN production was low, leading to defect of innate immune sensing of HIV-1 by MoDCs (6, 33, 76; reviewed in references 77 and 78). The defect of immune sensing of HIV-1 by DCs is due to HIV-1 capsid that prevents liberation of nucleic acid (79) and activation of DNA sensor cyclic GMP-AMP synthase (cGAS) (80, 81) in the cytosol before viral integration (80). The downregulation of SAMHD1 in the presence of CD4 T lymphocytes might favor reverse transcription of viral RNA, promoting the production of DNA retrotranscripts involved in DC sensing (80). The detection of IFN-α production in the presence of the protease inhibitor IDV further suggests that the final steps of viral assembly and maturation are not involved in DC sensing. Interestingly, DC-B lymphocyte cross talk did not induce IFN-α production, despite the increased HIV-1 replication and decreased SAMHD1 expression in DCs. This lack of IFN-α production during DC-B lymphocyte cross talk may be attributed to a paracrine feedback phenomenon. It has been shown that upregulation of the expression of costimulatory receptors on B lymphocytes by soluble CD23 and ICAM produced by HIV-1-infected macrophages enhanced HIV-1 infection of resting CD4+ T lymphocytes (82). The induced costimulatory molecules on B lymphocytes may provide some negative signaling pathways leading to the inhibition of IFN-α production by DCs. Another hypothesis is that nonactivated B lymphocytes may not be efficiently triggered by HIV-1, due to lack of high HIV-1 envelope protein gp120 affinity receptors. Conversely, gp120 may bind to and signal through α4β7 on B lymphocytes, resulting in increased expression of the immunosuppressive cytokine transforming growth factor β1 (TGF-β1) (83). This TGF-β1 production negatively regulated IFN-γ responses, as previously described for CD4+ T cells, by protein tyrosine phosphatase SHP-l, mitogen-activated protein kinase (MAPK), and extracellular signal-regulated kinase (ERK) pathways (84, 85). Thus, B lymphocytes may provide negative regulation of signaling pathways by TGF-β, leading to impairment of IFN-α production. These complex signaling pathways may explain the various degrees of SAMHD1 downregulation and HIV-1 replication observed, depending on the type of cells involved in the cross talk and on the type and degree of HIV-1 infection. Further investigation will be required to elucidate these distinct mechanisms.
SAMHD1 expression by DCs appeared to be donor dependent: the detection rates by flow cytometry vary between 20% and 80% in our study. Moreover, the levels of SAMHD1 vary according to the type of DCs studied in vitro and in vivo (86). Langerhans cells and memory T lymphocytes emigrating from human skin explants have been found to facilitate productive HIV-1 replication in vivo (87). In the context of HIV-1 transmission at mucosal site, HIV-infected cells efficiently transmit the virus across epithelial barriers through cell-to-cell contact (88–91). Therefore, modulation of SAMHD1-mediated retroviral restriction by cross talk with lymphocytes may be highly relevant in vivo. Consequently, the modulation of SAMHD1 expression in the various types of cells (such as macrophages, plasmacytoid and myeloid DCs, Langerhans and interstitial DCs, and lymphocytes) should be further investigated in coculture conditions and derived from in vivo studies. In addition, other restriction factors may also participate in the regulation of HIV-1 replication in DCs (92, 93). For example, APOBEC3A was recently reported to inhibit HIV-1 replication by interfering with the reverse transcription and hypermutating/accumulating nascent viral DNA (vDNA) in myeloid DCs (47, 50, 94, 95). MxB (MX2) was proposed to inhibit HIV-1 replication by targeting the viral capsid in monocytoid or permissive CD4+ T cell lines (96–98). These distinct host factors may play key cooperative effects in establishing HIV-1 control.
Our various analyses show that coculture with autologous lymphocytes decreases the SAMHD1 expression by DCs. This downregulation of SAMHD1 expression allows a significant increase of HIV-1 replication in DCs, which may favor the transfer of HIV-1 to T lymphocytes during early mucosal HIV-1 infection. Moreover, DC activation and innate immune sensing of HIV-1-infected immature DCs are observed under coculture conditions. However, once productively infected T cells are established, cross talk between DCs and infected T cells is reduced (73), therefore maintaining SAMHD1 expression (6). This impairment of efficient cross talk with HIV-1-infected T lymphocytes implies that during the chronic phase of HIV-1 infection, immune sensing by DCs may be restricted, therefore providing a likely explanation of physiological role of SAMHD1 antagonist Vpx in the control by the immune system of HIV-2 pathogenicity. Further studies of HIV-1 replication and sensing of HIV-1 in DCs should be performed in physiologically relevant models of DC-lymphocyte coculture. The modulation of DC-lymphocyte cross talk by pharmacological intervention may interfere with HIV-1 replication and immune responses and could prove to be a valuable preventive approach for restricting and/or promoting HIV-1 infection of DCs.
ACKNOWLEDGMENTS
We thank Olivier Schwartz and Françoise Porrot (Institut Pasteur, Unité Virus et Immunité) for the generous gift of antibody against human SAMHD1 and VLP-Vpx. We thank Fei Xiao (INSERM U1110) for critical discussions about this project.
The work was supported by grants from the EuroNeut41 (FP7-HELTH-2007-A-201038), Sidaction, Dormeur Investment Service, Ltd., and ANRS (French National Agency for Research on AIDS and Viral Hepatitis). B.S. was supported by a French fellowship from ANRS.
B.S. and M.E.B. performed the experiments, B.S. and C.M. analyzed the data, B.S., M.E.B., A.L., M.P., M.L., A.P., T.D., S.S., G.L., and C.M. contributed reagents, materials, and analysis tools, and B.S. and C.M. conceived the study, designed the experiments, and wrote the manuscript. All authors read and approved the final version of the manuscript.
The authors declare that they have no conflicting financial interests.
Footnotes
Published ahead of print 26 February 2014
REFERENCES
- 1.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman RM. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goujon C, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. 2006. With a little help from a friend: increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC). Gene Ther. 13:991–994. 10.1038/sj.gt.3302753 [DOI] [PubMed] [Google Scholar]
- 3.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657. 10.1038/nature10117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. 2011. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661. 10.1038/nature10195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger A, Sommer AF, Zwarg J, Hamdorf M, Welzel K, Esly N, Panitz S, Reuter A, Ramos I, Jatiani A, Mulder LC, Fernandez-Sesma A, Rutsch F, Simon V, Konig R, Flory E. 2011. SAMHD1-deficient CD14+ cells from individuals with Aicardi-Goutieres syndrome are highly susceptible to HIV-1 infection. PLoS Pathog. 7:e1002425. 10.1371/journal.ppat.1002425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puigdomenech I, Casartelli N, Porrot F, Schwartz O. 2013. SAMHD1 restricts HIV-1 cell-to-cell transmission and limits immune detection in monocyte-derived dendritic cells. J. Virol. 87:2846–2856. 10.1128/JVI.02514-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell RD, Holland PJ, Hollis T, Perrino FW. 2011. Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J. Biol. Chem. 286:43596–43600. 10.1074/jbc.C111.317628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF, Yap MW, de Carvalho LP, Stoye JP, Crow YJ, Taylor IA, Webb M. 2011. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480:379–382. 10.1038/nature10623 [DOI] [PubMed] [Google Scholar]
- 9.Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T, Pancino G, Priet S, Canard B, Laguette N, Benkirane M, Transy C, Landau NR, Kim B, Margottin-Goguet F. 2012. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat. Immunol. 13:223–228. 10.1038/ni.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welbourn S, Dutta SM, Semmes OJ, Strebel K. 2013. Restriction of virus infection but not catalytic dNTPase activity are regulated by phosphorylation of SAMHD1. J. Virol. 87:11516–11524. 10.1128/JVI.01642-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White TE, Brandariz-Nunez A, Valle-Casuso JC, Amie S, Nguyen LA, Kim B, Tuzova M, Diaz-Griffero F. 2013. The retroviral restriction ability of SAMHD1, but not its deoxynucleotide triphosphohydrolase activity, is regulated by phosphorylation. Cell Host Microbe 13:441–451. 10.1016/j.chom.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cribier A, Descours B, Valadao AL, Laguette N, Benkirane M. 2013. Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep. 3:1036–1043. 10.1016/j.celrep.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 13.Tristem M, Marshall C, Karpas A, Hill F. 1992. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J. 11:3405–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyader M, Emerman M, Sonigo P, Clavel F, Montagnier L, Alizon M. 1987. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature 326:662–669. 10.1038/326662a0 [DOI] [PubMed] [Google Scholar]
- 15.Gramberg T, Kahle T, Bloch N, Wittmann S, Mullers E, Daddacha W, Hofmann H, Kim B, Lindemann D, Landau NR. 2013. Restriction of diverse retroviruses by SAMHD1. Retrovirology 10:26. 10.1186/1742-4690-10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Gelais C, de Silva S, Amie SM, Coleman CM, Hoy H, Hollenbaugh JA, Kim B, Wu L. 2012. SAMHD1 restricts HIV-1 infection in dendritic cells (DCs) by dNTP depletion, but its expression in DCs and primary CD4+ T-lymphocytes cannot be upregulated by interferons. Retrovirology 9:105. 10.1186/1742-4690-9-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Descours B, Cribier A, Chable-Bessia C, Ayinde D, Rice G, Crow Y, Yatim A, Schwartz O, Laguette N, Benkirane M. 2012. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4(+) T-cells. Retrovirology 9:87. 10.1186/1742-4690-9-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger G, Turpin J, Cordeil S, Tartour K, Nguyen XN, Mahieux R, Cimarelli A. 2012. Functional analysis of the relationship between Vpx and the restriction factor SAMHD1. J. Biol. Chem. 287:41210–41217. 10.1074/jbc.M112.403816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldauf HM, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, Konig R, Fackler OT, Keppler OT. 2012. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat. Med. 18:1682–1689. 10.1038/nm.2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandariz-Nunez A, Valle-Casuso JC, White TE, Laguette N, Benkirane M, Brojatsch J, Diaz-Griffero F. 2012. Role of SAMHD1 nuclear localization in restriction of HIV-1 and SIVmac. Retrovirology 9:49. 10.1186/1742-4690-9-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunseri N, O'Brien M, Bhardwaj N, Landau NR. 2011. Human immunodeficiency virus type 1 modified to package simian immunodeficiency virus Vpx efficiently infects macrophages and dendritic cells. J. Virol. 85:6263–6274. 10.1128/JVI.00346-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaushik R, Zhu X, Stranska R, Wu Y, Stevenson M. 2009. A cellular restriction dictates the permissivity of nondividing monocytes/macrophages to lentivirus and gammaretrovirus infection. Cell Host Microbe 6:68–80. 10.1016/j.chom.2009.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tristem M, Purvis A, Quicke DL. 1998. Complex evolutionary history of primate lentiviral vpr genes. Virology 240:232–237. 10.1006/viro.1997.8929 [DOI] [PubMed] [Google Scholar]
- 24.Fregoso OI, Ahn J, Wang C, Mehrens J, Skowronski J, Emerman M. 2013. Evolutionary toggling of Vpx/Vpr specificity results in divergent recognition of the restriction factor SAMHD1. PLoS Pathog. 9:e1003496. 10.1371/journal.ppat.1003496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim ES, Fregoso OI, McCoy CO, Matsen FA, Malik HS, Emerman M. 2012. The ability of primate lentiviruses to degrade the monocyte restriction factor SAMHD1 preceded the birth of the viral accessory protein Vpx. Cell Host Microbe 11:194–204. 10.1016/j.chom.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spragg CJ, Emerman M. 2013. Antagonism of SAMHD1 is actively maintained in natural infections of simian immunodeficiency virus. Proc. Natl. Acad. Sci. U. S. A. 110:21136–21141. 10.1073/pnas.1316839110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou LJ, Tedder TF. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 93:2588–2592. 10.1073/pnas.93.6.2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto F, Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109–1118. 10.1084/jem.179.4.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su B, Xu K, Lederle A, Peressin M, Biedma ME, Laumond G, Schmidt S, Decoville T, Proust A, Lambotin M, Holl V, Moog C. 2012. Neutralizing antibodies inhibit HIV-1 transfer from primary dendritic cells to autologous CD4 T lymphocytes. Blood 120:3708–3717. 10.1182/blood-2012-03-418913 [DOI] [PubMed] [Google Scholar]
- 30.Holl V, Peressin M, Schmidt S, Decoville T, Zolla-Pazner S, Aubertin AM, Moog C. 2006. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood 107:4466–4474. 10.1182/blood-2005-08-3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smed-Sorensen A, Lore K, Walther-Jallow L, Andersson J, Spetz AL. 2004. HIV-1-infected dendritic cells up-regulate cell surface markers but fail to produce IL-12 p70 in response to CD40 ligand stimulation. Blood 104:2810–2817. 10.1182/blood-2003-07-2314 [DOI] [PubMed] [Google Scholar]
- 32.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. 2004. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc. Natl. Acad. Sci. U. S. A. 101:7669–7674. 10.1073/pnas.0402431101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fonteneau JF, Larsson M, Beignon AS, McKenna K, Dasilva I, Amara A, Liu YJ, Lifson JD, Littman DR, Bhardwaj N. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 78:5223–5232. 10.1128/JVI.78.10.5223-5232.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pertel T, Reinhard C, Luban J. 2011. Vpx rescues HIV-1 transduction of dendritic cells from the antiviral state established by type 1 interferon. Retrovirology 8:49. 10.1186/1742-4690-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. 2010. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467:214–217. 10.1038/nature09337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holl V, Xu K, Peressin M, Lederle A, Biedma ME, Delaporte M, Decoville T, Schmidt S, Laumond G, Aubertin AM, Moog C. 2010. Stimulation of HIV-1 replication in immature dendritic cells in contact with primary CD4 T or B lymphocytes. J. Virol. 84:4172–4182. 10.1128/JVI.01567-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Silva S, Wang F, Hake TS, Porcu P, Wong HK, Wu L. 2014. Downregulation of SAMHD1 expression correlates with promoter DNA methylation in Sezary syndrome patients. J. Investig. Dermatol. 134:562–565. 10.1038/jid.2013.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mascola JR, Louder MK, Winter C, Prabhakara R, De Rosa SC, Douek DC, Hill BJ, Gabuzda D, Roederer M. 2002. Human immunodeficiency virus type 1 neutralization measured by flow cytometric quantitation of single-round infection of primary human T cells. J. Virol. 76:4810–4821. 10.1128/JVI.76.10.4810-4821.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy EM, Gavegnano C, Nguyen L, Slater R, Lucas A, Fromentin E, Schinazi RF, Kim B. 2010. Ribonucleoside triphosphates as substrate of human immunodeficiency virus type 1 reverse transcriptase in human macrophages. J. Biol. Chem. 285:39380–39391. 10.1074/jbc.M110.178582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diamond TL, Roshal M, Jamburuthugoda VK, Reynolds HM, Merriam AR, Lee KY, Balakrishnan M, Bambara RA, Planelles V, Dewhurst S, Kim B. 2004. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J. Biol. Chem. 279:51545–51553. 10.1074/jbc.M408573200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Plata MT, Puigdomenech I, Izquierdo-Useros N, Puertas MC, Carrillo J, Erkizia I, Clotet B, Blanco J, Martinez-Picado J. 2013. The infectious synapse formed between mature dendritic cells and CD4(+) T cells is independent of the presence of the HIV-1 envelope glycoprotein. Retrovirology 10:42. 10.1186/1742-4690-10-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duncan CJ, Williams JP, Schiffner T, Gartner K, Ochsenbauer C, Kappes J, Russell RA, Frater J, Sattentau QJ. 2014. High multiplicity HIV-1 infection and neutralizing antibody evasion mediated by the macrophage-T cell virological synapse. J. Virol. 88:2025–2034. 10.1128/JVI.03245-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jolly C, Mitar I, Sattentau QJ. 2007. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J. Virol. 81:13916–13921. 10.1128/JVI.01585-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders RW, de Jong EC, Baldwin CE, Schuitemaker JH, Kapsenberg ML, Berkhout B. 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 76:7812–7821. 10.1128/JVI.76.15.7812-7821.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pertel T, Hausmann S, Morger D, Zuger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, Bisiaux A, Albert ML, Strambio-De-Castillia C, Mothes W, Pizzato M, Grutter MG, Luban J. 2011. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472:361–365. 10.1038/nature09976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coleman CM, Spearman P, Wu L. 2011. Tetherin does not significantly restrict dendritic cell-mediated HIV-1 transmission and its expression is upregulated by newly synthesized HIV-1 Nef. Retrovirology 8:26. 10.1186/1742-4690-8-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger G, Durand S, Fargier G, Nguyen XN, Cordeil S, Bouaziz S, Muriaux D, Darlix JL, Cimarelli A. 2011. APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog. 7:e1002221. 10.1371/journal.ppat.1002221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goujon C, Arfi V, Pertel T, Luban J, Lienard J, Rigal D, Darlix JL, Cimarelli A. 2008. Characterization of simian immunodeficiency virus SIVSM/human immunodeficiency virus type 2 Vpx function in human myeloid cells. J. Virol. 82:12335–12345. 10.1128/JVI.01181-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stopak KS, Chiu YL, Kropp J, Grant RM, Greene WC. 2007. Distinct patterns of cytokine regulation of APOBEC3G expression and activity in primary lymphocytes, macrophages, and dendritic cells. J. Biol. Chem. 282:3539–3546. 10.1074/jbc.M610138200 [DOI] [PubMed] [Google Scholar]
- 50.Peng G, Greenwell-Wild T, Nares S, Jin W, Lei KJ, Rangel ZG, Munson PJ, Wahl SM. 2007. Myeloid differentiation and susceptibility to HIV-1 are linked to APOBEC3 expression. Blood 110:393–400. 10.1182/blood-2006-10-051763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goujon C, Riviere L, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. 2007. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology 4:2. 10.1186/1742-4690-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pion M, Granelli-Piperno A, Mangeat B, Stalder R, Correa R, Steinman RM, Piguet V. 2006. APOBEC3G/3F mediates intrinsic resistance of monocyte-derived dendritic cells to HIV-1 infection. J. Exp. Med. 203:2887–2893. 10.1084/jem.20061519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blanco-Melo D, Venkatesh S, Bieniasz PD. 2012. Intrinsic cellular defenses against human immunodeficiency viruses. Immunity 37:399–411. 10.1016/j.immuni.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu HJ, Reuter MA, McDonald D. 2008. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog. 4:e1000134. 10.1371/journal.ppat.1000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavrois M, Neidleman J, Greene WC. 2008. The Achilles heel of the Trojan horse model of HIV-1 trans-infection. PLoS Pathog. 4:e1000051. 10.1371/journal.ppat.1000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295–1297. 10.1126/science.1084238 [DOI] [PubMed] [Google Scholar]
- 57.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135–144. 10.1016/S1074-7613(02)00259-5 [DOI] [PubMed] [Google Scholar]
- 58.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597. 10.1016/S0092-8674(00)80694-7 [DOI] [PubMed] [Google Scholar]
- 59.Blauvelt A, Asada H, Saville MW, Klaus-Kovtun V, Altman DJ, Yarchoan R, Katz SI. 1997. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Invest. 100:2043–2053. 10.1172/JCI119737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong P, Agosto LM, Ilinskaya A, Dorjbal B, Truong R, Derse D, Uchil PD, Heidecker G, Mothes W. 2013. Cell-to-cell transmission can overcome multiple donor and target cell barriers imposed on cell-free HIV. PLoS One 8:e53138. 10.1371/journal.pone.0053138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, Prevost MC, Estaquier J, Haase AT, Sol-Foulon N, Schwartz O. 2009. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J. Virol. 83:6234–6246. 10.1128/JVI.00282-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FY, Li XD, Asmuth DM, Huser T, Chen BK. 2009. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science 323:1743–1747. 10.1126/science.1167525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 199:283–293. 10.1084/jem.20030648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vasiliver-Shamis G, Dustin ML, Hioe CE. 2010. HIV-1 virological synapse is not simply a copycat of the immunological synapse. Viruses 2:1239–1260. 10.3390/v2051239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sattentau QJ. 2010. Cell-to-cell spread of retroviruses. Viruses 2:1306–1321. 10.3390/v2061306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jolly C, Sattentau QJ. 2004. Retroviral spread by induction of virological synapses. Traffic 5:643–650. 10.1111/j.1600-0854.2004.00209.x [DOI] [PubMed] [Google Scholar]
- 67.Feldmann J, Schwartz O. 2010. HIV-1 virological synapse: live imaging of transmission. Viruses 2:1666–1680. 10.3390/v2081666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shishkova Y, Harms H, Krohne G, Avota E, Schneider-Schaulies S. 2007. Immune synapses formed with measles virus-infected dendritic cells are unstable and fail to sustain T cell activation. Cell. Microbiol. 9:1974–1986. 10.1111/j.1462-5822.2007.00928.x [DOI] [PubMed] [Google Scholar]
- 69.Brossard C, Feuillet V, Schmitt A, Randriamampita C, Romao M, Raposo G, Trautmann A. 2005. Multifocal structure of the T cell-dendritic cell synapse. Eur. J. Immunol. 35:1741–1753. 10.1002/eji.200425857 [DOI] [PubMed] [Google Scholar]
- 70.Revy P, Sospedra M, Barbour B, Trautmann A. 2001. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat. Immunol. 2:925–931. 10.1038/ni713 [DOI] [PubMed] [Google Scholar]
- 71.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science 285:221–227. 10.1126/science.285.5425.221 [DOI] [PubMed] [Google Scholar]
- 72.Schindler M, Munch J, Kutsch O, Li H, Santiago ML, Bibollet-Ruche F, Muller-Trutwin MC, Novembre FJ, Peeters M, Courgnaud V, Bailes E, Roques P, Sodora DL, Silvestri G, Sharp PM, Hahn BH, Kirchhoff F. 2006. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell 125:1055–1067. 10.1016/j.cell.2006.04.033 [DOI] [PubMed] [Google Scholar]
- 73.Thoulouze MI, Sol-Foulon N, Blanchet F, Dautry-Varsat A, Schwartz O, Alcover A. 2006. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity 24:547–561. 10.1016/j.immuni.2006.02.016 [DOI] [PubMed] [Google Scholar]
- 74.Malim MH, Emerman M. 2008. HIV-1 accessory proteins—ensuring viral survival in a hostile environment. Cell Host Microbe 3:388–398. 10.1016/j.chom.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 75.Fackler OT, Alcover A, Schwartz O. 2007. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat. Rev. Immunol. 7:310–317. 10.1038/nri2041 [DOI] [PubMed] [Google Scholar]
- 76.Smed-Sorensen A, Lore K, Vasudevan J, Louder MK, Andersson J, Mascola JR, Spetz AL, Koup RA. 2005. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J. Virol. 79:8861–8869. 10.1128/JVI.79.14.8861-8869.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luban J. 2012. Innate immune sensing of HIV-1 by dendritic cells. Cell Host Microbe 12:408–418. 10.1016/j.chom.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manel N, Littman DR. 2011. Hiding in plain sight: how HIV evades innate immune responses. Cell 147:271–274. 10.1016/j.cell.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Invest. 115:3265–3275. 10.1172/JCI26032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lahaye X, Satoh T, Gentili M, Cerboni S, Conrad C, Hurbain I, El Marjou A, Lacabaratz C, Lelievre JD, Manel N. 2013. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity 39:1132–1142. 10.1016/j.immuni.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 81.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ. 2013. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341:903–906. 10.1126/science.1240933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swingler S, Brichacek B, Jacque JM, Ulich C, Zhou J, Stevenson M. 2003. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature 424:213–219. 10.1038/nature01749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jelicic K, Cimbro R, Nawaz F, Huang DW, Zheng X, Yang J, Lempicki RA, Pascuccio M, Van Ryk D, Schwing C, Hiatt J, Okwara N, Wei D, Roby G, David A, Hwang IY, Kehrl JH, Arthos J, Cicala C, Fauci AS. 2013. The HIV-1 envelope protein gp120 impairs B cell proliferation by inducing TGF-beta1 production and FcRL4 expression. Nat. Immunol. 14:1256–1265. 10.1038/ni.2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park IK, Letterio JJ, Gorham JD. 2007. TGF-beta 1 inhibition of IFN-gamma-induced signaling and Th1 gene expression in CD4+ T cells is Smad3 independent but MAP kinase dependent. Mol. Immunol. 44:3283–3290. 10.1016/j.molimm.2007.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park IK, Shultz LD, Letterio JJ, Gorham JD. 2005. TGF-beta1 inhibits T-bet induction by IFN-gamma in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1. J. Immunol. 175:5666–5674 [DOI] [PubMed] [Google Scholar]
- 86.Bloch N, O'Brien M, Norton TD, Polsky SB, Bhardwaj N, Landau NR. 2014. HIV-1 infection of plasmacytoid and myeloid dendritic cells is restricted by high levels of SAMHD1 that cannot be counteracted by Vpx. AIDS Res. Hum. Retroviruses 30:195–203. 10.1089/aid.2013.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pope M, Betjes MG, Romani N, Hirmand H, Cameron PU, Hoffman L, Gezelter S, Schuler G, Steinman RM. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389–398. 10.1016/0092-8674(94)90418-9 [DOI] [PubMed] [Google Scholar]
- 88.Klein K, Veazey RS, Warrier R, Hraber P, Doyle-Meyers LA, Buffa V, Liao HX, Haynes BF, Shaw GM, Shattock RJ. 2013. Neutralizing IgG at the portal of infection mediates protection against vaginal simian/human immunodeficiency virus challenge. J. Virol. 87:11604–11616. 10.1128/JVI.01361-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bernard-Stoecklin S, Gommet C, Corneau AB, Guenounou S, Torres C, Dejucq-Rainsford N, Cosma A, Dereuddre-Bosquet N, Le Grand R. 2013. Semen CD4(+) T cells and macrophages are productively infected at all stages of SIV infection in macaques. PLoS Pathog. 9:e1003810. 10.1371/journal.ppat.1003810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ganor Y, Zhou Z, Bodo J, Tudor D, Leibowitch J, Mathez D, Schmitt A, Vacher-Lavenu MC, Revol M, Bomsel M. 2013. The adult penile urethra is a novel entry site for HIV-1 that preferentially targets resident urethral macrophages. Mucosal. Immunol. 6:776–786. 10.1038/mi.2012.116 [DOI] [PubMed] [Google Scholar]
- 91.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257–270. 10.1016/j.immuni.2007.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goujon C, Schaller T, Galao RP, Amie SM, Kim B, Olivieri K, Neil SJ, Malim MH. 2013. Evidence for IFNalpha-induced, SAMHD1-independent inhibitors of early HIV-1 infection. Retrovirology 10:23. 10.1186/1742-4690-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fujita M, Nomaguchi M, Adachi A, Otsuka M. 2012. SAMHD1-dependent and -independent functions of HIV-2/SIV Vpx protein. Front. Microbiol. 3:297. 10.3389/fmicb.2012.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmitt K, Guo K, Katuwal M, Wilson D, Prochnow C, Bransteitter R, Chen XS, Santiago ML, Stephens EB. 2013. Lentivirus restriction by diverse primate APOBEC3A proteins. Virology 442:82–96. 10.1016/j.virol.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berger A, Munk C, Schweizer M, Cichutek K, Schule S, Flory E. 2010. Interaction of Vpx and apolipoprotein B mRNA-editing catalytic polypeptide 3 family member A (APOBEC3A) correlates with efficient lentivirus infection of monocytes. J. Biol. Chem. 285:12248–12254. 10.1074/jbc.M109.090977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Z, Pan Q, Ding S, Qian J, Xu F, Zhou J, Cen S, Guo F, Liang C. 2013. The interferon-inducible MxB protein inhibits HIV-1 infection. Cell Host Microbe 14:398–410. 10.1016/j.chom.2013.08.015 [DOI] [PubMed] [Google Scholar]
- 97.Kane M, Yadav SS, Bitzegeio J, Kutluay SB, Zang T, Wilson SJ, Schoggins JW, Rice CM, Yamashita M, Hatziioannou T, Bieniasz PD. 2013. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature 502:563–566. 10.1038/nature12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goujon C, Moncorge O, Bauby H, Doyle T, Ward CC, Schaller T, Hue S, Barclay WS, Schulz R, Malim MH. 2013. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 502:559–562. 10.1038/nature12542 [DOI] [PMC free article] [PubMed] [Google Scholar]