Abstract
The natural stilbene pawhuskin A has been shown to function as an opioid receptor antagonist, with preferential binding to the κ receptor. This finding encouraged assembly of a set of analogues to probe the importance of key structural features. Assays on these compounds determined that one (compound 29) shows potent opioid receptor binding activity and significantly improved selectivity for the κ receptor. These studies begin to illuminate the structural features of these non-nitrogenous opioid receptor antagonists that are required for activity.
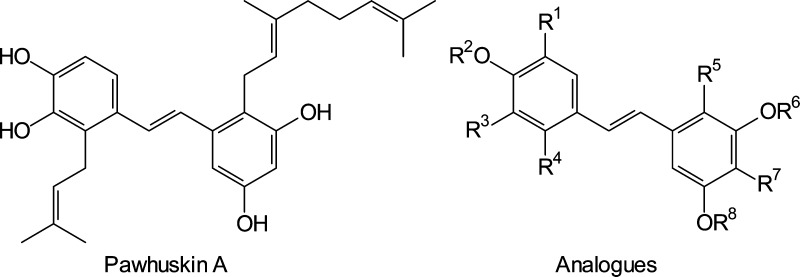
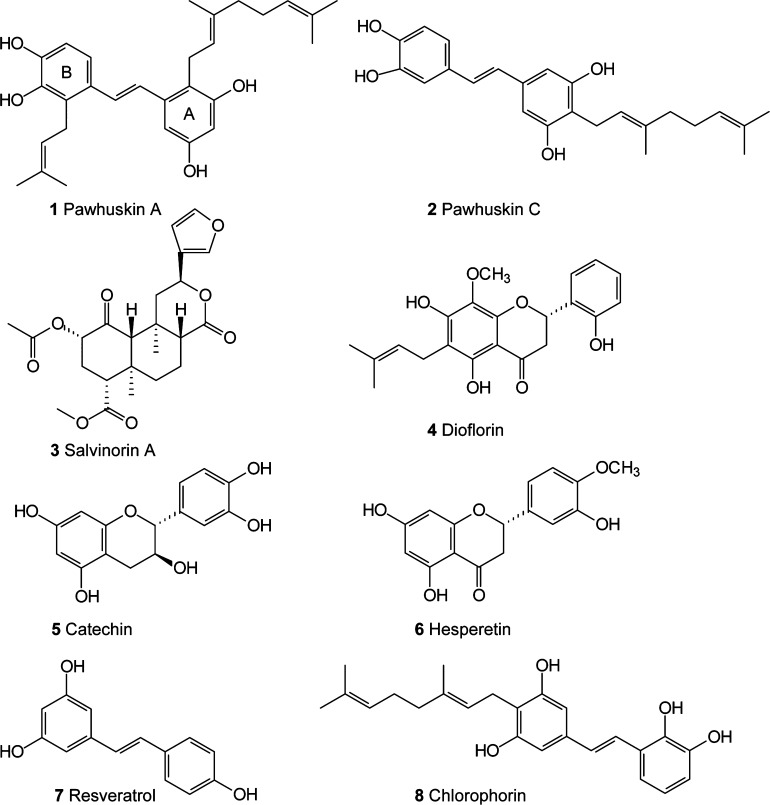
In 2004 Belofsky and co-workers reported a small set of prenylated stilbenes that they named the pawhuskins.1 This family of compounds, exemplified by pawhuskins A (1) and C (2), was isolated from the common North American purple prairie clover (Dalea purpurea) collected near Pawhuska, Oklahoma. Extracts of this plant reportedly have been made into teas and used by Native American peoples as a prophylactic and for treatment of various ailments.2 Belofsky’s findings support this ethnomedical use, since the pawhuskins were shown to modulate opioid receptors by displacement of a nonselective radioactive antagonist in rat brain striatal tissue.1 Pawhuskin A was the most potent member of the family, making it one of a small group of non-nitrogenous compounds with effects on the opiate receptor system. As part of an ongoing interest in natural prenylated stilbenes,3,4 we undertook studies to elucidate the character and receptor subtype selectivity of opioid modulation by pawhuskins. This effort already has led to the synthesis of both pawhuskins A5 and C,6 and here we report the results of our further studies on this class of compounds.
Several non-nitrogenous opioid receptor modulators have been isolated from natural sources. The most studied compound is salvinorin A (3), a potent hallucinogen isolated from Salvia divinorum.7 Salvinorin A has been shown to be a κ-opioid (KOP) receptor agonist, and KOP receptor ligands have become of interest with respect to studies of addiction and other disorders.8 Two total syntheses of salvinorin A have been reported,9,10 but modifications of the isolated natural product have driven more extensive structure–activity studies.11−17 The non-nitrogenous compound dioflorin (4), a prenylated flavonoid, was isolated from the Brazilian vine Dioclea grandiflora through activity-guided fractionation18−20 and shown to have analgesic activity.21 More extensive efforts to categorize the opioid receptor binding of dioflorin have not yet been reported. Bioassays with a series of other natural flavonoids including catechin (5) and hesperetin (6) have been conducted and demonstrate that this scaffold may have considerable potential for development of opioid receptor ligands.22 Other structural subtypes with opioid-binding activity are becoming more common,23−25 including stilbenoids more reminiscent of the pawhuskins such as resveratrol (7)26,27 and, more recently, chlorophorin (8).28
Salvinorin A (3) has been shown to be a functional agonist. Dioflorin (4) and other isolates of Dioclea display morphine-like analgesia that is inhibited by naloxone, a nonspecific opioid receptor antagonist, so they are presumably agonists as well.20 While the flavan-3-ol catechin (5) had good activity as an antagonist at the KOP receptor (Ke = 320 nM), the flavanone hesperetin (6) had no activity at the μ, δ, or κ receptors.22 The work of Sobolev and co-workers on peanut phytoalexins such as stilbene 8 determined the selectivity of these compounds against each opioid receptor, but these compounds have not yet been fully characterized using functional assays.28 Here we report the opioid receptor binding affinity and selectivity of pawhuskin A using a functional assay based on [35S]GTP-γ-S binding. We also report initial results of structure–activity relationship studies, which begin to illuminate the significance of the phenols and the prenyl group for activity.
Figure 1.
Structures of some non-nitrogenous opioid receptor modulators.
Results and Discussion
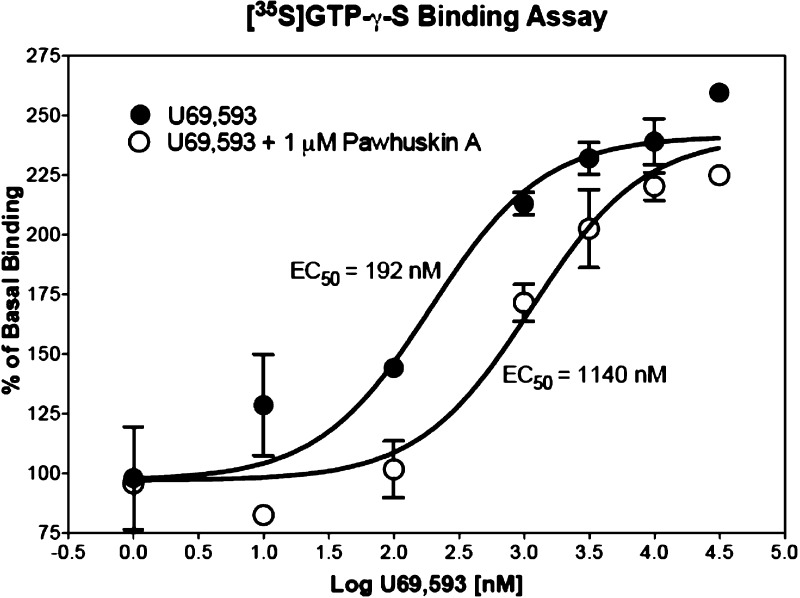
We began this exploration of pawhuskin opioid activity by probing the selectivity of pawhuskin A for human κ (KOP), μ (MOP), and δ (DOP) receptors. Even at a 10 μM concentration, pawhuskin A was found to have no intrinsic agonist activity at these receptors. However, further testing showed antagonist activity at all three of the opioid receptor subtypes. Furthermore, pawhuskin A caused a rightward shift in the agonist concentration response curve, and its antagonism was surmountable, suggesting a competitive mode of antagonism (Figure 2).29 Pawhuskin A is modestly selective for the κ receptor, with a Ke of 203 nM (δ/κ = 14.5, μ/κ = 2.9). Pawhuskin C (2) also displayed some antagonist activity at the KOP receptor, but was much less potent than compound 1.
Figure 2.
Representative graph of the antagonist activity of pawhuskin A in the KOP receptor affinity assay. Each data point represents the mean and SEM of duplicate samples.
While the natural product salvinorin A and many of its analogues are KOP receptor agonists, there are only limited examples of non-nitrogenous KOP receptor antagonists including some flavanoids.22 Pawhuskin A rivals the potency of the flavonoids, although catechin (5) displayed higher selectivity versus the other opioid receptors (μ/κ > 31). However improved KOP receptor selectivity might be uncovered by a synthetic exploration involving the pawhuskin’s stilbene scaffold. Synthetic efforts along these lines are encouraged by the recent interest in KOP receptor antagonists as potential treatments for stimulant abuse. Such agents might be of particular value as potential preventatives for relapse. While there has been some interest in using KOP agonists for treatment of substance abuse, compounds such as salvinorin A have been accompanied by serious side effects including potent hallucinogen activity. There is a significant relationship between relapse to stimulant abuse and stress.30 Indeed encounters with stressors, and even images that induce stress, have been shown to induce craving in stimulant abusers.31,32 The potent KOP receptor antagonist JDTic33 has been shown to block stress-induced cocaine seeking behavior and also has demonstrated antidepressant-like activity.34 This result was confirmed and expanded to show that pretreatment with the κ-opioid antagonist arodyn prevented stress-related induction of cocaine-conditioned place preference,35 which further heightens interest in κ-selective antagonists.
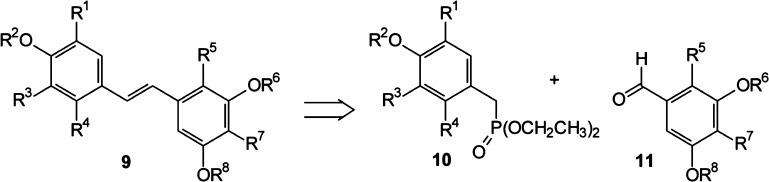
Our approach to exploration of the structure–activity relationships of pawhuskin A analogues took advantage of a core strategy used in the synthesis of other natural stilbenes (Figure 3).36−38 A disconnection of the central olefinic moiety (9) through a Horner–Wadsworth–Emmons transform allows choice of phosphonate coupling partners such as 10 and aldehydes such as 11, although the reversed pairing is also viable.39,40 This permits maximum convergence and provides for divergence through condensations of one aldehyde with several phosphonates or one phosphonate with several aldehydes.41 To begin exploration of the pharmacophore of pawhuskin A and the essential binding motifs for κ-selective antagonist activity, we undertook syntheses aimed at preparation of a small set of analogues through this strategy. Phenolic H-bonding is important to the KOP receptor selectivity of the antagonist JDTic and other members of the phenylpiperidine class of opioid receptor modulators.42 Furthermore, as in past studies of salvinorin A,43 the lack of a readily ionizable group that would form salt bridges with an opioid receptor suggested that attention should be directed at the H-bonding groups of pawhuskin A. Thus, we chose to prepare various methylated analogues to assess the importance of H-bond donation from the various hydroxy groups without a significant change in electron donation.
Figure 3.
Synthetic strategy for pawhuskin analogues.
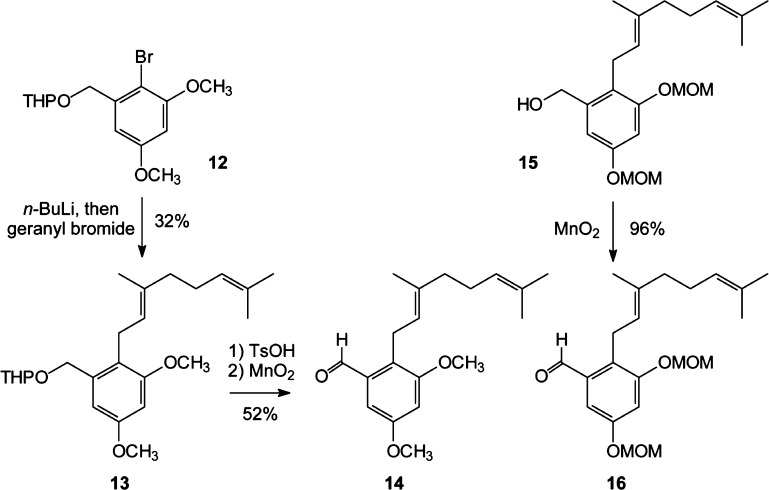
To allow efficient preparation of several analogues, as well as synthesis of regiospecifically methylated materials, the permethylated pawhuskin A analogue was pursued through preparation of both coupling partners aldehyde 14 (Scheme 1) and phosphonate 19 (Scheme 2) rather than methylation of the natural product. To access compound 14, halogen metal exchange was carried out on the known bromide 12.44,45 Treatment of the lithiated arene with geranyl bromide afforded the THP ether 13. Hydrolysis of the acetal protecting group and oxidation of the resulting benzylic alcohol with MnO2 gave aldehyde 14 in satisfactory yield. The alcohol 15 is known from our synthesis of pawhuskin A,5 and oxidation of that benzylic alcohol gave the methoxymethyl (MOM)-protected aldehyde 16.
Scheme 1. Synthesis of the Aldehydes 14 and 16.
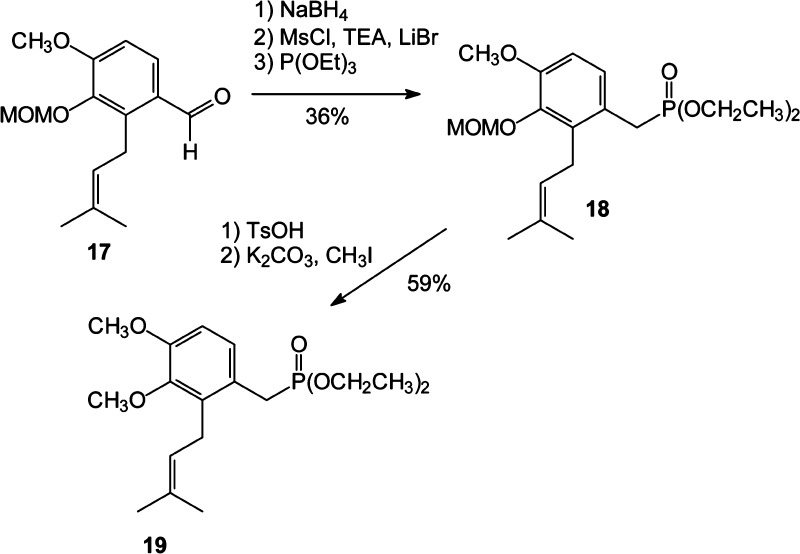
Scheme 2. Synthesis of Phosphonates 18 and 19.
To prepare the complementary phosphonate 19, the known aldehyde 17(46) was reduced to the corresponding alcohol, and the alcohol was treated with mesyl chloride and Et3N, then LiBr, and finally allowed to react with triethyl phosphite to obtain the phosphonate 18. After hydrolysis of the MOM protecting group, standard reaction with MeI and base afforded the dimethylated phosphonate 19.
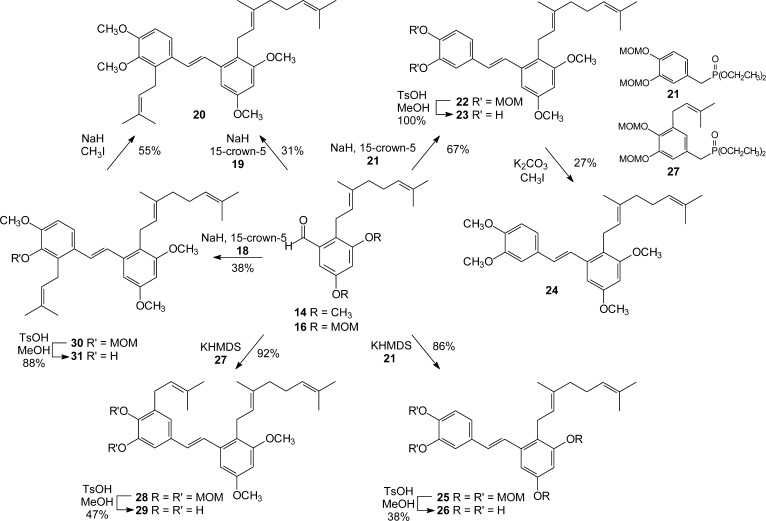
Coupling of aldehyde 14 and phosphonate 19 via Horner–Wadsworth–Emmons condensation afforded the fully methylated pawhuskin A analogue 20 in good yield (Scheme 3). With this compound in hand, we employed our small library of readily available phosphonates of type 10 to synthesize additional pawhuskin analogues. Thus, coupling of aldehyde 14 with known phosphonate 21(47) afforded the stilbene 22, which upon deprotection gave stilbene 23. Exhaustive methylation of compound 23 then gave the permethylated analogue 24. Condensation of compound 16 with phosphonate 21,38 followed by hydrolysis of the protecting groups in the resulting stilbene, gave the free phenolic pawhuskin A analogue 26. To reposition the prenyl group so that it is isomeric to pawhuskin A (1), phosphonate 27 was prepared from the corresponding benzylic alcohol under standard conditions. That prenylated benzylic alcohol could be prepared from bromovanillin through reactions parallel to those reported in a schweinfurthin synthesis.39 Condensation of phosphonate 27 with aldehyde 14 gave stilbene 28, and hydrolysis of the MOM groups gave the dimethylated analogue 29. Finally, condensation of aldehyde 14 with phosphonate 18 gave the selectively trimethylated pawhuskin A derivative 30. Hydrolysis of the MOM acetal gave the specific phenol 31. This compound was methylated to provide permethylated pawhuskin A (20) via a different route.
Scheme 3. Synthesis of Pawhuskin A Analogues 20, 23, 24, 26, 29, and 31.
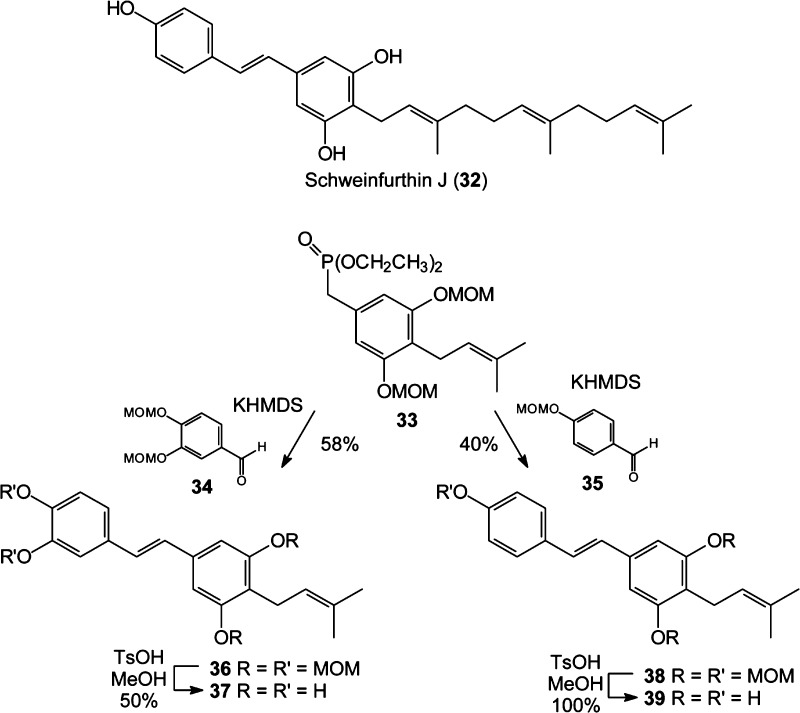
Because pawhuskin C (2) showed activity,1 we tested several analogues of this chemotype. This set includes the natural product schweinfurthin J (32, Scheme 4), which was isolated from the African plant Macaranga schweinfurthii(48,49) and can be viewed as lacking one phenolic hydroxy group and bearing a farnesyl side chain in relation to pawhuskin C. We then used the known phosphonate 33(50,51) to access the prenylated pawhuskin C analogue 37 and the natural product trans-arachidin-2 (39). Condensation of phosphonate 33 with the known aldehydes 34 and 35(52) gave the protected stilbenes 36 and 38, respectively. Hydrolysis of the four MOM acetals of compound 36 gave stilbene 37. Use of the MOM protecting group for all the hydroxy groups of compound 38 allows for deprotection to stilbene 39 in a single step, which is more efficient than the previous synthesis.53
Scheme 4. Pawhuskin C Analogues.
Of the various analogues studied for binding to opioid receptors, only the pawhuskin A analogue 29 and schweinfurthin J (32) demonstrated appreciable activity (Table 1). Schweinfurthin J with a 3 μM Ke for the MOP receptor and limited selectivity (δ/κ = 0.67, μ/κ = 0.33) is the only stilbene we have studied that shows selectivity for the μ-opioid receptor. Interestingly, schweinfurthin J is also closely related to chlorophorin (8), which was shown by Sobolev and co-workers to lower agonist binding to the κ and δ receptors to an equal extent but to have no substantial effect on the binding of agonists to the μ-opioid receptor.28
Table 1. Apparent Affinity of Compounds Tested.
| apparent
affinity of competitive antagonists (Ke) in μM |
selectivity |
||||
|---|---|---|---|---|---|
| compd | KOP | DOP | MOP | δ/κ | μ/κ |
| Paw A (1) | 0.20 | 2.9 | 0.57 | 14.5 | 2.9 |
| Paw C (2) | >10 | >10 | >10 | ||
| 20 | >10 | >10 | >10 | ||
| 23 | >10 | >10 | >10 | ||
| 24 | >10 | >10 | >10 | ||
| 26 | >10 | >10 | >10 | ||
| 29 | 0.15 | >10 | >10 | >67 | >67 |
| 31 | >10 | >10 | >10 | ||
| 32 | 9 | 6 | 3 | 0.67 | 0.33 |
| 37 | >10 | >10 | >10 | ||
| 39 | >10 | >10 | >10 | ||
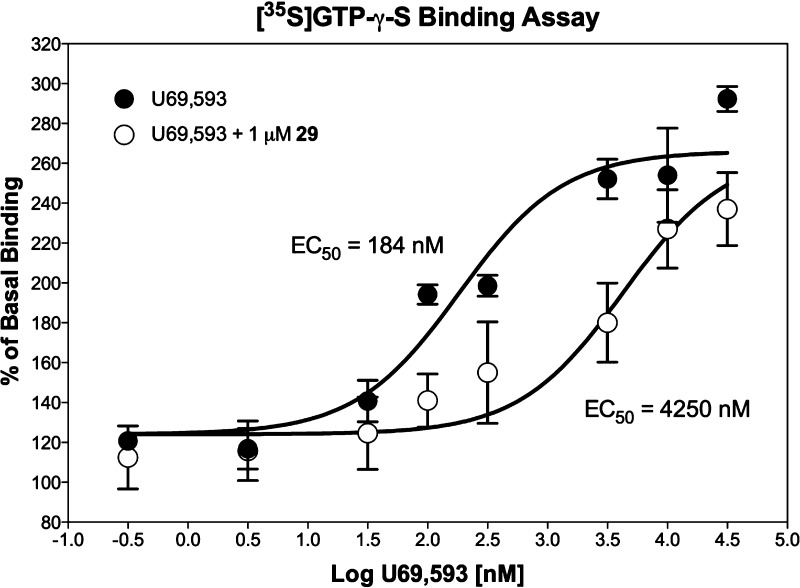
Of greater interest is analogue 29, which showed better binding affinity to the κ receptor than pawhuskin A and also demonstrated dramatically improved selectivity (δ/κ at least 4-fold larger and μ/κ at least 20-fold larger for compound 29 than for pawhuskin A). Indeed we could not find antagonist activity at the μ or δ receptors for compound 29 up to the highest concentrations typically tested (10 μM). This compound demonstrates that methylation of the malonate-derived hydroxy groups on the pawhuskin A scaffold does not abrogate the KOP receptor antagonist activity on this stilbene scaffold. Comparisons to compounds 20, 23, and 24 indicate that the presence and position of the prenyl substituent are important factors in binding to the KOP receptor. These results point to the importance of the shikimate-derived substructure and should allow further design with the aim of introducing more drug-like characteristics. Work on this strategy is currently under way.
Figure 4.
Representative graph of the antagonist activity of compound 29 in the KOP receptor affinity assay. Each data point represents the mean and SEM of duplicate samples.
Conclusions
This study has shown that the natural stilbene pawhuskin A is a competitive antagonist with selectivity for the KOP receptor. We also have shown that improved selectivity for the KOP versus the DOP and MOP receptors is possible within the constraints of the stilbene structure, which encourages further efforts to improve these molecules via synthesis. The isomeric pawhuskin A analogue 29 exhibited greater affinity and selectivity for the KOP receptor than pawhuskin A itself, indicating that the shikimate-derived ring is a key for κ-opioid receptor binding. Compound 29 shows significantly greater selectivity for the KOP than PF-04455242, which was advanced to phase 1 clinical trials for alcohol dependency, albeit with significantly lower potency (∼150 nM vs 3 nM).54,55
Thus far, none of the new compounds reported here have shown any agonist activity. While the study of KOP agonists for treatment of pain and addiction has been moving forward, their potential may be limited by side effects such as hallucinations and dysphoria. This makes the discovery of additional classes of KOP antagonists appealing. Therefore, this stilbene scaffold may present new opportunities for the discovery of compounds with utility in the treatment of addiction and depression.
Experimental Section
General Experimental Procedures
Both THF and Et2O were freshly distilled from Na/benzophenone. Both CH2Cl2 and Et3N were distilled from CaH2 prior to use. Solutions of n-BuLi were purchased from a commercial source and titrated with diphenylacetic acid prior to use. All other reagents and solvents were purchased from commercial sources and used without further purification. All reactions in nonaqueous solvents were conducted in flame-dried glassware under a positive pressure of Ar and with magnetic stirring. NMR spectra were obtained at 300–500 MHz for 1H and 75–125 MHz for 13C with CDCl3 or CD3OD as solvent and (CH3)4Si (1H, 0.00 ppm) or CDCl3 (13C, 77.0 ppm or 49.0 ppm) as internal standards unless otherwise noted. The 31P chemical shifts were reported in ppm relative to 85% H3PO4 (external standard). High-resolution mass spectra were obtained at the University of Iowa Mass Spectrometry Facility. Silica gel (60 Å, 0.040–0.063 mm) was used for flash chromatography.
2-Geranyl-3,5-dimethoxytetrahydropyranylbenzyl Alcohol (13)
To a stirred solution of TMEDA (0.58 mL, 3.9 mmol) and n-BuLi (2.47 M solution in hexanes, 1.5 mL, 3.6 mmol) in Et2O (20 mL) at −10 °C was added the bromide 12 (979 mg, 3.0 mmol) dissolved in Et2O (4 mL). After 45 min of stirring, CuI (742 mg, 3.9 mmol) was added and then geranyl bromide (840 mg, 3.9 mmol) was added slowly over 8 min to the reaction. After the mixture was stirred overnight, the reaction was quenched by addition of saturated aqueous NH4Cl. The resulting mixture was extracted with EtOAc, and the combined organic extracts were washed with brine, dried (MgSO4), filtered, and concentrated in vacuo. Final purification by flash column chromatography (4% EtOAc in hexanes) afforded compound 13 (364 mg, 32%) as a yellow oil: 1H NMR (500 MHz, CDCl3) δ 6.61 (d, J = 2.4 Hz, 1H), 6.42 (d, J = 2.5 Hz, 1H), 5.07–5.04 (m, 2H), 4.74 (d, J = 12.2 Hz, 1H), 4.70 (t, J = 3.7 Hz, 1H), 4.48 (d, J = 12.1 Hz, 1H), 3.94–3.90 (m, 1H), 3.80 (s, 3H), 3.79 (s, 3H), 3.37–3.29 (m, 2H), 2.06–1.80 (m, 5H), 1.80–1.54 (m, 5H), 1.75 (s, 3H), 1.65 (s, 3H), 1.57 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 158.6, 158.4, 138.0, 134.5, 131.2, 124.4, 123.3, 121.3, 105.0, 98.0 (2C), 66.9, 62.2, 55.7, 55.3, 39.8, 30.7, 26.8, 25.7, 25.5, 24.1, 19.5, 17.7, 16.1; HRMS (ESI) m/z calcd for C24H36O4Na (M + Na)+ 411.2511, found 411.2495.
2-Geranyl-3,5-dimethoxybenzaldehyde (14)
To a solution of the THP acetal 13 (364 mg, 0.9 mmol) in MeOH (8 mL) at room temperature was added TsOH (356 mg, 1.9 mmol). The solution was stirred for 2.5 h and quenched by addition of NaHCO3. The mixture was extracted with EtOAc, and the combined organic extracts were dried (MgSO4), filtered, and concentrated in vacuo to afford the benzylic alcohol as a yellow oil. This material was used in further reactions without additional purification: 1H NMR (300 MHz, CDCl3) δ 6.59 (d, J = 2.4 Hz, 1H), 6.43 (d, J = 2.2 Hz, 1H), 5.09–5.02 (m, 2H) 4.64 (d, J = 3.9 Hz, 2H), 3.81 (s, 3H), 3.80 (s, 3H), 3.35 (d, J = 6.8 Hz, 2H), 2.10–1.94 (m, 4H), 1.76 (s, 3H), 1.65 (s, 3H), 1.57 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 158.7, 158.3, 140.6, 135.0, 131.4, 124.1, 123.5, 120.3, 104.0, 97.9, 63.3, 55.6, 55.3, 39.6, 26.6, 25.6, 23.7, 17.6, 16.1; HRMS (EI) m/z calcd for C19H28O3 (M)+ 304.2038, found 304.2044.
To a stirred solution of the benzylic alcohol (285 mg, 0.9 mmol, assuming 100% conversion in the previous step) in CH2Cl2 (15 mL) was added activated MnO2 (815 mg, 9.4 mmol). The mixture was stirred overnight and subsequently was filtered and concentrated in vacuo. Final purification by flash column chromatography (12% EtOAc in hexanes) afforded aldehyde 14 (146 mg, 52% from 13) as a yellow oil: 1H NMR (300 MHz, CDCl3) δ 10.3 (s, 1H), 6.98 (d, J = 2.2 Hz, 1H), 6.68 (d, J = 1.9 Hz, 1H), 5.13–5.07 (m, 1H), 5.05–5.00 (m, 1H), 3.82 (s, 6H), 3.70 (d, J = 6.5 Hz, 2H), 2.24–1.90 (m, 4H), 1.76 (s, 3H), 1.64 (s, 3H), 1.56 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 191.8, 158.8, 158.6, 135.2, 134.9, 131.4, 127.3, 124.0, 123.4, 104.8, 101.9, 55.8, 55.5, 39.5, 26.5, 25.6, 22.5, 17.6, 16.2; HRMS (ESI) m/z calcd for C19H26O3Na (M + Na)+ 325.1780, found 325.1783.
2-Geranyl-3,5-bis(methoxymethoxy)benzaldehyde (16)
Activated MnO2 (644 mg, 7.1 equiv) was added to a solution of alcohol 15(5) (267 mg, 0.7 mmol) in CH2Cl2 (15 mL) at room temperature, and the mixture was stirred overnight. The mixture was filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (50% EtOAc in hexanes) afforded aldehyde 16 (256 mg, 96%) as a yellow oil: 1H NMR (300 MHz, CDCl3) δ 10.26 (s, 1H), 7.21 (d, J = 2.7 Hz, 1H), 7.04 (d, J = 2.2 Hz, 1H), 5.21 (s, 2H), 5.19 (s, 2H), 5.14–5.10 (m, 1H), 5.05–5.00 (m, 1H), 3.73 (d, J = 6.5 Hz, 2H), 3.48 (s, 6H), 2.08–1.93 (m, 4H), 1.86 (s, 3H), 1.73 (s, 3H), 1.65 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 191.6, 156.3, 156.2, 135.3 (2C), 131.5, 128.1, 124.1, 123.2, 109.3, 108.7, 94.7, 94.5, 56.2, 56.1, 39.6, 26.6, 25.6, 22.9, 17.6, 16.3; HRMS (ESI) m/z calcd for C21H30O5Na (M + Na)+ 385.1991, found 385.1983.
Diethyl{[4-methoxy-3-(methoxymethoxy)-2-(prenyl)phenyl]methyl} Phosphonate (18)
To a stirred solution of aldehyde 17 (1.16 g, 4.5 mmol) in MeOH (10 mL) at 0 °C was added NaBH4 (282 mg, 7.5 mmol) as a single aliquot. This solution was stirred for 30 min, and then H2O (50 mL) was added and the resulting solution was extracted with EtOAc. After concentration in vacuo, the resulting alcohol (1.07 g, 90%) was dissolved in THF (10 mL) and treated with Et3N (0.80 mL, 5.7 mmol) followed by methanesulfonyl chloride (0.31 mL, 4.01 mmol). After 15 min, LiBr (391 mg, 0.45 mmol) was added in THF (15 mL). The resulting solution was stirred for an additional 45 min and quenched by addition of H2O. This mixture was extracted with EtOAc, and then the combined organic extracts were washed with brine, dried (MgSO4), filtered, and concentrated in vacuo. The resulting yellow oil was dissolved in triethyl phosphite (5 mL) and heated at reflux for 5 days. Removal of excess triethyl phosphite in vacuo gave a yellow oil. Purification by flash column chromatography (100% EtOAc) afforded phosphonate 18 (622 mg, 40%) as a clear oil: 1H NMR (300 MHz, CDCl3) δ 6.97–6.93 (m, 1H), 6.67–6.63 (m, 1H), 4.95 (br s, 3H), 3.92–3.86 (m, 4H), 3.70 (s, 5H), 3.56 (s, 3H), 3.01 (d, JHP = 21 Hz, 2H), 1.70 (s, 3H), 1.58 (s, 3H), 1.16–1.10 (m, 6H); 13C NMR (75 MHz, CDCl3) δ 150.8 (d, JCP = 3.6 Hz), 143.7 (d, JCP = 3.7 Hz), 134.8 (d, JCP = 6.7 Hz), 131.3, 126.3 (d, JCP = 5.2 Hz), 122.7 (d, JCP = 9.3 Hz), 122.5, 109.4 (d, JCP = 3.9 Hz), 98.6, 61.6 (d, JCP = 7.1 Hz, 2C), 57.1, 55.2, 29.5 (d, JCP = 139 Hz), 25.3, 25.2, 17.6, 16.0 (d, JCP = 5.8 Hz, 2C); 31P NMR (122 MHz, CDCl3) 27.2; HRMS (EI) m/z calcd for C19H31O6P (M)+ 386.1858, found 386.1857.
Diethyl{[3,4-dimethoxy-2-(prenyl)phenyl]methyl} Phosphonate (19)
To a stirred solution of MOM ether 18 (103 mg, 0.3 mmol) in EtOH (2.5 mL) was added TsOH (152 mg, 0.8 mmol). The solution was stirred overnight, quenched by addition of saturated aqueous NH4Cl, and extracted with EtOAc. The combined organic extracts were washed with brine, dried (MgSO4), and concentrated in vacuo to afford the phenol as a yellow oil. To a stirred solution of the phenol (88 mg, 0.3 mmol) in acetone (6 mL) were added K2CO3 (242 mg, 1.8 mmol) and MeI (0.1 mL, 1.6 mmol). After the mixture was heated to reflux and stirred overnight, it was quenched by addition of H2O, and the mixture was extracted with EtOAc. The organic extracts were washed with 2 M NaOH, dried (MgSO4), and concentrated in vacuo. Without additional purification, methyl ether 19 (56 mg, 59%, 2 steps) was obtained as a yellow oil: 1H NMR (300 MHz, CDCl3) δ 6.96 (dd, J = 8.7 Hz, JHP = 3.2 Hz, 1H), 6.68 (d, J = 8.8 Hz, 1H), 4.96 (t, J = 6.5 Hz, 1H), 3.99–3.86 (m, 4H), 3.79 (s, 3H), 3.70 (s, 3H), 3.42 (d, J = 6.5 Hz, 2H), 3.04 (d, JHP = 21 Hz, 2H), 1.72 (s, 3H), 1.61 (s, 3H), 1.18 (td, J = 7.5 Hz, JHP = 3.7 Hz, 6H).
2-Geranyl-3,5,3′,4′-tetramethoxy-2′-prenyl-(E)-stilbene (20)
To a stirred suspension of NaH (60% dispersion in mineral oil, washed with hexanes, 33 mg, 0.8 mmol) in THF (2.5 mL) were added phosphonate 19 (56 mg, 0.2 mmol), aldehyde 14 (39 mg, 0.1 mmol), and 15-crown-5 (3 drops). The mixture was stirred for 2 h and quenched by addition of saturated aqueous NH4Cl. The resulting mixture was extracted with EtOAc, and the combined organic extracts were washed with brine, dried (MgSO4), and concentrated in vacuo. Final purification by flash column chromatography (10% EtOAc in hexanes) afforded stilbene 20 (20 mg, 31%) as a yellow oil: 1H NMR (500 MHz, CDCl3) δ 7.32 (d, J = 8.7 Hz, 1H), 7.12 (s, 2H), 6.80 (d, J = 8.8 Hz, 1H), 6.71 (d, J = 2.5 Hz, 1H), 6.21 (d, J = 2.5 Hz, 1H), 5.16–5.11 (m, 2H), 5.07–5.04 (m, 1H), 3.88 (s, 3H), 3.84 (s, 3H), 3.81 (s, 3H), 3.81 (s, 3H), 3.52–3.50 (m, 2H), 3.42 (d, J = 6.7 Hz, 2H), 2.07–2.03 (m, 2H), 1.98–1.92 (m, 2H), 1.81 (s, 3H), 1.78 (s, 3H), 1.67 (s, 3H), 1.62 (s, 3H), 1.55 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 158.5, 158.4, 152.3, 146.9, 138.4, 134.4, 134.0, 131.4, 131.2, 130.4, 128.0, 127.0, 124.4, 123.5, 123.3, 121.6, 121.1, 110.2, 101.6, 97.9, 60.7, 55.7 (2C), 55.3, 39.7, 26.8, 25.7, 25.6, 25.5, 24.3, 18.1, 17.6, 16.3; HRMS (EI) m/z calcd for C33H44O4 (M)+ 504.3240, found 504.3237.
2-Geranyl-3,5-dimethoxy-3′,4′-bis(methoxymethoxy)-(E)-stilbene (22)
To a stirred suspension of NaH (60% dispersion in mineral oil, washed with hexanes, 28 mg, 0.7 mmol) in THF (2.5 mL) were added aldehyde 14 (35 mg, 0.1 mmol), phosphonate 21(38) (49 mg, 0.1 mmol), and 15-crown-5 (3 drops). The mixture was stirred for 2 h and quenched with saturated aqueous NH4Cl. The resultant mixture was extracted with EtOAc, and the combined organic extracts were washed with brine, dried (MgSO4), and concentrated in vacuo. Final purification of the residue by flash column chromatography (10% EtOAc in hexanes) gave stilbene 22 (38 mg, 67%) as a yellow oil: 1H NMR (300 MHz, CDCl3) δ 7.32 (d, J = 1.6 Hz, 1H), 7.21 (d, J = 16.1 Hz, 1H), 7.15 (d, J = 8.4 Hz, 1H), 7.10 (dd, J = 8.5 Hz, 1.7 Hz, 1H), 6.89 (d, J = 16.2 Hz, 1H), 6.72 (d, J = 2.2 Hz, 1H), 6.42 (d, J = 2.1 Hz, 1H), 5.28 (s, 2H), 5.26 (s, 2H), 5.14–5.03 (m, 2H), 3.85 (s, 3H), 3.81 (s, 3H), 3.54 (s, 3H), 3.53 (s, 3H), 3.43 (d, J = 6.7 Hz, 2H), 2.08–1.95 (m, 4H), 1.81 (s, 3H), 1.61 (s, 3H), 1.54 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 158.5, 158.3, 147.3, 146.8 137.8, 134.3, 132.4, 131.2, 129.7, 125.8, 124.3, 123.5, 121.1, 120.8, 116.5, 114.6, 101.2, 98.0, 95.4, 95.3, 56.2 (2C), 55.6, 55.3, 39.7, 26.7, 25.6, 24.3, 17.6, 16.2; HRMS (ESI) m/z calcd for C30H41O6 (M + H)+ 497.2903, found 497.2918.
2-Geranyl-3,5-dimethoxy-3′,4′-dihydroxy-(E)-stilbene (23)
To a solution of bis(methoxymethyl) ether 22 (18 mg, 0.04 mmol) in MeOH (2 mL) was added TsOH (29 mg, 0.15 mmol). After the solution was stirred overnight, the reaction was quenched by addition of saturated aqueous NaHCO3. The resultant mixture was extracted with EtOAc, and the combined organic extracts were dried (MgSO4), filtered, and concentrated in vacuo. Final purification of a portion (25%) of the residual oil by preparative TLC (25% EtOAc in hexanes) afforded the stilbene 23 (5 mg, 100% by NMR) as a yellow oil; the remaining material (75%) was moved forward without additional purification. For compound 23: 1H NMR (300 MHz, CDCl3) δ 7.20–6.82 (m, 5H), 6.71 (m, 1H), 6.42 (m, 1H), 5.19–4.98 (m, 2H), 3.86 (s, 3H), 3.81 (s, 3H), 3.42 (d, J = 5.9 Hz, 2H), 2.07–1.97 (m, 4H), 1.80 (s, 3H), 1.62 (s, 3H), 1.55 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 158.5, 158.4, 143.8, 143.5, 137.9, 134.5, 131.3, 129.9, 125.1, 124.3, 123.5, 121.1, 119.9, 115.5, 113.1, 110.9, 101.4, 97.9, 55.7, 55.4, 39.7, 26.7, 25.6, 24.3, 17.7, 16.3; HRMS (ESI) m/z calcd for C26H33O4 (M + H)+ 409.2379, found 409.2374.
2-Geranyl-3,5,3′,4′-tetramethoxy-(E)-stilbene (24)
To a stirred solution of stilbene 23 in acetone (3 mL) was added K2CO3 (35 mg, 0.25 mmol) followed by MeI (38 μL, 0.61 mmol). The mixture was stirred for 2 days, and the reaction was quenched with H2O. The resulting mixture was extracted with EtOAc, and the combined organic extracts were washed with brine, dried (MgSO4), and concentrated in vacuo. Final purification by flash column chromatography (gradient of hexanes to 40% EtOAc in hexanes) provided stilbene 24 (4 mg, 27%) as a yellow oil: 1H NMR (300 MHz, CDCl3) δ 7.27–7.18 (m, 1H), 7.06–7.04 (m, 2H), 6.94–6.85 (m, 2H), 6.75 (d, J = 2.7 Hz, 1H), 6.43 (d, J = 2.4 Hz, 1H), 5.14 (t, J = 7.5 Hz, 1H), 5.06 (t, J = 7.5 Hz, 1H), 3.94 (s, 3H), 3.91 (s, 3H), 3.86 (s, 3H), 3.83 (s, 3H), 3.46–3.44 (m, 2H), 2.05–1.95 (m, 4H), 1.82 (s, 3H), 1.62 (s, 3H), 1.55 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 158.9, 149.1, 138.0, 134.2, 131.3, 130.8, 130.1, 125.2, 124.2, 123.4, 121.1, 119.9, 111..3, 108.8, 107.1, 105.8, 101.4, 97.9, 56.0, 55.8, 55.7, 55.4, 39.7, 26.8, 25.6, 24.4, 17.6, 16.3; HRMS (EI) m/z calcd for C28H36O4 (M)+ 436.2614, found 436.2606.
2-Geranyl-3,5,3′,4′-tetrakis(methoxymethoxy)-(E)-stilbene (25)
To a solution of potassium hexamethyldisilazane (KHMDS) (0.5 M solution in toluene, 2.3 mL, 1.16 mmol) in THF (1.5 mL) were added phosphonate 21 (46 mg, 0.13 mmol) and aldehyde 16 (35 mg, 0.10 mmol). After the solution was stirred for 4 h, the reaction was quenched by addition of NH4Cl. The resulting mixture was extracted with EtOAc, washed with brine, dried (MgSO4), and concentrated in vacuo. Final purification by flash column chromatography (7% EtOAc in hexanes) provided stilbene 25 (46 mg, 86%) as a yellow oil: 1H NMR (500 MHz, CDCl3) δ 7.31–7.11 (m, 4H), 6.96 (s, 1H), 6.88 (d, J = 15.7 Hz, 1H), 6.74 (s, 1H), 5.27–5.05 (m, 10H), 3.54–3.48 (m, 14H), 2.04–1.97 (m, 4H), 1.82 (s, 3H), 1.61 (s, 3H), 1.54 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 156.1, 155.8, 147.4, 146.9, 138.2, 134.5, 132.4, 131.3, 130.1, 125.5, 124.2, 123.3, 122.8, 120.9, 116.7, 114.8, 106.2, 102.9, 95.5, 95.4, 94.6 (2C), 56.2 (2C), 56.0 (2C), 39.7, 26.7, 25.6, 24.7, 17.6, 16.2; HRMS (EI) m/z calcd for C32H44O8 (M)+ 556.3036, found 556.3056.
2-Geranyl-3,5,3′,4′-tetrahydroxy-(E)-stilbene (26)
To a solution of stilbene 25 (23 mg, 0.04 mmol) in MeOH (4 mL) was added TsOH (63 mg, 0.33 mmol). The solution was stirred for 24 h, and the reaction was quenched by addition of NaHCO3. The resulting mixture was extracted with EtOAc, dried (MgSO4), and concentrated in vacuo. Final purification by preparative TLC (30% EtOAc in hexanes) afforded stilbene 26 (6 mg, 38%) as a yellow oil: 1H NMR (500 MHz, CD3OD) δ 6.96 (d, J = 16 Hz, 1H), 6.85 (d, J = 2.3 Hz, 1H), 6.71 (d, J = 2 Hz, 1H), 6.70 (d, J = 1.7 Hz, 1H), 6.65 (d, J = 15.8 Hz, 1H), 6.63 (d, J = 8.3 Hz, 1H), 6.44 (d, J = 2.1 Hz, 1H), 6.13 (d, J = 2.3 Hz, 1H), 5.01–5.00 (m, 1H), 4.95–4.92 (m, 1H), 3.27 (d, J = 6.6 Hz, 2H), 1.97–1.92 (m, 2H), 1.88–1.85 (m, 2H), 1.70 (s, 3H), 1.47 (s, 3H), 1.42 (s, 3H); 13C NMR (125 MHz, CD3OD) δ 157.0, 156.7, 146.5, 146.4, 139.8, 134.5, 132.1, 131.6, 130.7, 125.8, 125.4 (2C), 120.0, 119.1, 116.4, 114.0, 104.3, 102.6, 40.8, 27.8, 25.8, 25.1, 17.7, 16.5; HRMS (EI) m/z calcd for C24H28O4 (M)+ 380.1988, found 380.2014.
2-Geranyl-3,5-dimethoxy-3′-prenyl-4′,5′-bis(methoxymethoxy)-(E)-stilbene (28)
To a stirred solution of aldehyde 14 (21 mg, 0.07 mmol) and phosphonate 27 (51 mg, 0.12 mmol) in THF (1.4 mL) at 0 °C was added KHMDS (0.5 M in toluene, 0.69 mL, 0.35 mmol). The solution was stirred for 22 h at rt, and the reaction was quenched with NH4Cl. The resultant mixture was extracted with EtOAc, and the combined organic extracts were washed with brine, dried (MgSO4), filtered, and concentrated in vacuo. Final purification by preparative TLC (30% EtOAc in hexanes) gave stilbene 28 (36 mg, 92%) as a yellow oil: 1H NMR (500 MHz, CDCl3) δ 7.20 (d, J = 16.0 Hz, 1H), 7.14 (d, J = 1.8 Hz, 1H), 6.98 (d, J = 2.1 Hz, 1H), 6.87 (d, J = 15.7 Hz, 1H), 6.72 (d, J = 2.4 Hz, 1H), 6.41 (d, J = 2.6 Hz, 1H), 5.34–5.31 (m, 1H), 5.22 (s, 2H), 5.12 (s, 2H), 5.06–5.03 (m, 1H), 3.85 (s, 3H), 3.81 (s, 3H), 3.61 (s, 3H), 3.52 (s, 3H), 3.44–3.41 (m, 4H), 2.06–2.02 (m, 2H), 1.98–1.95 (m, 2H), 1.81 (s, 3H), 1.76 (s, 3H), 1.74 (s, 3H), 1.60 (s, 3H), 1.53 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 158.5, 158.4, 150.0, 144.4, 137.9, 136.1, 134.2, 133.9, 132.7, 131.2, 130.1, 126.3, 124.3, 123.5, 122.6, 121.5, 121.2, 112.1, 101.4, 99.1, 98.1, 95.2, 57.5, 56.2, 55.7, 55.4, 39.7, 28.6, 26.7, 25.8, 25.6, 24.4, 17.9, 17.6, 16.2; HRMS (ESI) m/z calcd for C35H49O6 (M + H)+ 565.3529, found 565.3524.
2-Geranyl-3,5-dimethoxy-3′-prenyl-4′,5′-dihydroxy-(E)-stilbene (29)
To a solution of bis-MOM acetal 28 (36 mg, 0.06 mmol) in MeOH (6.4 mL) was added TsOH (49 mg, 0.26 mmol). After the solution was stirred for 19.5 h at rt, additional TsOH (25 mg, 0.13 mmol) was added due to incomplete conversion to product. After the solution was stirred for an additional 22.5 h, the reaction was quenched by addition of NaHCO3. The resultant mixture was extracted with EtOAc, and the combined organic extracts were dried (MgSO4), filtered, and concentrated in vacuo. Final purification by preparative TLC (35% EtOAc in hexanes) provided stilbene 29 (14 mg, 47%) as a yellow oil: 1H NMR (500 MHz, CDCl3) δ 7.37 (d, J = 15.6 Hz, 1H), 6.94 (s, 1H), 6.84–6.80 (m, 2H), 6.71 (d, J = 2.1 Hz, 1H), 6.40 (d, J = 2.0 Hz, 1H), 5.45 (br s, 1H), 5.34 (t, J = 6.1 Hz, 1H), 5.11 (t, J = 6.7 Hz, 1H), 5.06–5.04 (m, 1H), 3.84 (s, 3H), 3.80 (s, 3H), 3.42 (d, J = 6.5 Hz, 2H), 3.37 (d, J = 7.1 Hz, 2H), 2.07–2.03 (m, 2H), 1.98–1.95 (m, 2H), 1.81–1.79 (m, 9H), 1.61 (s, 3H), 1.54 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 158.5, 158.4, 144.2, 141.9, 138.0, 135.3, 134.4, 131.3, 130.6, 130.1, 127.3, 125.2, 124.3, 123.6, 121.6, 121.1, 120.5, 110.9, 101.5, 97.9, 55.7, 55.4, 39.7, 29.9, 26.8, 25.8, 25.6, 24.4, 17.9, 17.6, 16.3; HRMS (ESI) m/z calcd for C31H41O4 (M)+ 477.3005, found 477.2994.
2-Geranyl-3,5-dimethoxy-2′-prenyl-3′-(methoxymethoxy)-4′-methoxy-(E)-stilbene (30)
To a stirred solution of aldehyde 14 (27 mg, 0.1 mmol) and 18 (51 mg, 0.1 mmol) in THF (1.5 mL) at room temperature were added NaH (60% dispersion in mineral oil, 22 mg, 0.6 mmol) and 15-crown-5 (2 drops). After the mixture was stirred overnight, the reaction was quenched by addition of NH4Cl. The resulting mixture was extracted with EtOAc, and then the combined organic extracts were dried (MgSO4), filtered, and concentrated in vacuo. Final purification by flash column chromatography (10% EtOAc in hexanes) provided 30 (18 mg) in 38% yield as a yellow oil: 1H NMR (300 MHz, CDCl3) δ 7.33 (d, J = 8.7 Hz, 1H), 7.11 (m, 2H), 6.80 (d, J = 8.6 Hz, 1H), 6.70 (d, J = 2.3 Hz, 1H), 6.41 (d, J = 2.5 Hz, 1H), 5.16–5.03 (m, 3H), 5.09 (s, 2H), 3.86 (s, 3H), 3.83 (s, 3H), 3.81 (s, 3H), 3.60 (s, 3H), 3.57 (d, J = 6.6 Hz, 2H), 3.42 (d, J = 6.7 Hz, 2H), 2.05–1.96 (m, 4H), 1.80 (s, 3H), 1.78 (s, 3H), 1.68 (s, 3H), 1.62 (s, 3H), 1.55 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 158.8, 158.7, 152.1, 144.1, 138.7, 134.8, 134.5, 131.6 (2C), 130.9, 128.4, 127.3, 124.7, 123.8, 123.6, 122.2, 121.4, 110.4, 101.8, 99.4, 98.2, 58.0, 56.1, 56.0, 55.6, 40.1, 30.0, 27.1, 26.1, 26.0 (2C), 24.7, 18.5, 18.0, 16.6; HRMS (EI) m/z calcd for C34H46O5 (M)+ 534.3345, found 534.3330.
2-Geranyl-3,5-dimethoxy-2′-prenyl-3′-hydroxy-4′-methoxy-(E)-stilbene (31)
To a stirred solution of MOM ether 30 (27 mg, 0.1 mmol) in MeOH (2.5 mL) was added TsOH (40 mg, 0.2 mmol). The solution was stirred overnight, and the reaction was quenched by addition of saturated aqueous NaHCO3. The resulting mixture was extracted with EtOAc, and the organic extracts were dried (MgSO4), filtered, and concentrated in vacuo. Stilbene 31 (22 mg, 88%) was obtained as a yellow oil: 1H NMR (300 MHz, CDCl3) δ 7.14 (m, 2H), 7.10 (m, 1H), 6.76 (m, 1H), 6.73 (d, J = 2.4 Hz, 1H), 6.42 (d, J = 2.2 Hz, 1H), 5.21–5.04 (m, 3H), 3.90 (s, 3H), 3.84 (s, 3H), 3.81 (s, 3H), 3.52 (d, J = 6.5 Hz, 2H), 3.43 (d, J = 6.4 Hz, 2H), 2.09–1.96 (m, 4H), 1.82 (s, 3H), 1.78 (s, 3H), 1.68 (s, 3H), 1.62 (s, 3H), 1.55 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 158.9, 158.1, 146.2, 143.6, 138.7, 134.7, 131.9, 131.5, 131.0, 128.5, 127.5, 126.1, 124.7, 123.9, 123.0, 121.5, 117.5, 108.8, 101.9, 98.3, 56.0, 55.7, 55.3, 39.8, 26.8, 25.7, 25.6, 25.1, 24.4, 18.1, 17.6, 16.3; HRMS (EI) m/z calcd for C32H42O4 (M + H)+ 490.3083, found 490.3087.
2-Geranyl-3,5,3′,4′-tetramethoxy-2′-prenyl-(E)-stilbene (20)
To a solution of stilbene 31 (11 mg, 0.02 mmol) in THF (3 mL) were added NaH (60% dispersion in mineral oil, 6 mg, 0.2 mmol) and MeI (2 drops). The mixture was stirred for 5 h, and the reaction was quenched by addition of H2O. The resultant mixture was extracted with EtOAc, and the combined organic extracts were washed with 2 M NaOH, dried (MgSO4), and concentrated in vacuo. Stilbene 20 (6 mg, 55%) was obtained as a yellow oil, with 1H NMR data that were identical to the data given above.
4-Prenyl-3,5,3′,4′-tetrakis(methoxymethoxy)-(E)-stilbene (36)
To a solution of KHMDS (0.5 M solution in toluene, 2.12 mL, 1.06 mmol) in THF (1.5 mL) were added phosphonate 23(51) (46 mg, 0.11 mmol) and aldehyde 34(52) (20 mg, 0.09 mmol). After the solution was stirred for 2 h, the reaction was quenched by addition of NH4Cl. The resulting mixture was extracted with EtOAc, washed with brine, dried (MgSO4), and concentrated in vacuo. Final purification by flash column chromatography provided stilbene 36 (25 mg, 58%) as a yellow oil: 1H NMR (500 MHz, CDCl3) δ 7.32 (d, J = 1.9 Hz, 1H), 7.14–7.09 (m, 2H), 6.98–6.89 (m, 4H), 5.29 (s, 2H), 5.25–5.21 (m, 7H), 3.56 (s, 3H), 3.53 (s, 3H), 3.50 (s, 6H), 3.39 (d, J = 7.2 Hz, 2H), 1.79 (s, 3H), 1.66 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 155.8 (2C), 147.4, 146.8, 136.4, 132.1, 131.0, 127.7 (2C), 122.7, 121.0, 119.7, 116.6, 114.3, 106.0 (2C), 95.4 (2C), 94.5 (2C), 56.2 (2C), 56.0 (2C), 25.7, 22.7, 17.7; HRMS (EI) m/z calcd for C27H36O8 (M)+ 488.2410, found 488.2416.
4-Prenyl-3,5,3′,4′-tetrahydroxy-(E)-stilbene (37)
After TsOH (40 mg, 0.21 mmol) was added to a solution of stilbene 36 (13 mg, 0.03 mmol) in MeOH (2.5 mL), the solution was stirred for 24 h. The reaction was quenched by addition of NaHCO3, and the resulting mixture was extracted with EtOAc, dried (MgSO4), and concentrated in vacuo. Final purification by preparative TLC (30% EtOAc in hexanes) gave stilbene 37 (4 mg, 50%) as a yellow oil. The 1H and 13C NMR spectra matched published data.56,57
4-Prenyl-3,5,4′-tris(methoxymethoxy)-(E)-stilbene (38)
To a solution of KHMDS (0.5 M solution in toluene, 4.62 mL, 2.31 mmol) in THF (1.5 mL) were added phosphonate 33 (99 mg, 0.24 mmol) and aldehyde 35(52) (32 mg, 0.19 mmol). After the solution was stirred for 3 h, the reaction was quenched by addition of NH4Cl. The resulting mixture was extracted with EtOAc, washed with brine, dried (MgSO4), and concentrated in vacuo. Final purification of the residue by flash column chromatography (4% EtOAc in hexanes) provided stilbene 38 (33 mg, 40%) as a yellow oil: 1H NMR (500 MHz, CDCl3) δ 7.44 (t, J = 2.7 Hz, 1H), 7.42 (t, J = 1.9 Hz, 1H), 7.03–7.01 (m, 2H), 6.98–6.95 (m, 1H), 6.92–6.90 (m, 1H), 6.92 (s, 2H), 5.24–5.19 (m, 5H), 5.19 (s, 2H), 3.50 (s, 6H), 3.49 (s, 3H), 3.39 (d, J = 7.2 Hz, 2H), 1.79 (s, 3H), 1.66 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 156.8, 155.8 (2C), 136.6, 131.3, 131.0, 127.7, 127.6 (2C), 127.2, 122.7, 119.6, 116.4 (2C), 106.0 (2C), 94.5 (2C), 94.4, 56.0 (3C), 25.8, 22.8, 17.8; HRMS (EI) m/z calcd for C25H32O6 (M)+ 428.2199, found 428.2191.
trans-Arachidin-2 (39)
To a solution of compound 38 (17 mg, 0.04 mmol) in MeOH (5 mL) was added TsOH (46 mg, 0.24 mmol). After the solution was stirred for 24 h, the reaction was quenched with NaHCO3. The resulting mixture was extracted with EtOAc, dried (MgSO4), and concentrated in vacuo. Final purification by flash column chromatography (12% EtOAc in hexanes) provided stilbene 39 (12 mg, 100%) as a yellow oil. Both the 1H and 13C data matched those of the known compound.56,57
Biological Assays
All compounds were initially screened for intrinsic and antagonist activity at 10 μM in the [35S]GTP-γ-S binding assay at the human κ and the μ and δ opioid receptors overexpressed in CHO cells. These cell lines were kindly provided by Dr. Liu-Chen (Temple University, κ) and Dr. Larry Toll (SRI International, μ and δ). Compounds were identified as antagonist characterized for functional antagonism (Ke) and selectivity by measuring the ability of the test compounds to inhibit stimulated [35S]GTP-γ-S binding produced by one of the selective agonists DAMGO (μ), DPDPE (δ), or U69,593 (κ). Agonist concentration–response curves were run in the presence or absence of a single concentration of test compound.
Briefly, the test compounds were assayed in duplicate in 1.4 mL polypropylene tubes in a 96-well format. CHO membrane homogenates (20–40 μg protein) were incubated with a positive control or the test compound, 0.1 nM [35S]GTP-γ-S, and 1 μM GDP in 50 mM HEPES buffer (pH 7.4) at room temperature for 1 h, after which bound radioligand was separated from free radioligand via rapid vacuum filtration over GF-B filters with a Brandel Scientific (Gaithersburg, MD, USA) 96-well harvester. Bound radioactivity is determined using a TopCount 12-detector instrument (Packard Instruments) using standard scintillation counting techniques. Bound radioactivity is normalized to samples containing vehicle (basal binding). A four-parameter logistic function was fit to these data to calculate the EC50 and Emax values using Prism (v. 6; Graph Pad Software, San Diego, CA, USA). The Ke values were calculated using the formula Ke = [L]/DR – 1, where [L] is the concentration of test compound, and DR is the ratio of agonist EC50 value in the presence or absence of test compound.
Acknowledgments
We thank Dr. R. J. Barney for his assistance with preparation of some early intermediates. Financial support from the National Institutes of Health (DA02-6573) is gratefully acknowledged. This research also was supported in part by the Intramural Research Program of NIH, National Cancer Institute, Center for Cancer Research, and the Roy J. Carver Charitable Trust as a Research Program of Excellence.
Supporting Information Available
Supplementary data including the 1H and 13C NMR spectra for the key intermediates and final products in this article are available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Dedication
We dedicate this contribution to our valued colleague Paul J. Klausmeyer, who isolated and characterized schweinfurthin J and who was killed in an automobile accident on January 22, 2013.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Belofsky G.; French A. N.; Wallace D. R.; Dodson S. L. J. Nat. Prod. 2004, 67, 26–30. [DOI] [PubMed] [Google Scholar]
- Gillmore M.Uses of Plants by the Indians of the Missouri River Region; University of Nebraska Press: Lincoln, NE, 1977. [Google Scholar]
- Topczewski J. J.; Kodet J. G.; Wiemer D. F. J. Org. Chem. 2011, 76, 909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topczewski J. J.; Wiemer D. F. Tetrahedron Lett. 2011, 52, 1628–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors J. D.; Buller M. J.; Boss K. D.; Wiemer D. F. J. Nat. Prod. 2008, 71, 1949–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors J. D.; Salnikova M. S.; Wiemer D. F. Tetrahedron Lett. 2005, 46, 1321–1324. [Google Scholar]
- Siebert D. J. J. Ethnopharmacol. 1994, 43, 53–56. [DOI] [PubMed] [Google Scholar]
- Lovell K. M.; Vasiljevik T.; Araya J. J.; Lozama A.; Prevatt-Smith K. M.; Day V. W.; Dersch C. M.; Rothman R. B.; Butelman E. R.; Kreek M. J.; Prisinzano T. E. Bioorg. Med. Chem. 2012, 20, 3100–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer J. R.; Lawrence J. F.; Wang G. C.; Evans D. A. J. Am. Chem. Soc. 2007, 129, 8968–8969. [DOI] [PubMed] [Google Scholar]
- Hagiwara H.; Suka Y.; Nojima T.; Hoshi T.; Suzuki T. Tetrahedron 2009, 65, 4820–4825. [Google Scholar]
- Harding W. W.; Schmidt M.; Tidgewell K.; Kannan P.; Holden K. G.; Dersch C. M.; Rothman R. B.; Prisinzano T. E. Bioorg. Med. Chem. Lett. 2006, 16, 3170–3174. [DOI] [PubMed] [Google Scholar]
- Harding W. W.; Schmidt M.; Tidgewell K.; Kannan P.; Holden K. G.; Gilmour B.; Navarro H.; Rothman R. B.; Prisinzano T. E. J. Nat. Prod. 2006, 69, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden K. G.; Tidgewell K.; Marquam A.; Rothman R. B.; Navarro H.; Prisinzano T. E. Bioorg. Med. Chem. Lett. 2007, 17, 6111–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. S.; Katavic P. L.; Lozama A.; Harding W. W.; Parrish D.; Deschamps J. R.; Dersch C. M.; Partilla J. S.; Rothman R. B.; Navarro H.; Prisinzano T. E. J. Med. Chem. 2007, 50, 3596–3603. [DOI] [PubMed] [Google Scholar]
- Tidgewell K.; Harding W. W.; Lozama A.; Cobb H.; Shah K.; Kannan P.; Dersch C. M.; Parrish D.; Deschamps J. R.; Rothman R. B.; Prisinzano T. E. J. Nat. Prod. 2006, 69, 914–918. [DOI] [PubMed] [Google Scholar]
- Lozama A.; Cunningham C. W.; Caspers M. J.; Douglas J. T.; Dersch C. M.; Rothman R. B.; Prisinzano T. E. J. Nat. Prod. 2011, 74, 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polepally P. R.; White K.; Vardy E.; Roth B. L.; Ferreira D.; Zjawiony J. K. Bioorg. Med. Chem. Lett. 2013, 23, 2860–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida E. R.; Almeida R. N.; Navarro D. S.; Bhattacharryya J.; Silva B. A.; Birnbaum J. S. J. Ethnopharmacol. 2003, 88, 1–4. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya J.; Majetich G.; Jenkins T. M.; Almeida R. N. J. Nat. Prod. 1998, 61, 413–414. [DOI] [PubMed] [Google Scholar]
- Batista J. S.; Almeida R. N.; Bhattacharyya J. J. Ethnopharmacol. 1995, 45, 207–210. [DOI] [PubMed] [Google Scholar]
- Almeida R. N.; Navarro D. S.; Almeida E. R.; Majetich G.; Bhattacharyya J. Pharm. Biol. 2000, 38, 394–395. [Google Scholar]
- Katavic P. L.; Lamb K.; Navarro H.; Prisinzano T. E. J. Nat. Prod. 2007, 70, 1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J. T.; Leon F.; Radwan M. M.; Dale O. R.; Husni A. S.; Manly S. P.; Lupien S.; Wang X. N.; Hill R. A.; Dugan F. M.; Cutler H. G.; Cutler S. J. J. Nat. Prod. 2011, 74, 1636–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J. T.; Radwan M. M.; Leon F.; Dale O. R.; Husni A. S.; Wu Y. S.; Lupien S.; Wang X. N.; Manly S. P.; Hill R. A.; Dugan F. M.; Cutler H. G.; Cutler S. J. J. Nat. Prod. 2013, 76, 824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell K. M.; Simpson D. S.; Cunningham C. W.; Prisinzano T. E. Future Med. Chem. 2009, 1, 285–301. [DOI] [PubMed] [Google Scholar]
- Jang M. S.; Cai E. N.; Udeani G. O.; Slowing K. V.; Thomas C. F.; Beecher C. W. W.; Fong H. H. S.; Farnsworth N. R.; Kinghorn A. D.; Mehta R. G.; Moon R. C.; Pezzuto J. M. Science 1997, 275, 218–220. [DOI] [PubMed] [Google Scholar]
- Gupta Y. K.; Sharma M.; Briyal S. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 667–672. [DOI] [PubMed] [Google Scholar]
- Sobolev V. S.; Khan S. I.; Tabanca N.; Wedge D. E.; Manly S. P.; Cutler S. J.; Coy M. R.; Becnel J. J.; Neff S. A.; Gloer J. B. J. Agric. Food Chem. 2011, 59, 1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunlakshana O.; Schild H. O. Br. J. Pharm. Chemother. 1959, 14, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek M. J.; Koob G. F. Drug Alcohol Depend. 1998, 51, 23–47. [DOI] [PubMed] [Google Scholar]
- Sinha R.; Fuse T.; Aubin L. R.; O’Malley S. S. Psychopharmacology (Berlin, Ger.) 2000, 152, 140–148. [DOI] [PubMed] [Google Scholar]
- McMahon R. C. J. Subst. Abuse Treat. 2001, 21, 77–87. [DOI] [PubMed] [Google Scholar]
- Thomas J. B.; Atkinson R. N.; Vinson N. A.; Catanzaro J. L.; Perretta C. L.; Fix S. E.; Mascarella S. W.; Rothman R. B.; Xu H.; Dersch C. M.; Cantrell B. E.; Zimmerman D. M.; Carroll F. I. J. Med. Chem. 2003, 46, 3127–3137. [DOI] [PubMed] [Google Scholar]
- Beardsley P. M.; Howard J. L.; Shelton K. L.; Carroll F. I. Psychopharmacology (Berlin, Ger.) 2005, 183, 118–126. [DOI] [PubMed] [Google Scholar]
- Carey A. N.; Borozny K.; Aldrich J. V.; McLaughlin J. P. Eur. J. Pharmacol. 2007, 569, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors J. D.; Beutler J. A.; Wiemer D. F. J. Org. Chem. 2005, 70, 925–931. [DOI] [PubMed] [Google Scholar]
- Kuder C. H.; Neighbors J. D.; Hohl R.; Wiemer D. F. Biorg. Med. Chem. 2009, 17, 4718–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors J. D.; Salnikova M. S.; Beutler J. A.; Wiemer D. F. Bioorg. Med. Chem. 2006, 14, 1771–1784. [DOI] [PubMed] [Google Scholar]
- Mente N. R.; Neighbors J. D.; Wiemer D. F. J. Org. Chem. 2008, 73, 7963–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodet J. G. Ph.D. Thesis, University of Iowa, 2010. [Google Scholar]
- Ulrich N. C.; Kodet J. G.; Mente N. R.; Kuder C. H.; Beutler J. A.; Hohl R. J.; Wiemer D. F. Bioorg. Med. Chem. 2010, 18, 1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. B.; Fix S. E.; Rothman R. B.; Mascarella S. W.; Dersch C. M.; Cantrell B. E.; Zimmerman D. M.; Carroll F. I. J. Med. Chem. 2004, 47, 1070–1073. [DOI] [PubMed] [Google Scholar]
- Prisinzano T. E. J. Med. Chem. 2013, 56, 3435–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. L.; White A. J. P.; Widdowson D. A.; Wilhelm R.; Williams D. J. J. Chem. Soc., Perkin Trans. 1 2001, 3269–3280. [Google Scholar]
- Zhou Q.; Snider B. B. J. Org. Chem. 2010, 75, 8224–8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. L.; Zhao Y. L. Chin. Chem. Lett. 1994, 5, 935–938. [Google Scholar]
- Cushman M.; Nagarathnam D.; Gopal D.; Chakraborti A. K.; Lin C. M.; Hamel E. J. Med. Chem. 1991, 34, 2579–2588. [DOI] [PubMed] [Google Scholar]
- Klausmeyer P.; Van Q. N.; Jato J.; McCloud T. G.; Beutler J. A. J. Nat. Prod. 2010, 73, 479–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.; Argade N. P. Synthesis 2012, 44, 2895–2902. [Google Scholar]
- Mente N. R.; Neighbors J. D.; Wiemer D. F. J. Org. Chem. 2008, 73, 7963–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mente N. R.; Wiemer A. J.; Neighbors J. D.; Beutler J. A.; Hohl R. J.; Wiemer D. F. Biorg. Med. Chem. Lett. 2007, 17, 911–915. [DOI] [PubMed] [Google Scholar]
- Heynekamp J. J.; Weber W. M.; Hunsaker L. A.; Gonzales A. M.; Orlando R. A.; Deck L. M.; Jagt D. L. V. J. Med. Chem. 2006, 49, 7182–7189. [DOI] [PubMed] [Google Scholar]
- Park B. H.; Lee H. J.; Lee Y. R. J. Nat. Prod. 2011, 74, 644–649. [DOI] [PubMed] [Google Scholar]
- Verhoest P. R.; Basak A. S.; Parikh V.; Hayward M.; Kauffman G. W.; Paradis V.; McHardy F.; McLean S.; Grimwood S.; Schmidt A. W.; Vanase-Frawley M.; Freeman J.; Van Deusen J.; Cox L.; Wong D.; Liras S. J. Med. Chem. 2011, 54, 5868–5877. [DOI] [PubMed] [Google Scholar]
- Carroll F. I.; Carlezon W. A. J. Med. Chem. 2013, 56, 2178–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.-C.; Lai Y.-H.; Djoko B.; Wu P.-L.; Liu C.-D.; Liu Y.-W.; Chiou R. Y. Y. J. Agric. Food Chem. 2006, 54, 10281–10287. [DOI] [PubMed] [Google Scholar]
- Huang C.-P.; Au L.-C.; Chiou R. Y. Y.; Chung P.-C.; Chen S.-Y.; Tang W.-C.; Chang C.-L.; Fang W.-H.; Lin S.-B. J. Agric. Food Chem. 2010, 58, 12123–12129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.