Abstract
Despite the widespread and devastating impact of depression on society, our current understanding of its pathogenesis is limited. Likewise, existing treatments are inadequate, providing relief to only a subset of people suffering from depression. The search for more effective antidepressant drugs includes the investigation of new molecular targets. Among them, current data suggests that sigma receptors are involved in multiple processes effecting antidepressant-like actions in vivo and in vitro. This review summarizes accumulated evidence supporting a role for sigma receptors in antidepressant effects and provides a conceptual framework for delineating their potential roles over the course of antidepressant treatment.
Keywords: Antidepressant, Glutamate, Serotonin, Sigma receptor, Signal transduction, Neuroplasticity, Neurogenesis
1. Introduction
Depression is one of the top ten causes of morbidity and mortality, afflicting up to 20% of the world’s population (Nestler et al., 2002; Berton and Nestler, 2006). In addition to its social toll, the economic burden of depression contributes approximately $44 billion in lost productivity annually in the United States (Stewart, 2003). The symptoms of depression are chronic, recurring, and life threatening (Berton and Nestler, 2006). Unfortunately, our current understanding of the etiology of depression is still rudimentary and current pharmacotherapeutic options are far from ideal.
Antidepressant drugs were discovered serendipitously in the 1950s (Berton and Nestler, 2006). The first two antidepressant drugs in widespread clinical use, iproniazid, a monoamine oxidase inhibitor (MAOI) and imipramine, a tricyclic antidepressant (TCA), were originally prescribed for other indications, but were found to possess potent antidepressant effects in humans (Deverteuil and Lehmann, 1958; Ball and Kiloh, 1959). They were subsequently shown to enhance central serotonin or norepinephrine neurotransmission, suggesting that their antidepressant actions could be attributed to this effect and by extension that depression was due to deficiencies in monoamine neurotransmission (Chaput et al., 1991; Blier and Bouchard, 1994; Slattery et al., 2004). Accordingly, the monoamine hypothesis of depression was established and continues to maintain a dominant role in antidepressant drug development (Prange, 1964; Schildkraut, 1965). The late 1980s were marked by the introduction of fluoxetine, a selective serotonin reuptake inhibitor (SSRI), which had a significantly reduced liability for unwanted side effects relative to the tricyclic antidepressants and MAOIs. Additional advances followed with the development of serotonin-norepinephrine reuptake inhibitors (SNRIs) and norepinephrine reuptake inhibitors (NRIs) which also produced antidepressant actions with fewer serious side effects than the classical tricyclic antidepressants and MAOIs. Despite these successes and the continued use of these medications, better therapeutic options are still needed (Slattery et al., 2004; Berton and Nestler, 2006; Racagni and Popoli, 2008; Lopez-Munoz and Alamo, 2009).
Major problems of existing antidepressant drugs include delayed clinical benefit, serious side effects, and a response in less than 50% of patients (Berton and Nestler, 2006). Consequently, there is still a great need for faster acting, safer, and more effective treatments for depression. In an effort to expand beyond classical monoamine based strategies, recent medication development activities have focused on neurotrophic factors, glutamatergic systems, the hypothalamic-pituitary axis (HPA) as well as a number of other less well characterized novel targets. Studies in these areas are ongoing and recent reviews are available on these topics (Castren and Rantamaki, 2008; Pariante and Lightman, 2008; Racagni and Popoli, 2008; Sen and Sanacora, 2008; aan het Rot et al., 2009). The present review focuses on another novel target for antidepressant therapeutic development, the sigma receptor.
In addition to the classical monoamines, and the more recently implicated novel receptor systems, sigma receptors have emerged as compelling targets for antidepressant drug development. Numerous in vivo studies indicate that sigma receptor agonists produce antidepressant-like effects in animals and humans (Matsuno et al., 1996; Ukai et al., 1998; Skuza and Rogoz, 2002; Skuza, 2003; Wang et al., 2007b). Further, many in vitro studies demonstrate that sigma receptor agonists modulate the activities of the same neurotransmitter systems, signaling pathways, and brain regions implicated in the pathophysiology of depression and the therapeutic effects of currently marketed therapeutics.
A number of excellent reviews have been published recently that provide updated accounts of sigma receptor pharmacology (Hashimoto and Ishiwata, 2006; Cobos et al., 2008; Maurice and Su, 2009; Tsai et al., 2009) or that specifically address the putative role of sigma receptors in neuropsychiatric disorders, including depression (Hayashi and Su, 2008; Stahl, 2008; Kulkarni and Dhir, 2009). This review focuses on sigma receptors as a potential target for the development of a new class of antidepressant drugs. The review begins with a summary of evidence implicating sigma receptors in the actions of antidepressant drugs. It then provides a conceptual framework for potential mechanism(s) of action of sigma-active antidepressant drugs and an account of recent studies supporting this conceptual framework.
1. Sigma receptors
Sigma receptors were initially proposed as a subtype of opioid receptor (Martin et al., 1976). Later studies demonstrated that they are unique proteins highly conserved across species, cell types, and organelles (Hanner et al., 1996; Kekuda et al., 1996; Seth et al., 1997; Seth et al., 1998; Mei and Pasternak, 2001). Sigma receptors are widely distributed in the body (Wolfe et al., 1989; Harada et al., 1994; Hellewell et al., 1994; Novakova et al., 1995; Wolfe et al., 1997). In the brain, they are found in significant concentrations in limbic and endocrine areas that have been implicated in the pathophysiology of depression (Drevets et al., 2008; aan het Rot et al., 2009), including the hippocampus, frontal cortex, hypothalamus, and olfactory bulb (Itzhak et al., 1985; Alonso et al., 2000).
The endogenous ligand(s) for sigma receptors have yet to be conclusively identified. However, a number of candidates have been proposed including some neuroactive steroids, sphingolipids (Su et al., 1988; Ramachandran et al., 2009), and most recently N,N-dimethyltryptamine (DMT) (Fontanilla et al., 2009). While neuroactive steroids undoubtedly play a significant role in brain physiology, the complexity of their interactions with multiple receptor systems and HPA function makes definitive assignment of their actions to specific processes a challenge. Nevertheless, cross pharmacology between neurosteroids and sigma ligands has been reported and antidepressant effects elicited by exogenously administered neurosteroids can be antagonized by sigma ligands. Studies of the role of neurosteroids in depression and as sigma receptor effectors are the subject of several reviews (van Broekhoven and Verkes, 2003; Maurice, 2004; Monnet and Maurice, 2006; Dhir and Kulkarni, 2008a). The roles of other putative endogenous sigma ligands are unknown at this time.
Two subtypes of sigma receptors, sigma-1 and sigma-2, have been identified, and are differentiated by their molecular weights, tissue distributions, and pharmacological profiles (Hellewell, 1990; Quirion et al., 1992; Hellewell et al., 1994; McCann et al., 1994). There is some evidence for additional subtypes of sigma receptors, but thus far they remain poorly characterized (Kovacs and Larson, 1995; Bergeron and Debonnel, 1997; Wolfe et al., 1997). Both currently identified subtypes appear to convey antidepressant properties, but research on sigma receptors has been biased toward the sigma-1 subtype primarily due to the availability of a significant number of ligands with high affinity and selectivity, and due to its successful cloning.
The sigma-1 receptor is a highly conserved 223 amino acid protein that has been cloned from several species, including rodents and humans (Hanner et al., 1996; Kekuda et al., 1996; Seth et al., 1997; Seth et al., 1998; Mei and Pasternak, 2001). Functionally, sigma-1 receptors appear to operate primarily via protein-protein interactions and have been shown to modulate the activity of various ion channels and signaling molecules (Maurice and Su, 2009). Sigma-1 receptors are expressed on the endoplasmic reticulum (ER) and can translocate between different cellular compartments in response to ligand binding (Morin-Surun et al., 1999; Hayashi and Su, 2003; Mavlyutov and Ruoho, 2007). Accordingly, the discrete activities that are ascribed to the sigma-1 receptor depend on its cellular location. Sigma-1 mediated modulation of ion channels on the plasmalemma (Yamamoto et al., 1995; Lupardus et al., 2000; Aydar et al., 2002; Tchedre et al., 2008; Johannessen et al., 2009) and control of intracellular calcium via interactions with inositol trisphosphate (IP3) receptors on the ER are well documented (Novakova et al., 1998; Hayashi et al., 2000; Hayashi and Su, 2001; Hayashi and Su, 2007; Wu and Bowen, 2008). Likewise, a recent report details a chaperone-like activity for sigma-1 receptors, whereby its association with IP3 receptors at the mitochondrial associated membrane (MAM) facilitates efficient Ca2+ signaling from the ER to the mitochondria, suggesting that sigma receptors play an important role in bioenergetics (Hayashi and Su, 2007).
The sigma-2 receptor has not yet been cloned but is thought to be 18–22 kDa protein that is enriched in lipid rafts, where it affects Ca2+ signaling through sphingolipid products (Crawford et al., 2002). The role of sigma-2 receptors in depression is not as well defined as that of sigma-1, although one report suggests that the sigma-2 selective ligand siramesine (Lu 28–179; 19-[4[1-(4-fluorophenyl)-1H-indol-3-yl]-1-butyl]spiro[isobenzofuran-1(3H),49-piperidine]) shows equivalent antidepressant activity compared to the established antidepressants citalopram and imipramine in a chronic mild stress model in mice (Sanchez and Papp, 2000).
3. Involvement of sigma receptors in the actions of antidepressant drugs
3.1. Interaction of antidepressant drugs at sigma receptors
The involvement of sigma receptors in the actions of antidepressant drugs was first suggested by observations that most marketed antidepressant drugs bind to these receptors, raising the possibility that some of their therapeutic effects may be mediated via these proteins (Schmidt et al., 1989; Itzhak and Kassim, 1990; Narita et al., 1996). Table 1 summarizes representative tricyclic antidepressants, MAOIs, SSRIs and newer generations of antidepressant drugs that have significant affinity for sigma receptors, particularly the sigma-1 subtype. In addition to the classical antidepressants, neurosteroids with antidepressant activity such as dehydroepiandrosterone (DHEA) and pregnenolone, bind to sigma receptors (Su et al., 1988; Su et al., 1990; Maurice, 2004) and the putative active constituent of St. John’s wort, an herbal treatment for depression (Mennini and Gobbi, 2004), has also been shown to possess significant affinity for sigma receptors (Raffa, 1998; Perfumi et al., 2001). More recently, direct evidence for the in vivo binding of the high affinity sigma ligand and SSRI, fluvoxamine, to sigma receptors, has been reported (Ishikawa et al., 2007). In this study, human volunteers were subjected to positron emission tomography with the high affinity sigma-1 selective radioligand [11C]SA 4503 (1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine dihydrochloride) prior to and following oral administration of fluvoxamine or paroxetine. The results conclusively show displacement of the radioimaging agent by fluvoxamine, but not by paroxetine, an SSRI which exhibits negligible affinity for the sigma-1 receptor (Ishikawa et al., 2007).
Table 1.
Representative compounds with affinity for sigma receptors.
| Compounds | Ki (nM)
|
||
|---|---|---|---|
| σ | σ1 | σ2 | |
| SSRIs | |||
| Fluvoxamine[1] | 36 | 8,439 | |
| Sertraline[1] | 57 | 5,297 | |
| S(+)-Fluoxetine[1] | 120 | 5,480 | |
| Citalopram[1] | 292 | 5,410 | |
| Paroxetine[1] | 1,893 | 22,870 | |
| TCAs | |||
| Opipramol[2] | 50 | ||
| Imipramine[1] | 343 | 2,107 | |
| Desipramine[1] | 1,987 | 11,430 | |
| MAOIs | |||
| Clorgyline[3] | 2.9 | 505 | |
| (+)Deprenyl[3] | 82 | 1,880 | |
| Harmaline[3] | 310 | 2,100 | |
| Ro11-1163[3] | 860 | >50,000 | |
| Acetylcholinesterase inhibitor | |||
| Donepezil[4]a | 14.6 | ||
| Steroids | |||
| Progesterone[5] | 24.6 | 15,700 | |
| Deoxycorticorsterone[6] | 938 | ||
| Dehydroepiandrosterone(DHEA)[7] | 3,700 | ||
| Sigma compounds | |||
| Haloperidol[8] | 0.90 | 7.93 | |
| BD 1047[9] | 0.93 | 9.15 | |
| NE-100[10]a | 1.5 | 84.6 | |
| (+)Pentazocine[8] | 1.62 | 728.4 | |
| SA4503[8] | 4.63 | 63.09 | |
| (+)SKF 10,047[11]a | 29 | 33,654 | |
| DTG[8] | 35.45 | 39.87 | |
| Igmesine (JO1784)[12]a | 39 | ||
| UMB23[13] | 41 | 32 | |
| OPC-14523[14]a | 47 | 56 | |
| St. John’s Wort | |||
| Hyperforin[15]a | 1,400 | ||
| Hypericine[15]a | 3,700 | ||
Note:
denotes IC50 in nM. σ denotes affinity for sigma receptors where affinity at individual subtypes has not been reported.
Narita, N., K. Hashimoto, et al. (1996) “Interactions of selective serotonin reuptake inhibitors with subtypes of σ receptors in rat brain.” Eur J Pharmacol. 307: 117–119.
Rao, T.S., J.A. Cler et al. (1990) “Neurochemical characterization of dopaminergic effects opipramol, a potent sigma receptor ligand, in vivo.” Neuropharmacology 12: 1191–1197.
Itzhak, Y., I, Stein, et al. (1991) “ Binding of sigma-ligands to C57BL/6 mouse brain membranes: effects of monoamine oxidase inhibitors and subcellular distribution studies suggest the existence of sigma-receptor subtypes.” J Pharmacol Exp Ther. 257:141–148.
Kato, K., H. Hayako, et al. (1999). “TAK-147, an acetylcholinesterase inhibitor, increases choline acetyltransferase activity in cultured rat septal cholinergic neurons.” Neurosci Lett 260(1): 5–8.
McCann, D.J., A. D., Weissman et al., (1994) “Sigma-1 and sigma-2 sites in rat brain: comparison of regional, ontogenetic, and subcellular patterns.” Synapse 17 (3): 182–189.
Su, T.P., E.D. London et al., (1988) “Steroid binding at σ receptors suggests a link between endocrine, nervous, and immune systems” 240: 219–221.
Takebayashi, M.,T., Hayashi, et al. (2004) “A perspective on the new mechanism of antidepressants: neuritogenesis through sigma1 receptors.” Pharmacopsychiatry 37 Suppl 3: s208–S213.
Lever, J.R., J.L. Gustafson et al., (2006) “σ1 and σ2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503” Synapse 59: 350–358.
Matsumoto, R.R., W.D. Bowen et al., (1995) “Characterization of two novel σ receptor ligands: antidystonic effects in rats suggest σ receptor antagonism” Eur J Pharmacol 280: 301–310.
Nakazato, A., K, Ohta et al., (1999) “Design, synthesis, structure-activity relationships, and biological characterization of novel arylalkoxyphenylalkylamine sigma ligands as potential antipsychotic drugs. J Med Chem 42(6): 1076–1087.
Bowen, W.D., B.R. de Costa et al. (1993) “[3H] –(+)-pentazocine: a potent and highly selective benzomorphan-based probe for sigma-1 receptors.” Mol Neuropharmacol 3: 117–126.
Earley, B., M., Burke et al., (1991) “Evidence for an anti-amnesic effect of JO 1784 in the rat: a potent and selective ligand for the sigma receptor.” Brain Res 546: 282–286.
Wang, J., A.L., Mack, et al, (2007) “Novel sigma (sigma) receptor agonists produce antidepressantlike effects in mice.” Eur. Neuropsychopharmacol., 17:708–716.
Tottori, K., T., Miwa, et al., (2001) “Antidepressant-like responses to the combined sigma and 5- HT1A receptor agonist OPC-14523.” Neuropharmacology 41: 976–988.
Gobbi, M., M., Moia et al. (2001) “In vitro binding studies with two Hypericum Perforatum extractshyperforin, hypercin and biapigenin-on 5-HT6, 5-HT7, GABAA/Benzodiazepine, sigma, NPY-Y1/Y2 receptors and dopamine transporters.” Pharmacopsychiatry 34 suppl 1:s45–s48.
3.2. Effects of a sigma receptor agonist in depressed humans
The sigma receptor agonist, igmesine hydrochloride [JO 1784; (+)-N-cyclopropylmethyl-N-methyl-1,4-diphenyl-1-ethylbut-3-en-1-ylamine hydrochloride] exhibits high affinity for the sigma-1 receptor (IC50 of 39 ± 8 nM in rat brain membrane). In an early Phase II open-label study, 31 severely depressed in-patients showed significant improvement for up to four weeks when treated with igmesine (Pande et al., 1999; Volz and Stoll, 2004). Following this study, igmesine was tested in a 6-week, multi-center, double blind, placebo-controlled study in 348 patients meeting the DSM-IV criteria for major depression (Pande et al., 1999; Volz and Stoll, 2004). In this study, igmesine (25 or 100 mg/day) was as effective as fluoxetine (qd, 20 mg/day), as measured using the Hamilton Depression Rating Scale (HAM-D score) (Pande et al., 1999; Volz and Stoll, 2004). Adverse events were reported in 50% and 62% of igmesine patients (25 and 100 mg/day, respectively), as compared to 66% and 53% of fluoxetine- and placebo-treated patients (Pande et al., 1999; Volz and Stoll, 2004). These data indicate that a selective sigma receptor agonist produces antidepressant effects in humans, with comparable efficacy to a well established, marketed medication.
3.3. Specificity of sigma agonist actions in producing antidepressant-like effects
Because most clinically-used antidepressant drugs interact with a variety of protein targets, appropriate tests of selective sigma receptor agonists were needed to conclusively associate sigma proteins with antidepressant effects. Accordingly, to determine if the activation of sigma receptors alone was sufficient to produce antidepressant actions, a series of preclinical studies was performed using the established rodent models, the forced swim test (FST) and the tail suspension test (TST) (Porsolt et al., 1977; Steru et al., 1985). The FST and TST are not depression models per se, but do exhibit robust and reproducible responses to currently known antidepressant treatments following acute dosing (Porsolt et al., 1977; Steru et al., 1985). In these tests, antidepressant-like effects were observed for several selective sigma receptor agonists and putative agonists including: di-o-tolylguanidine (DTG), igmesine, (+)-pentazocine, SA 4503, and UMB23 (1-(3-phenylpropyl)piperidine oxalate) (Matsuno et al., 1996; Wang et al., 2007a). Pre-treatment with the sigma receptor antagonists BD1047 (N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine) or NE-100 (N,N-dipropyl-2-[4-methoxy-3-(2-phenylethoxy)phenyl]ethylamine) abolished the antidepressant-like actions of the sigma receptor agonists, confirming the involvement of sigma receptors (Matsuno et al., 1996; Wang et al., 2007a). Studies with sigma-1 receptor knockout mice further support the role of these proteins in depression as these mice present a depression-like response in the FST (Sabino et al., 2009).
While the vast majority of clinically-used antidepressant drugs interact with sigma receptors, there are some exceptions. Paroxetine (SSRI) and desipramine (tricyclic antidepressant) have weak affinity for sigma receptors, yet are known to produce robust antidepressant effects in humans (Barringer, 1965; Caley and Weber, 1993; Narita et al., 1996). Therefore, activation of sigma receptors does not appear necessary for conveying therapeutic benefits. While not necessary, it is important to note that their activation alone appears sufficient for producing antidepressant-like effects.
Whereas many studies indicate that sigma receptor agonists evoke antidepressant actions, the specific mechanisms underlying these therapeutic effects have yet to be fully elucidated. Therefore, the remainder of this review summarizes data demonstrating that sigma receptor agonists promote processes and neural adaptations that elicit antidepressant effects.
4. Conceptual framework for the mechanism(s) of action of antidepressant drugs
No new, unique class of antidepressant drug has been introduced in the last 20 years (Berton and Nestler, 2006; Mathew et al., 2008). Yet, there have been significant advancements in our understanding of the basic neurobiology that underlies the actions of antidepressant medications (Manji, 2001; Nestler, 2006; Krishnan, 2008). The delayed onset of clinical effects (weeks to months) for drugs targeting monoaminergic systems suggest that antidepressant effects are not mediated solely by the acute alteration of neurotransmitter levels. Current theories take into consideration the complexity of changes in interneuronal transmission, intracellular signaling and neuronal structure that may accompany antidepressant treatment (Duman, 2004; Dranovsky and Hen, 2006; Warner-Schmidt and Duman, 2006; Dougherty and Rauch, 2007; Tanis and Duman, 2007; Tanis et al., 2007; McClung and Nestler, 2008). Consequently, the term “hypothesis of neuroplasticity” has been proposed to capture the diversity of these dynamic changes (Racagni and Popoli, 2008).
Figure 1 summarizes the hypothesized actions promoted by antidepressant medications, which are separated temporally into immediate, intermediate, and delayed effects. When antidepressant drugs bind to monoamine reuptake sites, sigma receptors, and other neurotransmitter receptors, immediate acute changes in neurotransmission are induced (Skuza, 2003; Berton and Nestler, 2006; Hayashi and Su, 2008; Mathew et al., 2008). The resulting activation of intracellular signaling cascades elicits a number of effects, including changes in Ca2+ levels, phosphorylation of effector proteins and transcription factors, and ultimately, modification of gene expression (Popoli, 2002; Tanis and Duman, 2007; Racagni and Popoli, 2008). These alterations may evoke persistent changes in the levels and activities of 1) neurotransmitters, 2) neurotransmitter receptors and transporters, 3) transcription factors such as cyclic adenosine monophosphate response element binding protein (CREB), and 4) growth factors, such as brain derived neurotrophic factor (BDNF) (McClung and Nestler, 2008; Racagni and Popoli, 2008). The resulting upregulation of gene function may also facilitate structural alterations that enhance synaptic plasticity, including increased synapse formation, spine density, neurite sprouting and elongation, and neurogenesis (Duman, 2001; Racagni and Popoli, 2008). The ability of antidepressant drugs to promote these structural/morphological changes in the brain may help to restore normal functions in depressed individuals where neuronal damage or dysregulated signaling has compromised function.
Figure 1. Hypothesized sequence of events for antidepressant actions.
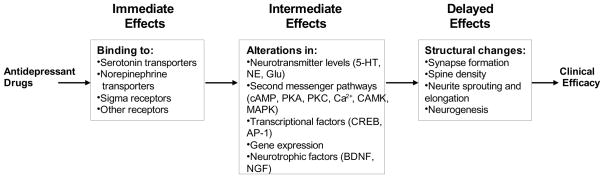
Classic antidepressant drugs produce effects which can be classified temporally into immediate, intermediate and delayed actions, with resulting functional changes associated at each stage.
Sigma receptor agonists have the ability to promote key neural adaptations that are characteristic of antidepressant drugs. Moreover, sigma-mediated events may occur at multiple points in the cascade of effects elicited by antidepressant medications. The specific mechanistic details through which these effects are elicited may differ somewhat from those currently attributed to existing antidepressant medications but the functional outcomes are hypothesized to be similar.
5. Mechanisms of antidepressant-like actions of sigma receptor agonists
5.1. Modulation of classical neurotransmitter systems
5.1.2. Glutamatergic modulation
Accumulating evidence indicates that NMDA receptor function, and glutamatergic signaling in general, are compromised in depression, and that modulation of glutamatergic neurotransmission contributes to the therapeutic effects of antidepressant drugs (Skolnick, 2002; Paul and Skolnick, 2003; Hashimoto, 2009; Machado-Vieira et al., 2009b; Skolnick et al., 2009). It is therefore significant that glutamatergic responses that are mediated through NMDA receptors can be modulated by sigma receptor ligands, where activation of sigma receptors results in enhanced NMDA neurotransmission (Iyengar et al., 1990; Monnet et al., 1990; Monnet et al., 1992b; Bergeron et al., 1993; Bergeron et al., 1995; Yamamoto et al., 1995; Debonnel and de Montigny, 1996; Gronier and Debonnel, 1999; Guitart et al., 2000; Bermack et al., 2002; Bermack and Debonnel, 2005; Martina et al., 2007). This enhanced NMDA neurotransmission may facilitate compensatory glutamatergic signaling in systems compromised by depressive pathology.
The potentiation of glutamatergic responses in hippocampal neurons in vivo via NMDA receptors following application of sigma-1 agonists is well established (Monnet et al., 1990; Monnet et al., 1992b; Bergeron et al., 1993; Bergeron et al., 1995). Recent investigations using whole cell patch techniques have identified specific currents that mediate these responses. In particular, small conductance Ca2+-activated K+ channels (SK channels) are implicated in the sigma-mediated potentiation of NMDA responses in CA1 pyramidal cells (Martina et al., 2007). Blockade of SK channels using the sigma receptor agonist (+)-pentazocine increases Ca2+ influx through NMDA receptors, resulting in enhanced NMDA-mediated responses and long-term potentiation (Martina et al., 2007). Control and antagonism studies further confirm that intracellular Ca2+ and Ca2+ influx through NMDA receptors is required for the (+)-pentazocine effect, which can be antagonized with haloperidol through non-dopaminergic, presumably sigma-mediated, mechanisms. Together with earlier studies demonstrating the ability of fluoxetine to block SK channels (Terstappen et al., 2003), and the importance of the hippocampus to depression pathophysiology (Duman et al., 1997; Nestler et al., 2002; Sheline et al., 2003; Stockmeier et al., 2004; Hercher et al., 2009), these data suggest a potential mechanism through which sigma receptors may promote therapeutically relevant effects.
Chronic administration of SA 4503, a putative sigma receptor agonist, elicits antidepressant-like effects in a variety of animal models; it also produces therapeutically relevant alterations in NMDA receptor expression in mice that have undergone an olfactory bulbectomy (OB) (Wang et al., 2007a). OB, a procedure that simulates many pathophysiological changes that characterize major depression in humans, also produces diminution of NMDA receptor expression in mice (Webster et al., 2000; Ho et al., 2001; Robichaud et al., 2001). Two weeks of chronic treatment with SA 4503 reverses the diminished expression of NMDA NR1 subunits in the prefrontal cortex, hippocampus, and amygdala of OB mice (Wang et al., 2007a). No effects on NR2A or NR2B subunits are observed (Wang et al., 2007a). The enhancement by SA 4503 of NR1 expression in OB mice is prevented with the sigma receptor antagonist, NE-100 (Wang et al., 2007a). In contrast, desipramine, an antidepressant drug that has weak affinity for sigma receptors does not show NR1 enhancing activity (Wang et al., 2007a). Together, these studies suggest that the enhancement of NMDA-mediated responses by sigma receptor agonists may be a contributing mechanism for sigma receptor elicited antidepressant-like effects.
A number of other effectors of glutamate neurotransmission have demonstrated antidepressant activity. NMDA antagonists such as ketamine have been reported to produce antidepressant effects in animal models and humans (Kugaya, 2005; Zarate et al., 2006; Machado-Vieira et al., 2009a), presumably through downstream activation of AMPA receptors. This is consistent with the antidepressant-like activity of positive AMPA modulators in animal models (O’Neill and Witkin, 2007). Antidepressant-like effects have also been observed in rodents following antagonism of metabotropic glutamate receptors (Kugaya, 2005; Pilc et al., 2008; Hashimoto, 2009). This includes antagonism of the presynaptic Group II autoreceptors (mGluR2 and mGluR3), which results in increased glutamatergic neurotransmission (Chaki et al., 2004). Reports of the antidepressant activity of the NMDA glycine binding site partial agonist, D-cycloserine, in humans (Crane, 1959) and rodents (Papp and Moryl, 1996; Lopes et al., 1997) also suggests a role for modulation of NMDA activity in antidepressant effects.
The complexity of glutamatergic signaling supports the possibility of multiple mechanisms that promote antidepressant activity. This may explain the seemingly contradictory antidepressant activities of reduced NMDA function via antagonists vs. enhanced NMDA transmission via sigma receptor activation. Additional studies to further characterize interactions between sigma receptors and NMDA, and non-NMDA glutamatergic mechanisms, are warranted.
5.1.2. Serotonergic modulation
The therapeutic effects of chronic administration of antidepressant drugs are associated with an enhancement of serotonin neurotransmission (Blier and Bouchard, 1994; Blier and de Montigny, 1994; Owens, 1996). Acute administration of antidepressant drugs, such as SSRIs, causes a reduction in the firing of serotonergic neurons, which recover with long term administration (Chaput et al., 1986; Beique et al., 2000). This recovery in firing of serotonin neurons is thought to develop after desensitization of somatodendritic 5-HT1A autoreceptors, activated in response to serotonin released from axon collaterals (Blier and de Montigny, 1994). This phenomenon has been proposed as a mechanism that explains the three to four week delay in clinical efficacy of antidepressant drugs (Chaput et al., 1986; Blier and de Montigny, 1994). In contrast to the delayed effects of SSRIs, the well established selective sigma receptor agonists DTG and (+)-pentazocine increase the firing of serotonergic neurons in the dorsal raphe nucleus (DRN) of anesthetized rats with only two days of treatment (Bermack and Debonnel, 2001). Further, this result was maintained following 21 days of sigma agonist treatment (Bermack and Debonnel, 2001). These rapid effects elicited by DTG and (+)-pentazocine appear to be mediated through sigma receptors because they can be prevented by co-administration of the sigma receptor antagonist, NE-100 (Bermack and Debonnel, 2001). The selective sigma receptor agonist SA 4503 also produces significant increases in the firing rate of DRN neurons after two days of treatment (Lucas et al., 2008). This sigma receptor-induced enhancement in serotonergic neuronal function has been proposed to facilitate a more rapid onset of antidepressant efficacy as compared to existing medications, and may also contribute to added efficacy by targeting new mechanisms (Bermack and Debonnel, 2001).
A compelling series of experiments suggests that a synergistic antidepressant effect can be elicited following simultaneous agonism of 5-HT1A and sigma receptors. Administration of a single oral dose of OPC-14523 ([1-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-5-methoxy-3,4-dihydro-2-quinolinone monomethanesulfonate), a combined SSRI/5-HT1A agonist/sigma-1 agonist, produces antidepressant-like effects in mice in the FST, whereas established antidepressant drugs such as fluoxetine or imipramine require four days to elicit a similar effect (Tottori et al., 2001). The behavioral effects of OPC-14523 are attenuated by either the sigma-1 receptor antagonist NE-100 or the selective 5-HT1A antagonist WAY-100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate), indicating that both receptor systems are important contributors to the observed effects (Tottori et al., 2001). More recent experiments confirm that the behavioral effects of OPC-14523 can be duplicated with the combined administration of sub-effective doses of the 5-HT1A agonist 8-OH-DPAT (8-hydroxy-N,N-dipropyl-2-aminotetralin) and a sigma receptor agonist such as DTG or SA 4503 (Skuza and Rogoz, 2007). In electrophysiological studies, OPC-14523 increases serotonergic neurotransmission in the DRN, and this effect is blocked with the sigma receptor antagonist NE-100 (Bermack et al., 2004). Together, the data demonstrate sigma receptor-mediated modulation of serotonergic functions. The enhanced effects following stimulation of both 5HT1A and sigma receptors suggest a potential novel pharmacotherapeutic strategy for future antidepressant development.
5.1.3. Catecholaminergic modulation
Current therapeutic strategies also exploit modulation of catecholaminergic systems to effect antidepressant activities. These strategies are supported by evidence for the involvement of dopamine and norepinephrine in the actions of SSRIs, MAOIs, and TCAs (Dailly et al., 2004; Nutt, 2006). Selective reuptake inhibitors for norepinephrine (NRIs) and combination reuptake inhibitors for serotonin/norepinephrine (SNRIs) and norepinephrine/dopamine (NDRIs) are also in clinical use. Therefore, it is noteworthy that the actions of two clinically relevant catecholaminergic-targeted antidepressant medications, bupropion and venlafaxine, can be modulated through sigma receptors (Dhir and Kulkarni, 2007; Dhir and Kulkarni, 2008b). Potential mechanisms through which sigma receptors modulate catecholaminergic neurotransmission have been studied in both in vitro and in vivo systems.
A number of in vitro studies have examined the role of sigma receptors on NMDA-stimulated release of catecholamines. The effects of sigma ligands on NMDA-stimulated release of [3H]norepinephrine from rat hippocampal slices produced conflicting results, with sigma agonists potentiating release in the hands of one research group (Monnet et al., 1992a; Monnet et al., 1996) and inhibiting release in the hands of another group (Gonzalez-Alvear and Werling, 1995b). The disparate results are likely due to differences in the amount of NMDA utilized for stimulation but together provide evidence that sigma ligands are capable of modulating norepinephrine release in the hippocampus with the caveat that the results observed are highly dependent on experimental conditions (Matsumoto et al., 2007). Similar studies have investigated sigma receptor-mediated modulation of NMDA-stimulated release of [3H]dopamine from rat and guinea pig striatal slices (Gonzalez-Alvear and Werling, 1994; Gonzalez-Alvear and Werling, 1995a; Gonzalez and Werling, 1997; Nuwayhid and Werling, 2003). The consensus from these studies is that sigma receptor agonists inhibit NMDA-stimulated release of striatal dopamine and that this effect can be reversed by application of sigma receptor antagonists (Gonzalez-Alvear and Werling, 1994; Gonzalez-Alvear and Werling, 1995a; Gonzalez and Werling, 1997; Nuwayhid and Werling, 2003).
In vivo studies also support the involvement of sigma receptors in the modulation of dopamine release in the rat brain. Microdialysis studies reveal increases in extracellular dopamine in the striatum of awake, freely moving animals following intraperitoneal (i.p.) injection of the sigma receptor agonists (+)-pentazocine or DTG (Patrick et al., 1993). Similarly, following subcutaneous (s.c.) injection, (+)-pentazocine or (+)-SKF 10,047 increases dopamine in the striatum and medial prefrontal cortex, but (−)-SKF 10,047 or DTG produces no changes (Gudelsky, 1995). The release of striatal dopamine appears to be sigma-mediated because NE-100 can antagonize the changes resulting from acute oral administration of SA 4503 (Kobayashi et al., 1997). The more direct application of (+)-pentazocine or DTG by intrastriatal infusion in rats produces a biphasic effect, with an initial increase in extracellular dopamine in the striatum in the first 30 minutes, followed by a decrease in the subsequent 90 minutes following continuous infusion (Gudelsky, 1999). These studies provide evidence that sigma receptors can modulate catecholamine release in specific areas of the brain; however, the mechanisms involved and the impact on depression pathology remain unknown.
5.2. Modulation of signaling pathways
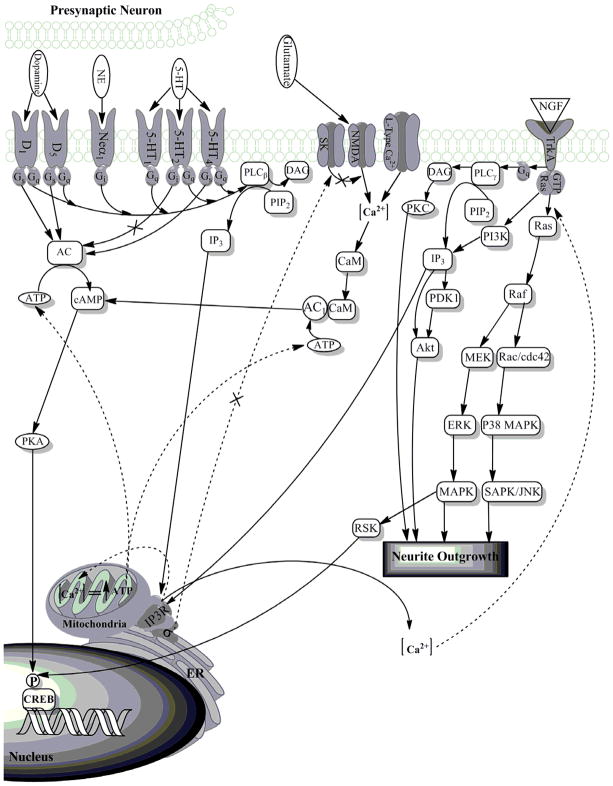
Intracellular second messenger signaling pathways are activated by classical neurotransmitters and neurotrophic factors via membrane associated receptors that can be modulated by sigma receptors. Activation of these signaling cascades affects a multitude of divergent but interrelated pathways which serve the common function of regulating downstream proteins such as transcription factors, which facilitate neuroplasticity and altered central nervous system functioning (Figure 2). The following sections summarize the major features of each of these systems and the potential role of sigma receptors.
Figure 2. Sigma receptors modulate numerous pathways implicated in the etiology or pathophysiology of depression.
It is believed that Ca2+ modulation through sigma receptors can act upon pathways involved in neurite outgrowth, as well as gene regulation, specifically, ERK, MAPK and Akt pathways. It is hypothesized that sigma receptor-mediated modulation of IP3Rs at the MAM, also contributes to an increase in ATP production from the mitochondria, which may have profound effects on signaling within the cell which have not yet been fully studied. Sigma receptors have also been shown to modulate PKA pathways; sigma agonists have been shown to modulate the phosphorylation of CREB, indicative of effects on gene transcription. Sigma receptors also have known effects on the transmission of signaling via neurotransmitters; however these pathways have not been fully elucidated. Dashed lines indicate known and hypothesized effects of sigma receptors on signaling pathways believed to be involved in depression.
5.2.1. Classical neurotransmitter signaling pathways
Classical neurotransmitters including glutamate, serotonin and norepinephrine, activate key signaling pathways implicated in the actions of antidepressant drugs, including the phosphoinositide second messenger system and the cyclic adenosine monophosphate (cAMP) pathway (Tanis and Duman, 2007). The initiation of these signaling cascades promotes the subsequent activation of protein kinases, including protein kinase C (PKC), protein kinase A (PKA), and calmodulin dependant protein kinase (CAMK) that phosphorylate downstream targets such as cytoskeletal proteins and transcription factors (Tanis and Duman, 2007). The activities of sigma receptor agonists appear to engage these same pathways by modulating intracellular Ca2+ or by affecting protein kinase activity.
The sigma-1 receptor modulates intracellular Ca2+ primarily through interactions with IP3 receptors on the ER and through interactions with Ca2+ channels on the plasma membrane (Hayashi et al., 2000; Hayashi and Su, 2007; Martina et al., 2007). Activation of sigma-1 receptors causes the dissociation of ankyrin B from IP3 receptors, resulting in disinhibition of IP3 receptors and potentiation of Ca2+ signaling from the ER to the cytosol (Hayashi and Su, 2001). As mentioned previously, sigma receptor-induced potentiation of Ca2+ entry into the cell can occur via sigma receptor agonist-stimulated enhancement of NMDA function, through the blockade of SK channels (Martina et al., 2007).
In addition to affecting the function of IP3 receptors, the sigma-1 receptor engages the phosphoinositide pathway through upregulation of phospholipase Cβ (PLCβ), and modulation of PKC activity (Romero et al., 2000; Monnet et al., 2003; Nuwayhid and Werling, 2003; Kim et al., 2008). The sigma receptor ligand E-5842, (4-[4-fluorophenyl]-1,2,3,6-tetrahydro-1-[4-{1,-2,4-triazol-1-1}butyl]pyridine citrate) can upregulate PLCβ in the frontal cortex with a concomitant increase in PLCβ activity (Romero et al., 2000). This increase in PLCβ activity correlates with an increase in phosphoinositide second messenger signaling. However, it has not been conclusively demonstrated that these effects are due to the activity of sigma receptors because no attempt has yet been made to antagonize this activity (Romero et al., 2000). Sigma-1 receptors are also involved in several processes mediated through PKC pathways. In rat hippocampal pyramidal neurons, the sigma-1 receptor agonist (+)-pentazocine potentiates glutamate elicited increases in intracellular Ca2+, an effect which can be inhibited with the sigma-1 antagonist NE-100 and the PKC inhibitor Gö-6976 (12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo[ 2,3-a]pyrrolo[3,4-c]carbazole) (Monnet et al., 2003). Inhibition of PKC was also shown to inhibit (+)-pentazocine-induced potentiation of NMDA stimulated [3H]dopamine release from rat striatal slices (Nuwayhid and Werling, 2003). Inhibition of phospholipase C in this same series of experiments also negated the effects of (+)-pentazocine while having no effect on NMDA stimulation in the absence of (+)-pentazocine (Nuwayhid and Werling, 2003).
The cellular consequences of sigma receptor-mediated modulation of signaling are extensive. Increases in intracellular Ca2+ can lead to increased neurotransmission, neurite outgrowth, and calmodulin signaling. Sigma receptor ligand-induced Ca2+ release from the ER may also impinge on signaling cascades activated by growth factor binding to Trk receptors. These include PKC, MAPK, SAPK/JNK, and Akt-mediated pathways (Nishimura et al., 2008). It has been demonstrated that sigma-1 receptor-mediated effects on these pathways enhance neurite outgrowth; however, the full extent of sigma receptor-mediated activation of these pathways and subsequent gene expression has yet to be determined (Nishimura et al., 2008).
One potential consequence of the activation of these Ca2+-dependant pathways is the modulation of gene expression by transcription factors such as CREB protein. A number of antidepressant drugs have been shown to regulate CREB (Carlezon et al., 2005; Nair and Vaidya, 2006; Tardito et al., 2006; Gass and Riva, 2007), which has been implicated in the expression of numerous genes involved in neuroplasticity and cell survival, including growth factors, which are further discussed in the next section.
Only one study specifically linking sigma receptors to the modulation of CREB has been reported to date. PPBP (4-phenyl-1-(4-phenylbutyl)piperidine), a prototypic sigma-1 receptor agonist, increases CREB phosphorylation (Yang et al., 2009). In this study, inhibitors for MAPK, MEK, CaMKII (Ca2+/calmodulin dependent kinase II) and PI3-K (phosphoinositide 3-kinase) were ineffective in blocking PPBP-potentiated CREB phosphorylation while the PKA inhibitor, H89 (N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline sulfonamide), and the sigma antagonist, rimcazole (9-{3-[(3R,5S)-3,5-dimethylpiperazin-1-yl]propyl}-9H-carbazole) prevented PPBP-mediated CREB phosphorylation (Yang et al., 2009). These results suggest that activation of sigma-1 receptors by PPBP stimulates CREB phosphorylation via a PKA-dependent pathway. Together, the data demonstrates the ability of sigma receptor ligands to modulate a variety of signaling cascades that are activated by classical neurotransmitter systems.
5.2.2. Neurotrophin and growth factor signaling pathways
Neurotrophins are extracellular signaling molecules with important roles in the development, growth, and maintenance of the central nervous system, promoting precursor proliferation, neuronal differentiation, and neuronal survival (Thomas and Peterson, 2008). In the brain, neurotrophins act through Trk receptors, with signaling achieved through PLC, Akt and MAP kinase pathways (Tanis and Duman, 2007; Tanis et al., 2007). Brain derived neurotrophic factor (BDNF) is the most widely studied neurotrophin in the context of depression research. In general, decreased levels of BDNF are associated with the pathophysiology of depression, while its upregulation is characteristic of antidepressant treatments (Racagni and Popoli, 2008).
A number of studies have demonstrated increased BDNF expression in vivo following chronic treatment with a variety of antidepressant drugs including MAOIs, SSRIs, and SNRIs (Duman and Monteggia, 2006). In vivo studies show that activation of TrkB, the primary receptor for BDNF, as well as TrkB-mediated PLCγ/IP3/Ca2+ signaling, are common mechanisms of antidepressant drugs (Rantamaki et al., 2007). Additional evidence supporting a role for BDNF in antidepressant activity is provided by the observation that antidepressant drugs are ineffective in the FST in BDNF knockout mice and in TrkB-T1 transgenic mice which overexpress a truncated TrkB receptor (Martinowich et al., 2007). Overall, BDNF signaling and activation of the TrkB receptor are thought to be required for the behavioral effects of antidepressant drugs in rodents (Saarelainen et al., 2003; Castren and Rantamaki, 2008).
Sigma receptors have been implicated in both the upregulation of BDNF expression and in the potentiation of BDNF-activated PLCγ/IP3/Ca2+ signaling pathways. Sigma-active antidepressant drugs such as imipramine and fluvoxamine potentiate BDNF-stimulated PLCγ activation in cultured cortical neurons, culminating in increased intracellular Ca2+ and glutamate release following chronic administration (Yagasaki et al., 2006). Evidence for the participation of sigma-1 receptors in these effects include: antagonism of imipramine-induced potentiation with the sigma-1 receptor antagonist BD1047, and enhancement of potentiation in cell cultures overexpressing sigma-1 receptors (Yagasaki et al., 2006).
Studies with the sigma-1 specific agonist, SA 4503, show that chronic, but not acute, dosing in adult rats increases the expression of BDNF in the hippocampus in a dose-dependent manner (Kikuchi-Utsumi and Nakaki, 2008). However, no increase of BDNF is observed in the striatum, midbrain, frontal cortex or thalamus in this study, suggesting that the BDNF changes are not a global effect of SA 4503 (Kikuchi-Utsumi and Nakaki, 2008).
In contrast to the effects of established antidepressant drugs, where increases in BDNF expression occur concomitantly with significant increases in hippocampal trkB mRNA and TrkB receptor expression (Nibuya et al., 1995), there appears to be a dissociation between the two endpoints for sigma receptor-mediated effects, where increases in hippocampal BDNF can be observed with no changes in TrkB receptor levels (Kikuchi-Utsumi and Nakaki, 2008). The impact and source of this difference in activities is unknown; however, the possibility that BDNF levels increase in response to sigma agonist-induced increases in glutamatergic neurotransmission has been proposed (Kikuchi-Utsumi and Nakaki, 2008).
An additional mechanism involving sigma receptors that potentially impacts levels of BDNF may be produced through activator protein 1 (AP-1). AP-1 is a transcription factor important in cellular differentiation, proliferation, and in the regulation of sigma-1 receptor expression (Seth et al., 1997; Prasad et al., 1998; Shaulian and Karin, 2001). Studies in a rat primary cerebellar neuron culture demonstrate that both AP-1 and cAMP-responsive element (CRE) dependent transcription can be stimulated by BDNF through cAMP independent pathways (Gaiddon et al., 1996). Furthermore, increased binding of AP-1 in the hippocampus has been observed following direct infusion of BDNF in rat dentate gyrus (Okamoto et al., 2003). Hence, upregulation of BDNF via antidepressant or sigma agonist treatment may contribute to increased levels of sigma-1 receptors, and CRE-related gene products, including BDNF, in a feed forward loop that does not require cAMP signaling.
5.4. Modulation of structural changes
The ability of antidepressant drugs to activate CREB and stimulate neurotrophic factor production and signaling may facilitate alterations in neuronal cell morphology and proliferation, which are described in detail in the next two sections.
5.4.1. Neurite sprouting
PC12 cells are a clonal rat adrenal pheochromocytoma line that differentiate in response to nerve growth factor (NGF), producing processes analogous to those formed by sympathetic neurons in primary cell culture. Thus, NGF-stimulated PC12 cultures serve as an accepted model for neuronal differentiation (Greene, 1976). The sigma-active antidepressant drugs fluvoxamine and imipramine enhance NGF-induced neurite sprouting in PC12 cells (Takebayashi et al., 2002). Similar effects are produced with the selective sigma-1 receptor agonist (+)-pentazocine, suggesting that activation of sigma receptors alone, particularly the sigma-1 subtype, can stimulate structural modifications that may impart antidepressant efficacy (Takebayashi et al., 2002). Similar neuroadaptations observed in cultured cortical rat neurons appear to involve modulation of BDNF signaling and glutamate release (Takebayashi et al., 2002; Yagasaki et al., 2006). Antagonists such as BD1047 and NE-100 attenuate these effects, as does an antisense oligonucleotide targeted to sigma-1, further implicating sigma receptors in these processes (Takebayashi et al., 2002; Yagasaki et al., 2006). Additional evidence for the involvement of sigma-1 receptors in the potentiation of NGF-induced neuronal growth includes: 1) upregulation of sigma-1 receptors following chronic antidepressant or (+)-pentazocine treatment, 2) enhanced neurite sprouting in cells overexpressing sigma-1, and 3) reduced NGF-induced neurite growth in sigma-1 antisense treated cells (Takebayashi et al., 2002).
More recent studies demonstrate similar potentiation of NGF-stimulated neurite growth for the sigma-1 specific ligand SA 4503, and implicate some of the specific signaling pathways involved. Antagonism of the actions of SA 4503 is effected with the sigma receptor antagonist NE-100, the IP3 receptor inhibitor xestospongin, as well as by specific inhibitors of signaling pathways downstream from the NGF receptor, TrkA (Nishimura et al., 2008). Reversal of the sigma-1-mediated effects with xestospongin is consistent with the involvement of a sigma receptor/IP3 interaction in the observed potentiation of NGF-induced neurite growth. Similarly, antagonism of the SA 4503-elicited effect by inhibition of proteins involved in downstream signaling from the TrkA receptor supports the inference that sigma receptor activation results in a positive modulatory effect on the TrkA pathway (Nishimura et al., 2008).
The sigma receptor has also been implicated in the potentiation of NGF-induced neurite outgrowth that is elicited by donepezil, an acetylcholinesterase inhibitor marketed for the treatment of Alzheimer’s associated dementia (Oda et al., 2007; Ishima et al., 2008). Donepezil has demonstrated efficacy in the FST in mice (Maurice et al., 2006) and displays high binding affinity (Ki = 14.6 nM) for the sigma-1 receptor, suggesting that its antidepressant effects are mediated, at least in part, by sigma receptors (Kato et al., 1999). As for SA 4503, antagonism of potentiation was achieved with either NE-100 or xestospongin (Ishima et al., 2008). Also consistent with the results observed with SA 4503, potentiation was associated with a concomitant increase in extracellular signal-regulated kinase (ERK) phosphorylation (Ishima et al., 2008), suggesting the involvement of the TrkA pathway. Thus, through activation of neurotrophin signaling pathways, sigma receptor ligands can promote neurite sprouting, a process which may aid in restoring synaptic function and cell morphology which has been compromised in depression.
5.5.2. Neurogenesis
Chronic treatment with antidepressant drugs upregulates expression of neurotrophic factors that support the growth of new neurons in the adult CNS (Duman and Monteggia, 2006; Martinowich et al., 2007; Balu et al., 2008). Neurogenesis is a complex multi-step process involving progenitor cell proliferation, differentiation, migration, neuronal maturation, and cell death (Balu, 2009). Hippocampal neurogenesis has been demonstrated following chronic antidepressant drug administration in rodents (Malberg et al., 2000) and tree shrews (Czeh et al., 2001), and following electroconvulsive shock in nonhuman primates (Perera et al., 2007). More recently, SSRI- and TCA-induced increases in neural progenitor cells in the dentate gyrus of humans was reported (Boldrini et al., 2009). Blocking neurogenesis can prevent the positive behavioral effects of antidepressants in animal models (Santarelli et al., 2003), suggesting that it can be an important component in the mechanism of antidepressant treatment (Perera et al., 2007; Sahay and Hen, 2007).
Continuous infusion of BDNF in the hippocampus of adult rats for two weeks results in the proliferation of newly formed hippocampal neurons (Scharfman et al., 2005). Therefore, the ability of antidepressant drugs to elicit neurogenesis may stem, at least in part, from their activation of neurotrophic factors. Because the sigma receptor agonist SA 4503 has been shown to upregulate BDNF in the hippocampus (Kikuchi-Utsumi and Nakaki, 2008), this may provide a mechanism through which sigma receptor agonists can promote neurogenesis.
As evidenced by increases in BrdU-labeled cells, cell proliferation has been observed in the hippocampus of rats treated chronically with representative antidepressant drugs such as tranylcypromine, reboxetine, fluoxetine, and fluvoxamine (Malberg et al., 2000; Manev, 2001). Similar studies have been performed with continuous administration of the sigma specific agonist SA 4503, eliciting significant increases in BrdU-staining cells in the subgranular zone (SGZ) of the hippocampus after three days of treatment (Lucas et al., 2008). Preliminary studies also confirm that administration of (+)-pentazocine, another sigma-1 receptor agonist, for 14 days, significantly augments the number of BrdU-positive cells in the hippocampus (Matsumoto and Yu, 2005).
Results of these studies provide evidence for the involvement of sigma receptors in neurogenesis. When considered as a whole, the data support an association between BDNF, neurogenesis, and the involvement of sigma receptors in the production of antidepressant-like actions.
6. Summary and conclusions
Antidepressant drugs stimulate a number of adaptive changes in the central nervous system. Among these changes are alterations in the function of neurotransmitter systems, particularly serotonin, norepinephrine, and glutamate. Antidepressant drugs also affect cellular plasticity by exerting actions through signaling molecules, transcription and neurotrophic factors. The importance of the ability of antidepressant drugs to promote structural changes, such as neuronal spouting and neurogenesis, in the CNS, has gained attention. With the growing acceptance that adaptive changes in the CNS are key contributors to the therapeutic actions of antidepressant drugs, the identification of new classes of compounds, such as sigma receptor agonists, that evoke such changes is of considerable interest, as they may represent novel antidepressant therapies.
Abbreviations
- Akt
protein kinase β
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid
- AP-1
activator protein 1
- BD1047
N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine
- BDNF
brain derived neurotrophic factor
- CaMK
calmodulin dependant protein kinase
- CaMKII
calmodulin kinase II
- cAMP
cyclic adenosine monophosphate
- CRE
cAMP-responsive element
- CREB
cAMP response element binding
- DHEA
dehydroepiandrosterone
- DMT
N,N-dimethyltryptamine
- DRN
dorsal raphe nucleus
- DTG
di-o-tolylguanidine
- EGF
epidermal growth factor
- ER
endoplasmic reticulum
- FST
forced swim test
- GSK-3
glycogen synthase kinase 3
- H89
N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline sulfonamide
- HAM-D
Hamilton Depression Rating Scale
- HPA
hypothalamic-pituitary axis
- IP3
inositol 1,4,5-trisphosphate
- MAM
mitochondrial associated membrane
- MAOI
monoamine oxidase inhibitor
- MAPK
mitogen activated protein kinase
- MEK
mitogen/extracellular signal regulated kinase
- NE-100
N,N-dipropyl-2-[4-methoxy-3-(2-phenylethoxy)phenyl]ethylamine
- NGF
nerve growth factor
- NMDA
N-methyl-D-aspartate
- NRI
norepinephrine reuptake inhibitor
- OB
olfactory bulbectomy
- PI3-K
phosphoinositol 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- PPBP
4-phenyl-1-(4-phenylbutyl) piperidine
- SA 4503
1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine dihydrochloride
- SNRI
serotonin-norepinephrine reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
- TCA
tricyclic antidepressant
- TST
tail suspension test
- UMB23
1-(3-phenylpropyl)piperidine oxalate
Footnotes
Declaration of interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. Cmaj. 2009;180:305–313. doi: 10.1503/cmaj.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Ball JR, Kiloh LG. A controlled trial of imipramine in treatment of depressive states. Br Med J. 1959;2:1052–1055. doi: 10.1136/bmj.2.5159.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. 2008;1211:37–43. doi: 10.1016/j.brainres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barringer TJ. Clinical Trial of Desipramine in the Treatment of Depression. Am J Psychiatry. 1965;121:1117–1119. doi: 10.1176/ajp.121.11.1117. [DOI] [PubMed] [Google Scholar]
- Beique JC, Blier P, de Montigny C, Debonnel G. Potentiation by (-)Pindolol of the activation of postsynaptic 5-HT(1A) receptors induced by venlafaxine. Neuropsychopharmacology. 2000;23:294–306. doi: 10.1016/S0893-133X(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Bergeron R, de Montigny C, Debonnel G. Biphasic effects of sigma ligands on the neuronal response to N-methyl-D-aspartate. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:252–260. doi: 10.1007/BF00233244. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Debonnel G. Effects of low and high doses of selective sigma ligands: further evidence suggesting the existence of different subtypes of sigma receptors. 1997;129:215–224. doi: 10.1007/s002130050183. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Debonnel G, De Montigny C. Modification of the N-methyl-D-aspartate response by antidepressant sigma receptor ligands. Eur J Pharmacol. 1993;240:319–323. doi: 10.1016/0014-2999(93)90918-8. [DOI] [PubMed] [Google Scholar]
- Bermack JE, Lavoie N, Dryver E, Debonnel G. Effects of sigma ligands on NMDA receptor function in the bulbectomy model of depression: a behavioural study in the rat. 2002;5:53–62. doi: 10.1017/S1461145701002760. [DOI] [PubMed] [Google Scholar]
- Bermack JE, Debonnel G. Modulation of serotonergic neurotransmission by short- and long-term treatments with sigma ligands. Br J Pharmacol. 2001;134:691–699. doi: 10.1038/sj.bjp.0704294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermack JE, Debonnel G. Distinct modulatory roles of sigma receptor subtypes on glutamatergic responses in the dorsal hippocampus. 2005;55:37–44. doi: 10.1002/syn.20085. [DOI] [PubMed] [Google Scholar]
- Bermack JE, Haddjeri N, Debonnel G. Effects of the potential antidepressant OPC-14523 [1-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-5-methoxy-3,4-dihydro-2-qu inolinone monomethanesulfonate] a combined sigma and 5-HT1A ligand: modulation of neuronal activity in the dorsal raphe nucleus. J Pharmacol Exp Ther. 2004;310:578–583. doi: 10.1124/jpet.104.066472. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Blier P, Bouchard C. Modulation of 5-HT release in the guinea-pig brain following long-term administration of antidepressant drugs. Br J Pharmacol. 1994;113:485–495. doi: 10.1111/j.1476-5381.1994.tb17015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WD, deCosta BR. [3H]-(+)pentazocine: a potent and highly selective benzomorphan based probe for sigma-1 receptors. Mol Neuropharmacol. 1993;3:117–176. [Google Scholar]
- Caley CF, Weber SS. Paroxetine: a selective serotonin reuptake inhibiting antidepressant. Ann Pharmacother. 1993;27:1212–1222. doi: 10.1177/106002809302701012. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Castren E, Rantamaki T. Neurotrophins in depression and antidepressant effects. 2008;289:43–52. doi: 10.1002/9780470751251.ch4. [DOI] [PubMed] [Google Scholar]
- Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, Yoshimizu T, Yasuhara A, Sakagami K, Okuyama S, Nakanishi S, Nakazato A. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004;46:457–467. doi: 10.1016/j.neuropharm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Presynaptic and postsynaptic modifications of the serotonin system by long-term administration of antidepressant treatments. An in vivo electrophysiologic study in the rat. Neuropsychopharmacology. 1991;5:219–229. [PubMed] [Google Scholar]
- Cobos EJ, Entrena JM, Nieto FR, Cendan CM, Del Pozo E. Pharmacology and therapeutic potential of sigma(1) receptor ligands. Curr Neuropharmacol. 2008;6:344–366. doi: 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane GE. Cyloserine as an antidepressant agent. Am J Psychiatry. 1959;115:1025–1026. doi: 10.1176/ajp.115.11.1025. [DOI] [PubMed] [Google Scholar]
- Crawford KW, Coop A, Bowen WD. Sigma(2) Receptors regulate changes in sphingolipid levels in breast tumor cells. Eur J Pharmacol. 2002;443:207–209. doi: 10.1016/s0014-2999(02)01581-9. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailly E, Chenu F, Renard CE, Bourin M. Dopamine, depression and antidepressants. Fundam Clin Pharmacol. 2004;18:601–607. doi: 10.1111/j.1472-8206.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- Debonnel G, de Montigny C. Modulation of NMDA and dopaminergic neurotransmissions by sigma ligands: possible implications for the treatment of psychiatric disorders. Life Sci. 1996;58:721–734. doi: 10.1016/0024-3205(95)02248-1. [DOI] [PubMed] [Google Scholar]
- Deverteuil RL, Lehmann HE. Therapeutic trial of iproniazid (marsilid) in depressed and apathetic patients. Can Med Assoc J. 1958;78:131–133. [PMC free article] [PubMed] [Google Scholar]
- Dhir A, Kulkarni S. Involvement of sigma (sigma1) receptors in modulating the anti-depressant effect of neurosteroids (dehydroepiandrosterone or pregnenolone) in mouse tail-suspension test. J Psychopharmacol. 2008a;22:691–696. doi: 10.1177/0269881107082771. [DOI] [PubMed] [Google Scholar]
- Dhir A, Kulkarni SK. Involvement of sigma-1 receptor modulation in the antidepressant action of venlafaxine. Neurosci Lett. 2007;420:204–208. doi: 10.1016/j.neulet.2007.04.055. [DOI] [PubMed] [Google Scholar]
- Dhir A, Kulkarni SK. Possible involvement of sigma-1 receptors in the anti-immobility action of bupropion, a dopamine reuptake inhibitor. Fundam Clin Pharmacol. 2008b;22:387–394. doi: 10.1111/j.1472-8206.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Rauch SL. Brain correlates of antidepressant treatment outcome from neuroimaging studies in depression. 2007;30:91–103. doi: 10.1016/j.psc.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology. 2001;25:836–844. doi: 10.1016/S0893-133X(01)00358-X. [DOI] [PubMed] [Google Scholar]
- Earley B, Burke M, Leonard BE, Gouret CJ, Junien JL. Evidence for an anti-amnesic effect of JO 1784 in the rat: a potent and selective ligand for the sigma receptor. Brain Res. 1991;546:282–286. doi: 10.1016/0006-8993(91)91492-j. [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiddon C, Loeffler JP, Larmet Y. Brain-derived neurotrophic factor stimulates AP-1 and cyclic AMP-responsive element dependent transcriptional activity in central nervous system neurons. J Neurochem. 1996;66:2279–2286. doi: 10.1046/j.1471-4159.1996.66062279.x. [DOI] [PubMed] [Google Scholar]
- Gass P, Riva MA. CREB, neurogenesis and depression. 2007;29:957–961. doi: 10.1002/bies.20658. [DOI] [PubMed] [Google Scholar]
- Gobbi M, Moia M, Pirona L, Morizzoni P, Mennini T. In vitro binding studies with two hypericum perforatum extracts--hyperforin, hypericin and biapigenin--on 5-HT6, 5-HT7, GABA(A)/benzodiazepine, sigma, NPY-Y1/Y2 receptors and dopamine transporters. Pharmacopsychiatry. 2001;34(Suppl 1):S45–48. doi: 10.1055/s-2001-15458. [DOI] [PubMed] [Google Scholar]
- Gonzalez GM, Werling LL. Release of [3H]dopamine from guinea pig striatal slices is modulated by sigma1 receptor agonists. 1997;356:455–461. doi: 10.1007/pl00005076. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alvear GM, Werling LL. Regulation of [3H]dopamine release from rat striatal slices by sigma receptor ligands. J Pharmacol Exp Ther. 1994;271:212–219. [PubMed] [Google Scholar]
- Gonzalez-Alvear GM, Werling LL. Sigma1 Receptors in rat striatum regulate NMDA-stimulated [3H]dopamine release via a presynaptic mechanism. Eur J Pharmacol. 1995a;294:713–719. doi: 10.1016/0014-2999(95)00617-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alvear GM, Werling LL. Sigma receptor regulation of norepinephrine release from rat hippocampal slices. Brain Res. 1995b;673:61–69. doi: 10.1016/0006-8993(94)01394-w. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic cloncal line of rate adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronier B, Debonnel G. Involvement of sigma receptors in the modulation of the glutamatergic/NMDA neurotransmission in the dopaminergic systems. 1999;368:183–196. doi: 10.1016/s0014-2999(99)00025-4. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA. Effects of sigma receptor ligands on the extracellular concentration of dopamine in the striatum and prefrontal cortex of the rat. Eur J Pharmacol. 1995;286:223–228. doi: 10.1016/0014-2999(95)00415-8. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA. Biphasic effect of sigma receptor ligands on the extracellular concentration of dopamine in the striatum of the rat. J Neural Transm. 1999;106:849–856. doi: 10.1007/s007020050205. [DOI] [PubMed] [Google Scholar]
- Guitart X, Mendez R, Ovalle S, Andreu F, Carceller A, Farre AJ, Zamanillo D. Regulation of ionotropic glutamate receptor subunits in different rat brain areas by a preferential sigma(1) receptor ligand and potential atypical antipsychotic. 2000;23:539–546. doi: 10.1016/S0893-133X(00)00142-1. [DOI] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y, Hara H, Sukamoto T. Characterization of specific (+)-[3H]N-allylnormetazocine and [3H]1,3-di(2-tolyl)guanidine binding sites in porcine gastric fundic mucosa. J Pharmacol Exp Ther. 1994;269:905–910. [PubMed] [Google Scholar]
- Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ishiwata K. Sigma receptor ligands: possible application as therapeutic drugs and as radiopharmaceuticals. Curr Pharm Des. 2006;12:3857–3876. doi: 10.2174/138161206778559614. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Maurice T, Su TP. Ca(2+) signaling via sigma(1)-receptors: novel regulatory mechanism affecting intracellular Ca(2+) concentration. J Pharmacol Exp Ther. 2000;293:788–798. [PubMed] [Google Scholar]
- Hayashi T, Su TP. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc Natl Acad Sci U S A. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108–15 cells. 2003;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. An update on the development of drugs for neuropsychiatric disorders: focusing on the sigma 1 receptor ligand. Expert Opin Ther Targets. 2008;12:45–58. doi: 10.1517/14728222.12.1.45. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: Decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that in guinea pig brain. Brain Res. 1990;527:244–253. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- Hercher C, Turecki G, Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J Psychiatr Res. 2009;43:947–961. doi: 10.1016/j.jpsychires.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Ho Y, Liu T, Tai M, Wen Z, Chow RS, Tsai Y, Wong C. Effects of olfactory bulbectomy on NMDA receptor density in the rat brain. Brain Res. 2001;900:214–218. doi: 10.1016/s0006-8993(01)02297-1. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Ishiwata K, Ishii K, Kimura Y, Sakata M, Naganawa M, Oda K, Miyatake R, Fujisaki M, Shimizu E, Shirayama Y, Iyo M, Hashimoto K. High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine: a positron emission tomography study using [11C]SA4503. Biol Psychiatry. 2007;62:878–883. doi: 10.1016/j.biopsych.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Ishima T, Nishimura T, Iyo M, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by donepezil: role of sigma-1 receptors and IP3 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1656–1659. doi: 10.1016/j.pnpbp.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Hiller JM, Simon EJ. Characterization of specific binding sites for [3H](d)-N-allylnormetazocine in rat brain membranes. 1985;27:46–52. [PubMed] [Google Scholar]
- Itzhak Y, Kassim CO. Clorgyline displays high affinity for sigma binding sites in C57BL/6 mouse brain. Eur J Pharmacol. 1990;176:107–108. doi: 10.1016/0014-2999(90)90139-w. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Stein I, Zhang SH, Kassim CO, Cristante D. Binding of sigma-ligands to C57BL/6 mouse brain membranes: effects of monoamine oxidase inhibitors and subcellular distribution studies suggest the existence of sigma-receptor subtypes. J Pharmacol Exp Ther. 1991;257:141–148. [PubMed] [Google Scholar]
- Iyengar S, Dilworth VM, Mick SJ, Contreras PC, Monahan JB, Rao TS, Wood PL. Sigma receptors modulate both A9 and A10 dopaminergic neurons in the rat brain: functional interaction with NMDA receptors. Brain Res. 1990;524:322–326. doi: 10.1016/0006-8993(90)90709-k. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Ramachandran S, Riemer L, Ramos-Serrano A, Ruoho AE, Jackson MB. Voltage-gated sodium channel modulation by sigma-receptors in cardiac myocytes and heterologous systems. Am J Physiol Cell Physiol. 2009;296:C1049–1057. doi: 10.1152/ajpcell.00431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Hayako H, Ishihara Y, Marui S, Iwane M, Miyamoto M. TAK-147, an acetylcholinesterase inhibitor, increases choline acetyltransferase activity in cultured rat septal cholinergic neurons. Neurosci Lett. 1999;260:5–8. doi: 10.1016/s0304-3940(98)00943-4. [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1) Biochem Biophys Res Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- Kikuchi-Utsumi K, Nakaki T. Chronic treatment with a selective ligand for the sigma-1 receptor chaperone, SA4503, up-regulates BDNF protein levels in the rat hippocampus. Neurosci Lett. 2008;440:19–22. doi: 10.1016/j.neulet.2008.05.055. [DOI] [PubMed] [Google Scholar]
- Kim HW, Roh DH, Yoon SY, Seo HS, Kwon YB, Han HJ, Kim KW, Beitz AJ, Lee JH. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br J Pharmacol. 2008;154:1125–1134. doi: 10.1038/bjp.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Matsuno K, Murai M, Mita S. Sigma 1 receptor subtype is involved in the facilitation of cortical dopaminergic transmission in the rat brain. 1997;22:1105–1109. doi: 10.1023/a:1027361101419. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Larson AA. Discrepancies in characterization of sigma sites in the mouse central nervous system. 1995;285:127–134. doi: 10.1016/0014-2999(95)00383-v. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugaya A, Sanacora G. Beyond monoamines: Glutamatergic function in mood disorders. CNS Spectrums. 2005;10:808–819. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Dhir A. sigma-1 receptors in major depression and anxiety. Expert Rev Neurother. 2009;9:1021–1034. doi: 10.1586/ern.09.40. [DOI] [PubMed] [Google Scholar]
- Lever JR, Gustafson JL, Xu R, Allmon RL, Lever SZ. Sigma1 and sigma2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse. 2006;59:350–358. doi: 10.1002/syn.20253. [DOI] [PubMed] [Google Scholar]
- Lopes T, Neubauer P, Boje KM. Chronic administration of NMDA glycine partial agonists induces tolerance in the Porsolt swim test. Pharmacol Biochem Behav. 1997;58:1059–1064. doi: 10.1016/s0091-3057(97)00302-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Munoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15:1563–1586. doi: 10.2174/138161209788168001. [DOI] [PubMed] [Google Scholar]
- Lucas G, Rymar VV, Sadikot AF, Debonnel G. Further evidence for an antidepressant potential of the selective sigma1 agonist SA 4503: electrophysiological, morphological and behavioural studies. Int J Neuropsychopharmacol. 2008;11:485–495. doi: 10.1017/S1461145708008547. [DOI] [PubMed] [Google Scholar]
- Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, Ruoho AE, Jackson MB. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol. 2000;526(Pt 3):527–539. doi: 10.1111/j.1469-7793.2000.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009a;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Ibrahim LA, Diaz-Granados N, Zarate CA., Jr Targeting glutamatergic signaling for the development of novel therapeutics for mood disorders. Curr Pharm Des. 2009b;15:1595–1611. doi: 10.2174/138161209788168010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Uz T, Smalheiser NR, Manev R. Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur J Pharmacol. 2001;411:67–70. doi: 10.1016/s0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nature Medicine. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Martina M, Turcotte ME, Halman S, Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Manji HK, Charney DS. Novel drugs and therapeutic targets for severe mood disorders. 2008;33:2080–2092. doi: 10.1038/sj.npp.1301652. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Yu Y, Mack A, Coop A, Ash J. Selective sigma receptor agonists produce antidepressant-like effects in mice. Experimental Biology. 2005:874. doi: 10.1016/j.euroneuro.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Su TP. Sigma receptors : chemistry, cell biology and clinical implications. Springer; New York: 2007. [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur J Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Kobayashi T, Tanaka MK, Mita S. Sigma 1 receptor subtype is involved in the relief of behavioral despair in the mouse forced swimming test. 1996;312:267–271. doi: 10.1016/0014-2999(96)00497-9. [DOI] [PubMed] [Google Scholar]
- Maurice T. Neurosteroids and sigma1 receptors, biochemical and behavioral relevance. Pharmacopsychiatry. 2004;37(Suppl 3):S171–182. doi: 10.1055/s-2004-832675. [DOI] [PubMed] [Google Scholar]
- Maurice T, Meunier J, Feng B, Ieni J, Monaghan DT. Interaction with sigma(1) protein, but not N-methyl-D-aspartate receptor, is involved in the pharmacological activity of donepezil. J Pharmacol Exp Ther. 2006;317:606–614. doi: 10.1124/jpet.105.097394. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009 doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Ruoho AE. Ligand-dependent localization and intracellular stability of sigma- 1 receptors in CHO-K1 cells. 2007;2:8. doi: 10.1186/1750-2187-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann DJ, Weissman AD, Su TP. Sigma-1 and sigma-2 sites in rat brain: comparison of regional, ontogenetic, and subcellular patterns. 1994;17:182–189. doi: 10.1002/syn.890170307. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- Mei J, Pasternak GW. Molecular cloning and pharmacological characterization of the rat sigma1 receptor. Biochem Pharmacol. 2001;62:349–355. doi: 10.1016/s0006-2952(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Mennini T, Gobbi M. The antidepressant mechanism of Hypericum perforatum. Life Sci. 2004;75:1021–1027. doi: 10.1016/j.lfs.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Blier P, Debonnel G, de Montigny C. Modulation by sigma ligands of N-methyl- D-aspartate-induced [3H]noradrenaline release in the rat hippocampus: G-protein dependency. 1992a;346:32–39. doi: 10.1007/BF00167567. [DOI] [PubMed] [Google Scholar]
- Monnet FP, De Costa BR, Bowen WD. Differentiation of sigma ligand-activated receptor subtypes that modulate NMDA-evoked [3H]-noradrenaline release in rat hippocampal slices. 1996;119:65–72. doi: 10.1111/j.1476-5381.1996.tb15678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet FP, Debonnel G, de Montigny C. In vivo electrophysiological evidence for a selective modulation of N-methyl-D-aspartate-induced neuronal activation in rat CA3 dorsal hippocampus by sigma ligands. 1992b;261:123–130. [PubMed] [Google Scholar]
- Monnet FP, Debonnel G, Junien JL, de Montigny C. N-methyl-D-aspartate-induced neuronal activation is selectively modulated by sigma receptors. 1990;179:441–445. doi: 10.1016/0014-2999(90)90186-a. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Maurice T. The sigma1 protein as a target for the non-genomic effects of neuro(active)steroids: molecular, physiological, and behavioral aspects. 2006;100:93–118. doi: 10.1254/jphs.cr0050032. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Morin-Surun MP, Leger J, Combettes L. Protein kinase C-dependent potentiation of intracellular calcium influx by sigma1 receptor agonists in rat hippocampal neurons. J Pharmacol Exp Ther. 2003;307:705–712. doi: 10.1124/jpet.103.053447. [DOI] [PubMed] [Google Scholar]
- Morin-Surun MP, Collin T, Denavit-Saubie M, Baulieu EE, Monnet FP. Intracellular sigma1 receptor modulates phospholipase C and protein kinase C activities in the brainstem. Proc Natl Acad Sci U S A. 1999;96:8196–8199. doi: 10.1073/pnas.96.14.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Vaidya VA. Cyclic AMP response element binding protein and brain-derived neurotrophic factor: molecules that modulate our mood? J Biosci. 2006;31:423–434. doi: 10.1007/BF02704114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato A, Ohta K, Sekiguchi Y, Okuyama S, Chaki S, Kawashima Y, Hatayama K. Design, synthesis, structure-activity relationships, and biological characterization of novel arylalkoxyphenylalkylamine sigma ligands as potential antipsychotic drugs. J Med Chem. 1999;42:1076–1087. doi: 10.1021/jm980212v. [DOI] [PubMed] [Google Scholar]
- Narita N, Hashimoto K, Tomitaka S, Minabe Y. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur J Pharmacol. 1996;307:117–119. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- Nestler EC, WA The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Ishima T, Iyo M, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth by fluvoxamine: role of sigma-1 receptors, IP3 receptors and cellular signaling pathways. PLoS ONE. 2008;3:e2558. doi: 10.1371/journal.pone.0002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova M, Ela C, Barg J, Vogel Z, Hasin Y, Eilam Y. Inotropic action of sigma receptor ligands in isolated cardiac myocytes from adult rats. Eur J Pharmacol. 1995;286:19–30. doi: 10.1016/0014-2999(95)00424-j. [DOI] [PubMed] [Google Scholar]
- Novakova M, Ela C, Bowen WD, Hasin Y, Eilam Y. Highly selective sigma receptor ligands elevate inositol 1,4,5-trisphosphate production in rat cardiac myocytes. Eur J Pharmacol. 1998;353:315–327. doi: 10.1016/s0014-2999(98)00398-7. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 2006;67(Suppl 6):3–8. [PubMed] [Google Scholar]
- Nuwayhid SJ, Werling LL. Sigma1 receptor agonist-mediated regulation of N-methyl-D-aspartate- stimulated [3H]dopamine release is dependent upon protein kinase C. J Pharmacol Exp Ther. 2003;304:364–369. doi: 10.1124/jpet.102.043398. [DOI] [PubMed] [Google Scholar]
- Oda T, Kume T, Katsuki H, Niidome T, Sugimoto H, Akaike A. Donepezil potentiates nerve growth factor-induced neurite outgrowth in PC12 cells. 2007;104:349–354. doi: 10.1254/jphs.fp0070563. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Shino Y, Hashimoto K, Kumakiri C, Shimizu E, Shirasawa H, Iyo M. Dynamic changes in AP-1 transcription factor DNA binding activity in rat brain following administration of antidepressant amitriptyline and brain-derived neurotrophic factor. Neuropharmacology. 2003;45:251–259. doi: 10.1016/s0028-3908(03)00148-5. [DOI] [PubMed] [Google Scholar]
- O’Neill MJ, Witkin JM. AMPA receptor potentiators: application for depression and Parkinson’s disease. Curr Drug Targets. 2007;8:603–620. doi: 10.2174/138945007780618517. [DOI] [PubMed] [Google Scholar]
- Owens MJ. Molecular and cellular mechanisms of antidepressant drugs. Depress Anxiety. 1996;4:153–159. doi: 10.1002/(SICI)1520-6394(1996)4:4<153::AID-DA1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Pande A, Geneve J, Scherrer B, Smith F, Leadbetter R, de Meynard C. A placebo-controlled trial of igmesine in the treatment of major depression. European Neuropsychopharmacology. 1999;9:138. [Google Scholar]
- Papp M, Moryl E. Antidepressant-like effects of 1-aminocyclopropanecarboxylic acid and D-cycloserine in an animal model of depression. Eur J Pharmacol. 1996;316:145–151. doi: 10.1016/s0014-2999(96)00675-9. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Patrick SL, Walker JM, Perkel JM, Lockwood M, Patrick RL. Increases in rat striatal extracellular dopamine and vacuous chewing produced by two sigma receptor ligands. Eur J Pharmacol. 1993;231:243–249. doi: 10.1016/0014-2999(93)90456-r. [DOI] [PubMed] [Google Scholar]
- Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfumi M, Panocka I, Ciccocioppo R, Vitali D, Froldi R, Massi M. Effects of a methanolic extract and a hyperforin-enriched CO2 extract of Hypericum perforatum on alcohol intake in rats. Alcohol Alcohol. 2001;36:199–206. doi: 10.1093/alcalc/36.3.199. [DOI] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Popoli M, Gennarelli M, Racagni G. Modulation of synaptic plasticity by stress and antidepressants. Bipolar Disord. 2002;4:166–182. doi: 10.1034/j.1399-5618.2002.01159.x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Prange AJ. The pharmacology and biochemistry of depression. Diseases of the Nervous System. 1964;25:217–221. [PubMed] [Google Scholar]
- Prasad PD, Li HW, Fei YJ, Ganapathy ME, Fujita T, Plumley LH, Yang-Feng TL, Leibach FH, Ganapathy V. Exon-intron structure, analysis of promoter region, and chromosomal localization of the human type 1 sigma receptor gene. 1998;70:443–451. doi: 10.1046/j.1471-4159.1998.70020443.x. [DOI] [PubMed] [Google Scholar]
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- Racagni G, Popoli M. Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin Neurosci. 2008;10:385–400. doi: 10.31887/DCNS.2008.10.4/gracagni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa RB. Screen of receptor and uptake-site activity of hypericin component of St. John’s wort reveals sigma receptor binding. Life Sci. 1998;62:PL265–270. doi: 10.1016/s0024-3205(98)00085-x. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Chu UB, Mavlyutov TA, Pal A, Pyne S, Ruoho AE. The sigma1 receptor interacts with N-alkyl amines and endogenous sphingolipids. Eur J Pharmacol. 2009;609:19–26. doi: 10.1016/j.ejphar.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantamaki T, Hendolin P, Kankaanpaa A, Mijatovic J, Piepponen P, Domenici E, Chao MV, Mannisto PT, Castren E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Rao TS, Cler JA, Mick SJ, Dilworth VM, Contreras PC, Iyengar S, Wood PL. Neurochemical characterization of dopaminergic effects of opipramol, a potent sigma receptor ligand, in vivo. Neuropharmacology. 1990;29:1191–1197. doi: 10.1016/0028-3908(90)90044-r. [DOI] [PubMed] [Google Scholar]
- Robichaud M, Beauchemin V, Lavoie N, Dennis T, Debonnel G. Effects of bilateral olfactory bulbectomy on N-methyl-D-aspartate receptor function: autoradiographic and behavioral studies in the rat. Synapse. 2001;42:95–103. doi: 10.1002/syn.1105. [DOI] [PubMed] [Google Scholar]
- Romero G, Perez MP, Carceller A, Monroy X, Farre AJ, Guitart X. Changes in phosphoinositide signalling activity and levels of the alpha subunit of G(q/11) protein in rat brain induced by E-5842, a sigma(1) receptor ligand and potential atypical antipsychotic. Neurosci Lett. 2000;290:189–192. doi: 10.1016/s0304-3940(00)01353-7. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009;198:472–476. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nature Neuroscience. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Papp M. The selective sigma2 ligand Lu 28–179 has an antidepressant-like profile in the rat chronic mild stress model of depression. Behav Pharmacol. 2000;11:117–124. doi: 10.1097/00008877-200004000-00003. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Lebel L, Koe BK, Seeger T, Heym J. Sertraline potently displaces (+)-[3H]3-PPP binding to sigma sites in rat brain. Eur J Pharmacol. 1989;165:335–336. doi: 10.1016/0014-2999(89)90734-6. [DOI] [PubMed] [Google Scholar]
- Sen S, Sanacora G. Major depression: emerging therapeutics. 2008;75:204–225. doi: 10.1002/msj.20043. [DOI] [PubMed] [Google Scholar]
- Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. Cloning and functional characterization of a sigma receptor from rat brain. J Neurochem. 1998;70:922–931. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun. 1997;241:535–540. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Skolnick P. Modulation of glutamate receptors: strategies for the development of novel antidepressants. Amino Acids. 2002;23:153–159. doi: 10.1007/s00726-001-0121-7. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Skuza G. Potential antidepressant activity of sigma ligands. Pol J Pharmacol. 2003;55:923–934. [PubMed] [Google Scholar]
- Skuza G, Rogoz Z. A potential antidepressant activity of SA4503, a selective sigma 1 receptor agonist. Behav Pharmacol. 2002;13:537–543. doi: 10.1097/00008877-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Skuza G, Rogoz Z. Antidepressant-like effect of combined treatment with selective sigma receptor agonists and a 5-HT1A receptor agonist in the forced swimming test in rats. Pharmacol Rep. 2007;59:773–777. [PubMed] [Google Scholar]
- Slattery DA, Hudson AL, Nutt DJ. Invited review: the evolution of antidepressant mechanisms. Fundam Clin Pharmacol. 2004;18:1–21. doi: 10.1111/j.1472-8206.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- Stahl SM. The sigma enigma: can sigma receptors provide a novel target for disorders of mood and cognition? J Clin Psychiatry. 2008;69:1673–1674. doi: 10.4088/jcp.v69n1101. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stewart W, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA. 2003;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Su TP, Shukla K, Gund T. Steroid binding at sigma receptors: CNS and immunological implications. Ciba Found Symp. 1990;153:107–113. doi: 10.1002/9780470513989.ch6. discussion 113–106. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. Nerve growth factor-induced neurite sprouting in PC12 cells involves sigma-1 receptors: implications for antidepressants. J Pharmacol Exp Ther. 2002;303:1227–1237. doi: 10.1124/jpet.102.041970. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. A perspective on the new mechanism of antidepressants: neuritogenesis through sigma-1 receptors. Pharmacopsychiatry. 2004;37(Suppl 3):S208–213. doi: 10.1055/s-2004-832679. [DOI] [PubMed] [Google Scholar]
- Tanis KQ, Duman RS. Intracellular signaling pathways pave roads to recovery for mood disorders. Ann Med. 2007;39:531–544. doi: 10.1080/07853890701483270. [DOI] [PubMed] [Google Scholar]
- Tanis KQ, Newton SS, Duman RS. Targeting neurotrophic/growth factor expression and signaling for antidepressant drug development. CNS Neurol Disord Drug Targets. 2007;6:151–160. doi: 10.2174/187152707780363276. [DOI] [PubMed] [Google Scholar]
- Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. 2006;58:115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- Tchedre KT, Huang RQ, Dibas A, Krishnamoorthy RR, Dillon GH, Yorio T. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest Ophthalmol Vis Sci. 2008;49:4993–5002. doi: 10.1167/iovs.08-1867. [DOI] [PubMed] [Google Scholar]
- Terstappen GC, Pellacani A, Aldegheri L, Graziani F, Carignani C, Pula G, Virginio C. The antidepressant fluoxetine blocks the human small conductance calcium-activated potassium channels SK1, SK2 and SK3. Neurosci Lett. 2003;346:85–88. doi: 10.1016/s0304-3940(03)00574-3. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Peterson DA. Even neural stem cells get the blues: evidence for a molecular link between modulation of adult neurogenesis and depression. 2008;14:183–193. [PMC free article] [PubMed] [Google Scholar]
- Tottori K, Miwa T, Uwahodo Y, Yamada S, Nakai M, Oshiro Y, Kikuchi T, Altar CA. Antidepressant-like responses to the combined sigma and 5-HT1A receptor agonist OPC-14523. Neuropharmacology. 2001;41:976–988. doi: 10.1016/s0028-3908(01)00147-2. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Hayashi T, Mori T, Su TP. Sigma-1 receptor chaperones and diseases. Cent Nerv Syst Agents Med Chem. 2009;9:184–189. doi: 10.2174/1871524910909030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukai M, Maeda H, Nanya Y, Kameyama T, Matsuno K. Beneficial effects of acute and repeated administrations of sigma receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol Biochem Behav. 1998;61:247–252. doi: 10.1016/s0091-3057(98)00093-8. [DOI] [PubMed] [Google Scholar]
- van Broekhoven F, Verkes RJ. Neurosteroids in depression: a review. Psychopharmacology (Berl) 2003;165:97–110. doi: 10.1007/s00213-002-1257-1. [DOI] [PubMed] [Google Scholar]
- Volz HP, Stoll KD. Clinical trials with sigma ligands. Pharmacopsychiatry. 2004;37(Suppl 3):S214– 220. doi: 10.1055/s-2004-832680. [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, Nitta A, Nabeshima T. Role of N-methyl-D-aspartate receptors in antidepressant-like effects of sigma 1 receptor agonist 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine dihydrochloride (SA-4503) in olfactory bulbectomized rats. J Pharmacol Exp Ther. 2007a;322:1305–1314. doi: 10.1124/jpet.107.124685. [DOI] [PubMed] [Google Scholar]
- Wang J, Mack AL, Coop A, Matsumoto RR. Novel sigma (sigma) receptor agonists produce antidepressant-like effects in mice. Eur Neuropsychopharmacol. 2007b;17:708–716. doi: 10.1016/j.euroneuro.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Webster HH, Flores G, Marcotte ER, Cecyre D, Quirion R, Srivastava LK. Olfactory bulbectomy alters NMDA receptor levels in the rat prefrontal cortex. Synapse. 2000;37:159–162. doi: 10.1002/1098-2396(200008)37:2<159::AID-SYN9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Jr, Culp SG, De Souza EB. Sigma-receptors in endocrine organs: identification, characterization, and autoradiographic localization in rat pituitary, adrenal, testis, and ovary. Endocrinology. 1989;124:1160–1172. doi: 10.1210/endo-124-3-1160. [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Jr, Ha BK, Whitlock BB, Saini P. Differential localization of three distinct binding sites for sigma receptor ligands in rat spleen. 1997;72:45–58. doi: 10.1016/s0165-5728(96)00140-3. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bowen WD. Role of sigma-1 receptor C-terminal segment in inositol 1,4,5- trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J Biol Chem. 2008;283:28198–28215. doi: 10.1074/jbc.M802099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su TP, Kunugi H. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J Biol Chem. 2006;281:12941–12949. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yamamoto T, Sagi N, Klenerova V, Goji K, Kawai N, Baba A, Takamori E, Moroji T. Sigma ligands indirectly modulate the NMDA receptor-ion channel complex on intact neuronal cells via sigma 1 site. 1995;15:731–736. doi: 10.1523/JNEUROSCI.15-01-00731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Alkayed NJ, Hurn PD, Kirsch JR. Cyclic adenosine monophosphate response element-binding protein phosphorylation and neuroprotection by 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) Anesth Analg. 2009;108:964–970. doi: 10.1213/ane.0b013e318192442c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]