Abstract
N-terminal pro-brain natriuretic peptide (NT-proBNP) is a routinely used prognostic parameter in patients with pre-capillary pulmonary hypertension (PH). As it accumulates in the presence of impaired renal function, the clinical utility of NT-proBNP in PH patients with concomitant renal insufficiency remains unclear. In a retrospective approach, patients with pre-capillary PH (group I or IV) and concomitant renal insufficiency at time of right heart catheterization (glomerular filtration rate (GFR) ≤60 ml/min/1.73 m2) were identified out of all prevalent pre-capillary PH patients treated at a single center. Forty patients with renal insufficiency (25.8%) were identified and matched regarding hemodynamic parameters with a control group of 56 PH patients with normal renal function (GFR >60 ml/min/1.73 m2). Correlations of NT-proBNP levels with hemodynamic and prognostic parameters (time to clinical worsening and overall survival) were assessed. Overall, GFR correlated inversely with NT-proBNP and had the strongest influence on NT-proBNP levels in a stepwise multiple linear regression model including hemodynamic parameters and age (r2 = 0.167). PH patients with renal insufficiency had significant higher levels of NT-proBNP (median: 1935 ng/l vs. 573 ng/l, p = 0.001). Nevertheless, NT-proBNP correlated with invasive hemodynamic parameters in these patients. Using higher cut-off values than in patients with preserved renal function, NT-proBNP levels were significantly associated with time to clinical worsening (>1660 ng/l, p = 0.001) and survival (>2212 ng/l, p = 0.047) in patients with renal insufficiency. Multivariate Cox’s proportional hazards analysis including established prognostic parameters, age and GFR confirmed NT-proBNP as an independent risk factor for clinical worsening in PH patients with renal insufficiency (hazard ratio 4.8, p = 0.007). Thus, in a retrospective analysis we showed that NT-proBNP levels correlated with hemodynamic parameters and outcome regardless of renal function. By using higher cut-off values, NT-proBNP seems to represent a valid clinical marker even in PH patients with renal insufficiency.
Introduction
Pulmonary hypertension (PH) is characterized by elevated pulmonary vascular resistance (PVR) leading to right ventricular overload, hypertrophy and dilation, and eventually causing right ventricular failure and death [1]. For accurate clinical decision-making robust (surrogate) markers are warranted that reflect hemodynamic status and predict disease progression and prognosis; ideally, such markers are of non-invasive nature. In patients with pre-capillary PH, non-invasive parameters include exercise capacity, World Health Organization functional class (WHO FC), echocardiographic signs and indices including pericardial effusion, right atrium size and left ventricular eccentricity [2]–[4]. In addition, different biomarkers obtained from patients’ blood have been investigated regarding their ability to reflect disease state [5].
Brain natriuretic peptide (BNP) and the N-terminal fragments of its pro-hormone, N-terminal pro-brain natriuretic peptide (NT-proBNP), have been implicated as functional biomarkers in a variety of cardiovascular diseases [6]. Levels of BNP and NT-proBNP are elevated following ventricular impairment and reflect the severity of hemodynamic dysfunction in heart disease [6]. BNP and NT-proBNP are released from ventricular myocytes in response to mechanical stretching, e.g. due to increased chamber pressure or volume overload [7], [8]. Physiologically, BNPs are involved in volume homeostasis, regulation of blood pressure and may control structural changes of the heart muscle in a paracrine manner [7], [8]. On the contrary, NT-proBNP exhibits no or only markedly reduced biological activity [8]. The plasma concentration of NT-proBNP is influenced by various factors such as gender, age, inflammation, exercise and even diurnal variations have been reported [6], [9]. Besides an increased release, elevated NT-proBNP levels may result from altered metabolism and/or decreased elimination from the circulation. The exact mechanism of NT-proBNP elimination remains controversial [8]. However, it has been shown that impaired renal function with a decreased glomerular filtration rate (GFR) leads to accumulation of NT-proBNP and might therefore hamper its prognostic utility [10], [11].
Previous studies have shown that levels of NT-proBNP correlate with invasive hemodynamic parameters, reflect right ventricular remodeling and predict survival in patients with pre-capillary PH and normal renal function [12]–[24]. However, it remains uncertain whether NT-proBNP is a useful clinical marker in PH patients with renal dysfunction, which may occur in up to 20% of patients [16]. In fact, Leuchte et al. observed a correlation of NT-proBNP levels with right atrial pressure only in patients with PH and concomitant renal insufficiency [16].
Our analysis aimed to assess (1) the impact of renal insufficiency on NT-proBNP levels and (2) the prognostic usefulness of NT-proBNP levels in patients with pre-capillary PH (patients with pulmonary arterial hypertension, PAH, or inoperable chronic thromboembolic PH, CTEPH) and concomitant renal insufficiency. In particular, we hypothesized that NT-proBNP levels measured at time of right heart catheterization can be used to assess disease severity and predict outcome in this subgroup of patients.
Materials and Methods
Patients’ Selection
All outpatients with pre-capillary PH (group I or IV), who showed concomitant renal insufficiency (defined as GFR ≤60 ml/min/1.73 m2) at time of last right heart catheterization, were identified retrospectively out of prevalent pre-capillary PH patients treated at a tertiary PH referral centre (University Medical Centre Hamburg-Eppendorf, Hamburg, Germany). These patients were retrospectively matched in regard to hemodynamic parameters with a control group of PH patients without renal insufficiency (GFR >60 ml/min/1.73 m2). All patients have been diagnosed in accordance to current guidelines [25]. Right heart catheters were performed between 2003 and 2012 during routine clinical workup and after informed consent. Catheters were performed either with diagnostic or prognostic purpose. All patients with CTEPH were referred to a center for thoracic surgery specialized on pulmonary endarterectomy (Kerckhoff Clinic, Bad Nauheim, Germany). Only patients considered as inoperable were offered PAH-specific pharmacological therapy in addition to anticoagulation. Patients’ data were anonymized and de-identified prior to analyses. Anonymous data were collected in a database that was generated only for the present analysis. Analyses has been carried out in agreement with local law (Hamburgisches Krankenhausgesetz, HmbKHG, § 12) and conducted according to the principles expressed in the Declaration of Helsinki. The database is not open-source; however, we may be contacted directly to enable access to the collected anonymous data.
Disease Parameters
For functional assessment including catheterization and laboratory findings patients were routinely admitted to our hospital for 3–5 days. All disease parameters were obtained retrospectively at the time of hospitalization for right heart catheterization (referred to as time of right heart catheterization). Hemodynamic parameters obtained from right heart catheterization included heart rate (/min, HR), mean right atrial pressure (mm Hg, RAP) mean pulmonary arterial pressure (mm Hg, mPAP) and pulmonary arterial wedge pressure (mm Hg, PAWP). Cardiac output (l/min, CO) was determined by the direct Fick method. PVR (dyne*s*cm5) and the cardiac index (l/min*m2, CI) were calculated secondary using standard formula. The 6-minute walking distance (6MWD) was obtained using a standardized protocol in accordance with the American Thoracic Society (ATS) statement (2002) [26]. Functional class according to the modified WHO classification was assessed by the attending physician. Serum-creatinine and NT-proBNP concentrations were measured using commercially available assays (Dimension Vista, Siemens Healthcare Diagnostics, IL, USA; Catalogue No. K6423A and K1033). GFR was estimated using the Modification of Diet in Renal Disease (ml/min/1.73 m2, MDRD) equation. Stage 3 to 5 of renal impairment (GFR ≤60 ml/min/1.73 m2) according to the National Kidney Foundation Practice Guidelines were defined as renal insufficiency [27]. A normalized ratio of NT-proBNP levels regarding age and gender was formed using reference data [28].
Follow-up Assessments and Outcome Parameters
Time to clinical worsening (TTCW) was defined as the time to the first event of clinical worsening after performing right heart catheterization. Patients were evaluated at least every three months in our outpatient department. Events were retrospectively identified from patients charts and were defined as (1) decrease of more than 15% in 6MWD (2) change in PAH-specific therapy due to signs and symptoms of progressive right heart failure (either add-on or substance switch, PAH patients only), (3) lung transplantation (PAH patients only), (4) PH-related hospitalization, or (5) death due to disease. TTCW for all patients was assessed in July 2012.
All-cause mortality of patients showing renal insufficiency was obtained by reviewing the patients’ charts or by contacting the respective general physician. Overall survival was assessed in January 2014.
Statistical Analysis
Differences of means between unpaired samples were assessed by Student t-test for parametric and Mann-Whitney-U test for non-parametric data; chi-square test was used to compare ordinal data. Spearman’s rank correlation was performed to assess correlation of non-parametric data. Time-dependent receiver-operating characteristic (ROC) analyses were performed across the ranges of NT-proBNP to assess cut-off values to predict clinical worsening and overall survival (highest sum of sensitivity and specificity). Kaplan-Meier and multivariate Cox’s proportional hazards analysis were performed to assess impact of NT-proBNP on TTCW and survival. For multivariate analysis the median of age, 6MWD and GFR in patients with renal insufficiency were used as cut-off values. All p-values were two-sided. P<0.05 was considered statistically significant. SPSS statistics 20 (IBM, Armonk, New York, United States) were used to perform all statistical analysis.
Results
Patients’ Characteristics
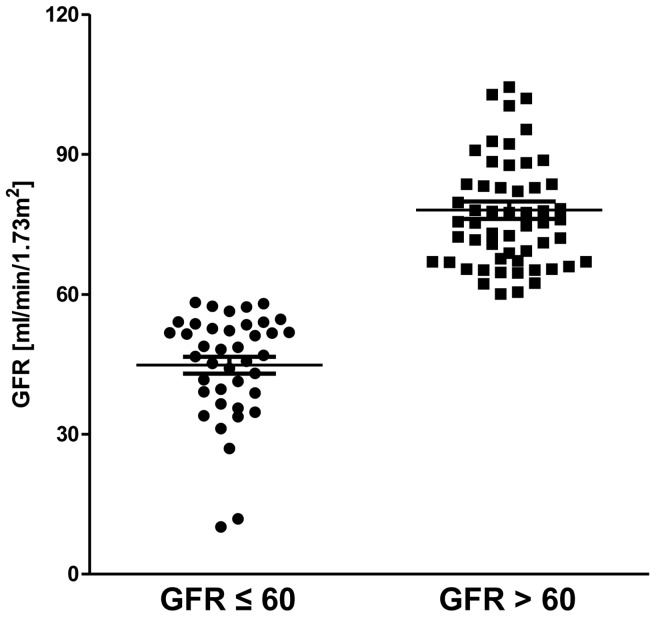
Forty patients (25.8%) with pre-capillary PH and concomitant renal insufficiency were identified out of 155 prevalent pre-capillary PH patients. As a control group 56 pre-capillary PH patients without renal insufficiency were retrospectively matched regarding hemodynamic parameters. In total, 96 PH patients were included in the present analysis. Patients’ characteristics at time of right heart catheterization are shown in table 1. In patients with renal insufficiency mean GFR was 45±11 ml/min/1.73 m2 (range: 10 to 58 ml/min/1.73 m2) compared to a mean GFR of 78±14 ml/min/1.73 m2 (range: 61 to 136 ml/min/1.73 m2) in patients with normal renal function (Figure 1). Mean of serum-creatinine was 1.5±0.8 mg/dl and 0.9±0.2 mg/dl, respectively. Patients with renal insufficiency were older, reached shorter 6MWD and tended to have higher WHO FC at time of right heart catheterization (Table 1). None of patients with renal insufficiency received renal replacement therapy. All patients with CTEPH were evaluated for pulmonary endarterectomy. CTEPH patients included in the study were either considered technically not operable or denied surgery. PAH-specific therapy at time of right heart catheterization according to group of disease, i.e. PAH or CTEPH, are given in table S1. Patients received either monotherapy, combination therapy or no PAH-specific pharmacological therapy.
Table 1. Patients’ characteristics.
| Parameter | Groups of patients | p-value | |||
| GFR >60 | GFR ≤60 | ||||
| n = 56 | n = 40 | ||||
| Age [yr] | 60±11.7 | 69±10 | <0.001& | ||
| Gender | Female | 39 | 31 | 0.39§ | |
| Male | 17 | 9 | |||
| BMI [kg/m2] | 27.1±5.8 | 25±5.4 | 0.34 | ||
| PH class | IPAH | 25 | 20 | 0.45§ | |
| APAH | 15 | 13 | |||
| CTD | 7 | 8 | |||
| Other# | 8 | 5 | |||
| CTEPH | 16 | 7 | |||
| WHO FC | I | 3 | – | 0.05§ | |
| II | 11 | 3 | |||
| III | 37 | 36 | |||
| IV | 5 | 1 | |||
| 6MWD [m] | 366±121.8 | 285.6±122.8 | 0.002 | ||
| Mean PAP [mm Hg] | 42.2±14.4 | 40.9±13.9 | 0.67 | ||
| RAP [mm Hg] | 7.5±4.3 | 9.2±5.4 | 0.16 | ||
| PAWP [mm Hg] | 12±5 | 13±6.5 | 0.15 | ||
| PVR [dyne*s*cm5] | 707.2±462.7 | 711.2±472 | 0.97 | ||
| CI [L/min*m2] | 2.3±0.7 | 2.3±0.7 | 0.92 | ||
Data are presented as mean ± SD or numbers.
Comparison of means between GFR-groups are performed by Student’s T, Mann-Whitney-U& or Chi-Square test§. CTD = connective tissue disease. # Other includes HIV, porto-pulmonary hypertension and congenital heart diseases.
Figure 1. Glomerular filtration rate (GFR).
Distribution of GFR (ml/min/1.73 m2) among patients’ groups. Mean GFR was 45±11 ml/min/1.73 m2 (range: 10 to 58 ml/min/1.73 m2) and 78±14 ml/min/1.73 m2 (ranging from 61 to 136 ml/min/1.73 m2).
Correlation of NT-proBNP Levels with Renal Function
Overall, the level of NT-proBNP correlated inversely with GFR (r = −0.36, p<0.001). In a stepwise multiple linear regression model including mPAP, RAP, PVR, CI and age, GFR had the strongest influence on NT-proBNP levels (r2 = 0.167).
Comparison of NT-proBNP Levels between PH Patients with and without Renal Insufficiency
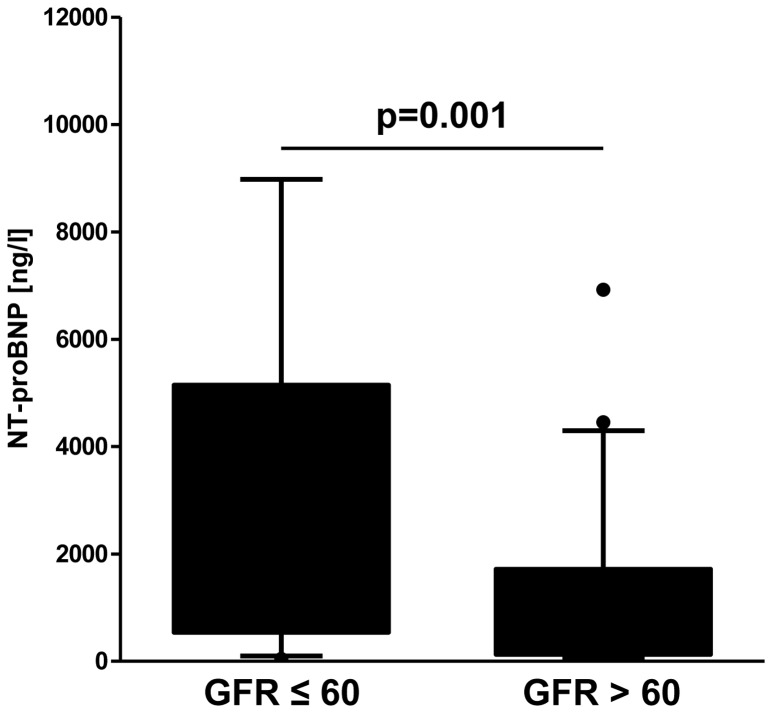
Levels of NT-proBNP were significantly higher in patients with renal insufficiency compared to hemodynamically matched control patients with normal renal function (p = 0.001, Mann-Whitney-U; Figure 2). Median NT-proBNP concentration was 1935 ng/l (range: 44 to 14,534 ng/l) in patients with renal insufficiency and 573 ng/l (range: 47 to 6924 ng/l) in patients with normal renal function. Moreover, NT-proBNP levels were significantly increased in patients with renal insufficiency when comparing only patients with WHO FC III/IV (p = 0.017, Mann-Whitney-U) and of older age (older than the mean age of 64 years, p<0.001, Mann-Whitney-U). However, older patients with renal insufficiency had more impaired hemodynamics compared to older patients with preserved renal function (mPAP 38±13 vs. 34±8 mmHg, p = 0.003; PVR 714±523 vs. 503±203 dyne*s*cm5, p = 0.004; T-tests).
Figure 2. N-terminal pro-brain natriuretic peptide (NT-proBNP).
In patients with renal insufficiency (defined as glomerular filtration rate (GFR) ≤60 ml/min/1.73 m2) the levels of NT-proBNP were significantly higher (Mann-Whitney-U-test). Median NT-proBNP concentration was 1935 ng/l (range: 44 to 14,534 ng/l) in patients with renal insufficiency and 573 ng/l (range: 47 to 6924 ng/l) in patients with normal renal function.
Correlation of NT-proBNP with Hemodynamic Parameters
In patients with normal renal function, NT-proBNP levels correlated with all hemodynamic parameters but RAP. In patients with renal insufficiency, NT-proBNP levels were significantly associated with all hemodynamic parameters including RAP, PVR and CI. Although statistical significant, correlations were generally moderate. An age and gender normalized ratio of NT-proBNP could not markedly improve correlation in patients with or without renal insufficiency (Table S2).
Prediction of Clinical Worsening by NT-proBNP Levels
During a mean follow-up time of 20.6 months (range: 0 to 106 months) clinical worsening occurred in 44 out of 96 (46%) patients at a mean time of 8.8 months (range: 0 to 84 months). Causes for clinical worsening were as following: Decrease of more than 15% in 6MWD (n = 16 cases, 36%), hospitalization for progressive right heart failure (n = 14 patients, 32%), escalation of PAH-specific medication (n = 14, 32%) and two patients died during follow-up assessment regarding TTCW. From this cohort, no patient underwent lung transplantation during the follow-up period.
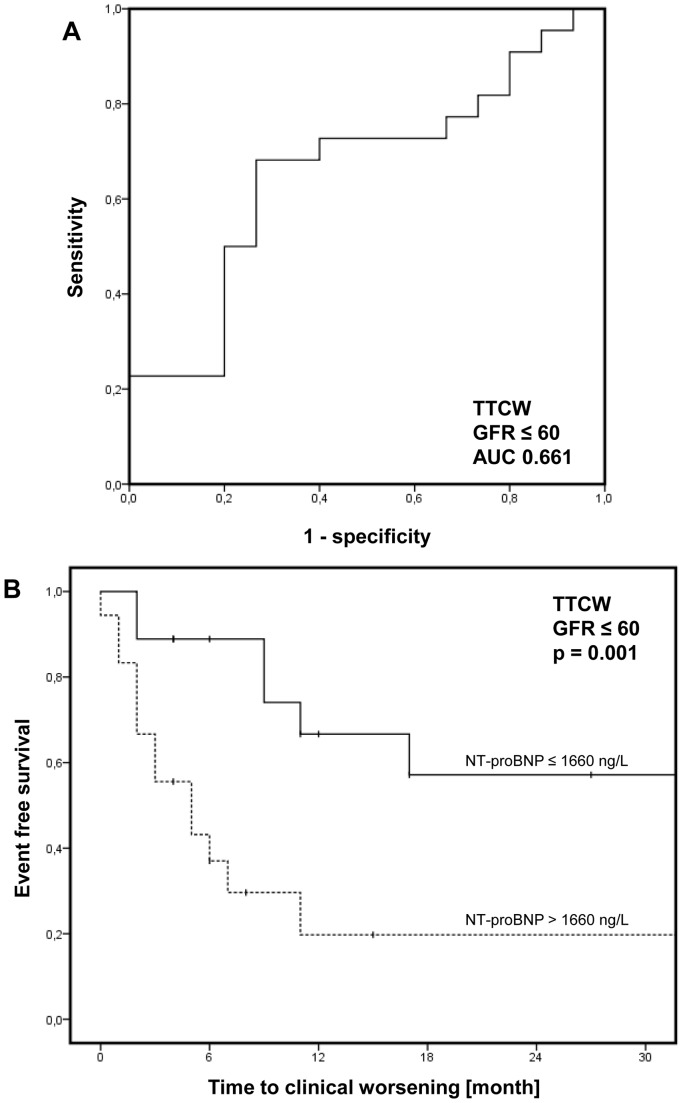
Cut-off NT-proBNP values to predict TTCW were assessed by ROC analysis. Using a cut-off value of 1292 ng/l in patients with normal renal function (sensitivity 50%, specificity 83%; AUC = 0.603; Figure S1) higher levels of NT-proBNP were significantly associated with early clinical worsening in Kaplan-Meier analysis (p = 0.02, log-rank; Figure S1). In PH patients with renal insufficiency a cut-off value of 1660 ng/l (sensitivity 68%, specificity 73%; AUC = 0.661; Figure 3A) was identified and NT-proBNP levels above this threshold were significantly associated with unfavorable clinical outcome (p = 0.001, log-rank; Figure 3B). Of note, no difference occurred regarding GFR if patients were stratified according to NT-proBNP cut-offs. GFR was 48±7 in patients with renal insufficiency and NT-proBNP levels beneath or equal to and 43±12 above 1660 ng/l (p = 0.11, t-test).
Figure 3. Time to clinical worsening (TTCW).
Receiver operating characteristic (ROC) analysis to determine the cut-off value in patients with renal insufficiency (defined as glomerular filtration rate (GFR) ≤60 ml/min/1.73 m2; A). In Kaplan-Meier analysis, higher levels of n-terminal pro-brain natriuretic peptide (NT-proBNP) were significantly associated with early clinical worsening (B; p = 0.001, log-rank).
Most interestingly, among patients with renal insufficiency, NT-proBNP levels above 1660 ng/l were independently associated with early clinical worsening in a multivariate Cox’s proportional hazards analysis including age, gender, renal function (GFR) as wells as non-invasive prognostic parameter such as 6MWD and WHO FC (Hazard ratio 4.8, 95% CI 1.6–14.9, p = 0.007; Table 2).
Table 2. Multivariate Cox’s proportional hazards analysis assessing the predictive value of n-terminal pro-brain natriuretic peptide (NT-proBNP) levels on clinical worsening in 40 PH patients with concomitant renal insufficiency (defined as glomerular filtration rate (GFR) ≤60 ml/min/1.73 m2) in a model with further established non-invasive parameters, age and renal function.
| Parameter | Hazard ratio | 95% confidence interval | p-value | |
| Lower | Upper | |||
| Age >71 yr | 1.3 | 0.5 | 3.8 | 0.58 |
| Male gender | 0.7 | 0.1 | 3.5 | 0.66 |
| GFR <48 ml/min/1.73 m2 | 1.5 | 0.5 | 4.5 | 0.44 |
| 6MWD <279 m | 2.3 | 0.7 | 7,4 | 0.17 |
| WHO FC | 15.1 | 1.2 | 196.1 | 0.038 |
| NT-proBNP>1660 ng/l | 4.8 | 1.6 | 14.9 | 0.007 |
Median of age, 6MWD and GFR in patients with renal insufficiency were used as cut-off values.
Prediction of Survival by NT-proBNP levels in PH Patients with Renal Insufficiency
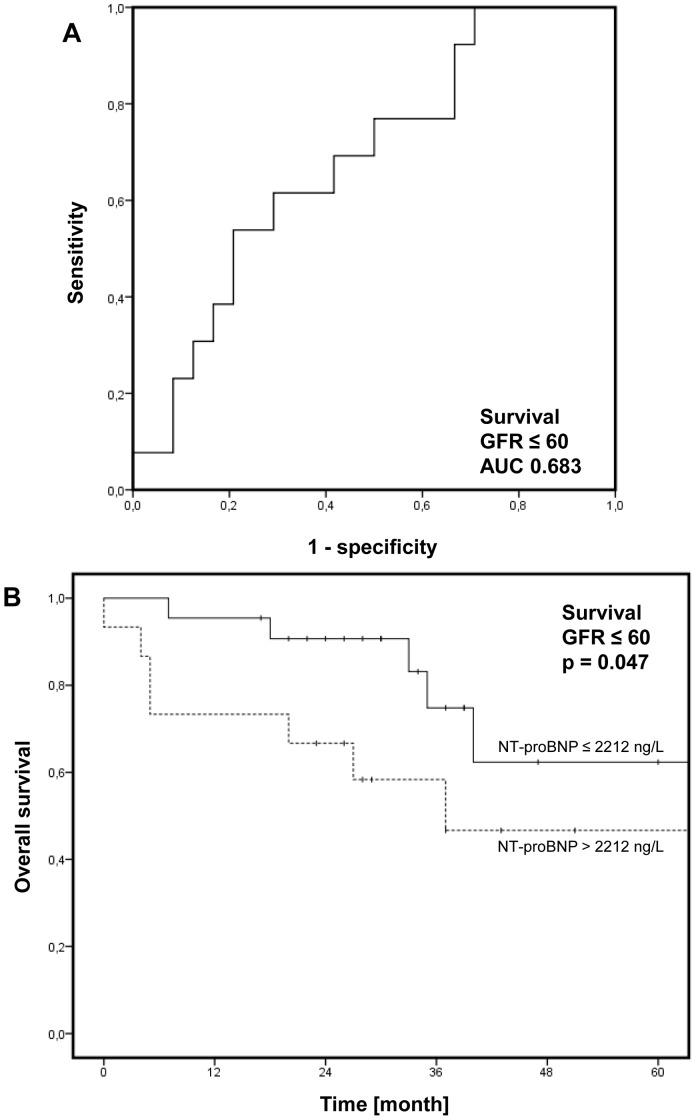
During a mean follow-up time of 39 months (range: 0 to 126) 13 out of 40 (33%) patients with renal insufficiency died. In ROC analysis, a NT-proBNP cut-off value of 2212 ng/l (sensitivity 62%, specificity 71%; AUC = 0.68; Figure 4A) was identified and levels above this threshold were significantly associated with unfavorable overall survival (p = 0.047, log-rank; Figure 4B). One-year survival rates of patients were 95% and 73% and five-year survival rates 67% and 43%. Again, GFR did not differ between patients with higher (>2212 ng/l; 43±7 ml/min/1.73 m2) and lower NT-proBNP levels (≤2212 ng/l; 47±13 ml/min/1.73 m2, p = 0.21, t-test).
Figure 4. Overall Survival.
Receiver operating characteristic (ROC) analysis to determine the cut-off value in patients with renal insufficiency (defined as glomerular filtration rate (GFR) ≤60 ml/min/1.73 m2; A). In Kaplan-Meier analysis, higher levels of n-terminal pro-brain natriuretic peptide (NT-proBNP) were significantly associated with poor survival (B; p = 0.047, log-rank).
In a multivariate Cox’s proportional hazards analysis NT-proBNP levels above 2212 ng/l were not independently associated with survival if age, gender, renal function (GFR), 6MWD as well as WHO FC were included in the analysis (Hazard ratio 2.226, 95% CI 0.61–8.016, p = 0.22).
Discussion
According to our data in pre-capillary PH patients, NT-proBNP levels are significantly affected by the renal function and accumulate in the presence of renal impairment. Nevertheless, in pre-capillary PH patients with concomitant renal insufficiency NT-proBNP levels at time of right heart catheterization correlate with relevant invasive hemodynamic parameters and are associated with clinical worsening as well as survival.
Renal insufficiency displays a crucial co-morbidity in patients with pre-capillary PH and may develop due to hemodynamic failure with low cardiac output or may exist independently [16]. In the US-based observational REVEAL registry (Registry to EValuate Early And Long-term PAH), for instance, renal insufficiency was found in only 4% of patients at time of enrolment. However, it was significantly associated with unfavorable one-year overall mortality (hazard ratio 1.9) [29]. Others have observed renal insufficiency more frequently in around 20% of PH patients [16]. Furthermore, two studies have reported that a preserved renal function was associated with better in-hospital and short-term survival in PH patients who have been hospitalized due to right heart failure (HF) [30], [31].
The elimination of NT-proBNP from the circulation lacks known active clearance mechanisms and relies presumably on passive excretion by organ beds with large degrees of blood flow including the kidneys [8]. In patients with acute or chronic left HF it seems evident that NT-proBNP levels are higher in those with renal dysfunction suggesting that the clearance depends on renal elimination [32]. Despite this association, it has been shown that NT-proBNP levels stratify outcome in patients with chronic left HF and concomittant renal dysfunction [33]. In addition, Hori et al. have reported an even better survival stratification by NT-proBNP as compared to BNP in chronic left HF patients with severe renal insufficiency (GFR <30 ml/min/1.73 m2) [34]. In the latter study cut-off values varied widely based on renal function (range: 258 to 5809 ng/l) [32], [34]. Thus, on an individual level uncertainty remains in the interpretation of NT-proBNP in patients with renal dysfunction.
In patients with pre-capillary PH, NT-proBNP levels have been demonstrated to correlate with invasive hemodynamic parameters such as RAP, mPAP, PVR and CI [12]–[14], [16], [17], [19], [20], [24], [35], as well as non-invasive parameters of disease severity such as 6MWD and WHO FC [14], [17], [19]–[21], [24]. Moreover, base-line NT-proBNP values have been shown to stratify patients’ survival in numerous studies [14]–[16], [20], [23]. Mathai et al. have reported an association between NT-proBNP levels and survival in a cohort of 98 PAH patients independent of renal function [19]. Estimated renal function, however, was mostly normal in these patients (mean GFR of 82±31 ml/min/1.73 m2) [19]. The cut-off values for NT-proBNP in studies assessing the prognostic utility of NT-proBNP in PH patients have ranged between 1256 to 1800 ng/l. Although larger outcome studies are still required to verify the optimal cut-off level, a NT-proBNP level below 1400 ng/l seems useful for identification of PAH patients with favorable prognosis [25]. Furthermore, a “normalization” of BNP or NT-proBNP level has recently been suggested as a potential treatment goal in PAH [36].
We show that renal insufficiency can be observed in a significant number of patients with pre-capillary PH. In these patients elevation of NT-proBNP due to renal dysfunction has to be taken into account if NT-proBNP level is used for risk-stratification or even as treatment goal. According to our data, differences in NT-proBNP as predictor in ROC analysis regarding short-term clinical worsening are relatively small between patient with (1292 ng/l) and without renal insufficiency (1660 ng/l). In contrast, in patients with renal insufficiency a higher cut-off (2212 ng/l) is required for stratification of survival.
There are some limitations to our study - first and foremost the study’s retrospective design: Observational periods and medication history (i.e. use, type, and duration) are varying. Furthermore, patients with CTEPH and PAH may behave differently if PAH-specific medication is given. However, patients with inoperable CTEPH have been suggested to show overlapping pathophysiological features with PAH patients, e.g. small vessel arteriopathy regardless of thromboembolism [37]. The number of included patients in our study is relatively small. Thus, our multivariate analyses may perform in a different way than others (e.g. REVEAL). The measurement of renal function at time of right heart catheterization displays a “snap-shot” and variations over time are possible and very likely. Nevertheless, our observation reflects routine clinical management of patients with these rare diseases in a PH referral centre.
In a retrospective study with two hemodynamically matched groups of PH patients with and without renal insufficiency, we show that, although NT-proBNP levels are significantly affected by renal function, this marker correlates with relevant hemodynamic parameters and is associated with clinical outcome and survival even in patients with renal insufficiency if higher cut-off are used. Thus, in these patients NT-proBNP seems to represent valid clinical marker and facilitate clinical decision-making.
Supporting Information
Time to clinical worsening (TTCW): Receiver operating characteristic (ROC) analysis to determine the cut-off value in patients with preserved renal function (defined as glomerular filtration rate (GFR) >60 ml/min/1.73 m2; A). In Kaplan-Meier analysis, higher levels of n-terminal pro-brain natriuretic peptide (NT-proBNP) were significantly associated with early clinical worsening (B).
(PDF)
Patients’ PAH-specific medication at time of right heart catheterization.
(PDF)
Correlation of n-terminal pro-brain natriuretic peptide (NT-proBNP) with hemodynamic parameters in PH patients with and without renal insufficiency.
(PDF)
Acknowledgments
The authors are very thankful to Anja Paulsen, Ute Dickschas and Carmen Grüner for their organizational and technical support.
Funding Statement
The work was funded by the University Medical Center Hamburg-Eppendorf. Furthermore, this work was supported by a grant from the Deutsche Forschungsgemeinschaft to JKH (He 6855/1-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rabinovitch M (2012) Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 122: 4306–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, et al. (2002) Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 39: 1214–1219. [DOI] [PubMed] [Google Scholar]
- 3. Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, et al. (2000) Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 161: 487–492. [DOI] [PubMed] [Google Scholar]
- 4. McLaughlin VV, McGoon MD (2006) Pulmonary arterial hypertension. Circulation 114: 1417–1431. [DOI] [PubMed] [Google Scholar]
- 5. Cracowski JL, Leuchte HH (2012) The potential of biomarkers in pulmonary arterial hypertension. Am J Cardiol 110: 32S–38S. [DOI] [PubMed] [Google Scholar]
- 6. Battistoni A, Rubattu S, Volpe M (2012) Circulating biomarkers with preventive, diagnostic and prognostic implications in cardiovascular diseases. Int J Cardiol 157: 160–168. [DOI] [PubMed] [Google Scholar]
- 7. Hall C (2004) Essential biochemistry and physiology of (NT-pro)BNP. Eur J Heart Fail 6: 257–260. [DOI] [PubMed] [Google Scholar]
- 8. Martinez-Rumayor A, Richards AM, Burnett JC, Januzzi JL Jr (2008) Biology of the natriuretic peptides. Am J Cardiol 101: 3–8. [DOI] [PubMed] [Google Scholar]
- 9. Wu AH, Smith A, Wieczorek S, Mather JF, Duncan B, et al. (2003) Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol 92: 628–631. [DOI] [PubMed] [Google Scholar]
- 10. Srisawasdi P, Vanavanan S, Charoenpanichkit C, Kroll MH (2010) The effect of renal dysfunction on BNP, NT-proBNP, and their ratio. Am J Clin Pathol 133: 14–23. [DOI] [PubMed] [Google Scholar]
- 11. Anwaruddin S, Lloyd-Jones DM, Baggish A, Chen A, Krauser D, et al. (2006) Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. J Am Coll Cardiol 47: 91–97. [DOI] [PubMed] [Google Scholar]
- 12. Blyth KG, Groenning BA, Mark PB, Martin TN, Foster JE, et al. (2007) NT-proBNP can be used to detect right ventricular systolic dysfunction in pulmonary hypertension. Eur Respir J 29: 737–744. [DOI] [PubMed] [Google Scholar]
- 13. Souza R, Bogossian HB, Humbert M, Jardim C, Rabelo R, et al. (2005) N-terminal-pro-brain natriuretic peptide as a haemodynamic marker in idiopathic pulmonary arterial hypertension. Eur Respir J 25: 509–513. [DOI] [PubMed] [Google Scholar]
- 14. Andreassen AK, Wergeland R, Simonsen S, Geiran O, Guevara C, et al. (2006) N-terminal pro-B-type natriuretic peptide as an indicator of disease severity in a heterogeneous group of patients with chronic precapillary pulmonary hypertension. Am J Cardiol 98: 525–529. [DOI] [PubMed] [Google Scholar]
- 15. Fijalkowska A, Kurzyna M, Torbicki A, Szewczyk G, Florczyk M, et al. (2006) Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest 129: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 16. Leuchte HH, El Nounou M, Tuerpe JC, Hartmann B, Baumgartner RA, et al. (2007) N-terminal pro-brain natriuretic peptide and renal insufficiency as predictors of mortality in pulmonary hypertension. Chest 131: 402–409. [DOI] [PubMed] [Google Scholar]
- 17. Souza R, Jardim C, Julio Cesar Fernandes C, Silveira Lapa M, Rabelo R, et al. (2007) NT-proBNP as a tool to stratify disease severity in pulmonary arterial hypertension. Respir Med 101: 69–75. [DOI] [PubMed] [Google Scholar]
- 18. Bernal V, Pascual I, Esquivias P, Garcia-Gil A, Mateo JM, et al. (2009) N-terminal brain natriuretic peptide as a diagnostic test in cirrhotic patients with pulmonary arterial hypertension. Transplant Proc 41: 987–988. [DOI] [PubMed] [Google Scholar]
- 19. Mathai SC, Bueso M, Hummers LK, Boyce D, Lechtzin N, et al. (2010) Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. Eur Respir J 35: 95–104. [DOI] [PubMed] [Google Scholar]
- 20. Mauritz GJ, Rizopoulos D, Groepenhoff H, Tiede H, Felix J, et al. (2011) Usefulness of serial N-terminal pro-B-type natriuretic peptide measurements for determining prognosis in patients with pulmonary arterial hypertension. Am J Cardiol 108: 1645–1650. [DOI] [PubMed] [Google Scholar]
- 21. Frantz RP, McDevitt S, Walker S (2012) Baseline NT-proBNP correlates with change in 6-minute walk distance in patients with pulmonary arterial hypertension in the pivotal inhaled treprostinil study TRIUMPH-1. J Heart Lung Transplant 31: 811–816. [DOI] [PubMed] [Google Scholar]
- 22. Yeager ME, Nguyen CM, Belchenko DD, Colvin KL, Takatsuki S, et al. (2012) Circulating fibrocytes are increased in children and young adults with pulmonary hypertension. Eur Respir J 39: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nickel N, Golpon H, Greer M, Knudsen L, Olsson K, et al. (2012) The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 39: 589–596. [DOI] [PubMed] [Google Scholar]
- 24. Suntharalingam J, Goldsmith K, Toshner M, Doughty N, Sheares KK, et al. (2007) Role of NT-proBNP and 6MWD in chronic thromboembolic pulmonary hypertension. Respir Med 101: 2254–2262. [DOI] [PubMed] [Google Scholar]
- 25. Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, et al. (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 26. ATS statement (2002) Guidelines for the six-minute walk test. Am J Respir Crit Care Med 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 27. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, et al. (2003) National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139: 137–147. [DOI] [PubMed] [Google Scholar]
- 28. Galasko GI, Lahiri A, Barnes SC, Collinson P, Senior R (2005) What is the normal range for N-terminal pro-brain natriuretic peptide? How well does this normal range screen for cardiovascular disease? Eur Heart J 26: 2269–2276. [DOI] [PubMed] [Google Scholar]
- 29. Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, et al. (2012) The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 141: 354–362. [DOI] [PubMed] [Google Scholar]
- 30. Haddad F, Fuh E, Peterson T, Skhiri M, Kudelko KT, et al. (2011) Incidence, correlates, and consequences of acute kidney injury in patients with pulmonary arterial hypertension hospitalized with acute right-side heart failure. J Card Fail 17: 533–539. [DOI] [PubMed] [Google Scholar]
- 31. Mielniczuk LM, Chandy G, Stewart D, Contreras-Dominguez V, Haddad H, et al. (2012) Worsening renal function and prognosis in pulmonary hypertension patients hospitalized for right heart failure. Congest Heart Fail 18: 151–157. [DOI] [PubMed] [Google Scholar]
- 32. Sato Y (2013) Diagnostic and prognostic property of NT-proBNP in patients with renal dysfunction. Journal of cardiology 61: 446–447. [DOI] [PubMed] [Google Scholar]
- 33. van Kimmenade RR, Januzzi JL Jr, Baggish AL, Lainchbury JG, Bayes-Genis A, et al. (2006) Amino-terminal pro-brain natriuretic Peptide, renal function, and outcomes in acute heart failure: redefining the cardiorenal interaction? J Am Coll Cardiol 48: 1621–1627. [DOI] [PubMed] [Google Scholar]
- 34. Horii M, Matsumoto T, Uemura S, Sugawara Y, Takitsume A, et al. (2013) Prognostic value of B-type natriuretic peptide and its amino-terminal proBNP fragment for cardiovascular events with stratification by renal function. Journal of cardiology 61: 410–416. [DOI] [PubMed] [Google Scholar]
- 35. Gan CT, McCann GP, Marcus JT, van Wolferen SA, Twisk JW, et al. (2006) NT-proBNP reflects right ventricular structure and function in pulmonary hypertension. Eur Respir J 28: 1190–1194. [DOI] [PubMed] [Google Scholar]
- 36. McLaughlin VV, Gaine SP, Howard LS, Leuchte HH, Mathier MA, et al. (2013) Treatment goals of pulmonary hypertension. J Am Coll Cardiol 62: D73–81. [DOI] [PubMed] [Google Scholar]
- 37. Humbert M (2010) Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: pathophysiology. Eur Respir Rev 19: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time to clinical worsening (TTCW): Receiver operating characteristic (ROC) analysis to determine the cut-off value in patients with preserved renal function (defined as glomerular filtration rate (GFR) >60 ml/min/1.73 m2; A). In Kaplan-Meier analysis, higher levels of n-terminal pro-brain natriuretic peptide (NT-proBNP) were significantly associated with early clinical worsening (B).
(PDF)
Patients’ PAH-specific medication at time of right heart catheterization.
(PDF)
Correlation of n-terminal pro-brain natriuretic peptide (NT-proBNP) with hemodynamic parameters in PH patients with and without renal insufficiency.
(PDF)