Abstract
Objectives
The co-occurrence of rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) is well described in rheumatoid arthritis (RA). However, the mechanisms underlying the potential interaction between these two distinct autoantibodies has not been well defined. We sought to evaluate the epidemiologic and molecular interaction of ACPA and RF with both disease activity as well as measures of RA-associated inflammation.
Methods
In a cohort of 1,488 Veterans with RA, measures of disease activity and levels of serum cytokines and multiplex ACPA were compared between the double-negative (aCCP-/RF-), ACPA-positive and RF-negative (aCCP+/RF-), ACPA-negative and RF-positive (aCCP-/RF+), or double-positive (aCCP+/RF+) subgroups. Studies were additionally performed using an in vitro immune complex (IC) stimulation assay in which macrophages were incubated with ACPA-ICs in the presence or absence of monoclonal IgM RF, and TNFα production measured as readout of macrophage activation.
Results
Compared with the double negative (as well each single positive) subgroup, the aCCP+/RF+ subgroup exhibited higher disease activity, serum CRP, and inflammatory cytokines (all P<0.001). In vitro stimulation of macrophages by ACPA-ICs induced increased cytokine production with the addition of monoclonal IgM RF as compared to ACPA-ICs alone (P=0.003)
Conclusions
The combined presence of ACPA and IgM RF mediates increased proinflammatory cytokine production in vitro, and is associated with increased systemic inflammation and disease activity in RA. Our data suggest that IgM RF enhances the capacity of ACPA-ICs to stimulate macrophage cytokine production, thereby providing a mechanistic link by which RF enhances the pathogenicity of ACPA-ICs in RA.
Introduction
Rheumatoid arthritis (RA) is often characterized by the presence of circulating autoantibodies. Described over 50 years ago1-3, rheumatoid factor (RF) is detectable in nearly 70% of patients although its presence is not specific for RA 4 5. Nevertheless, whether RF plays a role in RA pathogenesis has remained unclear. More recently, attention has been given to anti-citrullinated protein antibodies (ACPA). Commonly tested for using proprietary anti-cyclic citrullinated peptide (aCCP) assays, ACPA are detectable in approximately 70% of RA patients and are highly specific to RA6. In addition to their use in diagnostic criteria, RF and ACPA portend important prognostic information.
Multiple studies have examined the diagnostic utility of RF and ACPA 7 8, and multiple studies have demonstrated that higher concentrations of both autoantibodies are associated with a more aggressive disease course marked by increased disease activity and reduced rates of remission 9-11. However, there has been little investigation into the mechanisms by which these autoantibodies could interact and/or contribute to RA pathogenesis. Although several in vitro 12-14 and in vivo 15 studies have identified a potential role for ACPA in disease pathogenesis, the role of RF in RA pathogenesis remains elusive. In this study we sought to investigate the role of RF as a contributor to RA inflammatory burden both independently and in synergy with ACPA.
Materials and Methods
Patient samples and clinical measures
Study subjects included U.S. Veterans enrolled in the Veterans Affairs Rheumatoid Arthritis (VARA) registry with sites across the U.S. 16. The registry has received Institutional Review Board approval at each site, and the Stanford Institutional Review Board approved the biomarker and in vitro analyses performed on RA samples. All patients satisfied 1987 American College of Rheumatology (ACR) classification criteria for RA 17 and provided informed written consent and HIPAA authorization. Subjects included in this study include a cohort of 1,488 Veterans with RA (89% male), and detailed demographics are presented in Table 1. Banked serum was available for a representative population of 1,466 subjects and was used for multiplex cytokine and autoantibody analysis.
Table 1.
Characteristics of RA Study Patients at the Time of Study Enrollment.
| Concordant seronegative n = 206 |
RF-/ aCCP+ n = 102 |
RF+/ aCCP- n = 134 |
Concordant seropositive n = 1,046 |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean or % | S.D. | Mean or % | S.D. | Mean or % | S.D. | Mean or % | S.D. | |
| Sociodemographics and comorbidity | ||||||||
| Age, yrs | 64.5 | 12.2 | 61.2 | 11.9 | 63.3 | 11.9 | 63.0 | 11.2 |
| Male gender | 89.3 | 88.2 | 91.8 | 91.1 | ||||
| Race / ethnicity | ||||||||
| Caucasian | 79.6 | 75.5 | 76.9 | 76.7 | ||||
| African American | 15.1 | 16.7 | 15.7 | 16.7 | ||||
| Other | 5.3 | 7.8 | 7.5 | 6.6 | ||||
| ≥ High school education | 86.7 | 86.7 | 85.6 | 84.0 | ||||
| Comorbidity count | 2.6 | 1.6 | 2.2 | 1.5 | 2.4 | 1.5 | 2.3 | 1.6 |
| RA factors | ||||||||
| Ever smoking** | 73.2 | 73.4 | 73.9 | 82.7 | ||||
| HLA-DRB1 SE pos.** | 54.6 | 77.8 | 54.4 | 76.5 | ||||
| HLA-DRB1 SE – 2 alleles** | 8.7 | 23.2 | 6.5 | 24.7 | ||||
| HLA-DR3** | 25.6 | 14.1 | 32.6 | 12.8 | ||||
| Age at diagnosis, yrs** | 55.9 | 15.0 | 50.7 | 13.7 | 53.8 | 13.7 | 51.8 | 13.6 |
| Disease duration, yrs* | 8.7 | 10.1 | 10.5 | 10.7 | 9.5 | 10.7 | 11.2 | 11.4 |
| Prednisone use | 35.0 | 40.7 | 40.7 | 45.2 | ||||
| Methotrexate use* | 45.4 | 64.8 | 51.2 | 49.7 | ||||
| Biological use | 16.9 | 24.2 | 23.6 | 21.1 | ||||
| Nodules** | 12.1 | 24.5 | 26.1 | 33.8 | ||||
overall ANOVA; P<0.01;
P<0.001
In addition to banked sera, VARA collects clinical data including baseline and longitudinal DAS28 scores, as well as baseline measurement of ACPA using the second generation CCP2 antibody ELISA (Diastat, Axis-Shield Diagnostics Ltd., UK, positivity ≥ 5 U/ml). VARA also collects RF test (positivity ≥ 15 IU/ml) and high sensitivity C-reactive protein (hs-CRP) both as determined by nephelometry (Siemens Healthcare Diagnostics, Germany). Hs-CRP concentrations were not available at follow-up visits. While VARA is a multicenter project, standardized autoantibodies are measured in a single investigator's laboratory (GMT). HLA-DRB1 genotyping was conducted as previously described18. Patients were categorized as being positive or negative for HLA-DRB1 shared epitope-containing alleles and HLA-DR3 alleles.
Follow-up measures include tender and swollen joint counts (0-28), erythrocyte sedimentation rate (ESR, mm/h), pain (0-10), multidimensional Health Assessment Questionnaire (MD-HAQ, 0-3) 19, patient and provider global assessments (0-100), and treatments. A comorbidity count (0-9) was calculated for each patient using administrative codes 20. Because we were investigating the association of autoantibodies with follow-up clinical parameters, patients were excluded if: autoantibody or 28-joint Disease Activity Score (DAS28) data was unavailable; only a single clinical observation was recorded; or if the total follow-up duration was < 6 months.
Multiplex cytokine analysis
Multiplex analysis of cytokines and chemokines in human serum was performed by using the 17 cytokine Bio-Plex ProHuman Cytokine Assay (BioRad) run on the Luminex 200 system according to the manufacturer's instructions with the exception that a proprietary BioRad assay dilution buffer was modified to contain reagents demonstrated to reduce the effects of heterophilic antibodies in multiplex immunoassays as previously described 21. Data processing was performed by using Bio-Plex Manager 5.0, and analyte concentrations (in picograms per milliliter) were interpolated from standard curves.
Multiplex ACPA antigen arrays
Antibodies targeting 37 putative rheumatoid arthritis-associated autoantigens were measured using a custom bead-based immunoassay on the BioPlex platform as previously described 21 22. Of the 37 antigens, 30 are citrullinated and 7 are native (native histone 2A, histone 2B, ApoA1, filaggrin 48-65 peptide, vimentin, fibrinogen, and ApoA1 231-248 peptide). Briefly, serum was diluted and mixed with spectrally distinct florescent beads conjugated with putative rheumatoid arthritis-associated autoantigens followed by incubation with anti-human phycoerythrin antibody and analysis on a Luminex 200 instrument.
Data analysis
Patients were categorized into subgroups including double-negative (aCCP-/RF-; n=204), aCCP+/RF- (n=96), aCCP-/RF+ (n=135), or double-positive (aCCP+/RF+; n=1031). Comparisons of patient characteristics were examined by autoantibody subgroup using chi-square tests or analysis of variance (ANOVA). Unadjusted comparisons of the 8 continuous disease activity measures assessed at enrollment were examined using one-way ANOVA with Tukey's post-test to compare each of the 4 subgroups. Levels of each cytokine as well as hs-CRP, were compared between the double-negative, aCCP+/RF-, aCCP-/RF+, or double-positive subgroups using the Kruskal Wallis test with Dunn's post-test. ESR was log-transformed prior to analysis to render a more normal distribution. Given skewed distributions, joint counts were dichotomized into 0 tender/swollen joints and ≥ 1 tender/swollen joints, with comparisons examining the probability of having a joint count > 0. Continuous joint counts were reported for descriptive purposes.
We examined whether the associations observed between autoantibody status and disease activity at enrollment were independent of other covariates and whether these associations were apparent over an extended period of observation. For multivariable analyses, concordant negative cases served as the referent population. Generalized linear mixed models (GLMM) were used to evaluate multivariable associations of autoantibody group with disease activity assessed over follow-up. The GLMM models adjusted for the random effects from sites and correlations between the outcomes from the same patient over time via a compound symmetry correlation structure. Analyses were completed using STATA v12 (StataCorp, College Station, TX), SAS v9.3 (SAS Institute, Cary, NC), and Prism GraphPad v5 (GraphPad Software, La Jolla, CA).
Additional comparisons were performed on multiplex cytokine as well as multiplex analysis of autoantibodies using SAM (Significance Analysis of Microarrays (SAM) Version 3.08)23. Output was sorted based on false discovery rates (FDRs) in order to identify antigens with the greatest differences in autoantibody reactivity between serologic subgroups. The use of FDRs obviates the need to adjust for multiple comparisons. Hierarchical clustering was performed using Cluster® 3.0 to arrange the SAM results according to similarities among autoantibody specificities, and results were displayed using Java Treeview® (Version 1.1.3). For all heatmap displays, values are reported as fluorescence intensity relative to the average of the evaluated cohort.
In vitro ACPA-IC stimulation assays
ACPA-IC stimulation of human macrophages was performed as previously described12. Rabbit polyclonal anti-fibrinogen IgG (Pierce) or human IgG derived from patients with ACPA-positive RA used to generate plate-bound citrullinated fibrinogen-containing immune complexes (cFb-IC). RA-IgG was from 3 pooled plasma samples shown by ELISA to contain high levels of anti-cFb antibodies was purified by affinity chromatography on protein G columns, according to the instructions of the manufacturer (Pierce).
Monoclonal IgM RF derived from a lymphoblastoid cell line generated from a RA patient (as previously described) 24 was a generous gift of Drs. Michael Steinitz and Reuven Laskov (Hebrew University, Jerusalem, Israel). IgM-RF was isolated from cell culture supernatant by ammonium sulfate precipitation, washed, and dialyzed against PBS. The resultant product was found to contain primarily IgM with no contamination of human IgG. This antibody has been demonstrated to bind human and rabbit IgG but not IgM and, when bound to immune complexes, fails to bind complement24. Additional monoclonal IgM antibodies with in vitro RF activity isolated from patients with mixed cryoglobulinemia were generously provided by Dr. Mariana Newkirk (McGill University, Montreal, Canada).
The purified RA-IgG and monoclonal RF were separately concentrated by centrifugation over a 100 kD molecular weight filter column with buffer exchange to PBS (Amicon Ultra; Millipore) and were depleted of endotoxin by filtration through a polymyxin B column (Pierce Detoxigel). RA-IgG and RF IgM concentrations were estimated according to optical density at 280 nm, were aliquoted, and stored at -80°C. For generation of cFb-IC, flat-bottomed 96-well culture plates were coated overnight at 4°C with 50 µl of cFb (20 µg/ml), washed in PBS containing 0.05% Tween 20, and then incubated for 2 hours at 4°C with 100 µl of rabbit polyclonal anti-fibrinogen antibody (50 ug/ml), 100 ul of anti-cFb–positive IgG (10 mg/ml), or, 100 ul of anti-cFb-positive IgG preincubated with monoclonal IgM RF (stock concentration 5 mg/ml used at 1:20 or 1:100 dilution) or, as a control, with PBS alone. Wells were again washed in PBS containing 0.05% Tween 20, and macrophages (50,000/well) in 200 µl RMPI containing 5% FCS were then added to the wells and incubated for 16 hours at which time levels of TNF in culture supernatant were measured by ELISA (Peprotech). All in vitro experiments were performed in triplicate and in at least 2 separate experiments. Levels of TNFα production from in vitro macrophage stimulation assays were compared using an unpaired Students T-test.
Results
ACPA and RF in VARA Cohort
Patient (n = 1,488) characteristics are summarized in Table 1. Patients had a mean follow-up of 3.6 ± 2.8 years with 16,822 encounters and 5,284 patient-years of observation. A majority were males (91%) with a mean age of ∼63 years. Most (70%) were concordant for exhibiting positive RF and aCCP antibodies, with an additional 14% being concordant negative for aCCP and RF antibodies. Nine percent were aCCP-/RF+ and 6.9% were aCCP+/RF-. There were significant differences across autoantibody groups in: age at diagnosis, disease duration, the presence of nodules, and methotrexate use. Compared to the others, concordant negative patients were older at diagnosis, had shorter disease durations, a lower prevalence of nodules, and were less likely to be receiving methotrexate.
HLA-DRB1 shared epitope, HLA-DR3, and smoking frequencies are shown in Table 1. Ever smoking was more common among concordant autoantibody positive cases (83%) compared to others (73-74%; p < 0.001). HLA-DRB1 SE positivity was higher in groups characterized by aCCP positivity (P < 0.001), irrespective of RF. HLA-DR3 positivity was less common in those with a positive aCCP antibody (P < 0.001 across groups). The average titer for anti-CCP2 was nearly identical in the CCP+/RF- and the CCP+/RF+ (275.85 AU vs. 273.64 AU), and similarly the average RF titer in the CCP-/RF+ group was nearly identical to the average RF titer in the CCP+/RF+ group (329.03 IU vs. 328.12 IU).
Concurrent presence of ACPA and RF is associated with increased RA disease activity
Compared with the double-negative subgroup, the double-positive subgroup (aCCP+/RF+; possessing both ACPA and RF) exhibited significantly higher levels of RA disease activity with a mean baseline DAS28 score of 4.2 (±1.6) as compared with 3.7 (±1.6) in the double-negative, 3.4 (±1.6), in the APCA single positive, and 3.9 (±1.7) in the RF single positive (Overall ANOVA, P<0.001) (Table 2). After adjusting for multiple comparisons across 4 groups, ESR, hs-CRP, and DAS28 values at the time of enrollment were higher in the concordant positive group compared to others. Post-test comparisons between each pair of groups demonstrated a significantly higher levels ESR and CRP among the concordant seropositive (aCCP+/RF+) compared to the double negative, aCCP+/RF- or aCCP-/RF+ and DAS28 was significantly higher in the aCCP+/RF+ subgroup compared to the double negative and CCP+/RF- subgroup with a non-significant trend compared to the aCCP-/RF+ subgroup.
Table 2. Measures of disease activity in rheumatoid arthritis patients at the time of study enrollment by autoantibody status.
| Concordant seronegative n = 206 |
RF-/ aCCP+ n = 102 |
RF+/ aCCP- n = 134 |
Concordant seropositive n = 1,046 |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| Pain score (0-10) | 5.1 | 2.8 | 4.3 | 2.7 | 4.8 | 2.7 | 4.8 | 2.8 |
| MD-HAQ (0-3) | 0.94 | 0.63 | 0.81 | 0.62 | 0.96 | 0.58 | 0.98 | 0.60 |
| Tender joints (0-28) | 6.0 | 7.5 | 4.5 | 7.0 | 5.5 | 6.7 | 6.3 | 7.3 |
| Swollen joints (0-28) | 4.1 | 5.4 | 4.0 | 5.7 | 5.3 | 6.6 | 5.8 | 6.4 |
| Patient global (0-100) | 45.1 | 27.0 | 35.8 | 23.2 | 43.4 | 26.1 | 43.8 | 25.8 |
| Provider global (0-100) | 39.9 | 24.5 | 29.1 | 19.8 | 32.0 | 25.2 | 37.8 | 23.3 |
| ESR, mm/hr a | 19.4 | 19.6 | 21.2 | 21.9 | 24.8 | 24.7 | 30.2 | 24.0 |
| CRP, mg/L b | 0.92 | 1.78 | 0.92 | 0.74 | 1.20 | 1.78 | 1.36 | 2.13 |
| DAS-28 c | 3.7 | 1.6 | 3.4 | 1.6 | 3.9 | 1.7 | 4.2 | 1.6 |
Note. MD-HAQ = Multidimensional Health Assessment Questionnaire, ESR = erythrocyte sedimentation rate, DAS = Disease Activity Score; Group comparisons used transformation of outcome variable (joint counts dichotomized as 0 vs. > 0 and ESR log-transformed); statistical significance defined as P value < 0.00625
overall ANOVA P value < 0.001, p-value for concordant seropositive vs. concordant seronegative < 0.001, P value for concordant seropositive vs. discordant RF+ = 0.007, P value for concordant seropositive vs. discordant aCCP+ = 0.001.
overall ANOVA P=value <0.001, P value for concordant seropositive vs. concordant seronegative < 0.001, p-value for concordant seropositive vs. discordant RF+ = 0.01, P value for concordant seropositive vs. discordant aCCP+ = 0.<001.
overall ANOVA P value < 0.001, p-value for concordant seropositive vs. concordant seronegative = 0.001, P value for concordant seropositive vs. discordant aCCP+ = 0.001.
Similarly, in multivariable models, the presence of dual aCCP+/RF+ autoantibody status was associated with longitudinal measurements of clinical and laboratory measures of disease activity including swollen joint count, ESR, and DAS28 when compared to the double negative or aCCP+/RF- subgroups while only a similar trend was noted when compared to the aCCP-/RF+ subgroup (Table 3).
Table 3. Multivariable associations of dual anti-CCP/RF autoantibody status with measures of disease activity in rheumatoid arthritis patients over follow-up (referent to concordant seronegative patients)*.
| Discordant RF-/ aCCP+ |
Discordant RF+/ aCCP- |
Concordant RF+/ aCCP+ |
||||
|---|---|---|---|---|---|---|
| β-Coefficient | P value | β-Coefficient | P value | β-Coefficient | P value | |
| Pain score (0-10) | -0.489 | 0.094 | -0.008 | 0.972 | -0.288 | 0.097 |
| MD-HAQ (0-3) | -0.161 | 0.034 | -0.000 | 0.996 | -0.015 | 0.725 |
| Tender joints (0 vs. > 0) | -0.251 | 0.181 | 0.326 | 0.042 | 0.052 | 0.624 |
| Swollen joints (0 vs. > 0) a | 0.100 | 0.562 | 0.619 | <0.001 | 0.507 | <0.001 |
| Patient global (0-100mm) | -6.088 | 0.015 | -0.212 | 0.925 | -1.588 | 0.315 |
| Provider global (0-100mm) | -3.804 | 0.069 | 2.597 | 0.191 | 2.418 | 0.069 |
| Log-ESR, mm/hr b | 0.444 | <0.001 | 0.410 | <0.001 | 0.652 | <0.001 |
| DAS-28 c | -0.003 | 0.987 | 0.387 | 0.005 | 0.418 | <0.001 |
Multivariable models adjusted for age, gender, disease duration, comorbidity score, race/ethnicity, and treatments including the use of methotrexate, prednisone, or biologicals
P value for concordant seropositive vs. discordant RF+ = 0.426, P value for concordant seropositive vs. discordant aCCP+ = 0.008.
p-value for concordant seropositive vs. discordant RF+ = 0.005, P value for concordant seropositive vs. discordant aCCP+ = 0.036.
p-value for concordant seropositive vs. discordant RF+ = 0.786, P value for concordant seropositive vs. discordant aCCP+ = 0.002.
Concurrent presence of ACPA and RF is predictive of RA-associated inflammation
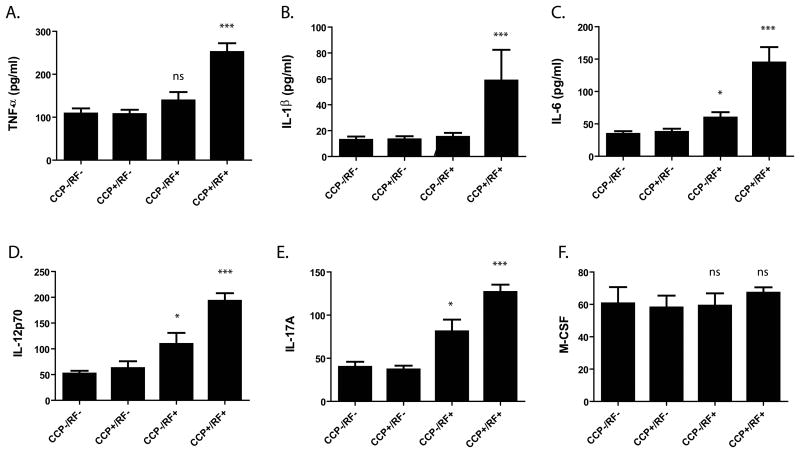
Compared with the double-negative subgroup, the double-positive subgroup (aCCP+/RF+) exhibited significantly higher levels of ESR, hs-CRP as well as multiple circulating inflammatory cytokines including TNFα, IL-1β, IL-6, IL12p70, and IL-17A (Figure 1; all P<0.001 by ANOVA). Although it is likely that many cytokines, including the ones most upregulated in the concordant aCCP+/RF+ subgroups, are highly related and thus not subject to a high risk for type I error with multiplex cytokine analysis, stringent implementation of Bonferroni's correction for 17 potentially independent cytokines would require a P value of 0.0029 to declare significance and this is achieved for all the cytokines represented in Figure 1.
Figure 1. RF and ACPA double-positive RA patients exhibit higher serum levels of RA-associated cytokines.
Patients were categorized into subgroups including double-negative (aCCP-/RF-; n=204), aCCP+/RF- (n=96), aCCP-/RF+ (n=135), or double-positive (aCCP+/RF+; n=1031) and levels of serum cytokines measured by multiplex immunoassay. Comparisons between groups were performed using the Kruskal Wallis test with Tukey's multiple comparison post-test. Note is made of significantly elevated levels of many (A-E), but not all (F), RA-associated serum cytokines *P<0.01, **P<0.001. ***P<0.0001. Statistics reported in the figure are in comparison to the double-negative subgroup.
Additionally, levels for all cytokines were compared between the double negative (aCCP-/RF-), aCCP-/RF+, and a set of concordant seropositive (aCCP+/RF+) patients matched by age, gender, and disease duration. Three-way group comparisons were made using SAM (Significance Analysis of Microarrays (SAM) Version 3.08)23 and output was sorted based on false discovery rates (FDRs). Notably, use of FDR obviates the need to adjust for multiple comparisons. Supplemental Figure 1 is a heatmap again demonstrating significant upregulation in 10 of 17 cytokines in the aCCP+/RF+ subgroup compared to the aCCP-/RF-, as well as compared to both the the aCCP+/RF- and aCCP-/RF+ subgroups.
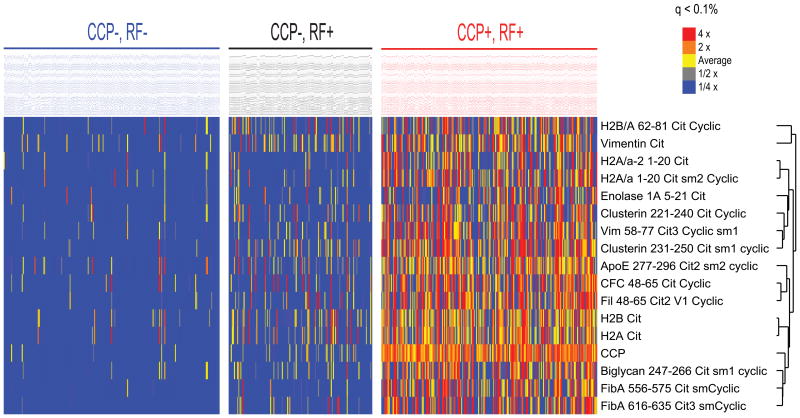
A smaller but, in many cases statistically significant, elevation in cytokines (as well as some clinical measures of disease activity) was noted in the RF single positive subgroup compared to the double-negative and aCCP+/RF- subgroups. We hypothesized that elevations among the aCCP-/RF+ subgroup may reflect the presence in some subjects of physiologic ACPA but not detected by or below the range of the commercial anti-CCP2 ELISA. To support this hypothesis, we used a multiplex antigen array to assess for ACPA sub-specificities which might be present in the aCCP-/RF+ subgroup. Figure 2 demonstrates that 11 of 30 ACPA (but none of the non-citrullinated controls) were elevated among the aCCP-/RF+ subjects as compared to the aCCP-/RF- subgroup. Thus, we hypothesize that low levels of ACPA which are not strongly represented by the anti-CCP2 assay may synergize with RF to induce RA-associated inflammation.
Figure 2. Increased ACPA are identified among the aCCP-/RF+ cohort compared to the concordant double-negative (aCCP-/RF-) population.
Levels of autoantibodies against 37 putative targets of the RA immune response compared between the aCCP+/RF- (n=96) and aCCP-/RF+ (n=135) using a multiplex antigen microarray. Using SAM, output was sorted based on false discovery rates (FDRs) in order to identify antigens with the greatest differences in autoantibody reactivity between serologic subgroups. Values are reported as fluorescence intensity relative to the average of the evaluated cohort.
Monoclonal RF augments the stimulatory capacity of ACPA-immune complexes
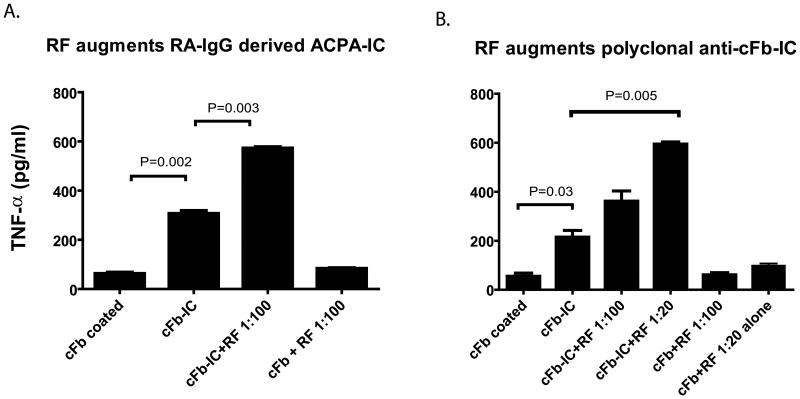
We previously demonstrated the ability of ACPA-ICs to stimulate macrophage cytokine production via co-stimulation of FcγR and the innate immune receptor TLR412. To evaluate the effect of RF on ACPA, we pre-incubated ACPA-containing RA-IgG with monoclonal IgM RF before formation of ACPA-IC. As previously described, in vitro stimulation of monocyte-derived macrophages by ACPA-ICs demonstrated increased cytokine production (compared with cFb alone P=0.002) and the addition of monoclonal IgM RF resulted in a further significant increase in macrophage TNFα production as compared to ACPA-ICs alone (P=0.003) (Figure 2A). Notably, we additionally tested 2 monoclonal IgM antibodies with in vitro RF activities which were isolated from patients with mixed cryoglobulinemia. However, in our assay, addition of these IgM antibodies at 20 ug/ml, 100 ug/ml, and 200 ug/ml failed to augment macrophage TNF secretion in response to ACPA-ICs (data not shown).
Given the potential inclusion of IgG (or IgM) RF in our ACPA positive IgG preparation, we further investigated the ability of monoclonal IgM RF to enhance the stimulatory activity of anti-citrullinated fibrinogen ICs, this time formed with a rabbit polyclonal anti-fibrinogen antibody previously demonstrated to bind native and citrullinated fibrinogen12 and which could be targeted by our monoclonal IgM RF by ELISA and western blot (data not shown). In results analogous to those in previous studies, we observed the ability of anti-citrullinated fibrinogen ICs to stimulate macrophage cytokine production and, as with human RA-derived IgG preparations, we observed the ability of monoclonal IgM RF to augment anti-citrullinated fibrinogen IC-induced macrophage activation as evidenced by increased cytokine production (Figure 2B).
Discussion
Though there are several studies demonstrating increased disease activity in the presence of either aCCP or RF, to our knowledge, this is the first study identifying a synergistic role for ACPA and RF in mediating RA-associated inflammation and disease activity. We have shown that baseline autoantibody status was associated with select measures of disease activity, both at presentation and over time. Specifically, joint swelling and higher baseline DAS28 values were more commonly observed in the concordant seropositive RA population and, to a lesser extent RF positivity, regardless of aCCP status. Interestingly, ESR values were similarly increased in all autoantibody positive groups (both concordant positive and discordant patients) as compared to seronegative patients even after accounting for the numerically higher frequency of RA-related treatments used by seropositive cases; however, values were further increased in the concordant seropositive population as compared to the each single positive subgroup. The observed increases in disease activity and clinical inflammatory markers are similarly paralleled by a nearly identical pattern of elevation among several inflammatory cytokines previously associated with RA pathophysiology25 including those we and others have previously demonstrated to be produced in response to stimulation of macrophage by ACPA ICs12 13.
The increased inflammation and disease activity observed with the concordant double-seropositive subgroup is supportive of a potential role for these RA-associated autoantibodies in mediating the pathogenesis of RA. However, association doesn't prove causality. Thus we utilized an in vitro model of ACPA-IC mediated inflammation to identify a novel mechanism by which the interaction of ACPA and RF may act in concert, together contributing to the pathophysiology of RA inflammation and disease activity. We thus demonstrate a unifying mechanism by which the two overtly distinct autoantibody types which characterize RA can interact to promote RA disease pathogenesis.
First identified by the ability of RA serum to agglutinate IgG coated sheep red blood cells and subsequently defined as an immunoglobulin targeting the Fc portion of IgG3, RF is now considered characteristic for the presence of RA, and has been associated with increased disease severity9 11. However, the pathogenic role of RF has been questioned and, to date, poorly defined. In 1961, Ragan stated “the significance of rheumatoid factor in the pathogenesis of rheumatoid arthritis remains conjectural”3. Over 50 years later, our understanding has progressed little. Previous studies have suggested the ability of RF accelerate experimental models of immune complex mediated vascular damage26, and although there is evidence for the ability of RF to fix complement27 28 other studies to suggest that RF may in fact prevent complement activation by IgG immune complexes thus leading the authors to speculate that the effects of RF are in fact mediated by attachment to the Fc region of the IgG molecule rather than complement activation29. As such, this study offers, to our knowledge, the first mechanistic role for RF in RA disease propagation. Additionally, it has long been known that RF is present in the setting of non-RA immune activation, including most notably, viral and bacterial infections. Prior studies have identified the ability of RF-expressing B cells to recognize ICs (but not monomeric IgG) and to present the retained antigen to T cells30, thus enhancing the immune response to foreign antigen. Thus the generation of RF may have a teleological purpose in its potential ability stabilize protective immune complexes. We propose that soluble RF may have developed to offer a protective evolutionary advantage against infectious pathogens. As the immune response associated with RA and similar autoimmune conditions is also associated with the presence of RF, we hypothesize that inflammatory disease pathogenesis has co-opted the beneficial role of IC stabilization, thus enhancing the pathogenic capacity of disease-associated autoantibodies and resultant ICs.
There are several limitations to this study. Given the large proportion of older men examined, reflective of current VA beneficiaries, caution should be used before applying these results to a broader RA patient population. Also, given reports highlighting the pathogenic role that ACPA might play in propagating RA-related joint damage14, further analyses examining the relationship of autoantibody status with clinical and radiographic outcomes in RA is important to examine. Finally despite use of methods optimized to minimize the effects of heterophilic antibodies, our laboratory studies do not eliminate the potential of heterophiic antibodies such as RF to induce erroneously elevated signal in sandwich immunoassays31 32. Thus, there remains a risk that the observed elevation of cytokines in the concordant seropositive subgroup is in part a result of such bias. However, the relative lack of signal in the RF single positive subgroup, as well as supporting clinical and ESR data, argue against a significant contribution of such confounding in this study. Finally, there remains great complexity and heterogeneity of IgM RF. Though most monoclonal RF characterized to date target the Cγ2–Cγ3 interface in the Fc region on the IgG molecule33, the variations in exact binding sites as well as the role of various post-translational modifications34 35 to effect RF binding, as well as the typically polyclonal nature of RF in vivo, may limit the generalizability of our IC data generated with a single monoclonal IgM RF.
In summary, our results demonstrate a significantly increased level of disease activity as well as increased markers of systemic inflammation associated with the concordant presence of both RF and ACPA in multivariate analysis accounting for a variety of other potentially associated clinical variables. In this manuscript, we demonstrate a mechanism by which the IgM RF/ACPA interaction may directly contribute to RA disease pathogenesis. These results provide not only useful information for prognosticating RA severity, but, by identifying a mechanistic interaction between ACPA and IgM RF, advance our understanding of the role of RA-associated autoantibodies in mediating the pathogenesis of RA.
Supplementary Material
Supplemental Figure 1. Concordant seropositive (aCCP+/RF+) RA patients exhibit higher serum levels of serum cytokines than either the double negative (aCCP-/RF-) or the aCCP-/RF+ subgroups. A subgroup of aCCP+/RF+ patients was matched to aCCP-/RF- by age, gender, and disease duration (n=204). Levels of 17 cytokines were compared across groups and SAM output was sorted based on false discovery rates (FDRs) in order to identify antigens with the greatest differences in autoantibody reactivity between the three serologic subgroups. Values are reported as fluorescence intensity relative to the average of the evaluated cohort. SAM identified significant elevation of 10 of 17 cytokines among the concordant seropositive subgroup compared to either the aCCP-/RF+ (n=135) or aCCP-/RF- subgroups (n=204).
Supplemental Table 1: Average anti-CCP2 and RF titers by subgroup
Figure 3. IgM rheumatoid factor augments macrophage activation by ACPA immune complexes in vitro.
A, Human monocyte-derived macrophages were added to plates pre-coated with human cFb-IC formed in the presence or absence of monoclonal IgM RF and macrophage stimulation assessed as measured by the level of TNFα in harvested cell culture supernatants. B, Human monocyte–derived macrophages were added to plates pre-coated with cFb-IC formed using polyclonal rabbit anti-human fibrinogen antibody in the presence or absence of monoclonal IgM RF, and macrophage stimulation assessed as measured by the level of TNFα in harvested cell culture supernatants. Results are representative of experiments performed at least twice. Values are the mean/SEM from triplicate cultures.
Acknowledgments
This work was funded by NIH NIAMS R01- AR0636763 and U01-AI101981 (WHR), Department of Veterans Affairs Research funding (JS, TRM, WHR) American College of Rheumatology REF Within-Our-Reach Award (TRM, WHR), and an Arthritis Foundation Innovative Research Award (JS).
References
- 1.Rose HM, Ragan C, et al. Differential agglutination of normal and sensitized sheep erythrocytes by sera of patients with rheumatoid arthritis. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1948;68(1):1–6. doi: 10.3181/00379727-68-16375. [DOI] [PubMed] [Google Scholar]
- 2.Franklin EC, Holman HR, Muller-Eberhard HJ, Kunkel HG. An unusual protein component of high molecular weight in the serum of certain patients with rheumatoid arthritis. J Exp Med. 1957;105(5):425–38. doi: 10.1084/jem.105.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ragan C. The history of the rheumatoid factor. Arthritis Rheum. 1961;4:571–3. doi: 10.1002/art.1780040602. [DOI] [PubMed] [Google Scholar]
- 4.Nell VP, Machold KP, Stamm TA, Eberl G, Heinzl H, Uffmann M, et al. Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Ann Rheum Dis. 2005;64(12):1731–6. doi: 10.1136/ard.2005.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146(11):797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 6.Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43(1):155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Greiner A, Plischke H, Kellner H, Gruber R. Association of anti-cyclic citrullinated peptide antibodies, anti-citrullin antibodies, and IgM and IgA rheumatoid factors with serological parameters of disease activity in rheumatoid arthritis. Ann N Y Acad Sci. 2005;1050:295–303. doi: 10.1196/annals.1313.031. [DOI] [PubMed] [Google Scholar]
- 8.Silveira IG, Burlingame RW, von Muhlen CA, Bender AL, Staub HL. Anti-CCP antibodies have more diagnostic impact than rheumatoid factor (RF) in a population tested for RF. Clin Rheumatol. 2007;26(11):1883–9. doi: 10.1007/s10067-007-0601-6. [DOI] [PubMed] [Google Scholar]
- 9.Miriovsky BJ, Michaud K, Thiele GM, O'Dell J, Cannon GW, Kerr G, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease burden in U.S. veterans with rheumatoid arthritis. Ann Rheum Dis. 2010;69:1292–7. doi: 10.1136/ard.2009.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DM, Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis. 2003;62(9):870–4. doi: 10.1136/ard.62.9.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hueber W, Utz PJ, Robinson WH. Autoantibodies in early arthritis: advances in diagnosis and prognostication. Clin Exp Rheumatol. 2003;21(5 Suppl 31):S59–64. [PubMed] [Google Scholar]
- 12.Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis Rheum. 2011;63(1):53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clavel C, Nogueira L, Laurent L, Iobagiu C, Vincent C, Sebbag M, et al. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58(3):678–88. doi: 10.1002/art.23284. [DOI] [PubMed] [Google Scholar]
- 14.Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122(5):1791–802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116(4):961–73. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikuls TR, Kazi S, Cipher D, Hooker R, Kerr GS, Richards JS, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34(7):1480–4. [PubMed] [Google Scholar]
- 17.Arnett F, Edworthy S, Bloch D, McShane D, Fries J, Cooper N, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Mikuls TR, Gould KA, Bynote KK, Yu F, Levan TD, Thiele GM, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione s-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12(6):R213. doi: 10.1186/ar3190. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum. 1999;42(10):2220–30. doi: 10.1002/1529-0131(199910)42:10<2220::AID-ANR26>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Mikuls TR, Padala PR, Sayles HR, Yu F, Michaud K, Caplan L, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65(2):227–34. doi: 10.1002/acr.21778. [DOI] [PubMed] [Google Scholar]
- 21.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. Plos ONE. 2012;7(5):e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokolove J, Lindstrom TM, Robinson WH. Development and deployment of antigen arrays for investigation of B-cell fine specificity in autoimmune disease. Front Biosci (Elite Ed) 2012;4:320–30. doi: 10.2741/379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98(9):5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinitz M, Tamir S. Human monoclonal autoimmune antibody produced in vitro: rheumatoid factor generated by Epstein-Barr virus-transformed cell line. Eur J Immunol. 1982;12(2):126–33. doi: 10.1002/eji.1830120206. [DOI] [PubMed] [Google Scholar]
- 25.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 26.Baum J, Stastny P, Ziff M. Effects of the Rheumatoid Factor and Antigen-Antibody Complexes on the Vessels of the Rat Mesentery. J Immunol. 1964;93:985–92. [PubMed] [Google Scholar]
- 27.Bianco NE, Dobkin LW, Schur PH. Immunological properties of isolated IgG and IgM anti-gamma-globulins (rheumatoid factors) Clinical and experimental immunology. 1974;17(1):91–101. [PMC free article] [PubMed] [Google Scholar]
- 28.Sabharwal UK, Vaughan JH, Fong S, Bennett PH, Carson DA, Curd JG. Activation of the classical pathway of complement by rheumatoid factors. Assessment by radioimmunoassay for C4. Arthritis Rheum. 1982;25(2):161–7. doi: 10.1002/art.1780250208. [DOI] [PubMed] [Google Scholar]
- 29.Balestrieri G, Tincani A, Migliorini P, Ferri C, Cattaneo R, Bombardieri S. Inhibitory effect of IgM rheumatoid factor on immune complex solubilization capacity and inhibition of immune precipitation. Arthritis Rheum. 1984;27(10):1130–6. doi: 10.1002/art.1780271008. [DOI] [PubMed] [Google Scholar]
- 30.Roosnek E, Lanzavecchia A. Efficient and selective presentation of antigen-antibody complexes by rheumatoid factor B cells. J Exp Med. 1991;173(2):487–9. doi: 10.1084/jem.173.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hueber W, Tomooka BH, Zhao X, Kidd BA, Drijfhout JW, Fries JF, et al. Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with up regulation of proinflammatory cytokines. Ann Rheum Dis. 2007;66(6):712–9. doi: 10.1136/ard.2006.054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todd DJ, Knowlton N, Amato M, Frank MB, Schur PH, Izmailova ES, et al. Erroneous augmentation of multiplex assay measurements in patients with rheumatoid arthritis due to heterophilic binding by serum rheumatoid factor. Arthritis Rheum. 2011;63(4):894–903. doi: 10.1002/art.30213. [DOI] [PubMed] [Google Scholar]
- 33.Artandi SE, Calame KL, Morrison SL, Bonagura VR. Monoclonal IgM rheumatoid factors bind IgG at a discontinuous epitope comprised of amino acid loops from heavy-chain constant-region domains 2 and 3. Proc Natl Acad Sci U S A. 1992;89(1):94–8. doi: 10.1073/pnas.89.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonagura VR, Artandi SE, Davidson A, Randen I, Agostino N, Thompson K, et al. Mapping studies reveal unique epitopes on IgG recognized by rheumatoid arthritis-derived monoclonal rheumatoid factors. J Immunol. 1993;151(7):3840–52. [PubMed] [Google Scholar]
- 35.Newkirk MM, Goldbach-Mansky R, Lee J, Hoxworth J, McCoy A, Yarboro C, et al. Advanced glycation end-product (AGE)-damaged IgG and IgM autoantibodies to IgG-AGE in patients with early synovitis. Arthritis Res Ther. 2003;5(2):R82–90. doi: 10.1186/ar622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Concordant seropositive (aCCP+/RF+) RA patients exhibit higher serum levels of serum cytokines than either the double negative (aCCP-/RF-) or the aCCP-/RF+ subgroups. A subgroup of aCCP+/RF+ patients was matched to aCCP-/RF- by age, gender, and disease duration (n=204). Levels of 17 cytokines were compared across groups and SAM output was sorted based on false discovery rates (FDRs) in order to identify antigens with the greatest differences in autoantibody reactivity between the three serologic subgroups. Values are reported as fluorescence intensity relative to the average of the evaluated cohort. SAM identified significant elevation of 10 of 17 cytokines among the concordant seropositive subgroup compared to either the aCCP-/RF+ (n=135) or aCCP-/RF- subgroups (n=204).
Supplemental Table 1: Average anti-CCP2 and RF titers by subgroup