Abstract
Objective
Colonic mucosa-associated Escherichia coli are increased in Crohn's disease (CD) and colorectal cancer (CRC). They variously haemagglutinate, invade epithelial cell lines, replicate within macrophages, translocate across M (microfold) cells and damage DNA. We investigated genes responsible for these effects and their co-association in colonic mucosal isolates.
Design
A fosmid library yielding 968 clones was prepared in E coli EPI300-T1 using DNA from a haemagglutinating CRC isolate, and resulting haemagglutinating clones were 454-pyrosequenced. PCR screening was performed on 281 colonic E coli isolates from inflammatory bowel disease (IBD) (35 patients), CRC (21) and controls (24; sporadic polyps or irritable bowel syndrome).
Results
454-Pyrosequencing of fosmids from the haemagglutinating clones (n=8) identified the afimbrial adhesin afa-1 operon. Transfection of afa-1 into E coli K-12 predictably conferred diffuse adherence plus invasion of HEp-2 and I-407 epithelial cells, and upregulation of vascular endothelial growth factor. E coli expressing afaC were common in CRC (14/21, p=0.0009) and CD (9/14, p=0.005) but not ulcerative colitis (UC; 8/21) compared with controls (4/24). E coli expressing both afaC and lpfA (relevant to M-cell translocation) were common in CD (8/14, p=0.0019) and CRC (14/21, p=0.0001), but not UC (6/21) compared with controls (2/24). E coli expressing both afaC and pks (genotoxic) were common in CRC (11/21, p=0.0015) and UC (8/21, p=0.022), but not CD (4/14) compared with controls (2/24). All isolates expressed dsbA and htrA relevant to intra-macrophage replication, and 242/281 expressed fimH encoding type-1 fimbrial adhesin.
Conclusions
IBD and CRC commonly have colonic mucosal E coli that express genes that confer properties relevant to pathogenesis including M-cell translocation, angiogenesis and genotoxicity.
Keywords: BACTERIAL ADHERENCE, BACTERIAL INTERACTIONS, BACTERIAL PATHOGENESIS, GUT INFLAMMATION, E. COLI
Significance of this study.
What is already known about on this subject?
Mucosa-associated E coli are increased in CD and colon cancer.
These E coli adhere to and invade intestinal epithelial cells in culture, and replicate within macrophages.
These properties have led to them being designated AIEC. However, no genotype is consistent across all AIEC isolates.
What are the new findings?
There is an increased prevalence of afaC+ DAEC isolates associated with the colonic mucosa in CD and colon cancer
The presence of the afimbrial adhesin operon correlates with diffuse adherence to, invasion of, and increased VEGF expression in intestinal epithelial cells
Colonic mucosal E coli that are diffusely adherent and that bear key genes potentially relevant to CD (conferring mucosal invasion and translocation and replication in macrophages), as well as to colon cancer pathogenesis (conferring the ability to induce angiogenesis and genotoxicity) are strongly associated with colon cancer and IBD, and could be relevant to pathogenesis.
How might it impact on clinical practice in the foreseeable future?
Interventions that either reduce colonisation by DAEC or block their interaction with the mucosa may have preventive or therapeutic effects in colon cancer and CD.
Introduction
It is accepted that bacteria are involved in inflammatory bowel disease (IBD) pathogenesis but the mechanisms are poorly understood.1 An increase in mucosa-associated Escherichia coli in Crohn's disease (CD) has been found in both ileum2 3 and colon.4–7 Increased mucosa-associated E coli have also been reported in colorectal cancer (CRC)5 8 and to a lesser extent in ulcerative colitis (UC).9–11 These E coli typically adhere to and invade intestinal epithelial cells in culture and replicate within macrophages.12 13 Gentamicin treatment of CD intestinal biopsies followed by lysis and culture implies the presence of intracellular E coli,5 8 14 and E coli DNA has been demonstrated within a majority of CD granulomas.15
Designated as ‘adherent, invasive E coli’ (AIEC), some of the properties of these E coli have been associated with specific genes, particularly in studies of the ‘paradigm’ ileal AIEC, LF82.16 These include high-temperature requirement-A (htrA) and oxidoreductase disulfide bond-A protein (dsbA), which support intra-macrophage survival17 18 and long polar fimbriae (lpfA) involved in translocation across M cells of the follicle-associated epithelium (FAE).19 There is, however, no genotype that is consistent across all AIEC. Moreover, their invasion of epithelial cell lines in vitro varies between cell lines and is a property found in other E coli including diffusely adherent E coli (DAEC) and uropathogenic E coli (UPEC).20 21 We have previously shown that colonic mucosa-associated E coli from CD and CRC commonly expressed haemagglutinins and that this correlated with their ability to adhere to and invade epithelial cell lines.5
Recent studies show that E coli possessing the polyketide synthase gene complex (pks) responsible for producing the genotoxin colibactin induce inflammation-associated CRC in mice22 and are commonly mucosa associated in sporadic CRC.22 23
Here we have used a fosmid-clone library, based on genomic DNA derived from a colon cancer mucosal AIEC, to investigate the nature of the haemagglutinin gene(s), its relevance to the AIEC phenotype, and distribution among mucosal E coli from IBD, CRC and controls. We also screened these isolates for htrA and dsbA, relevant to replication within macrophages, lpfA and fimH relevant to M-cell translocation, a likely portal of entry for mucosal invasion in CD and for the pks gene complex, relevant to CRC pathogenesis.
Methods
Cell culture
The human colon adenocarcinoma cell-line Caco2 (#86010202), Burkitt's lymphoma cell-line Raji-B (#85011429) and J774A.1 murine macrophages (#91051511) were from ECACC (Wiltshire, UK). Human I-407 (CCL-6) and HEp-2 (CCL-23) cells were from the American Type Culture Collection (LGC Standard; Teddington, UK). Caco2-cl1 cells, kindly provided by Dr Elisabet Gullberg (Linköping University, Sweden), were originally obtained from Dr Maria Rescigno (European Institute of Oncology; Milan, Italy).24 Caco2, Caco2-cl1 and I-407 were cultured in DMEM, HEp-2 in MEM, Raji-B and J774A.1 in RPMI-1640; supplemented with 10% FBS, 4 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and maintained at 37°C in 5% carbon dioxide.
Generation of an E coli library from a colonic mucosa-associated haemagglutinating E coli with the AIEC phenotype
Using E coli HM358, a haemagglutination-positive isolate from a CRC patient5 as the parent strain, a randomly sheared, non-biased fosmid library was constructed (CopyControl fosmid library kit; Epicentre, Wisconsin, USA). Genomic DNA extracted from HM358 (Genomix Cell/Tissue kit: Talent; Italy) was sheared, end-repaired with 5′-phosphorylated blunt ends, 30–50 kb DNA fragments ligated to phosphatase-treated fosmid pCC1FOS and introduced into phage T1-resistant E coli K-12 derivate EPI300-T1. E coli clones were selected on Luria–Bertani chloramphenicol (12.5 μg/mL) agar.
Haemagglutination assay
E coli clones were screened for haemagglutination of 1% group O human erythrocytes (NBS; Liverpool, UK)5 with haemagglutination-positive E coli HM358 and haemagglutination-negative E coli EPI300-T1/pCC1FOS as controls.
454-Pyrosequencing
Following the induction of high copy number of fosmids during bacterial growth (Epicentre copy-control auto-induction solution), clones from haemagglutination-positive E coli were extracted using Qiafilter midi-kits (Qiagen; Crawley, UK). 454-Sequencing was performed using the GS-FLX Titanium Series (Roche-454 Life Science; Branford, USA). 454-Reads were assembled with Newblerv2.0 and protein-coding sequences identified with GeneMark-Pv2.4 (http://exon.biology.gatech.edu/). Predicted open-reading frames and translated amino acids were subjected to BLASTP to identify homologies to operons. Gene representation was performed using Artemisv13 (http://www.sanger.ac.uk/resources/software/artemis/).
Following sequence analysis, E coli HM358 and all fosmid-containing haemagglutination-positive E coli were screened by PCR for afimbrial adhesin (afaC), P-fimbriae (papC), type-1 pili (fimA/fimC1/fimH), outer-membrane protein-C (ompC) and flagellin (fliC). Primers and conditions are detailed in supplementary file S1 (available online only).
Presence of afa operon in colonic mucosal E coli strains
A panel of 281 colonic mucosal E coli previously isolated from 80 patients was studied;5 71 from 14 CD patients, 51 from 21 UC patients, 120 from 21 CRC patients and 39 from 24 controls. IBD and control isolates were obtained from colonoscopic biopsy of non-ulcerated sigmoid colon, CRC isolates from biopsy of resection specimens.5 PCR to detect all afa strains, irrespective of afaE subtype, was performed using primers to amplify afaC, present in all operons of the afimbrial adhesin family (afa-1,-2,-3,-5,-7,-8 and daa).25 DAEC C1845, provided by Professor Alain Servin (INSERM-UMR756, Châtenay-Malabry, France), which carries the daa operon26 and CD AIEC LF82 lacking afa,16 were used as controls. Additional ileal CD isolates LF10, LF11, LF86 and LF13, provided by Professor Arlette Darfeuille-Michaud (INSERM-UMR1071, Clermont-Ferrand, France),2 were also screened for the afa operon.
Generation of an afa-1-expressing recombinant E coli
Following sequence identification of Afa-1 as the putative adhesin responsible for haemagglutination, purified fosmid was extracted from haemagglutination-positive clone E coli 8H8. The complete afa-1 operon (6.8 kb) was isolated by XbaI/SpeI restriction enzyme digest, purified by gel electrophoresis, ligated into pUC18 using T4 DNA-ligase and propagated in E coli One-ShotTOP10 (Invitrogen) selected on 100 µg/mL ampicillin agar. pUCAfa was then used to transform chemically competent E coli EPI300-T1 containing empty fosmid, to allow direct comparison with E coli 8H8. The presence and orientation of afa-1 within pUCAfa were confirmed by PCR. Functional adhesin was confirmed by haemagglutination and adhesion to HEp-2 cells (see supplementary file S2, available online only).
Diffuse adherence to HEp-2 cells
The ability of E coli isolates, fosmid and pUCAfa-transformed constructs to mediate diffuse adherence to HEp-2 was assessed in the presence of 1% methyl-α-d-mannopyranoside to exclude type-1 fimbriae-mediated adhesion,27 with DAEC C1845 included as positive control.26
Adherence and invasion to intestinal epithelial cell lines
Adherence to, and invasion of, E coli to HEp-2, I-407 and differentiated Caco2 cells (15d post-confluent) was assessed by gentamicin protection assay in the presence of methyl-α-d-mannopyranoside.5 Bacteria were cultured overnight on Luria–Bertani agar, with adherence and invasion calculated as the percentage of the original inoculum, and data expressed relative to wild-type AIEC HM358.
Real-time PCR for VEGF
Confluent I-407 cells (8×105 cells/well) were serum-starved for 24 h and then infected for 4 h Multiplicity of infection 20 (MOI 20) with either CRC AIEC HM358, E coli EPI300-T1/pCC1FOS transformed with afa-1 operon (pUCAfa) or vector alone (pUC18), or DAEC C1845 (known to upregulate vascular endothelial growth factor (VEGF)).20 Total RNA was isolated (RNeasy kit; Qiagen) and quantified by NanoDrop. VEGF messenger RNA was assessed by quantitative PCR of first-strand synthesised complementary DNA (Roche, Burgess Hill, UK) in a Lightcycler480 system using human VEGF or β-actin specific primers (Eurogentec) and probes from the Roche Universal Probe Library (see online supplementary file S1, available online only).
Replication of E coli within J774A.1 macrophages
Macrophages were seeded onto 24-well plates (105 cells/well) for 24 h and infected (MOI 10) with either E coli HM358, library clones or E coli constructs in antibiotic-free media. Intra-macrophage replication was determined by recovery of intracellular bacteria from lysed gentamicin-treated cells after 6 h or 24 h, relative to intracellular numbers at 3 h.13
Translocation through M cells in culture
Translocation through M cells, generated by co-culture of Caco2-cl1 and Raji-B cells, was conducted as previously described.28 Successful M-cell generation was confirmed by translocation of CD E coli HM605 and 0.5 µm yellow-green FluoSpheres (Invitrogen). Trans-epithelial electrical resistance was monitored throughout.
Screening for intra-macrophage replication genes htrA and dsbA, lpfA and the genotoxin-producing pks pathogenicity island in colonic mucosal E coli
PCR assays for genes relevant to AIEC intramacrophage survival and replication, dsbA and htrA,17 18 and the two major lpf operons (lpfAShigella and lpfALF82) identified in ileal CD AIEC,19 were performed. PCR for pks prevalent in patients with IBD and CRC, was performed previously.22 Primers detailed in supplementary file S1 (available online only).
Statistics.
N is the total number of independent experiments performed, with n replicates for each treatment group. Independent groups were assessed for normality and equality of variance, and analysed using Mann–Whitney U or Kruskal–Wallis followed by pair-wise comparison of treatment means (StatsDirectv2.6.2; Sale, UK). Comparing PCR datasets, Fisher's exact and χ2 tests were utilised as appropriate, in which N is the number of patients, and n is E coli. Differences were considered significant at p<0.05.
Results
Fosmids extracted from haemagglutinin-positive E coli in the clone library derived from CRC mucosal E coli HM358 share a common region containing the afimbrial adhesin afa-1 operon.
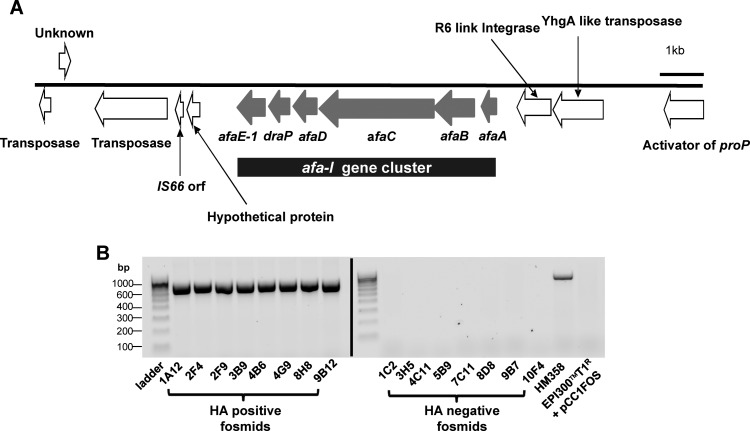
A total of 968 fosmid clones was generated from randomly sheared 30–50 kb DNA fragments of E coli HM358, a haemagglutination-positive CRC colonic mucosal isolate. Eight of the resulting 968 E coli library clones were strongly haemagglutination positive. Analysis of 454-sequence data identified only the full afa-1 operon, coding for afimbrial adhesin Afa-1, as common to the haemagglutination-positive fosmids, plus some additional surrounding transposase and integrase elements (figure 1A; see supplementary file S3, available online only). The afa operon identified contained six open-reading frames encoding for AfaA (a transcriptional regulator), AfaB (a periplasmic chaperone), AfaC (an usher), AfaD (an invasin), DraP (a linker element found in the dra operon) and AfaE-1 (the mannose-resistant adhesin); figure 1A. PCR targeting a 672 bp amplicon of afaC confirmed the afa operon within the parent E coli HM358 and all eight haemagglutination-positive fosmid clones, whereas eight haemagglutination-negative clones chosen at random from the library and E coli EPI300-T1/pCC1FOS were afa negative (figure 1B). The nucleotide sequence of the HM358 afa-1 cluster was deposited in GenBank; accession no. JN688153.
Figure 1.
Haemagglutinin-positive E coli in the fosmid clone library derived from E coli HM358 share a common region containing the afimbrial adhesin afa-1 operon. (A) Genomic organisation of the common region shared by haemagglutination (HA)-positive clones includes afaA (encoding a transciptional regulator); afaB (chaperone); afaC (usher); afaD (invasin); draP (linker element) and afaE-1 (an adhesin). (B) PCR for afaC. A 672 bp fragment is detected in all eight haemagglutination-positive clones and E coli HM358. E coli EPI300-T1/pCC1FOS and eight haemagglutination-negative fosmids lacked afaC.
PCR identified that all eight fosmid-containing haemagglutination-positive E coli were positive for fliC, fimA, fimC1, fimH and ompC but negative for papC and lpfA.
Increased prevalence of afa in mucosal E coli isolates from CD and CRC patients
PCR for afaC (present in all operons of the afimbrial adhesin family) in a large panel of colonic mucosal E coli isolates showed increased prevalence of afa among isolates from CD (nine of 14 patients; p=0.005, Fisher's exact test) and CRC (14 of 21; p=0.0009), but not UC (eight of 21), compared with controls (four of 24); table 1). When expressed using E coli as denominator, the presence of afaC was also more common among isolates from CD (39 of 71 isolates (54%)), UC (28/51 (54%)) and CRC (73/120 (60%)) compared with controls (11/39 (28%)); all p≤0.02, with CRC versus controls p=0.0008, χ2; table 2. Like AIEC LF82, ileal CD isolates LF11 and LF86 were negative for afaC, whereas ileal isolates LF10 and LF13 were positive.
Table 1.
Prevalence of afaC positive E coli in patients with CD, colitis and colon cancer compared with controls (using total number of patients as the denominator)†
| Total no. of patients | afaC+ | p Value* | |
|---|---|---|---|
| CD | 14 | 9 | 0.005 |
| CRC | 21 | 14 | 0.0009 |
| UC | 21 | 8 | NS |
| Controls | 24 | 4 |
*p Values obtained using Fishers exact test (2P component).
†Presence or absence of afaC is based on PCR assay.
afaC, gene encoding afimbrial adhesin outer membrane usher protein; CD, Crohn's disease; CRC, colorectal cancer; UC, ulcerative colitis.
Table 2.
Presence of afaC in E coli isolated from patients with CD, colitis and colon cancer compared with controls (using total number of E coli as the denominator)†
| afaC+ | afaC− | p Value* | |
|---|---|---|---|
| CD | 39 | 32 | 0.0127 |
| CRC | 73 | 47 | 0.0008 |
| UC | 28 | 23 | 0.0204 |
| Controls | 11 | 28 |
*p Values obtained using χ2 test (Yates-corrected).
†Presence or absence of afaC is based on PCR assay.
afaC, gene encoding afimbrial adhesin outer membrane usher protein; CD, Crohn's disease; CRC, colorectal cancer; UC, ulcerative colitis.
Presence of the afa-1 operon correlates with diffuse adherence to and invasion of HEp-2 cells
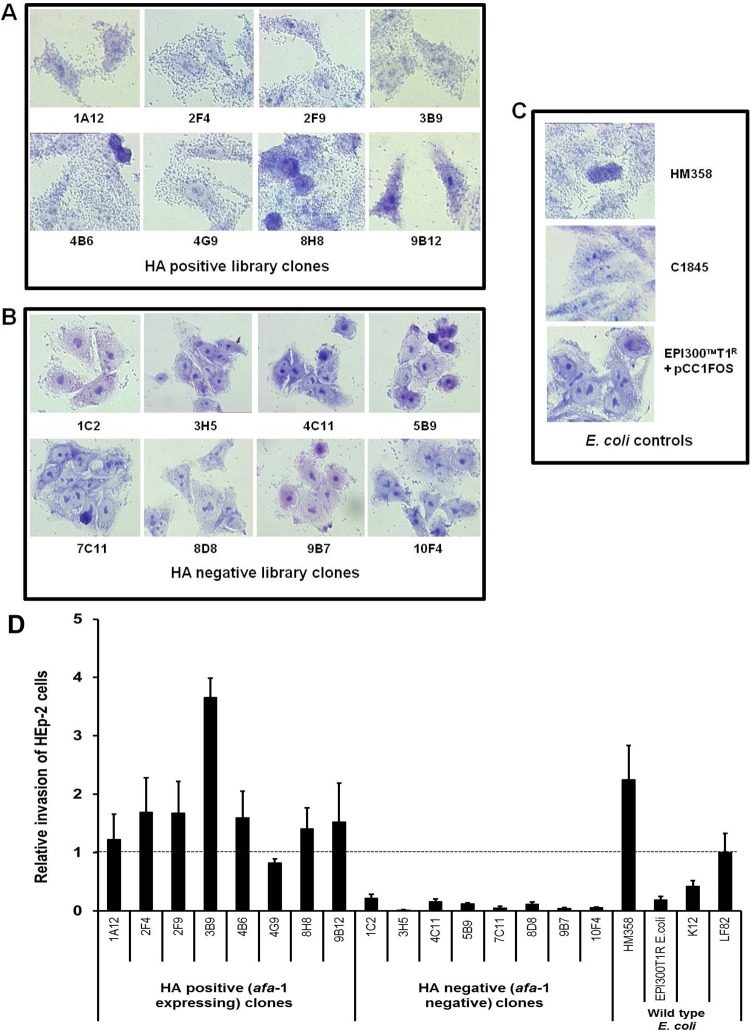
After 6 h infection of HEp-2 cells, AIEC HM358 and all eight haemagglutination-positive (afa-1 possessing) library clones demonstrated diffuse adherence to HEp-2 characteristic of DAEC (figure 2A–C). No adherence to HEp-2 was observed with eight randomly-selected haemagglutination-negative clones, nor with the K-12 plating strain E coli EPI300-T1/pCC1FOS. All the haemagglutination-positive (afa-1 possessing) E coli invaded HEp-2 cells, at levels similar to those observed for E coli HM358. The haemagglutination-negative (afa-1 negative) clones, afa-negative AIEC LF82, and the K-12-derived EPI300-T1 plating strain, were all substantially less invasive to HEp-2; p<0.0001; Kruskal–Wallis (figure 2D).
Figure 2.
The presence of the afa-1 operon in haemagglutinating E coli correlates with diffuse adherence and invasion to HEp-2 epithelial cells. Giemsa stain of HEp-2 cells infected with E coli strains. (A) All eight haemagglutinin-positive library clones showed diffuse adherence to cell cultures. (B) Eight haemagglutination (HA)-negative fosmid clones chosen at random from the library were non-adherent. (C) Colonic mucosally associated E coli HM358 exhibited a diffusely adherent pattern as per diffusely adherent E coli C1845. The E coli K12 plating strain EPI300T1 containing pCC1Fos was non-adherent. (D) The eight haemagglutination-positive fosmid library clones possessing the afa-1 gene cluster, exhibiting diffuse adherence, showed increased ability to invade Hep-2 cells compared to haemagglutination-negative clones. Invasion calculated as percentage of the original inoculums (multiplicity of infection 10) and expressed relative to E coli LF82 previously shown to be invasive in this cell line.2 * p<0.05 and *** p<0.001 when compared to the non-invasive plating strain EPI300-T1 containing pCC1Fos alone (mean±SEM; N=3 experiments, each performed with n=3 replicates; Kruskal–Wallis).
Presence of the afa-1 operon correlates with the ability of E coli to adhere to and invade intestinal epithelial cells
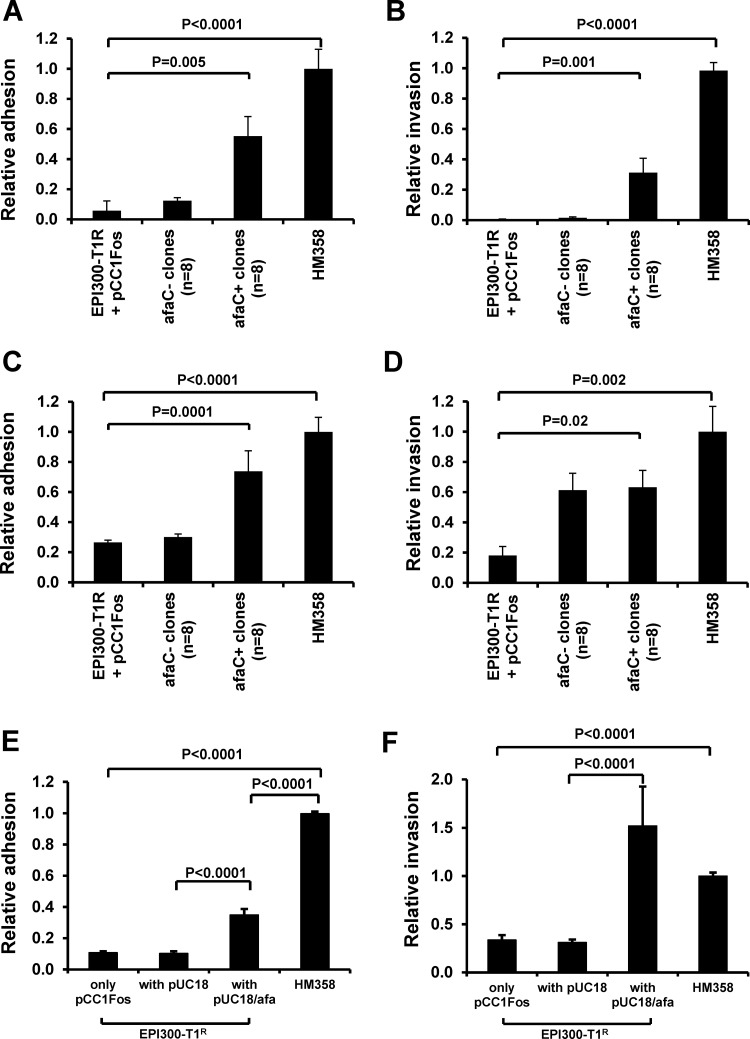
The eight haemagglutination-positive E coli clones possessing afa-1 were all shown to adhere to undifferentiated I-407 cells (adhesion relative to HM358 was 0.55±0.13) (mean±SEM) in contrast to non-haemagglutinating, afa-negative E coli, 0.12±0.02; n=4 p<0.0001, Kruskal–Wallis (figure 3A). Similarly, haemagglutination-positive, afa-1-possessing E coli invaded I-407 cells (invasion relative to HM358 was 0.31±0.13) in contrast to haemagglutination-negative, afa-negative clones (0.01±0.01); p<0.0001 (figure 3B). Haemagglutination-positive E coli clones possessing afa-1 also demonstrated greater adhesion to fully differentiated Caco2 cells (afa-positive clones, 0.74±0.14 compared to afa-negative clones, 0.3±0.02); n=4, p<0.0001 (figure 3C). Of note though, three of eight haemagglutination-negative (afa-1 negative) E coli were able to invade differentiated Caco2 (figure 3D). The plating strain EPI300-T1/pCC1FOS showed negligible adhesion to and invasion of both cell lines.
Figure 3.
The presence of afa-1 in haemagglutinating E coli correlates with ability to adhere to and invade intestinal epithelial cells. Relative ability of eight haemagglutination-positive library clones possessing afa-1 (afaC+ as determined by PCR) and haemagglutination-negative clones (n=8) to (A) adhere to and (B) invade I-407 cells. Data mean (±SEM) relative to that observed by E coli HM358; n=4. Increased (C) adherence to, and (D) invasion of differentiated Caco2 cells by afa-1-possessing clones was observed. (E and F) E coli EPI300-T1/pCC1FOS transformed with afa-1 (pUCAfa) adheres to and invades I-407 cells. Data expressed relative to plating strain; N=3, each n=3–5 replicates). p Values determined by Kruskal–Wallis. Afa, afimbrial adhesin; afa-1, afimbrial adhesin operon 1; afaC, gene encoding afimbrial adhesin outer membrane usher protein.
Transfection of the full afa-1 operon (pUCAfa) into the plating strain resulted in increased adhesion to and invasion of I-407 cells compared to the plating strain transformed with pUC18; p<0.0001, Kruskal–Wallis (figure 3E,F). Similar results were obtained using differentiated Caco2 cells (data not shown).
Assessment of the afaC status of 24 colonic mucosal isolates previously assessed for adhesion to and invasion into I-407 epithelial cells5 showed that nine of 13 afaC-positive isolates were invasive to I-407 cells. However, various afaC-negative isolates, including LF82, were also observed to be invasive for this cell line.
Presence of afa-1 in E coli upregulates VEGF expression by intestinal epithelial cells
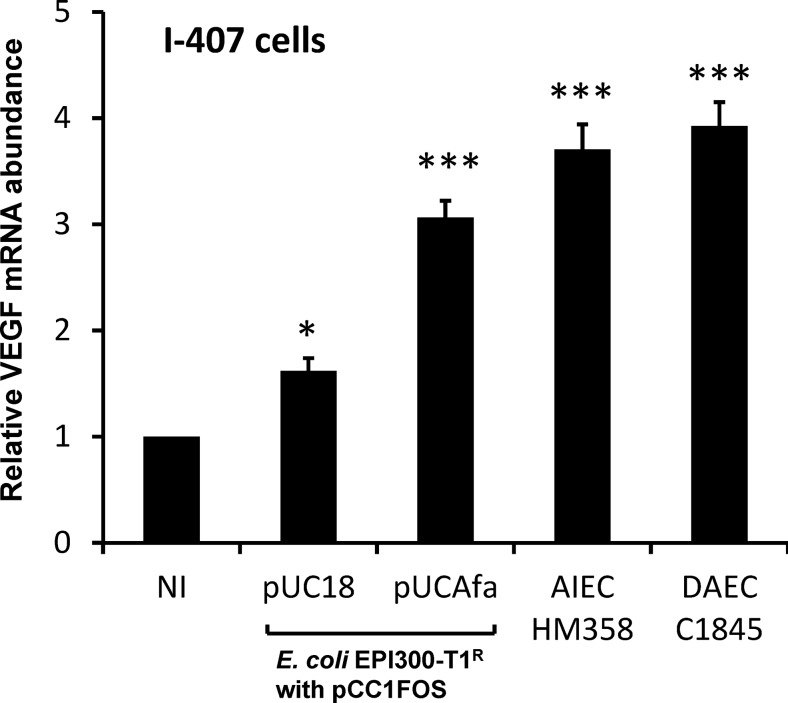
VEGF mRNA was upregulated 3.07±0.16-fold in I-407 cells infected with E coli EPI300-T1/pCC1FOS transformed with the afa-1 operon (pUCAfa) and 3.71±0.22-fold with AIEC HM358, compared to uninfected cells (N=4, n=3; p<0.001 Kruskal–Wallis). The level of response was similar in cells infected with DAEC C1845 (N=2, n=3; p<0.001); figure 4.
Figure 4.
The presence of afa in E coli upregulates vascular endothelial growth factor (VEGF) expression in cultured intestinal epithelial cells. Confluent serum-starved I-407 cells (8×105 cells/well) were infected for 4 h with either wild-type colorectal cancer adherent, invasive E coli HM358, Afa/Dr diffusely adhering E coli C1845 or E coli EPI300-T1/pCC1FOS transformed with afa-1 operon (pUCAfa) or vector alone (pUC18) and total RNA extracted. VEGF mRNA measured by quantitative PCR relative to β actin. Data mean (±SEM) relative to non-infected cells (set at 100%); N=2–4; each n=3 replicates). *p<0.05, *** p<0.001 determined by Kruskal–Wallis. AIEC, adherent, invasive E coli; DAEC, diffusely adherent E coli.
The presence of Afa-1 in mucosally associated E coli does not confer ability to replicate within macrophages or translocate through M cells
In contrast to the parent isolate HM358, no significant intra-macrophage replication was seen with any of the eight haemagglutination-positive (afa-1 possessing) E coli clones (see supplementary file S4, available online only). Likewise, seven of the eight haemagglutination-negative clones tested (excepting 4C11), and the E coli EP1300-T1 plating strain containing pUCAfa, were unable to replicate within macrophages. Moreover, following initial infection and gentamicin treatment, fewer recombinant E coli containing afa-1 were internalised within macrophages (0.6±0.1×104 CFU/well) compared with E coli containing pUC18 alone (11.0±2.1×104 CFU/well; p<0.0001), suggesting that possession of Afa-1 inhibits phagocytosis (table 3). It was also observed that haemagglutination-positive fosmid library clones possessing the afa-1 operon, showed no significant increased ability above haemagglutination-negative (afa-1 negative) clones to release tumour necrosis factor alpha (TNFα) from macrophages (see supplementary file S4, available online only). Similar TNFα levels were released from macrophages by AIEC HM358 (1712±236 pg TNFα/mL) and E coli K-12 strain EPI300-T1/pCC1FOS expressing Afa-1 from pUCAfa (1742±31 pg/mL) compared to uninfected controls (16±5 pg/mL; n=3). All 281 colonic mucosal E coli isolates (including HM358) expressed dsbA and htrA, relevant to intramacrophage replication.
Table 3.
E coli uptake and replication within J774-A1 murine macrophages
| Strain | Uptake of bacteria* (CFU/well×104) | Fold replication 6 h/3 h† | Fold replication 24 h/3 h† |
|---|---|---|---|
| EPI300-T1 | |||
| + pCC1FOS | 9.3±1.6 | 1.70±0.23 | 0.69±0.16 |
| + pCC1FOS/pUC18 | 11.0±2.1 | 0.86±0.05 | 1.21±0.12 |
| + pCC1FOS/pUC Afa | 0.6±0.1‡ | 1.07±0.06 | 0.27±0.10 |
| HM358 | 5.36±1.1 | 17.66±3.80§ | 11.26±2.67§ |
Data expressed as means±SEM, determined from N=2–7 independent experiments, with each experiment performed with n=2–3 replicates.
*CFU recovered from lysed macrophages after 3 h (2 h infection followed by 1 h gentamicin treatment).
†Recovered intracellular bacteria from lysed macrophages after 6 h or 24 h, relative to intracellular numbers at 3 h. Statistical analysis was performed using Mann–Whitney U with Bonferroni correction.
‡Significantly different from EPI300-T1/pCC1FOS+pUC18; p<0.0001.
§Significantly different from EPI300-T1/pCC1FOS; p<0.0001.
CFU, colony-forming units.
While HM358 (which expresses lpfA) was observed to translocate across M cells, no significant translocation was seen for E coli EPI300-T1 pUCAfa, indicating that possession of Afa-1 adhesin does not support FAE transcytosis (see supplementary file S5, available online only).
Increased prevalence in CD and CRC of DAEC possessing lpfA and fimH relevant to M cell translocation
Screening of 281 colonic mucosally associated E coli demonstrated a striking increased prevalence in CD and CRC of isolates possessing afa together with lpfA (tables 4–7; see supplementary file S6, available online only). Most isolates (242/281) also expressed fimH (61/71 CD, 97/120 CRC, 46/51 UC and 38/39 controls). Only six CD isolates and one cancer isolate expressing both afaC and lpfA were negative for fimH.
Table 4.
Prevalence of afaC+, lpfA+ E coli in patients with CD, colitis and colon cancer compared with controls (with total patients as the denominator)†
| afaC+, lpfA+ | Total no of patients | p Value* | |
|---|---|---|---|
| CD | 8 | 14 | 0.0019 |
| CRC | 14 | 21 | 0.0001 |
| UC | 6 | 21 | NS |
| Controls | 2 | 24 |
*p Values obtained using Fishers exact test (2P component).
†Presence or absence of genes is based on PCR assays.
afaC, gene encoding afimbrial adhesin outer membrane usher protein; CD, Crohn's disease; CRC, colorectal cancer; lpfA, gene encoding long polar fimbrial protein; UC, ulcerative colitis.
Table 5.
Presence of afaC+, lpfA+ E coli in CD, colitis and colon cancer compared with controls (using total number of E coli as the denominator)†
| afaC+, lpfA+ | Total no of E coli | p Value* | |
|---|---|---|---|
| CD | 30 | 71 | 0.0011 |
| CRC | 51 | 120 | 0.0005 |
| UC | 15 | 51 | NS |
| Controls | 4 | 39 |
*p Values obtained using χ2 test (Yates-corrected).
†Presence or absence of genes is based on PCR assays.
afaC, gene encoding afimbrial adhesin outer membrane usher protein; CD, Crohn's disease; CRC, colorectal cancer; lpfA, gene encoding long polar fimbrial protein; UC, ulcerative colitis.
Table 6.
Prevalence of afaC+, pks+ E coli in patients with CD, colitis and colon cancer compared with controls (using total number of patients as the denominator)†
| Total no of patients | afaC+, pks+ | p Value* | |
|---|---|---|---|
| CD | 14 | 4 | NS |
| CRC | 21 | 11 | 0.0015 |
| UC | 21 | 8 | 0.0222 |
| Controls | 24 | 2 |
*p Values obtained using Fisher's exact test (2P component).
†Presence or absence of afaC and pks is based on PCR assay.
afaC, gene encoding afimbrial adhesin outer membrane usher protein; CRC, colorectal cancer; pks, polyketide synthase gene complex; UC, ulcerative colitis.
Table 7.
Presence of afaC+, pks+ E coli in patients with CD, colitis and colon cancer compared with controls (using total number of E coli as the denominator)†
| afaC+, pks+ | afaC−, pks− | p Value* | |
|---|---|---|---|
| CD | 13 | 58 | NS |
| CRC | 35 | 85 | 0.0119 |
| UC | 17 | 34 | 0.0082 |
| Controls | 3 | 36 |
*p Values obtained using χ2 test (Yates-corrected).
†Presence or absence of afaC and pks is based on PCR assay.
afaC, gene encoding afimbrial adhesin outer membrane usher protein; CD, Crohn's disease; CRC, colorectal cancer; pks, polyketide synthase gene complex; UC, ulcerative colitis.
Increased prevalence in CRC and UC of DAEC (afa positive E coli) possessing the pks genotoxicity island relevant to carcinogenesis
We have recently shown that colonic mucosal E coli from sporadic CRC and IBD commonly express the pks genotoxicity island that confers the ability to induce experimental CRC. Screening of E coli for co-expression of afaC and pks shows a marked increase in afaC+/pks+ E coli in association with CRC and with UC but not CD (tables 4–7).
Discussion
This study shows that colonic mucosa-associated afa-1-positive DAEC are increased in CD and CRC. They adhere to and invade intestinal epithelial cells in culture but also commonly express lpfA relevant to M-cell translocation that is more likely to be the major initial route for invasion in vivo.19 Colonic mucosal DAEC isolates also possess htrA and dsbA relevant to survival within macrophages,17 18 part of the characteristic phenotype of CD-associated AIEC.12
Afa-expression confers the ability to induce VEGF expression by epithelial cells, relevant to angiogenesis and tumour development29 30 and this is confirmed here. We have recently shown that colonic mucosal E coli from patients with IBD and CRC more commonly express the pks pathogenicity island whose gene products result in the formation of the metabolite colibactin, a genotoxin with the ability to cause epithelial DNA damage and induce tumours in a mouse model of inflammation-associated CRC.22 Here we show that colonic mucosal E coli isolates from CRC and UC commonly express both pks and afaC together. CD afaC-expressing E coli isolates, however, did not commonly express pks. This may relate to the lack of the increased risk of CRC seen in CD in the absence of colonic involvement, and also raises the possibility that increased pks expression by mucosa-associated E coli might be a consequence of colitis, because the presence of pks has been shown not to affect E coli-induced inflammation in the mouse IBD model.22
Possession of the afa-1 operon defines a subgroup of E coli that have a characteristic diffuse adherence pattern to HEp-2 epithelial cells and that includes UPEC and diarrhoeagenic DAEC strains.20 21 27 31 Sequencing revealed that the afa-1 operon identified in our colonic mucosal isolate shares the same linker element draP as in the Dr operon, which encodes the Afa-related Dr adhesin. Transfection of the afa-1 operon into a non-pathogenic E coli EPI300-T1 (K-12) strain conferred the ability not only to adhere to HEp-2 but also to invade this and other cell lines, thus conferring part of the CD AIEC phenotype. The wild-type strain HM358 shows still greater ability to adhere to and invade than the afa-1-transfected E coli K-12 strain, implying that other adhesins/invasins are also involved and this is confirmed by invasion to I-407 seen in occasional afa-negative isolates.
The afa-1 operon does not confer the other phenotypic property reported for AIEC and relevant to CD pathogenesis, that is, replication within macrophages. Two genes htrA and dsbA are already known to support AIEC LF82 replication within macrophages, both encoding stress tolerance proteins that reduce bacterial killing within phagolysosomes.17 18 Colonic mucosal DAEC isolates from CD and CRC patients are shown here also to possess htrA and dsbA needed to complete the AIEC phenotype.
UPEC with the DAEC phenotype have previously been shown to invade epithelial cells in vitro but it has been uncertain whether or not they invade fully differentiated intestinal cells.32 It should be noted that the same also applies to AIEC isolates from CD, which have not been convincingly seen within intestinal epithelial cells in human mucosal samples. Indeed, even bona fide intestinal pathogens such as Salmonella spp., Shigella spp., Mycobacteria and Cholera vibrios require, as their initial portal of entry, translocation across the specialised M cells that overlie Peyer's patches and lymphoid follicles.33 It seems likely that for CD-associated E coli also, initial invasion occurs in vivo via M cells, a process that probably requires possession both of LpfA, shown here to be common among CD DAEC, and FimH.19 34 This accords with evidence that Peyer's patches in the distal ileum and lymphoid follicles in the colon are the sites for the earliest lesions (aphthoid ulcers) in CD.34 35 It is intriguing that Peyer's patches and lymphoid follicles affected by early Crohn's lesions have surrounding neovascularisation that may be seen as a ‘red ring sign’ on colonoscopy36 and enhanced by previous fluorescein injection,37 a phenomenon that could plausibly reflect angiogenesis driven by Afa-positive E coli.
Mucosa-associated AIEC have been reported particularly in the ileum of CD patients,2 3 14 although they also occur in the colon,4–6 and studies that include ileal and colonic samples from the same individuals have shown mucosa-associated E coli, if present, throughout the terminal ileum and colon.7 Their adhesion to ileal mucosa requires overexpression of CEACAM6, occurring in response to inflammation.38 39 The ‘paradigm’ ileal AIEC LF82,2 is afaC negative,16 although other ileal isolates tested were found to be afaC positive. It seems likely from the studies presented here, that DAEC possessing afa-1 may be better suited to colonisation of the colonic environment.
Members of the Afa/Dr family commonly use a glycosylphosphatidylinositol-anchored protein, the decay-accelerating factor (DAF), as a cellular receptor.20 DAF is apically localised in differentiated Caco2 cells.40 Afa/Dr adhesins also bind variably to CEACAM-1, CEACAM-5 (CEA) and CEACAM-6.41 DAEC binding induces the recruitment of DAF and CEA family receptors around adhering bacteria by a lipid raft-dependent mechanism, which initiates internalisation and cell signalling.29 40–43 Both CEACAM-6 and DAF are upregulated in IBD and CRC,44 45 and thus could favour Afa/Dr-expressing E coli colonisation. DAEC infection of the human colonic T84 cell line promotes IL-8 release and neutrophil transepithelial migration, which in turn induces TNFα and IL-1β-dependent upregulation of DAF.46
It has been shown previously that DAEC are partly resistant to phagocytosis by neutrophils.47 Recombinant E coli expressing Afa/Dr adhesins, including Afa-1, Afa-III, Dr and F1845, adhere to neutrophils but remain extracellular.48 The present study suggests that Afa also confers resistance to uptake by macrophages.
It is interesting that the presence of Afa-1, although conferring the ability to invade some epithelial cell lines, does not confer the ability to translocate across M cells. Although this has been shown here using an in vitro M-cell model, we have previously shown excellent correlation between results obtained in this model for human colonic mucosa-associated E coli and their ability to translocate across human FAE in ileal explants cultured in Ussing chambers.28 M cells are phagocytic in vitro, even for inert particles, and it may be that translocation across M cells bears more relationship to phagocytosis than to invasion. Translocation is dependent not only on the possession of LpfA but also on interaction between FimH and its receptor, GP2, selectively expressed by M cells.19 34 The majority of the E coli isolates shown here to possess afaC, dsbA, htrA and lpfA, also possess fimH. It is intriguing that circulating anti-pancreatic antibodies found in CD patients have GP2 as their epitope.49 It seems plausible that a combination of bacterial protein and attached GP2 may be presented as foreign antigen in a manner analogous to the co-presentation of gliadin peptide and tissue transglutaminase in the development of the celiac-associated autoantibody.
Our data show a higher prevalence of colonic mucosal afaC-positive E coli in patients with ileal and/or colonic CD and CRC than in controls. Previous studies of E coli isolates from patients with CD did not find significant differences in afaE-3 or afa/draBC prevalence in CD patients nor in afa/draBC frequency between AIEC/non-AIEC isolates.10 11 However, the same research group recently reported afaE-3 association in approximately 24% of E coli isolates of newly diagnosed CD patients.50 One major difference with those studies is the choice of primers used to screen isolates. The primers used in the present study target all afa strains, irrespective of the afaE subtype.25
DAEC not only promote VEGF secretion by epithelial cells but also induce epithelial–mesenchymal transition (EMT)29 implicated in carcinoma progression.51 EMT contributes to intestinal fibrosis in a mouse model of CD, and EMT markers have also been detected in Crohn's fistulas.52 53 VEGF/VEGFR2 signalling similarly links between inflammation and colitis-associated cancer and promotes tumour growth in vivo.30
The association between colonic mucosal DAEC and CRC adds to growing evidence linking bacteria with CRC pathogenesis. We have previously speculated that bacterial–epithelial interactions might be particularly important in progression from dysplasia to cancer.54 Dysplastic mucosa is usually goblet cell depleted and lacks overlying mucus. Moreover, the underlying glycocalyx is sparse. It is therefore much easier for bacteria to gain direct contact with the mucosal surface, a location that is relatively sterile in the normal colon. This would allow interaction between bacterial components and Toll-like receptors, with subsequent downstream signalling via MyD88 to nuclear factor κ B activation. Epithelial nuclear factor κ B activation, rather than histological inflammation, has been implicated as the mechanism for inflammation-associated CRC,55 moreover MyD88-deficient mice cross-bred onto Apcmin mice show markedly reduced tumour formation.56 The ability of pks-expressing E coli to damage DNA probably makes these bacteria particularly dangerous to the host if they become established in close contact with the colonic epithelium. If epithelial-associated bacteria such as DAEC play a causative role in CRC and CD then dietary consumption of soluble plant fibres that prevent mucosal recruitment of bacteria5 28 may be protective against both conditions.
The strong association between colonic mucosal afa-positive DAEC and both CD and sporadic CRC suggests a possible role for DAEC in the pathogenesis of both conditions, but does not imply that the mechanisms involved will be the same for both. Therefore, co-expression of lpfA, important for M-cell translocation, is relevant to CD but unlikely to be relevant to CRC. Co-expression of the genotoxicity island pks, possession of which confers the ability to induce breaks in double-stranded DNA, is relevant to CRC but not obviously to IBD. Therefore, the possible mechanisms for E coli-induced carcinogenesis may be independent from any effects on IBD pathogenesis. The link with Afa expression probably relates to the propensity for DAEC to colonise the colonic mucosa although studies have yet to be performed to address this directly. Intervention studies will ultimately be needed to assess the role of DAEC in the pathogenesis of CD and CRC.
Supplementary Material
Footnotes
Contributors: BJC and JMR contributed equally. BJC, JMR and CW obtained funding and with JRM, designed research. AA, BJC, CC, CLR, FS, MKF, MP-H, PKF, PK and NH performed experiments. BJC, CW, MP-H, MKF, JRM and JMR performed analyses and interpretation of data. BJC, JMR and MP-H drafted the manuscript, with critical revision by all authors.
Funding: MPH, FS, PKF and PK were supported by the NIHR Biomedical Research Centre for Microbial Diseases, Liverpool (01CD1). MKF was funded by Crohn's and Colitis UK (M/08/1). AA was supported by Dammam University, Saudi Arabia. JRM and CC were supported by Science Foundation Ireland. BJC acknowledges funding from the North West Cancer Research Fund, UK (CR727) and the support of the European Science Foundation, in the framework of the Research Networking Programme, the European Network for Gastrointestinal Health Research. The sponsors had no role in the study design, nor in the collection and interpretation of data.
Competing interests: JMR is/has been a member of advisory boards for Atlantic, Procter & Gamble and Falk, has received speaking honoraria from Abbott, Falk, Ferring, Glaxo Smith Kline, Procter & Gamble and Schering Plough and, with the University of Liverpool and Provexis PLC, holds a patent for use of a soluble fibre preparation as maintenance therapy for Crohn's disease. BJC has received a speaking honorarium from Amgen Inc.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We affirm that all data necessary for a reader of Gut to understand and evaluate the conclusions of the paper will be archived in an approved database and made available to any reader. After publication, all reasonable requests for materials and data will be fulfilled. There is a University of Liverpool MTA regarding the human mucosal E coli. We can confirm that to date all requests from researchers within the international scientific community for isolates from the Liverpool archive have been granted and materials supplied.
Accession number: The nucleotide sequence of the afa-1 operon from E coli HM358 has been submitted to GenBank (http://www.ncbi.nlm.nih.gov/genbank/); accession number JN688153.
References
- 1.Flanagan P, Campbell BJ, Rhodes JM. Bacteria in the pathogenesis of inflammatory bowel disease. Biochem Soc Trans 2011;39:1067–72 [DOI] [PubMed] [Google Scholar]
- 2.Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 1998;115:1405–13 [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, et al. Abnormal microbiota composition in the ileocolonic mucosa of Crohn's disease patients as revealed by PCR-denaturing gradient gel electrophoresis. Inflamm Bowel Dis 2006;12:1136–45 [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Medina M, Aldeguer X, Lopez-Siles M, et al. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn's disease. Inflamm Bowel Dis 2009;15:872–82 [DOI] [PubMed] [Google Scholar]
- 5.Martin HM, Campbell BJ, Hart CA, et al. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology 2004;127:80–93 [DOI] [PubMed] [Google Scholar]
- 6.Mylonaki M, Rayment NB, Rampton DS, et al. Molecular characterization of rectal mucosa-associated bacterial flora in inflammatory bowel disease. Inflamm Bowel Dis 2005;11:481–7 [DOI] [PubMed] [Google Scholar]
- 7.Willing B, Halfvarson J, Dicksved J, et al. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm Bowel Dis 2009;15:653–60 [DOI] [PubMed] [Google Scholar]
- 8.Swidsinski A, Khilkin M, Kerjaschki D, et al. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology 1998;115:281–6 [DOI] [PubMed] [Google Scholar]
- 9.Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002;122:44–54 [DOI] [PubMed] [Google Scholar]
- 10.Kotlowski R, Bernstein CN, Sepheri S, et al. High prevalence of Escherichia coli belonging to the B2 and D phylogenetic groups in inflammatory bowel disease. Gut 2007;56:669–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sepehri S, Kotlowski R, Bernstein CN, et al. Microbial diversity of inflamed and non-inflamed gut biopsy tissues in inflammatory bowel disease. Inflamm Bowel Dis 2007;13:675–83 [DOI] [PubMed] [Google Scholar]
- 12.Glasser AL, Boudeau J, Barnich N, et al. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect Immun 2001;69:5529–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian S, Roberts CL, Hart CA, et al. Replication of colonic Crohn's disease mucosal Escherichia coli isolates within macrophages and their susceptibility to antibiotics. Antimicrob Agents Chemother 2008;52:427–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 2004;127:412–21 [DOI] [PubMed] [Google Scholar]
- 15.Ryan P, Kelly RG, Lee G, et al. Bacterial DNA within granulomas of patients with Crohn's disease—detection by laser capture microdissection and PCR. Am J Gastroenterol 2004;99:1539–43 [DOI] [PubMed] [Google Scholar]
- 16.Miquel S, Peyretaillade E, Claret L, et al. Complete genome sequence of Crohn's disease-associated adherent-invasive E. coli strain LF82. PLoS ONE 2010;5:e12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bringer MA, Barnich N, Glasser AL, et al. HtrA stress protein is involved in intramacrophagic replication of adherent, invasive Escherichia coli strain LF82 isolated from a patient with Crohn's disease. Infect Immun 2005;73:712–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bringer MA, Rolhion N, Glasser AL, et al. The oxidoreductase DsbA plays a key role in the ability of the Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 to resist macrophage killing. J Bacteriol 2007;189:4860–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chassaing B, Rolhion N, Vallee A, et al. Crohn disease-associated adherent-invasive E. coli bacteria target mouse and human Peyer's patches via long polar fimbriae. J Clin Invest 2011;121:966–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Servin AL. Pathogenesis of Afa/Dr diffusely-adhering Escherichia coli. Clin Microbiol Rev 2005;18:264–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulvey MA. Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol 2002;4:257–71 [DOI] [PubMed] [Google Scholar]
- 22.Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012;338:120–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buc E, Dubois D, Sauvanet P, et al. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 2013;8:e56964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001;2:361–7 [DOI] [PubMed] [Google Scholar]
- 25.Bilge SS, Clausen CR, Lau W, et al. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol 1989;171:4281–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Bouguenec C, Lalioui L, du Merle L, et al. Characterization of AfaE adhesins produced by extraintestinal and intestinal human Escherichia coli isolates: PCR assays for detection of Afa adhesins that do or do not recognize Dr blood group antigens. J Clin Microbiol 2001;39:1738–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nataro JP, Kaper JB, Robins-Browne R, et al. Patterns of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J 1987;6:829–31 [DOI] [PubMed] [Google Scholar]
- 28.Roberts CL, Keita AV, Duncan SH, et al. Translocation of Crohn's disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut 2010;59:1331–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cane G, Ginouves A, Marchetti S, et al. HIF-1alpha mediates the induction of IL-8 and VEGF expression on infection with Afa/Dr diffusely adhering E. coli and promotes EMT-like behaviour. Cell Microbiol 2010;12:640–53 [DOI] [PubMed] [Google Scholar]
- 30.Waldner MJ, Wirtz S, Jefremow A, et al. VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. J Exp Med 2010;207:2855–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labigne-Roussel A, Schmidt MA, Walz W, et al. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol 1985;162:1285–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Bouguenec C, Servin AL. Diffusely adherent Escherichia coli strains expressing Afa/Dr adhesins (Afa/Dr DAEC): hitherto unrecognized pathogens. FEMS Microbiol Letts 2006;256:185–94 [DOI] [PubMed] [Google Scholar]
- 33.Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin Immunol 1999;11:193–203 [DOI] [PubMed] [Google Scholar]
- 34.Hase K, Kawano K, Nochi T, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M-cells initiates mucosal immune response. Nature 2009;462:226–30 [DOI] [PubMed] [Google Scholar]
- 35.Fujimura Y, Kamoi R, Iida M. Pathogenesis of aphthoid ulcers in Crohn's disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut 1996;38:724–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krauss A, Agaimy A, Neumann Het al. Characterization of lymphoid follicles with red ring signs as first manifestation of early Crohn's disease by conventional histopathology and confocal laser endomicroscopy. Int J Clin Exp Pathol 2012;5:411–21 [PMC free article] [PubMed] [Google Scholar]
- 37.Maunory V, Mordon S, Geboes Ket al. Early vascular changes in Crohn's disease: an endoscopic fluorescence study. Endoscopy 2000;32:700–5 [DOI] [PubMed] [Google Scholar]
- 38.Barnich N, Darfeuille-Michaud A. Abnormal CEACAM6 expression in Crohn disease patients favors gut colonization and inflammation by adherent-invasive E. coli. Virulence 2010;1:281–2 [DOI] [PubMed] [Google Scholar]
- 39.Barnich N, Carvalho FA, Glasser AL, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn's disease. J Clin Invest 2007;117:1566–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernet-Camard MF, Coconnier MH, Hudault S, et al. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut 1996;38:248–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korotkova N, Yarova-Yarovaya Y, Tchesnokova V, et al. Escherichia coli DraE adhesin-associated bacterial internalization by epithelial cells is promoted independently by decay-accelerating factor and carcinoembryonic antigen-related cell adhesion molecule binding and does not require the DraD invasin. Infect Immun 2008;76:3869–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goluszko P, Popov V, Selvarangan R, et al. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell-line. J Infect Dis 1997;176:158–67 [DOI] [PubMed] [Google Scholar]
- 43.Guignot J, Peiffer I, Bernet-Camard MF, et al. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect Immun 2000;68:3554–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berstad AE, Brandtzaeg P. Expression of cell membrane complement regulatory glycoproteins along the normal and diseased human gastrointestinal tract. Gut 1998;42:522–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koretz K, Bruderlein S, Henne C, et al. Decay-accelerating factor (DAF, CD55) in normal colorectal mucosa, adenomas and carcinomas. Br J Cancer 1992;66:810–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Betis F, Brest P, Hofman V, et al. Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesins. Infect Immun 2003;71:1774–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brest P, Betis F, Cuburu N, et al. Increased rate of apoptosis and diminished phagocytic ability of human neutrophils infected with Afa/Dr diffusely adhering Escherichia coli strains. Infect Immun 2004;72:5741–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson JR, Skubitz KM, Nowicki BJ, et al. Nonlethal adherence to human neutrophils mediated by Dr antigen-specific adhesins of Escherichia coli. Infect Immun 1995;63:309–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roggenbuck D, Hausdorf G, Martinez-Gamboa L, et al. Identification of GP2, the major zymogen granule membrane glycoprotein, as the autoantigen of pancreatic antibodies in Crohn's disease. Gut 2009;58:1620–28 [DOI] [PubMed] [Google Scholar]
- 50.Sepehri S, Khafipour E, Bernstein CN, et al. Characterization of Escherichia coli isolated from gut biopsies of newly diagnosed patients with inflammatory bowel disease. Inflamm Bowel Dis 2011;17:1451–63 [DOI] [PubMed] [Google Scholar]
- 51.Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871–90 [DOI] [PubMed] [Google Scholar]
- 52.Bataille F, Rohrmeier C, Bates R, et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn's disease. Inflamm Bowel Dis 2008;14:1514–27 [DOI] [PubMed] [Google Scholar]
- 53.Flier SN, Tanjore H, Kokkotou EG, et al. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem 2010;285:20202–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med 2002;8:10–16 [DOI] [PubMed] [Google Scholar]
- 55.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004;118:285–96 [DOI] [PubMed] [Google Scholar]
- 56.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 2007;317:124–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.