Abstract
Coding sequences for major trichome regulatory genes, including the positive regulators GLABRA 1(GL1), GLABRA 2 (GL2), ENHANCER OF GLABRA 3 (EGL3), and TRANSPARENT TESTA GLABRA 1 (TTG1) and the negative regulator TRIPTYCHON (TRY), were cloned from wild Brassica villosa, which is characterized by dense trichome coverage over most of the plant. Transcript (FPKM) levels from RNA sequencing indicated much higher expression of the GL2 and TTG1 regulatory genes in B. villosa leaves compared with expression levels of GL1 and EGL3 genes in either B. villosa or the reference genome species, glabrous B. oleracea; however, cotyledon TTG1 expression was high in both species. RNA sequencing and Q-PCR also revealed an unusual expression pattern for the negative regulators TRY and CPC, which were much more highly expressed in trichome-rich B. villosa leaves than in glabrous B. oleracea leaves and in glabrous cotyledons from both species. The B. villosa TRY expression pattern also contrasted with TRY expression patterns in two diploid Brassica species, and with the Arabidopsis model for expression of negative regulators of trichome development. Further unique sequence polymorphisms, protein characteristics, and gene evolution studies highlighted specific amino acids in GL1 and GL2 coding sequences that distinguished glabrous species from hairy species and several variants that were specific for each B. villosa gene. Positive selection was observed for GL1 between hairy and non-hairy plants, and as expected the origin of the four expressed positive trichome regulatory genes in B. villosa was predicted to be from B. oleracea. In particular the unpredicted expression patterns for TRY and CPC in B. villosa suggest additional characterization is needed to determine the function of the expanded families of trichome regulatory genes in more complex polyploid species within the Brassicaceae.
Introduction
Trichomes are protruding structures which protect plant surfaces against dehydration and insect pests, and provide a storage organ to handle metal toxicity [1], [2]. Trichome development has been studied extensively at the molecular level in non-glandular trichomes of the model plant, Arabidopsis thaliana, in which trichome regulation is controlled by multiple transcription factors. Among these, a number of major positive regulatory genes for trichome initiation have been studied in detail, including GLABRA 1(GL1), GLABRA 2 (GL2), GLABRA 3 (GL3), ENHANCER OF GLABRA 3 (EGL3), and TRANSPARENT TESTA GLABRA 1 (TTG1) [3]. GL1 was isolated by gene tagging [4] and is a member of the R2R3 activator MYB gene family in A. thaliana [5]. GL3 and EGL3 encode members of an IIIf subfamily of basic Helix-Loop-Helix (bHLH) proteins [6]. TTG1 encodes a WD40 domain protein [7]. GL1, TTG1 and GL3/EGL3 are positive patterning proteins which form an activator MYB-bHLH-WD40 (MBW) tri-protein complex that induces the expression of an immediate downstream target gene, GLABRA 2 (GL2). GL2 encodes a homeobox transcription factor and its induction is required both for trichome cell specification and for subsequent phases of trichome morphogenesis such as cell expansion, branching, and cell wall maturation [8]–[10]. Mutations in any of these four above-mentioned genes reduce trichome initiation and the density of trichome patterning in A. thaliana [11]–[14] A yeast two hybrid assay demonstrated a direct physical interaction between GL3 and GL1, TTG1, and itself; GL1 and GL3 co-overexpression confirmed their interaction, but GL1 and TTG1 do not physically interact [15]. Mutations in GL3 modestly reduce trichome density, branching, DNA endoduplication, and trichoblast size [11], [15]. Egl3 mutant plants have no obvious trichome defects, but gl3egl3 double mutants show a complete glabrous phenotype [14] due to some functional overlap between the two genes [16].
TRIPTYCHON (TRY) is the first identified negative regulator of Arabidopsis trichome initiation [11] and encodes a small single R3-MYB repeat protein which lacks the R2 activation domain [6], [17]. The activator proteins of the Arabidopsis MBW tri-protein complex are assumed to locally activate their own expression and that of TRY [18]. TRY moves through plasmodesmata into adjacent cells where it and several other potent inhibitor proteins such as CAPRICE (CPC), ENHANCER OF TRY and CPC1 and 2 (ETC1 and ETC3) can competitively bind to the MBW complex to release GL1 [19]–[23]. This release of GL1 renders the MYB complex inactive at stimulating GL2 expression and prevents trichome initiation in neighbouring cells [2], [18]. At the same time, the GL3 protein “traps” and reduces the mobility of TTG1 proteins (which can move from neighboring cells into trichome-initiating cells) by binding to them in trichome-initiating cells [3], [24], [25].
Trichomes are found on other species within the Brassicaceae, but very few details are known about Brassica trichome genes and their regulation compared with the model Arabidopsis. Brassica napus is an amphidiploid species originating from a cross between Brassica rapa (of the A genome) and Brassica oleracea (of the C genome) [26]. Leaf trichomes are found in substantial numbers on many lines of B. rapa, but not on B. oleracea, and the distribution on B. rapa is patchy rather than even, the patterning is light, and occurs mainly on only a few tissues (eg. leaves and stems) [27]. Leaf trichomes are mainly found on the adaxial side of the A. thaliana and B. rapa leaves. In contrast, B. napus is practically devoid of trichomes.
Trichome genes that have been characterized from the Brassicas only include B. rapa TTG1, GL1, and GL2, but analysis of a full set of regulatory genes has never been conducted within a single species. An orthologue, BrTTG1, isolated from a brown-seeded hairy B. rapa genotype, was found to functionally complement (rescue) an A. thaliana ttg1 mutant [28], while the orthologue isolated from B. rapa yellow-seeded glabrous germplasm was not functional. B. rapa leaf hairiness was associated with nucleotide polymorphisms in the DNA-binding domain of the BrGL1 gene [29], and several BrGL1 alleles with varying impact on phenotype have been found. B. rapa harbouring the B-allele of BrGL1 produces hairy plants when transformed with the A-allele and hairless plants with the C-allele [30]. GL2 promoter regions in A. thaliana and B. napus have low homology and confer differential expression patterns, such that the BnGL2 promoter introduced into A. thaliana is only expressed at an early stage of trichome development, whereas the native AtGL2 promoter is expressed throughout the whole process of trichome development [31]. Moreover, glabrous B. napus plants expressing the A. thaliana GL3 from a constitutive promoter develop an extremely dense coverage of trichomes on stems and young leaves [32] compared with overexpression in Arabidopsis. These findings indicate differences between the Brassica and A. thaliana genes that can impact trichome function and trichome patterning.
Brassica villosa is a wild C genome relative of glabrous B. oleracea, yet B. villosa is even more densely covered, with evenly distributed trichomes over most surfaces of the plant, than B. napus transformed with the AtGL3 gene [32], [33]. Like other Brassica species, B. villosa trichomes are without branches, whereas trichomes on the model species, A. thaliana, are multi-branched. The limited information available for Brassica trichome genes and the dense trichome patterning on B. villosa organs prompted us to dissect the trichome regulatory gene network in this species. In this regard, we successfully amplified B. villosa orthologues to the GL1, GL2, EGL3, TTG1 and TRY coding sequences and compared their expression patterns and sequence structure with corresponding genes available from B. rapa, B. oleracea, B. napus, and A. thaliana.
Materials and Methods
Plant Material
A. thaliana (Columbia), Brassica oleracea (T01000 DH3), and Brassica napus (cv. Westar) seeds were obtained from germplasm collections at the Saskatoon Research Centre, Saskatoon, Canada. Brassica villosa Biv. subsp. drepanensis seeds were obtained from the Centre for Genetic Resources, Wageningen, The Netherlands. Seeds of B. rapa (cv. Echo) were obtained from Plant Gene Resources Canada, Saskatoon, Canada. All seeds were grown in soil-less potting mixture in a controlled greenhouse environment (16 h light/8 h dark, 20/17°C) supplemented with halogen lights at Agriculture and Agri-Food Canada, Saskatoon, Canada.
Amplification and Sequence Analysis of Five Major Trichome Genes from B. villosa
Specific primers to amplify Brassica villosa (Bv) orthologues to GL1, GL2, EGL3, TTG1 and TRY were designed from coding sequences from B. napus and B. rapa (Table 1). The orthologues were amplified from cDNA developed from B. villosa seedling leaf RNA (isolated as described under gene expression profiling). At the time of amplification, the B. rapa EGL3 (accession HM208589.1) was mis-annotated as GL3 in NCBI, but the nomenclature was recently corrected. PCR was conducted using 32 cycles of 94°C for 30 sec, 56°C for 30 sec, and 72°C for up to 3 minutes (based on the length of the template cDNA), a final extension time of 5 minutes at 72°C, and PFU Ultra II fusion HotStart DNA polymerase enzyme (Stratagene, USA). Primers were designed starting at the translational start and ending with the 3′ ends of the B. napus and B. rapa cDNA sequences (including restriction sites) except for TRY, for which the forward primer was designed from the 72nd residue upstream from the translational start site (Table 1). At the time of cloning, only an unannotated EST was available for TRY rather than a cDNA with a verified translational start site. Amplified sequences were cloned into the pGEM-Teasy vector (Promega, Madison, WI, USA), and then several amplicons (clones) per gene were sequenced in the DNA Technologies Laboratory of National Research Council, Saskatoon, and used to search the NCBI database using BLASTN to determine gene identity.
Table 1. Primers used to isolate/analyze GL1, GL2, GL3, TTG1 and TRY genes from B. villosa.
| Primers | NCBI accession | Sequence (5′ to 3′) |
| Coding Sequences from B. napus 1 | ||
| GL1-F | HQ162473.1 | GTCAGGATCCATGAGAACGAGGAGAAGAACAGA |
| GL1-R | GTCACTGCAGCTAGAGACAGTAGCCAGTATCA | |
| GL2-F | EU826520.1 | GTCAGGATCCATGTCAATGGCCGTCGAGATGTCA |
| GL2-R | ATATCTGCAGTGTTGTGCAGCGTGACAGAGACGA | |
| TRY-F | EE451172.1 | AGCTGGATCCGCTTGCATTCTCCAACT |
| TRY-R | CGCACTGCAGGCAATTTCGTTATGCTATATG | |
| Coding Sequences from B. rapa 1 | ||
| EGL3-F | HM208589.1 | GTCAGGATCCATGGCTGCTGTAGAAAACAG |
| EGL3-R | GCAGCTGCAGAGTGCATCTTGAATCATTCCT | |
| TTG1-F | HM208590.1 | TAGAGGATCCATGGACAACGCAGCTCCGGACT |
| TTG1-R | AGTCGGTACCTCAAACTCTAAGGAGCTGCA | |
| Conserved Regions (for Q-PCR) 2 | ||
| GL1-F | ACTGGGCTGAAGAGGTGTGGA | |
| GL1-R | GAGATGAGTGTTCCAGTGA | |
| GL2-F | CGCTGGCCGGGAGAAAGAGC | |
| GL2-R | GGAGGTTTTTTCTGGATGAA | |
| EGL3-F | ACATTCAATGGAGTTACGGA | |
| EGL3-R | AGAGATTCGTAAAGCTCTCT | |
| TTG1-F | CTCTGGGAGGTCAACGAA | |
| TTG1-R | ATGCTGCACGTGCCTAAC | |
| TRY-F | CATCACTCCTCTTCTCACA | |
| TRY-R | TGTGGTGGGGAAGAAAACAGA | |
| BnEF1F (B. napus) | CCCATTCGTCCCCATCTCTGGA | |
| BnEF1R (B. napus) | ACGGAGGGGCTTGTCCGAGG | |
Forward (F) primers were designed at the 5′ coding end and reverse (R) primers to the 3′ end of cDNA sequences from B. napus for GL1 and GL2 and from B. rapa for EGL3 and TTG1. Forward primers for TRY were designed 72 nucleotides before coding region and reverse primer at 3′ end of the cDNA from B. rapa. Underlined sequences indicate incorporated restriction enzyme sites; BamHI and PstI sites in the GL1, GL2, EGL3 and TRY forward and reverse primers, respectively; BamHI and KpnI sites in the TTG1 forward and reverse primers, respectively.
Primers for Q-PCR were designed to conserved regions based on alignments between the A. thaliana homologue and the homologues from four Brassica species. B nEF1F/BnEF1R are endogenous reference gene primers.
Construction of Transgenic Expression Vectors and Transformation into the Arabidopsis Wild Type
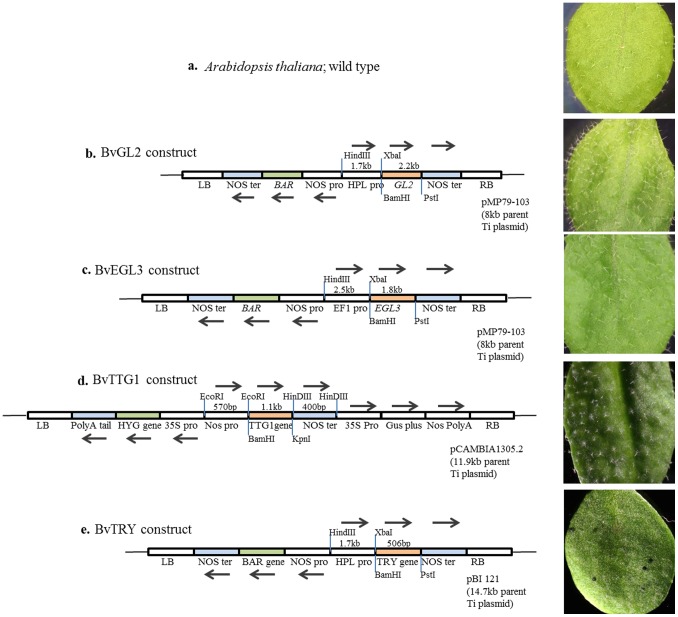
Binary vectors pMP79-103 and pBI121 were initially modified by inserting a 1.7 kb Hydroperoxide Lyase (HPL) [34] promoter between HindIII/XbaI sites, and then a 2.2 kb BvGL2 and 506 bp BvTRY cDNA sequence were cloned, respectively, downstream of the promoter using BamHI/KpnI restriction sites. In a separate construct, pMP79-103 vector was modified by inserting a 2.5 kb Elongation factor 1(EF1) promoter between HindIII/XbaI sites, and then a 1.8 kb BvEGL3 cDNA sequence was cloned using BamHI/PstI restriction sites. pCAMBIA1305.2 vector was modified by cloning a cassette carrying 570 bp NOS promoter, 1.1 kb cDNA clone of TTG1 and 400 bp NOS terminator between EcoRI and HindIII restriction sites. All of these binary constructs were individually transformed into Agrobacterium tumefaciens strain GV3101 by electroporation. Arabidopsis plants were transformed with respective plasmids using the floral dip method [34]. Transformed plants were selected on the basis of respective vector based resistance markers. Pictures were taken from T1 generation plants three weeks after germination.
RNA Sequencing for Verification and Expression Profiling of Individual Gene Copies
Fresh-frozen cotyledons and first true-leaves (before the second true leaf emergence) of B. villosa and B. oleracea were collected for RNA sequencing from seedlings (5–7 plants per replicate) for 3 biological replicates. RNA was extracted using standard methods, then developed into libraries using a TruSeq RNA sample preparation kit (Illumina, San Diego, CA, USA). Library sequencing (100 cycles) was conducted from both ends on an Illumina HiSeq 2000. A total of 51 Gb of B.oleracea reads (Cotyledons; 26 Gb and true leaves; 25 Gb) and 60 Gb of B.villosa reads (Cotyledons; 33 Gb and true leaves; 27 Gb) were obtained. The RNAseq data was trimmed using trimmomatic ver.0.30 with minimum quality score of 15, removing the first 12 bp, and a minimum length of 20, which was then aligned to the genome of B. oleracea with TopHat ver. 2.0.7 (with parameters, including minimum intron length (i) of 20, maximum intron length (I) of 11000, and distance between pair ends (r) of 30) [35]. The aligned RNAseq reads were assembled into transcripts and their relative abundance was estimated as fragments per kilobase of exon per million fragments mapped (FPKM) using Cufflinks software [35]. Differential expression and statistical analysis of individual gene copies was conducted using Cummerbund package in R; http://www.r-project.org/. Raw data from the RNAseq experiment was deposited to NCBI with SRA accession number, SRP035213.
Q-PCR for Total Gene Expression Profiling Relative to B. napus
First true leaves were collected separately from three individual plants at the same stage as mentioned above for B. villosa for each of the four other species mentioned under plant material. Total RNA was extracted from the seedling leaves (three independent preparations per species) with a commercial RNA-Easy mini kit (Qiagen, Valencia, CA, USA) and cDNA synthesized using Superscript II™ (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. Quantitative real time-PCR (Q-PCR) was conducted using a Platinum SYBR Green Super Mix-UDG kit (Invitrogen), a CFX96 Real-Time PCR system (BioRad, Hercules, CA, USA), and primers to conserved regions for GL1, GL2, EGL3, TTG1 and TRY within all five species (Table 1). Even though the B. napus EGL3 coding sequence was not available, the primers to EGL3-specific conserved regions in other species amplified the B. napus transcripts in Q-PCR reactions. For each pair of gene-specific primers, melting curve analysis was conducted to determine the melting temperature and to ensure a single PCR amplicon of the expected length. Three independent RNA preparations were assayed per species and data was analyzed using CFX Manager Software (BioRad). The expression level of each mRNA was determined using the mean cycle threshold (ΔCT) value normalized to an endogenous reference gene, BnEF1 (Table 1). Mean values with corresponding standard errors were expressed relative to glabrous B. napus leaf tissue and analysed by one-way analysis of variance (ANOVA) using PROC GLM in SAS ver. 9.2 (SAS, 2008) and a completely randomized design (CRD) (with genes and species as the two main factors). Significantly different means were detected by Fisher’s protected Least Significant Difference (LSD) tests.
Bioinformatic Analysis of Brassica Trichome Regulatory Genes
A. thaliana nucleotide and protein sequence information for GL1, GL2, EGL3, TTG1, and TRY was collected from http://www.arabidopsis.org. B. villosa sequences were obtained as above. B. napus sequences for GL1, GL2, TTG-2 and TRY were obtained from EST databases available at http://rapa.agr.gc.ca and http://blast.ncbi.nlm.nih.gov. B. napus EST databases were searched at http://napus.agr.gc.ca/aped (B. napus EGL3 sequence was unavailable). B. rapa sequence information was collected from http://brassicadb.org/brad, http://www.plantgdb.org/BrGDB, and http://www.brassica-rapa.org/BRGP/index.jsp. B. oleracea sequences were collected from the B. oleracea genome database at Agriculture and Agri-Food Canada, Saskatoon (Parkin, unpublished). Nucleotide and translated protein sequences were aligned using Clustal-W in Vector NTI 9 (Invitrogen). Molecular weight (Mr) and isoelectric point [35] were determined on translated protein sequences using http://web.expasy.org/compute_pi/. Amino acid frequencies were determined using http://emboss.bioinformatics.nl/cgi-bin/emboss/fuzzpro. Phylogenetic analysis was carried out using Molecular Evolutionary Genetics Analysis software (MEGA v5.1) and the neighbour joining method of phylogenetic inference with a bootstrap parameter of 1000 replications [36]. Initially, all six copies (ESTs) for BnTTG1 and the short (<100 bp) B. rapa sequences for BrTRY2 and BrTRY3 were used in phylogeny testing, but later only the longer overlapping BnTTG1 consensus sequence and the longer BrTRY1 were used with the other orthologues to improve the confidence (bootstrap values) of phylogenetic relationships above 50%. Protein conserved domain recognition was determined at http://www.ebi.ac.uk/Tools/pfa/iprscan/.
Evolutionary analysis was performed to obtain more information on similarity and variation between the five major trichome regulatory genes of B. villosa and orthologues within three other Brassicas and A. thaliana. A pairwise comparison of GL1, GL2, EGL3, TTG1 and TRY coding region of the orthologous genes was used to calculate the ratio of non-synonymous amino acid substitution rate (Ka) to synonymous substitution rate (Ks) using the maximum likelihood algorithm implemented in Phylogenetic Analysis by Maximum Likelihood (PAML) [37]. As with the phylogenetic analysis, all the B. napus TTG1 coding sequences and the very short B. rapa TRY2 and TRY3 sequences were initially aligned with other sequences, but later excluded from the evolutionary analysis due to lack of sufficient overlapping sequence. Generally, a Ka/Ks of 1 indicates neutral selection, Ka/Ks <1 indicates a functional constraint with purifying selection, and Ka/Ks >1 shows accelerated evolution with positive selection [37].
Results
Trichome Regulatory Gene Amplification in B. villosa
Amplification of the coding regions for B. villosa orthologues to the GL1, GL2, EGL3, TTG1 and TRY regulatory genes using primers based on available B. napus and B. rapa sequences gave single PCR bands (in some cases amplifying multiple copies). The bands were cloned, sequenced and used to search the NCBI database to confirm gene identity and to determine copy number. Three independent clones each were sequenced for the BvGL1, BvGL2, and BvTTG1 genes from B. villosa, and this was expanded to 10 clones for BvEGL3, and thirty clones for BvTRY. Alignments of the cloned sequences to private (B. oleracea) and public (B. rapa, B. napus) databases, plus RNAseq data, indicated that B. villosa contained a single copy each for GL1 and GL2 and two unique sequences for EGL3 (95% amino acid identity) (Table 2; Figure S1, protein sequence shown). Only one TTG1 copy could be isolated from B. villosa using the B. rapa primers in spite of a second copy (with only 45% nucleotide identity) in the B. oleracea database (Parkin, unpublished). Sequencing of 30 TRY clones indicated two copies from B. villosa with 100% amino acid identity (Figure S1). RNAseq suggested a 3rd copy for B. villosa TRY not seen within the 30 amplicons (Table 2).
Table 2. Database accession numbers for orthologues to the major trichome regulatory genes (GL1, Gl2, EGL3, TTG1 and TRY) in B. villosa.
| Gene | B. villosa (NCBI) | B. oleracea (Unpublished data, Dr. I. Parkin) | B. rapa (BRAD) | B. napus (NCBI) | Arabidopsis(TAIR) | Function in Overexpression And Knockdown studies in B. rapa |
| GL1 | KF188209 | Bo7g090950 | Bra025311-1 Bra039065-2 | HQ162473.1 | AT3G27920, Myb-like protein, helps in induction of trichome development | Like Arabidopsis |
| GL2 | KF188210 | Bo6g046840 | Bra003535 | EU826521.1-1 EU826520.1-2 | AT1G79840, Homeodomain proteinaffects trichomes initiation & development | Unknown |
| EGL3 | KF188207-1 KF188208-2 | Bo9g029230-1 Bo9g035460-2 | Bra027653-1 Bra027796-2 | NA | AT1G63650, a bHLH transcription factor 1, mutant has reduced trichomes | Unknown |
| TTG1 | KF188213 | Bo7g096780-1 Bo2g159360-2 | Bra009770 | EF175932.1-1 EF175931.1-2 EU192031.1-3 EF175929.1-4 EF175930.1-5 EU192030.1-6 | AT5G24520, WD-40 protein involved in trichome development | Like Arabidopsis |
| TRY | KF188211-1 KF188212-2 | Bo2g046050-1 Bo3g022870-2 Bo9g110930-3 | Bra022637-1 Bra026297-2 Bra029089-3 | EE451172(EST) | AT5G53200, Myb-like protein, mutation leads to glabrous leaves | Unknown |
Genes in the same row are closest orthologues to each other. NA = Sequence not available.
Phylogeny of B. villosa Trichome Regulatory Genes
In general, the amplified B. villosa translated coding sequences were over 90% identical to other Brassica orthologues, especially GL2 and TTG1 (Table S1), with only a few exceptions. B. villosa GL1 was only 82% similar to B. rapa GL1-2 and 75% similar to AtGL3. B. villosa EGL3-1 was 87% similar to B. oleracea, B. rapa, and Arabidopsis EGL3-2, while B. villosa EGL3-2 was 82% similar to the three latter sequences. B. villosa TRY-1 and TRY-2 were 86% and 87% similar to B. oleracea TRY-3 and B. rapa TRY-1, respectively. In contrast, B. villosa TTG1-1 and all other orthologues were only 48% similar to B. oleracea TTG1-2.
Phylogenetic trees using translated protein sequences indicate that GL1 and GL2 from hairy B. villosa are closest to several sequences from the two non-hairy species B. napus and B. oleracea (Figure 1). The two BvEGL3 sequences both showed strong relationships to EGL3-1 from B. oleracea and B. rapa, and were less close to B. rapa and B. oleracea EGL3-2 sequences, which were closer to the Arabidopsis EGL3 (B. napus EGL3 sequence was not available). BvTTG1 sequence was intermediate between Arabidopsis and B. napus, BoTTG1-1, and B. rapa TTG1 sequences, and farthest from BoTTG1-2 (which was only 45% similar to BoTTG1-1). BvTRY-1 and BvTRY-2 paired together and fell nearest to the pairing between the B. napus consensus sequence and the B. oleracea TRY-3 sequences. Initially, all six copies (ESTs) for BnTTG1 and the short (<100 bp) B. rapa sequences for BrTRY2 and BrTRY3 were used in this phylogeny testing. Later (due to differences in sequence length), only the longer overlapping BnTTG1 consensus sequence and longer BrTRY1 were compared with the other orthologues, and this improved the confidence (bootstrap values) of the phylogenetic relationships.
Figure 1. Phylogenetic relationships for the five major trichome regulatory genes present in Brassica and A. thaliana.
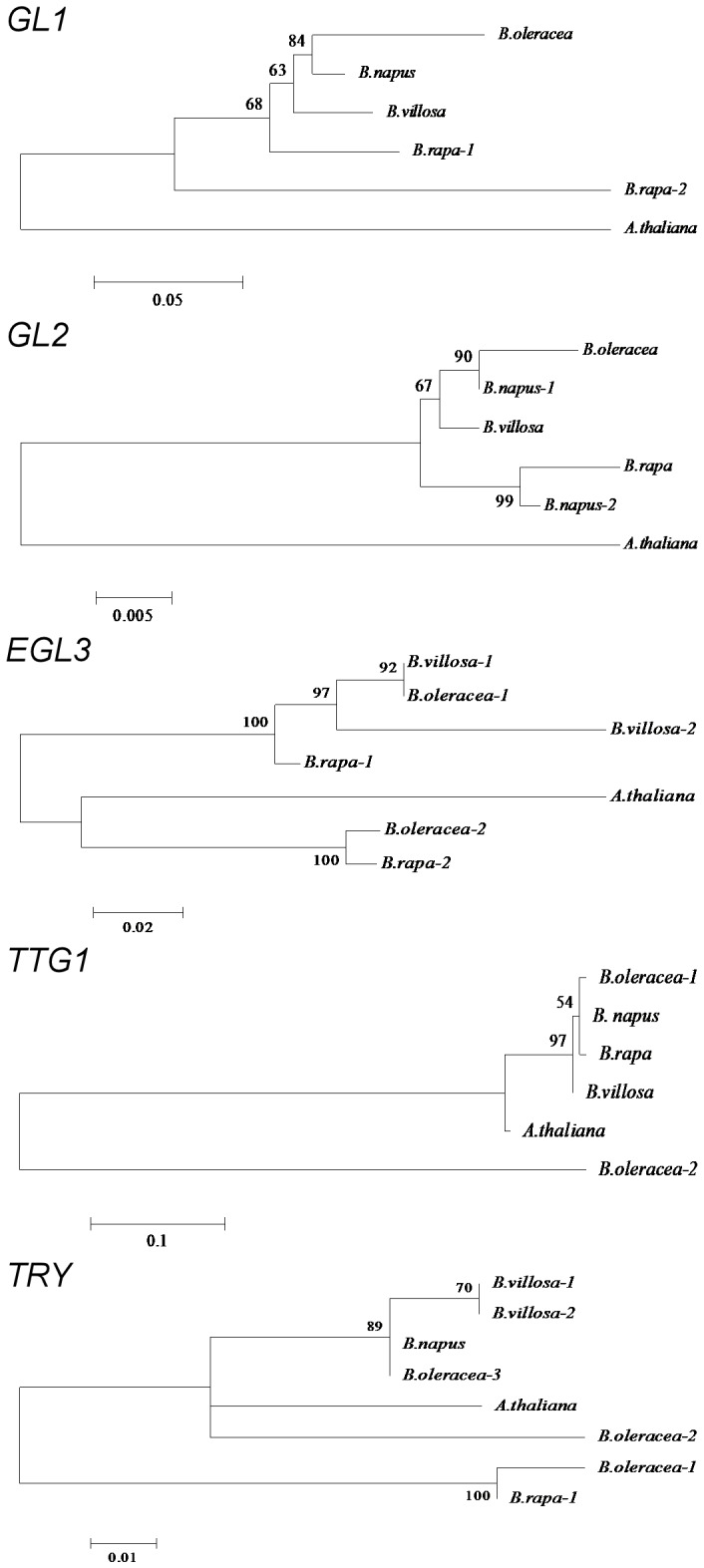
Sequences were analysed by the maximum likelihood method with bootstrap values (%) indicated (100% is implicit in vacant branching positions). Scale indicates amino acid substitutions per site. A consensus sequence based on six B. napus TTG1 copies in NCBI was used for more robust analysis rather than the individual BnTTG1 copies, which each gave very weak associations due to limited overlapping sequence. B. rapa TRY-2 and TRY-3 were also not included since their small size gave spurious weak associations of <50%. Although three copies for TRY exist in B. oleracea and B. villosa (Fig. 2), TRY-3 could not be cloned from B. villosa cDNA due to low expression.
Total Trichome Regulatory Gene Expression in Leaves of Hairy B. villosa, Diploid Brassica, and A. thaliana, Relative to Glabrous B. napus
Alignment of the B. villosa nucleotide sequences with other known Brassica and A. thaliana trichome regulatory sequences in public and private databases (data not shown) indicated conserved regions from which we designed Q-PCR primers (Table 1) to compare total expression levels of the five genes relative to orthologues of glabrous B. napus. In a preliminary Q-PCR expression experiment, all five trichome regulatory genes were highly expressed in trichome-bearing first true leaves of A. thaliana (with GL1, GL2, and EGL3 being the highest and TTG1 and TRY being the lowest) (Figure S2, insert). In contrast, expression of these orthologues in the first leaf of B. villosa, B. oleracea, B. rapa and B. napus (set at 1) (was dramatically lower than in Arabidopsis (Figure S2). GL1 and GL2 transcription was highest in B. villosa when only the four Brassica species were compared. B. villosa expression was proportionately more similar to B. rapa expression (in leaves bearing a light coverage of leaf trichomes) when total relative expression patterns of each of the four positive regulatory genes were compared across the Brassica species. Moreover, the relative expression pattern was highly distinct for the two hairy Brassica species compared with the glabrous B. oleracea and B. napus. Both hairy lines showed proportionately lower total leaf expression for EGL3 and TTG1 than GL1 and GL2, but TRY expression in B. rapa was much lower. In contrast, B. villosa with its dense leaf trichome coverage showed high expression for TRY.
Copy-specific Trichome Regulatory Gene Expression in B. villosa and its C Genome Relative, B. oleracea
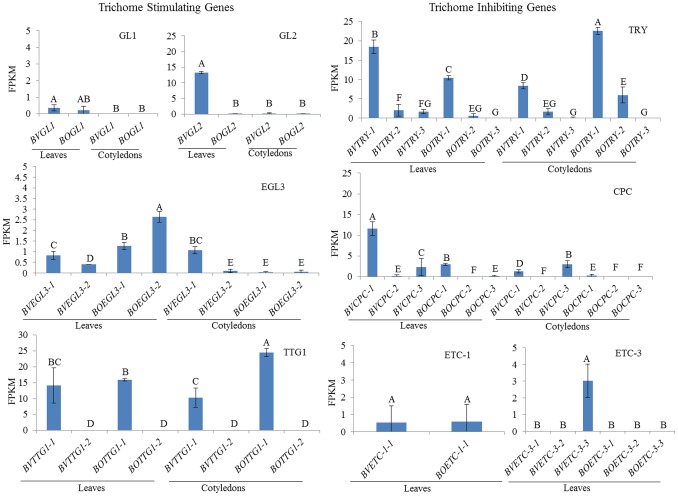
Since total overall transcript levels for GL1, GL2 and unexpectedly for TRY were very high in B. villosa leaves relative to the other Brassica species in the Q-PCR analysis, expression levels were examined for the four trichome positive regulatory genes, and TRY by RNA seq. This analyses was expanded to include three other major negative regulatory genes (CPC, ETC1 and ETC3) to determine whether any particular copy was more prominently expressed in hairy B. villosa first true leaves (and glabrous cotyledons). To do this, we took advantage of a new reference genome in the glabrous C genome relative, B. oleracea (Parkin, unpublished). Expression of two copies were detected for EGL3 and TTG1 and three copies for TRY and CPC in B. villosa by mapping to the B. oleracea genome. RNA seq showed that the single-copy BvGL2 gene and BvTTG1-1 and BoTTG1 have very high transcript levels in hairy B. villosa leaves compared with the GL1 and EGL3 positive regulatory genes. Transcripts of the single BoGL2 were undetectable in glabrous B. oleracea leaves and similarly TTG1-2 was undetectable in glabrous cotyledons of both species (Figure 2). Expression of the single copy GL1 gene was very low (<0.5 FPKM) in leaves and equivalent between the two species, and undetectable in cotyledons. Both copies of EGL3 were expressed at a slightly higher levels in leaves (ranging from 0.3 to 2.5 FPKM) than cotyledons (ranging from 0.05 to 1 FPKM), but the transcript levels were reduced in B. villosa leaves compared with B. oleracea leaves (Figure 2). Curiously, BvEGL3-1 had higher leaf transcript levels proportionally to BvEGL3-2 in B. villosa, whereas the converse was true in B. oleracea. In cotyledons, BvEGL3-1 had expression (equivalent to leaf), and both genes were almost undetectable in B. oleracea cotyledons (Figure 2). The single copy B.villosa GL3 has a similar pattern of expression to that of BvEGL3-1, with higher expression of BvGL3 in cotyledons compared to BoGL3, whereas higher expression of BoGL3 in true leaves compared to BvGL3 (data not shown). The B. villosa single copy of GL2, copy 1 of EGL3 and TTG1 all proved to be functional proteins, since their binary expression constructs enhanced trichome density in Arabidopsis (Figure 3). Preliminary analysis of knockdown lines for these genes in both Arabidopsis and B. napus resulted in no observed phenotype changes (Nayidu and Gruber, unpublished).
Figure 2. Transcript levels for individual gene copies of the four trichome positive regulatory genes and four negative regulatory genes in B. villosa compared with B. oleracea.
RNAseq data is expressed as fragments per kilobase of exon per million fragments mapped (FPKM). Within each panel, different letters represent significantly different means (± standard error) for 3 independent RNA extractions (1st true leaves or cotyledons from up to 10 plants per extraction) at p≤0.05.
Figure 3. Trichome phenotypes of Arabidopsis transgenic plants overexpressing trichome related genes from Brassica villosa.
a. Expanded leaf of wild type Arabidopsis (Columbia) showing normal trichome pattern. b. BvGL2 over-expressed transgenic leaf showing increased trichome number. c. BvEGL3 over-expressed transgenic leaf showing increased trichome number. d. BvTTG1 over-expressed transgenic leaf showing increased trichome number. e. BvTRY over-expressed transgenic leaf showing glabrous phenotype. (All photographs were taken with 10X magnification by a compound microscope at 3 weeks after germination).
Transcripts for three different copies of the TRY negative regulatory gene and two copies of BoTRY were detected (Figure 2). Very high leaf and cotyledon expression was detected for BvTRY-1 in B. villosa (18–20 FPKM) and BoTRY-1 in B. oleracea (9–10 FPKM), while BvTRY-2/BoTRY-2 and BvTRY-3/BoTRY-3 transcript levels were much lower in both leaves and cotyledons (Figure 2). The ranking and proportionate expression of individual TRY gene copies for B. oleracea was similar to B. villosa, but 50% lower in leaves and much higher in cotyledons. The higher combined expression level (FPKM value) for the three TRY genes in trichome-rich B. villosa leaves correlated with the overall higher total TRY expression level measured in the B. villosa leaves by Q-PCR. The fact that the relative expression for all three TRY genes was quite similar in both species and that BvTRY-1 and BoTRY-1 expression was equally high regardless of the extreme differences in leaf trichome density between B. villosa and B. oleracea was inconsistent with the Arabidopsis model of TRY being a negative regulator in B. villosa, even though Arabidopsis leaf trichomes were eliminated when Arabidopsis was transformed with a BvTRY-1 expression construct (Figure 3).
Since the expression pattern of TRY-1 was higher in trichome-covered B. villosa leaves, we took advantage of the new B. oleracea genome database to examine individual copies of several other prominent negative regulatory genes, CPC, ETC1, and ETC3 ([23]; Table 2). Only one copy existed for ETC1 in both B. villosa and B. oleracea and the expression level of both was very low (0.05 FPKM) in leaves (Figure 2) and undetected in cotyledons of both species (data not shown). ETC3 had three copies in both species, but only BvETC3-3 was expressed (2–4 FPKM) in B. villosa leaves (Figure 2), and the other copies were undetectable in either species and in cotyledons (data not shown). In contrast, all three copies of the CPC gene were expressed in leaves of both species, but expression of CPC-1 was predominant in hairy B. villosa leaves and 6-fold higher than BoTRY-1 in glabrous B. oleracea leaves. The other two CPC copies had low transcript levels in leaves and cotyledons, and BvCPC-3 expression (2.5 FPKM) was predominant in cotyledons.
Sequence Comparisons between B. villosa, three other Brassicas and Arabidopsis
Since gene function impacts phenotype from a combination of transcript level and translated protein sequence, amino acid sequence diversity was determined for translated sequences of the five cloned regulatory genes in B. villosa, the three other Brassica species and A. thaliana (Figure S1). Overall, sequences for B. villosa were quite similar to those of the other Brassicas and A. thaliana in length, Mr and pI, with the following exceptions (Table 3). BvEGL3-2 was 20 kDa shorter than BvEGL3-1 from B. villosa and the other EGL3 sequences from B. oleracea, B. rapa, and Arabidopsis. The Mr of BvTRY-1 was most similar to that of B. oleracea, B. napus, and Arabidopsis, while BvTRY-2 was smaller. Isoelectric points for proteins from B. villosa were similar to those of orthologues in other Brassica species and Arabidopsis and usually acidic, except in the case of GL1 (Table 3). Here, the B. villosa orthologue BvGL1 was neutral and had a pI closest to B. rapa BrGL1-1 rather than to alkaline pI of BnGL1-1, BoGL1-1, BrGL1-2, BoTTG1-2 and most of the TRY sequences.
Table 3. Theoretical protein size (Mr/Number of amino acid) and isoelectric point (pI) of trichome regulatory coding sequences in the Brassicaceae.
| B. napus (Mr) | B. oleracea (Mr) | B. rapa (Mr) | B. villosa (Mr) | A. thaliana (Mr) | B. napus (pI) | B. oleracea (pI) | B. rapa (pI) | B. villosa (pI) | A. thaliana (pI) | |
| GL1-1 GL1-2 | 26.0/225 | 24.7/212 | 26.1/22522.7/199 | 26.1/225 | 26.3/228 | 8.80 | 9.42 | 7.67 9.22 | 7.69 | 6.66 |
| GL2-1 GL2-2 | 83.7/75083.7/750 | 83.6/748 | 83.8/750 | 83.5/748 | 86.5/776 | 6.58 6.83 | 6.58 | 6.33 | 6.56 | 6.38 |
| EGL3-1 EGL3-2 | ND | 64.0/57367.6/604 | 66.3/59667.8/606 | 66.5/59746.6/421 | 66.6/616 | ND | 5.59 5.15 | 5.47 5.14 | 5.52 6.20 | 5.06 |
| TTG1-1 TTG1-2 TTG1-3 TTG1-4 TTG1-5 TTG1-6 | 37.3/337 37.2/33737.2/337 37.3/33737.4/338 37.2/337 | 37.2/33724.5/212 | 37.3/337 | 37.3/337 | 37.9/341 | 4.66 4.66 4.66 4.66 4.66 4.70 | 4.66 10.77 | 4.66 | 4.66 | 4.71 |
| TRY-1 TRY-2 TRY-3 | 13.1/107 | 13.0/10713.0/10619.0/160 | 9.07/756.51/546.34/51 | 13.0/1079.02/75 | 13.0/106 | 9.58 | 9.519.249.02 | 9.299.515.28 | 9.219.39 | 9.51 |
ND, not determined (sequence not available). Closest orthologues between the species are positioned within the same row. Data represents all known orthologues and homologues for each species.
Many amino acid substitutions, additions and deletions were apparent within each of the five trichome genes when comparisons were made between B. villosa and the four other species. In total, there were 58 amino acid changes (26.2%) out of a total of 237 consensus amino acids (CAA) for GL1, 30 (3.8%) out of 786 CAA for GL2, 92 (15.1%) out of 608 CAA for EGL3-1, 132 (27.2%) out of 485 CAA for EGL3-2, and 111(31.2%) out of 356 CAA for TTG1. TRY genes were the smallest compared to the positive regulatory genes and had the most amino acid differences between the species: 99 (84.6%) out of 117 CAA for TRY-1 and 111 (69.4%) out of 160 CAA for TRY-2 (Figure S1). Out of the total amino acid differences observed, those which were unique to B. villosa or distinguished hairy germplasm from glabrous germplasm were more closely examined (Table 4, Figure S1). Several particularly noteworthy variations between hairy and glabrous germplasm involved more extreme charge and hydrophobicity differences that potentially could affect the molecular structure and functional properties of a protein. These included consensus amino acid (CAA) position 223 in the 3′ end of GL1, where the aromatic phenylalanine [38] in B. villosa and B. rapa and the hydrophobic residue leucine (Leu) in A. thaliana and B. rapa [hydrophobicity values of 2.8 (F) and 3.8 (L), respectively [39]] were replaced by the slightly hydrophilic serine (Ser, value of −0.8) in glabrous B. napus and B. oleracea (Table 4). CAA position 273 in GL2 included a glutamine (Gln) residue in hairy B. villosa, B. napus (one out of two copies), and the hairy B. rapa sequences, and a negatively charged glutamate acid (Glu) residue in A. thaliana. These were replaced by a positively charged imidazole ring (histidine, His) in the other copy of glabrous B. napus and in glabrous B. oleracea sequences. CAA position 439 in GL2-1 included an alanine conserved between hairy B. villosa, A. thaliana, and B. rapa proteins and replaced by a hydrophobic valine in GL2 of glabrous B. napus and B. oleracea. Finally, three positions in BvGL1, two in BvGL2, two in EGL3, one in BvTTG1, and one in TRY were unique to B. villosa. In particularly, the serine in CAA position 154 of all BvTRY sequences was replaced by an arginine in TRY genes from all other species.
Table 4. Specific amino acids in five trichome regulatory genes within the Brassicaceae.
| *Consensus position | A. thaliana hairy | B. rapa AA hairy | B. napus AC glabrous | B.oleracea CC glabrous | B. villosa CC hairy | |
| GL1 | 137* | Pro | Pro | Pro | Pro | Thr |
| 154* | Gln | Gln | Gln | Gln | Glu | |
| 202* | Asn | Asn | Asn | Asn | Asp | |
| 223(1) 223(2) | Leu NA | Phe Leu | Ser NA | Ser NA | Phe NA | |
| GL2 | 273(1) 273(2) | Glu NA | Gln NA | His Gln | His NA | Gln NA |
| 282* | Tyr | Tyr | Tyr | Tyr | Phe | |
| 287* | Ala | Ala | Ala | Ala | Ser | |
| 439-(1) 439-(2) | Ala | Ala | Val | Val | Ala | |
| EGL3 | 171(1) * 171(2) | Val NA | Val Val | ND ND | Val Val | Ala Val |
| 552* | Leu | Leu | ND | Leu | Val | |
| TTG1 | 4* | Ser | Ser | Ser | Ser | Ala |
| TRY | 154(1) * 154(2) * 154(3) * | Arg NA NA | Arg NIL NIL | Arg NA NA | Arg Arg Arg | Ser Ser NA |
*Selected positions in the aligned consensus amino acid sequence (CAA) were selected from Figure S1 if they distinguished hairy from glabrous germplasm (dark arrows in Fig. S1) or were unique to B. villosa (*red arrows in Fig. S1). ND, not determined (sequence not available). NIL, missing amino acid. NA, not applicable. Note: Multiple gene copies are only indicated if an amino acid differed between the copies.
Each of the four trichome initiation genes and TRY had domains which were conserved within all the Brassica species and A. thaliana, but they also showed species distinctions not correlated with a trichome phenotype (Figure S1). GL1 consisted of well known conserved R2 and R3 MYB DNA binding domains, but B. rapa GL1 was missing CAAs 1-29 and the BoGL1 had a nonsense mutation at the end of the sequence and five unique amino acids (CAA position 119–123) in a conserved region immediately downstream of the R3MYB domain. GL2 showed no significant differences in the conserved homeobox domain and had the most consistent amino acid profile across all species compared to the other regulatory genes (Figure S3). However, CAA positions 114 to 118 present in the conserved A. thaliana START domain and the unique 29 amino acid (aa) Arabidopsis leader sequence were missing in GL2 from the four Brassica species (Figure S1). EGL3 translated sequences were mainly equivalent in their bHLH-DNA binding domains, but variable between CAA 391-3 in all orthologues (Figure S1). Noteworthy was an entirely different sequence post-CAA 426 for B. villosa EGL3-2, whereas all other EGL3s were missing aa from 426–455 and 485 to the stop codon, aa 456–484 were completely different, and sequences for B. oleracea EGL3s were missing CAAs 164–187. BoTTG1-2 and TRY also showed diversity. TRY was completely conserved in the R3 MYB binding domain but diverse in other areas (Figure S1). BvTRY-2, BrTRY-1 and BrTRY-2 sequences were each missing CAA 53–85, B. rapa TRY-3 was missing aa 105–160 within and beyond the R3 binding region, TRY-3 of B. oleracea had a unique 52 aa leader sequence, while B. rapa TRY-2 and 3 had the smallest R3 MYB binding regions. Except for CAA 1–85 missing in BvTRY-2, BvTRY-1 and BvTRY-2 were identical, but the very low expression of BvTRY-3 prevented evaluation.
Ka/Ks Amino Acid Substitutions
Leucine (L), serine (S) and arginine (R) were the most abundant amino acids within all five trichome regulatory genes for B. villosa as well as the other four species (Figure S3). However, cysteine was completely absent in TRY orthologues (except for B. oleracea and B. rapa TRY-2, each of which had one cysteine at CAA 95). Moreover, B. villosa TRY-2 and B. rapa TRY-1 were under-represented in V, F, S, T, D, H, K, and R compared with BvTRY-1 and TRY in other species examined (Figure S3). This led us to determine Ka/Ks amino acid substitution values for the five trichome genes to assess whether sufficiently different amino acid substitutions had occurred to potentially change protein function.
Ka/Ks values were much less than one for most pair-wise comparisons between homologues and orthologues, especially for GL2, TTG1, and TRY, indicating mainly synonymous substitutions that would not impact protein structure (Table S1). Ka/Ks values for EGL3 sequence comparisons were higher in general than values for GL2, TTG1, and TRY comparisons, but less than one and lower than many of the Ka/Ks values determined for GL1 sequences (Table 5; Table S1). Most pairwise comparisons between GL1 in B. villosa and each Brassica species and Arabidopsis consistently showed synonymous substitutions. However, Ka/Ks ratios for BvGL1 and BoGL1 were much closer to one, indicating an evolutionary tendency towards significant change. This was even more extreme between GL1 from B. villosa and B. rapa (1.04) and from B. villosa and B. napus (1.87) (Table 5), indicating large substitution differences that may have the potential for functional changes.
Table 5. Ka/Ks * ratios for trichome regulatory gene comparisons between B. villosa and three other Brassica species and Arabidopsis.
| Pairwise Comparison | GL1 | GL2 | EGL3 | TTG1 | TRY | |
| B. napus | B. villosa | 1.87 | 0.09 | NA | 0.03 | 0.23 |
| B. villosa | B. rapa-1 | 1.04 | 0.06 | 0.42 | 0.02 | 0.19 |
| B. oleracea | B. villosa | 0.78 | 0.14 | 0.51 | 0.1 | 0.06 |
| B. villosa | B. rapa-2 | 0.21 | – | 0.31 | – | –+ |
| B.villosa | A. thaliana | 0.24 | 0.07 | 0.2 | 0.01 | 0.06 |
| B.napus-2 | B.villosa | 0.09 | 0.09 | 0.09 | 0.09 | – |
| B.villosa-1 | B.villosa-2 | – | – | 0.86 | – | 0.00 |
| B.villosa-1 | B.oleracea-2 | – | – | 0.30 | 0.27 | 0.15 |
| B.oleracea-1 | B.villosa-2 | – | – | 0.86 | – | 0.06 |
| B.villosa-2 | B.oleracea-2 | – | – | 0.40 | – | 0.15 |
| B.villosa-2 | B.rapa-2 | – | – | 0.49 | – | –+ |
| B.villosa-2 | A.thaliana | – | – | 0.21 | – | 0.06 |
| B.napus-3 | B.villosa | – | – | – | 0.05 | – |
| B.napus-4 | B.villosa | – | – | – | 0.03 | – |
| B.napus-5 | B.villosa | – | – | – | 0.03 | – |
| B.napus-6 | B.villosa | – | – | – | 0.03 | – |
| B.villosa-1 | B.oleracea-3 | – | – | – | – | 0.19 |
| B.villosa-2 | B.oleracea-3 | – | – | – | – | 0.19 |
*Ka/KS (Yang Z, 1997): Ka, non-synonymous nucleotide substitution. Ks, synonymous nucleotide substitution value.
B. rapa-2 (BRTRY-2) and B. rapa-3 (BRTRY-3) amino acid sequences were too short to be included. NA, B. napus sequence not available.
Discussion
Brassica villosa is a weedy C genome species of the Brassicaceae and is of interest to molecular evolutionists and plant breeders for its dense trichome patterning on most tissues [33]. In the present study, we compared expression patterns and coding sequences for key trichome regulatory genes of B. villosa with those of orthologues from A. thaliana and several species within Brassica A and C genomes, as a means of distinguishing unique B. villosa sequence patterns from those of more modest trichome-bearing species and glabrous germplasm. Phylogenetic trees indicated that GL1, GL2, EGL3 and TRY sequences from hairy B. villosa are closest to several orthologues from the two non-hairy species B. napus and B. oleracea. These data confirm the closer relationship of these B. villosa positive regulatory trichome genes to those of B. oleracea and the C genome of B. napus than to Arabidopsis regardless of the trichome density differences. Li and co-workers showed pairing of GL1 sequences from hairy Brassica incana (related to B. villosa) with those from non-hairy B. napus lines (with a 92–94% sequence identity) [29].
Generally, RNA sequencing showed that transcript levels of one of the multiple copies of EGL3, TTG1, TRY, CPC, and ETC3 were predominant compared to levels of the other copies in B. villosa. Expression for the trichome stimulating genes, BvGL2 and BvTTG1, and the trichome inhibiting genes, BvTRY-1 and BvCPC-1, was very high in young true leaves of B. villosa (where dense trichome coverage is found) and low in cotyledons compared with GL1, EGL3, ETC1, and ETC3 genes. This B. villosa RNAseq pattern was distinct and somewhat unexpected since the BvEGL3 expression was lower than in glabrous B. oleracea leaves and the BvTTG1 expression was quite similar in level to BoTTG1 expression. Expression of the BvTRY-1 and BvCPC-1 was also high in hairy B. villosa, and expression of BoTRY-1 was high in glabrous B. oleracea leaves. This was inconsistent with the Arabidopsis model of TRY and CPC as negative regulators of trichome initiation, where enhanced leaf trichome density phenotype occurred when TRY expression was knocked down in Arabidopsis try mutants [40]. The data implies that BvTRY-1, BvCPC-1, and (potentially) BvETC3-1 genes may not behave as negative regulators of trichome initiation in B. villosa. Protein coding sequences for BvTRY-1 and BvTRY-2 genes were closest to those of non-hairy BoTRY-3 and BnTRY, and all four of these are closer to each other and to A. thaliana than to the other Brassica TRY genes. Redundant trichome negative regulatory genes exist in the A. thaliana model [25] and functional redundancy can speed up gene evolution [41]–[43]. Hence, B villosa may use these R3 regulatory genes with high expression for a different purpose in the densely covered B. villosa true leaves. This hypothesis is supported by the insertion of BvTRY-1 into B. napus, yielding transgenic plants in which trichome density is not affected even though the same binary construct was used to depress Arabidopsis trichome development (Nayidu and Gruber, unpublished and Figure 3 respectively). In the future, it will be particularly useful to express these B villosa genes in a range of other Brassica species and to develop knock-out RNAi lines in B. villosa to solve the mystery of their true function. This will depend on the development of a transformation system for B. villosa, such as the protocols that now exist for B. napus, B. rapa, B. oleracea, and B. carinata [44]–[47]. Additional analysis of B. villosa gene structure is also necessary for a complete understanding of their introns, untranslated regions, and promoters. For example, intron 1 and 30 non-coding nucleotides in A. thaliana are important for the expression of the GL1 gene [5], [48], [49]. A 620 bp fragment of the TRY promoter contains sequences that mediate the repression of its own expression, and deletion of this promoter region can rescue the A. thaliana try mutant phenotype [50]. Moreover, the Arabidopsis TRY promoter contains sequences regulated by microRNA-regulated “SQUAMOSA PROMOTER BINDING PROTEIN LIKE” (SPL) genes [50], and enhanced expression of the SPL-regulator miR156b in Arabidopsis increases trichome density [51].
The high expression pattern for GL2 and TRY-1 in B. villosa leaves led us to compare the coding sequences of the five trichome regulatory genes we had isolated from B. villosa with those in B. napus, two diploid Brassica species, and A. thaliana. Extreme trichome coverage in B. villosa leaves may be due (at least in part) to polymorphism and evolutionary differences that impact on protein function rather than solely to specific gene transcript levels, which are dictated by promoter “strength” and intra-gene regulatory structures. Although analysis showed few differences between most orthologues, overall evolutionary selection was detected between GL1 proteins of hairy (B. villosa, B. rapa) and glabrous (B. napus) genotypes by their high pairwise Ka/Ks values (>1), potentially due to sites involved in adaptive change. Adaptive changes may occur at surprisingly few sites; consequently, the overall Ka/Ks ratio for an entire protein may remain dominated by non-adaptive changes and be substantially lower than unity (reviewed in [52]) as seen by the Ka/Ks scores for GL2, EGL3, TTG1, and TRY. Moreover, once a protein achieves a new advantageous function, the frequency of non-synonymous substitutions at the adapted sites will be reduced by new functional constraints [52].
A substantial proportion of the differences that distinguished the four Brassica trichome regulatory sequences occurred outside of conserved domains. Several of these individual amino acid differences were sufficiently dramatic to potentially change the molecular and functional properties of these proteins. Particularly, three variable positions in GL1 and GL2 translated sequences distinguished hairy B. villosa and B. rapa from glabrous B. napus and B. oleracea germplasm, and all the B. villosa genes had a minimum of one unique site (and usually more) compared with the other species. Bloomer [53] reported on the effects of natural variation in GL1 from Arabidopsis and suggested that qualitative differences in trichome phenotypes (glabrous or hairy) might have arisen independently several times by three unique protein coding changes and a whole locus deletion. The same authors also suggested that quantitative variation (in trichome density) might have arisen because of completely linked amino acid replacements and mutations in a known (as yet uncharacterized) enhancer region within the AtGL1 locus. In addition, Li et al. proposed that a 5-bp deletion in exon 3 of the B. rapa GL1 gene (starting at CAA 110 in the present study) is the basis of a hairless phenotype that arose from a normally hairy double haploid brown-seeded line [29]. Hence, the extremely dense trichome coverage of B. villosa could be due to a combination of relatively higher transcription of GL2, hydrophobic amino acids and evolutionary changes in GL1, as well as the replacement of serine in all BvTRY sequences, and potentially a different function for TRY-1, CPC-1, and ETC3-3 (consistent with their higher B. villosa leaf transcript levels). Moreover, the glabrous leaves of the C genome relative B. oleracea could result from two non-synonymous substitutions (from asparagine to serine (at CAA 26 and 112), five continuous amino acid replacements (at CAA 119–123), one nonsense mutation (at CAA 224) leading to a shortened GL1 amino acid sequence, and a missing aa stretch in the bHLH DNA binding region of BoEGL3-1 and BoEGL3-2. Our study adds additional sequence variation data to a previous report detailing two 1 bp deletions and 1 bp insertion in exon 3 of the B. oleracea GL1 sequence [29]. Changes in protein function with individual amino acid modifications are also seen in other species and genes. Yeam et al. (2007) showed that a G/R polymorphism at aa 107 of the Capsicum eukaryotic translation initiation factor 4E protein (eIF4E) is sufficient for the acquisition of resistance against several Capsicum and tobacco etch potyvirus strains by expressing the amino acid substituted gene in potato (Solanum lycopersicum) [54].
Likely, the genetic variation we have uncovered is the “tip of the iceberg” in terms of variation that affects the function of Brassica trichome genes, since the trichome pathway in the simpler A. thaliana genome is already considered to be an integrated hierarchy of regulation by complex cell cycle status, transcriptional control and cytoskeletal function [55]. This is confirmed by the ever increasing number of trichome genes being discovered in A. thaliana mutant populations (>80 genes known in TAIR) [38] (Taheri et al., 2014 submitted manuscript from the same lab) and by the on-going discovery of cis-regulatory sequences that provide greater diversification in gene function, eg. for GL1 and MYB23 [56]. The constant improvement in genomic-scale sequencing technology, SNP analysis, and computation can now be applied to characterize large within-accession and within-species variance in trichome gene patterning in the Brassicaceae and identify statistically robust associations hitherto undetectable [57], [58].
Conclusion
The present study utilized expression profiling and bioinformatics to compare B. villosa trichome regulatory genes with their orthologues in B. oleracea, B. rapa, B napus, and A. thaliana. In doing so, we discovered the potential for positive evolutionary selection in the GL1 gene between B. villosa, B. rapa and B. napus. Several point mutations found within GL1 and GL2 protein sequences correlated with hairy and glabrous phenotypes within these five species and have the potential to be predictive factors. We also discovered high transcript levels for BvGL2, BvTTG1, BvTRY-1, and BvCPC-1 in B. villosa leaves with densely covered trichomes. This B. villosa gene expression pattern is contrary to the trichome gene models in Arabidopsis in which cells are glabrous when expression of trichome R3 MYB inhibitor genes is high and trichomes are present in greater density and clustered when these genes are knocked down by mutation [23]. These B. villosa genes are now being tested in comparative over-expression studies in Arabidopsis and B. napus. Investigations on these genes should also be expanded to include a much broader range of hairy and glabrous Brassica germplasm within the Triangle of U [26].
Supporting Information
Alignment of translated protein sequences for four major trichome positive regulatory genes and one negative regulatory gene in the Brassicaceae. GL1: Conserved R2 and R3 MYB DNA binding domains are boxed. A unique 5 aa variable sequence and a unique 14 aa 3′ deleted sequence are designated in the B. oleracea sequence by ovals. GL2: The conserved HDZip homeobox domain and a large START domain are boxed. A unique A. thaliana 29 aa leader sequence is delineated by an oval. GL3: The conserved bHLH protein binding domain is boxed. TTG1: Two conserved WD40 protein domains are boxed. A unique 44 aa leader sequence in B. oleracea is designated by an oval. TRY: The conserved R2 MYB DNA binding domains are boxed. A 33 aa unique deletion in the B. rapa gene is designated by an oval. For all 5 genes, unique amino acid modifications are designated for B. villosa (red arrows) and for hairy vs. glabrous plants (blue arrows).
(PDF)
Relative expression of four trichome regulatory genes and one negative regulatory gene in leaves of hairy Brassica villosa, three other Brassica species, and A. thaliana. Main panel shows expressed transcripts from four Brassica species. Insert panel shows A. thaliana orthologues which are much more highly expressed compared with the Brassica species. Expression (Q-PCR) in both panels is relative to glabrous B. napus cv. Westar (set at 1). Different letters in both panels indicate significant differences of the means (± standard error) at p≤0.05 in an LSD test (SAS, 2008).
(TIF)
Amino acid profiles for five major trichome regulatory sequences from Brassica villosa, three other Brassica species, and A. thaliana.
(TIF)
*Ka/Ks values from pairwise comparisons of all trichome regulatory gene orthologues and homologues within four Brassica species and Arabidopsis.
(DOCX)
Acknowledgments
The authors thank Dr. L. Visser at the Center for Genetic Resources, Wageningen, The Netherlands, for the gift of B. villosa seeds. Dr. N. Nayidu and Dr. A. Taheri were recipients of Visiting Fellowships to a Canadian Government Laboratory.
Funding Statement
1. Agriculture and Agri-Food Canada, 2. Canola Council of Canada, 3. The Saskatchewan Agricultural Development Fund, and 4. The Saskatchewan Canola Development Commission. POS Bio-Sciences provided support in the form of a salary for author TSW-G, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Broadhurst CL, Chaney RL, Angle JS, Maugel TK, Erbe EF, et al. (2004) Simultaneous hyperaccumulation of nickel, manganese, and calcium in Alyssum leaf trichomes. Environmental Science & Technology 38: 5797–5802. [DOI] [PubMed] [Google Scholar]
- 2. Larkin JC, Brown ML (2003) Schiefelbein J (2003) How do cells know what they want to be when they grow up? Lessons from epidermal patterning in Arabidopsis. Annual Review of Plant Biology 54: 403–430. [DOI] [PubMed] [Google Scholar]
- 3. Balkunde R, Pesch M, Hülskamp M (2010) Chapter Ten-Trichome Patterning in Arabidopsis thaliana: From Genetic to Molecular Models. Current Topics in Developmental Biology 91: 299–321. [DOI] [PubMed] [Google Scholar]
- 4. Herman PL, Marks MD (1989) Trichome development in Arabidopsis thaliana. II. Isolation and complementation of the GLABROUS1 gene. The Plant Cell Online 1: 1051–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oppenheimer DG, Herman PL, Sivakumaran S, Esch J, Marks MD (1991) A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell 67: 483–493. [DOI] [PubMed] [Google Scholar]
- 6. Zhao H, Wang X, Zhu D, Cui S, Li X, et al. (2012) A Single Amino Acid Substitution in IIIf Subfamily of Basic Helix-Loop-Helix Transcription Factor AtMYC1 Leads to Trichome and Root Hair Patterning Defects by Abolishing Its Interaction with Partner Proteins in Arabidopsis. Journal of Biological Chemistry 287: 14109–14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, et al. (1999) The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. The Plant Cell Online 11: 1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cristina M, Sessa G, Dolan L, Linstead P, Baima S, et al. (2002) The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. The Plant Journal 10: 393–402. [DOI] [PubMed] [Google Scholar]
- 9. Rerie WG, Feldmann KA, Marks MD (1994) The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes & Development 8: 1388–1399. [DOI] [PubMed] [Google Scholar]
- 10. Szymanski DB, Jilk RA, Pollock SM, Marks MD (1998) Control of GL2 expression in Arabidopsis leaves and trichomes. Development 125: 1161–1171. [DOI] [PubMed] [Google Scholar]
- 11. Hülskamp M, Miséra S, Jürgens G (1994) Genetic dissection of trichome cell development in Arabidopsis. Cell 76: 555–566. [DOI] [PubMed] [Google Scholar]
- 12. Larkin JC, Walker JD, Bolognesi-Winfield AC, Gray JC, Walker AR (1999) Allele-specific interactions between ttg and gl1 during trichome development in Arabidopsis thaliana . Genetics 151: 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schellmann S, Hulskamp M (2005) Epidermal differentiation: trichomes in Arabidopsis as a model system. International Journal of Developmental Biology 49: 579. [DOI] [PubMed] [Google Scholar]
- 14. Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A (2003) A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130: 4859–4869. [DOI] [PubMed] [Google Scholar]
- 15. Payne CT, Zhang F, Lloyd AM (2000) GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics 156: 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morohashi K, Zhao M, Yang M, Read B, Lloyd A, et al. (2007) Participation of the Arabidopsis bHLH factor GL3 in trichome initiation regulatory events. Plant Physiology 145: 736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, et al. (2002) TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. The EMBO Journal 21: 5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishida T, Kurata T, Okada K, Wada T (2008) A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 59: 365–386. [DOI] [PubMed] [Google Scholar]
- 19.Digiuni S, Schellmann S, Geier F, Greese B, Pesch M, et al.. (2008) A competitive complex formation mechanism underlies trichome patterning on Arabidopsis leaves. Molecular Systems Biology 4. [DOI] [PMC free article] [PubMed]
- 20. Esch JJ, Chen M, Sanders M, Hillestad M, Ndkium S, et al. (2003) A contradictory GLABRA3 allele helps define gene interactions controlling trichome development in Arabidopsis. Development 130: 5885–5894. [DOI] [PubMed] [Google Scholar]
- 21. Kurata T, Ishida T, Kawabata-Awai C, Noguchi M, Hattori S, et al. (2005) Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development 132: 5387–5398. [DOI] [PubMed] [Google Scholar]
- 22. Wang S, Kwak S-H, Zeng Q, Ellis BE, Chen X-Y, et al. (2007) TRICHOMELESS1 regulates trichome patterning by suppressing GLABRA1 in Arabidopsis. Development 134: 3873–3882. [DOI] [PubMed] [Google Scholar]
- 23. Wester K, Digiuni S, Geier F, Timmer J, Fleck C, et al. (2009) Functional diversity of R3 single-repeat genes in trichome development. Development 136: 1487–1496. [DOI] [PubMed] [Google Scholar]
- 24. Bouyer D, Geier F, Kragler F, Schnittger A, Pesch M, et al. (2008) Two-dimensional patterning by a trapping/depletion mechanism: the role of TTG1 and GL3 in Arabidopsis trichome formation. PLoS Biology 6: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pesch M, Hülskamp M (2009) One, two, three… models for trichome patterning in Arabidopsis? Current Opinion in Plant Biology 12: 587–592. [DOI] [PubMed] [Google Scholar]
- 26. U N (1935) Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Japanese Journal of Botany 7: 389–452. [Google Scholar]
- 27.Agren J, Schemske DW (1994) Evolution of trichome number in a naturalized population of Brassica rapa. American Naturalist: 1–13.
- 28. Zhang J, Lu Y, Yuan Y, Zhang X, Geng J, et al. (2009) Map-based cloning and characterization of a gene controlling hairiness and seed coat color traits in Brassica rapa . Plant Molecular Biology 69: 553–563. [DOI] [PubMed] [Google Scholar]
- 29. Li F, Kitashiba H, Nishio T (2011) Association of sequence variation in BrassicaGLABRA1 orthologs with leaf hairiness. Molecular Breeding 28: 577–584. [Google Scholar]
- 30.Li F, Zou Z, Yong HY, Kitashiba H, Nishio T (2013) Nucleotide sequence variation of GLABRA1 contributing to phenotypic variation of leaf hairiness in Brassicaceae vegetables. Theoretical and applied genetics. [DOI] [PubMed]
- 31. ZeTao B, GuoHua C, Lei S, LiMing X, ChengAng W, et al. (2010) Isolation and functional characterization of GLABRA2 promoter related to trichome development in Brassica napus . Journal of Agricultural Biotechnology 18: 210–217. [Google Scholar]
- 32. Gruber MY, Wang S, Ethier S, Holowachuk J, Bonham-Smith PC, et al. (2006) “HAIRY CANOLA”–Arabidopsis GL3 Induces a Dense Covering of Trichomes on Brassica napus Seedlings. Plant Molecular Biology 60: 679–698. [DOI] [PubMed] [Google Scholar]
- 33. Palaniswamy P, Bodnaryk RP (1994) A wild Brassica from Sicily provides trichome-based resistance against flea beetles, Phyllotreta cruciferae (Goeze)(Coleoptera: Chrysomelidae). The Canadian Entomologist 126: 1119–1130. [Google Scholar]
- 34. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation ofArabidopsis thaliana. The Plant Journal 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 35. Trapnell C, Roberts A, Goff L, Pertea G, Kim D, et al. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols 7: 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings in Bioinformatics 9: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang Z (1997) PAML: a program package for phylogenetic analysis by maximum likelihood. Computer Applications in the Biosciences: CABIOS 13: 555–556. [DOI] [PubMed] [Google Scholar]
- 38. Robinson SJ, Tang LH, Mooney BAG, McKay SJ, Clarke WE, et al. (2009) An archived activation tagged population of Arabidopsis thaliana to facilitate forward genetics approaches. BMC Plant Biology 9: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. Journal of Molecular Biology 157: 105–132. [DOI] [PubMed] [Google Scholar]
- 40. Kirik V, Simon M, Wester K (2004) Schiefelbein J, Hulskamp M (2004) ENHANCER of TRYand CPC 2 (ETC2) reveals redundancy in the region-specific control of trichome development of Arabidopsis. Plant molecular biology 55: 389–398. [DOI] [PubMed] [Google Scholar]
- 41. Blanc G, Wolfe KH (2004) Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. The Plant Cell Online 16: 1679–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conant GC, Wolfe KH (2008) Turning a hobby into a job: how duplicated genes find new functions. Nature Reviews Genetics 9: 938–950. [DOI] [PubMed] [Google Scholar]
- 43. Cusack BP, Wolfe KH (2007) When gene marriages don’t work out: divorce by subfunctionalization. Trends in Genetics 23: 270–272. [DOI] [PubMed] [Google Scholar]
- 44. Babic V, Datla RS, Scoles GJ, Keller WA (1998) Development of an efficient Agrobacterium-mediated transformation system for Brassica carinata. Plant Cell Reports 17: 183–188. [DOI] [PubMed] [Google Scholar]
- 45. Bhalla PL, Singh MB (2008) Agrobacterium-mediated transformation of Brassica napus and Brassica oleracea. Nature protocols 3: 181–189. [DOI] [PubMed] [Google Scholar]
- 46. Poulsen GB (1996) Genetic transformation of Brassica. Plant Breeding 115: 209–225. [Google Scholar]
- 47. Radke SE, Turner JC, Facciotti D (1992) Transformation and regeneration of Brassica rapa using Agrobacterium tumefaciens. Plant Cell Reports 11: 499–505. [DOI] [PubMed] [Google Scholar]
- 48. Larkin JC, Oppenheimer DG, Pollock S, Marks MD (1993) Arabidopsis GLABROUS1 gene requires downstream sequences for function. The Plant Cell Online 5: 1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang S, Wang JW, Yu N, Li CH, Luo B, et al. (2004) Control of plant trichome development by a cotton fiber MYB gene. The Plant Cell Online 16: 2323–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pesch M, Hülskamp M (2011) Role of TRIPTYCHON in trichome patterning in Arabidopsis. BMC Plant Biology 11: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wei S, Gruber MY, Yu B, Gao M-J, Khachatourians GG, et al. (2012) Arabidopsis mutant sk156 reveals complex regulation of SPL15 in a miR156-controlled gene network. BMC plant biology 12: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patthy L (2009) Protein evolution: Wiley-Blackwell.
- 53.Bloomer RH, Juenger TE, Symonds VV (2012) Natural variation in GL1 and its effects on trichome density in Arabidopsis thaliana. Molecular Ecology. [DOI] [PubMed]
- 54. Yeam I, Cavatorta JR, Ripoll DR, Kang BC, Jahn MM (2007) Functional dissection of naturally occurring amino acid substitutions in eIF4E that confers recessive potyvirus resistance in plants. The Plant Cell Online 19: 2913–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Szymanski DB, Lloyd AM, Marks MD (2000) Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis. Trends in Plant Science 5: 214–219. [DOI] [PubMed] [Google Scholar]
- 56. Koornneeff M, Dellaert LWM, Van der Veen JH (1982) EMS-and relation-induced mutation frequencies at individual loci in Arabidopsis thaliana(L.) Heynh. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 93: 109–123. [DOI] [PubMed] [Google Scholar]
- 57. Hauser MT, Harr B, Schlötterer C (2001) Trichome distribution in Arabidopsis thaliana and its close relative Arabidopsis lyrata: molecular analysis of the candidate gene GLABROUS1 . Molecular Biology and Evolution 18: 1754–1763. [DOI] [PubMed] [Google Scholar]
- 58. Larkin JC, Young N, Prigge M, Marks MD (1996) The control of trichome spacing and number in Arabidopsis. Development 122: 997–1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of translated protein sequences for four major trichome positive regulatory genes and one negative regulatory gene in the Brassicaceae. GL1: Conserved R2 and R3 MYB DNA binding domains are boxed. A unique 5 aa variable sequence and a unique 14 aa 3′ deleted sequence are designated in the B. oleracea sequence by ovals. GL2: The conserved HDZip homeobox domain and a large START domain are boxed. A unique A. thaliana 29 aa leader sequence is delineated by an oval. GL3: The conserved bHLH protein binding domain is boxed. TTG1: Two conserved WD40 protein domains are boxed. A unique 44 aa leader sequence in B. oleracea is designated by an oval. TRY: The conserved R2 MYB DNA binding domains are boxed. A 33 aa unique deletion in the B. rapa gene is designated by an oval. For all 5 genes, unique amino acid modifications are designated for B. villosa (red arrows) and for hairy vs. glabrous plants (blue arrows).
(PDF)
Relative expression of four trichome regulatory genes and one negative regulatory gene in leaves of hairy Brassica villosa, three other Brassica species, and A. thaliana. Main panel shows expressed transcripts from four Brassica species. Insert panel shows A. thaliana orthologues which are much more highly expressed compared with the Brassica species. Expression (Q-PCR) in both panels is relative to glabrous B. napus cv. Westar (set at 1). Different letters in both panels indicate significant differences of the means (± standard error) at p≤0.05 in an LSD test (SAS, 2008).
(TIF)
Amino acid profiles for five major trichome regulatory sequences from Brassica villosa, three other Brassica species, and A. thaliana.
(TIF)
*Ka/Ks values from pairwise comparisons of all trichome regulatory gene orthologues and homologues within four Brassica species and Arabidopsis.
(DOCX)