Abstract
A major unmet medical need is the lack of treatments to prevent (or modify) epilepsy in patients at risk, for example, after epileptogenic brain insults such as traumatic brain injury, stroke, or prolonged acute symptomatic seizures like complex febrile seizures or status epilepticus. Typically, following such brain insults there is a seizure-free interval (“latent period”), lasting months to years before the onset of spontaneous recurrent epileptic seizures. The latent period after a brain insult offers a window of opportunity in which an appropriate treatment may prevent or modify the epileptogenic process induced by a brain insult. A similar latent period occurs in patients with epileptogenic gene mutations. Studies using animal models of epilepsy have led to a greater understanding of the factors underlying epileptogenesis and have provided significant insight into potential targets by which the development of epilepsy may be prevented or modified. This review focuses largely on some of the most common animal models of epileptogenesis and their potential utility for evaluating proposed antiepileptogenic therapies and identifying useful biomarkers. The authors also describe some of the limitations of using animal models in the search for therapies that move beyond the symptomatic treatment of epilepsy. Promising results of previous studies designed to evaluate antiepileptogenesis and the role of monotherapy versus polytherapy approaches are also discussed. Recent data from both models of genetic and acquired epilepsies strongly indicate that it is possible to prevent or modify epileptogenesis, and, hopefully, such promising results can ultimately be translated into the clinic.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0250-1) contains supplementary material, which is available to authorized users.
Keywords: Anti-seizure drug, Antiepileptic drug, Epilepsy, Animal models of epilepsy, Disease modification
Introduction
Animal models have, since 1937, been the foundation on which many new therapies have been identified for the treatment of symptomatic epilepsy [1–3]. The successful identification of several new antiepileptic (or anti-seizure) drugs (AEDs) since the 1990s clearly supports the value of animal models in the early identification of promising new drugs for the patient with epilepsy [1]. Unfortunately, despite this success, approximately 30 % of patients with epilepsy fail to achieve full seizure control or suffer intolerable adverse events [2]. As such, no one would argue that there is a clear need for more effective and better tolerated therapies for the treatment of symptomatic epilepsy.
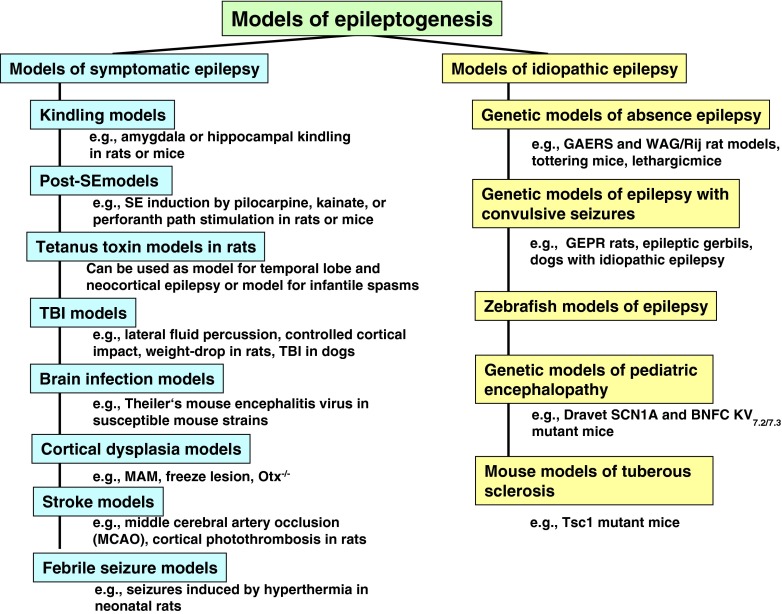
In addition to the development of better therapies for the symptomatic treatment of epilepsy, the availability of a therapy that would prevent or delay the development of epilepsy, or the associated cognitive comorbidities would represent a substantial advance in the overall management of epilepsy [3–6]. As epilepsy is progressive and the seizures and comorbidities can worsen with continued duration, such a disease-modifying or antiepileptogenic therapy, once available, could also have a disease-modifying effect when epilepsy is already established, for example by reducing or preventing the emergence of more severe seizures, pharmacoresistance, or comorbidities. Unfortunately, there are many substantive challenges at the preclinical and clinical level that must first be overcome before a “disease-modifying” therapy or “cure” can be realized. For example, it would be important to demonstrate that any proposed therapy is effective in one or more of the animal models of epileptogenesis summarized in Fig. 1, and that there is a clear path for the clinical development of a purported disease-modifying or antiepileptogenic therapy. Unfortunately, unlike the animal models that have successfully identified a number of new therapies for the symptomatic treatment of epilepsy, the animal models of epileptogenesis have not been validated clinically [4, 6]. To this point, clinical validation will not be provided until that first truly “antiepileptic” or “disease-modifying” therapy, identified in a specific animal model, is proven effective in an appropriately designed clinical trial. Having said this, the community should not be discouraged from pursing this approach, but should be aware of the limitations of the existing models and employ caution when designing preclinical studies and interpreting the results obtained. Many of the issues around the design, interpretation, and reporting of results from antiepileptogenesis and disease-modifying preclinical studies briefly discussed herein are highlighted in a special issue of Epilepsia [7].
Fig. 1.
Important models of epileptogenesis. Please note that the models shown are not exclusive, but represent rather examples of models that have been used in the past for studying epileptogenesis. Not all of these models have been used as yet for studying drug effects on epileptogenesis. SE = status epilepticus; TBI = traumatic brain injury; MAM = methylazoxymethanol acetate; GAERS = Genetic Absence Epileptic Rat of Strasbourg; GEPR = genetically epilepsy-prone rat; BNFC = benign familial neonatal convulsions; KV = voltage-gated Kv potassium channels; WAG/Rij = Wistar Albino Glaxo/Rij
This review will focus largely on the different animal models of epileptogenesis and their potential utility for evaluating proposed antiepileptogenic therapies and identifying useful biomarkers. We also discuss the results of previous studies of antiepileptogenesis and the role of monotherapy versus polytherapy approaches for identifying promising antiepileptogenic therapies.
Animal Models of Acquired Epilepsy
In much the same way that animal models are used to identify therapies for the symptomatic treatment of epilepsy, a number of experimental epilepsy models have emerged that could be used in the search for a potential “disease-modifying” or “antiepileptogenic” therapy. In addition to the models of acquired (symptomatic) epilepsy, a number of genetic models with mutations known to be associated with a particular seizure type or epilepsy syndrome have been described (Fig. 1).
By definition, acquired epilepsy results from a brain insult that is followed by a “latent period” that can be months or years in duration [4, 6]. As discussed below, this latent period provides a “window of opportunity” wherein a potential antiepileptogenic or disease-modifying therapy could be administered in an effort to prevent, delay, or modify the epileptogenic process unleashed by the initial brain insult. Several of the available animal models listed in Fig. 1 display many of the features of human epilepsy, and may play a role in the testing and discovery of novel disease-modifying or antiepileptogenic therapies for one or more of the acquired or genetic epilepsies. One would also hope that the information gleaned from the preclinical studies utilizing these models would guide the design of any subsequent clinical trials.
Of the myriad available rodent models of acquired epilepsy, the kindling, status epilepticus (SE), and traumatic brain injury (TBI) models represent the three models most often employed in the search for antiepileptogenic and disease-modifying therapies [4, 6]. With that said, a number of etiologically relevant models have emerged that display important properties of human acquired epilepsy (Fig. 1). These include the tetanus toxin model, which, depending on injection site and age of the animals, can be used as a model of infantile spasms and temporal lobe or neocortical epilepsy, the neonatal hypoxia–ischemia model of hypoxic–ischemic injury, neonatal hyperpyrexia model of febrile seizures, models of infantile spasms, models of cortical dysplasia, stroke, and an emerging model of viral encephalitis (see White [6] for a review and references). With the exception of the kindling models, each of these models is associated with a latent period of varying length, an altered seizure threshold, the development of spasms in the case of infantile spasms, or the emergence of unprovoked seizures. Underlying the phenotypic features of these models is a number of histopathologic and functional abnormalities that includes cell loss, synaptic reorganization, and network hyperexcitability. From a translational perspective, the hope would be that those therapies found effective in preventing or modifying the epileptogenic process at the preclinical level could be translated to the human population at risk for developing epilepsy. It is important to note that the underlying pathology would likely be different amongst these and other models of acquired epilepsy, and, as such, drugs found effective in one model would not necessarily be effective in other models. Indeed, the underlying biological substrates that contribute to epileptogenesis in one etiological model of epilepsy would not necessarily be the same in another epilepsy model; for example, epileptogenesis following stroke would likely involve excitotoxic cell death, inflammation, gliosis, microglial activation, and other alterations, whereas epilepsy that is associated with models of tuberosclerosis would more likely respond to therapies that target the mammalian target of rapamycin (mTOR) pathway. As a consequence, a pharmacological agent being effective in a model of tuberosclerosis may be an interesting clinical candidate for preventing development of epilepsy associated with this condition but would not automatically be antiepileptogenic in other conditions. Nonetheless, each model of acquired epilepsy provides a unique platform for testing proposed “disease-modifying” or “antiepileptogenic” therapies.
A useful animal model for seeking mechanisms or pharmacological agents for antiepileptogenic or disease-modifying therapies should have at least the following properties: 1) a brain insult that is known to result in acquired epilepsy in humans; 2) a latent period following the brain insult that allows to interfere with epileptogenesis; 3) long-term consequences (e.g., spontaneous seizures, neurodegeneration, and behavioral and cognitive alterations) occurring after the latent period that are similar to those observed in humans following such brain insults; 4) a high frequency of spontaneous recurrent seizures (SRS), so that any antiepileptogenic or disease-modifying effect of an investigational drug can be easily determined by video/electroencephalography (EEG) monitoring of SRS; 5) similar as in humans, the brain insult should not result in epilepsy in the majority of animals, but only a proportion (e.g., 50 %) of the animals should develop SRS—this would allow the investigator to determine which biomarkers, if any, separate between animals developing or not developing SRS after the insult; such biomarkers would be of significant value for evaluating the therapeutic response to a potential antiepileptogenic or disease modifying therapy (see below).
Genetic Models of Human Epilepsy
As summarized in Fig. 1, several genetic rodent models with known mutations have emerged that recapitulate many important characteristics of human epilepsy. These models are providing important insight into the role of a specific mutation in ictogenesis and epileptogenesis. Furthermore, many of these models are emerging as important tools for validating novel targets for the treatment and prevention of epilepsy. The potential of a genetic model for evaluating new therapies for the treatment of human epilepsy is best exemplified by the WAG/Rij rat and the Genetic Absence Epileptic Rat of Strasbourg (GAERS). These two genetic models represent two important phenotypic models of human absence epilepsy that display an electrographic and pharmacologic profile consistent with primary generalized epilepsy [8]. They have been utilized for years for evaluating the therapeutic potential of an investigational drug for the treatment of primary generalized epilepsy. As discussed below, several studies have now been completed utilizing the WAG/Rij and other genetic models to evaluate the disease modifying/ antiepileptogenic potential of targeted therapies.
Furthermore, various genetic mouse models of epilepsy, including Scn1A knockout mice, gamma-aminobutyric acid-Aγ2(R43Q) mice, Tsc1 mutant mice, Kv7.2/7.3 mice, and Fmr1 knockout mice, have been described in recent years that possess many of the features of human genetic epilepsies resulting from a specific defect in a specific receptor or voltage-gated ion channel [6]. Such models present valuable opportunities to isolate and experimentally reproduce gene mutations for human seizure disorders, to test molecular mechanisms of epileptogenesis, and to explore strategies to correct early hyperexcitability defects in the developing brain. However, as yet, genetic mouse models of epilepsy are only rarely used to test investigational drugs for antiepileptogenic or disease-modifying properties.
In addition to the myriad of rodent seizure and epilepsy models, zebrafish are emerging as a potentially important non-rodent platform system for the early evaluation of anti-seizure activity (see, e.g., [9] and [10]). The ease with which seizure activity, both behavioral and electrographic, induced by either chemoconvulsants or genetic mutations, can be quantitated makes the zebrafish an extremely attractive model for the rapid screening of investigational drugs. Because of their genetic tractability, the zebrafish model is also potentially useful for the study of monogenic epilepsy syndromes. For example, Baraban and colleagues [10, 11] are using the zebrafish system to model some of pediatric epilepsies such as Dravet syndrome, which results from mutations in Scn1a. The rapidity with which know human mutations can be expressed in zebrafish may offer a unique mechanism for assessing purported antiepileptogenic drug and genetic therapies.
A comprehensive review of the pathophysiological and phenotypical features of all of the models summarized in Fig. 1 is outside the scope of this review (see White [6] for a review and references), and the remainder of this article will focus the some of the factors that should be considered when embarking on an intervention study using one or more of these models.
Important Considerations When Using Animal Models in the Search for Antiepileptogenic Drugs
The validity of any scientific investigation depends, in a large part, on the rigor that was applied in the design and conduct of the study; for example, “Were the outcome measures and statistical procedures clearly defined before the initiation of the study?”, “Was the study powered sufficiently to detect a difference?”, “Were the experimenters blinded during treatment and analysis phases of the experiment?”, “Were animals randomly assigned to the experimental groups?”. These, and other, factors, which have recently been the subject of extensive discussion, can certainly affect the outcome of a study and lead to unsubstantiated conclusions and non-reproducible results [see [4] and [12]).
In addition to the above considerations, a number of other factors can play an important role in the outcome and interpretation of experimental results obtained from an intervention study. These include, but are not limited to:
Intent of the study; for example, is it designed to demonstrate an antiepileptogenic, disease-modifying, or neuroprotective effect of a putative therapy?
Treatment initiation and duration; Is it coincident with the therapeutic window of the selected model?
Is dosing based on some knowledge of the pharmacokinetic properties of the therapy, that is, Adsorption, Distribution, Metabolism, and Excretion (ADME)?
Are the observed outcomes due to the “anti-seizure” or “antiepileptogenic” properties of the treatment?
Definitions
The intent of any intervention study should be defined clearly at the outset as it will certainly play an important role in selecting the outcome measures. In a 2013 review by Pitkänen et al. [12], “epileptogenesis refers to the development and extension of tissue capable of generating spontaneous seizures, resulting in (1) development of an epileptic condition and/or (2) progression after the condition is established”, whereas “disease modification has two components: antiepileptogenesis (AEG) and comorbidity modification”. Accordingly, an antiepileptogenic study would evaluate the ability of a test compound to alter one or more of the underlying processes that contribute to the development or progression of an epileptic condition, whereas a disease-modifying study would evaluate the ability of a therapy, when administered prior to epilepsy onset, to affect the frequency, duration, and/or severity of the seizures when they do occur. When administered after the onset of spontaneous seizures, a disease-modifying therapy can “alleviate seizure severity, prevent or reduce the progression of epilepsy, or change the seizures from drug resistant to drug sensitive” [12]. Given these definitions, a therapy that is thought to be antiepileptogenic could easily be shown to be disease-modifying; however, this conclusion could not be reached if the study is not designed in a way that would allow for prolonged monitoring after the nth seizure is observed. For example, given the short nature associated with the “latent period” in some epilepsy models, a failed antiepileptogenic study could conclude at some time point after the nth seizure (defined before study initiation). After all, it is much easier to demonstrate that a drug is not antiepileptogenic than to prove it is! If the study design allows for prolonged monitoring, one would then know whether the treatment actually “modified” the outcome by shortening the duration, modifying the frequency, or altering the severity of the subsequent seizures. Neuroprotection refers to the preservation of neuronal cells and/or function. The ability to correlate histopathology and network function through imaging, electrophysiology, or other noninvasive measures would play an important role in the development and validation of in vivo biomarkers. This is becoming more and more important given the evidence suggesting that epilepsy can develop in the absence of marked histopathological damage [4].
Treatment Initiation and Duration: Is it Coincident with the Therapeutic Window of the Selected Model?
When should treatment with the investigational antiepileptogenic therapy be initiated and how long should it continue? The answer to this question relies, in a large part, on accurate knowledge of the “latent period” of the model that is being employed by the investigator. Thus far, the majority of intervention studies have been conducted in one of the SE models, where the “latent period” can be extremely short (hours to a few days) to 20 or more days [4]. Importantly, it can also be very variable between animals, thereby making it extremely difficult to design a study that will minimize variability and maximize reproducibility. As a result, efforts to reduce variability by halting the SE after a pre-determined time have been attempted in an effort to “clamp” the duration of convulsive SE; however, such efforts have been employed with varying degrees of success. The largest concern over studies that do not employ video-EEG to monitor the duration of the initial SE is that the investigator may successfully limit the convulsive SE without necessarily affecting the duration of nonconvulsive SE: an effect that can contribute to variability in the extent of neuronal damage and the “latent period” [4]. In an ideal situation, treatment with the “putative” antiepileptogenic compound would begin at the same time after SE onset and continue for the duration of the “latent period”. This leads to the next question: How long should animals be monitored after discontinuation of treatment? Again, this depends on the initial purpose of the study. If the study was designed to assess whether or not the treatment was antiepileptogenic, then monitoring could be discontinued (in theory) after the nth observed seizure. If the study permitted prolonged monitoring after the nth seizure, then it would be possible to ascertain whether the treatment was disease-modifying. For the purposes of this discussion, monitoring refers to continuous (24/7) video-EEG; anything else would certainly compromise the interpretation of the results.
Is Dosing Based on Some Knowledge of the Pharmacokinetic Properties of the Therapy (ADME)?
Many of the intervention studies conducted in the past have been done with very little information about the ADME and or pharmacokinetics/pharmacodynamics of the investigational drug. This becomes particularly important when one considers that epilepsy is a progressive disease and that treatment may need to continue for weeks to months. Depending on the question being asked, it may be necessary for there to be constant brain exposure to the investigational therapy for prolonged periods of time. To this point, there should be some evidence that the purported drug target is altered following the brain insult and that the therapy is able to engage the target for the duration of the treatment. This discussion implies that there is a mechanism to deliver the therapy for the duration of the study in such a way that the drug achieves and maintains steady state blood and brain levels. Because steady state dosing depends on the half-life of the drug, it is important to know something about the metabolism of the drug so that an optimized dosing protocol can be designed [13]. Unfortunately, rodents are generally rapid metabolizers of most drugs and the half-life of AEDs, except phenobarbital, is short necessitating the need for multiple daily drug administrations. In an ideal situation, a drug would be administered without animal handling, as this can often precipitate a seizure in an epileptic rodent; however, this is difficult to do if the drug cannot be administered in the food, water, or through a mini-osmotic pump (or similar devices). Depending on the dose, solubility, and stability of the drug, it may be possible to achieve adequate exposure by one of these three preferred routes. The importance of this issue cannot be overstated, as a failed study could be the result of a number of factors related to ADME, including lack of brain penetration and target engagement for the duration of the study, development of pharmacokinetic and pharmacodynamic tolerance with chronic exposure, and inappropriate dose (too low or too high) [13].
Are the Observed Outcomes due to the ‘Anti-seizure’ or ‘Antiepileptogenic’ Properties of the Treatment?
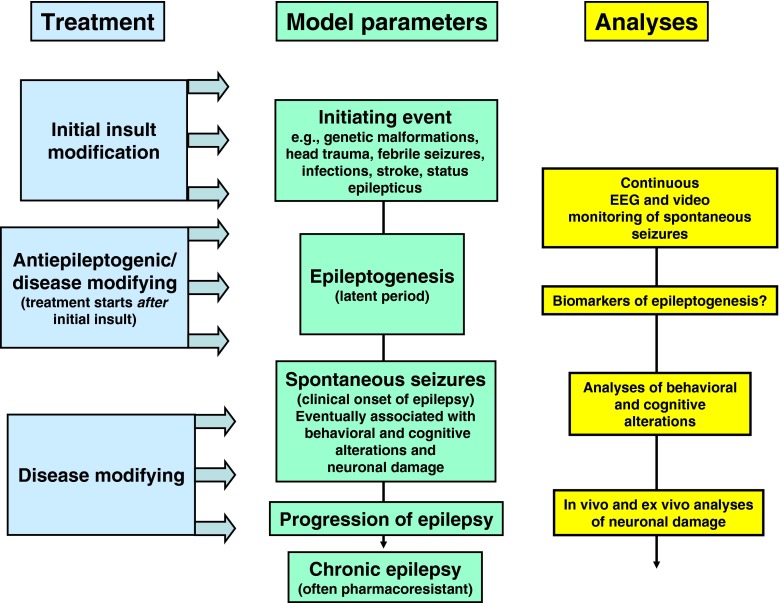
With the availability of a number of SE models, it has become increasingly apparent that the duration of SE can affect the degree of brain damage and that the degree of damage can contribute to the development and severity of epilepsy. Thus, anything that might modify the initial insult can have dramatic effects on the outcome and could lead to an erroneous conclusion regarding the antiepileptogenic or disease-modifying properties of the investigational drug (Fig. 2). In this regard, it is extremely important to rule out the possibility that a “positive” outcome is not the result of a drug’s ability to modify the “insult” or initial SE. This is best done by video-EEG monitoring for the duration of the SE to insure that the duration and severity of the initial SE is consistent between the vehicle control and experimental groups. Furthermore, when a new vehicle is employed, it is also important to verify that the vehicle does not possess any untoward effects that could compromise the interpretation of the study. An important example here is ethanol, which is often used to dissolve hydrophobic drugs, but may exert anticonvulsant effects of its own and synergistically enhance the anticonvulsant (and various other) effects of drugs dissolved in this vehicle [14–17].
Fig. 2.
Concepts and model parameters in studying drug effects on epileptogenesis. EEG = electroencephalography
Early results with the mTOR inhibitor rapamycin suggested that treatment with this immunosuppressant was disease-modifying in two different models of SE-induced epilepsy [18, 19]. Furthermore, Zeng et al. [20] found that rapamycin exerted an anti-seizure effect in a mouse model of tuberous sclerosis—a disease in which there is strong evidence supporting a role for the hyperactivation of the mTOR pathway in the pathology of tuberous sclerosis. Unfortunately, the initial observations suggesting that rapamycin prevents SE-induced mossy fiber sprouting were contingent upon the continued exposure to rapamycin. More recently, Heng et al. [21] confirmed that rapamycin treatment prevented mossy fiber sprouting in the pilocarpine SE model of temporal lobe epilepsy; however, rapamycin treatment failed to prevent the development of epilepsy. Based on the studies conducted to date, rapamycin treatment does appear to possess the ability to limit mossy fiber sprouting. However, it also appears to possess a prominent anti-seizure effect—an action that could contribute to its ability to prevent mossy fiber sprouting. Interestingly, this is another study suggesting that SE-induced epilepsy can occur in the absence of marked mossy fiber sprouting and other types of morphological alterations in the hippocampus (see Löscher and Brandt [4] for further discussion).
One strategy that can be used to exclude the possibility that the effect of an investigational drug on development of SRS following brain insult is secondary to an anti-seizure (or initial insult-modifying) effect is to determine whether the agent exerts anti-seizure effects after acute or repeated treatment in naïve animals (using electrical or chemical seizure induction). Indeed, the lack of anti-seizure efficacy in such acute seizure models coupled with clear-cut reduction in the development of SRS in chronic models of epileptogenesis by the same treatment could argue for truly antiepileptogenic effect. However, depending on the epileptogenic brain insult, the mechanism of an initial insult-modifying effect in a chronic model of epileptogenesis may differ from mechanism(s) of anti-seizure effects in acute seizure models, so that, as illustrated by rapamycin, it is not a trivial task to differentiate between insult modifying and antiepileptogenic drug effects.
Comorbidity Modification
The ability to prevent the development of epilepsy in the susceptible individual represents the “Holy Grail” of epilepsy research; however, preventing the cognitive decline and the emergence of other debilitating comorbidities associated with epilepsy would represent a significant step forward for the patient with epilepsy [4]. To this end, employing the currently available models to evaluate the cognitive sparing potential of a new therapy could provide the patient with an improved quality of life in the near term while the search for a “cure” continues.
Biomarkers for Epileptogenesis and Antiepileptogenesis
Given the heterogeneity and variability associated with different brain insults, it may take many years before a patient develops epilepsy after injury. As such, the duration of any intervention trial could easily exceed available resources, thereby making most antiepileptogenesis studies highly impracticable. The availability of biomarkers that track with the onset and progression of epilepsy in a susceptible patient would be enormously valuable for evaluating the therapeutic response to a potential antiepileptogenic or disease-modifying therapy. Validated biomarkers of epileptogenesis and ictogenesis, as suggested by Engel et al. [22], could be used to “(a) predict the development of an epilepsy condition, (b) identify the presence and severity of tissue capable of generating spontaneous seizures, (c) measure progression after the condition is established, (d) create animal models for more cost-effective screening of potential antiepileptogenic and anti-seizure drugs and devices, and (e) reduce the cost of clinical trials of potential antiepileptogenic interventions by enriching the trial population with patients at high risk for developing epilepsy”. In this regard, the wide range of etiologically relevant animal epilepsy models could play an important role in the identification and validation of biomarkers. Information gleaned from animal studies could be translated to the appropriate human population. Similarly, coincident development of biomarkers in patients with epilepsy can then be back-translated to animal studies and utilized for therapy discovery. The importance of having access to validated biomarkers cannot be understated, and efforts at both the clinical and preclinical level to achieve this goal are clearly required.
As discussed by other authors in this issue, a number of biomarkers have emerged that may provide predictive insight into the process of epileptogenesis. These include alterations in hippocampal magnetic resonance images, as suggested by the FEBSTAT study, presence of interictal spikes and high-frequency oscillations in the EEG, altered seizure threshold, and the presence of blood markers suggestive of brain inflammation or neurodegeneration [3, 22]. In addition, genetic biomarkers have the potential to predict outcome and inform treatment for the selected patient population.
It is clear that the success of future antiepileptogenic trials could benefit from the continued development and validation of these and other emerging biomarkers. For example, the availability of a biomarker that could successfully identify the patient at risk for developing epilepsy would allow for pre-emptive treatment with a potential antiepileptogenic therapy. This, when coupled with the availability of a biomarker that tracks with the progression of epilepsy, would revolutionize current thoughts regarding the design and execution of an intervention study.
Previous Studies on Antiepileptogenesis
Recently, Löscher and Brandt [4] have discussed extensively previous preclinical studies on antiepileptogenesis in animal models and the few clinical studies in patients with TBI, so that discussion will not be repeated here, but the interested reader is encouraged to read their review. What is important, however, for the topic of this review is to note the diverse strategies that have been used preclinically to modify the development of epilepsy after brain insults such as SE or TBI, including prevention (or modification) of the insult (by treating the animals before inducing the insult), initial insult modification by reducing the severity or duration of the insult (by treating the animals during the insult, e.g., SE), and antiepileptogenesis (or disease modification) by treating the animals after an insult of enough severity and duration to induce epileptogenesis in the absence of any treatment (see Fig. 2). Only a few studies have examined whether treatment of rodents after occurrence of the first SRS can modify the subsequent progression of epilepsy. In the following, we will only discuss studies that have tested compounds for “true” antiepileptogenic or disease-modifying efficacy by administering the compounds after an epileptogenic brain insult. As shown in Fig. 2, and discussed above, in addition to recording SRS by continuous (24/7) video-EEG recording as a primary endpoint in antiepileptogenesis or disease-modification studies, the analyses of behavioral and cognitive alterations associated with epileptogenesis and neuronal damage provide additional endpoints that can be assessed in such studies [4]. Here, it is interesting to note that the majority of preclinical studies on potential antiepileptogenic or disease-modifying treatments that found effects reported reduced behavioral or neurodegenerative alterations, or reduced frequency of SRS, rather than prevention of SRS [4]. One reason for this finding may be that the brain insults that are used in rodents to induce epileptogenesis are much too severe to allow for the prevention of epilepsy by treating the animals after the insult. This view is supported by the fact that often the latent period in such models is very short (or even not existent), so that there might be no window of opportunity to interfere with epileptogenesis [23, 24]. Thus, one important goal for the future is to modify the available animal models in order to reduce the severity of the brain insult, thereby increasing the duration of the latent period and reducing the percentage of animals that develop epilepsy (which, typically, is >90 % in the SE models currently used). Otherwise, we will continue to produce negative findings with potentially interesting compounds in preclinical antiepileptogenesis trials.
Studies with AEDS
Almost all available AEDs, which are clinically used for symptomatic suppression of SRS in patients with epilepsy, have been evaluated for antiepileptogenic or disease-modifying effects in animal models of epileptogenesis [4]. Although these drugs were developed for control of seizures and not for prevention of epilepsy after brain insults, many of them possess mechanisms of action that could also interfere with epileptogenesis [25]. It is therefore not surprising that some of these AEDs, particularly valproate, levetiracetam, and topiramate, were reported to exert disease-modifying effects in rat SE models of epileptogenesis [4]. More recently, we used valproate, which we found previously to exert disease-modifying and neuroprotective effects in an electrical SE model of epileptogenesis [26], to determine the therapeutic window of this effect. By using a protocol that allowed us to effectively interrupt SE and compare various treatment protocols with valproate administered after SE, we found that continuous infusion of valproate for 24 h immediately after the SE was the most effective neuroprotective treatment, preventing most of the neuronal damage in the hippocampal formation, including the dentate hilus [27]. This suggests that the therapeutic window may be open for a shorter period than commonly assumed, and as recently suggested [28, 29]. Our positive result stands in contrast to many examples of experimental treatments that did not seem to have any significant effect when the treatment was started at the end of the insult [30]. As stated by Sloviter [30], “the work of Löscher and colleagues is a significant step forward […] because it reminds us that reasonable certainty about the ineffectiveness of a given treatment requires reasonable certainty that the right dose was administered by the right route, in the right concentration, at the right time, and for the right duration”. False-negative conclusions are probably a significant factor in this field [30].
Whereas most previous studies designed to assess AED effects on epileptogenesis have used post-SE models of epilepsy, few studies have used genetic models of generalized epilepsy [4]. In the WAG/Rij rat model of absence epilepsy, early prophylactic treatment with ethosuximide, levetiracetam, or zonisamide (but not carbamazepine) before the onset of spike-wave discharges in the EEG suppressed the development of such absence-like seizures [31–33], which was also subsequently observed in the GAERS model of absence epilepsy [34]. These findings suggest that models are available in which epileptogenesis can be controlled and that early treatment during development may provide a strategy for preventing genetic epilepsy in susceptible individuals.
In addition to SE and genetic models, amygdala kindling has been widely used to evaluate antiepileptogenic effects of AEDs [4]. This model, in which repeated administration of an initially subconvulsive electrical stimulus via a depth electrode in the basolateral amygdala results in the development of seizures with increasing severity and duration, has been criticized in recent years because the model is characterized by induced seizures instead of SRS, and drugs given during kindling acquisition may suppress kindling merely by an anticonvulsant effect rather than exerting an antiepileptogenic effect [4]. However, the latter problem can be easily overcome by using a protocol in which treatment of rats during kindling is followed by a wash-out phase without treatment and subsequent continuation of kindling in the absence of drug [35]. By using this experimental design, valproate and levetiracetam were shown to retard kindling after drug withdrawal [35, 36], whereas various other AEDs were ineffective in this regard [4]. Based on findings of functional (prokindling) alterations after depth electrode implantation in the absence of electrical stimulation in rats, Löscher [37] has proposed that amygdala kindling may present a model of TBI, in which the consequences of penetrating brain injury (by the depth electrode) are facilitated by electrical stimulation. More recently, D’Ambrosio and colleagues [38, 39] have used rostral parasagittal fluid percussion injury as a model of TBI in rats and started to evaluate AEDs and other treatments for antiepileptogenic effects in this model.
In apparent contrast to the promising findings with valproate in preclinical models of epileptogenesis, a clinical trial with this AED in patients with TBI failed to demonstrate any antiepileptogenic or disease-modifying effect, which is similar to previous trials with other AEDs (e.g., phenobarbital, phenytoin, carbamazepine) in such patients [40]. However, the limitations of current technology to assist in antiepileptogenesis trials must be acknowledged [41], so that important effects of treatment may have been missed in such post-traumatic epilepsy prevention trials. Furthermore, assuming that the window of opportunity following brain insults in patients is as short as in animal models, which is supported by findings with neuroprotective agents (e.g., tissue plasminogen activator) in stroke [42], the treatment of TBI patients with AEDs such as valproate may have missed this window in at least some of the patients, thus contributing to the negative outcome of such studies. In an excellent review, Mani et al. [43] have recently described what an ideal human antiepileptogenesis trial should look like to enhance the change of identifying clinically meaningful drug effects.
Studies with Experimental Compounds Targeting Mechanisms of Epileptogenesis
Prompted by the apparently negative outcome of most antiepileptogenesis studies with AEDs and the increasing understanding of the molecular and functional brain alterations possibly underlying epileptogenesis, many researchers started to use compounds directly targeting such alterations in animal models of epileptogenesis [4]. However, similar to studies with AEDs, most studies using such experimental compounds, including neuroprotective, neuromodulatory, or anti-inflammatory drugs, failed to prevent the development of SRS, but, at best, exerted disease-modifying effects [4]. One remarkable exception was recently reported by Liu et al. [44]. By using a mouse model of SE-induced epilepsy, in which kainate is injected into the basolateral amygdala, leading to limbic SE (which is interrupted after 40 mins by diazepam and lorazepam) and development of SRS after a seizure-free latent period of several days, inhibition of the SE-induced activation of brain-derived neurotrophic factor receptor TrkB was shown to prevent SRS, ameliorate anxiety-like behavior, and limit loss of hippocampal neurons when tested weeks to months later [44]. This study thus provides a proof-of-concept that antiepileptogenesis is possible, provided that models are used that avoid the massive brain alterations associated with induction of SE by systemic administration of convulsants such as kainate or pilocarpine. Such proof-of-concept was also reported in a study that used a TBI model in rats, in which mild passive focal cooling following rostral parasagittal fluid percussion injury in rats was associated with potent and persistent prevention and modification of epileptic seizures [39]. In contrast, the experimental AED carisbamate was without effect on epileptogenesis in this model [38].
Single Treatment Versus Combinatorial Treatment Strategies
Most previous studies on antiepileptogenesis used single drug treatment during the latent period in animal models of epilepsy [4]. However, epileptogenesis is a multifactorial process, so that it is unlikely that drugs acting via a specific target, for example an ion channel or neurotransmitter receptor, can halt this process. This may also explain why compounds such as valproate, which display a multitude of mechanisms of action, are more likely to exert disease-modifying effects than other, more selective AEDs. Combinatorial treatment strategies, in which several drugs are combined in an effort to engage different targets presumed to be involved in the epileptogenic network, may be a more effective strategy than treatment with single, highly specific drugs [3].
Concept of Network Pharmacology
Most epilepsies develop not from alterations of a single target, but rather from complex alterations that result in an epileptic network in the brain [3, 45, 46]. The only existing cure of epilepsy in many patients is resective surgery in which the regional epileptic network, or part of this network, is removed [47]. Thus, single-target treatments focused exclusively on a single protein, or an individual biochemical pathway, may be less effective than multiple-target treatments targeting different proteins or pathways involved in the network. The latter approach has been recently termed “network pharmacology”, and relates to principles of systems biology [48, 49]. The principle of network pharmacology is to develop combinations (“cocktails”) of existing or novel drugs that would, when administered together, modulate several mechanisms, in an effort to regulate different targets within a biological network. This strategy may turn out to be particularly useful for diseases like the epilepsies where the etiology is complex and do not sufficiently respond to single target treatments, or for which no treatment exists. Systems biology-based approaches of network pharmacology have recently been proposed for developing antiepileptic and antiepileptogenic treatments [4, 50, 51]. In addition to combining drugs in network pharmacology, one target that modulates several pathways is an alternative option as illustrated by the mTOR pathway [52] or transcription factors such as Nrf2 [53].
Examples for Synergistic Drug Combinations
Twenty years ago, we reported that the anticonvulsant effect of 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(F)quinoxaline (NBQX), which acts on the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subtype of glutamate receptors, can be potentiated by extremely low doses (0.0001–0.1000 mg/kg) of the N-methyl-D-aspartate (NMDA) receptor antagonist MK-801 (dizocilpine) in the amygdala kindling model [54]. Similar over-additive effects were seen when NBQX was combined with the competitive NMDA antagonist CGP39551 (the carboxyethylester of DL-(E)-2-amino-4-methyl-5-phosphono-3-pentenoic acid) or the low-affinity, rapidly channel blocking NMDA receptor antagonist memantine [54, 55]. Adverse effects were not potentiated by combining low doses of NMDA antagonists with NBQX. These data suggested that both non-NMDA and NMDA glutamate receptors are critically involved in the kindled state, and that combinations of AMPA and NMDA receptor antagonists provide a new strategy for treatment of epileptic seizures. However, it took almost 20 years before the first AMPA antagonist, perampanel, was approved for treatment of epilepsy [25]. We are currently evaluating combinations of clinically-approved NMDA antagonists (ketamine, memantine) and perampanel in models of difficult-to-treat seizures. Furthermore, in view of the fact that both glutamate receptor subtypes are critically involved in epileptogenesis [56], combinations of NMDA and AMPA antagonists are interesting candidates for antiepileptogenesis.
Another interesting example is the combination of phenobarbital with the diuretic bumetanide [57]. Bumetanide inhibits the neuronal chloride cotransporter NKCC1, which acts as a chloride importer. Gamma-aminobutyric acid-mediated excitation has been observed when expression of NKCC1 is higher than expression of the chloride exporter KCC2; e.g., early during development and in the hippocampus of adults with temporal lobe epilepsy [58, 59]. Based on the hypothesis that increased expression of NKCC1 may be involved in the development of neuronal hyperexcitability during epileptogenesis in adults, we evaluated whether treatment with bumetanide after pilocarpine-induced SE alters the long-term consequences of the brain insult [60]. Based on promising effects of combinations of bumetanide and phenobarbital in neonatal seizures [61], treatment with bumetanide alone was compared with treatment with phenobarbital alone and with combinations of the two drugs. None of the treatments prevented development of SRS, but the combined treatment counteracted the development of behavioral alterations associated with epilepsy, indicating a disease-modifying effect [60]. As bumetanide is rapidly eliminated in rodents and only poorly penetrates into the brain [60], we are currently trying to overcome these disadvantages by either administering more lipophilic prodrugs of bumetanide or inhibiting its metabolism in rodents, or both. Interestingly, the lack of any significant effects of single drug treatment with bumetanide in our study [60] may be due to the model (pilocarpine) used because in a more recent study by Koyama et al. [62] bumetanide was shown to prevent the development of epilepsy after febrile seizures in rats. Thus, again, the type and severity of epileptogenic brain insult is important when studying the antiepileptogenic efficacy of compounds. Positive findings with such compounds in a model should not be quickly extrapolated to the clinic but await replication in other models.
Increasing evidence suggests an important role for inflammatory processes in epileptogenesis [63]. Three main inflammatory pathways, namely the interleukin-1 (IL-1) receptor/Toll-like receptor signaling, cyclooxygenase-2 (COX-2), and the transforming growth factor β signaling, provide interesting targets for antiepileptogenic intervention [3, 63]. However, targeting each of these pathways alone may not be sufficient. Kwon et al. [64] selected anti-inflammatory drugs directed against IL-1 receptors (IL-1ra), a COX-2 inhibitor (CAY 10404), and an antagonist of microglia activation of caspase-1 (minocycline), and tested them for neuroprotective and antiepileptogenic effects in a SE model in 2–3-week-old rats. None of these drugs was effective in attenuating neuronal damage or in limiting the development of spontaneous seizures when administered individually. When empiric binary combinations of these drugs were tried, the combined targeting of IL-1ra and COX-2 resulted in attenuation of acute brain injury, reduced the development of SRS, and limited the extent of mossy fiber sprouting. The authors concluded that “deployment of an empirically designed ‘drug cocktail’ targeting multiple inflammatory signaling pathways for a limited duration after an initial insult like SE may provide a practical approach to neuroprotection and anti-epileptogenic therapy”. However, treatment with anti-inflammatory agents was started before injecting pilocarpine to induce SE, and the combination treatment of COX-2 inhibitor plus IL-1ra significantly delayed the latency to pilocarpine-induced SE onset, indicating initial insult modification [64]. Thus, experiments in which this combination is administered after the SE (or other epileptogenic brain insults) are needed. Nevertheless, these examples strongly indicate that combinatorial treatment strategies are the way to go.
Conclusions
Animal seizure and epilepsy models continue to play an important role in the early discovery of new therapies for the symptomatic treatment of epilepsy. The success of the current screening approach is indisputable, and millions of patients worldwide have benefited from the process employed by the National Institute of Neurological Disorders and Stroke (NINDS) Anticonvulsant Screening Program and other early discovery programs. Furthermore, studies using animal models have led to a greater understanding of the factors underlying ictogenesis and epileptogenesis, and provided significant insight into the mechanisms of how AEDs act to suppress seizures. Unfortunately, the current approach has done little to identify therapies that will prevent or modify the epilepsies in people at risk, largely because of a lack of consensus within the epilepsy research community regarding which, if any, animal model and experimental approach would be best suited for an antiepileptogenesis and/or disease-modification study.
This review focuses largely on some of the most common animal models of epileptogenesis and their potential utility for evaluating proposed antiepileptogenic therapies and identifying useful biomarkers. We also describe some of the limitations of using animal models in the search for therapies that move beyond the symptomatic treatment of epilepsy. Promising results of previous studies designed to evaluate antiepileptogenesis and the role of mono- versus polytherapy approaches are also discussed. Importantly, results obtained with ethosuximide and levetiracetam in models of primary generalized epilepsy certainly suggest that it may be possible to prevent, or at least modify, the development of epilepsy in this patient population. Furthermore, our knowledge of the targets involved in the epileptogenic process has matured to the point that targeted studies are not only possible, but are beginning to yield important data supporting the concept that a clinical trial is within reach.
As discussed in this review, exciting new data from the McNamara laboratory [44], using a pharmacological and genetic approach, suggests that inhibition of one such target, that is, TrkB, shortly after initiation of SE prevents SRS and anxiety-like behavior and limits hippocampal neuronal loss. Other potential targets include NKCC1 [57, 62] and modulators of neuroinflammation, that is, IL-1, COX-2, and transforming growth factor β [63]. Further interesting findings (not discussed in detail here) include 1) administration of decoy oliognucleotides limiting the transcriptional repressor, neuron-restrictive silencer factor (NRSF), which is initiated after SE, resulted in a 70 % reduction in the number of SRS during the ensuing 2 weeks [65]; 2) pharmacological depletion of a microRNA, miR-134, initiated after SE reduced the occurrence of SRS when tested weeks later [66]; and 3) treatment with atipamezole, an α2-adrenergic receptor antagonist, starting 1 week after SE, reduced frequency and severity of SRS tested after wash-out from atipamezole, indicating a disease-modifying effect [67]. Whether it will be possible to prevent epilepsy with just a single drug is still not clear and well-designed studies that compare this approach to a “cocktail” approach are clearly warranted.
In summary, the animal models and experimental approaches employed in the search for therapies that may possess antiepileptogenic and/or disease-modifying properties have certainly matured in recent years to the point that larger scaled preclinical studies are warranted wherein the most promising observations can be quickly replicated. Furthermore, enhanced communication between basic and clinical scientists is leading to more interdisciplinary research that will lead, ultimately, to the design and execution of clinical trials in the susceptible patient population. Only then, will it be possible to determine whether promising preclinical results can be translated into the development of a disease-modifying or preventive therapy.
Electronic supplementary material
(PDF 1225 kb)
(PDF 1224 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Contributor Information
H. Steve White, Email: swhite@hsc.utah.edu.
Wolfgang Löscher, Email: wolfgang.loescher@tiho-hannover.de.
References
- 1.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9:68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- 2.Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia. 2011;52:657–678. doi: 10.1111/j.1528-1167.2011.03024.x. [DOI] [PubMed] [Google Scholar]
- 3.Löscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for antiepileptic drug discovery and development. A joint endeavor of academia and industry. Nat Rev Drug Discov. 2013;12:757–776. doi: 10.1038/nrd4126. [DOI] [PubMed] [Google Scholar]
- 4.Löscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010;62:668–700. doi: 10.1124/pr.110.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitkänen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 6.White HS. Animal models for evaluating antiepileptogenesis. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s basic mechanisms of the epilepsies. 4. New York: Oxford University Press; 2012. pp. 1041–1054. [Google Scholar]
- 7.Galanopoulou AS, Simonato M, French JA, O’Brien TJ. Joint AES/ILAE translational workshop to optimize preclinical epilepsy research. Epilepsia. 2013;54(Suppl. 4):1–2. doi: 10.1111/epi.12293. [DOI] [PubMed] [Google Scholar]
- 8.Coenen AM, Van Luijtelaar EL. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33:635–655. doi: 10.1023/A:1026179013847. [DOI] [PubMed] [Google Scholar]
- 9.Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 2005;131:759–768. doi: 10.1016/j.neuroscience.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Baraban SC, Dinday MT, Hortopan GA. Drug screening and transcriptomic analysis in Scn1a zebrafish mutants identifies potential lead compound for Dravet Syndrome. Nature Comm. 2013;4:2410. doi: 10.1038/ncomms3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baraban SC, Löscher W. New approaches to model epilepsy and identify promising drug treatments. In: Scharfman H, Buckmaster PS, editors. Issues in clinical epileptology. A view from the bench. Heidelberg: Springer; 2013. [Google Scholar]
- 12.Pitkänen A, Nehlig A, Brooks-Kayal AR, Dudek FE, Friedman D, Galanopoulou AS, et al. Issues related to development of antiepileptogenic therapies. Epilepsia. 2013;54(Suppl. 4):35–43. doi: 10.1111/epi.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Löscher W. The pharmacokinetics of antiepileptic drugs in rats: consequences for maintaining effective drug levels during prolonged drug administration in rat models of epilepsy. Epilepsia. 2007;48:1245–1258. doi: 10.1111/j.1528-1167.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 14.Simmonds MA, Turner JP. Potentiators of responses to activation of gamma-aminobutyric acid (GABAA) receptors. Neuropharmacology. 1987;26:923–930. doi: 10.1016/0028-3908(87)90071-2. [DOI] [PubMed] [Google Scholar]
- 15.Löscher W, Nolting B, Fassbender CP. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. I. The influence of administration vehicles. Epilepsy Res. 1990;7:173–181. doi: 10.1016/0920-1211(90)90013-L. [DOI] [PubMed] [Google Scholar]
- 16.Costa E, Guidotti A. Benzodiazepines on trial: A research strategy for their rehabilitation. Trends Pharmacol Sci. 1996;17:192–200. doi: 10.1016/0165-6147(96)10015-8. [DOI] [PubMed] [Google Scholar]
- 17.Voss J, Sanchez C, Michelsen S, Ebert B. Rotarod studies in the rat of the GABAA receptor agonist gaboxadol: lack of ethanol potentiation and benzodiazepine cross-tolerance. Eur J Pharmacol. 2003;482:215–222. doi: 10.1016/j.ejphar.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29:8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heng K, Haney MM, Buckmaster PS. High-dose rapamycin blocks mossy fiber sprouting but not seizures in a mouse model of temporal lobe epilepsy. Epilepsia. 2013;54:1535–1541. doi: 10.1111/epi.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engel J, Jr, Pitkänen A, Loeb JA, Dudek FE, Bertram EH, III, Cole AJ, et al. Epilepsy biomarkers. Epilepsia. 2013;54(Suppl. 4):61–69. doi: 10.1111/epi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sloviter RS. Hippocampal epileptogenesis in animal models of mesial temporal lobe epilepsy with hippocampal sclerosis: the importance of the “latent period” and other concepts. Epilepsia. 2008;49(Suppl. 9):85–92. doi: 10.1111/j.1528-1167.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 24.Sloviter RS, Bumanglag AV. Defining “epileptogenesis” and identifying “antiepileptogenic targets” in animal models of acquired temporal lobe epilepsy is not as simple as it might seem. Neuropharmacology. 2013;69:3–15. doi: 10.1016/j.neuropharm.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Löscher W, Schmidt D. Epilepsy: Perampanel-new promise for refractory epilepsy? Nat Rev Neurol. 2012;8:661–662. doi: 10.1038/nrneurol.2012.222. [DOI] [PubMed] [Google Scholar]
- 26.Brandt C, Gastens AM, Sun MZ, Hausknecht M, Löscher W. Treatment with valproate after status epilepticus: Effect on neuronal damage, epileptogenesis, and behavioral alterations in rats. Neuropharmacology. 2006;51:789–804. doi: 10.1016/j.neuropharm.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Langer M, Brandt C, Zellinger C, Löscher W. Therapeutic window of opportunity for the neuroprotective effect of valproate versus the competitive AMPA receptor antagonist NS1209 following status epilepticus in rats. Neuropharmacology. 2011;61:1033–1047. doi: 10.1016/j.neuropharm.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Bumanglag AV, Sloviter RS. Minimal latency to hippocampal epileptogenesis and clinical epilepsy after perforant pathway stimulation-induced status epilepticus in awake rats. J Comp Neurol. 2008;510:561–580. doi: 10.1002/cne.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstrong C, Morgan RJ, Soltesz I. Pursuing paradoxical proconvulsant prophylaxis for epileptogenesis. Epilepsia. 2009;50:1657–1669. doi: 10.1111/j.1528-1167.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloviter RS. Progress on the issue of excitotoxic injury modification vs. real neuroprotection; implications for post-traumatic epilepsy. Neuropharmacology. 2011;61:1048–1050. doi: 10.1016/j.neuropharm.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 31.Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, et al. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia. 2008;49:400–409. doi: 10.1111/j.1528-1167.2007.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo E, Citraro R, Scicchitano F, De Fazio S, Di Paola ED, Constanti A, et al. Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia. 2010;51:1560–1569. doi: 10.1111/j.1528-1167.2009.02400.x. [DOI] [PubMed] [Google Scholar]
- 33.Russo E, Citraro R, Scicchitano F, De Fazio S, Perrotta I, Di Paola ED, et al. Effects of early long-term treatment with antiepileptic drugs on development of seizures and depressive-like behavior in a rat genetic absence epilepsy model. Epilepsia. 2011;52:1341–1350. doi: 10.1111/j.1528-1167.2011.03112.x. [DOI] [PubMed] [Google Scholar]
- 34.Dezsi G, Ozturk E, Stanic D, Powell KL, Blumenfeld H, O’Brien TJ, et al. Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy. Epilepsia. 2013;54:635–643. doi: 10.1111/epi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silver JM, Shin C, McNamara JO. Antiepileptogenic effects of conventional anticonvulsants in the kindling model of epilepsy. Ann Neurol. 1991;29:356–363. doi: 10.1002/ana.410290404. [DOI] [PubMed] [Google Scholar]
- 36.Löscher W, Hönack D, Rundfeldt C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther. 1998;284:474–479. [PubMed] [Google Scholar]
- 37.Löscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50:105–123. doi: 10.1016/S0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- 38.Eastman CL, Verley DR, Fender JS, Stewart TH, Nov E, Curia G, et al. Antiepileptic and antiepileptogenic performance of carisbamate after head injury in the rat: blind and randomized studies. J Pharmacol Exp Ther. 2011;336:779–790. doi: 10.1124/jpet.110.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Ambrosio R, Eastman CL, Darvas F, Fender JS, Verley DR, Farin FM, et al. Mild passive focal cooling prevents epileptic seizures after head injury in rats. Ann Neurol. 2013;73:199–209. doi: 10.1002/ana.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50(Suppl. 2):10–13. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 41.Dichter MA. Posttraumatic epilepsy: the challenge of translating discoveries in the laboratory to pathways to a cure. Epilepsia. 2009;50(Suppl. 2):41–45. doi: 10.1111/j.1528-1167.2008.02009.x. [DOI] [PubMed] [Google Scholar]
- 42.Furlan AJ. Challenges in acute ischemic stroke clinical trials. Curr Cardiol Rep. 2012;14:761–766. doi: 10.1007/s11886-012-0311-9. [DOI] [PubMed] [Google Scholar]
- 43.Mani R, Pollard J, Dichter MA. Human clinical trails in antiepileptogenesis. Neurosci Lett. 2011;497:251–256. doi: 10.1016/j.neulet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu G, Gu B, He XP, Joshi RB, Wackerle HD, Rodriguiz RM, et al. Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe epilepsy. Neuron. 2013;79:31–38. doi: 10.1016/j.neuron.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertram EH, Zhang DX, Mangan P, Fountain N, Rempe D. Functional anatomy of limbic epilepsy: a proposal for central synchronization of a diffusely hyperexcitable network. Epilepsy Res. 1998;32:194–205. doi: 10.1016/S0920-1211(98)00051-5. [DOI] [PubMed] [Google Scholar]
- 46.Engel J, Jr, Thompson PM, Stern JM, Staba RJ, Bragin A, et al. Connectomics and epilepsy. Curr Opin Neurol. 2013;26:186–194. doi: 10.1097/WCO.0b013e32835ee5b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiebe S, Jette N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat Rev Neurol. 2012;8:669–677. doi: 10.1038/nrneurol.2012.181. [DOI] [PubMed] [Google Scholar]
- 48.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 49.Ainsworth C. Networking for new drugs. Nat Med. 2011;17:1166–1168. doi: 10.1038/nm1011-1166. [DOI] [PubMed] [Google Scholar]
- 50.Loeb JA. Identifying targets for preventing epilepsy using systems biology. Neurosci Lett. 2011;497:205–212. doi: 10.1016/j.neulet.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Margineanu DG. Systems biology impact on antiepileptic drug discovery. Epilepsy Res. 2012;98:104–115. doi: 10.1016/j.eplepsyres.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Vezzani A. Before epilepsy unfolds: finding the epileptogenesis switch. Nat Med. 2012;18:1626–1627. doi: 10.1038/nm.2982. [DOI] [PubMed] [Google Scholar]
- 53.Mazzuferi M, Kumar G, van Eyll J, Danis B, Foerch P, Kaminski RM. Nrf2 defense pathway: Experimental evidence for its protective role in epilepsy. Ann Neurol. 2013;74:560–568. doi: 10.1002/ana.23940. [DOI] [PubMed] [Google Scholar]
- 54.Löscher W, Rundfeldt C, Hönack D. Low doses of NMDA receptor antagonists synergistically increase the anticonvulsant effect of the AMPA receptor antagonist NBQX in the kindling model of epilepsy. Eur J Neurosci. 1993;5:1545–1550. doi: 10.1111/j.1460-9568.1993.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 55.Löscher W, Hönack D. Over-additive anticonvulsant effect of memantine and NBQX in kindled rats. Eur J Pharmacol. 1994;259:R3–R5. doi: 10.1016/0014-2999(94)90514-2. [DOI] [PubMed] [Google Scholar]
- 56.Rogawski MA, Donevan SD. AMPA receptors in epilepsy and as targets for antiepileptic drugs. Adv Neurol. 1999;79:947–963. [PubMed] [Google Scholar]
- 57.Löscher W, Puskarjov M, Kaila K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology. 2013;69:62–74. doi: 10.1016/j.neuropharm.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 58.Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, et al. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- 59.Miles R, Blaesse P, Huberfeld G, Wittner L, Kaila K. Chloride homeostasis and GABA signaling in temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. jasper’s basic mechanisms of the epilepsies. 4. New York: Oxford University Press; 2012. pp. 581–590. [PubMed] [Google Scholar]
- 60.Brandt C, Nozadze M, Heuchert N, Rattka M, Löscher W. Disease-modifying effects of phenobarbital and the NKCC1 inhibitor bumetanide in the pilocarpine model of temporal lobe epilepsy. J Neurosci. 2010;30:8602–8612. doi: 10.1523/JNEUROSCI.0633-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kahle KT, Staley KJ. The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures. Neurosurg Focus. 2008;25:1–8. doi: 10.3171/FOC/2008/25/9/E22. [DOI] [PubMed] [Google Scholar]
- 62.Koyama R, Tao K, Sasaki T, Ichikawa J, Miyamoto D, Muramatsu R, et al. GABAergic excitation after febrile seizures induces ectopic granule cells and adult epilepsy. Nat Med. 2012;18:1271–1278. doi: 10.1038/nm.2850. [DOI] [PubMed] [Google Scholar]
- 63.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2013;69:16–24. doi: 10.1016/j.neuropharm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwon YS, Pineda E, Auvin S, Shin D, Mazarati A, Sankar R. Neuroprotective and antiepileptogenic effects of combination of anti-inflammatory drugs in the immature brain. J Neuroinflammation. 2013;10:30. doi: 10.1186/1742-2094-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McClelland S, Flynn C, Dube C, Richichi C, Zha Q, Ghestem A, et al. Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann Neurol. 2011;70:454–464. doi: 10.1002/ana.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jimenez-Mateos EM, Engel T, Merino-Serrais P, McKiernan RC, Tanaka K, Mouri G, et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med. 2012;18:1087–1094. doi: 10.1038/nm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitkänen A, Narkilahti S, Bezvenyuk Z, Haapalinna A, Nissinen J. Atipamezole, an alpha(2)-adrenoceptor antagonist, has disease modifying effects on epileptogenesis in rats. Epilepsy Res. 2004;61:119–140. doi: 10.1016/j.eplepsyres.2004.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)
(PDF 1224 kb)