Abstract
Neuroactive steroids are endogenous neuromodulators capable of altering neuronal activity and behavior. In rodents, systemic administration of endogenous or synthetic neuroactive steroids reduces ethanol self-administration. We hypothesized this effect arises from actions within mesolimbic brain regions that we targeted by viral gene delivery. Cytochrome P450 side chain cleavage (P450scc) converts cholesterol to pregnenolone, the rate-limiting enzymatic reaction in neurosteroidogenesis. Therefore, we constructed a recombinant adeno-associated serotype 2 viral vector (rAAV2), which drives P450scc expression and neuroactive steroid synthesis. The P450scc-expressing vector (rAAV2-P450scc) or control GFP-expressing vector (rAAV2-GFP) were injected bilaterally into the ventral tegmental area (VTA) or nucleus accumbens (NAc) of alcohol preferring (P) rats trained to self-administer ethanol. P450scc overexpression in the VTA significantly reduced ethanol self-administration by 20% over the 3 week test period. P450scc overexpression in the NAc, however, did not alter ethanol self-administration. Locomotor activity was unaltered by vector administration to either region. P450scc overexpression produced a 36% increase in (3α,5α)-3-hydroxypregnan-20-one (3α,5α-THP, allopregnanolone)-positive cells in the VTA, but did not increase 3α,5α-THP immunoreactivity in NAc. These results suggest that P450scc overexpression and the resultant increase of 3α,5α-THP-positive cells in the VTA reduces ethanol reinforcement. 3α,5α-THP is localized to neurons in the VTA, including tyrosine hydroxylase neurons, but not astrocytes. Overall, the results demonstrate that using gene delivery to modulate neuroactive steroids shows promise for examining the neuronal mechanisms of moderate ethanol drinking, which could be extended to other behavioral paradigms and neuropsychiatric pathology.
Keywords: alcohol, allopregnanolone, neuroactive steroid, neurosteroid, P450scc, ventral tegmental area
Introduction
Neuroactive steroids are neuromodulators synthesized in the brain that modulate neuronal activity and influence motivation and emotional behaviors. The GABAergic neuroactive steroid (3α,5α)-3-hydroxypregnan-20-one (3α,5α-THP or allopregnanolone) is a potent positive allosteric modulator of GABA type A (GABAA) receptors. GABAA receptors are the primary inhibitory receptors in the brain and mediate many of the behavioral effects of ethanol. Ethanol-induced increases of 3α,5α-THP contribute to many of the neurophysiological and behavioral effects of ethanol in rats (VanDoren et al., 2000; Hirani et al., 2002, 2005) as well as subjective effects of alcohol in humans (Pierucci-Lagha et al., 2005). Furthermore, human alcoholics have reduced blood plasma levels of 3α,5α-THP during alcohol withdrawal (Romeo et al., 1996). Moreover, increasing evidence suggests that modulating GABAergic neuroactive steroid levels may have therapeutic value for treating multiple neurologic and psychiatric disorders (Marx et al., 2006; Morrow, 2007; Rupprecht et al., 2010; Brinton, 2013).
Neuroactive steroids alter both ethanol reinforcement and ethanol consumption in rodents. For example, pregnenolone (Besheer et al., 2010a) and the synthetic GABAergic neuroactive steroid 3α,5β-20-oxo-pregnane-3-carboxylic acid (O'Dell et al., 2005) dose dependently reduce ethanol self-administration without producing sedation. Administration of 3α,5α-THP or the longer acting synthetic analog of 3α,5α-THP, ganaxolone, produces biphasic effects on ethanol self-administration (Janak et al., 1998; Ford et al., 2005; Besheer et al., 2010a). However, there are concerns that 3α,5α-THP and ganaxolone produce sedation (Belelli et al., 1989; Besheer et al., 2010a), limiting therapeutic effectiveness. Furthermore, the therapeutic potential of exogenously administered 3α,5α-THP may also be limited by rapid metabolism (Purdy et al., 1990).
The mesolimbic pathway is strongly implicated in ethanol reinforcement and consumption (Koob, 1992; McBride et al., 1999; Gonzales et al., 2004), and intracerebroventricular administration of 3α,5α-THP alters mesolimbic dopamine release (Motzo et al., 1996; Rougé-Pont et al., 2002). Therefore, we hypothesized that neuroactive steroids reduce ethanol self-administration via actions in the nucleus accumbens (NAc) and/or the ventral tegmental area (VTA). To circumvent limitations of exogenous 3α,5α-THP administration, such as rapid metabolism and sedation, we used viral vector-mediated gene delivery to increase neuroactive steroid production in the NAc or the VTA. The synthesis of neuroactive steroids requires cholesterol conversion to pregnenolone by the mitochondrial cytochrome P450 side chain cleavage (P450scc) enzyme, the rate-limiting enzymatic reaction in steroid synthesis. Therefore, we constructed a recombinant adeno-associated serotype 2 (rAAV2) vector that overexpresses P450scc in vivo, which should lead to long-term elevations of neuroactive steroids selectively in cells that contain the requisite biosynthetic enzymes. Not only did the viral vector manipulation result in chronically elevated P450scc expression, pregnenolone synthesis, and levels of 3α,5α-THP, but regionally specific P450scc overexpression significantly influenced long-term operant ethanol self-administration. We also used confocal imaging to determine cell type-specific localization of 3α,5α-THP in the VTA.
Materials and Methods
Animals
Adult male Wistar rats (∼275 g) were obtained from Harlan Laboratories and used for characterizing viral vectors (n = 16 for in vivo confirmation of P450scc overexpression in the NAc Shell; n = 8 to examine effects of vector infusion on 3α,5α-THP immunoreactivity in the NAc). Male rats were chosen for these studies to eliminate the potential confounding variable of fluctuating neuroactive steroid levels during the estrous cycle. The effects of rAAV2-P450scc transduction in the NAc on operant ethanol self-administration were assessed using adult male alcohol-preferring (P) rats bred in-house at the University of North Carolina at Chapel Hill (UNC). This stock of P rats was derived from breeders of the selected line of P rats provided by Indiana University (courtesy of Dr. T.K. Li). The effects of rAAV2-P450scc transduction in the VTA on operant ethanol self-administration and the double-labeling experiments were performed using two cohorts of adult male P rats obtained from Indiana University. Baseline operant ethanol self-administration resulted in moderate ethanol consumption and was comparable in all groups of P rats used for these studies. Animals were double housed until surgery and single housed thereafter. Home cages were Plexiglas containing corn cob bedding and food and water was available ad libitum unless otherwise stated. The colony room was maintained on a normal 12 h light/dark cycle (light onset at 0700 h). Procedures followed National Institutes of Health (NIH) Guidelines under UNC Institutional Animal Care and Use Committee approved protocols. Viral vector use was approved by the UNC Department of Environmental Health and Safety Biosafety Committee.
Apparatus
Self-administration chambers.
Operant ethanol self-administration was performed in conditioning chambers measuring 30.5 × 24.1 cm (Med Associates) located inside sound-attenuating cubicles. Cubicles were equipped with an exhaust fan for ventilation, which also masked external sounds. Both the left and right walls of the chambers contain a lever and liquid receptacle (i.e., 2 per chamber). The appropriate number of lever press responses simultaneously activated a stimulus light over the lever and a syringe pump (Med Associates) that delivered 0.1 ml of liquid solution into a receptacle over a 1.66 s duration. Lever responses during reinforcer delivery were counted, but did not result in programmed consequences. The operant chambers were connected to a computer programmed to control sessions and record the resulting data.
Locomotor chambers.
Clear Plexiglas chambers (43.2 × 43.2 cm; Med Associates) with 16 photobeams per axis were used to measure locomotor activity in the same animals used in the NAc and VTA operant self-administration studies. Open field tests took place at 26 d post vector infusion for the NAc animals and at 4 weeks postinfusion in the VTA animals. Total horizontal distance traveled (centimeters) was determined by the number of photobeam breaks during 30 min. Photobeam breaks were collected via computer interface in 2 min time intervals using Activity Monitor locomotor activity software (Med Associates).
Operant ethanol self-administration
Lever press training.
The day before initial training, animals were water deprived for ∼24 h. Immediately following water deprivation, animals were placed in the operant chambers for a 16 h lever press training session where 0.1 ml of sucrose (10%, w/v) or water were concurrently available contingent on lever press responses. Lever press responses were initially maintained on a concurrent fixed-ratio 1 (CONC FR1 FR1) schedule of reinforcement and were gradually increased to CONC FR2 FR2 following delivery of 4 reinforcers, followed by a CONC FR4 FR4 schedule after delivery of 10 reinforcers. Following the 16 h training session animals were returned to their home cage for a period of 24 h with ad libitum water access thereafter.
Sucrose fading, operant baseline sessions, and operant self-administration testing.
After the initial training session, rats began daily (M–F) 30 min sessions on a CONC FR4 FR4 schedule. To facilitate lever pressing for ethanol, a modified sucrose fading procedure (Samson, 1986) was used as previously described (Hodge et al., 1993; Besheer et al., 2010b). Briefly, ethanol was gradually added to the 10% (w/v) sucrose solution and sucrose was faded out until only 15% (v/v) ethanol maintained lever responding. The sequence of sucrose/ethanol solutions were as follows: 10% sucrose/2% ethanol (10S/2E), 10S/5E, 10S/10E, 5S/10E, 5S/15E, 2S/15E, and 0S/15E, with two sessions conducted at each concentration. Following sucrose fading, animals experienced a minimum of 28 baseline operant self-administration sessions with 15% ethanol versus water. One week following surgery, animals that received rAAV infusion in the NAc underwent 14 d of operant testing sessions. Animals that received rAAV infusion in the VTA underwent 21 d of operant sessions. Ethanol (95%, w/v, Pharmco-AAPER) was diluted in distilled water to 15% (v/v). Ethanol intake (g/kg) was determined from the animal's body weight and number of reinforcers received during a session. Using this paradigm we have previously reported baseline blood ethanol concentrations of ∼80 mg/dl in P rats with similar response rates (Besheer et al., 2008).
P450scc AAV plasmid construction and virus packaging
An AAV vector plasmid containing P450scc was created similarly as described previously (Choi et al., 2006; McCown, 2006). In brief, the P450scc sequence was amplified from mRNA isolated from the rat olfactory bulbs and converted to cDNA using a SuperScript II reverse-transcriptase kit (Life Technologies). The primers were designed to incorporate AgeI and NotI restriction sites in the 5′ and 3′ portion of the sequences, respectively. The sequences of the primers were as follows: (1) 5′-ACCGGTATGCTGGCAAAAGGTCTT-3′ and (2) 5′-GCGGCCGCTCATACAGTGTCGCCTTTTCTG-3′. The amplified PCR product was ligated into a TOPO TA vector as an intermediate step. Digestion of this intermediate vector with AgeI and NotI resulted in a P450scc cDNA fragment that was subsequently ligated into an AAV plasmid containing a chicken β-actin (CBA) promoter 5′ to the AgeI site to drive P450scc gene expression. Inverted terminal repeats (ITRs) flanked the β-actin promoter and P450scc cDNA sequence (Fig. 1B). This P450scc clone was sequenced to verify the integrity of the start and stop codons as well as the cDNA sequence. A single point mutation was noted that resulted in a nonpolar valine to the smaller nonpolar alanine at amino acid position 151. This mutation was further determined to be inconsequential as verified by increased mRNA and protein expression by reverse-transcriptase PCR and Western blot, respectively, in transient transfected fibroblasts as well as assessment of function via conversion of 22-hydroxycholesterol to pregnenolone via radioimmunoassay (RIA). Subsequently, the recombinant AAV2- P450scc (rAAV2-P450scc) viral vector was packaged by the UNC Viral Vector Core (7.5 × 1011 viral particles/ml). AAV serotype 2 was used due to its previous success in infecting neuronal cells, long-term gene expression, and minimal immune response. Control rAAV2-GFP vectors were also obtained from the UNC Vector Core (5 × 1011 to 1 × 1012 viral particles/ml).
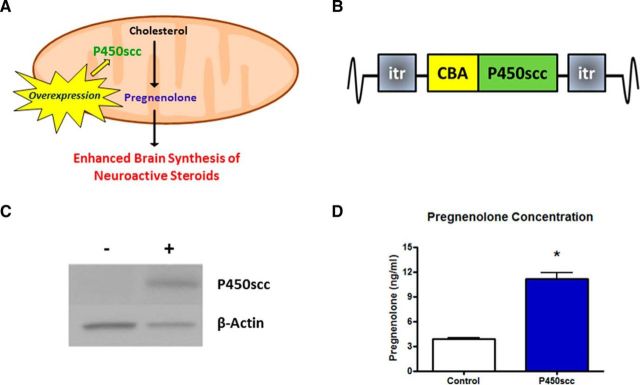
Figure 1.
The P450scc construct increases functional P450scc expression resulting in elevated pregnenolone levels. A, Model to enhance neuroactive steroid synthesis by overexpressing P450scc, which performs the limiting enzymatic reaction in steroidogenesis. B, P450scc construct consisting of the P450scc gene driven by the ubiquitous CBA promoter flanked by ITRs. C, Transient transfection of mouse (Ltk−) fibroblast cells increased P450scc by ∼10-fold (p < 0.001) 48 h post-transfection. Representative immunoblot probed for P450scc with β-actin used as a loading control: + indicates transfection with P450scc construct, − indicates control cells. D, Pregnenolone levels were increased in the cell media (p < 0.0001), measured by RIA; *p < 0.0001 compared with control.
Cell culture
Mouse L(tk−) fibroblast cells were used as described previously (Harris et al., 1995), since transfection efficiency is higher in non-neuronal cells. Briefly, cells were grown in flasks previously coated with poly-l-lysine using DMEM with high glucose (Life Technologies) along with 10% fetal bovine serum and penicillin-streptomycin. Cells were maintained in a 5% CO2 humidified incubator. To test vector construct, cells were transiently transfected using Lipofectamine (Invitrogen).
P450scc mRNA and protein analysis
Cell and tissue preparation.
Cell culture samples were washed with ice-cold PBS, scraped, and pelleted down by low-speed centrifugation. Rats received either rAAV2-P450scc or rAAV2-GFP infusions into the NAc or NAc shell and were killed at 1 week postsurgery for in vivo characterization of P450scc overexpression (NAc shell) or at 4 weeks postsurgery (NAc) following operant self-administration and open field studies. Brains were rapidly removed, flash frozen using isopentane, and stored at −80C° until processed. One animal in the NAc operant self-administration experiments was excluded because the full rAAV2-P450scc infusion was not delivered, and an increase in P450scc transcript indicative of rAAV2-P450scc transduction (>100%) was not observed.
mRNA analysis.
Total RNA was isolated from microdissected tissue using TRIzol and converted to cDNA using SuperScript II (Invitrogen) with random hexamers. cDNA was amplified using the primer pair listed above under plasmid construction with a three-step PCR program. Samples were subsequently separated using a 1.0% Tris/EDTA gel. cDNA samples were re-amplified using primers specific to β-actin (Whitman et al., 2013) for normalization. Gel images were acquired using a Fotodyne imaging cabinet (Fotodyne), and quantified using NIH Image 1.57. Data were analyzed using Student's t test.
Western blot analysis.
Microdissected NAc shell tissue was homogenized in a whole-cell lysate buffer containing 1% SDS, 1 mm EDTA, and 10 mm Tris. Protein concentrations were determined by a bicinchoninic acid protein assay. Samples were subsequently denatured and separated using SDS-PAGE and transferred to polyvinylidene difluoride membranes (Invitrogen). Membranes were probed with an antibody against P450scc (Millipore). Bands were visualized using enhanced chemiluminescence (GE Healthcare) under nonsaturating conditions. Blots were then re-exposed to an actin-specific antibody (Millipore) for normalization. Densitometric analysis was conducted using NIH Image 1.57 and data were analyzed using Student's t test.
Surgery and viral vector infusion.
Animals were anesthetized with isoflurane and placed into a stereotaxic apparatus (David Kopf Instruments). The rAAV2-P450scc or control rAAV2-GFP vectors were infused bilaterally with a syringe pump (Harvard Apparatus) and 10 μl gastight syringes (Hamilton Company), connected with polyethylene tubing to needles made from stainless steel 30 gauge tubing (Smallparts). Needles and tubing were coated with a 10% pluronic F-68 solution (Sigma-Aldrich) to minimize stainless steel binding of virus. Virus was infused at 0.2 μl/min at a total volume of 2–4 μl/hemisphere. Needles were left in place at the infusion site for an additional 5 min to allow for virus diffusion. Infusion coordinates relative to bregma were based on the rat brain atlas (Paxinos and Watson, 1998) as follows: NAc shell (AP + 1.6, ML ± 0.7, DV −7.5), NAc (AP + 1.6, ML ± 1.5, DV −7.5), VTA (AP − 5.5, ML ± 2.0, DV −8.3 with 10° angle). The optimal infusion volume for each brain region was determined with pilot surgeries using increasing volumes of the rAAV2-GFP vector. Optimal volumes were determined by GFP visualization that was limited to the target brain region. Animals were given 1 week to recover from surgery before resuming operant self-administration. VTA needle placements were confirmed using immunohistochemistry (IHC) for glial fibrillary acid protein (GFAP). In the NAc experiments brain tissue was freshly frozen. Therefore, infusions were confirmed by P450scc mRNA increases in the NAc indicative of successful rAAV2-P450scc transduction (>100% increase in P450scc mRNA). In the NAc shell experiments for in vivo vector characterization all animals were included in the statistical analysis.
IHC
Tissue preparation.
Animals were anesthetized with pentobarbital sodium (150 mg/kg, i.p.; Professional Compounding Centers of America) and transcardially perfused with PBS followed by 4% paraformaldehyde. Tissue was postfixed in 4% paraformaldehyde for 24 h at 4°C, sectioned coronally at 40 μm on a vibrating microtome, and stored at −30°C in cryoprotectant until further processing.
3α,5α-THP IHC.
3α,5α-THP immunohistochemical assays with 3,3′-diaminobenzidine (DAB) detection were performed as previously described in detail (Cook et al., 2014a). No detergents or organic solvents were used to prevent steroid leaching and (three to four sections/animal/brain region) were analyzed. For fluorescent detection, tissue was rinsed, blocked and incubated in 3α,5α-THP antiserum for 48 h at 4°C (1:500) followed by an Alexa Fluor 488 secondary antibody (Life Technologies).
3α,5α-THP IHC cell counts.
3α,5α-THP immunoreactivity with DAB detection was visualized using an Olympus CX41 light microscope (Olympus America) and images were captured with a digital camera (Regita model; QImaging). Image analysis software (Bioquant Life Sciences version 8.00.20) that uses linear integrated optical density was used for determining positive cell counts. The microscope, camera, and software were background corrected and normalized to preset light levels to ensure fidelity of data acquisition. Positive cell count measurements were calculated from a defined region (e.g., brain region), divided by the area of the region in square millimeters, and expressed as cell counts/mm2. Data were acquired from three to four sections/animal/brain region, and averaged within a brain region for an individual animal to obtain one value per subject. Immunoreactivity was analyzed using Student's t test (Prism; GraphPad Software). Brain region analyses were performed using histological coordinates as follows: mPFC (four sections corresponding to +3.20, +3.00, +2.70, and +2.20 AP), NAc (three to four sections corresponding to +2.20, +1.70, +1.60, or + 1.20 AP), and VTA (four sections corresponding to −5.20/−5.30, −5.60, −5.80, and −6.04 AP). All analyses were based on coordinates relative to bregma in the Rat Brain Atlas (Paxinos and Watson, 1998).
GFP and GFAP epifluorescence.
Enhanced GFP (eGFP) immunofluorescence was used to determine infection efficiency of the rAAV2 vectors. Sections were mounted on microslides, rinsed, blocked, and incubated with eGFP primary antibody (1:1000; Millipore). Following rinses, slides were incubated with Alexa Fluor 488 secondary antibody (Life Technologies) for 1 h at room temperature, rinsed, and finally incubated with a fluorescent red Nissl stain (Life Technologies) to identify neuroanatomical landmarks. GFAP immunofluorescence was used on free-floating sections (1:3000; Dako) to determine needle placement of virus injections in the VTA of P rats (Fig. 5). Following GFAP primary antibody incubation, sections were rinsed and incubated with Alexa Fluor 594 secondary antibody (Life Technologies). Immunofluorescence was visualized with a Leica DMIRB inverted microscope and images were captured with a QImaging MicroPublisher camera and computer software.
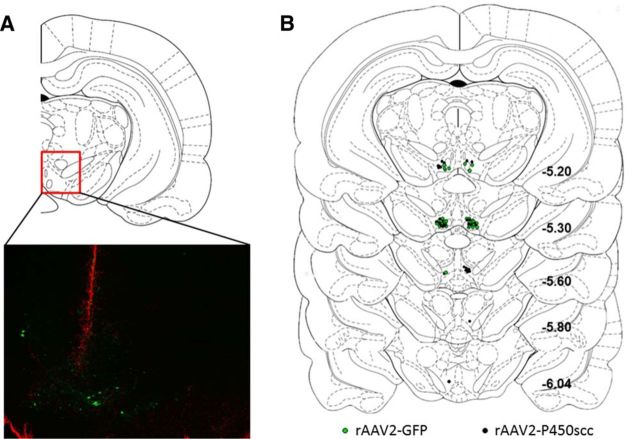
Figure 5.
Localization of viral vector infusions was determined using GFAP immunofluorescence to visualize needle tracts. A, Representative image showing GFAP immunofluorescence (red) at the needle tract and GFP-positive cells (green) in the VTA 4 weeks following rAAV2-GFP infusion. The red box indicates the location of the representative image (−5.20 mm relative to bregma) within the VTA. B, Location within the VTA of rAAV2-GFP (green circles) or rAAV2–P450scc (black circles) infusions, relative to bregma.
Double immunofluorescent labeling and confocal microscopy.
Tissue from P rats (n = 3) not used in the self-administration studies was used for double-labeling experiments. Free-floating sections were rinsed, blocked, and incubated in primary antibody for cell-type specific markers: TH (1:1000; ImmunoStar), NeuN (1:200; clone 60; Millipore), or GFAP (1:3000) for 24 h at 4°C. Next, sections were rinsed, blocked, and incubated with 3α,5α-THP primary antibody for 48 h at 4°C. Then, sections were rinsed and incubated with secondary antibody (Alexa Fluor 488 for 3α,5α-THP visualization and Alexa Fluor 594 for cell-type specific markers; Life Technologies). Immunofluorescence was visualized using a Leica SP2 laser scanning confocal microscope and computer software. Cell-type markers and 3α,5α-THP immunofluorescence were imaged sequentially to prevent fluorophore bleed-through.
RIA
Pregnenolone concentrations were measured using a procedure previously described in detail (Porcu et al., 2006), modified for use with cell media. Briefly, pregnenolone was extracted from cell media (spiked with 1000 counts per minute of [3H] pregnenolone for recovery) three times with 3 ml of ethyl acetate. The dried extracts were reconstituted in 2 ml of assay buffer, and 0.5 ml aliquots were used for the assay (run in duplicate) and for recovery measurement. Pregnenolone antibody was purchased from MP Biomedicals. Pregnenolone values are expressed as ng/ml cell media.
Results
P450scc gene delivery increases P450scc and the neuroactive steroid pregnenolone in L(tk−) cells
Overexpression of P450scc increases the rate-limiting enzyme in steroid synthesis, thereby driving neuroactive steroid expression (Fig. 1A). To achieve this goal in vivo, P450scc gene expression was driven by a ubiquitous CBA promoter in the context of an AAV vector (Fig. 1B). To confirm that the P450scc construct results in increases of P450scc and steroidogenesis in vitro, we transiently transfected mouse L(tk−) fibroblast cells and measured P450scc protein levels as well as pregnenolone levels in the cell media. Transfection of fibroblasts with the P450scc construct resulted in a 10-fold increase in P450scc protein expression at 48 h post-transfection (t(4) = 11.74, p < 0.001; Fig. 1C), as well as an increase in extracellular pregnenolone levels (t(12) = 8.360, p < 0.0001; Fig. 1D).
Infusion of rAAV2-P450scc in the NAc shell increases P450scc
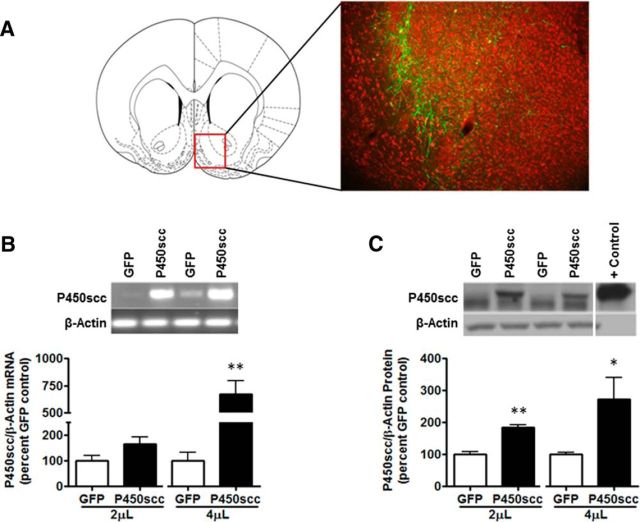
To verify that rAAV2-P450scc increases P450scc in vivo, we infused the rAAV2-P450scc or rAAV2-GFP vectors at 2 and 4 μl into the NAc shell of Wistar rats and measured mRNA and protein levels 1 week following surgery. The infection efficiency of the rAAV2-GFP vector (2 μl) and location of transduction within the NAc shell (Fig. 2A) was determined 1 week postinfusion. The 4 μl rAAV2-P450scc infusion increased P450scc mRNA by 573 ± 122% (t(6) = 4.532, p < 0.005; Fig. 2B) and protein levels were increased by 84 ± 9% following the 2 μl infusion (t(6) = 6.847, p < 0.005) and by 172 ± 69% following the 4 μl infusion (t(6) = 2.479, p < 0.05; Fig. 2C).
Figure 2.
rAAV2–P450scc transduction in the NAc shell increases P450scc mRNA and protein expression dependent on the volume of virus infused. A, Representative photomicrograph (10×) of rAAV2-GFP infection efficiency in the NAc shell 1 week after 2 μl rAAV2-GFP infusion (green, GFP; red, Nissl stain). The red box indicates the location of the representative photomicrograph (+1.60 mm relative to bregma) within the NAc. B, The 4 μl infusion of rAAV2–P450scc significantly increased P450scc mRNA (p < 0.005) at 1 week postsurgery. Representative gel showing P450scc mRNA following 4 μl rAAV2–P450scc or rAAV2-GFP infusion in the NAc shell. β-Actin was used as a loading control. C, rAAV2–P450scc transduction increased protein levels of P450scc following both the 2 μl (p < 0.005) and 4 μl (p < 0.05) infusions at 1 week postsurgery. Representative immunoblot probed for P450scc following 4 μl rAAV2–P450scc or rAAV2-GFP infusion in the NAc shell. P450scc is the uppermost band that was increased following rAAV2–P450scc transduction. β-Actin was used as a loading control, but is not seen in the positive control (rat adrenal) due to the small amount of protein loaded (1 μg) compared with brain samples;*p < 0.05 compared with GFP control, **p < 0.005 compared with GFP control.
Overexpression of P450scc in the NAc does not alter 3α,5α-THP or operant ethanol self-administration
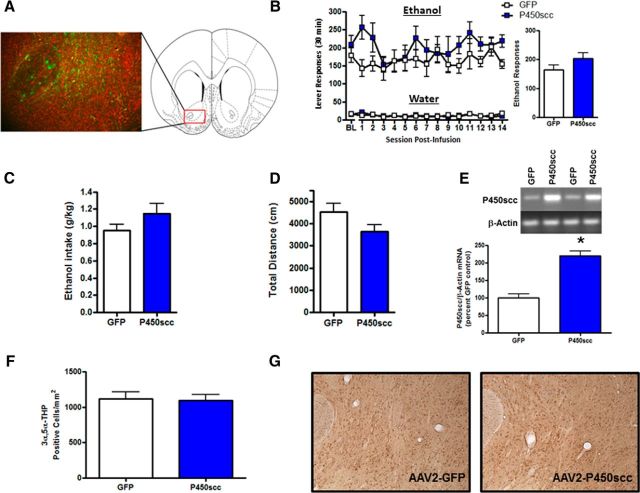
To determine whether P450scc overexpression in the NAc alters ethanol reinforcement, we infused the rAAV2-P450scc or rAAV2-GFP vectors (3 μl) into the NAc of P rats trained to self-administer ethanol. We have previously shown that ethanol decreases levels of cellular 3α,5α-THP in the NAc “shore” (core-shell border; Cook et al., 2014a), and others have shown that dopamine is released in the NAc shore during operant self-administration of ethanol (Howard et al., 2009). Therefore, we aimed our viral infusions (3 μl) at the NAc shore, which is also centralized within the NAc and thereby limits viral infection of surrounding brain regions (Fig. 3A). All ethanol self-administration data were analyzed using two-way repeated-measures ANOVA across all test sessions. P450scc overexpression in the NAc did not alter operant ethanol responding (main effect of rAAV2-P450scc treatment; F(1,156) = 2.297, p = 0.1555; Fig. 3B). There was no difference in ethanol intake (main effect of rAAV2-P450scc treatment; F(1,156) = 2.186, p = 0.1651), which averaged 1.14 ± 0.12 g/kg for the rAAV2-P450scc group and 0.94 ± 0.07 g/kg for the rAAV2-GFP group during the 14 d of testing. Furthermore, there was no difference in water-responding (F(1,156) = 0.0048, p = 0.9457; Fig. 3B) or general locomotor activity (t(12) = 1.813, p = 0.0948; Fig. 3D) between the rAAV-P450scc and rAAV-GFP groups. The rAAV-P450scc vector did, however, increase P450scc mRNA levels in the NAc 4 weeks following infusion (t(12) = 6.690, p < 0.0001; Fig. 3E). To determine whether rAAV2-P450scc infusion in the NAc increases 3α,5α-THP, we infused the rAAV2-P450scc or control vector into the NAc of a separate group of Wistar rats and performed 3α,5α-THP IHC at 1 week following infusion. IHC analysis revealed that rAAV2-P450scc did not increase 3α,5α-THP in the NAc (t(6) = 0.1893, p = 0.86; Fig. 3G).
Figure 3.
rAAV2–P450scc transduction in the NAc increases P450scc mRNA but does not alter operant ethanol self-administration or cellular 3α,5α-THP. A, Representative photomicrograph (10×) of rAAV2-GFP infection efficiency in the NAc 1 week after 3 μl of rAAV2-GFP infusion (green, GFP; red, Nissl stain). The red box indicates the location of the representative photomicrograph (+1.60 mm relative to bregma) within the NAc. B, rAAV2–P450scc transduction in the NAc did not alter operant ethanol responding or water responding, compared with rAAV2-GFP controls. Ethanol responding over the 14 d of test sessions is collapsed in the bar graph. C, rAAV2–P450scc transduction in the NAc did not alter mean ethanol intake (g/kg) over the 14 d of test sessions, compared with rAAV2-GFP controls. D, rAAV2–P450scc transduction in the NAc did not alter total distance traveled (centimeters) in the open field test at 26 d following vector infusions. E, The infusion of rAAV2–P450scc (3 μl) significantly increased P450scc mRNA in the NAc at 4 weeks postsurgery. Representative gel showing P450scc mRNA level following 3 μl rAAV2–P450scc or rAAV2-GFP infusion in the NAc. β-Actin was used as a loading control. F, Infusion of rAAV2–P450scc (3 μl) in the NAc of Wistar rats did not alter 3α,5α-THP-positive cells in the NAc at 1 week postsurgery. G, Representative photomicrographs (10×) of cellular 3α,5α-THP immunoreactivity in the NAc 1 week following 3 μl rAAV2-GFP or rAAV2–P450scc infusion in the NAc. Baseline (BL) represents 1 week average of ethanol responding during the week before surgery; *p < 0.0001 compared with GFP control.
Overexpression of P450scc in the VTA reduces long-term operant ethanol self-administration
To test whether P450scc overexpression in the VTA alters ethanol reinforcement, we infused the rAAV2-P450scc or rAAV2-GFP vectors (2 μl) into the VTA of P rats trained to self-administer ethanol. rAAV2-P450scc infusion in the VTA reduced ethanol responding by 20% (main effect of rAAV2-P450scc treatment: F(1,540) = 13.67, p < 0.005; main effect of time: F(20,540) = 2.785, p < 0.0001; Fig. 4B) and ethanol intake by 14% (main effect of rAAV2-P450scc treatment: F(1,540) = 8.174, p < 0.01; main effect of time: F(20,540) = 3.092, p < 0.0001; Fig. 4C) over the 21 d of testing. Viral infusion did not affect water responding (F(1,540) = 0.4870, p = 0.4912; Fig. 4B) or general locomotor activity (t(27) = 0.5534, p = 0.5845; Fig. 4D). There was a main effect of time on water responding (F(20,540) = 1.643, p < 0.05) due to higher water responding on the initial days following surgery, which over the 3 weeks stabilized at a lower level for both groups. The reduction in ethanol reinforcement and consumption was associated with an increase in 3α,5α-THP-positive cells in the VTA of animals that received rAAV2-P450scc infusion (t(27) = 3.104, p < 0.005;Fig. 4E,F). Since viral infusions may increase 3α,5α-THP in projection sites of the VTA, we also measured 3α,5α-THP immunoreactivity in the NAc and mPFC. There was no difference in 3α,5α-THP-positive cells in the NAc (t(13) = 1.108, p < 0.28) or mPFC (t(13) = 0.7394, p < 0.47) of animals that received rAAV2-P450scc infusion in the VTA (data not shown). Needle placements were determined by using GFAP immunohistochemistry (Fig. 5).
Figure 4.
rAAV2–P450scc transduction in the VTA produces long-term reductions in operant ethanol self-administration and increases 3α,5α-THP-positive cells. A, Representative photomicrograph (10×) of rAAV2-GFP infection efficiency in the VTA 1 week after 2 μl rAAV2-GFP infusion (green, GFP; red, Nissl stain). The red box indicates the location of the representative photomicrograph (−5.80 mm relative to bregma) within the VTA. B, rAAV2–P450scc transduction in the VTA reduced operant ethanol (p < 0.005) but not water responding over the 21 d of test sessions, compared with rAAV2-GFP controls. Mean ethanol responding over the 21 d of test sessions is collapsed in the bar graph. C, rAAV2–P450scc transduction in the VTA reduced mean ethanol intake (g/kg; p < 0.01) over the 21 d of test sessions, compared with rAAV2-GFP controls. D, rAAV2–P450scc transduction in the VTA did not alter total distance traveled (centimeters) in the open field test at 4 weeks following vector infusions. E, Infusion of rAAV2–P450scc (2 μl) in the VTA increased 3α,5α-THP-positive cells in the VTA (p < 0.005) at 4 weeks postsurgery. F, Representative photomicrographs (10×) of cellular 3α,5α-THP immunoreactivity in the VTA 4 weeks following 2 μl rAAV2-GFP or rAAV2–P450scc infusion in the VTA. Baseline (BL) represents 1 week average of ethanol responding during the week before surgery; *p < 0.01 and **p < 0.005 compared with control values.
3α,5α-THP colocalizes with TH and NeuN in the VTA
To identify cell types in which 3α,5α-THP is localized in the VTA, we used scanning laser confocal microscopy and double labeled with NeuN (neuronal marker), GFAP (astrocyte marker), or TH (putative dopamine cell marker) in P rats (n = 3) not included in behavioral studies. We found that 3α,5α-THP is located in neurons in the VTA (Fig. 6A), but does not appear to be present in astrocytes (Fig. 6B). 3α,5α-THP was located in all TH-positive neurons examined (294 cells) as well as TH-negative neurons (Fig. 6C, arrows).
Figure 6.
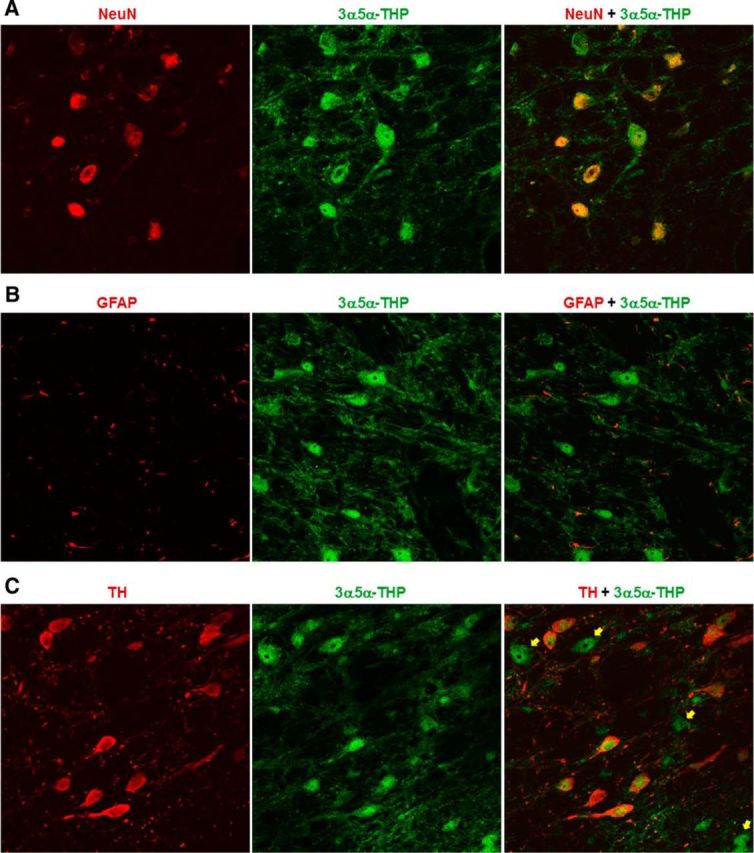
Confocal scanning microscopy revealed that 3α,5α-THP colocalizes with NeuN-positive neurons and TH-positive neurons, but not GFAP-positive astrocytes in the VTA of P rats. A, 3α,5α-THP (green) colocalizes with NeuN (red) in the VTA. B, 3α,5α-THP (green) does not colocalize with GFAP (red) in the VTA. C, 3α,5α-THP (green) colocalized with all TH (red)-positive cells examined in the VTA (294 cells). 3α,5α-THP is also located in TH-negative cells in the VTA (yellow arrows).
Discussion
In the present study we developed a viral vector to overexpress P450scc, and drive long-term neurosteroid production in neurons that contain the necessary biosynthetic enzymes. We showed that P450scc overexpression in the NAc does not alter ethanol self-administration or 3α,5α-THP levels. However, rAAV2-P450scc infusion in the VTA produced persistent reductions in ethanol self-administration over the 21 d of testing. The effects of rAAV2-P450scc transduction in the VTA appear to be specific to ethanol responding since there was no effect on water-responding or motor activity. Furthermore, reduced ethanol self-administration was associated with a 36% increase in 3α,5α-THP-positive cells in the VTA. However, 3α,5α-THP immunoreactivity was not altered downstream in the NAc or the mPFC. It appears that rAAV2-P450scc infusion in the VTA produces long-term reductions in ethanol reinforcement by increasing neurosteroids, including 3α,5α-THP in the VTA. Further investigation revealed that 3α,5α-THP is localized to both TH-positive and TH-negative VTA neurons, but does not appear to be present in cells labeled with GFAP.
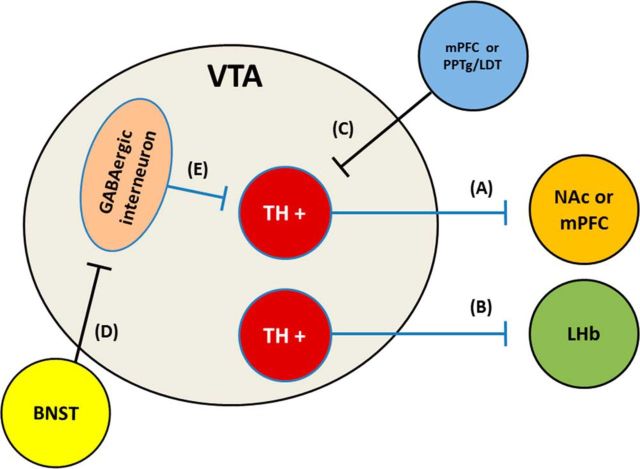
The ability of rAAV2-P450scc transduction of VTA neurons to modulate ethanol self-administration is likely due to modulation of neural circuitry via GABAA receptor-mediated neuronal inhibition. The available data suggest that increasing 3α,5α-THP within a cell reduces the excitability of that particular cell (Saalmann et al., 2006; Akk et al., 2007; Tokuda et al., 2010, 2011). Therefore, rAAV2-P450scc transduction of a cell most likely produces a presynaptic inhibitory effect. It is not clear how 3α,5α-THP accesses the neuroactive steroid transmembrane binding sites on GABAA receptors, but it has been proposed to occur via intracellular (i.e., presynaptic) lateral diffusion through the cell membrane (Akk et al., 2007) or by a paracrine or autocrine mechanism (Herd et al., 2007), as no active release mechanism has been identified. In the present study, we ascertained that 3α,5α-THP is located in TH-positive dopamine neurons in the VTA, but we cannot rule out the possibility that glutamatergic or GABAergic neurons were also transduced. It is important to note that TH-positive dopamine cells in the VTA co-release glutamate in the NAc shell during optical stimulation (Stuber et al., 2010), therefore, rAAV2-P450scc transduction of TH-positive neurons in the VTA may reduce glutamatergic as well as dopaminergic activity. Furthermore, a recently characterized group of TH-positive VTA neurons that project to the lateral habenula (LHb) have been shown to produce reward when optically stimulated (Stamatakis et al., 2013). Therefore, it is possible that ethanol reinforcement was reduced by neuroactive steroid elevations in these LHb projecting cells of the VTA. Further experimentation will be needed to understand how local increases of 3α,5α-THP in the VTA alter mesolimbic activity and identify cell types important in these effects.
It is also likely that rAAV2-P450scc transduction of VTA neurons altered their response to ethanol. Since ethanol is thought to disinhibit dopamine neurons (Spanagel and Weiss, 1999), increased 3α,5α-THP in these cells may alter the ability of ethanol to produce this effect. Indeed increased 3α,5α-THP in the VTA may shift the dose–response of ethanol, increasing ethanol sensitivity, since 3α,5α-THP and ethanol both modulate GABAA receptors. In addition, rAAV2-P450scc transduction of the VTA may have also influenced 3α,5α-THP in glutamatergic afferent projection neurons from the PFC or pedunculopontine tegmental nucleus (PPTg)/laterodorsal tegmental nucleus (LDT), for example. These neurons provide excitatory control over dopamine neurons, and if inhibited by increased 3α,5α-THP, a decrease in dopamine neuron activity could be a possible outcome. In contrast, if 3α,5α-THP is increased in the glutamatergic cells that synapse onto the GABAergic interneurons, which normally inhibit dopamine neurons, we would expect an increase in dopaminergic activity. The bed nucleus of the stria terminalis (BNST) sends both GABAergic and glutamatergic afferent projections to the VTA that predominately synapse on TH-negative GABAergic neurons (Kudo et al., 2012). Optical stimulation of these projections produce opposing effects on reward and aversion (Jennings et al., 2013), with stimulation of the GABAergic projection producing reward and stimulation of the glutamatergic projection producing aversive behavior. Therefore, although it is unclear how, increases of 3α,5α-THP in the VTA from BNST afferent projections may play a role in our behavioral results. There are several ways that increased intracellular 3α,5α-THP could diminish ethanol-induced VTA activity and/or excitability via local intracellular-mediated neuronal inhibition, which may consequently result in reduced ethanol self-administration (Fig. 7).
Figure 7.
Simplified schematic of potential mechanisms of rAAV2–P450scc transduction-induced effects on VTA neurons believed to regulate ethanol reinforcement and consumption or in which optical stimulation is rewarding. Transduction of TH+ neurons may reduce activity in cells that project to either the (A) NAc, mPFC, and/or the (B) LHb. Transduction of afferent projections from (C) PFC or PPTg/LDT may decrease activation of TH+ projection neurons. Transduction of (D) BNST afferent projections onto GABAergic neurons have the potential to influence rewarding and/or aversive behavior. Transduction of inhibitory interneurons in the VTA is not expected to have any effect since biosynthetic enzymatic machinery is absent in these cells, but (E) increased extracellular levels of 3α,5α-THP may act via GABAergic interneurons to increase activity of TH+ neurons.
Alternatively, 3α,5α-THP could be “released” or diffuse to produce extracellular actions. If 3α,5α-THP is released from VTA neurons, the main theoretical consequence would be inhibition of interneurons. In contrast to reducing VTA dopamine cell activity, 3α,5α-THP inhibition of GABAergic interneurons would likely increase dopamine activity (Tan et al., 2010). Pharmacologic manipulations in the NAc and VTA suggest reduced dopamine activity is associated with a reduction in the maintenance of operant ethanol self-administration in nondependent rats (for review see, Gonzales et al., 2004). Therefore, it is unlikely that increased release of 3α,5α-THP was the predominant effect of rAAV2-P450scc transduction in the VTA. Finally, we cannot rule out the possibility that rAAV2-P450scc transduction of VTA involves several of these mechanisms resulting in the overall behavioral sequelae observed. Ultimately, using a viral vector to target neuron types selectively may identify cell types/circuitry in the VTA that contribute to the current behavioral findings.
The inability of rAAV2-P450scc transduction in the NAc to alter operant ethanol responding may be due to the lack of increase of 3α,5α-THP in NAc neurons that regulate ethanol self-administration. It is surprising that rAAV2-P450scc transduction in the NAc did not increase cellular 3α,5α-THP levels. Previous studies have shown dense cellular staining of 3α,5α-THP in the striatum (Saalmann et al., 2007; Cook et al., 2014a,b), suggesting that the necessary biosynthetic enzymes are present in NAc neurons. However, we have recently shown acute ethanol administration reduces 3α,5α-THP immunoreactivity in the NAc shore, in contrast to many other brain regions that display an ethanol-induced increase of 3α,5α-THP (Cook et al., 2014a,b). Therefore, regulation of 3α,5α-THP synthesis and/or metabolism may differ in the NAc, compared with other brain regions. Nonetheless, the observation that rAAV2-P450scc transduction of NAc did not alter local 3α,5α-THP levels or ethanol self-administration supports the idea that 3α,5α-THP may be requisite for the effects of rAAV2-P450scc transduction of the VTA.
Limitations of the current study include the lack of measurement of other neurosteroids, and the lack of a palatable control reinforcer. It is likely that other neurosteroids, including progesterone, deoxycorticosterone, and additional neuroactive metabolites were increased in the VTA, which may have contributed to the results. Transfection of the P450scc construct increases pregnenolone, and systemic pregnenolone administration increases many neuroactive steroids (Porcu et al., 2009). Pregnenolone administration also reduces ethanol reinforcement (Besheer et al., 2010a). It is important to note that water responding was low, indicating a floor effect may apply to this measure. Therefore, sucrose or saccharin self-administration would be a more optimal reinforcer to determine the specificity of the effects of rAAV2-P450scc.
The results in the current studies underscore the importance of endogenous GABAergic neuroactive steroids in the regulation of neurotransmission across the brain, and potential for therapeutic manipulation. Since these steroids are synthesized in brain circuitry and other endocrine organs, they provide a mechanism for interactions between many organ systems, integrating effects of all the neuroendocrine axes. Therefore, it is not surprising that alcohol use disorders are impacted by stress, gender, and neuroimmune processes that are all affected by neuroactive steroids. Since plasma 3α,5α-THP levels are reduced during withdrawal in human alcoholics (Romeo et al., 1996), this target merits further investigation.
Genetic regulation of neurosteroid levels has multiple advantages over systemic administration of neuroactive steroids or their analogs. First, the effects of rAAV transduction persist in the CNS at least 2 years in rats (Klein et al., 2002), 8 years in rhesus monkeys (Hadaczek et al., 2010), and is considered a permanent episomal modification of the genetic content of the cell. Indeed, the effects on ethanol self-administration in the present study persisted for 3 weeks, and showed no sign of diminishing. This is a critical factor for biomedical research examining chronic diseases such as addictive disorders. Next, it is possible to minimize unwanted side effects by producing steroidogenesis at sites exhibiting pathological activity, avoiding sites that confer unwanted neuroactive steroid effects. Indeed, we have recently found that chronic intermittent ethanol administration reduces 3α,5α-THP immunolabeling in the VTA, but not the NAc shell (Maldonado-Devincci et al., 2014), and this effect is associated with increased ethanol consumption (Becker and Lopez, 2004). Thus, the present data converge with other studies upon the conclusion that modulation of VTA neuroactive steroids regulates ethanol reinforcement and drinking. Therefore, the combination of P450scc gene delivery with studies of brain activity could allow targeted manipulation of abnormal cellular activity that may underlie alcohol use disorders or other neurological or psychiatric disease.
Footnotes
This work was supported by R37-AA10564, U01-AA016672, and U01-AA020935 (A.L.M.); R37-AA014983 (C.W.H.); P60-AA011605 (A.L.M. and C.W.H.), T32-AA007573, and T32-ES007126; and the University of North Carolina (UNC) Bowles Center for Alcohol Studies. We would like to acknowledge Stephanie Nelli, Mackenzie Neighbors, Alex Fetzer, and Ana Maria Dumitru for technical assistance. Dr. Garret Stuber and Alice Stamatakis provided helpful advice and valuable scientific discussions. We thank Dr. Michael Chua and Dr. Neal Kramarcy with the Michael Hooker Microscopy facility at UNC for their invaluable microscopy advice, assistance, and discussions. We thank Indiana University School of Medicine for providing the P rats, supported by Research Award Grant (R24 AA015512). We acknowledge the late Robert H. Purdy for providing the affinity purified antibody used to label 3α,5α-THP immunoreactivity in this study.
The authors declare no conflict of interest.
References
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABA(A) receptors. Pharmacol Ther. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.ALC.0000149977.95306.3A. [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:209–221. doi: 10.1111/j.1530-0277.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lindsay TG, O'Buckley TK, Hodge CW, Morrow AL. Pregnenolone and ganaxolone reduce operant ethanol self-administration in alcohol-preferring P rats. Alcohol Clin Exp Res. 2010a;34:2044–2052. doi: 10.1111/j.1530-0277.2010.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010b;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol. 2013;9:241–250. doi: 10.1038/nrendo.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi VW, Asokan A, Haberman RA, McCown TJ, Samulski RJ. Production of recombinant adeno-associated viral vectors and use for in vitro and in vivo administration. Curr Protoc Neurosci Chapter. 2006;4 doi: 10.1002/0471142301.ns0417s35. Unit 4.17. [DOI] [PubMed] [Google Scholar]
- Cook JB, Dumitru AM, O'Buckley TK, Morrow AL. Ethanol administration produces divergent changes in GABAergic neuroactive steroid immunohistochemistry in the rat brain. Alcohol Clin Exp Res. 2014a;38:90–99. doi: 10.1111/acer.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JB, Nelli SM, Neighbors MR, Morrow DH, O'Buckley TK, Maldonado-Devincci AM, Morrow AL. Ethanol alters local cellular levels of (3α,5α)-3-hydroxypregnan-20-one (3α,5α-THP) independent of the adrenals in subcortical brain regions. Neuropsychopharmacology. 2014b doi: 10.1038/npp.2014.46. doi: 10.1038/npp.2014.46. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Hadaczek P, Eberling JL, Pivirotto P, Bringas J, Forsayeth J, Bankiewicz KS. Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2-hAADC. Mol Ther. 2010;18:1458–1461. doi: 10.1038/mt.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Proctor WR, McQuilkin SJ, Klein RL, Mascia MP, Whatley V, Whiting PJ, Dunwiddie TV. Ethanol increases GABAA responses in cells stably transfected with receptor subunits. Alcohol Clin Exp Res. 1995;19:226–232. doi: 10.1111/j.1530-0277.1995.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt's forced swim test: modulation by 3α-hydroxy-5α-pregnan-20-one. Neuropharmacology. 2002;43:1339–1350. doi: 10.1016/S0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hirani K, Sharma AN, Jain NS, Ugale RR, Chopde CT. Evaluation of GABAergic neuroactive steroid 3α-hydroxy-5α-pregnane-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology. 2005;180:267–278. doi: 10.1007/s00213-005-2169-7. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Lewis RS, Erickson HL. Specific decreases in ethanol-but not water-reinforced responding produced by the 5-HT3 antagonist ICS 205–930. Alcohol. 1993;10:191–196. doi: 10.1016/0741-8329(93)90034-L. [DOI] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Gonzales RA. The dopamine response in the nucleus accumbens core-shell border differs from that in the core and shell during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:1355–1365. doi: 10.1111/j.1530-0277.2009.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. doi: 10.1111/j.1530-0277.1998.tb03708.x. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Hamby ME, Gong Y, Hirko AC, Wang S, Hughes JA, King MA, Meyer EM. Dose and promoter effects of adeno-associated viral vector for green fluorescent protein expression in the rat brain. Exp Neurol. 2002;176:66–74. doi: 10.1006/exnr.2002.7942. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M, Watanabe M. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J Neurosci. 2012;32:18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Cook JB, O'Buckley TK, Morrow DH, McKinley RE, Morrow AL. Chronic intermittent ethanol exposure and withdrawal alters (3α,5α)-3-hydroxy-pregnan-20-one immunostaining in mesocorticolimbic regions of C57BL/6J mice. 2014 doi: 10.1111/acer.12530. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31:1249–1263. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/S0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- McCown TJ. Adeno-associated virus-mediated expression and constitutive secretion of galanin suppresses limbic seizure activity in vivo. Mol Ther. 2006;14:63–68. doi: 10.1016/j.ymthe.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Morrow AL. Recent developments in the significance and therapeutic relevance of neuroactive steroids-Introduction to the special issue. Pharmacol Ther. 2007;116:1–6. doi: 10.1016/j.pharmthera.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzo C, Porceddu ML, Maira G, Flore G, Concas A, Dazzi L, Biggio G. Inhibition of basal and stress-induced dopamine release in the cerebral cortex and nucleus accumbens of freely moving rats by the neurosteroid allopregnanolone. J Psychopharmacol. 1996;10:266–272. doi: 10.1177/026988119601000402. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Purdy RH, Covey DF, Richardson HN, Roberto M, Koob GF. Epipregnanolone and a novel synthetic neuroactive steroid reduce alcohol self-administration in rats. Pharmacol Biochem Behav. 2005;81:543–550. doi: 10.1016/j.pbb.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain. San Diego: Academic; 1998. [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Porcu P, Rogers LS, Morrow AL, Grant KA. Plasma pregnenolone levels in cynomolgus monkeys following pharmacological challenges of the hypothalamic-pituitary-adrenal axis. Pharmacol Biochem Behav. 2006;84:618–627. doi: 10.1016/j.pbb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. Simultaneous quantification of GABAergic 3α,5α/3α,5β neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Blinn JR, Paul SM. Synthesis, metabolism, and pharmacological activity of 3α-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J Med Chem. 1990;33:1572–1581. doi: 10.1021/jm00168a008. [DOI] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin Neuropharmacol. 1996;19:366–369. doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Rougé-Pont F, Mayo W, Marinelli M, Gingras M, Le Moal M, Piazza PV. The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens. Eur J Neurosci. 2002;16:169–173. doi: 10.1046/j.1460-9568.2002.02084.x. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Morgan IG, Calford MB. Neurosteroids involved in regulating inhibition in the inferior colliculus. J Neurophysiol. 2006;96:3064–3073. doi: 10.1152/jn.00786.2006. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kirkcaldie MT, Waldron S, Calford MB. Cellular distribution of the GABAA receptor-modulating 3alpha-hydroxy, 5alpha-reduced pregnane steroids in the adult rat brain. J Neuroendocrinol. 2007;19:272–284. doi: 10.1111/j.1365-2826.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/S0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, O'Dell KA, Izumi Y, Zorumski CF. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J Neurosci. 2010;30:16788–16795. doi: 10.1523/JNEUROSCI.4101-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J Neurosci. 2011;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman BA, Knapp DJ, Werner DF, Crews FT, Breese GR. The cytokine mRNA increase induced by withdrawal from chronic ethanol in the sterile environment of brain is mediated by CRF and HMGB1 release. Alcohol Clin Exp Res. 2013;37:2086–2097. doi: 10.1111/acer.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]