Abstract
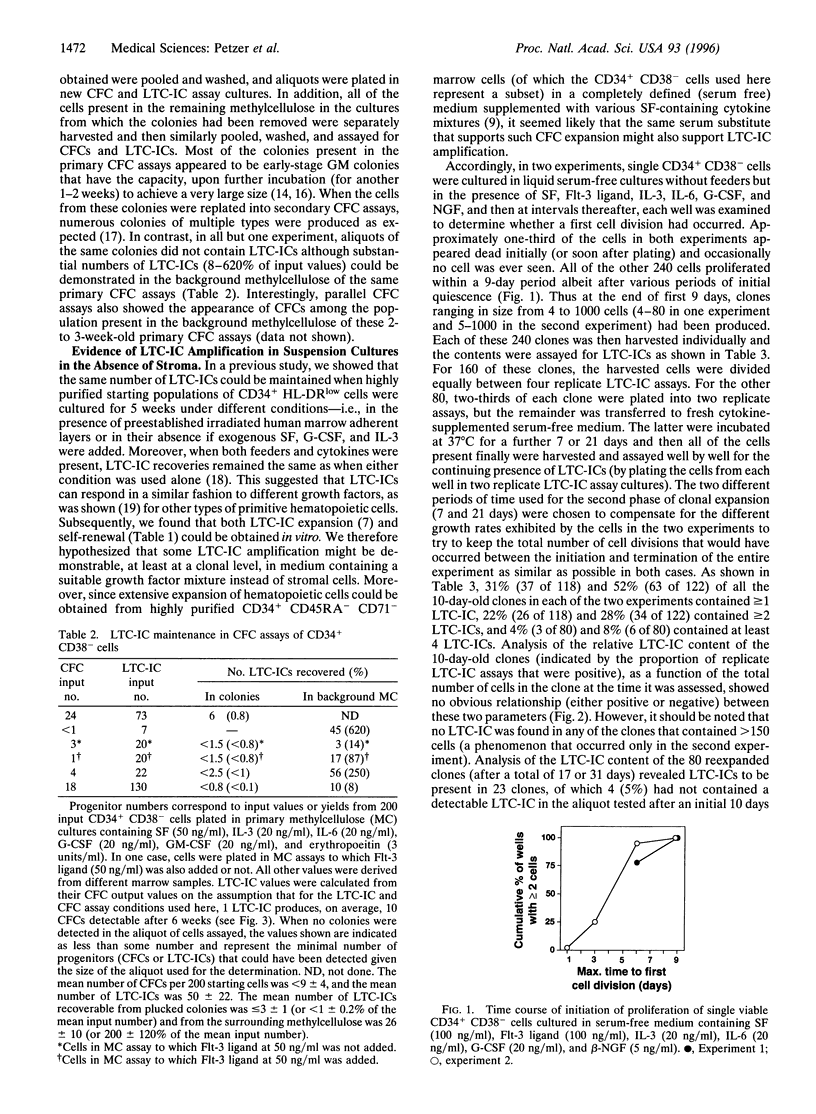
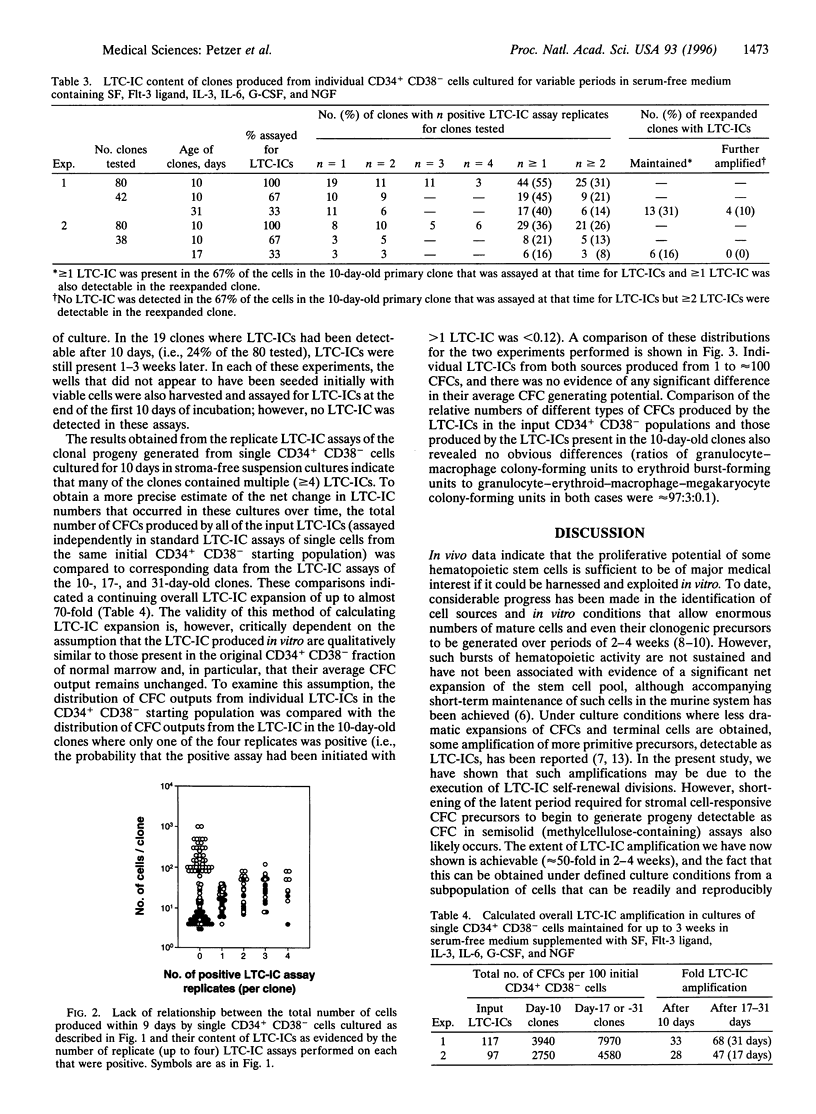
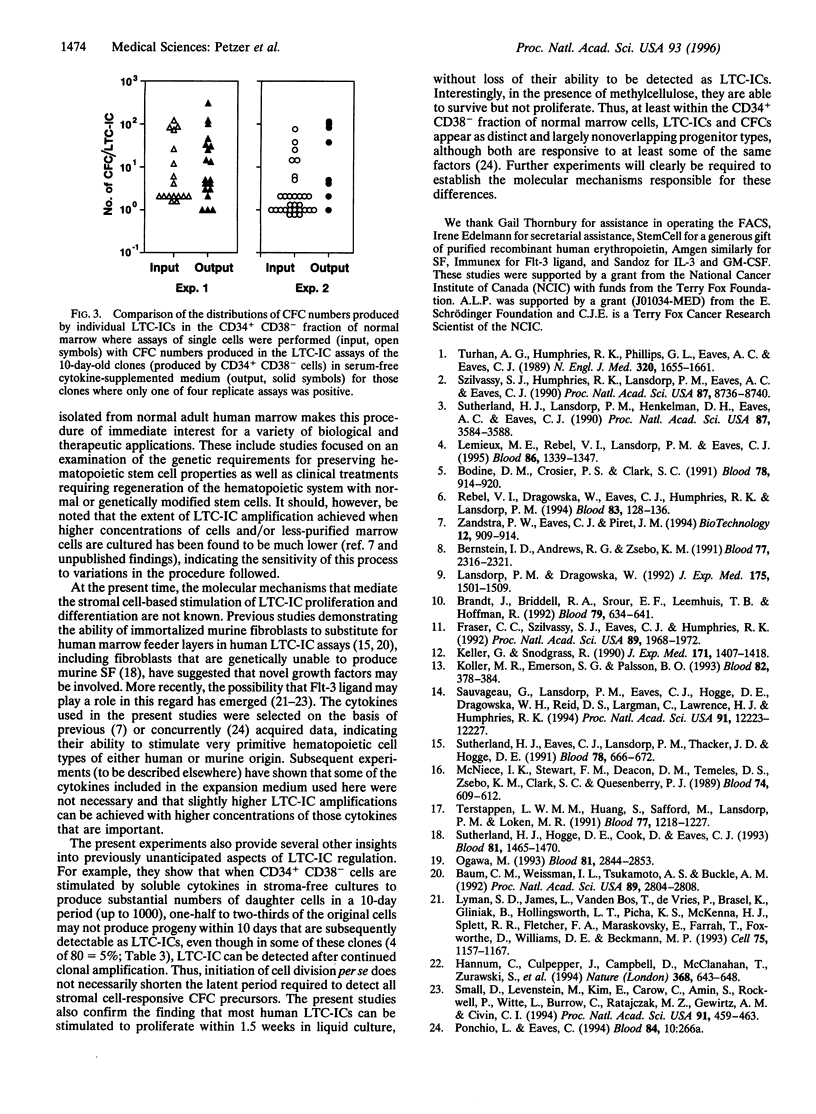
A major goal of experimental and clinical hematology is the identification of mechanisms and conditions that support the expansion of transplantable hematopoietic stem cells. In normal marrow, such cells appear to be identical to (or represent a subset of) a population referred to as long-term-culture-initiating cells (LTC-ICs) so-named because of their ability to produce colony-forming cell (CFC) progeny for > or = 5 weeks when cocultured with stromal fibroblasts. Some expansion of LTC-ICs in vitro has recently been described, but identification of the factors required and whether LTC-IC self-renewal divisions are involved have remained unresolved issues. To address these issues, we examined the maintenance and/or generation of LTC-ICs from single CD34+ CD38- cells cultured for variable periods under different culture conditions. Analysis of the progeny obtained from cultures containing a feeder layer of murine fibroblasts engineered to produce steel factor, interleukin (IL)-3, and granulocyte colony-stimulating factor showed that approximately 20% of the input LTC-ICs (representing approximately 2% of the original CD34+ CD38- cells) executed self-renewal divisions within a 6-week period. Incubation of the same CD34+ CD38- starting populations as single cells in a defined (serum free) liquid medium supplemented with Flt-3 ligand, steel factor, IL-3, IL-6, granulocyte colony-stimulating factor, and nerve growth factor resulted in the proliferation of initial cells to produce clones of from 4 to 1000 cells within 10 days, approximately 40% of which included > or = 1 LTC-IC. In contrast, in similar cultures containing methylcellulose, input LTC-ICs appeared to persist but not divide. Overall the LTC-IC expansion in the liquid cultures was 30-fold in the first 10 days and 50-fold by the end of another 1-3 weeks. Documentation of human LTC-IC self-renewal in vitro and identification of defined conditions that permit their extensive and rapid amplification should facilitate analysis of the molecular mechanisms underlying these processes and their exploitation for a variety of therapeutic applications.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum C. M., Weissman I. L., Tsukamoto A. S., Buckle A. M., Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein I. D., Andrews R. G., Zsebo K. M. Recombinant human stem cell factor enhances the formation of colonies by CD34+ and CD34+lin- cells, and the generation of colony-forming cell progeny from CD34+lin- cells cultured with interleukin-3, granulocyte colony-stimulating factor, or granulocyte-macrophage colony-stimulating factor. Blood. 1991 Jun 1;77(11):2316–2321. [PubMed] [Google Scholar]
- Bodine D. M., Crosier P. S., Clark S. C. Effects of hematopoietic growth factors on the survival of primitive stem cells in liquid suspension culture. Blood. 1991 Aug 15;78(4):914–920. [PubMed] [Google Scholar]
- Brandt J., Briddell R. A., Srour E. F., Leemhuis T. B., Hoffman R. Role of c-kit ligand in the expansion of human hematopoietic progenitor cells. Blood. 1992 Feb 1;79(3):634–641. [PubMed] [Google Scholar]
- Fraser C. C., Szilvassy S. J., Eaves C. J., Humphries R. K. Proliferation of totipotent hematopoietic stem cells in vitro with retention of long-term competitive in vivo reconstituting ability. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1968–1972. doi: 10.1073/pnas.89.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum C., Culpepper J., Campbell D., McClanahan T., Zurawski S., Bazan J. F., Kastelein R., Hudak S., Wagner J., Mattson J. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 1994 Apr 14;368(6472):643–648. doi: 10.1038/368643a0. [DOI] [PubMed] [Google Scholar]
- Keller G., Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990 May 1;171(5):1407–1418. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller M. R., Emerson S. G., Palsson B. O. Large-scale expansion of human stem and progenitor cells from bone marrow mononuclear cells in continuous perfusion cultures. Blood. 1993 Jul 15;82(2):378–384. [PubMed] [Google Scholar]
- Lansdorp P. M., Dragowska W. Long-term erythropoiesis from constant numbers of CD34+ cells in serum-free cultures initiated with highly purified progenitor cells from human bone marrow. J Exp Med. 1992 Jun 1;175(6):1501–1509. doi: 10.1084/jem.175.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux M. E., Rebel V. I., Lansdorp P. M., Eaves C. J. Characterization and purification of a primitive hematopoietic cell type in adult mouse marrow capable of lymphomyeloid differentiation in long-term marrow "switch" cultures. Blood. 1995 Aug 15;86(4):1339–1347. [PubMed] [Google Scholar]
- Lyman S. D., James L., Vanden Bos T., de Vries P., Brasel K., Gliniak B., Hollingsworth L. T., Picha K. S., McKenna H. J., Splett R. R. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993 Dec 17;75(6):1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- McNiece I. K., Stewart F. M., Deacon D. M., Temeles D. S., Zsebo K. M., Clark S. C., Quesenberry P. J. Detection of a human CFC with a high proliferative potential. Blood. 1989 Aug 1;74(2):609–612. [PubMed] [Google Scholar]
- Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993 Jun 1;81(11):2844–2853. [PubMed] [Google Scholar]
- Rebel V. I., Dragowska W., Eaves C. J., Humphries R. K., Lansdorp P. M. Amplification of Sca-1+ Lin- WGA+ cells in serum-free cultures containing steel factor, interleukin-6, and erythropoietin with maintenance of cells with long-term in vivo reconstituting potential. Blood. 1994 Jan 1;83(1):128–136. [PubMed] [Google Scholar]
- Sauvageau G., Lansdorp P. M., Eaves C. J., Hogge D. E., Dragowska W. H., Reid D. S., Largman C., Lawrence H. J., Humphries R. K. Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12223–12227. doi: 10.1073/pnas.91.25.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D., Levenstein M., Kim E., Carow C., Amin S., Rockwell P., Witte L., Burrow C., Ratajczak M. Z., Gewirtz A. M. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):459–463. doi: 10.1073/pnas.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Lansdorp P. M., Thacker J. D., Hogge D. E. Differential regulation of primitive human hematopoietic cells in long-term cultures maintained on genetically engineered murine stromal cells. Blood. 1991 Aug 1;78(3):666–672. [PubMed] [Google Scholar]
- Sutherland H. J., Hogge D. E., Cook D., Eaves C. J. Alternative mechanisms with and without steel factor support primitive human hematopoiesis. Blood. 1993 Mar 15;81(6):1465–1470. [PubMed] [Google Scholar]
- Sutherland H. J., Lansdorp P. M., Henkelman D. H., Eaves A. C., Eaves C. J. Functional characterization of individual human hematopoietic stem cells cultured at limiting dilution on supportive marrow stromal layers. Proc Natl Acad Sci U S A. 1990 May;87(9):3584–3588. doi: 10.1073/pnas.87.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvassy S. J., Humphries R. K., Lansdorp P. M., Eaves A. C., Eaves C. J. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terstappen L. W., Huang S., Safford M., Lansdorp P. M., Loken M. R. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38- progenitor cells. Blood. 1991 Mar 15;77(6):1218–1227. [PubMed] [Google Scholar]
- Turhan A. G., Humphries R. K., Phillips G. L., Eaves A. C., Eaves C. J. Clonal hematopoiesis demonstrated by X-linked DNA polymorphisms after allogeneic bone marrow transplantation. N Engl J Med. 1989 Jun 22;320(25):1655–1661. doi: 10.1056/NEJM198906223202504. [DOI] [PubMed] [Google Scholar]
- Zandstra P. W., Eaves C. J., Piret J. M. Expansion of hematopoietic progenitor cell populations in stirred suspension bioreactors of normal human bone marrow cells. Biotechnology (N Y) 1994 Sep;12(9):909–914. doi: 10.1038/nbt0994-909. [DOI] [PubMed] [Google Scholar]



