Abstract
Previously, we reported that viable but nonculturable (VBNC) Vibrio cholerae was converted into a culturable state by coculture with several eukaryotic cell lines including HT-29 cells. In this study, we found that a factor converting VBNC V. cholerae into a culturable state (FCVC) existed in cell extracts of eukaryotic cells. FCVC was nondialyzable, proteinase K-sensitive, and stable to heating at <60°C for 5 min. We prepared thiosulfate citrate bile salts sucrose (TCBS) plates with FCVC (F-TCBS plates). After confirming that VBNC V. cholerae O1 and O139 formed typical yellow colonies on F-TCBS plates, we tried to isolate cholera toxin gene-positive VBNC V. cholerae from environmental water samples collected in urban slum areas of Kolkata, India and succeeded in isolating V. cholerae O1 El Tor variant strains harboring a gene for the cholera toxin. The possible importance of VBNC V. cholerae O1 as a source of cholera outbreaks is discussed.
Keywords: Factor converting VBNC into culturable, VBNCVibrio cholerae
Introduction
Cholera caused by Vibrio cholerae O1 and O139 continues to be a major cause of severe diarrhea, especially in developing countries (World Health Organization 2012). The infectious materials are water and food contaminated by V. cholerae O1 and O139. In cholera endemic areas like Kolkata, India (Nair et al. 2010) where clean drinking water is not easily available, large numbers of people use environmental water from lakes, ponds, and rivers as domestic water for washing vegetables, fish and dishes, and sometimes even as drinking water. Thus, it has been suggested that the cholera endemic continues because environmental water is contaminated by V. cholerae O1 and O139. However, the frequencies of isolating V. cholerae O1 and O139 from environmental water samples in cholera endemic areas are quite low even in epidemic seasons (Huq et al. 1990; Islam et al. 1994) and the numbers of the organisms, if isolated, are not sufficient to explain the cause of the disease (Levine et al. 1988). Moreover, between epidemic seasons V. cholerae O1 and O139 are very rarely isolated (Roszak and Colwell 1987).
Many investigators have reported the existence of viable but nonculturable (VBNC) V. cholerae O1 and O139 in environmental water in cholera endemic areas (Roszak and Colwell 1987; Alam et al. 2006), and even between epidemic seasons VBNC V. cholerae O1 is present in a biofilm form (Faruque et al. 2006; Alam et al. 2007). These data strongly suggest that VBNC V. cholerae O1 and O139 are the sources of infection in these areas. To confirm this hypothesis, we tried to isolate VBNC V. cholerae from environmental water samples in a culturable form and report our successful results in this article.
Material and Methods
Bacterial strains and culture media
V. cholerae O1 (N16961) and V. cholerae O139 (VC-280) were taken from stock cultures in the laboratory repository at the National Institute of Cholera and Enteric Diseases. The culture media used were alkaline peptone water, pH 8.8 (APW) (Eiken, Tokyo, Japan), nutrient agar (NA; Difco, Franklin Lakes, NJ) supplemented with 1% NaCl (NA) and thiosulfate citrate bile salts sucrose agar (TCBS) (Eiken).
Culture of eukaryotic cells
HT-29 cells (human colon adenocarcinoma grade II cells) were cultured in Dulbecco's modified Eagle's medium (DMEM; catalogue number: 12800-017; Gibco Life Science, Paisley, Scotland, UK) supplemented with 1.5 g L−1 NaHCO3 (Sigma, St. Louis, MO), 3.56 g L−1 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (Sigma), and 10% fetal bovine serum (FBS; catalogue number 10082-147; Gibco Life Science). CHO cell (Chinese hamster ovary cells) and HeLa cells (human cervical cancer cells) were cultured in minimum essential medium (MEM; catalogue number: 61100-061; Gibco Life Science) supplemented with 10% FBS, while Caco-2 cells (human epithelial colorectal adenocarcinoma cells) were cultured in MEM supplemented with 20% FBS. T84 cell (human colonic adenocarcinoma cells) and Intestine 407 cells (human jejunum and ileum cells) were cultured in DMEM supplemented with 10% FBS. All media were supplemented with 100 μg mL−1 streptomycin and 100 UmL−1 penicillin. All cell cultures were carried out in a CO2 incubator at 37°C under 5% CO2.
Preparation of VBNC V. cholerae
VBNC V. cholerae O1 (N16961) and O139 (VC-280) were prepared as described previously (Senoh et al. 2010, 2012). Each V. cholerae strain was inoculated into APW and incubated at 37°C for 16 h, after which the cells were collected by centrifugation at 5000g for 10 min at 25°C (Heraeus Biofuge Stratos; Kendro, Langenselbold, Germany), washed twice with a VBNC microcosm buffer, comprising 1% sterile solution of artificial seawater (1% Instant Ocean; Aquarium Systems, Mentor, OH), and suspended in 200 mL of VBNC microcosm buffer in a 1-L flask to a final concentration of ∼1 × 108 cells mL−1. The cells in VBNC microcosm buffer were incubated at 4°C in the dark without shaking. After incubation for 11 weeks, no culturable cells were observed following incubation of each VBNC microcosm buffer in APW at 37°C for 16 h. Thus, the VBNC microcosm buffer after incubation for 11 weeks contained VBNC V. cholerae O1 (N16961) and O139 (VC-280).
Enumeration of culturable V. cholerae in the microcosm
A 0.1 mL aliquot of VBNC microcosm buffer containing VBNC V. cholerae was serially diluted by twofold, and 0.1 mL of each dilution was inoculated into a mixture of 0.3 mL of 1.5-fold-condensed APW and 0.05 mL of phosphate-buffered saline, pH 7.0 (PBS). After incubation at 37°C for 16 h without shaking, the turbidity of each APW was observed by the naked eye and the highest dilution of the microcosm buffer that became turbid was recorded. Assuming that one culturable cell produced turbidity by growth, the number of culturable cells per milliliter of the original microcosm buffer was calculated.
Preparation of cell extracts of eukaryotic cells
Eukaryotic cells were cultured as described above. Confluent cells in 10-cm-diameter petri dishes were washed with PBS, scrapped off, and centrifuged at 3000g for 5 min at 25°C. The collected cells were resuspended in 0.5 mL of PBS, mixed with 0.1-mm-diameter glass beads, and disrupted by shaking for 90 sec using a ShakeMaster (BioMedical Science, Tokyo, Japan). After centrifugation of each mixture at 20,000g for 5 min at 4°C, the supernatant was passed through a 0.20-μm membrane filter (EMD Millipore Corporation, Billerica, MA). The obtained cell extract without further dilution (∼0.5 mL) was designated a solution of a factor converting VBNC to a culturable state (FCVC).
Examination of V. cholerae conversion with FCVC solution
A 0.1-mL aliquot of a VBNC V. cholerae preparation and 0.05 mL of FCVC were added to 0.3 mL of 1.5-fold-condensed APW and incubated at 37°C for 16 h without shaking. When the APW became turbid, a 0.1-mL aliquot was inoculated onto TCBS plates and incubated at 37°C for 16 h. The yellow colonies that appeared were inoculated onto NA plates and incubated at 37°C for 16 h. The serotypes of the colonies on NA plates were confirmed by appropriate typing sera (Denka, Tokyo, Japan).
Dialysis of FCVC
A 0.5-mL aliquot of FCVC was placed in a dialysis bag (SnakeSkin®; MWCO: 10K, Takara Bio Inc., Shiga, Japan) and dialysis was carried out against 2 L of 20 mmol/L Tris-HCl (PH 8.5) at 4°C for 16 h.
Treatment of FCVC with proteinase K
A 1-μL aliquot of 25 mg mL−1 of Proteinase K (Sigma) in PBS was added to 98 μL of FCVC and incubated at 37°C for 1 h. To inactivate the proteinase K activity for further assays with VBNC V. cholerae, 1 μL of 50 mg mL−1 proteinase K inhibitor (pefabloc SC; Roche Diagnostics, Mannheim, Germany) in PBS was added to 99 μL of proteinase K-treated FCVC and incubated at 37°C for 1 h.
Treatment with proteinase K and proteinase K inhibitor
A mixture of 1 μL of 25 mg mL−1 of Proteinase K in PBS and 1 μL of 50 mg mL−1 Pefabloc SC in PBS was incubated at 37°C for 1 h, followed by addition of the mixture to 98 μL of FCVC, and incubated at 37°C for 1 h.
Examination of heat stability of FCVC
Aliquots (0.1 mL) of FCVC were treated at different temperatures for various time intervals and then subjected to 10-fold serial dilution. A 0.05-mL aliquot of each FCVC dilution and 0.1 mL of VBNC V. cholerae preparation were added to 0.3 mL of 1.5-fold-condensed APW. After incubation at 37°C for 16 h, the turbidity of the APW was observed by the naked eye and confirmation of V. cholerae was carried out as described above.
Preparation of TCBS plates with FCVC
TCBS plates with FCVC (F-TCBS plates) were prepared as follows. TCBS medium was dissolved in water and boiled in accordance with the manufacturer's instructions. When the TCBS had cooled to about 60°C, ∼20 mL was poured into a 9-cm-diameter petri dish together with 1.0 mL of previously added nondiluted FCVC from HT-29 cells. The solutions were mixed thoroughly and allowed to solidify.
Collection of environmental water samples
Four sampling points were identified in urban slum areas of Kolkata (Fig. 1). Points A and B were different sites in a small pond of about 2500 m2. Point C was at a backwater in another slum area. Point D was at a riverside. At each of the sampling points, 50 mL of water was collected twice a month, with at least 2-week intervals, for 1 year (October 2010–September 2011).
Figure 1.
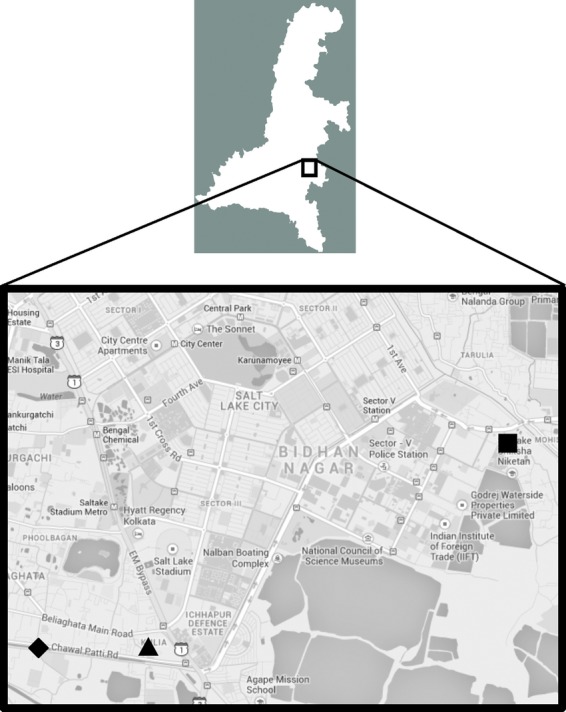
Map (from Google Maps) showing the sampling points in Kolkata, India. (▪), Points A and B; (▴), Point C; (♦), Point D.
Examination of environmental water samples for existing VBNC V. cholerae
Aliquots (0.1 mL) of the collected environmental water samples were inoculated directly on both F-TCBS and TCBS plates and incubated at 37°C for 16 h. When the total numbers of colonies formed on the plates were <10, the water samples were condensed by 10-fold. Aliquots (0.1 mL) of the condensed samples were then used for direct plating on F-TCBS and TCBS plates.
Polymerase chain reaction
To identify the environmental isolates as cholera toxin-producing V. cholerae polymerase chain reaction (PCR) for a V. cholerae-specific gene, ompW and a cholera toxin-specific gene, ctx was carried out as described previously (Shirai et al. 1991; Nandi et al. 2000). Mismatch amplification mutation assay (MAMA) PCR was carried out as described by Morita et al. (2008).
Results
Preparation of VBNC V. cholerae
A VBNC microcosm buffer containing ∼1 × 108 cells mL−1 of either V. cholerae O1 (N16961) or V. cholerae O139 (VC-280) was incubated at 4°C in the dark without shaking. Culturable cells in each VBNC microcosm buffer were enumerated periodically as described in the Material and Methods. As shown in Figure 2, no culturable cells of either V. cholerae O1 (N16961) or O139 (VC-280) were observed after incubation for 11 weeks. Furthermore, 0.1-mL aliquots of these VBNC microcosm buffers did not produce any colonies on either NA or TCBS plates. In further experiments, these VBNC microcosm buffers were used as VBNC V. cholerae O1 (N16961) and V. cholerae O139 (VC-280).
Figure 2.
Reduction in the number of culturable cells of Vibrio cholerae in a VBNC microcosm buffer. Approximately 1 × 108 cells mL−1 of V. cholerae O1 (N16961) and V. cholerae O139 (VC-280) in VBNC microcosm buffer were incubated at 4°C in the dark without shaking. The number of culturable cells in each VBNC microcosm buffer was determined in duplicate. Representative results of three experiments are presented. (•), V. cholerae O1 (N16961); (▴), V. cholerae O139 (VC-280).
Conversion of VBNC V. cholerae O139 to a culturable state by extracts of eukaryotic cells
Cell extracts of various eukaryotic cells converted VBNC V. cholerae O139 (VC-280) to a culturable state (Table 1). In this experiment, twofold serially diluted cell extracts of various eukaryotic cells were added to APW. After inoculation of VBNC V. cholerae O139 (VC-280) into the APW, incubation was carried out at 37°C for 16 h without shaking. The conversion was confirmed by turbidity of the APW. Growth of VBNC V. cholerae O139 (VC-280) was observed after addition of the cell extracts of various eukaryotic cells, while no growth of VBNC V. cholerae O139 (VC-280) was observed without addition of the cell extracts. Among the eukaryotic cells examined, the cell extract of HT-29 cells showed the highest conversion activity. These results indicate that the cell extracts of eukaryotic cells contained a factor that converts VBNC V. cholrae into a culturable state. We named this factor FCVC.
Table 1.
Conversion of VBNC Vibrio cholerae O139 (VC-280) into culturable state by cell extract of various eukaryotic cells.
| Source of cell extract | HT-29 | T84 | Caco-2 | Intestine 407 | HeLa | CHO |
|---|---|---|---|---|---|---|
| With cell extract of eukaryotic cells | 1:101 | 1:4 | 1:1.8 | 1:2.5 | 1:0.6 | 1:4 |
| Without cell extract of eukaryotic cells | –2 | – | – | – | – | – |
Caco-2, human epithelial colorectal adenocarcinoma cells; HeLa, human cervical cancer cells.
Highest dilution of cell extract at which VBNC V. cholerae O139 (VC-280) was converted into culturable state. Each experiment was repeated five times.
No conversion was observed. Each experiment was repeated five times.
Some properties of FCVC
Some properties of the VBNC V. cholerae conversion activity of FCVC from HT-29 are presented in Table 2. After FCVC was dialyzed in a dialysis bag (SnakeSkin®; MWCO: 10K) the conversion activity was retained in the dialysis bag, indicating that the molecular weight of FCVC was more than 10,000. Regarding the effect of proteinase K on the VBNC V. cholerae conversion activity of FCVC from HT-29 cells, FCVC lost its conversion activity after the proteinase treatment, and this effect was inhibited by treatment with proteinase K inhibitor.
Table 2.
Some properties of FCVC from HT-29 cells.
| Dialysis | |
| Before | 1:161 |
| After | 1:16 |
| Treatment with proteinase K | |
| None | 1:16 |
| Treatment with proteinase | –2 |
| Treatment with proteinase and proteinase inhibitor | 1:16 |
Highest dilution of FCVC at which VBNC Vibrio cholerae O139 (VC-280) was converted into a culturable state.
No conversion was observed.
The heat stability of the VBNC V. cholerae conversion activity of FCVC from HT-29 cells was examined and the results are shown in Figure 3A. FCVC was stable during incubation at less than 60°C for 5 min, but lost its activity after incubation at 70°C or higher. As shown in Figure 3B, when FCVC was incubated at 37°C, it retained its activity for 6 days. After 7 days, the activity decreased to one-half, but subsequently remained the same for at least for additional 4 days.
Figure 3.
Heat stability of FCVC from HT-29 cells. (A) Aliquots (0.1 mL) of FCVC from HT-29 cells were treated at various temperatures for 5 min and the treated samples were subjected to 10-fold serial dilution. An aliquot (0.05 mL) of each diluted sample was added to 0.3 mL of 1.5-fold-condensed APW, to which 0.1 mL of VBNC Vibrio cholerae O139 (VC-280) had been added. After incubation at 37°C for 16 h, turbidity was observed by the naked eye and recorded. When the alkaline peptone water (APW) became turbid, it was plated on NA plates and randomly selected colonies were confirmed to be V. cholerae O139 by serotyping. Each experiment was repeated three times. (B) Aliquots (0.1 mL) of FCVC from HT-29 cells were incubated at 37°C for the indicated time periods and the incubated samples were subjected to 10-fold serial dilution. Further experimental procedures were as described in (A).
These results suggest that the FCVC is a heat-stable protein with a molecular weight of more than 10,000.
Conversion of VBNC V. cholerae on TCBS plates with FCVC
We examined whether VBNC V. cholerae O139 (VC-280) was converted to a culturable form on F-TCBS plates. As shown in Figure 4A, VBNC V. cholerae O139 formed yellow colonies on F-TCBS plates, while no colonies appeared on TCBS plates without FCVC (Fig. 4B). These results indicated that VBNC V. cholerae O139 was converted to a culturable state on F-TCBS plates, and thus formed colonies.
Figure 4.
Colony formation of VBNC Vibrio cholerae O139 on F-TCBS plates. Aliquots (0.1 mL) of VBNC V. cholerae O139 (VC-280) were plated on F-TCBS (A) and TCBS (B) plates, and incubated at 37°C for 16 h.
Isolation of VBNC V. cholerae from environmental water samples
Using F-TCBS plates, we tried to isolate VBNC V. cholerae O1 and O139 from environmental water samples collected in urban slum areas of Kolkata, India. As shown in Table 3, the number of colonies that appeared on F-TCBS plates (total: 14,978) was significantly higher than the number that appeared on TCBS plates without FCVC (total: 10,669). As yellow colonies appeared on both F-TCBS and TCBS plates, typical Vibrio-like colonies observed by the naked eye were subjected to PCR for a V. cholerae-specific gene, ompW. About 19 times more ompW-positive strains were identified from colonies on F-TCBS plates (total: 1894) compared with the number from TCBS plates without FCVC (total: 101). All ompW-positive strains were analyzed for whether they harbored a cholera toxin gene, ctx. A total of 120 ctx-positive strains were identified from F-TCBS plates, while only six were identified from TCBS plates without FCVC. These ctx-positive V. cholerae strains were only detected at sampling points A and B. All ompW-positive, ctx-positive V. cholerae strains were confirmed to be serotype O1, biotype El Tor variant by their polymyxin B sensitivity and to harbor classical ctxB by MAMA-PCR.
Table 3.
Number of VBNC Vibrio cholerae isolated from environmental water samples.
| FCVC | Total number of colonies1 | Total number of yellow colonies | Total number of typical Vibrio-like colonies | Total number of ompW-positive strains | Total number of Ctx-positive strains |
|---|---|---|---|---|---|
| Point A | |||||
| + | 4359 | 1540 | 1053 | 626 | 36 |
| − | 2976 | 899 | 52 | 37 | 4 |
| Point B | |||||
| + | 4292 | 1483 | 1149 | 750 | 84 |
| − | 2961 | 1159 | 58 | 28 | 2 |
| Point C | |||||
| + | 3199 | 1158 | 678 | 341 | 0 |
| − | 2562 | 593 | 92 | 21 | 0 |
| Point D | |||||
| + | 3128 | 953 | 504 | 177 | 0 |
| − | 2170 | 520 | 37 | 15 | 0 |
| Total | |||||
| + | 14,978 | 5134 | 3384 | 1894 | 120 |
| − | 10,669 | 3171 | 239 | 101 | 6 |
Data are the sums of the numbers observed for all environmental samples collected twice a month for 1 year.
The seasonal distribution of VBNC V. cholerae strains isolated from environmental water samples is shown in Figure 5. The ompW-positive VBNC V. cholerae strains were isolated throughout the year, although there was a peak of isolation from April to June, with the highest isolation in May. On the other hand, most of the ompW-positive, ctx-positive VBNC V. cholerae strains were isolated in April. In September, only one ompW-positive, ctx-positive VBNC V. cholerae strain was isolated.
Figure 5.
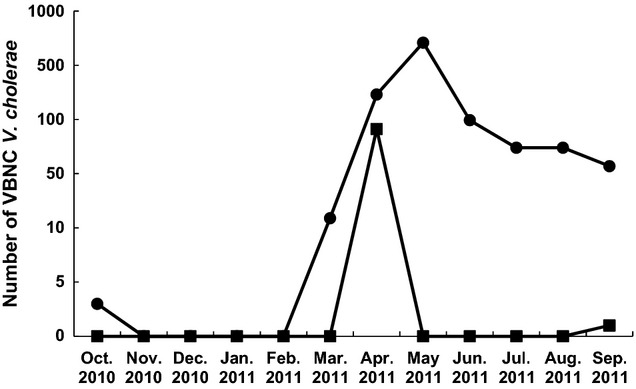
Monthly isolation of VBNC Vibrio cholerae from environmental water samples in slum areas of Kolkata. The sums of the numbers of ompW-positiveV. cholerae (•) and ompW-positive, ctx-positive V. cholerae O1 (▪) observed during each month were plotted.
Discussion
The VBNC state in bacteria is defined as bacteria that remain viable but are unable to grow or divide on, or in, routinely used bacterial media. As the first report by Xu et al. (1982) on VBNC Escherichia coli and V. cholerae, the existence of VBNC states in the environment of various bacteria, such as V. cholerae (Brayton et al. 1987; Aulet et al. 2007), E. coli (Garcia-Armisen and Servais 2004; Lleo et al. 2005; Zimmerman et al. 2009), Enterococcus faecalis (Lleo et al. 2005), and Arcobacter spp. (Fera et al. 2010), has been reported.
Conversion of VBNC bacteria to a culturable state has been reported under several conditions, such as temperature upshift (Wai et al. 1996; Gupte et al. 2003), incubation in phosphate buffer (Dukan et al. 1997), supplementation with catalase or sodium pyruvate (Mizunoe et al. 2000), addition of heat-stable autoinducer of growth (Reissbrodt et al. 2002), addition of resuscitation-promoting factor (Panutdaporn et al. 2006), and presence of Acanthamoeba castellannii (Steinert et al. 1997). However, none of these conditions succeeded in converting VBNC bacteria from environmental samples to a culturable state. Only recently, Faruque et al. (2006) and Alam et al. (2007) reported successful isolation of VBNC V. cholerae from environmental water samples by passage through rabbit ileal loops.
Previously, we reported the conversion of VBNC V. cholera and other enteric bacteria into a culturable state by coculture with several eukaryotic cell lines including HT-29 (Senoh et al., 2010; Senoh et al. 2012). In this study, we demonstrated that a factor converting VBNC bacteria into a culturable state (FCVC) existed in cell extracts of various cultured eukaryotic cells. As FCVC was resistant to heating at 60°C, we prepared TCBS plates containing FCVC (F-TCBS plates). When VBNC V. cholerae O139 (VC-280) was inoculated on F-TCBS plates, colonies of V. cholerae O139 were formed, suggesting that the VBNC bacteria were converted into a culturable state. By using F-TCBS plates, we successfully isolated VBNC V. cholerae from environmental water samples in urban slum areas of Kolkata, India. Quite large numbers of V. cholerae O1 strains carrying the ctx gene formed typical yellowish colonies on F-TCBS plates. Not only during the cholera epidemic season (April), but also between epidemic seasons (September and October), VBNC V. cholerae O1 was isolated from environmental water samples, although the frequency of its isolation between epidemic seasons was much lower than that during the epidemic season.
The evidence presented in this study that ctx-positive VBNC V. cholerae O1 exists in environmental water within cholera endemic areas strongly supports the hypothesis that the aquatic environment of cholera endemic areas is the source of cholera outbreaks. Further systematic epidemiology of the geographical and seasonal distributions of ctx-positive VBNC V. cholerae O1 and O139 in the environment of cholera endemic areas like Kolkata using F-TCBS plates will strengthen the above hypothesis.
Acknowledgments
This study was supported by the Japan Initiative for Global Research Network on Infectious Diseases, Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of Interest
None declared.
Funding Information
This study was supported by the Japan Initiative for Global Research Network on Infectious Diseases, Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Alam M, Sultana M, Nair GB, Sack RB, Sack DA, Siddique AK. Toxigenic Vibrio cholerae in the aquatic environment of Mathbaria, Bangladesh. Appl. Environ. Microbiol. 2006;72:2849–2855. doi: 10.1128/AEM.72.4.2849-2855.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M, Sultana M, Nair GB, Siddique AK, Hasan NA, Sack RB. Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc. Natl. Acad. Sci. USA. 2007;104:17801–17806. doi: 10.1073/pnas.0705599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulet O, Silva C, Fraga SG, Pichel M, Cangemi R, Gaudioso C. Detection of viable and viable nonculturable Vibrio cholerae O1 through cultures and immunofluorescence in the Tucumán rivers, Argentina. Rev. Soc. Bras. Med. Trop. 2007;40:385–390. doi: 10.1590/s0037-86822007000400002. [DOI] [PubMed] [Google Scholar]
- Brayton PR, Tamplin ML, Huq A, Colwell RR. Enumeration of Vibrio cholerae O1 in Bangladesh waters by fluorescent-antibody direct viable count. Appl. Environ. Microbiol. 1987;53:2862–2865. doi: 10.1128/aem.53.12.2862-2865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukan S, Lévi Y, Touati D. Recovery of culturability of an HOCl-stressed population of Escherichia coli after incubation in phosphate buffer: resuscitation or regrowth? Appl. Environ. Microbiol. 1997;63:4204–4209. doi: 10.1128/aem.63.11.4204-4209.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque SM, Biswas K, Udden SM, Ahmad QS, Sack DA, Nair GB. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc. Natl. Acad. Sci. USA. 2006;103:6350–6355. doi: 10.1073/pnas.0601277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fera MT, Gugliandolo C, Lentini V, Favaloro A, Bonanno D, La Camera E. Specific detection of Arcobacter spp. in estuarine waters of Southern Italy by PCR and fluorescent in situ hybridization. Lett. Appl. Microbiol. 2010;50:65–70. doi: 10.1111/j.1472-765X.2009.02767.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Armisen T, Servais P. Enumeration of viable E. coli in rivers and wastewaters by fluorescent in situ hybridization. J. Microbiol. Methods. 2004;58:269–279. doi: 10.1016/j.mimet.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Gupte AR, Joseph CLE, de Rezende SW. Induction and resuscitation of viable but nonculturable Salmonella enterica serovar Typhimurium DT104. Appl. Environ. Microbiol. 2003;69:6669–6675. doi: 10.1128/AEM.69.11.6669-6675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A, Colwell RR, Rahman R, Ali A, Chowdhury MA, Parveen S. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl. Environ. Microbiol. 1990;56:2370–2373. doi: 10.1128/aem.56.8.2370-2373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Hasan MK, Miah MA, Yunus M, Zaman K, Albert MJ. Isolation of Vibrio cholerae O139 synonym Bengal from the aquatic environment in Bangladesh: implications for disease transmission. Appl. Environ. Microbiol. 1994;60:1684–1686. doi: 10.1128/aem.60.5.1684-1686.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM, Kaper JB, Herrington D, Losonsky G, Morris JG, Clements ML. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect. Immun. 1988;56:161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo MM, Bonato B, Tafi MC, Signoretto C, Pruzzo C, Canepari P. Molecular vs culture methods for the detection of bacterial faecal indicators in groundwater for human use. Lett. Appl. Microbiol. 2005;40:289–294. doi: 10.1111/j.1472-765X.2005.01666.x. [DOI] [PubMed] [Google Scholar]
- Mizunoe Y, Wai SN, Ishikawa T, Takade A, Yoshida S. Resuscitation of viable but nonculturable cells of Vibrio parahaemolyticus induced at low temperature under starvation. FEMS Microbiol. Lett. 2000;186:115–120. doi: 10.1111/j.1574-6968.2000.tb09091.x. [DOI] [PubMed] [Google Scholar]
- Morita M, Ohnishi M, Arakawa E, Bhuiyan NA, Nursin S, Alam M. Development and validation of a mismatch amplification mutation PCR assay to monitor the dissemination of an emerging variant of Vibrio cholerae O1 biotype El Tor. Microbiol. Immunol. 2008;52:314–317. doi: 10.1111/j.1348-0421.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- Nair GB, Ramamurthy T, Bhattaleftya MK, Krishnan T, Ganguly S, Saha DR. Emerging trends in the etiology of enteric pathogens as evidenced from an active surveillance of hospitalized diarrhoeal patients in Kolkata, India. Gut Pathog. 2010;2:4. doi: 10.1186/1757-4749-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi B, Nandy R, Mukhopadhyay S, Nair GB, Shimada T, Ghose AC. Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein OmpW. J. Clin. Microbiol. 2000;38:4145–4151. doi: 10.1128/jcm.38.11.4145-4151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panutdaporn N, Kawamoto K, Asakura H, Makino S. Resuscitation of the viable but non-culturable state of Salmonella enterica serovar Oranienburg by recombinant resuscitation-promoting factor derived from Salmonella Typhimurium strain LT2. Int. J. Food Microbiol. 2006;106:241–247. doi: 10.1016/j.ijfoodmicro.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Reissbrodt R, Rienaecker I, Romanova JM, Freestone PP, Haigh RD, Lyte M. Resuscitation of Salmonella enterica serovar Typhimurium and enterohemorragic Escherichia coli from the viable but nonculturable state by heat-stable enterobacterial autoinducer. Appl. Environ. Microbiol. 2002;68:4788–4794. doi: 10.1128/AEM.68.10.4788-4794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak DB, Colwell RR. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoh M, Ghosh-Banerjee J, Ramamurthy T, Hamabata T, Kurakawa T, Takeda M. Conversion of viable but nonculturable Vibrio cholerae to the culturable state by co-culture with eukaryotic cells. Microbiol. Immunol. 2010;54:502–507. doi: 10.1111/j.1348-0421.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- Senoh M, Ghosh-Banerjee J, Ramamurthy T, Colwell RR, Miyoshi S, Nair GB. Conversion of viable but nonculturable enteric bacteria to culturable by co-culture with eukaryotic cells. Microbiol. Immunol. 2012;56:342–345. doi: 10.1111/j.1348-0421.2012.00440.x. [DOI] [PubMed] [Google Scholar]
- Shirai H, Nishibuchi M, Ramamurthy T, Bhattaleftya SK, Pal SC, Takeda Y. Polymerase chain reaction for detection of the cholera enterotoxin operon of Vibrio cholerae. J. Clin. Microbiol. 1991;29:2517–2521. doi: 10.1128/jcm.29.11.2517-2521.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert M, Emödy L, Amann R, Hacker J. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl. Environ. Microbiol. 1997;63:2047–2053. doi: 10.1128/aem.63.5.2047-2053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai SN, Moriya T, Kondo K, Misumi H, Amako K. Resuscitation of Vibrio cholerae O1 strain TSI-4 from a viable but nonculturable state by heat shock. FEMS Microbiol. Lett. 1996;136:187–191. doi: 10.1111/j.1574-6968.1996.tb08047.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Cholera 2011. Wkly Epidemiol. Rec. 2012;87:289–304. [Google Scholar]
- Xu HS, Roberts N, Singleton FL, Attwell RW, Grimes DJ, Colwell RR. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 1982;8:313–323. doi: 10.1007/BF02010671. [DOI] [PubMed] [Google Scholar]
- Zimmerman AM, Rebarchik DM, Flowers AR, Williams JL, Grimes DJ. Escherichia coli detection using mTEC agar and fluorescent antibody direct viable counting on coastal recreational water samples. Lett. Appl. Microbiol. 2009;49:478–483. doi: 10.1111/j.1472-765X.2009.02693.x. [DOI] [PubMed] [Google Scholar]


