Abstract
Diseases are an emerging threat to ocean ecosystems. Coral reefs, in particular, are experiencing a worldwide decline because of disease and bleaching, which have been exacerbated by rising seawater temperatures. Yet, the ecological mechanisms behind most coral diseases remain unidentified. Here, we demonstrate that a coral pathogen, Vibrio coralliilyticus, uses chemotaxis and chemokinesis to target the mucus of its coral host, Pocillopora damicornis. A primary driver of this response is the host metabolite dimethylsulfoniopropionate (DMSP), a key element in the global sulfur cycle and a potent foraging cue throughout the marine food web. Coral mucus is rich in DMSP, and we found that DMSP alone elicits chemotactic responses of comparable intensity to whole mucus. Furthermore, in heat-stressed coral fragments, DMSP concentrations increased fivefold and the pathogen's chemotactic response was correspondingly enhanced. Intriguingly, despite being a rich source of carbon and sulfur, DMSP is not metabolized by the pathogen, suggesting that it is used purely as an infochemical for host location. These results reveal a new role for DMSP in coral disease, demonstrate the importance of chemical signaling and swimming behavior in the recruitment of pathogens to corals and highlight the impact of increased seawater temperatures on disease pathways.
Keywords: Vibrio, microfluidics, chemotaxis, DMSP, chemical signaling, motility
Introduction
The globally distributed marine bacterium Vibrio coralliilyticus (Pollock et al., 2010) causes bleaching and tissue loss in reef-building corals (Ben-Haim et al., 2003). Despite the widespread loss of corals to diseases (Harvell et al., 2009), little is known about their onset, and fundamental questions, such as how a pathogen finds its host, have remained largely unanswered (Bourne et al., 2009). Among human enteric pathogens, the ability to swim (motility) and guide movement in response to chemical gradients (chemotaxis) is a common phenotype in the infection process (Boin et al., 2004; Croxen et al., 2006). In the ocean, we found that motility is universal among putative coral pathogens (Supplementary Table S1). This prevalence, together with the presence of strong chemical gradients that can extend over 2 mm from the coral surface (Kühl et al., 1995; Mass et al., 2010), suggests that motile responses to chemical cues may be a pervasive mechanism for coral pathogens to locate and colonize their hosts. Yet, beyond evidence that motility and chemotaxis are involved in Vibrio-induced bleaching (Banin et al., 2001; Meron et al., 2009), there has been no direct, real-time observation of the motile behavior of pathogens, nor any insight into the specific chemical triggers of chemotaxis or its dependence on the host's physiological state. By integrating microfluidic experiments with the collection of coral exudates, we found that V. coralliilyticus (Pollock et al., 2010) markedly changes its motility behavior in response to the mucus of its host, Pocillopora damicornis, to rapidly target the source of the cue.
The surface of a coral is lined with mucus of variable viscosity, which is continuously excreted for cleansing, feeding and defense (Brown and Bythell, 2005). This mucus contains a broad range of chemicals, including water-soluble glycoproteins, amino acids and metabolites (Brown and Bythell, 2005). In the mucus of many coral species, the sulfur compound dimethylsulfoniopropionate (DMSP) reaches concentrations (1–62 μM) orders of a magnitude higher than in the surrounding seawater (6–11 nM) (Broadbent and Jones, 2004; Van Alstyne et al., 2006). For corals, this molecule might act as an antioxidant (Sunda et al., 2002) or as an overflow system for the symbiotic zooxanthellae to excrete excess sulfur (Stefels, 2000). DMSP has also been shown to be a potent chemoattractant for several marine micro- and macro-organisms (Debose and Nevitt, 2007; Seymour et al., 2010). Here, we show that DMSP is a primary chemical cue for V. coralliilyticus' behavioral responses to the mucus of P. damicornis and that its increased production under heat stress enhances the attraction of the pathogen.
Materials and methods
Organism growth conditions and laboratory mucus collection
All experiments were conducted using V. coralliilyticus, strain BAA-450, acquired from the American Type Culture Collection (www.atcc.org, Manassas, VA, USA) and grown in 0.2 μM filtered, autoclaved seawater (FASW) with 1% 2216 media (BD Difco) in a shaking incubator at 30°C. Small colonies of the coral P. damicornis (from the Birch Aquarium at Scripps, La Jolla, CA, USA) were cultured at 25 °C in artificial seawater (Instant Ocean, Spectrum Brands Company, Cincinnati, OH, USA) on a 12-h light–dark cycle. Mucus was collected from the colonies by exposing them to air for 3 min. Owing to volume requirements of the microfluidic assays, the mucus was then diluted to 1:2 in FASW and vortexed for 10 s to mix thoroughly.
Mucus collection on Davies Reef (Great Barrier Reef)
Small colonies of the coral P. damicornis and Acropora millepora were collected from Davies Reef, Great Barrier Reef, Australia (18°05′ S/147°39′ E) and transferred to the outdoor aquarium facility of the Australian Institute of Marine Science (Townsville, QLD, Australia). Mucus was collected from the colonies by removing them from the water, shaking off excess water for 10 s and then holding them upside down collecting dripping mucus with a syringe. Freshly collected mucus was then homogenized and divided in two: one half was flash-frozen in liquid nitrogen; the second half was directly extracted with 40 ml of high-performance liquid chromatography-grade methanol for DMSP quantification. The frozen portion was later used in chemotaxis assays.
Metabolism and DMSP measurements
DMSP metabolism
Two different basal media were used to determine the DMSP metabolic capabilities of V. coralliilyticus: a modified marine ammonium salt medium (Raina et al., 2009) lacking any carbon source, and a modified basal salt medium lacking any sulfur source (Fuse et al., 2000). DMSP was added to both the media (1 mM final concentration) and acted either as a sole carbon source or as a sole sulfur source. The pH was adjusted to 8.2. To account for the potential cometabolism of DMSP with other compounds present in coral mucus, mucus was collected as described above, homogenized, filtered twice (0.2 μm) and sonicated for 10 min. Five milliliters of marine ammonium salt medium, modified basal salt medium or sterile mucus were inoculated in triplicate from single V. coralliilyticus colonies and incubated at 28 °C between 1 and 6 days with shaking in gas-tight vials. Control bottles containing only the basal media and DMSP were used to account for the possible chemical breakdown of DMSP. Results from these experiments were confirmed using an alternative V. coralliilyticus strain, LMG 23696 (Sussman et al., 2008). Bottles inoculated with the Pseudovibrio sp. P12 (an alphaproteobacterium isolated from healthy P. damicornis) grown under identical conditions acted as the positive control.
Acrylate metabolism
Marine ammonium salt medium lacking carbon was used to investigate the ability of strain BAA-450 to degrade acrylate (1 mM, final concentration). Five milliliters of marine ammonium salt medium were inoculated in triplicate from single BAA-450 colonies and incubated at 28 °C between 1 and 6 days with shaking. Control bottles containing only the basal medium and acrylate were set up, along with the ones inoculated with BAA-450, to account for its possible chemical breakdown. Bottles inoculated with the Pseudovibrio sp. P12 grown under identical conditions served as a positive control.
NMR measurements
DMSP metabolism assays and DMSP quantification were performed by 1H nuclear magnetic resonance (NMR) (Tapiolas et al., 2013). Briefly, the headspace of each gas-tight vial was first sampled with a syringe. Methanol (40 ml) was then added to each culture tube to extract DMSP and acrylate, and the mixtures were subsequently dried in vacuo using a rotary evaporator (Buchi, Flawil, Switzerland). The dried extracts were resuspended in a mixture of deuterated methanol (CD3OD, D 99.8%, 750 μl) and deuterium oxide (D2O, D 99.8%, 250 μl) (Cambridge Isotope Laboratories, Andover, MA, USA). A 750-μl aliquot of the particulate-free extract was transferred into a 5-mm Norell tube (Norell Inc., Landisville, NJ, USA) and analyzed immediately by 1H NMR. Spectra were recorded on a Bruker Avance 600 MHz NMR spectrometer (Billerica, MA, USA) with a TXI 5 mm probe and quantification was performed using the Electronic REference To access In vivo Concentrations method (ERETIC) (Tapiolas et al., 2013). No DMSP degradation, acrylate by-products or dimethylsulfide smell were present in the DMSP medium experiments for V. coralliilyticus or the negative control, whereas all were present in the Pseudovibrio positive control. In the acrylate medium experiments, acrylate was degraded by both V. coralliilyticus and the Pseudovibrio positive control, but not the no-bacteria negative control. The same NMR protocol was also used to quantify the amount of DMSP present in coral mucus from P. damicornis and A. millepora.
Chemotactic index (IC)
We quantified the magnitude of the chemotactic response using the chemotactic index, IC, which measures the enhancement in the cell concentration within the region initially occupied by the mucus (that is, the central band in Supplementary Figure S1), relative to the cell concentration outside that area, minus 1. IC=0 thus corresponds to a uniform cell distribution (that is, no chemotaxis). See Seymour et al. (2010) for more details. For each experiment, triplicate 0.2 μm FASW control trials were run first, wherein the same FASW used to grow the cells and to make the DMSP dilutions was injected into the microfluidic device in lieu of an attractant. All IC curves for a given attractant were normalized to their FASW control by subtracting the mean IC among the three FASW trials.
To compare the IC values observed in this study with values observed by Stocker et al. (2008) for Escherichia coli and Pseudoalteromonas haloplanktis in a similar (but not identical) experimental setup, data were extrapolated from Figure 2b of that manuscript and converted from the hot spot index (H) to IC. The hotspot index was defined by Stocker et al. as the mean concentration of bacteria within the central, WC=300 μm wide region of the microchannel relative to the mean concentration over the entire channel width, W=1200 μm. The data were converted to IC using the following conversion formula: IC=((W−WC)/((W/H)−WC))−1.
Diffusive gradient microfluidic experiments
Microinjector device for chemotaxis assays
A 2.8-mm wide microchannel with a 400-μm wide injector (Supplementary Figure S1) was fabricated using soft lithography techniques described previously (Seymour et al., 2008) to establish diffusive gradients for chemotaxis assays. Briefly, the attractant was injected into the microchannel (Supplementary Figure S1; inlet B) as a 400-μm wide band equidistant from the channel's side walls, whereas the cells were injected in the channel on either side of the band (Supplementary Figure S1; inlet A). The cells and attractant were flowed into the channel and then the flow was stopped to allow the attractant to diffuse laterally and the cells to respond to the gradient. DMSP (DMSP·HCl; C5H10SO2·HCl; TCI) was freshly prepared with FASW to make 15 μM, 45 μM and 61 μM working solutions that closely corresponded to the amount of DMSP measured in the P. damicornis and A. millepora mucus samples. A. millepora was chosen as a second species to test because V. coralliilyticus is known to infect it as well (Sussman et al., 2009). These freshly prepared DMSP solutions as well as P. damicornis mucus collected from Davies Reef (Great Barrier Reef; preserved at −80 °C (as described in the mucus collection section) and thawed on ice directly before experimental use; measured to contain 12–15 μM DMSP) and from corals maintained in the laboratory at MIT, A. millepora mucus from the Great Barrier Reef (containing 45–62 μM DMSP) and a FASW control were tested against overnight cultures of V. coralliilyticus.
The channel was loaded at moderate flow rates (2 μl min−1) to establish an initial experimental condition where the cells and the attractant were in discrete bands (Supplementary Figure S1B). To begin the experiment, the flow was stopped and the channel was imaged directly downstream of the end of the microinjector using phase-contrast video microscopy on a Nikon Ti microscope (Tokyo, Japan) equipped with an Andor Neo CCD camera (6.5 μm/pixel; Belfast, UK) at 1 frame per second for 6 min. Five replicates of each experiment were conducted, and the microchannel was flushed for 30 s with fresh cells and attractant between replicates. Flushing with FASW lasted for 2 min in between different attractants. Each video was analyzed for cell positions using an automated image segmentation software developed in-house with MATLAB (MathWorks, Natick, MA, USA). Background subtraction and cross-correlation functions were used to detect non-motile cells or other particles from the mucus, which were excluded from the cumulative cell distribution across the channel. The resulting time series of cell distributions are presented for P. damicornis (Figure 1) and for A. millepora (Supplementary Figure S2).
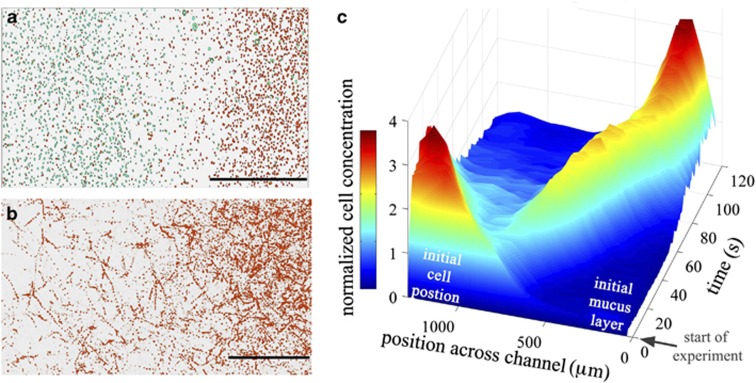
Figure 1.
V. coralliilyticus is strongly attracted to coral mucus. (a) Positions and (b) trajectories of individual V. coralliilyticus cells exposed to a diffusing coral mucus gradient in a microfluidic channel (Supplementary Figure S1). A 400-μm thick layer of mucus, harvested from laboratory-cultured P. damicornis corals, was created in a microchannel (half of the layer is shown) and allowed to diffuse. The scale bars are 200 μm. In (a), cell positions at the start of the experiment and after two minutes are colored teal and red, respectively, and overlaid. In (b), trajectories acquired between 100 and 115 s after the start of the experiment are shown. The two panels show the strong shift in the cells' position and their intense accumulation into the mucus layer (the right side of the images). Also see Supplementary Movie S1. (c) The full time series of the spatial distribution of the pathogen population across the width of the microfluidic channel. Color and height both measure the local, instantaneous concentration of bacteria, normalized to a mean of one. Note the intense wave of bacteria actively migrating into the mucus layer.
Temperature stress experiment on Heron Island
To test the response of V. coralliilyticus to mucus from corals under high-temperature stress, a field experiment was carried out on Heron Island, Great Barrier Reef, Australia (23°26′ 37′′S/151°54′ 44′′E). Three colonies of P. damicornis were collected from the reef flat in front of the Heron Island Research Station, fragmented into 48 nubbins and allowed to recover and acclimate in a flow-through seawater tank pulling water from the reef flat for 8 days. Fragments were then distributed evenly into six tanks with three fragments from each donor colony in each tank. A randomized sample design for both fragment placement within the tanks and treatment assignment to each tank was employed. Three tanks were maintained at ambient seawater temperature (22 °C) for the duration of the experiment, and the other three tanks began at ambient temperature and then were slowly ramped by 1.5 °C per day for 7 days. All fragments were sampled for mucus by air exposure as described above at the initial time and after 7 days, when the temperature-treated tanks reached 31 °C. One-third of the mucus samples were preserved for DMSP measurements (described above) by adding 600 μl of methanol and freezing at −20 °C. Clonal replication is essential for comparing responses because DMSP concentration can vary with irradiance, zooxanthellae density and seawater temperature (Sunda et al., 2002; Van Alstyne et al., 2006). The rest of the samples were immediately frozen at −80 °C unaltered and shipped to MIT, where they were used in microfluidic chemotaxis experiments with the microinjector setup (Supplementary Figure S1). Replicate mucus samples from the heat-stress experiment were tested on three different days in the lab with freshly grown V. coralliilyticus cells. All trials yielded comparable results to those shown in the main text (Figure 2b; Supplementary Figure S6).
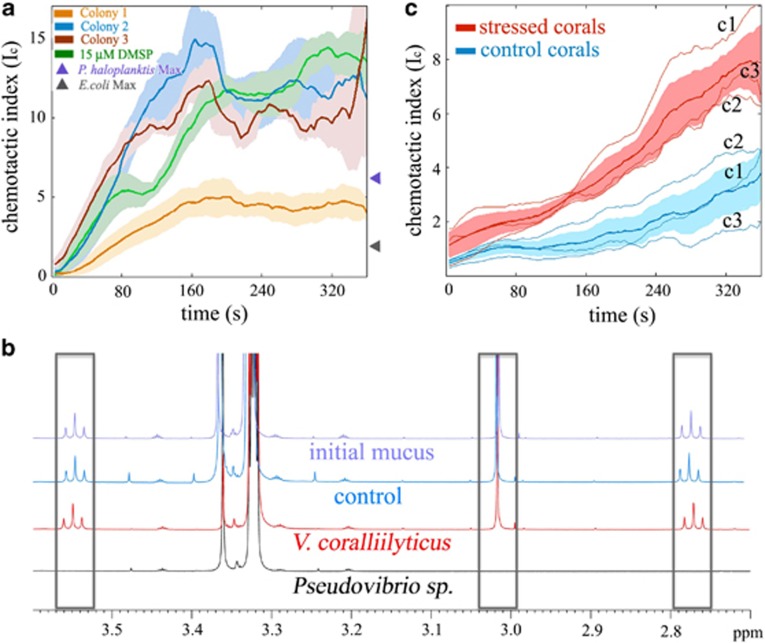
Figure 2.
The pathogen's chemotaxis is primarily triggered by DMSP and is enhanced by heat stress of the host. (a) Time series of the chemotactic index, IC, (a measure of the strength of cell accumulation) of V. coralliilyticus in response to a 400-μm thick layer (Supplementary Figure S1) of coral mucus (Colonies 1–3) or 15 μM DMSP (green line). Solid lines and shading represent the mean and s.e. of three replicate experiments. Mucus was collected from three different colonies of P. damicornis on Heron Island and contained 11.9–14.8 μM DMSP (Supplementary Information). The pathogen responds with comparable intensity to DMSP and mucus. Shown for reference are also the maximum chemotactic indices (suitably converted from Stocker et al. (2008) attained over 15 min by E. coli responding to a mixture of two of its most potent chemoattractants at near-optimal concentrations (serine and aspartate, 10 μM each; gray triangle) and by P. haloplanktis responding to algal exudates (purple triangle). All data were normalized against the respective no-attractant controls. (b) Profiles from quantitative NMR of freshly collected P. damicornis mucus from Heron Island (initial mucus, purple) reveal distinct peaks for DMSP (gray boxes). Twenty-four hours of incubation of whole mucus with V. coralliilyticus (red) resulted in no measurable DMSP degradation, akin to the no-bacteria control (blue), whereas the positive control strain Pseudovibrio spp. degraded DMSP entirely, as evidenced by the disappearance of the DMSP peaks (black). (c) Time series of V. coralliilyticus' IC, in response to coral mucus from a clonally replicated temperature-stress experiment (maximum=31 °C) performed on Heron Island. Chemotaxis was twice as strong toward mucus from stressed coral fragments (red) as compared with fragments from the same colonies maintained at ambient temperature (blue). Thin lines show each of the three individual colonies (c1–c3), bold lines show their mean and shading represents the s.e. All curves were normalized against seawater controls.
Mathematical model of simultaneous chemotaxis and chemokinesis
We modeled the chemotaxis and chemokinesis of V. coralliilyticus using an existing modeling framework for bacterial chemotaxis (Brown and Berg, 1974; Jackson, 1987; Kiørboe and Jackson, 2001), augmented by a concentration-dependent swimming speed that was based on our experimental observations (Figure 3a; Supplementary Information; Supplementary Figures S4 and S9). For simplicity and based on results from Figure 3a, we modeled chemokinesis as a 24% increase in swimming speed, from 66 μm s−1 at a relative chemoattractant concentrations of C ⩽20% of pure attractant to 82 μm s−1 for C >20%. From the spatial distribution of 3000 cells across the channel at each time point, we computed the time series of IC (as detailed in the chemotactic index section of Materials and Methods). This was done twice: once in the presence of chemokinesis, and once in the absence of chemokinesis, in which case the swimming speed was uniformly equal to 66 μm s−1. Results are presented in Figure 3b.
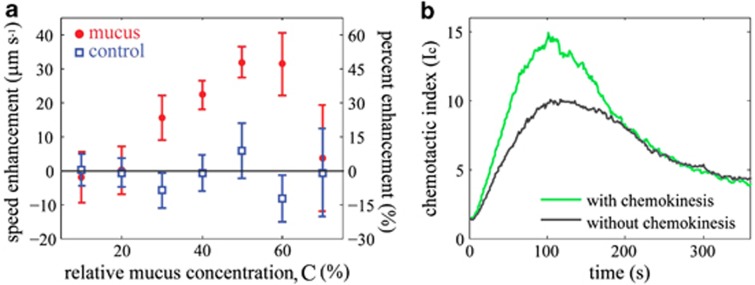
Figure 3.
V. coralliilyticus exhibits chemokinesis that markedly increases the strength and speed of its response to coral mucus. (a) Swimming speed enhancement as a function of the mucus concentration instantaneously experienced by cells (Supplementary Figure S3). The enhancement is relative to the mean swimming speed in the absence of mucus (66 μm s−1) and is expressed as both a speed difference (left axis) and a percent difference (right axis). Mucus concentrations, predicted from solution of the diffusion equation (Supplementary Information), are expressed relative to full mucus (i.e., the initial mucus concentration in the microchannel). Error bars represent the s.e. (b) A mathematical model of bacterial motility (Supplementary Information) shows that IC,MAX is 50% higher when chemokinesis in included (green) compared with the case of chemotaxis alone (gray), and that IC,MAX without chemokinesis is reached in <50% of the time when chemokinesis is present. Chemokinesis was modeled as a 24% enhancement of the mean swimming speed in regions with mucus concentrations greater than 20% of full mucus, based on the observed speed–concentration relationship (panel a).
Results and discussion
To examine the ecological mechanism behind coral infection by V. coralliilyticus, we performed chemotaxis experiments using a microfluidic assay. V. coralliilyticus responded to coral mucus with remarkable speed and directionality. A microfluidic device was used to create a 400-μm thick layer of mucus adjacent to a 1-mm thick seawater suspension of V. coralliilyticus, and we imaged the spatial distribution of the pathogen population with high-temporal-resolution (15 frames s−1) video microscopy (Figure 1; Supplementary Figure S1). Within 10 s of exposure to the mucus, bacteria began swimming up the associated chemical gradient. Within 60 s, >50% of cells had migrated into the 400-μm thick layer of mucus (Figure 1; Supplementary Movie S1). We quantified the magnitude of the pathogen's chemotactic response with the chemotactic index, IC (Seymour et al., 2010), which measures the enhancement in cell concentration within the initial mucus layer relative to the cell concentration outside that layer (IC=0 corresponds to no chemotaxis). V. coralliilyticus reached IC >14 within 3 min of being exposed to mucus (Figure 2a), a much more intense response than previously observed for either enteric or marine bacteria (Figure 2a). The strength of this response was confirmed by tracking individual bacteria and quantifying their mean chemotactic velocity (Supplementary Figures S3–S5), which reached 36% of the average swimming speed, considerably higher than the 5–15% typical of the model organism for bacterial chemotaxis, E. coli (Ahmed and Stocker, 2008).
To determine the chemical signal responsible for this response, we analyzed coral mucus using quantitative NMR (Tapiolas et al., 2013). The DMSP concentrations in mucus from healthy colonies collected on Davies Reef, were high, ranging from 11.9–14.8 (±1.2) μM for P. damicornis and up to 62.2 (±2.0) μM for A. millepora, another coral species susceptible to V. coralliilyticus infection (Sussman et al., 2009). Additional chemotaxis experiments revealed that DMSP (15 μM), when used as the sole attractant, elicited a chemotactic response of comparable magnitude to P. damicornis mucus (IC,MAX∼14; Figure 2a). The pathogen's response varied somewhat from colony to colony, as is expected owing to the natural variability in mucus composition among colonies. This variability notwithstanding, all responses observed with DMSP at comparable concentrations to those found in coral mucus were consistent with it being a primary driver of the chemotaxis. In addition, all responses observed were substantially faster and stronger than previously observed chemotactic responses of the enteric bacterium E. coli to its strongest chemoattractants and of the marine bacterium P. haloplanktis to algal exudates (Figure 2a). The same was true for A. millepora mucus when chemotactic responses to DMSP alone in mucus-equivalent concentrations were compared with responses to whole mucus (Supplementary Figure S2). These results demonstrate that DMSP within coral mucus is a major driver of the pathogen's behavior.
Chemotaxis by marine bacteria toward DMSP has previously been ascribed to DMSP's value as a rich carbon and sulfur source, as bacteria may obtain up to 15% of their carbon and most of their sulfur from DMSP (Zubkov et al., 2001). Intriguingly, despite its vigorous swimming response toward DMSP, V. coralliilyticus does not detectably metabolize the compound. NMR analysis following a 6-day incubation of the pathogen in minimal media, where DMSP was either the sole carbon or the sole sulfur source, showed no degradation of DMSP by V. coralliilyticus (Supplementary Figure S7). The bacterium similarly did not degrade DMSP within whole-coral mucus (Figure 2b), confirming that it cannot cometabolize DMSP with other mucus-derived molecules. V. coralliilyticus' inability to metabolize DMSP, combined with its lack of genes homologous to any known DMSP degradation gene (Supplementary Table S2), indicates that DMSP is used by this pathogen purely as an infochemical, a function that DMSP and its derivatives also serve among pelagic reef fish (Debose and Nevitt, 2007) and marine protists (Seymour et al., 2010; Garcés et al., 2013). By demonstrating that the origin of the DMSP cue for V. coralliilyticus is its host, these results provide the first evidence that DMSP has a signaling role in the onset of a bacterial disease by aiding in the detection of a suitable host.
The importance of DMSP as a signaling cue, together with evidence that stressed corals are more susceptible to bacterial disease (Bruno et al., 2007), prompted us to examine whether the mucus of stressed corals contains more DMSP and elicits stronger responses in V. coralliilyticus compared with unstressed controls. We performed a clonally replicated high-temperature stress experiment (temperature raised 1.5 °C/day over 6 days) on Heron Island. Analysis by quantitative NMR demonstrated a fivefold increase in DMSP concentration in the mucus of heat-stressed P. damicornis fragments (31 °C) compared with control fragments from the same colonies (maintained at 22 °C). No other molecule was substantially enriched in the stressed coral mucus compared with the controls, with the exception of DMSP's main degradation product, dimethylsulfide, toward which V. coralliilyticus chemotaxes only very weakly (Supplementary Figure S8). This result shows that DMSP may therefore be a strong cue for V. coralliilyticus to detect stressed or susceptible coral hosts. This latter hypothesis was supported by further chemotaxis experiments, which yielded a twofold higher IC in response to mucus from stressed fragments (IC,MAX=8) relative to mucus from control fragments (IC,MAX=4) (Figure 2c; Supplementary Figure S6). The stress-induced enhancement of host-released cues and the simultaneous intensification of the pathogen's response strongly suggest that chemical signaling and active behavior represent important components in the pathway to disease. Furthermore, the widespread increase in susceptibility to bacterial infection of stressed animals (Mydlarz et al., 2006; Verbrugghe et al., 2012) suggests that chemical interactions such as these may be a recurring element in other marine diseases.
The behavioral response of V. coralliilyticus to coral mucus and DMSP is not limited to chemotaxis, but includes a second, powerful behavioral adaptation: chemokinesis. Whereas chemotaxis is the ability to bias swimming direction in response to a chemical gradient, chemokinesis is the ability to change swimming speed in response to a change in chemical concentration. By analyzing trajectories of individual swimming cells (Supplementary Figure S3), we found that V. coralliilyticus exhibits a strong chemokinetic response to coral mucus (Supplementary Figures S4 and S9). V. coralliilyticus increases its mean swimming speed by 24% (from 66 μm s−1 to 82 μm s−1) in regions with C >20% (where C is the local mucus concentration as a percentage of full mucus) and by up to 48% (98 μm s−1) where C≈60% (Figure 3a). At even higher concentrations, cells slowed down (70 μm s−1) possibly to retain their position near the source.
The rare ability of V. coralliilyticus to simultaneously employ chemotaxis and chemokinesis represents a powerful adaptation for responding to the chemical signals emanating from its coral host. Chemokinesis alone would result in the dispersion of cells away from regions of high chemical concentration because faster swimming cells have higher dispersal rates (Schnitzer, 1990). However, a mathematical model (Supplementary Information) reveals that, when paired with the ability to sense gradients, chemokinesis can substantially increase the chemotactic velocity and therefore reduce the time required to traverse a chemical gradient. A modest concentration-dependent increase in swimming speed (24%) nearly halved the timescale of the response to mucus (55 s vs 105 s to reach IC=10) and increased the peak response intensity by 50% (IC,MAX of 15 vs 10; Figure 3b; Supplementary Information). Although chemokinesis has been observed in a range of bacteria (Barbara and Mitchell, 2003; Seymour et al., 2010), neither its disproportionate contribution to a bacterium's ability to climb chemical gradients nor its potential importance in an infection process have been previously reported. This observation indicates that chemokinesis is a powerful behavioral adaptation in V. coralliilyticus's response to host-derived chemical signals and that bacterial infection of corals may be driven by considerably more specific adaptations than a binary presence or absence of motility (Meron et al., 2009).
If DMSP represents a steady signal that seemingly provides ample time to locate the coral surface, why does V. coralliilyticus exhibit multiple, energy-intensive (Taylor and Stocker, 2012) motility adaptations of such magnitude? We hypothesize that the reason lies in the highly dynamic environment at the coral surface, where resident microbes contend for the best niches (Ritchie, 2006), mucus is periodically shed (Garren and Azam, 2012), surface cilia deter the attachment of fouling organisms (Wahl et al., 1998) and external flows on the order of millimeters per second sweep over the colony (Lesser et al., 1994). The residence time next to the surface is likely to be short, offering the pathogen only limited windows of opportunity for colonization of the host. In this complex environment, the ability to swim with high directionality (chemotaxis) and accelerate when accelerate when heightened concentrations are detected (chemokinesis) could aid V. coralliilyticus substantially in reaching the coral mucus.
Once a cell is within the mucus layer, it is only a short distance away from the coral tissue, and mucus itself is unlikely to slow its progress toward the tissue. This is supported by mathematical models (Spagnolie et al., 2013) that predict that even when the medium is viscoelastic and has twice the viscosity of seawater, a bacterium with a single polar flagellum, such as V. coralliilyticus, will actually experience a minor increase (<5%) in swimming speed. The mucus layer is furthermore often very thin (a few hundred micrometers; Jatkar et al., 2009), including for P. damicornis (Garren and Azam, 2012), requiring only seconds to traverse at typical swimming speeds. Finally, our experiments on chemokinesis, conducted with a 333 μm thick layer of whole mucus, showed no evidence for any decrease in motility. Rather these experiments demonstrated an up to 48% increase in speed at intermediate mucus concentrations. As a result, we expect that bacterial pathogens will have no physical difficulty penetrating the mucus layer.
Our findings reveal a previously unrecognized role of DMSP in the pathway to coral disease. The discovery that DMSP is involved in a pathogen's response to its host broadens the diversity of roles that this molecule has in the ocean (Kirst, 1989; Kirst et al., 1991; Stefels, 2000; Sunda et al., 2002; Debose and Nevitt, 2007; Simó et al., 2009; Seymour et al., 2010) to include that of a kairomone (a chemical that benefits the receiving but not the producing organism) and, together with the recent observation of a marine parasitoid using dimethylsulfide to locate its dinoflagellate host (Garcés et al., 2013), suggests that sulfur compounds may be involved in host recognition in a broader class of marine infections. The surprising observation that V. coralliilyticus was unable to degrade DMSP, which is otherwise a rich carbon and sulfur source for bacteria (Zubkov et al., 2001), suggests that the pathogen either has an unknown pathway for utilizing DMSP at exceedingly slow rates or it uses DMSP solely as a strong infochemical—our metabolic analyses point toward the latter explanation. The finding that hosts under heat stress exude mucus that is richer in DMSP and triggers heightened pathogen responses indicates that V. coralliilyticus may use this chemical cue to target stressed hosts. This emphasizes the risks posed to corals by warming waters and contributes to understanding the mechanisms underlying disease outbreaks associated with increasing seawater temperatures. Taken together, these observations unveil a strong role of microscale chemical ecology and microbial behavior in coral disease (Figure 4), emphasizing that the mechanistic drivers of many ecosystem processes may best be understood at the microscale level.
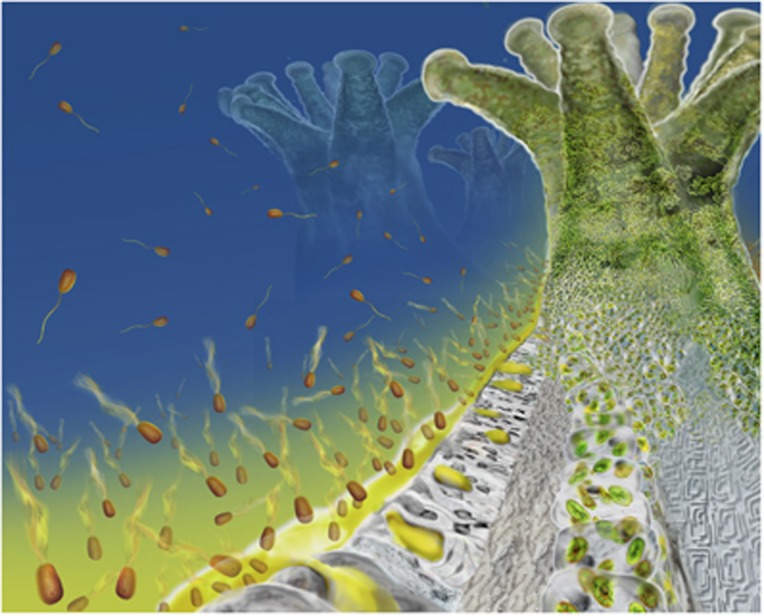
Figure 4.
A new model for host detection by coral pathogens. The coral surface represents an intense source of molecules, such as DMSP, that diffuse (yellow gradient) away from the surface and out into the surrounding water, thereby establishing chemical gradients that motile bacterial pathogens (not to scale) can use to navigate toward their host. The striking prevalence of motility among putative coral pathogens (Supplementary Table S1), together with the strength of the chemical signals at the coral surface relative to typical signals in the water column, indicate that the advanced motility adaptations described here could be a widespread phenotype associated with coral disease.
Acknowledgments
We thank JS Guasto, C Motti, F Nosratpour, PJ Ralph, T Santiano-McHatton and the Birch Aquarium at Scripps for assistance. Credit for Figure 4: G Gorick, M Garren and R Stocker. This work was supported by the Human Frontiers in Science Program award no. RGY0089 to RS and JRS to a Gordon and Betty Moore Foundation Investigator Grant to RS, by the Australian Research Council Grant DP110103091 to JRS, by a Samsung Scholarship to KS, by funds through the marine microbiology program at AIMS to DGB, by the post-graduate award from the Department of Environmental Science and Climate Change Cluster at the University of Technology Sydney and the Australian Coral Reef Society Terry Walker award 2012 to JT and by NSF awards OCE-0744641-CAREER, CBET-1066566 and CBET-0966000 to RS. We are grateful to the Great Barrier Reef Marine Park Authority for coral collection permits G09/31733.1 (PJ Ralph, University of Technology Sydney) and G12/35236.1 (Australian Institute of Marine Science).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Ahmed T, Stocker R. Experimental verification of the behavioral foundation of bacterial transport parameters using microfluidics. Biophys J. 2008;95:4481–4493. doi: 10.1529/biophysj.108.134510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin E, Israely T, Fine M, Loya Y, Rosenberg E. Role of endosymbiotic zooxanthellae and coral mucus in the adhesion of the coral-bleaching pathogen Vibrio shiloi to its host. FEMS Microbiol Lett. 2001;199:33–37. doi: 10.1111/j.1574-6968.2001.tb10647.x. [DOI] [PubMed] [Google Scholar]
- Barbara GM, Mitchell JG. Marine bacterial organisation around point-like sources of amino acids. FEMS Microbiol Ecol. 2003;43:99–109. doi: 10.1111/j.1574-6941.2003.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Ben-Haim Y, Zicherman-Keren M, Rosenberg E. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl Environ Microbiol. 2003;69:4236–4242. doi: 10.1128/AEM.69.7.4236-4242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boin MA, Austin MJ, Hase CC. Chemotaxis in Vibrio cholerae. FEMS Microbiol Lett. 2004;239:1–8. doi: 10.1016/j.femsle.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Bourne DG, Garren M, Work TM, Rosenberg E, Smith GW, Harvell CD. Microbial disease and the coral holobiont. Trends Microbiol. 2009;17:554–562. doi: 10.1016/j.tim.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Broadbent AD, Jones GB. DIMS and DMSP in mucus ropes, coral mucus, surface films and sediment pore waters from coral reefs in the Great Barrier Reef. Marine Freshwater Res. 2004;55:849–855. [Google Scholar]
- Brown BE, Bythell JC. Perspectives on mucus secretion in reef corals. Marine Ecol-Prog Ser. 2005;296:291–309. [Google Scholar]
- Brown DA, Berg HC. Temporal simulation of chemotaxis in Escherichia coli. Proc Natl Acad Sci USA. 1974;71:1388–1392. doi: 10.1073/pnas.71.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, Harvell CD, et al. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 2007;5:1220–1227. doi: 10.1371/journal.pbio.0050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxen MA, Sisson G, Melano R, Hoffman PS. The Helicobacter pylori chemotaxis receptor TlpB (HP0103) is required for pH taxis and for colonization of the gastric mucosa. J Bacteriol. 2006;188:2656–2665. doi: 10.1128/JB.188.7.2656-2665.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debose JL, Nevitt GA. Investigating the association between pelagic fish and dimethylsulfoniopropionate in a natural coral reef system. Marine Freshwater Res. 2007;58:720–724. [Google Scholar]
- Fuse H, Takimura O, Murakami K, Yamaoka Y, Omori T. Utilization of dimethyl sulfide as a sulfur source with the aid of light by Marinobacterium sp. strain DMS-S1. Appl Environ Microbiol. 2000;66:5527–5532. doi: 10.1128/aem.66.12.5527-5532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcés E, Alacid E, Rene A, Petrou K, Simó R. Host-released dimethylsulphide activates the dinoflagellate parasitoid Parvilucifera sinerae. ISME J. 2013;7:1065–1068. doi: 10.1038/ismej.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garren M, Azam F. Corals shed bacteria as a potential mechanism of resilience to organic matter enrichment. ISME J. 2012;6:1159–1165. doi: 10.1038/ismej.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvell D, Altizer S, Cattadori IM, Harrington L, Weil E. Climate change and wildlife diseases: when does the host matter the most. Ecology. 2009;90:912–920. doi: 10.1890/08-0616.1. [DOI] [PubMed] [Google Scholar]
- Jackson GA. Simulating chemosensory response of marine microorganisms. Limnol Oceanogr. 1987;32:1253–1266. [Google Scholar]
- Jatkar AA, Brown BE, Bythell JC, Guppy R, Morris NJ, Pearson JP. Measuring mucus thickness in reef corals using a technique devised for vertebrate applications. Marine Biol. 2009;2:261–267. [Google Scholar]
- Kiørboe T, Jackson GA. Marine snow, organic solute plumes, and optimal chemosensory behavior of bacteria. Limnol Oceanogr. 2001;46:1309–1318. [Google Scholar]
- Kirst G. Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:21–53. [Google Scholar]
- Kirst GO, Thiel C, Woll TH, Nothnagel J, Wanzek M, Ulmke R. Dimethylsulfoniopropionate (DMSP) in ice algae and its possible biological role. Marine Chem. 1991;35:381–388. [Google Scholar]
- Kühl M, Cohen Y, Dalsgaard T, Jørgensen BB, Revsbech NP. Microenvironment and photosynthesis of zooxanthellae in sceractinian corals studied with microsensors for O2, pH, and light. Marine Ecol-Prog Ser. 1995;117:159–172. [Google Scholar]
- Lesser MP, Weis VM, Patterson MR, Jokiel PL. Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis- diffusion barriers, inorganic carbon limiation, and biochemical plasticity. J Exp Marine Biol Ecol. 1994;178:153–179. [Google Scholar]
- Mass T, Genin A, Shavit U, Grinstein M, Tchernov D. Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proc Natl Acad Sci USA. 2010;107:2527–2531. doi: 10.1073/pnas.0912348107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meron D, Efrony R, Johnson WR, Schaefer AL, Morris PJ, Rosenberg E, et al. Role of flagella in virulence of the coral pathogen Vibrio coralliilyticus. Appl Environ Microbiol. 2009;75:5704–5707. doi: 10.1128/AEM.00198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mydlarz LD, Jones LE, Harvell CD. Innate immunity environmental drivers and disease ecology of marine and freshwater invertebrates. Annu Rev Ecol Evol Syst. 2006;37:251–288. [Google Scholar]
- Pollock FJ, Wilson B, Johnson WR, Morris PJ, Willis BL, Bourne DG. Phylogeny of the coral pathogen Vibrio coralliilyticus. Environ Microbiol Rep. 2010;2:172–178. doi: 10.1111/j.1758-2229.2009.00131.x. [DOI] [PubMed] [Google Scholar]
- Raina JB, Tapiolas D, Willis BL, Bourne DG. Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Appl Environ Microbiol. 2009;75:3492–3501. doi: 10.1128/AEM.02567-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie KB. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Marine Ecol-Prog Ser. 2006;322:1–14. [Google Scholar]
- Schnitzer MJ. Strategies for chemotaxis. Symp Soc Gen Microbiol. 1990;46:15–34. [Google Scholar]
- Seymour JR, Ahmed T, Marcos, Stocker R. A microfluidic chemotaxis assay to study microbial behavior in diffusing nutrient patches. Limnol Oceanogr-Meth. 2008;6:477–488. [Google Scholar]
- Seymour JR, Simó R, Ahmed T, Stocker R. Chemoattraction to dimethylsulfoniopropionate throughout the marine microbial food web. Science. 2010;329:342–345. doi: 10.1126/science.1188418. [DOI] [PubMed] [Google Scholar]
- Simó R, Vila-Costa M, Alonso-Sáez L, Cardelús C, Guadayol O, et al. Annual DMSP contribution to S and C fluxes through phytoplankton and bacterioplankton in a NS Mediterranean coastal site. Aquat Microb Ecol. 2009;57:43–55. [Google Scholar]
- Spagnolie S, Liu B, Powers TR. Locomotion of helical bodies in viscoelastic fluids: Enhanced swimming at large helical amplitudes. Phys Rev Lett. 2013;111:068101. doi: 10.1103/PhysRevLett.111.068101. [DOI] [PubMed] [Google Scholar]
- Stefels J. Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. J Sea Res. 2000;43:183–197. [Google Scholar]
- Stocker R, Seymour JR, Samadani A, Hunt DE, Polz MF. Rapid chemotactic response enables marine bacteria to exploit ephemeral microscale nutrient patches. Proc Natl Acad Sci USA. 2008;105:4209–4214. doi: 10.1073/pnas.0709765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunda W, Kieber DJ, Kiene RP, Huntsman S. An antioxidant function for DMSP and DMS in marine algae. Nature. 2002;418:317–320. doi: 10.1038/nature00851. [DOI] [PubMed] [Google Scholar]
- Sussman M, Mieog JC, Doyle J, Victor S, Willis BL, Bourne DG. Vibrio zinc-metalloprotease causes photoinactivation of coral endosymbionts and coral tissue lesions. PLoS One. 2009;4:e4511. doi: 10.1371/journal.pone.0004511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M, Willis BL, Victor S, Bourne DG. Coral pathogens identified for White Syndrome (WS) epizootics in the Indo-Pacific. PLoS One. 2008;3:e2393. doi: 10.1371/journal.pone.0002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiolas DM, Raina JB, Lutz A, Willis BL, Motti CA. Direct measurement of dimethysulfoniopropionate (DMSP) in reef-building corals using quantitative nuclear magnetic resonance (qNMR) spectroscopy. J Exp Marine Biol Ecol. 2013;443:85–89. [Google Scholar]
- Taylor JR, Stocker R. Trade-offs of chemotactic foraging in turbulent water. Science. 2012;338:675–679. doi: 10.1126/science.1219417. [DOI] [PubMed] [Google Scholar]
- Van Alstyne KL, Schupp P, Slattery M. The distribution of dimethylsulfoniopropionate in tropical Pacific coral reef invertebrates. Coral Reefs. 2006;25:321–327. [Google Scholar]
- Verbrugghe E, Boyen F, Gaastra W, Bekhuis L, Leyman B, Van Parys A, et al. The complex interplay between stress and bacterial infections in animals. Vet Microbiol. 2012;155:115–127. doi: 10.1016/j.vetmic.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Wahl M, Kroger K, Lenz M. Non-toxic protection against epibiosis. Biofouling. 1998;12:205–226. [Google Scholar]
- Zubkov MV, Fuchs BM, Archer SD, Kiene RP, Amann R, Burkill PH. Linking the composition of bacterioplankton to rapid turnover of dissolved dimethylsulphoniopropionate in an algal bloom in the North Sea. Environ Microbiol. 2001;3:304–311. doi: 10.1046/j.1462-2920.2001.00196.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.