Abstract
Compaction of the eukaryotic genome into the confined space of the cell nucleus must occur faithfully throughout each cell cycle to retain gene expression fidelity. For decades, experimental limitations to study the structural organization of the interphase nucleus restricted our understanding of its contributions towards gene regulation and disease. However, within the past few years, our capability to visualize chromosomes in vivo with sophisticated fluorescence microscopy, and to characterize chromosomal regulatory environments via massively-parallel sequencing methodologies have drastically changed how we currently understand epigenetic gene control within the context of three-dimensional nuclear structure. The rapid rate at which information on nuclear structure is unfolding brings challenges to compare and contrast recent observations with historic findings. In this review, we discuss experimental breakthroughs that have influenced how we understand and explore the dynamic structure and function of the nucleus, and how we can incorporate historical perspectives with insights acquired from the ever-evolving advances in molecular biology and pathology.
Keywords: Gene regulation, Chromosomal architecture, Chromosomal territories, Epigenetics, Nuclear matrix
THE LORE OF THE CELL NUCLEUS: An Historical Perspective
In the late 1870’s, Oscar Hertwig published his observations on sea urchin fertilization, describing for the first time fusion of the sperm and oocyte nuclei (Hertwig and Campbell, 1895). These initial observations, which followed the discoveries of mitosis and Mendelian inheritance, contributed to the understanding that the nucleus houses the genetic material that governs heritable traits. In 1944, Oswald Avery proved that genetic information is encoded within DNA (Avery et al., 1944). This discovery subsequently led to the definitive description of the double helical model of DNA by James Watson and Francis Crick (Watson and Crick, 1953). At the time, the apparently simple, code-like nature of DNA led to Crick’s concept of “the central dogma of molecular biology”, which postulated that genetic information is transferred residue by residue from DNA to RNA to protein (Crick, 1958). The central dogma, although challenged many times, was fundamental to how genetics was understood and taught for decades. The original postulate was that protein could not transfer information to DNA or RNA in a heritable manner. This concept therefore inadvertently relegated the nucleus and nuclear proteins to merely structural components that functioned to compartmentalize genetic information from other cellular organelles without direct engagement in controlling gene “read-outs”.
Advances in technology and experimental approaches are now directly challenging the classical diagrams of the interphase cell nucleus, where DNA is illustrated as a disorganized tangle of cords, and depicts the RNA polymerase holo-enzyme tracking along a linear DNA strand to transcribe mRNA. Today, we recognize that the cell nucleus is a highly organized structure that contributes to the genetic and epigenetic control of genes. Recently, the field of genomics has made huge strides in sequencing whole genomes, global characterization of gene regulatory marks, and profiling of the nuclear ultrastructure in different cell and tissue types. Importantly, sequences and genome-wide regulatory marks for human diseases are being mapped. Our appreciation for the combined informational content is expanding significantly. The use of high-throughput methods to query regulatory networks has drastically altered how we tackle concepts developed only 20 years ago. As a consequence, paradigms in gene regulation are constantly evolving.
The tides of information
In 2000, Jim Kent at the University of California, Santa Cruz brought the UCSC Genome Browser online. This browser began as a resource to combine and visualize datasets produced by the Human Genome Project (Kent et al., 2002), but has since expanded to encompass a wealth of information gained from the ENCyclopedia Of DNA Elements (ENCODE) Project, launched in 2003 (Birney et al., 2007; Schmutz et al., 2004). In 2007, the ENCODE Consortium published a pilot study surveying a small component of the human genome to demonstrate the applicability of high-throughput workflows for the unbiased discovery of regulatory elements (Birney et al., 2007). This report not only validated early concepts of chromatin structure, but also revealed previously unknown aspects of chromatin architecture. The project, publically available on the UCSC Genome Browser, has now expanded to include whole-genomes and regulatory elements of human and mouse. Along with the Model Organism ENCylopedia Of DNA Elements (modENCODE) project, which surveys functional elements of Drosophila melanogaster and Caenorhabditis elegans (Celniker et al., 2009), the ENCODE datasets and the UCSC genome browser are highly valuable resources for studying a wide array of biological profiles including, but not limited to: 1) sequence conservation among multiple species, 2) presence of regulatory regions in diverse tissues, cell types, and disease states, 3) single-nucleotide polymorphisms, 4) epigenetic marks encompassing DNA methylation, histone modifications, and three-dimensional interactions, 5) RNA expression profiles, and 6) select transcription factor binding profiles.
The ENCODE Project has corroborated many historical observations, but it has also become increasingly difficult to assign biological significance to the wealth of information deposited in the continually expanding information cloud. This is especially true when high-throughput data conflict with long-held notions of how genes are regulated. When both the human genome project and the ENCODE project began, the goals were to collate genomic information such that applied bioinformatics would not only explain how genes are regulated, but could also predict how they will be regulated in a given biological context. However, it quickly became evident that the immediate aim should be to evaluate the depth at which this wealth of information can enrich our understanding of biology. This problem can become hyperbolically complicated when considering that the model systems already represented by the ENCODE data sets span several disease states, developmental models, and even engineered systems.
How do we then negotiate the large amount of information provided by high-throughput studies to support, reject, or modify long-held concepts? Perhaps the integrated interpretation of both high-resolution and more global studies with classical molecular biology studies is needed to understand the true biology. Such a comprehensive approach is necessary especially since controversial ideas that are initially rejected, can be and are accepted concepts later. The goal of this review is to illustrate how our understanding of gene transcription has gradually unfolded, how the collective interpretation of biology, genomics, gene regulation, and epigenetics contributes to the modern concepts of a dynamic 3-dimensional nucleus, and how it continues to evolve with technological advances. We will focus on the evolution of major concepts of nuclear ultrastructure, as well as its impact on gene regulation, and will discuss some of the tools that helped to establish our understanding of the hierarchical organization of the nucleus, and how diseases can be characterized, identified, and potentially treated, through similarly integrated approaches.
ESTABLISHING THE NUCLEAR COMPARTMENTS OF THE GENOME: The Lay of the Land
The compact nucleus: accommodating regulatory machinery
Compacted within the limited confines of the human somatic cell nucleus and distributed among 23 pairs of chromosomes, are over 3 billion base pairs of DNA, measuring approximately 2.3 meters. How DNA is organized within the nucleus has been a subject of investigation since its discovery. The idea that DNA condenses in an orderly fashion was based on early observations that mitotic chromosomes faithfully compact during every cellular division (reviewed in Paweletz, 2001). Whether interphase chromosomes also exhibited structured organization was less known, since visualization via early light microscopy techniques was not sufficient in resolving such order. The discovery that DNA was packed into nucleosomes consisting of histones, suggested an architectural organization of chromosomes (Billett and Barry, 1974; Littau et al., 1965). Yet, an ordered compartmentalization within the nucleus remained a point of conjecture for several decades. However, as visualization methods improved, and evidence for an organized nucleus mounted, the idea that there is an ordered compartmentalization of regulatory machinery within the nucleus was ultimately accepted. Functional outcomes of compartmentalization are best illustrated by studies in the process of development.
Dynamic chromatinization during embryogenesis
In metazoans, compartmentalization of the somatic genome begins at the fusion of zygotic nuclei during fertilization. In contrast to somatic cells, where chromosomes are bound by nucleosomes, the paternal genome within the sperm nucleus is much more compact and is bound by protamines (Ward and Coffey, 1991). Since there is no active transcription occurring in the sperm (Ward and Zalensky, 1996), the efficient and stable compaction of the paternal genome is likely favored over a histone bound configuration that is required for active transcription. Interestingly, it has been shown that about 4% of the human sperm genome remains associated with nucleosomes (Brykczynska et al., 2010; Hammoud et al., 2009). Although zygotically silent, it is thought that these positions, act to carry epigenetic marks in the paternal genome to the embryo (Carone et al., 2010; Ng et al., 2010). Directly following fusion, the haploid DNA of the sperm is de-compacted and protamines are replaced by maternal histones of the egg. Only when properly loaded with histones can the paternal DNA adopt a compact conformation. For example, the Histone Cell Cycle Regulation Defective Homolog A (Hira) and Yemanuclein (Yem) genes in the Drosophila are required to package histone H3 (H3.3) to the paternal genome. Loss of HIRA or YEM leads to an irregular paternal pronucleus and the subsequent loss of the paternal genome (Bonnefoy et al., 2007; Orsi et al., 2013).
Fertilization is then followed by the process of embryo blastogenesis, the early stages of embryogenesis that is marked by formation of the blastula. The blastula contains cells of the inner cell mass population that harbor the ability to differentiate into various cell types. These non-differentiated pluripotent cells are characteristically more de-condensed than differentiated cells (Bartova et al., 2008). In normal somatic cells, chromosome regions are not homogenously compacted. Interphase chromosomes exist as highly compacted “closed” heterochromatin and less compacted “open” euchromatin. These regions are very distinct and were first visualized cytologically, where heterochomatin stained with higher intensity (Heitz, 1928). Consistent with these observations, advances in electron microscopy during the early 1960s, revealed that heterochromatic regions could be visualized by more electron dense nuclear domains (Davies, 1967; Goodman and Spiro, 1962; Hay and Revel, 1963). Euchromatin and heterochromatin can also be distinguished by how sensitive they are to enzymatic digestion by DNase I, micrococcal nuclease, or mungbean nuclease.
Differentiation-dependent genomic remodeling was observed and hypothesized early on to be concomitant with the reprogramming of the pluripotent cell towards a terminally differentiated cell fate. Among the earliest demonstration of this process was the rearrangement and condensation of chromatin during the process of myogenesis (Chaly et al., 1996). Additionally, during the retinoic acid induced differentiation of human embryonic stem cells, chromosomes 6 and 8 are shown to condense approximately 30% and 54% of their initial volumes, respectively (Bartova et al., 2008). Although the genome-wide rearrangement of chromosomes likely established the cell for proper transcriptional control, the functional dependency of the 3-dimensional nuclear organization on cell fate and identity remained underexplored.
Compartmenting active and silent genes
It is still unclear whether the functional priority for nuclear organization is to efficiently compact and decompact the genome during mitosis such that transcriptionally silent regions remain in heterochromatic states. Although this hypothesis has yet to be directly tested, there is ample evidence to suggest that chromosomes are folded in three-dimensional space to achieve compaction efficiency. For example, chromosomal conformation analyses suggest that interactions among linearly proximal sequences occur more frequently than those among distal sequences, (Lieberman-Aiden et al., 2009; Yaffe and Tanay, 2011). Contrary to this notion, the clustering of heterochromatic regions at the nuclear periphery and localization of euchromatic regions towards the interior of the nucleus (Cavalli and Misteli, 2013) argues that occupancy in three-dimensional space is crucial for gene regulation.
In mammals and flies, specific genomic regions physically associate with the nuclear periphery at the nuclear lamina, a protein network that resides at the inner nuclear membrane (illustrated in Fig. 2 and reviewed in Guelen et al., 2008; Kind and van Steensel, 2010). In most cell types, the nuclear envelope is lined with heterochromatin containing gene-poor or transcriptionally silenced regions. Transcriptional silencing is mediated by lamina-associated sequences present near genes (Zullo et al., 2012), and experimental approaches that tether active genes to the nuclear periphery generally result in repression or reduced gene activity (Finlan et al., 2008; Reddy et al., 2008; Zullo et al., 2012). It is noteworthy that labeling experiments have identified sites of active transcription near the periphery; conversely inactive genes can be found in the nuclear interior (Misteli, 2013). Interestingly, early DNase I nuclear digestion experiments demonstrated the preferential localization of DNase-sensitive, open regions at the nuclear periphery (Hutchison and Weintraub, 1985). Although these results somewhat contradicted other findings at the time, they likely revealed DNase hypersensitive regions within heterochromatic regions that act to confer structural information at lamin-associated domains.
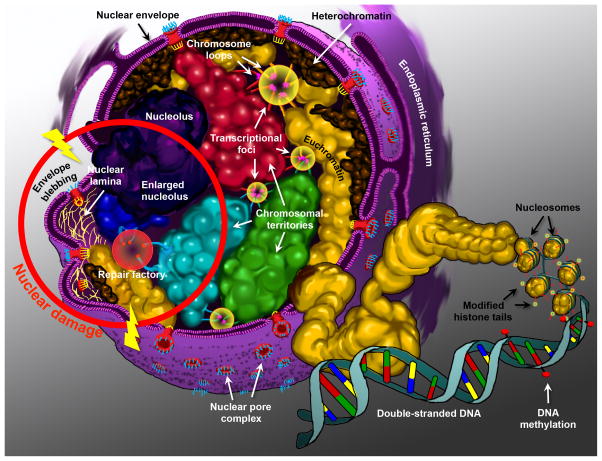
Fig. 2.
Diagram of the nucleus. Highlighting the structural components that contribute to the epigenetic control of genes. Chromosomes are arranged as non-random chromosomal territories (designated by different colors: red, green, cyan, and blue), and are composed of fractal globules of chromosomal folds that establish their ultrastructure. High concentrations of regulatory complexes reside within the interchromosomal space and constitute the nuclear matrix. Highlighted here are the transcriptional foci or factories (yellow circles), where loops of chromosomes can be recruited away from their territories to interact with regulatory elements in trans (red, green, cyan, and gold loops). Chromosomes are also classified into euchromatin (open and more transcriptionally active) (gold domains, plus the red, green, cyan, and blue territories), and heterochromatin (closed and more transcriptionally silent) (brown domains). Heterochromatin is predominantly located at the nuclear periphery. Chromatin is compacted via the winding of DNA around nucleosomes (gold spheroids). Nucleosomes are themselves composed of histone octamers that are epigenetically modified on their histone tails (red and yellow features). DNA methylation (red lollipops) at CpG islands also contributes to the epigenetic control of genes. The nucleolus, the most cytologically prominent feature of the nucleus is illustrated in purple. Perturbations in the nuclear structure that can be attributed to mutations, gene dysregulation, or environmental damage are illustrated within the red ring. Double-strand break repair occurs within repair factories (red circle), which are conducive to translocation events between neighboring chromosomal regions (blue and cyan chromosomes). In laminopathies, the disruption of the nuclear lamina (yellow fibrils) cause nuclear envelope blebbing, and the loss of peripheral localization of certain chromosomal regions. The nuclei of cancer cells are characterized by abnormal, enlarged, and/or fragmented nucleolar morphology.
Despite the ample evidence for inactive gene localization to the nuclear periphery, this is not true in all biological conditions. For example, heterochromatin is located in the interior of terminally differentiated rod photoreceptor cells in the nucleus of mammals adapted to nocturnal life, while euchromatin is located at the nuclear periphery (Solovei et al., 2009). By contrast, mammals with diurnal lifestyles do not show this rearrangement. Since heterochromatin exhibits a higher refractive index than euchromatin, the inverted nuclei act as micro-lenses in the eyes of nocturnal mammals (Solovei et al., 2009). It would be interesting to address whether there are inherent differences between the transcriptional profiles of rod cells in nocturnal versus diurnal mammals.
Bookmarking for restoring transcription after mitosis
The cell faithfully synthesizes DNA during S-phase and chromatin undergoes mitotic compaction during M-phase. As the cell exits mitosis, chromosomes de-compact and the transcriptional network is presumably preserved in the progeny nucleus. Several recent studies within the past decade have shown that a subset of genes is bookmarked by transcription factors on mitotic chromosomes, while other transcription factors are displaced from genes (Delcuve et al., 2008). Mitotic bookmarking factors allows for the rapid transcription of genes following mitotic de-compaction. For example, ribosomal RNA genes are poised for by the osteogenic RUNX2 transcription factor expression when osteoblast cells emerge from mitosis (Stein et al., 2009). Currently, the most notable bookmarking factors are Forkhead box protein A1 (FOXA1), the master regulator of androgen receptor activity (Augello et al., 2011; Caravaca et al., 2013; Zaret et al., 2010); GATA1 a factor that is essential for hematopoietic development (Caiulo et al., 1991; Kadauke et al., 2012); and the Runt-related transcription factor 2 (RUNX2), the master regulator of osteoblast development (Lian et al., 2004). The list of transcription factors that are capable of interacting with mitotic chromosomes to bookmark genes remains incomplete. To date, over 30 transcription factors are known to exhibit bookmarking activity by virtue of being at least partially retained on mitotic chromosomes (Kadauke and Blobel, 2013). Whether other lineage “master regulators” are capable of binding mitotic chromosomes has yet to be fully addressed. However, not all “master gene” factors are retained on mitotic chromosomes. For example, the T-box transcription factor 2 (TBX2), the master regulator of organogenesis, is largely evicted from DNA during mitosis (Bilican and Goding, 2006)
Bookmarking of genes may play a much larger role than simply marking highly expressed genes after mitosis. One of the more intriguing questions in biology is how the expression profile of a differentiated cell is retained after the majority of transcription factors are either degraded or displaced and newly synthesized nucleosomes are incorporated. More specifically, how do pluripotent stem cells vs. differentiated cells retain their unique transcriptional memory? The involvement of epigenetic mechanisms underlying cell fate and lineage commitment is hinted at by the mitotic retention of tissue-specific regulatory proteins such as RUNX2 on promoters that are functionally linked to the establishment and maintenance of the cell phenotype (Zaidi et al., 2010). Whether a coordinated effort by combinations of bookmarking factors, or bookmark code, which acts in a tissue and temporal-dependent manner to uniquely reestablish the interphase nuclear architecture, has yet to be fully explored.
CHROMOSOMAL TERRITORIES: Defining the Genetic Boundaries
Visualizing chromosomal clustering by microscopy
In 1885, Carl Rabl first described that interphase chromosomes were organized into discrete territories in the salamander Salamandra maculate (Rabl, 1885). A little later, Theodor Boveri observed by light microscopy that during the blastomere stages of the roundworm Parascaris equorum, chromatin order was stably maintained, occupying distinct regions of the nuclear space following cellular division and reformation of the nucleus (Boveri, 1909). He suggested that each chromosome visible in mitosis retained its individuality during interphase. However, the development of the electron microscope in 1950 and subsequent electron micrography studies failed to support the idea that interphase chromosomes resided in exclusive nuclear territories (Wischnitzer, 1973). Although well-defined structures like nucleoli and electron-dense heterochromatic domains at the nuclear periphery were well-documented, the notion that interphase chromosomes were individually partitioned in an organized fashion stood unproven for several decades. Biological approaches that showed stained clumps of condensed chromatin, achieved by squashing fixed Chinese hamster cells in acetic acid and treating air-dried cells to a sodium hydroxide solution, were the first physical indications that interphase chromatin resided within distinct territories (Stack et al., 1977). Although these experimental results were initially criticized as being precipitation artifacts, they did correlate well with the concept that chromatin was organized into territories. Following these studies, Thomas Cremer and colleagues showed that focally UV-damaged DNA was localized to a small subset of DNA fibers when de-compacted, and not dispersed widely and randomly (Zorn et al., 1979), suggesting that chromosomes were confined in territories. However, direct visual evidence of chromosomal territories was not possible until fluorescence in situ hybridization (FISH) methods were developed in the late 1970s (Cheung et al., 1977; van Driel et al., 1995), and were able to demonstrate that individual chromosomes occupied a spatially restricted territory that is 2–4 microns in diameter (illustrated in Fig. 1 and 2 reviewed in Cavalli and Misteli, 2013).
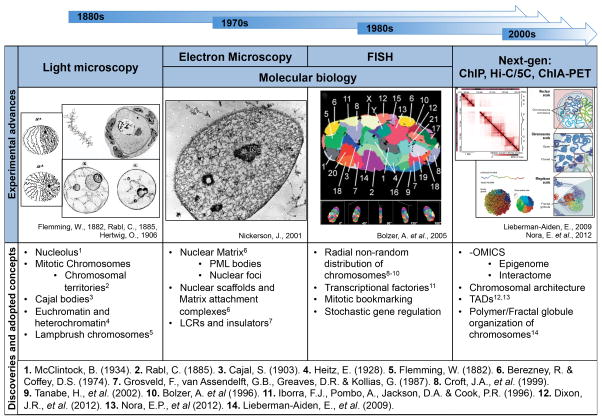
Fig. 1.
Timeline of experimental advances. Illustrating the seminal discoveries and adopted concepts that were developed as a result of evolving methodologies.
Earlier observations of chromosomal distribution have now been supported by more elaborate three-dimensional multi-color FISH techniques that allowed for the coloration of multiple chromosomes (Bolzer et al., 2005). These methods have shown that chromosomes are radially dispersed and are positionally defined by parameters including transcriptional activity, replication timing, and the GC content. For example, using painted probes, the gene-dense human chromosome 19 was consistently found in the interior of human lymphocyte and fibroblast nuclei, while the gene-poor chromosome 18 was consistently located at the nuclear periphery (Cremer et al., 2003; Croft et al., 1999; Tanabe et al., 2002). The pattern of radial distribution also seems to be specific to certain cell types (Hepperger et al., 2008).
High-throughput methodologies shed light on chromosomal territories
The ability to characterize chromatin structure by 3D-FISH alone has been met with considerable technical issues. For example, differences in fixation procedures and probe-labeling efficiency of chromosome pairs may impact chromosomal territory delineation (Cremer et al., 2008; Ronneberger et al., 2008), thus leading to ambiguous interpretation of chromosomal territory plasticity. The introduction of high-throughput methodologies has greatly benefited how we view three-dimensional chromosomal interactions. Solid-phase PCR, the basis for the massively parallel sequencing (high-throughput sequencing) platforms, was first introduced in the early- to mid-1990s (Adessi et al., 2000). The high-throughput sequencing methodology then led to an explosion of technical developments that allowed for the exploration of gene regulation on a global scale when conjugated to preexisting assays used to address various aspects of gene regulation. One of these techniques, chromosome conformation capture (3C), applies the systematic querying of chromosomal interaction frequencies of ligated chromatin fragments (Dekker et al., 2002). This technique was originally developed to qualitatively detect interactions fixed in 3-dimensional space between several individual ligation products by locus specific primers and PCR. The first 3C experiments confirmed that regulatory regions or far-distal enhancers looped into cognate basal promoter sequences (Miele and Dekker, 2008), an assumed behavior of enhancers that had yet to be formally proven. It didn’t take long for the 3C method to be coupled with microarray and massively-parallel sequencing strategies to yield several technique variants. Notably, carbon-copy chromosome conformation capture (5C) and high-throughput genome-wide 3C (Hi-C) helped to reintroduce the concept of chromosome territories, where they have largely supported the notion that open and closed chromatin regions are spatially segregated. Additionally these methods continue to demonstrate that interchromosomal interactions take place, albeit less frequently than intrachromosomal interactions, thus helping to enrich the concept of chromosomal territories to include territorial intermingling, another concept that light and fluorescence microscopy methods hinted at (Dekker et al., 2013).
Studies using 5C and Hi-C have now allowed for the 3-dimensional “polymer” modeling of non-random and non-homogenous compaction of chromatin into “fractal globules”, a polymer configuration that can attain knot-free, maximal compaction where smaller proximally located globules defined by several looping interactions can serve as monomers for larger globules. Thus, the chromosomal territory is in essence composed of globules-of-globules-of-globules of sequence (Bau and Marti-Renom, 2011; Dekker et al., 2013; Lieberman-Aiden et al., 2009). The fractal globule would thus be facilitative of rapid unfolding and refolding necessary during entry and exit of mitosis, as well as during the melting of condensed chromatin at transcriptionally activated gene regions (Lieberman-Aiden et al., 2009). On the whole, these significant advances provide strong support for concepts that were developed decades before (reviewed in Cremer et al., 2008). In fact, fractal-like looping of chromatin was observed in lampbrush chromosomes, where early light and electron microscopy demonstrated that chromosomes loop in the shape of lamp brushes, bringing long-ranging sequence in close proximity with one another (Anderson and Smith, 1978; Flemming, 1882; Gall et al., 1991). Importantly, 5C studies provide quantifiable support for the notion that favored interactions occur among regions of chromosomes that lie closer in linear proximity to each other, but interaction frequencies exponentially decay with increasing genomic distance (Dekker et al., 2013).
The changing of paradigms
The profiling of genomic topography via FISH microscopy and massively-parallel sequencing methodologies have provided novel insight into gene regulation. When transcription factors involved in the activation of bacterial operons were discovered, the prediction was that gene regulation likely worked in a very similar manner in higher eukaryotes. When the complex behaviors of eukaryotic promoters and enhancers were later discovered, the idea that enhancers could activate multiple promoters within and between chromosomes was still unknown. One of the first examples of gene regulation via interchromsomsomal interactions were provided in the 1950s by Edward Lewis in studies with the bithorax complex in Drosophila. He observed that, promoters and enhancers acted in trans at homologously paired chromosomes in a process called transvection (Lewis, 1954). Although initially thought to be artifacts during the pachytene stages of meiosis, these observations stimulated interest in non-homologous chromosomal interactions in mammalian systems. With the advent of FISH methodologies, examples of regulatory interactions occuring in trans were revealed. For example, at the mouse odorant genes, a single regulatory H-element enhancer region coordinates the expression of multiple olfactory-receptor genes (Lomvardas et al., 2006). Additionally, circular 3C (4C) experiments that use inverse PCR to generate genome-wide interaction profiles at single loci have demonstrated that an NF-κB responsive enhanceosome complex establishes inter-chromosomal interactions with the interferon B-gene (Apostolou and Thanos, 2008; Zhao et al., 2006).
There is now surprising, yet compelling evidence, that gene promoters can act as enhancers for neighboring genes (Li et al., 2012). These interactions likely occur among co-regulated genes, where distally located promoters can potentiate the transcriptional output of proximal promoters. In addition, enhancers themselves often bind RNA polymerase II and produce non-coding RNAs (De Santa et al., 2010; Djebali et al., 2012a; Mousavi et al., 2013; Wang et al., 2011), further increasing the complexity of eukaryotic gene regulation.
Chromosomal structure at the single-gene scale has also been shown to impact gene regulation. Initially observed in yeast, regions spanning the transcriptional start sites of genes loop with the transcriptional termination site (O’Sullivan et al., 2004). These looping events are also conserved at certain mammalian promoters, and are thought to allow for an efficient recycling or reactivation of RNA polymerase II (Tan-Wong et al., 2008; Tan-Wong et al., 2009), and at least for the transcriptional processing of the H4 gene, 5′ to 3′ looping is observed during the abbreviated G1 phase of hES cells (Medina et al., 2012).
Although 3C-based methods have proven to be a powerful tool for characterizing chromatin folding, these methods simply reveal the relative interaction frequencies of chromosomal regions within a cell population. Limitations of the approach include, but are not limited to, the following: 1) 3C does not distinguish functional from non-functional associations (Dekker et al., 2013), 2) chromosomal interactions can result from direct co-localization of regions sharing a nuclear matrix component such as the nuclear lamina, nucleolus, a transcription factory, or simply due to non-specific packing of the chromatin fiber (Dekker et al., 2013), and 3) methods based on 3C do not reveal mechanisms that lead to genomic interactions, with the possible exception of ChIP-coupled with high-throughput paired-end sequencing of interacting fragments (ChIA-PET) (Fullwood et al., 2009).
Looping for regulation
While 5C and Hi-C methods have demonstrated that chromosomes are highly structured, the extent to which each interaction is attributed to the control of gene expression is not fully understood. However, evidence from the increasing number of 3C-based studies has demonstrated how of chromosomal structure impacts gene regulation. One of the best-studied examples regards the mammalian β-globin locus, where transcriptionally reliant long-range interactions occur among a powerful distal enhancer, a locus control region (LCR), and a set of distal promoters located 40–80 kb away. These events are mediated by specific transcription factors including KLF1 and GATA1 (Deng et al., 2012; Drissen et al., 2004; Vakoc et al., 2005). Additionally, many looping events occur between active gene promoters and distal elements that bypass one or more neighboring genes. These observations suggest that the positional arrangement of genes and elements along the chromosome arm is a poor predictor of their structural interactions and that higher-order organization serves a functional role beyond compaction (Dekker et al., 2013). Furthermore, genes can interact with multiple distal elements, and elements can interact with multiple genes. There are many examples of gene regulatory switches involving changes in loop architecture to bring different enhancers in contact with a promoter. One such example is the Homeobox (Hox) gene cluster, in which individual genes are sequentially activated along the rostral-to-caudal body axis to organize the body plan during mammalian development (Kmita and Duboule, 2003). By multiplex chromosome conformation capture-on-chip (4C) to generate interaction profiles of the Hox locus, the organization of the HoxD cluster was shown to change upon gene activation (Noordermeer et al., 2011; Simonis et al., 2006). Analysis of the human HOXA gene cluster by 3C shows similar 3-dimensional architectural changes during differentiation (Fraser et al., 2009; Noordermeer et al., 2011).
Single genes that exhibit dynamic control in various cell types or even under variable stimuli may be regulated via its promoter interacting with a primary enhancer, while a secondary enhancer acts to confer additional transcriptional control (Perry et al., 2010; Perry et al., 2011). For example, the transcriptional control of the Muscle Creatine Kinase gene relies on an upstream enhancer that acts to promote fast-twitch muscle expression while an intronic enhancer promotes expression in slow-twitch muscle (Tai et al., 2011). Some genes utilize multiple enhancer regions to establish developmentally regulated expression. For example, enhancer usage at the MRF4 and Myf5 gene locus remains the classical example of dynamic regulatory control of promoters between multiple enhancer regions during embryogenesis (Carvajal et al., 2008; Hadchouel et al., 2003). However, chromatin loops are not limited to active genes. Looping can also occur at genes prior to their full activation (Palstra et al., 2003; Vernimmen et al., 2007). In these examples, the onset of transcription is concomitant with additional looping interactions. These dynamic looping mechanisms may be related to the priming of transcription while maintaining a configuration conducive to gene silencing. Interestingly, upon repression of the Kit gene, the loss of an enhancer-promoter loop is followed by a de novo loop formation within the gene body (Jing et al., 2008).
Molecular glues within the nucleus
A large body of work has established mechanisms that maintain chromosomal folds in the interphase nucleus. Factors such as CTCF, cohesin, and SATB1 play essential roles in establishing three-dimensional nuclear architecture (Degner et al., 2011; Guo et al., 2012; Junier et al., 2012). Initially, CTCF was identified as a negative regulator that bound three direct repeats of the CCCTC-motif at the chicken myc-c locus (Lobanenkov et al., 1990). Subsequent studies identified CTCF as a boundary and insulator element factor, a component in establishing chromatin loops, and a transcriptional activator (Hou et al., 2012; Phillips and Corces, 2009; Phillips-Cremins et al., 2013), indicating that CTCF serves as a multifunctional molecular glue within the nucleus to establish long-range interactions between regulatory regions (Ohlsson et al., 2010). Interestingly, some 5C studies in human cells have shown that, enhancers skip many sites bound by CTCF in contacting their cognate distal promoters. Thus, further work in this field of study is required to fully grasp the actions of CTCF.
Recently, a study focused on the profiling of chromosomal loops involving CTCF using ChIA-PET (Fullwood et al., 2009; Li et al., 2012) showed that, CTCF was centered at 1,480 cis and 336 trans interactions in mouse ES cells. In this same study, the role of CTCF in anchoring chromosomes to the nuclear lamina was also supported. Although the predominant interactions centered at CTCF-bound regions are intrachromosomal, ChIA-PET experiments on the other hand show that two-thirds of the contacts involving the presumed transcriptionally linked ERα foci are trans interactions and do not act on the same chromosome (Fullwood et al., 2009), suggesting that actively transcribed units may make several trans interactions when compared to typical DNA segments. This is reminiscent of previous studies that demonstrated that transcriptionally active foci were excluded from chromosomal territories within inter-chromosomal domains (Visser et al., 2000).
In addition to chromosomal looping, CTCF appears to play an important role in the recruitment of cohesin, a protein originally identified in the attachment of sister chromatids during mitosis (Parelho et al., 2008; Rubio et al., 2008). Cohesin has been recently implicated in the epigenetic regulation of genes through the establishment of chromosomal organization in interphase and the nucleation of transcriptional hubs via bookmarking (Yan et al., 2013). Furthermore, the knockdown of Scm1, a subunit of the cohesin complex, or Med12, a mediator complex member, results in the disruption of chromosomal architecture and the misexpression of cohesin-targeted genes (Phillips-Cremins et al., 2013).
TRANSCRIPTIONAL MICROENVIRONMENTS: Coming Together, Keeping Together, Working Together
Focal sites of active transcription
As early as the 1990s, the concept of nuclear microenvironments in higher eukaryotes consisting of high concentrations of replication, transcription, and repair factors was taking hold (Jackson and Pombo, 1998; Ma et al., 1998). At the time, chromosomes were theorized to be constrained by their physical association with nuclear bodies that make up the nuclear matrix, a ribonuclear protein scaffold that anchors and regulates nuclear functions (Berezney and Coffey, 1974; Berezney et al., 1995; Getzenberg, 1994; He et al., 1991; Stuurman et al., 1990). The nucleolus, the first well-documented nuclear regulatory microenvironment (reviewed in Boisvert et al., 2007; McKeown and Shaw, 2009), is the most prominent cytological feature within the nucleus and is the site for ribosomal RNA (rRNA) expression. Ribosomal genes are among the most abundant transcripts in the eukaryotic cell, and are predominantly transcribed by RNA Pol I within the nucleolus. In humans, chromosomes 13, 14, 15, 21, and 22 each carry tandem copies of the rRNA gene. Likely, the localization of active ribosomal transcription originating from multiple chromosomes within the nucleolus is due, in part, to the high concentration of RNA Pol I and associated factors within this structure (Papantonis and Cook, 2013). In addition, the promyelocytic leukemia (PML) nuclear bodies, structures of 0.1–1.0 μm in diameter found in most cell lines and tissue types, interact concurrently with several genomic sites (Stuurman et al., 1990). Cajal bodies, discovered by Santiago Ramon y Cajal in 1903 by silver-stained neuronal cells (Cajal, 1903) and rediscovered as “coiled bodies” within the nucleus by electron microscopy (Monneron and Bernhard, 1969), are proposed processing centers for histones and small nuclear RNAs. Clustering of Cajal bodies also hinted at the static compartmentalization of the nucleus (Brasch and Ochs, 1992). These early observations prompted the question whether genes sharing similar expression profiles or controlled by the same regulatory protein complexes are also localized into discrete transcriptional microenvironments enriched with factors that allow coordinated levels of gene expression.
The concept of active DNA polymerases being immobilized within discrete and stable environments during DNA replication was first described in 1962, and was supported by the finding that nascent DNA was tightly associated with a nuclear matrix (Berezney and Coffey, 1975; Jacob and Brenner, 1963). Later, immunofluorescence experiments showing that S-phase cells contained discrete nuclear foci of active polymerases (Nakamura et al., 1986) definitively proved that DNA polymerases are immobilized when active. Yet, the presence of focally immobilized transcriptional regions remained unproven. The first evidence for the existence of fixed RNA polymerases came from experiments using detergent and 1M NaCl to isolate nucleiods comprised of rosettes of DNA attached to a cluster of bound polymerases (reviewed in Papantonis and Cook, 2013), initially suggesting that RNA polymerases were the molecular ties that attached chromosomal loops to the matrix. Indeed, Pol II and Pol III reside in exclusive interchromosomal domains within the nucleus, where RNA Pol II produces the majority of protein coding transcripts, and RNA Pol III produces the class of small RNAs including tRNAs and a subset of miRNAs (Pombo et al., 1999b). However, positional exclusion of Pol I and Pol II was not enough evidence to suggest that transcription occurred in discrete foci, and the specialization of transcriptional nuclear bodies beyond RNA Pol distribution into territories remained poorly understood.
The concept of nuclear scaffolds
Aside from the nucleolus, there is ample evidence suggesting that combinatorial assembly and organization of nuclear microenvironments is mediated by scaffolding proteins, which associate with target gene promoters and reside in subnuclear domains (reviewed in Stein et al., 2000; Zaidi et al., 2007). The discovery of DNA anchorage sites in the enhancers and intronic sequences of genes was among the first indications that genes were tethered to larger protein scaffolding complexes (Brooks et al., 1994; Chen et al., 1993; Cockerill et al., 1987; Dickinson et al., 1992). These sequences are termed matrix-associated regions (MARs) or scaffold attachment regions (SARs), and are approximately 200 base pairs in length (Getzenberg, 1994). They are characterized by AT-rich sequence, although other sequence motifs exist. MARs have been shown to functionally confer increased transcriptional activity in genes, suggesting their association with nuclear factors (Getzenberg, 1994; Stief et al., 1989; van Wijnen et al., 1993). Among the first MARs associating factors identified were: poly(ADP-ribosyl) polymerase (PARP-1) (Levy-Wilson, 1981), which is better known for its function in DNA damage repair and involved in the recruitment of other transcriptional activators and the recruitment of histone modifying enzymes (reviewed in Gluch et al., 2008); special AT-rich DNA binding protein 1 (SATB1), the first cell-type-restricted MARs associated factor (Dickinson et al., 1992); and YY1 (at the time identified as NMP-1), which binds to the nuclear matrix attachment regions upstream of the human H4 histone gene promoter (Dworetzky et al., 1992; Guo et al., 1995). The high-affinity binding events of steroid receptors to the nuclear-matrix in many estrogen and androgen responsive tissues were also demonstrated early on (Barrack, 1987).
Most recently, 3C-based analyses have supported the notion that nuclear scaffolds act to establish transcriptional environments. For example, there is compelling evidence that promoter interactions can be specifically induced by expression of scaffolding factors. Experiments utilizing 4C show that the inactive SAMD4A gene made relatively few cis interactions. However, upon activation by TNFα, the SAMD4A promoter initiated contacts with the TNFAIP2 promoter and with other active genes on different chromosomes, consistent with these genes sharing a scaffold (Papantonis et al., 2012). In addition, the inhibition of transcriptional elongation via treatment with a chemical inhibitor does not completely disrupt chromosomal contacts at 3C-defined interacting regions (Mitchell and Fraser, 2008; Palstra et al., 2008), indicating that genes are scaffolded by auxiliary complexes and form independently of RNA Pol II recruitment.
Factor and gene specific factories
Although there is compelling evidence that sequence-tethered chromatin is held in physical space within the nuclear matrix by scaffolding factors, what practical benefits are gained from the recruitment of genes into discrete transcriptional foci? The nuclear matrix differs in protein composition in a tissue-specific manner and can change in response to hormonal levels, suggesting that matrix components either alter or reflect the activity of the regulatory network (Getzenberg and Coffey, 1990). This suggests that the composition of scaffolded transcriptional complexes can also be tissue-specific.
The concept of factor-defined transcriptional complexes may best be reflected in the work by Peter Cook and colleagues, who have shown that the promoter sequences of genes can help position chromosomes within the nucleus during active transcription (Papantonis and Cook, 2013). Thus, gene promoters tether to static transcription factories, where the transcription of two or more genes is carried out at locations of high factor concentration (illustrated in Fig. 2 and reviewed in Papantonis and Cook, 2013; Sutherland and Bickmore, 2009). This model suggests that the transcriptional machinery is not completely diffusible. Rather, promoters are recruited to transcription factories that are fixed in space, exhibit cell type-specific composition and are responsive to upstream signals. This model is supported by the fluorescence microscopy-based observation that DNA can diffuse freely, and that activated genes are likely localized to transcription factories (Bornfleth et al., 1999; Edelmann et al., 2001; Marshall et al., 1997). Furthermore, it has long been shown that certain gene regions are targeted to specific foci within the nucleus, suggesting that sequence motifs are not simply binding sites for transcription factors, but can be conceptually thought of as regulatory sequences that carry zip codes (Brickner et al., 2012).
One of the earliest examples of DNA content conferring positional information within the nucleus was the characterization of nucleolar organization regions (NORs). NORs are specific genomic foci that act to promote the formation of the nucleolus and were first identified by Barbara McClintock in maize (McClintock, 1934). It was shown later that the key RNA Pol I transcription factor UBF acts to sequester NORs into nucleoli (Nemeth et al., 2010). Domains that lack bound UBF are not initially incorporated into functional nucleoli (Roussel et al., 1996), suggesting that regulatory elements including promoters, when recognized by transcription factors, act as zip codes which position genomic regions to transcriptional machinery. Additionally, early work demonstrated that genes encoding transcripts with characteristic 3′-stem loop structures but lacking introns or poly(A) tails (histones, small nuclear RNAs, U1–4 U11 and U12) are all transcribed on the surface of Cajal bodies (Jacobs et al., 1999). This observation suggests that different factories process different sets of transcripts. Consequently, 4C analysis shows that a subset of non-coding genes also tends to co-localize (Robyr et al., 2011).
Recently, convincing demonstration that genes are transcribed within specific transcription factories was achieved using engineered mini-chromosome constructs, plasmids that contain eukaryotic origins of replication and promoter sequences of interest. When introduced into cells, mini-chromosomes were recruited to specific foci within the nucleus in a promoter-specific manner (Xu and Cook, 2008). Mini-chromosomes carrying identical (intron-less) transcription units were transcribed in the same factories, but those with intronic sequence were targeted to and transcribed in a different factory (Larkin et al., 2013a; Xu and Cook, 2008). In support of this finding, ChIA-PET experiments show that intron-less genes often contact each other (Li et al., 2012). Similarly in yeast, the association of genes with nuclear pores appears to be dependent on targeting sequences within the 5′-ends of genes that sequester them from the nuclear interior to the nuclear pore, further supporting the notion that eukaryotic promoter sequences can act as zip codes.
Despite accumulating evidence that transcription factories within microenvironments recruit actively transcribed genes, the concept of transcriptional factories was mainly hindered by the ability to accurately detect subnuclear structures via immuno-labeling and fluorescence or electron microscopy methods (Papantonis and Cook, 2013). The first low-resolution method of detecting transcription factories involved pulsing cells with [3H] uridine and visualizing the location of nascent RNA by autoradiography (reviewed in Papantonis and Cook, 2013). Higher resolution methods include the use of fluorescent approaches by tagging newly synthesized transcripts with nucleotide analogues and detection by indirect immuno-labeling (Faro-Trindade and Cook, 2006a; Faro-Trindade and Cook, 2006b; Iborra et al., 1996a; Iborra et al., 1996b). By these methods, a wide range of nucleoplasmic factories have been detected (Papantonis and Cook, 2013; Pombo et al., 1999a), varying between ~3,000 to 33,000 factories per cell, depending on the cell type and species. RNA FISH probes targeting two independent transcripts within one factory showed that factories are typically ~46 to 54 nm in diameter, regardless of cell type (Larkin et al., 2012; Larkin et al., 2013b; Papantonis et al., 2010). It is predicted that all transcription essentially occurs in factories (Papantonis and Cook, 2013) and recently, the protein compositions of transcription factories organized by Pol I, Pol II and Pol III are being defined (Melnik et al., 2011).
Immunofluorescence analyses within recent years have revealed that nuclear microenvironments tend to be highly dynamic structures. During erythroid differentiation, which is marked by dynamic transcriptional changes, the β-globin locus relocates away from the nuclear periphery and moves centrally into transcriptional foci (Bender et al., 2012). This relocation and subsequent Pol II elongation appears to depend on the activity of the β-globin LCR. Individual deletion of DNase-hypersensitive sites within the LCR suggested that the β-globin LCR is an array of four enhancers that act independently to output near continuous Pol II elongation (Bender et al., 2012). The release of paused Pol II is therefore not a binary process, but is dynamically adjusted in response to differentiation cues and environmental changes. Therefore, the association of genes via enhancer elements within discrete transcription factories may help mediate the output of finely controlled gene expression. The recruitment of transcriptional complexes, which includes post-transcriptional speckles (Fox et al., 2002), to dynamic microenvironments may act to co-regulate groups of genes. In fact, the most compelling evidence that transcription occurs within tightly defined microenvironments is that “trans-splicing” occurs extensively in mammals. Additionally, these trans-splicing events have been shown to coincide with 3C-enriched interactions (Djebali et al., 2012b; Gingeras, 2009; Li and Heermann, 2013).
Transcriptional repression
Much focus has been placed on how gene transcription is activated and maintained. However, gene repression is just as critical for the establishment of cellular identity. The X-chromosome inactivation is the first documented large-scale architectural change that mediates transcriptional repression (Barr and Bertram, 1949; Lyon, 1962). The main cytological difference between the inactive X-chromosome (Xi) and the active X-chromosome (Xa) or other autosomes is that Xi is more highly compacted (Eils et al., 1996). Also known as the Barr body, Xi is generally located at the nuclear periphery. As stated before, demonstration by FISH and 3C analyses show that silent regions cluster together at the nuclear periphery (Simonis and de Laat, 2008). The localizing of the Barr body and other heterochromatic regions in three-dimensional space prompted interest into how the nuclear structure impacts gene suppression.
Advances in experimental methods are continually shedding light on how genomic landscapes establish suppressed gene regions. The first well-studied genomic elements that controlled spurious transcriptional effects were LCRs and insulator regions. LCRs were first identified by their ability to regulate far distal enhancer regions so that they were only able to interact with their designated promoter, and not flanking genes. It was known that the inclusion of LCRs conferred copy number and positional control in ectopic chromatin sites in transgenic animals (Grosveld et al., 1987). Similarly, insulator elements were shown to prevent spurious enhancer activity (Burgess-Beusse et al., 2002). However, the mechanistic understanding of insulators was unknown prior to the discovery of CTCF. By binding CTCF factors, which are also known to regulate LCR activity, insulator elements were thought to establish transcriptional barriers between gene domains (Sexton et al., 2012). Genome-wide studies combining CTCF ChIA-PET, ChIP-seq, and Hi-C data suggest that LCRs and CTCF-bound insulator regions are likely similar. Recently, LCRs have been shown to associate with the nuclear periphery, suggesting that they function to establish territorial information by shuttling gene loci to specific genomic locations to be transcribed or suppressed. The loss of LCR elements leads to mislocalization, and consequently misexpression of genes (Bender et al., 2012). Further characterization of differentially regulated blocks of chromatin established by LCR or insulators will be critical to understand how structure impacts expression.
Interestingly, the action of LCRs is strikingly similar to the emerging concept of topologically associating domains (TAD) identified by recent 5C and Hi-C experiments. TADs exhibit high-interaction frequency that can span hundreds of kilobases, and are partially defined by genomic boundary elements that also recruit CTCF and cohesin (Dixon et al., 2012; Nora et al., 2012). Loci within a TAD interact frequently with each other and infrequently with loci located outside of the TAD (Dekker et al., 2013). These observations suggested that enhancer and promoter interactions are particularly frequent within TADs, perhaps acting to establish domains or to limit the range of regulatory elements that blocks of genes can survey and interact with, a concept similar to CTCF defined insulator elements and LCRs.
MERGING CONCEPTS OF THE STABLE NUCLEUS VERSUS THE DYNAMIC NUCLEUS: Even a Clear Concept Has Only a Limited Range of Applicability
The probabilistic nucleus
Historically, studies that probe nuclear organization often had conflicting outcomes. Ultimately, the recent advances in techniques to probe the genomic landscape have allowed investigators to observe for the first time how our genome is structured in three-dimensional space, although it is still difficult to extrapolate the function of every fold observed. The mapping of chromosomal interactions based on 5C, Hi-C, and other 3C-based methods have shown that the genome exists in highly ordered fractal globules within chromosomal territories. Furthermore, compelling live-cell imaging demonstrates that the motion of genes and chromosomes during interphase is constrained, suggesting that the nucleus is topologically constant (Chubb et al., 2002). However, the genome must be dynamically and differentially ordered to produce physiological levels of gene expression. This notion is evident by the observation that chromatin domains de-condense and move from the chromosomal territory into interchromosomal space to form long-ranging contacts during gene activation (Morey et al., 2007; Morey et al., 2009). Conformational data suggest that some of this reorganization drives concerted gene expression. For example, 5C models have revealed statistically significant interactions in the form of transcription foci among highly expressed and functionally related genes (Dekker et al., 2013). Similarly, using predominantly single-cell FISH analyses, Tom Misteli and others have described non-random proximity patterns in the cell nuclei of various tissues (Parada et al., 2004; Parada et al., 2002; Roix et al., 2003). However, cell-to-cell variations of chromosomal arrangements have been found for all cell types queried thus far, and suggest that even homogenous cell culture models do not exhibit uniform chromosomal organization. What these findings demonstrate is that chromosome conformation capture methods are limited in that they represent the chromosomal interactions of millions of cells and that interaction mapping generated from population-based methods is a summation of many different genomic landscapes. On the other hand, single-cell FISH represents snap-shots of individual nuclei, thus yielding non-uniform proximity patterns of a dynamic system.
Cell-type specific or transient interactions at transcription factories may represent low frequency interactions. Variability in biological behavior between individual cells comes from the stochastic activity of genes (Raj and van Oudenaarden, 2008). The gene expression profile of a population of cells in response to a dosage dependent signal could reflect a subpopulation of cells that may only express a subset of genes, while another may express a different subset. Stochastic gene expression is also concomitant with stochastic gene interaction. An early demonstration of this behavior was termed “gene kissing”, where 3D-FISH provided evidence for transient spatial association of the Angelman syndrome/Prader-Willi syndrome (AS/PWS) loci during late S phase (LaSalle and Lalande, 1996; Teller et al., 2007). In summary, transcriptional profiles of individual cells within a population can range widely, and reflect a stochastic behavior of promoters where genes are expressed in bursts rather than truly continuous (Chubb et al., 2006; Larson et al., 2009; Raj et al., 2006). This is best illustrated by the cooperative action of the multiple hypersensitive sites of the β-globin LCR that act to achieve near continuous transcriptional activity, but the deletion of one or more sites, leads to fewer transcriptional events (Bender et al., 2012).
Evaluating three-dimensional interactions
As studies delve deeper into understanding how chromosomal structure regulates cell identity, the gap between our ability to observe structural elements and our ability to relate these observations to biological processes will narrow. Notably, when interpreting how chromatin interactions defines the nucleus, it is important to consider that repressed domains tend to interact with other inactive domains on the same chromosomal arm and are likely more stable, while active domains can dynamically and stochastically interact with multiple active regions on the same or on different chromosomes. These observations suggest that repressed domains predominantly define the framework of chromosomal territories. Due to the biased nature of interacting chromosomal regions, sequences that are “inactive” may be detected at higher frequencies. Similarly, most investigations tend to focus on how global looping events can explain global gene regulation, despite the fact that DNA replication, DNA repair, and other nuclear processes occur simultaneously and also define chromosomal organization.
Recently, the massively parallel sequencing of Okazaki fragments on a genome-wide scale allowed for the construction of the replication map for eukaryotic genomes (McGuffee et al., 2013). Consequently, 5C and Hi-C approaches have also identified clustering of origins of early replication (Duan et al., 2010). Together, such studies allow profiling of origins of DNA replication throughout S-phase and can help define hubs enriched for replication machinery. These studies and similar approaches will undoubtedly bring concepts predicted a few decades back, stating that DNA replication occurs at centralized factories (Berezney and Coffey, 1975; Berezney and Wei, 1998) to a full circle.
Interestingly, simultaneous labeling of replication and transcription sites in mammalian cells followed by three dimensional microscopy and computer imaging techniques demonstrated that replication and transcription sites are clustered into separate higher-order domains or zones in the cell nucleus (Wei et al., 1998). However, it has been suggested the transcriptional zones are rezoned for replication, demonstrating that nuclear foci may exhibit multilayered functionality (Cook, 1998). Ultimately, the interpretation of 5C and Hi-C analyses performed in asynchronous cells need to consider that interactions may reflect organization related to mitotic packing or interactions with static DNA replication factories.
Functional testing of regulatory regions
One perplexing problem that arises from the concept of transcription factories relates to how one interprets transfection and reporter gene assays that have remained a staple for understanding promoter and enhancer activity for nearly three decades (Dennis and Berg, 1985; Lee et al., 1987). Regulatory regions of interest that drive reporter constructs can be tested by single base pair mutational analysis, which in turn, can yield quantifiable changes in transcriptional activity. If promoters in native contexts tether to specific factories within the nucleus, why do parameters such as element spacing, base pair deletions, or point mutations have a significant impact on reporter gene activity? Perhaps one explanation is that there are soluble transcription factors that freely diffuse within the nucleus, and upon recognition of an open or poised regulatory sequence, can either initiate or help to nucleate new transcription factories via de novo transcription factory formation. Likely, the high plasmid concentrations usually transfected into cells in these experiments may nucleate nascent transcription factories with properties that are unique to the promoter-enhancer sequences present on the plasmid construct. This notion is supported by early experiments showing that plasmids can coalesce in vitro in a sequence- and factor-specific manner (Dunaway and Droge, 1989; Mahmoudi et al., 2002; Mueller-Storm et al., 1989). Although plasmid DNA have been shown to recruit endogenously expressed histones (Shi et al., 2004), it is still unclear the extent to which plasmids can mimic endogenous chromosomal landscapes to promote the nucleation of matrix factors.
Similarly, transgenic studies have been susceptible to the phenomenon of transgenic variegation (Dobie et al., 1996). Copy number and insertion location of transgenic reporters are known to affect the activity of promoter transgenes. It is now known that epigenetic effects of flanking chromatin at sites of integration can lead to transgene variegation. These concepts raise a very interesting question: can the simple introduction of exogenous sequence affect the transcription of endogenous genes via formation of nascent transcription factories. Thus far, there is no evidence to suggest that such a phenomenon can occur; however, the ever-changing paradigm of transcriptional activation and repression may potentially alter how reporter gene constructs are designed, tested, and interpreted.
EPIGENETIC GENE CONTROL AND THE CHROMOSOMAL LANDSCAPE: The Structure of Inheritance
Chromosomal architecture as a heritable entity
Epigenetic regulation relates to heritable mechanisms driving gene expression that are not mediated by DNA sequence. These encompass DNA methylation as well as histone modifications, which include acetylation, methylation, phosphorylation, sumoylation, and ubiquitination. However, does the three-dimensional structure of chromosomes itself also confer epigenetic and heritable gene control in a tissue- or cell type-dependent manner? Evidence presented thus far suggests that the organization of chromosomes alone cannot define cellular identity. Conversely, cellular identity cannot fully predict chromosomal structure. Yet, several studies have linked epigenetic marks to chromosomal organization.
From yeast to human, the relationship between DNA methylation, histone modifications, and chromosome architecture has been studied in depth (Taverna et al., 2007). For example, DNA compaction is dependent on the composition of histones within bound nucleosomes. The inactivated X-chromosome is marked by H3K9 methylation, and low levels of histone acetylation and H3K4 tri-methylation (Chow et al., 2005). Large-scale analysis of chromatin structure, histone modifications, and expression profiles show that genomes are partitioned into well-defined, actively or repressively marked domains that strongly correlate with transcriptional activity. Furthermore, these domains are separated by sharp boundaries marked by specific histone modification patterns that overlap binding sites for chromatin insulator proteins (Dixon et al., 2012; Handoko et al., 2011; Nora et al., 2012). Notably, mono/tri-methylated lysine 4 on histone H3 (H3K4) generally marks active or poised gene promoters and enhancers, lysine 9 (H3K9) acetylation marks active genes, and di/tri-methylated lysine 27 (H3K27) and H3K9 are correlated with silenced gene domains. These marks, in turn, can act to recruit transcription factors that favor specific classes of histone modification: Chromodomain proteins recognize a wide range of H3 methylation marks, Tudor domain proteins can recognize H3K3me3, methylated lysine 20 on histone H4, and di-methylation arginine 3 on histone H4 (H4R3me2), and Bromodomain proteins recognize H3 lysine acetylation. Combinatorial marks also determine how a gene is transcribed. For instance, the bivalent association of H3K4me3 and H3K27me3 at gene promoters is thought to mark developmental genes (Bernstein et al., 2006). The combination of H3 arginine 17 mono-methylation, asymmetric di-methylation (H3R17me1/me2a), and H3 lysine 18 and 23 acetylation (H3K18/23ac) marks estrogen-stimulated transcription (Daujat et al., 2002). In addition to histone marks in mammals, methylation at CpG islands marks transcriptional silencing and abrogation of transcription factor binding.
Histone modification and DNA methylation have all been proven to be heritable modifications throughout any given cell lineage. There is also a tremendous body of work relating histone modifications to chromosomal re-arrangements, suggesting a strong link between chromosomal organization and epigenetic control. For example, the genome-wide profiling of different mammalian cells shows that large heterochromatic blocks of hundreds of kilobases known as LOCKs (Large Organized Chromatin K9 modifications) carry the repressive histone marks H3K9me2/3 and overlap with LADs (Lamin Associated Domains) (Guelen et al., 2008). These regions are interspersed with small euchromatic islands that are hypersensitive to DNase I, bind CTCF, and carry active histone marks (H3K4me3, H3K9ac). The current challenge is to now perform comparative analysis with ChIP-seq of active and repressive histone marks with Hi-C or 5C data sets to definitively determine a relationship between histone composition and nuclear organization. Single locus 3C or 4C models are at the forefront of demonstrating these types of relationships. For example, during the dynamic three-dimensional transitions at the Hox cluster, the chromatin microenvironment is altered from a repressive histone H3K27me3, to a transcription-permissive histone H3K4me3 state (Noordermeer et al., 2011).
Cellular Pluripotency
Lineage commitment and differentiation is marked by large changes in chromatin organization. However, one of the most intriguing questions concerning stem cell pluripotency relates to how embryonic stem cells (ESCs) maintain an open genome state to allow for maximum plasticity. How the nuclear environment impacts the expression of pluripotency genes is still emerging. In general, ESC chromosomes are characterized by an abundance of active chromatin marks, and have a relatively lower level of repressive marks (Mikkelsen et al., 2007). During differentiation, these marks disappear and chromosomes largely begin to condense. Conversely, genome-wide studies show that genes associated with the nuclear lamina in ESCs lose their interactions with the nuclear lamina during differentiation (Peric-Hupkes et al., 2010). Therefore, the extent to which nuclear architecture affects cellular plasticity has yet to be fully understood.
For many years, investigators sought to understand how regulatory mechanisms can be altered on a genome-wide scale to reconfigure terminally differentiated cells into a more stem-like state. Some of the earliest work in this regard involved cellular de-differentiation using 5′-azacytidine to prevent de novo DNA methylation (Razin and Cedar, 1991). However, the 5′-azacytidine method failed to obtain a true pluripotent cell population. Between 2007 and 2008, Shinya Yamanaka and colleagues were able to induce pluripotency (iPS) in terminally differentiated somatic cells using a cocktail of transcription factors: OCT4, SOX2, MYC, and KLF4 (Takahashi and Yamanaka, 2006). The ability of these factors to epigenetically convert terminally differentiated cells into pluripotent cells is partly attributed to their role as “pioneer” factors, a subset of transcription factors that can access silent chromatin during embryonic development and direct the binding of other transcription factors (Cirillo et al., 2002; Zaret and Carroll, 2011). Pluripotency factors can thus bind to the distal elements of many genes required for reprogramming, through bypassing and reorganizing the preexisting chromatin topology (Gao et al., 2013).
The ability of the pluripotentcy factors to transform the genetic landscape is fascinating. Considering that terminally differentiated cells are defined by pre-existing transcription factories, how does the cell accommodate the exogenous expression of a pluripotency factor? Do new factory complexes form and rapidly change the transcriptional profile of the cell? How is the chromosomal architecture rearranged? Genome-wide occupancy maps of ES and iPS cells show that OCT4, SOX2, and KLF4 factors are central to the core pluripotency network. By contrast, c-Myc binds to the promoters of genes involved in cell growth and survival, and indirectly enhance reprogramming by allowing more chances for stochastic transcriptional events to occur at bound OCT4, SOX2, and KLF4 regions (Hanna et al., 2009; Kim et al., 2008; Knoepfler, 2008; Sridharan et al., 2009). Thus, central to the epigenetic conversion of the nuclear structure is the ability of Oct4, Sox2, and KLF factors to bind chromatin lacking detectable open histone marks and to permit c-Myc to gain access to its target sites (Soufi et al., 2012). In this regard, response to pluripotency factor stimulation is a stepwise process. Binding initially occurs at distal conserved elements or enhancers preceding marked transcriptional activation and is followed by recruitment to promoters. Promotion of gene activity in this manner recapitulates the recruitment of pioneer factors at enhancers during early development of progenitor cells (Soufi et al., 2012). Perhaps then, pluripotency factors do nucleate new factories or at least modulate pre-existing ones. Further work is required to characterize how the nuclear ultrastructure is altered during iPS reprogramming.
NUCLEAR INSTABILITY AND GENETIC DISEASES: A Thing Is Right When It Tends to Preserve Stability of the Biotic Community, It Is Wrong When It Tends Otherwise
The Almanac of Genetic Stability
How have evolving concepts in nuclear structure altered our understanding and treatment of diseases? This section will summarize a few clinical examples that demonstrate how perturbations of the nuclear structure can directly affect the transcriptional regulation of genes, thus leading to disease phenotypes. We will also describe developing trends that have great potential for the epigenetic manipulation of the genome to treat disease.
Progeria: disruption of the nuclear lamina
One of the best-studied pathologies associated with perturbations in the nuclear architecture is the progeroid class of diseases caused by mutations in the LaminA/C (LMNA) gene (Capell and Collins, 2006). LMNA produces two protein isoforms that are critical components of the nuclear lamina. Interestingly, mutations throughout LMNA can manifest in a diversity of diseases that affect many tissue types: Emery-Dreifuss muscular dystrophy (muscular), Charcot-Marie-Tooth disease (neuronal), limb girdle muscular dystrophy (muscular), Hutchinson-Gilford progeria (systemic), and familial partial lipodystrophy (subcutaneous fat) (Rankin and Ellard, 2006). The range of mutant phenotypes caused by LMNA defects suggests that perturbations of the nuclear lamina dysregulates multiple target genes in a tissue or cell-type specific manner. In the case of Hutchinson-Gilford progeria, some genes lose their association with the nuclear lamina, however these genes did not exhibit drastic changes in gene expression (Kubben et al., 2012). Yet, during astrocyte differentiation, inactive genes are readily upregulated when displaced from the nuclear lamina (Peric-Hupkes et al., 2010). Currently, how LMNA mutations act to alter epigenetic gene regulation is not fully understood.
Perturbation of chromosomal interactions in Cornelia de Lange syndrome
Cornelia de Lange syndrome (CdLS) is a genetic disorder characterized by delayed growth, abnormal facial features, upper limb development abnormalities, and cognitive retardation (Liu and Krantz, 2008). The disease is predominately caused by dysregulated genes encoding proteins that are in the cohesin complex or interact with it. However, the most prominent cause is via mutations within the NIPBL (nipped-B-like) gene (Krantz et al., 2004). NIPBL is not a core component of cohesin, but is important for loading the cohesin complex onto chromatin during S-phase, where cohesin functions to hold sister chromatids together. Paradoxically, CdLS cell lines do not consistently exhibit premature sister chromatid separation or related dysfunctions during mitosis. The phenotypes observed in CdLS individuals suggest a defect in gene regulation rather than in chromatid cohesion during cell division (Liu et al., 2009). CdLS cell lines where NIPBL is mutated exhibit a large number of moderately misexpressed genes and by FISH analysis, a disrupted interphase chromatin structure. Loss of NIPBL causes widespread decompaction that is most pronounced at regions with high gene density (Nolen et al., 2013). Interestingly, a recent report attributes a role for mitotic bookmarking to cohesin, where cohesin contributes to the genome-wide retention of transcriptional memory (Yan et al., 2013). Disruption of cohesin function leads to an alteration of transcription factor clustering at enhancers and promoters, and a global reduction of DNase hypersensitivity, further supporting a model in which CdLS is caused by aberrations in gene expression.
Polycomb repression in Facioscapulohumeral muscular dystrophy
Facioscapulohumeral muscular dystrophy (FSHD) is an autosomal dominant disease characterized by the progressive wasting of the facial, upper arm, and shoulder girdle muscles. FSHD is unique in that it is caused by deletions that reduce the copy number of the tandemly repeated 3.3kb D4Z4 sequence at the subtelomeric 4q chromosomal arm (Cabianca and Gabellini, 2010). These deletions are thought to cause the inappropriate de-repression of several 4q35 genes. The D4Z4 region shares several features with repressive polycomb/ trithorax response elements (PREs/TREs) that act to epigenetically silence genes by the recruitment of Polycomb group (PcG) proteins, which in turn repress the expression of nearby genes (Schuettengruber et al., 2007). In the non-disease state, the D4Z4 region is extensively bound by repressive PcG proteins leading to the spreading of DNA methylation, histone de-acetylation, and chromatin compaction at the 4q35 region. FSHD patients harbor a loss of repressive marks and the de-repression of 4q35 genes (Lemmers et al., 2010). One model that has been put forth is that D4Z4 deletion leads to insufficient binding of PcG due to the loss of D4Z4 repeat regions, causing the production of DBE-T, a long ncRNA embedded within the D4Z4 repeat (Cabianca et al., 2012). DBE-T is thought to promote a topological reorganization of the FSHD locus leading to de-repression of the 4q35 genes, and a positive feedback loop that sustains 4q35 gene de-repression.
Translocations in cancer models
In 1973, Janet Rowley proposed that the chronic myelogenous leukemia (CML) was caused by a chromosomal translocation event between chromosomes 9 and 22 t(9;22), resulting in the Philadelphia chromosome discovered previously by Peter Nowell in 1960 (Nowell and Hungerford, 1960; Rowley, 1973b). The fusion of the ABL gene encoding tyrosine kinase on chromosome 9 and the BCR gene on chromosome 22 leads to a chimeric fusion protein with constitutive oncogenic kinase activity (Heisterkamp et al., 1985). This was a seminal demonstration that chromosomal defects could cause cancer. Along with Rowley’s discovery of the 8;21 translocation in acute myelogenous leukemia, which produces the AML1-ETO fusion of the known nuclear scaffolding protein RUNX1/CBFA2 gene at 21q22, and the MTG8 encoded protein ETO at 8q22 (Erickson et al., 1992; Miyoshi et al., 1991; Rowley, 1973a), many other translocation events have been characterized (Zhang and Rowley, 2006). The AML1-ETO fusion protein results in the loss of the RUNX1 nuclear targeting signal and as a result, is mislocalized within nuclear microenvironments that are distinct from those in which wild-type RUNX1 resides (Barseguian et al., 2002; McNeil et al., 1999; Zaidi et al., 2007). This results in the aberrant recruitment of the N-CoR repressor complex, further exacerbating the disease phenotype by altering the nuclear environment and affecting the expression of off target genes (Okuda et al., 1996).
Translocation events require double-strand breaks (DSB), physical association with broken ends, and the misjoining of the partner chromosomes. Interestingly, translocation events occur at hotspots in the genome. For example, the t(8;21) translocation event is prevalent in acute myelegenous leukemias (Zhang and Rowley, 2006) and always takes place at breakpoints in the RUNX1 gene that is nuclease accessible. Why do translocation hot spots exist? The idea that chromosomal DSB ends are sequestered to DNA damage repair factories has been a longstanding hypothesis (Meister et al., 2003). The observation that domains of DSBs can undergo long-range movements argues for the existence of damage factories (Soutoglou et al., 2007). The increase in focal concentration likely improves efficiency of repair and the spatial proximity of breaks may promote misjoining. For example in flies, damaged regions of heterochromatin exit the chromosomal territory for repair (Chiolo et al., 2011). Within mammals, evidence shows that although DSB ends migrate to repair centers, they have limited mobility (Girst et al., 2013), suggesting that structural components that establish chromosomal positioning and proximity in a cell-type structural factors contribute strongly to translocation frequency.
Numerous FISH studies have underscored a strong correlation between the spatial proximity of chromosomes or genes and their translocation frequencies (Bickmore and Teague, 2002; Parada et al., 2002). In the case of mouse lymphocytes, breakpoints in chromosomes 12 and 15 frequently translocate and are proximally located, whereas this translocation does not occur in hepatocytes (Parada et al., 2004). Instead, chromosomes 5 and 6 are often translocated in hepatomas, where these breakpoints are proximally located, but are not in lymphocytes. Thus, the physical proximity between genomic regions likely drives translocation events. The recent development of high-throughput, genome-wide translocation sequencing (HTGTS) has confirmed the notion that translocations occur strongly based on the proximity of chromosomal positions (Chiarle et al., 2011).
Nuclear architecture as a diagnostic readout for disease
With the increased understanding of the importance of nuclear architecture as well as the three-dimensional organization of chromatin for gene regulation, it becomes pertinent to use advances in experimental approaches to discover alterations in nuclear architecture for diagnosis and treatment of disease. Namely, cancer cells show a highly modified nuclear architecture, suggesting a functional relationship between nuclear organization and gene expression that can facilitate tumor diagnosis. Proteins that reside in sub-nuclear domains are also being used to diagnose cancer. Cancer-related changes to the compartmentalization of PML bodies, Runx and acute lymphoblastic leukemia 1 (ALL1) regulatory domains, changes in the number, size, and localization of nucleoli, aberrations of the perinucleolar compartment (PNC), PCNA and BRCA I nuclear foci are all hallmarks that can benefit cancer prognoses (Fischer et al., 2004; Zaidi et al., 2007). Similarly, aneuploidy and the fragmentation of chromosomes, which are characteristics of transformed cell nuclei, undoubtedly affect territorial organization. To diagnose these genomic abnormalities, spectral karyotyping (SKY or M-FISH) and comparative genomic hybridization can be utilized to characterize many chromosomal aberrations of hematological diseases and solid tumors (Knutsen et al., 2005). It is also becoming increasingly plausible to use high-throughput tools for the diagnosis and treatment of disease. The use of massively-parallel genome sequencing and global-scale DNase-hypersensitivity methods to map nuclear architecture for personalized medicine are on-the-horizon approaches to screen for changes or aberrations of regulatory DNA elements and SARs at gene boundaries (Metzker, 2010; Schweiger et al., 2013).
Gene therapy tools to epigenetically manipulate gene expression
The development of gene therapy strategies has always been an endpoint goal for the treatment of genetic diseases. As mechanisms of epigenetic gene control continues to become unraveled, bold new approaches for epigenetic therapies are also being developed. A recent example for such strategies involved whole-chromosome silencing by the transgenic insertion of an XIST target transgene into chromosome 21 of a trisomy Down’s syndrome fibroblast cell line (Jiang et al., 2013). This led to the generation of a chromosome 21 Barr body, which effectively resulted in the in vitro phenotypic reversal. This study demonstrated the plausibility of modifying the higher-order architecture of a disease-causing chromosome and perhaps represents a turning point for therapeutic strategies. Since epigenetic manipulation can be heritable, it in theory circumvents the requirement of a dosing regimen of therapeutic products. Furthermore, epigenetic therapy does not require gene editing at the mutated gene, as this may not always be a viable option. Similar to this strategy is the development of both the TALEN and the CRISPR approaches for targeted gene editing (Cong et al., 2013). The recent use of customizable TAL effectors fused to Fok1 nuclease has become a revolutionary method to design and utilize sequence-specific nucleases for genome editing (Bogdanove and Voytas, 2011). Fusion of TAL effectors to either DNA methyltransferases, histone demethylases, or deacetylases to epigenetically modulate gene expression are promising approaches to correct gene misexpression (Bogdanove and Voytas, 2011). The most recent demonstration of this concept is the fusion of TAL-effectors to LSD1, an H3K4 demethylase, to transcriptionally silent gene expression (Mendenhall et al., 2013). Similarly, promoter silencing using a CRISPR-mediated RNA-guidance of a kinase-dead Cas9 domain (dcas9) (Gilbert et al., 2013) is yet another potential strategy for targeted epigenetic therapy. Since both TAL-effectors and CRISPR-mediated methodologies can theoretically be conjugated to any modulatory catalytic domain, genome–wide epigenetic manipulation is likely achievable.
THE ALMANAC: Concluding Thoughts
In this review, we have discussed how our understanding of nuclear organization has evolved with the development of increasingly sophisticated assay techniques, and how this relates to the diagnosis and treatment of disease. These continually shifting paradigms have led us to embrace the analogy of the almanac. The traditional almanac functioned as an annual publication that provided important information about technology trends, covered practical ways of living for the coming year, and were largely based on divination. Although modern almanacs still tend to reference astrological charts, they now contain meteorologically backed weather forecasts and descriptions of healthy living based on trends in medical findings. It is always left to the discretion of the reader to embrace information gained through modern, traditional, or both sources. As an almanac essentially aims to predict and interpret the outcomes of observable events while adapting old theories to new technologies, it seems fitting for the field of genomics to publish an all-encompassing genomic almanac that details integrated knowledge, sourced both from classical studies and those gained from next-generation methodologies.
Here, we have highlighted many studies that have focused on nuclear organization: from early cytological observations, to the bioinformatic dissection of the genome, to the impact of nuclear instability on disease. Since its discovery, chromosome biology has undergone several waves of paradigm changing concepts. Despite these changes, it is satisfying that the shifts in paradigm often result in the resolution and complementation, rather than the rejection, of standing notions. Because there is always value in considering historical studies when interpreting data that rely on current high-throughput or high-resolution methodologies, it may be necessary to begin publishing an annual Genomic Almanac. The fact that reviews are continually being published which explore new discoveries and how they impact the interpretation of historic studies reflects the desire for such a publication to be established. In this manner, we can truly appreciate and learn from the diversity of the approaches used to study genomic, epigenetic and gene regulatory networks.
Acknowledgments
Contract Grant Sponsor: National Institutes of Health: P01 CA082834 (to GSS), P01 AR48818 (to GSS), R01 AR039588 (to GSS and JBL), and R37DE012528 (to JBL).
The authors thank C. Himeda for critical reading of the manuscript, and J. Diaz for editorial assistance with preparation of the manuscript.
Footnotes
The authors have no conflicts of interest.
LITERATURE CITED
- Adessi C, Matton G, Ayala G, Turcatti G, Mermod JJ, Mayer P, Kawashima E. Solid phase DNA amplification: characterisation of primer attachment and amplification mechanisms. Nucleic Acids Res. 2000;28:E87. doi: 10.1093/nar/28.20.e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Smith LD. Patterns of synthesis and accumulation of heterogeneous RNA in lampbrush stage oocytes of Xenopus laevis (Daudin) Dev Biol. 1978;67:274–285. doi: 10.1016/0012-1606(78)90199-9. [DOI] [PubMed] [Google Scholar]
- Apostolou E, Thanos D. Virus Infection Induces NF-kappaB-dependent interchromosomal associations mediating monoallelic IFN-beta gene expression. Cell. 2008;134:85–96. doi: 10.1016/j.cell.2008.05.052. [DOI] [PubMed] [Google Scholar]
- Augello MA, Hickey TE, Knudsen KE. FOXA1: master of steroid receptor function in cancer. EMBO J. 2011;30:3885–3894. doi: 10.1038/emboj.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery OT, Macleod CM, McCarty M. Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types: Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type Iii. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr ML, Bertram EG. A morphological distinction between neurones of the male and female, and the behaviour of the nucleolar satellite during accelerated nucleoprotein synthesis. Nature. 1949;163:676. doi: 10.1038/163676a0. [DOI] [PubMed] [Google Scholar]
- Barrack ER. Steroid hormone receptor localization in the nuclear matrix: interaction with acceptor sites. J Steroid Biochem. 1987;27:115–121. doi: 10.1016/0022-4731(87)90302-5. [DOI] [PubMed] [Google Scholar]
- Barseguian K, Lutterbach B, Hiebert SW, Nickerson J, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Multiple subnuclear targeting signals of the leukemia-related AML1/ETO and ETO repressor proteins. Proc Natl Acad Sci U S A. 2002;99:15434–15439. doi: 10.1073/pnas.242588499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartova E, Krejci J, Harnicarova A, Kozubek S. Differentiation of human embryonic stem cells induces condensation of chromosome territories and formation of heterochromatin protein 1 foci. Differentiation. 2008;76:24–32. doi: 10.1111/j.1432-0436.2007.00192.x. [DOI] [PubMed] [Google Scholar]
- Bau D, Marti-Renom MA. Structure determination of genomic domains by satisfaction of spatial restraints. Chromosome Res. 2011;19:25–35. doi: 10.1007/s10577-010-9167-2. [DOI] [PubMed] [Google Scholar]
- Bender MA, Ragoczy T, Lee J, Byron R, Telling A, Dean A, Groudine M. The hypersensitive sites of the murine beta-globin locus control region act independently to affect nuclear localization and transcriptional elongation. Blood. 2012;119:3820–3827. doi: 10.1182/blood-2011-09-380485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R, Coffey DS. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974;60:1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berezney R, Coffey DS. Nuclear protein matrix: association with newly synthesized DNA. Science. 1975;189:291–293. doi: 10.1126/science.1145202. [DOI] [PubMed] [Google Scholar]
- Berezney R, Mortillaro MJ, Ma H, Wei X, Samarabandu J. The nuclear matrix: a structural milieu for genomic function. Int Rev Cytol. 1995;162A:1–65. doi: 10.1016/s0074-7696(08)61228-0. [DOI] [PubMed] [Google Scholar]
- Berezney R, Wei X. The new paradigm: integrating genomic function and nuclear architecture. J Cell Biochem Suppl. 1998;30–31:238–242. [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Bickmore WA, Teague P. Influences of chromosome size, gene density and nuclear position on the frequency of constitutional translocations in the human population. Chromosome Res. 2002;10:707–715. doi: 10.1023/a:1021589031769. [DOI] [PubMed] [Google Scholar]
- Bilican B, Goding CR. Cell cycle regulation of the T-box transcription factor tbx2. Exp Cell Res. 2006;312:2358–2366. doi: 10.1016/j.yexcr.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Billett MA, Barry JM. Role of histones in chromatin condensation. Eur J Biochem. 1974;49:477–484. doi: 10.1111/j.1432-1033.1974.tb03852.x. [DOI] [PubMed] [Google Scholar]
- Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clelland GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, Haydock A, Humbert R, James KD, Johnson BE, Johnson EM, Frum TT, Rosenzweig ER, Karnani N, Lee K, Lefebvre GC, Navas PA, Neri F, Parker SC, Sabo PJ, Sandstrom R, Shafer A, Vetrie D, Weaver M, Wilcox S, Yu M, Collins FS, Dekker J, Lieb JD, Tullius TD, Crawford GE, Sunyaev S, Noble WS, Dunham I, Denoeud F, Reymond A, Kapranov P, Rozowsky J, Zheng D, Castelo R, Frankish A, Harrow J, Ghosh S, Sandelin A, Hofacker IL, Baertsch R, Keefe D, Dike S, Cheng J, Hirsch HA, Sekinger EA, Lagarde J, Abril JF, Shahab A, Flamm C, Fried C, Hackermuller J, Hertel J, Lindemeyer M, Missal K, Tanzer A, Washietl S, Korbel J, Emanuelsson O, Pedersen JS, Holroyd N, Taylor R, Swarbreck D, Matthews N, Dickson MC, Thomas DJ, Weirauch MT, Gilbert J, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanove AJ, Voytas DF. TAL effectors: customizable proteins for DNA targeting. Science. 2011;333:1843–1846. doi: 10.1126/science.1204094. [DOI] [PubMed] [Google Scholar]
- Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8:574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- Bolzer A, Kreth G, Solovei I, Koehler D, Saracoglu K, Fauth C, Muller S, Eils R, Cremer C, Speicher MR, Cremer T. Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol. 2005;3:e157. doi: 10.1371/journal.pbio.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy E, Orsi GA, Couble P, Loppin B. The essential role of Drosophila HIRA for de novo assembly of paternal chromatin at fertilization. PLoS Genet. 2007;3:1991–2006. doi: 10.1371/journal.pgen.0030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfleth H, Edelmann P, Zink D, Cremer T, Cremer C. Quantitative motion analysis of subchromosomal foci in living cells using four-dimensional microscopy. Biophys J. 1999;77:2871–2886. doi: 10.1016/S0006-3495(99)77119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveri T. Die Blastomerenkerne von Ascaris megalocephala und die Theorie der Chromosomenindividualitat. Arch Zellforsch. 1909;3:181–268. [Google Scholar]
- Brasch K, Ochs RL. Nuclear bodies (NBs): a newly “rediscovered” organelle. Exp Cell Res. 1992;202:211–223. doi: 10.1016/0014-4827(92)90068-j. [DOI] [PubMed] [Google Scholar]
- Brickner DG, Ahmed S, Meldi L, Thompson A, Light W, Young M, Hickman TL, Chu F, Fabre E, Brickner JH. Transcription factor binding to a DNA zip code controls interchromosomal clustering at the nuclear periphery. Dev Cell. 2012;22:1234–1246. doi: 10.1016/j.devcel.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AR, Nagy BP, Taylor S, Simonet WS, Taylor JM, Levy-Wilson B. Sequences containing the second-intron enhancer are essential for transcription of the human apolipoprotein B gene in the livers of transgenic mice. Mol Cell Biol. 1994;14:2243–2256. doi: 10.1128/mcb.14.4.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brykczynska U, Hisano M, Erkek S, Ramos L, Oakeley EJ, Roloff TC, Beisel C, Schubeler D, Stadler MB, Peters AH. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol. 2010;17:679–687. doi: 10.1038/nsmb.1821. [DOI] [PubMed] [Google Scholar]
- Burgess-Beusse B, Farrell C, Gaszner M, Litt M, Mutskov V, Recillas-Targa F, Simpson M, West A, Felsenfeld G. The insulation of genes from external enhancers and silencing chromatin. Proc Natl Acad Sci U S A. 2002;99(Suppl 4):16433–16437. doi: 10.1073/pnas.162342499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabianca DS, Casa V, Bodega B, Xynos A, Ginelli E, Tanaka Y, Gabellini D. A long ncRNA links copy number variation to a polycomb/trithorax epigenetic switch in FSHD muscular dystrophy. Cell. 2012;149:819–831. doi: 10.1016/j.cell.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabianca DS, Gabellini D. The cell biology of disease: FSHD: copy number variations on the theme of muscular dystrophy. J Cell Biol. 2010;191:1049–1060. doi: 10.1083/jcb.201007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiulo A, Nicolis S, Bianchi P, Zuffardi O, Bardoni B, Maraschio P, Ottolenghi S, Camerino G, Giglioni B. Mapping the gene encoding the human erythroid transcriptional factor NFE1-GF1 to Xp11.23. Hum Genet. 1991;86:388–390. doi: 10.1007/BF00201840. [DOI] [PubMed] [Google Scholar]
- Cajal S. Un sencillo metodo de coloracion selectiva del reticulo protoplasmico y sus efectos en los diversos organos nerviosos de vertebrados e invertebrados. Trab Lab Investig Biol Univ Madr. 1903;2:129–221. [Google Scholar]
- Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nat Rev Genet. 2006;7:940–952. doi: 10.1038/nrg1906. [DOI] [PubMed] [Google Scholar]
- Caravaca JM, Donahue G, Becker JS, He X, Vinson C, Zaret KS. Bookmarking by specific and nonspecific binding of FoxA1 pioneer factor to mitotic chromosomes. Genes Dev. 2013;27:251–260. doi: 10.1101/gad.206458.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, Meissner A, Weng Z, Hofmann HA, Friedman N, Rando OJ. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal JJ, Keith A, Rigby PW. Global transcriptional regulation of the locus encoding the skeletal muscle determination genes Mrf4 and Myf5. Genes Dev. 2008;22:265–276. doi: 10.1101/gad.442408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G, Misteli T. Functional implications of genome topology. Nat Struct Mol Biol. 2013;20:290–299. doi: 10.1038/nsmb.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker SE, Dillon LA, Gerstein MB, Gunsalus KC, Henikoff S, Karpen GH, Kellis M, Lai EC, Lieb JD, MacAlpine DM, Micklem G, Piano F, Snyder M, Stein L, White KP, Waterston RH. Unlocking the secrets of the genome. Nature. 2009;459:927–930. doi: 10.1038/459927a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaly N, Munro SB, Swallow MA. Remodelling of the nuclear periphery during muscle cell differentiation in vitro. J Cell Biochem. 1996;62:76–89. doi: 10.1002/(sici)1097-4644(199607)62:1<76::aid-jcb9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Chen J, Young F, Bottaro A, Stewart V, Smith RK, Alt FW. Mutations of the intronic IgH enhancer and its flanking sequences differentially affect accessibility of the JH locus. EMBO J. 1993;12:4635–4645. doi: 10.1002/j.1460-2075.1993.tb06152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung SW, Tishler PV, Atkins L, Sengupta SK, Modest EJ, Forget BG. Gene mapping by fluorescent in situ hybridization. Cell Biol Int Rep. 1977;1:255–262. doi: 10.1016/0309-1651(77)90050-9. [DOI] [PubMed] [Google Scholar]
- Chiarle R, Zhang Y, Frock RL, Lewis SM, Molinie B, Ho YJ, Myers DR, Choi VW, Compagno M, Malkin DJ, Neuberg D, Monti S, Giallourakis CC, Gostissa M, Alt FW. Genome-wide translocation sequencing reveals mechanisms of chromosome breaks and rearrangements in B cells. Cell. 2011;147:107–119. doi: 10.1016/j.cell.2011.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiolo I, Minoda A, Colmenares SU, Polyzos A, Costes SV, Karpen GH. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell. 2011;144:732–744. doi: 10.1016/j.cell.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JC, Yen Z, Ziesche SM, Brown CJ. Silencing of the mammalian X chromosome. Annu Rev Genomics Hum Genet. 2005;6:69–92. doi: 10.1146/annurev.genom.6.080604.162350. [DOI] [PubMed] [Google Scholar]
- Chubb JR, Boyle S, Perry P, Bickmore WA. Chromatin motion is constrained by association with nuclear compartments in human cells. Curr Biol. 2002;12:439–445. doi: 10.1016/s0960-9822(02)00695-4. [DOI] [PubMed] [Google Scholar]
- Chubb JR, Trcek T, Shenoy SM, Singer RH. Transcriptional pulsing of a developmental gene. Curr Biol. 2006;16:1018–1025. doi: 10.1016/j.cub.2006.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9:279–289. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- Cockerill PN, Yuen MH, Garrard WT. The enhancer of the immunoglobulin heavy chain locus is flanked by presumptive chromosomal loop anchorage elements. J Biol Chem. 1987;262:5394–5397. [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. Duplicating a tangled genome. Science. 1998;281:1466–1467. doi: 10.1126/science.281.5382.1466. [DOI] [PubMed] [Google Scholar]
- Cremer M, Grasser F, Lanctot C, Muller S, Neusser M, Zinner R, Solovei I, Cremer T. Multicolor 3D fluorescence in situ hybridization for imaging interphase chromosomes. Methods Mol Biol. 2008;463:205–239. doi: 10.1007/978-1-59745-406-3_15. [DOI] [PubMed] [Google Scholar]
- Cremer M, Kupper K, Wagler B, Wizelman L, von Hase J, Weiland Y, Kreja L, Diebold J, Speicher MR, Cremer T. Inheritance of gene density-related higher order chromatin arrangements in normal and tumor cell nuclei. J Cell Biol. 2003;162:809–820. doi: 10.1083/jcb.200304096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FH. On protein synthesis. Symp Soc Exp Biol. 1958;12:138–163. [PubMed] [Google Scholar]
- Croft JA, Bridger JM, Boyle S, Perry P, Teague P, Bickmore WA. Differences in the localization and morphology of chromosomes in the human nucleus. J Cell Biol. 1999;145:1119–1131. doi: 10.1083/jcb.145.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daujat S, Bauer UM, Shah V, Turner B, Berger S, Kouzarides T. Crosstalk between CARM1 methylation and CBP acetylation on histone H3. Curr Biol. 2002;12:2090–2097. doi: 10.1016/s0960-9822(02)01387-8. [DOI] [PubMed] [Google Scholar]
- Davies HG. Fine structure of heterochromatin in certain cell nuclei. Nature. 1967;214:208–210. doi: 10.1038/214208a0. [DOI] [PubMed] [Google Scholar]
- De Santa F, Barozzi I, Mietton F, Ghisletti S, Polletti S, Tusi BK, Muller H, Ragoussis J, Wei CL, Natoli G. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010;8:e1000384. doi: 10.1371/journal.pbio.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degner SC, Verma-Gaur J, Wong TP, Bossen C, Iverson GM, Torkamani A, Vettermann C, Lin YC, Ju Z, Schulz D, Murre CS, Birshtein BK, Schork NJ, Schlissel MS, Riblet R, Murre C, Feeney AJ. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc Natl Acad Sci U S A. 2011;108:9566–9571. doi: 10.1073/pnas.1019391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Marti-Renom MA, Mirny LA. Exploring the three-dimensional organization of genomes: interpreting chromatin interaction data. Nat Rev Genet. 2013;14:390–403. doi: 10.1038/nrg3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- Delcuve GP, He S, Davie JR. Mitotic partitioning of transcription factors. J Cell Biochem. 2008;105:1–8. doi: 10.1002/jcb.21806. [DOI] [PubMed] [Google Scholar]
- Deng W, Lee J, Wang H, Miller J, Reik A, Gregory PD, Dean A, Blobel GA. Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell. 2012;149:1233–1244. doi: 10.1016/j.cell.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E, Berg P. Transcription from a plant gene promoter in animal cells. Nucleic Acids Res. 1985;13:7945–7957. doi: 10.1093/nar/13.22.7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson LA, Joh T, Kohwi Y, Kohwi-Shigematsu T. A tissue-specific MAR/SAR DNA-binding protein with unusual binding site recognition. Cell. 1992;70:631–645. doi: 10.1016/0092-8674(92)90432-c. [DOI] [PubMed] [Google Scholar]
- Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Roder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigo R, Gingeras TR. Landscape of transcription in human cells. Nature. 2012a;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebali S, Lagarde J, Kapranov P, Lacroix V, Borel C, Mudge JM, Howald C, Foissac S, Ucla C, Chrast J, Ribeca P, Martin D, Murray RR, Yang X, Ghamsari L, Lin C, Bell I, Dumais E, Drenkow J, Tress ML, Gelpi JL, Orozco M, Valencia A, van Berkum NL, Lajoie BR, Vidal M, Stamatoyannopoulos J, Batut P, Dobin A, Harrow J, Hubbard T, Dekker J, Frankish A, Salehi-Ashtiani K, Reymond A, Antonarakis SE, Guigo R, Gingeras TR. Evidence for transcript networks composed of chimeric RNAs in human cells. PLoS One. 2012b;7:e28213. doi: 10.1371/journal.pone.0028213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobie KW, Lee M, Fantes JA, Graham E, Clark AJ, Springbett A, Lathe R, McClenaghan M. Variegated transgene expression in mouse mammary gland is determined by the transgene integration locus. Proc Natl Acad Sci U S A. 1996;93:6659–6664. doi: 10.1073/pnas.93.13.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drissen R, Palstra RJ, Gillemans N, Splinter E, Grosveld F, Philipsen S, de Laat W. The active spatial organization of the beta-globin locus requires the transcription factor EKLF. Genes Dev. 2004;18:2485–2490. doi: 10.1101/gad.317004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z, Andronescu M, Schutz K, McIlwain S, Kim YJ, Lee C, Shendure J, Fields S, Blau CA, Noble WS. A three-dimensional model of the yeast genome. Nature. 2010;465:363–367. doi: 10.1038/nature08973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaway M, Droge P. Transactivation of the Xenopus rRNA gene promoter by its enhancer. Nature. 1989;341:657–659. doi: 10.1038/341657a0. [DOI] [PubMed] [Google Scholar]
- Dworetzky SI, Wright KL, Fey EG, Penman S, Lian JB, Stein JL, Stein GS. Sequence-specific DNA-binding proteins are components of a nuclear matrix-attachment site. Proc Natl Acad Sci U S A. 1992;89:4178–4182. doi: 10.1073/pnas.89.9.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann P, Bornfleth H, Zink D, Cremer T, Cremer C. Morphology and dynamics of chromosome territories in living cells. Biochim Biophys Acta. 2001;1551:M29–39. doi: 10.1016/s0304-419x(01)00023-3. [DOI] [PubMed] [Google Scholar]
- Eils R, Dietzel S, Bertin E, Schrock E, Speicher MR, Ried T, Robert-Nicoud M, Cremer C, Cremer T. Three-dimensional reconstruction of painted human interphase chromosomes: active and inactive X chromosome territories have similar volumes but differ in shape and surface structure. J Cell Biol. 1996;135:1427–1440. doi: 10.1083/jcb.135.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson P, Gao J, Chang KS, Look T, Whisenant E, Raimondi S, Lasher R, Trujillo J, Rowley J, Drabkin H. Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood. 1992;80:1825–1831. [PubMed] [Google Scholar]
- Faro-Trindade I, Cook PR. A conserved organization of transcription during embryonic stem cell differentiation and in cells with high C value. Mol Biol Cell. 2006a;17:2910–2920. doi: 10.1091/mbc.E05-11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faro-Trindade I, Cook PR. Transcription factories: structures conserved during differentiation and evolution. Biochem Soc Trans. 2006b;34:1133–1137. doi: 10.1042/BST0341133. [DOI] [PubMed] [Google Scholar]
- Finlan LE, Sproul D, Thomson I, Boyle S, Kerr E, Perry P, Ylstra B, Chubb JR, Bickmore WA. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 2008;4:e1000039. doi: 10.1371/journal.pgen.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AH, Bardarov S, Jr, Jiang Z. Molecular aspects of diagnostic nucleolar and nuclear envelope changes in prostate cancer. J Cell Biochem. 2004;91:170–184. doi: 10.1002/jcb.10735. [DOI] [PubMed] [Google Scholar]
- Flemming W. Vogel, FCW. 1882. Zellsubstanz, Kern und Zelltheilung. [Google Scholar]
- Fox AH, Lam YW, Leung AK, Lyon CE, Andersen J, Mann M, Lamond AI. Paraspeckles: a novel nuclear domain. Curr Biol. 2002;12:13–25. doi: 10.1016/s0960-9822(01)00632-7. [DOI] [PubMed] [Google Scholar]
- Fraser J, Rousseau M, Shenker S, Ferraiuolo MA, Hayashizaki Y, Blanchette M, Dostie J. Chromatin conformation signatures of cellular differentiation. Genome Biol. 2009;10:R37. doi: 10.1186/gb-2009-10-4-r37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH, Chew EG, Huang PY, Welboren WJ, Han Y, Ooi HS, Ariyaratne PN, Vega VB, Luo Y, Tan PY, Choy PY, Wansa KD, Zhao B, Lim KS, Leow SC, Yow JS, Joseph R, Li H, Desai KV, Thomsen JS, Lee YK, Karuturi RK, Herve T, Bourque G, Stunnenberg HG, Ruan X, Cacheux-Rataboul V, Sung WK, Liu ET, Wei CL, Cheung E, Ruan Y. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462:58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG, Murphy C, Callan HG, Wu ZA. Lampbrush chromosomes. Methods Cell Biol. 1991;36:149–166. [PubMed] [Google Scholar]
- Gao F, Wei Z, An W, Wang K, Lu W. The interactomes of POU5F1 and SOX2 enhancers in human embryonic stem cells. Sci Rep. 2013;3:1588. doi: 10.1038/srep01588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getzenberg RH. Nuclear matrix and the regulation of gene expression: tissue specificity. J Cell Biochem. 1994;55:22–31. doi: 10.1002/jcb.240550105. [DOI] [PubMed] [Google Scholar]
- Getzenberg RH, Coffey DS. Tissue specificity of the hormonal response in sex accessory tissues is associated with nuclear matrix protein patterns. Mol Endocrinol. 1990;4:1336–1342. doi: 10.1210/mend-4-9-1336. [DOI] [PubMed] [Google Scholar]
- Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS. CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras TR. Implications of chimaeric non-co-linear transcripts. Nature. 2009;461:206–211. doi: 10.1038/nature08452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girst S, Hable V, Drexler GA, Greubel C, Siebenwirth C, Haum M, Friedl AA, Dollinger G. Subdiffusion supports joining of correct ends during repair of DNA double-strand breaks. Sci Rep. 2013;3:2511. doi: 10.1038/srep02511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluch A, Vidakovic M, Bode J. Scaffold/matrix attachment regions (S/MARs): relevance for disease and therapy. Handb Exp Pharmacol. 2008:67–103. doi: 10.1007/978-3-540-72843-6_4. [DOI] [PubMed] [Google Scholar]
- Goodman RH, Spiro D. Electron microscopic studies of giant salivary gland chromosomes. Exp Cell Res. 1962;27:359–363. doi: 10.1016/0014-4827(62)90246-x. [DOI] [PubMed] [Google Scholar]
- Grosveld F, van Assendelft GB, Greaves DR, Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, van Steensel B. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–951. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- Guo B, Odgren PR, van Wijnen AJ, Last TJ, Nickerson J, Penman S, Lian JB, Stein JL, Stein GS. The nuclear matrix protein NMP-1 is the transcription factor YY1. Proc Natl Acad Sci U S A. 1995;92:10526–10530. doi: 10.1073/pnas.92.23.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Monahan K, Wu H, Gertz J, Varley KE, Li W, Myers RM, Maniatis T, Wu Q. CTCF/cohesin-mediated DNA looping is required for protocadherin alpha promoter choice. Proc Natl Acad Sci U S A. 2012;109:21081–21086. doi: 10.1073/pnas.1219280110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadchouel J, Carvajal JJ, Daubas P, Bajard L, Chang T, Rocancourt D, Cox D, Summerbell D, Tajbakhsh S, Rigby PW, Buckingham M. Analysis of a key regulatory region upstream of the Myf5 gene reveals multiple phases of myogenesis, orchestrated at each site by a combination of elements dispersed throughout the locus. Development. 2003;130:3415–3426. doi: 10.1242/dev.00552. [DOI] [PubMed] [Google Scholar]
- Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handoko L, Xu H, Li G, Ngan CY, Chew E, Schnapp M, Lee CW, Ye C, Ping JL, Mulawadi F, Wong E, Sheng J, Zhang Y, Poh T, Chan CS, Kunarso G, Shahab A, Bourque G, Cacheux-Rataboul V, Sung WK, Ruan Y, Wei CL. CTCF-mediated functional chromatin interactome in pluripotent cells. Nat Genet. 2011;43:630–638. doi: 10.1038/ng.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, van Oudenaarden A, Jaenisch R. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED, Revel JP. The fine structure of the DNP component of the nucleus. An electron microscopic study utilizing autoradiography to localize DNA synthesis. J Cell Biol. 1963;16:29–51. doi: 10.1083/jcb.16.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DC, Martin T, Penman S. Localization of heterogeneous nuclear ribonucleoprotein in the interphase nuclear matrix core filaments and on perichromosomal filaments at mitosis. Proc Natl Acad Sci U S A. 1991;88:7469–7473. doi: 10.1073/pnas.88.17.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N, Stam K, Groffen J, de Klein A, Grosveld G. Structural organization of the bcr gene and its role in the Ph’ translocation. Nature. 1985;315:758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Heitz E. Das heterochromatin der Moose. Jehrb Wiss Botanik. 1928;69:762–818. [Google Scholar]
- Hepperger C, Mannes A, Merz J, Peters J, Dietzel S. Three-dimensional positioning of genes in mouse cell nuclei. Chromosoma. 2008;117:535–551. doi: 10.1007/s00412-008-0168-2. [DOI] [PubMed] [Google Scholar]
- Hertwig O, Campbell HJ. The cell; outlines of general anatomy and physiology. xvi. Sonnenschein; London: 1895. p. 368. [Google Scholar]
- Hou C, Li L, Qin ZS, Corces VG. Gene density, transcription, and insulators contribute to the partition of the Drosophila genome into physical domains. Mol Cell. 2012;48:471–484. doi: 10.1016/j.molcel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison N, Weintraub H. Localization of DNAase I-sensitive sequences to specific regions of interphase nuclei. Cell. 1985;43:471–482. doi: 10.1016/0092-8674(85)90177-1. [DOI] [PubMed] [Google Scholar]
- Iborra FJ, Pombo A, Jackson DA, Cook PR. Active RNA polymerases are localized within discrete transcription “factories’ in human nuclei. J Cell Sci. 1996a;109 (Pt 6):1427–1436. doi: 10.1242/jcs.109.6.1427. [DOI] [PubMed] [Google Scholar]
- Iborra FJ, Pombo A, McManus J, Jackson DA, Cook PR. The topology of transcription by immobilized polymerases. Exp Cell Res. 1996b;229:167–173. doi: 10.1006/excr.1996.0355. [DOI] [PubMed] [Google Scholar]
- Jackson DA, Pombo A. Replicon clusters are stable units of chromosome structure: evidence that nuclear organization contributes to the efficient activation and propagation of S phase in human cells. J Cell Biol. 1998;140:1285–1295. doi: 10.1083/jcb.140.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F, Brenner S. On the regulation of DNA synthesis in bacteria: the hypothesis of the replicon. C R Hebd Seances Acad Sci. 1963;256:298–300. [PubMed] [Google Scholar]
- Jacobs EY, Frey MR, Wu W, Ingledue TC, Gebuhr TC, Gao L, Marzluff WF, Matera AG. Coiled bodies preferentially associate with U4, U11, and U12 small nuclear RNA genes in interphase HeLa cells but not with U6 and U7 genes. Mol Biol Cell. 1999;10:1653–1663. doi: 10.1091/mbc.10.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Jing Y, Cost GJ, Chiang JC, Kolpa HJ, Cotton AM, Carone DM, Carone BR, Shivak DA, Guschin DY, Pearl JR, Rebar EJ, Byron M, Gregory PD, Brown CJ, Urnov FD, Hall LL, Lawrence JB. Translating dosage compensation to trisomy 21. Nature. 2013 doi: 10.1038/nature12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Vakoc CR, Ying L, Mandat S, Wang H, Zheng X, Blobel GA. Exchange of GATA factors mediates transitions in looped chromatin organization at a developmentally regulated gene locus. Mol Cell. 2008;29:232–242. doi: 10.1016/j.molcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junier I, Dale RK, Hou C, Kepes F, Dean A. CTCF-mediated transcriptional regulation through cell type-specific chromosome organization in the beta-globin locus. Nucleic Acids Res. 2012;40:7718–7727. doi: 10.1093/nar/gks536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadauke S, Blobel GA. Mitotic bookmarking by transcription factors. Epigenetics Chromatin. 2013;6:6. doi: 10.1186/1756-8935-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadauke S, Udugama MI, Pawlicki JM, Achtman JC, Jain DP, Cheng Y, Hardison RC, Blobel GA. Tissue-specific mitotic bookmarking by hematopoietic transcription factor GATA1. Cell. 2012;150:725–737. doi: 10.1016/j.cell.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind J, van Steensel B. Genome-nuclear lamina interactions and gene regulation. Curr Opin Cell Biol. 2010;22:320–325. doi: 10.1016/j.ceb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Kmita M, Duboule D. Organizing axes in time and space; 25 years of colinear tinkering. Science. 2003;301:331–333. doi: 10.1126/science.1085753. [DOI] [PubMed] [Google Scholar]
- Knoepfler PS. Why myc? An unexpected ingredient in the stem cell cocktail. Cell Stem Cell. 2008;2:18–21. doi: 10.1016/j.stem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Knutsen T, Gobu V, Knaus R, Padilla-Nash H, Augustus M, Strausberg RL, Kirsch IR, Sirotkin K, Ried T. The interactive online SKY/M-FISH & CGH database and the Entrez cancer chromosomes search database: linkage of chromosomal aberrations with the genome sequence. Genes Chromosomes Cancer. 2005;44:52–64. doi: 10.1002/gcc.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris CA, Nowaczyk MJ, Toriello H, Bamshad MJ, Carey JC, Rappaport E, Kawauchi S, Lander AD, Calof AL, Li HH, Devoto M, Jackson LG. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet. 2004;36:631–635. doi: 10.1038/ng1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubben N, Adriaens M, Meuleman W, Voncken JW, van Steensel B, Misteli T. Mapping of lamin A- and progerin-interacting genome regions. Chromosoma. 2012;121:447–464. doi: 10.1007/s00412-012-0376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JD, Cook PR, Papantonis A. Dynamic reconfiguration of long human genes during one transcription cycle. Mol Cell Biol. 2012;32:2738–2747. doi: 10.1128/MCB.00179-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JD, Papantonis A, Cook PR. Promoter type influences transcriptional topography by targeting genes to distinct nucleoplasmic sites. J Cell Sci. 2013a;126:2052–2059. doi: 10.1242/jcs.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JD, Papantonis A, Cook PR, Marenduzzo D. Space exploration by the promoter of a long human gene during one transcription cycle. Nucleic Acids Res. 2013b;41:2216–2227. doi: 10.1093/nar/gks1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson DR, Singer RH, Zenklusen D. A single molecule view of gene expression. Trends Cell Biol. 2009;19:630–637. doi: 10.1016/j.tcb.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSalle JM, Lalande M. Homologous association of oppositely imprinted chromosomal domains. Science. 1996;272:725–728. doi: 10.1126/science.272.5262.725. [DOI] [PubMed] [Google Scholar]
- Lee W, Mitchell P, Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987;49:741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lemmers RJ, van der Vliet PJ, Klooster R, Sacconi S, Camano P, Dauwerse JG, Snider L, Straasheijm KR, van Ommen GJ, Padberg GW, Miller DG, Tapscott SJ, Tawil R, Frants RR, van der Maarel SM. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science. 2010;329:1650–1653. doi: 10.1126/science.1189044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Wilson B. ADP-ribosylation of trout testis chromosomal proteins: distribution of ADP-ribosylated proteins among DNase I-sensitive and -resistant chromatin domains. Arch Biochem Biophys. 1981;208:528–534. doi: 10.1016/0003-9861(81)90541-5. [DOI] [PubMed] [Google Scholar]
- Lewis E. The Theory and Application of a New Method of Detecting Chromosomal Rearrangements in Drosophila melanogaster. The American Naturalist. 1954;88:225–239. [Google Scholar]
- Li G, Ruan X, Auerbach RK, Sandhu KS, Zheng M, Wang P, Poh HM, Goh Y, Lim J, Zhang J, Sim HS, Peh SQ, Mulawadi FH, Ong CT, Orlov YL, Hong S, Zhang Z, Landt S, Raha D, Euskirchen G, Wei CL, Ge W, Wang H, Davis C, Fisher-Aylor KI, Mortazavi A, Gerstein M, Gingeras T, Wold B, Sun Y, Fullwood MJ, Cheung E, Liu E, Sung WK, Snyder M, Ruan Y. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell. 2012;148:84–98. doi: 10.1016/j.cell.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Heermann DW. Using chimaeric expression sequence tag as the reference to identify three-dimensional chromosome contacts. DNA Res. 2013;20:45–53. doi: 10.1093/dnares/dss032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, Sandstrom R, Bernstein B, Bender MA, Groudine M, Gnirke A, Stamatoyannopoulos J, Mirny LA, Lander ES, Dekker J. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littau VC, Burdick CJ, Allfrey VG, Mirsky SA. The role of histones in the maintenance of chromatin structure. Proc Natl Acad Sci U S A. 1965;54:1204–1212. doi: 10.1073/pnas.54.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Krantz ID. Cohesin and human disease. Annu Rev Genomics Hum Genet. 2008;9:303–320. doi: 10.1146/annurev.genom.9.081307.164211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang Z, Bando M, Itoh T, Deardorff MA, Clark D, Kaur M, Tandy S, Kondoh T, Rappaport E, Spinner NB, Vega H, Jackson LG, Shirahige K, Krantz ID. Transcriptional dysregulation in NIPBL and cohesin mutant human cells. PLoS Biol. 2009;7:e1000119. doi: 10.1371/journal.pbio.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobanenkov VV, Nicolas RH, Adler VV, Paterson H, Klenova EM, Polotskaja AV, Goodwin GH. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5′-flanking sequence of the chicken c-myc gene. Oncogene. 1990;5:1743–1753. [PubMed] [Google Scholar]
- Lomvardas S, Barnea G, Pisapia DJ, Mendelsohn M, Kirkland J, Axel R. Interchromosomal interactions and olfactory receptor choice. Cell. 2006;126:403–413. doi: 10.1016/j.cell.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Lyon MF. Sex chromatin and gene action in the mammalian X-chromosome. Am J Hum Genet. 1962;14:135–148. [PMC free article] [PubMed] [Google Scholar]
- Ma H, Samarabandu J, Devdhar RS, Acharya R, Cheng PC, Meng C, Berezney R. Spatial and temporal dynamics of DNA replication sites in mammalian cells. J Cell Biol. 1998;143:1415–1425. doi: 10.1083/jcb.143.6.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi T, Katsani KR, Verrijzer CP. GAGA can mediate enhancer function in trans by linking two separate DNA molecules. EMBO J. 2002;21:1775–1781. doi: 10.1093/emboj/21.7.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Straight A, Marko JF, Swedlow J, Dernburg A, Belmont A, Murray AW, Agard DA, Sedat JW. Interphase chromosomes undergo constrained diffusional motion in living cells. Curr Biol. 1997;7:930–939. doi: 10.1016/s0960-9822(06)00412-x. [DOI] [PubMed] [Google Scholar]
- McClintock B. The relation of a particular chromosomal element to the development of the nucleoli in Zea mays. Zeitschrift fur Zellforschung und mikroskopische Anatomie. 1934;2:294–328. [Google Scholar]
- McGuffee SR, Smith DJ, Whitehouse I. Quantitative, genome-wide analysis of eukaryotic replication initiation and termination. Mol Cell. 2013;50:123–135. doi: 10.1016/j.molcel.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown PC, Shaw PJ. Chromatin: linking structure and function in the nucleolus. Chromosoma. 2009;118:11–23. doi: 10.1007/s00412-008-0184-2. [DOI] [PubMed] [Google Scholar]
- McNeil S, Zeng C, Harrington KS, Hiebert S, Lian JB, Stein JL, van Wijnen AJ, Stein GS. The t(8;21) chromosomal translocation in acute myelogenous leukemia modifies intranuclear targeting of the AML1/CBFalpha2 transcription factor. Proc Natl Acad Sci U S A. 1999;96:14882–14887. doi: 10.1073/pnas.96.26.14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina R, Ghule PN, Cruzat F, Barutcu AR, Montecino M, Stein JL, van Wijnen AJ, Stein GS. Epigenetic control of cell cycle-dependent histone gene expression is a principal component of the abbreviated pluripotent cell cycle. Mol Cell Biol. 2012;32:3860–3871. doi: 10.1128/MCB.00736-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister P, Poidevin M, Francesconi S, Tratner I, Zarzov P, Baldacci G. Nuclear factories for signalling and repairing DNA double strand breaks in living fission yeast. Nucleic Acids Res. 2003;31:5064–5073. doi: 10.1093/nar/gkg719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnik S, Deng B, Papantonis A, Baboo S, Carr IM, Cook PR. The proteomes of transcription factories containing RNA polymerases I, II or III. Nat Methods. 2011;8:963–968. doi: 10.1038/nmeth.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall EM, Williamson KE, Reyon D, Zou JY, Ram O, Joung JK, Bernstein BE. Locus-specific editing of histone modifications at endogenous enhancers. Nat Biotechnol. 2013 doi: 10.1038/nbt.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- Miele A, Dekker J. Long-range chromosomal interactions and gene regulation. Mol Biosyst. 2008;4:1046–1057. doi: 10.1039/b803580f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O’Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T. The cell biology of genomes: bringing the double helix to life. Cell. 2013;152:1209–1212. doi: 10.1016/j.cell.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JA, Fraser P. Transcription factories are nuclear subcompartments that remain in the absence of transcription. Genes Dev. 2008;22:20–25. doi: 10.1101/gad.454008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Shimizu K, Kozu T, Maseki N, Kaneko Y, Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc Natl Acad Sci U S A. 1991;88:10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneron A, Bernhard W. Fine structural organization of the interphase nucleus in some mammalian cells. J Ultrastruct Res. 1969;27:266–288. doi: 10.1016/s0022-5320(69)80017-1. [DOI] [PubMed] [Google Scholar]
- Morey C, Da Silva NR, Perry P, Bickmore WA. Nuclear reorganisation and chromatin decondensation are conserved, but distinct, mechanisms linked to Hox gene activation. Development. 2007;134:909–919. doi: 10.1242/dev.02779. [DOI] [PubMed] [Google Scholar]
- Morey C, Kress C, Bickmore WA. Lack of bystander activation shows that localization exterior to chromosome territories is not sufficient to up-regulate gene expression. Genome Res. 2009;19:1184–1194. doi: 10.1101/gr.089045.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi K, Zare H, Dell’orso S, Grontved L, Gutierrez-Cruz G, Derfoul A, Hager GL, Sartorelli V. eRNAs Promote Transcription by Establishing Chromatin Accessibility at Defined Genomic Loci. Mol Cell. 2013;51:606–617. doi: 10.1016/j.molcel.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Storm HP, Sogo JM, Schaffner W. An enhancer stimulates transcription in trans when attached to the promoter via a protein bridge. Cell. 1989;58:767–777. doi: 10.1016/0092-8674(89)90110-4. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Morita T, Sato C. Structural organizations of replicon domains during DNA synthetic phase in the mammalian nucleus. Exp Cell Res. 1986;165:291–297. doi: 10.1016/0014-4827(86)90583-5. [DOI] [PubMed] [Google Scholar]
- Nemeth A, Conesa A, Santoyo-Lopez J, Medina I, Montaner D, Peterfia B, Solovei I, Cremer T, Dopazo J, Langst G. Initial genomics of the human nucleolus. PLoS Genet. 2010;6:e1000889. doi: 10.1371/journal.pgen.1000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- Nolen LD, Boyle S, Ansari M, Pritchard E, Bickmore WA. Regional chromatin decompaction in Cornelia de Lange syndrome associated with NIPBL disruption can be uncoupled from cohesin and CTCF. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer D, Leleu M, Splinter E, Rougemont J, De Laat W, Duboule D. The dynamic architecture of Hox gene clusters. Science. 2011;334:222–225. doi: 10.1126/science.1207194. [DOI] [PubMed] [Google Scholar]
- Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, Piolot T, van Berkum NL, Meisig J, Sedat J, Gribnau J, Barillot E, Bluthgen N, Dekker J, Heard E. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85–109. [PubMed] [Google Scholar]
- O’Sullivan JM, Tan-Wong SM, Morillon A, Lee B, Coles J, Mellor J, Proudfoot NJ. Gene loops juxtapose promoters and terminators in yeast. Nat Genet. 2004;36:1014–1018. doi: 10.1038/ng1411. [DOI] [PubMed] [Google Scholar]
- Ohlsson R, Bartkuhn M, Renkawitz R. CTCF shapes chromatin by multiple mechanisms: the impact of 20 years of CTCF research on understanding the workings of chromatin. Chromosoma. 2010;119:351–360. doi: 10.1007/s00412-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- Orsi GA, Algazeery A, Meyer RE, Capri M, Sapey-Triomphe LM, Horard B, Gruffat H, Couble P, Ait-Ahmed O, Loppin B. Drosophila Yemanuclein and HIRA cooperate for de novo assembly of H3.3-containing nucleosomes in the male pronucleus. PLoS Genet. 2013;9:e1003285. doi: 10.1371/journal.pgen.1003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palstra RJ, Simonis M, Klous P, Brasset E, Eijkelkamp B, de Laat W. Maintenance of long-range DNA interactions after inhibition of ongoing RNA polymerase II transcription. PLoS One. 2008;3:e1661. doi: 10.1371/journal.pone.0001661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palstra RJ, Tolhuis B, Splinter E, Nijmeijer R, Grosveld F, de Laat W. The beta-globin nuclear compartment in development and erythroid differentiation. Nat Genet. 2003;35:190–194. doi: 10.1038/ng1244. [DOI] [PubMed] [Google Scholar]
- Papantonis A, Cook PR. Transcription Factories: Genome Organization and Gene Regulation. Chem Rev. 2013 doi: 10.1021/cr300513p. [DOI] [PubMed] [Google Scholar]
- Papantonis A, Kohro T, Baboo S, Larkin JD, Deng B, Short P, Tsutsumi S, Taylor S, Kanki Y, Kobayashi M, Li G, Poh HM, Ruan X, Aburatani H, Ruan Y, Kodama T, Wada Y, Cook PR. TNFalpha signals through specialized factories where responsive coding and miRNA genes are transcribed. EMBO J. 2012;31:4404–4414. doi: 10.1038/emboj.2012.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papantonis A, Larkin JD, Wada Y, Ohta Y, Ihara S, Kodama T, Cook PR. Active RNA polymerases: mobile or immobile molecular machines? PLoS Biol. 2010;8:e1000419. doi: 10.1371/journal.pbio.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada LA, McQueen PG, Misteli T. Tissue-specific spatial organization of genomes. Genome Biol. 2004;5:R44. doi: 10.1186/gb-2004-5-7-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada LA, McQueen PG, Munson PJ, Misteli T. Conservation of relative chromosome positioning in normal and cancer cells. Curr Biol. 2002;12:1692–1697. doi: 10.1016/s0960-9822(02)01166-1. [DOI] [PubMed] [Google Scholar]
- Parelho V, Hadjur S, Spivakov M, Leleu M, Sauer S, Gregson HC, Jarmuz A, Canzonetta C, Webster Z, Nesterova T, Cobb BS, Yokomori K, Dillon N, Aragon L, Fisher AG, Merkenschlager M. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell. 2008;132:422–433. doi: 10.1016/j.cell.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Paweletz N. Walther Flemming: pioneer of mitosis research. Nat Rev Mol Cell Biol. 2001;2:72–75. doi: 10.1038/35048077. [DOI] [PubMed] [Google Scholar]
- Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SW, Solovei I, Brugman W, Graf S, Flicek P, Kerkhoven RM, van Lohuizen M, Reinders M, Wessels L, van Steensel B. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38:603–613. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MW, Boettiger AN, Bothma JP, Levine M. Shadow enhancers foster robustness of Drosophila gastrulation. Curr Biol. 2010;20:1562–1567. doi: 10.1016/j.cub.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MW, Boettiger AN, Levine M. Multiple enhancers ensure precision of gap gene-expression patterns in the Drosophila embryo. Proc Natl Acad Sci U S A. 2011;108:13570–13575. doi: 10.1073/pnas.1109873108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Cremins JE, Sauria ME, Sanyal A, Gerasimova TI, Lajoie BR, Bell JS, Ong CT, Hookway TA, Guo C, Sun Y, Bland MJ, Wagstaff W, Dalton S, McDevitt TC, Sen R, Dekker J, Taylor J, Corces VG. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell. 2013;153:1281–1295. doi: 10.1016/j.cell.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombo A, Hollinshead M, Cook PR. Bridging the resolution gap: Imaging the same transcription factories in cryosections by light and electron microscopy. J Histochem Cytochem. 1999a;47:471–480. doi: 10.1177/002215549904700405. [DOI] [PubMed] [Google Scholar]
- Pombo A, Jackson DA, Hollinshead M, Wang Z, Roeder RG, Cook PR. Regional specialization in human nuclei: visualization of discrete sites of transcription by RNA polymerase III. EMBO J. 1999b;18:2241–2253. doi: 10.1093/emboj/18.8.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl C. Uber Zelltheilung. Morph Jb. 1885;10:214–330. [Google Scholar]
- Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 2006;4:e309. doi: 10.1371/journal.pbio.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell. 2008;135:216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J, Ellard S. The laminopathies: a clinical review. Clin Genet. 2006;70:261–274. doi: 10.1111/j.1399-0004.2006.00677.x. [DOI] [PubMed] [Google Scholar]
- Razin A, Cedar H. DNA methylation and gene expression. Microbiol Rev. 1991;55:451–458. doi: 10.1128/mr.55.3.451-458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- Robyr D, Friedli M, Gehrig C, Arcangeli M, Marin M, Guipponi M, Farinelli L, Barde I, Verp S, Trono D, Antonarakis SE. Chromosome conformation capture uncovers potential genome-wide interactions between human conserved non-coding sequences. PLoS One. 2011;6:e17634. doi: 10.1371/journal.pone.0017634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roix JJ, McQueen PG, Munson PJ, Parada LA, Misteli T. Spatial proximity of translocation-prone gene loci in human lymphomas. Nat Genet. 2003;34:287–291. doi: 10.1038/ng1177. [DOI] [PubMed] [Google Scholar]
- Ronneberger O, Baddeley D, Scheipl F, Verveer PJ, Burkhardt H, Cremer C, Fahrmeir L, Cremer T, Joffe B. Spatial quantitative analysis of fluorescently labeled nuclear structures: problems, methods, pitfalls. Chromosome Res. 2008;16:523–562. doi: 10.1007/s10577-008-1236-4. [DOI] [PubMed] [Google Scholar]
- Roussel P, Andre C, Comai L, Hernandez-Verdun D. The rDNA transcription machinery is assembled during mitosis in active NORs and absent in inactive NORs. J Cell Biol. 1996;133:235–246. doi: 10.1083/jcb.133.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JD. Identificaton of a translocation with quinacrine fluorescence in a patient with acute leukemia. Ann Genet. 1973a;16:109–112. [PubMed] [Google Scholar]
- Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973b;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Rubio ED, Reiss DJ, Welcsh PL, Disteche CM, Filippova GN, Baliga NS, Aebersold R, Ranish JA, Krumm A. CTCF physically links cohesin to chromatin. Proc Natl Acad Sci U S A. 2008;105:8309–8314. doi: 10.1073/pnas.0801273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz J, Wheeler J, Grimwood J, Dickson M, Yang J, Caoile C, Bajorek E, Black S, Chan YM, Denys M, Escobar J, Flowers D, Fotopulos D, Garcia C, Gomez M, Gonzales E, Haydu L, Lopez F, Ramirez L, Retterer J, Rodriguez A, Rogers S, Salazar A, Tsai M, Myers RM. Quality assessment of the human genome sequence. Nature. 2004;429:365–368. doi: 10.1038/nature02390. [DOI] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Schweiger MR, Barmeyer C, Timmermann B. Genomics and epigenomics: new promises of personalized medicine for cancer patients. Brief Funct Genomics. 2013;12:411–421. doi: 10.1093/bfgp/elt024. [DOI] [PubMed] [Google Scholar]
- Sexton T, Yaffe E, Kenigsberg E, Bantignies F, Leblanc B, Hoichman M, Parrinello H, Tanay A, Cavalli G. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell. 2012;148:458–472. doi: 10.1016/j.cell.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Simonis M, de Laat W. FISH-eyed and genome-wide views on the spatial organisation of gene expression. Biochim Biophys Acta. 2008;1783:2052–2060. doi: 10.1016/j.bbamcr.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Simonis M, Klous P, Splinter E, Moshkin Y, Willemsen R, de Wit E, van Steensel B, de Laat W. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C) Nat Genet. 2006;38:1348–1354. doi: 10.1038/ng1896. [DOI] [PubMed] [Google Scholar]
- Solovei I, Kreysing M, Lanctot C, Kosem S, Peichl L, Cremer T, Guck J, Joffe B. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell. 2009;137:356–368. doi: 10.1016/j.cell.2009.01.052. [DOI] [PubMed] [Google Scholar]
- Soufi A, Donahue G, Zaret KS. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutoglou E, Dorn JF, Sengupta K, Jasin M, Nussenzweig A, Ried T, Danuser G, Misteli T. Positional stability of single double-strand breaks in mammalian cells. Nat Cell Biol. 2007;9:675–682. doi: 10.1038/ncb1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan R, Tchieu J, Mason MJ, Yachechko R, Kuoy E, Horvath S, Zhou Q, Plath K. Role of the murine reprogramming factors in the induction of pluripotency. Cell. 2009;136:364–377. doi: 10.1016/j.cell.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack SM, Brown DB, Dewey WC. Visualization of interphase chromosomes. J Cell Sci. 1977;26:281–299. doi: 10.1242/jcs.26.1.281. [DOI] [PubMed] [Google Scholar]
- Stein GS, van Wijnen AJ, Stein JL, Lian JB, Montecino M, Choi J, Zaidi K, Javed A. Intranuclear trafficking of transcription factors: implications for biological control. J Cell Sci. 2000;113 (Pt 14):2527–2533. doi: 10.1242/jcs.113.14.2527. [DOI] [PubMed] [Google Scholar]
- Stein GS, Zaidi SK, Stein JL, Lian JB, van Wijnen AJ, Montecino M, Young DW, Javed A, Pratap J, Choi JY, Ali SA, Pande S, Hassan MQ. Transcription-factor-mediated epigenetic control of cell fate and lineage commitment. Biochem Cell Biol. 2009;87:1–6. doi: 10.1139/o08-094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stief A, Winter DM, Stratling WH, Sippel AE. A nuclear DNA attachment element mediates elevated and position-independent gene activity. Nature. 1989;341:343–345. doi: 10.1038/341343a0. [DOI] [PubMed] [Google Scholar]
- Stuurman N, Meijne AM, van der Pol AJ, de Jong L, van Driel R, van Renswoude J. The nuclear matrix from cells of different origin. Evidence for a common set of matrix proteins. J Biol Chem. 1990;265:5460–5465. [PubMed] [Google Scholar]
- Sutherland H, Bickmore WA. Transcription factories: gene expression in unions? Nat Rev Genet. 2009;10:457–466. doi: 10.1038/nrg2592. [DOI] [PubMed] [Google Scholar]
- Tai PW, Fisher-Aylor KI, Himeda CL, Smith CL, Mackenzie AP, Helterline DL, Angello JC, Welikson RE, Wold BJ, Hauschka SD. Differentiation and fiber type-specific activity of a muscle creatine kinase intronic enhancer. Skelet Muscle. 2011;1:25. doi: 10.1186/2044-5040-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tan-Wong SM, French JD, Proudfoot NJ, Brown MA. Dynamic interactions between the promoter and terminator regions of the mammalian BRCA1 gene. Proc Natl Acad Sci U S A. 2008;105:5160–5165. doi: 10.1073/pnas.0801048105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan-Wong SM, Wijayatilake HD, Proudfoot NJ. Gene loops function to maintain transcriptional memory through interaction with the nuclear pore complex. Genes Dev. 2009;23:2610–2624. doi: 10.1101/gad.1823209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe H, Muller S, Neusser M, von Hase J, Calcagno E, Cremer M, Solovei I, Cremer C, Cremer T. Evolutionary conservation of chromosome territory arrangements in cell nuclei from higher primates. Proc Natl Acad Sci U S A. 2002;99:4424–4429. doi: 10.1073/pnas.072618599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat Struct Mol Biol. 2007;14:1025–1040. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller K, Solovei I, Buiting K, Horsthemke B, Cremer T. Maintenance of imprinting and nuclear architecture in cycling cells. Proc Natl Acad Sci U S A. 2007;104:14970–14975. doi: 10.1073/pnas.0704285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M, Weiss MJ, Dekker J, Blobel GA. Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol Cell. 2005;17:453–462. doi: 10.1016/j.molcel.2004.12.028. [DOI] [PubMed] [Google Scholar]
- van Driel R, Wansink DG, van Steensel B, Grande MA, Schul W, de Jong L. Nuclear domains and the nuclear matrix. Int Rev Cytol. 1995;162A:151–189. doi: 10.1016/s0074-7696(08)61231-0. [DOI] [PubMed] [Google Scholar]
- van Wijnen AJ, Bidwell JP, Fey EG, Penman S, Lian JB, Stein JL, Stein GS. Nuclear matrix association of multiple sequence-specific DNA binding activities related to SP-1, ATF, CCAAT, C/EBP, OCT-1, and AP-1. Biochemistry. 1993;32:8397–8402. doi: 10.1021/bi00084a003. [DOI] [PubMed] [Google Scholar]
- Vernimmen D, De Gobbi M, Sloane-Stanley JA, Wood WG, Higgs DR. Long-range chromosomal interactions regulate the timing of the transition between poised and active gene expression. EMBO J. 2007;26:2041–2051. doi: 10.1038/sj.emboj.7601654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser AE, Jaunin F, Fakan S, Aten JA. High resolution analysis of interphase chromosome domains. J Cell Sci. 2000;113 (Pt 14):2585–2593. doi: 10.1242/jcs.113.14.2585. [DOI] [PubMed] [Google Scholar]
- Wang D, Garcia-Bassets I, Benner C, Li W, Su X, Zhou Y, Qiu J, Liu W, Kaikkonen MU, Ohgi KA, Glass CK, Rosenfeld MG, Fu XD. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature. 2011;474:390–394. doi: 10.1038/nature10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WS, Coffey DS. DNA packaging and organization in mammalian spermatozoa: comparison with somatic cells. Biol Reprod. 1991;44:569–574. doi: 10.1095/biolreprod44.4.569. [DOI] [PubMed] [Google Scholar]
- Ward WS, Zalensky AO. The unique, complex organization of the transcriptionally silent sperm chromatin. Crit Rev Eukaryot Gene Expr. 1996;6:139–147. doi: 10.1615/critreveukargeneexpr.v6.i2-3.30. [DOI] [PubMed] [Google Scholar]
- Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- Wei X, Samarabandu J, Devdhar RS, Siegel AJ, Acharya R, Berezney R. Segregation of transcription and replication sites into higher order domains. Science. 1998;281:1502–1506. doi: 10.1126/science.281.5382.1502. [DOI] [PubMed] [Google Scholar]
- Wischnitzer S. The submicroscopic morphology of the interphase nucleus. Int Rev Cytol. 1973;34:1–48. doi: 10.1016/s0074-7696(08)61933-6. [DOI] [PubMed] [Google Scholar]
- Xu M, Cook PR. Similar active genes cluster in specialized transcription factories. J Cell Biol. 2008;181:615–623. doi: 10.1083/jcb.200710053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe E, Tanay A. Probabilistic modeling of Hi-C contact maps eliminates systematic biases to characterize global chromosomal architecture. Nat Genet. 2011;43:1059–1065. doi: 10.1038/ng.947. [DOI] [PubMed] [Google Scholar]
- Yan J, Enge M, Whitington T, Dave K, Liu J, Sur I, Schmierer B, Jolma A, Kivioja T, Taipale M, Taipale J. Transcription Factor Binding in Human Cells Occurs in Dense Clusters Formed around Cohesin Anchor Sites. Cell. 2013;154:801–813. doi: 10.1016/j.cell.2013.07.034. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Young DW, Javed A, Pratap J, Montecino M, van Wijnen A, Lian JB, Stein JL, Stein GS. Nuclear microenvironments in biological control and cancer. Nat Rev Cancer. 2007;7:454–463. doi: 10.1038/nrc2149. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Young DW, Montecino MA, Lian JB, van Wijnen AJ, Stein JL, Stein GS. Mitotic bookmarking of genes: a novel dimension to epigenetic control. Nat Rev Genet. 2010;11:583–589. doi: 10.1038/nrg2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Caravaca JM, Tulin A, Sekiya T. Nuclear mobility and mitotic chromosome binding: similarities between pioneer transcription factor FoxA and linker histone H1. Cold Spring Harb Symp Quant Biol. 2010;75:219–226. doi: 10.1101/sqb.2010.75.061. [DOI] [PubMed] [Google Scholar]
- Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rowley JD. Chromatin structural elements and chromosomal translocations in leukemia. DNA Repair (Amst) 2006;5:1282–1297. doi: 10.1016/j.dnarep.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Tavoosidana G, Sjolinder M, Gondor A, Mariano P, Wang S, Kanduri C, Lezcano M, Sandhu KS, Singh U, Pant V, Tiwari V, Kurukuti S, Ohlsson R. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet. 2006;38:1341–1347. doi: 10.1038/ng1891. [DOI] [PubMed] [Google Scholar]
- Zorn C, Cremer C, Cremer T, Zimmer J. Unscheduled DNA synthesis after partial UV irradiation of the cell nucleus. Distribution in interphase and metaphase. Exp Cell Res. 1979;124:111–119. doi: 10.1016/0014-4827(79)90261-1. [DOI] [PubMed] [Google Scholar]
- Zullo JM, Demarco IA, Pique-Regi R, Gaffney DJ, Epstein CB, Spooner CJ, Luperchio TR, Bernstein BE, Pritchard JK, Reddy KL, Singh H. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell. 2012;149:1474–1487. doi: 10.1016/j.cell.2012.04.035. [DOI] [PubMed] [Google Scholar]