Abstract
Retinal angiogenesis is a major cause of blindness in ischemic retinopathies including diabetic retinopathy and retinopathy of prematurity. Integrin αvβ3 is a promising therapeutic target for ocular angiogenesis, modulating the pro-angiogenic actions of multiple growth factors. In this study, we sought to determine the effects of the integrin αvβ3 antagonist tetra-iodothyroacetic acid (tetrac) on the angiogenic actions of VEGF and erythropoietin (EPO) in cultured human retinal endothelial cells. In addition, we investigated the effect of tetrac and a nanoparticulate formulation of tetrac on retinal angiogenesis in vivo, in the mouse oxygen-induced retinopathy (OIR) model. Tetrac inhibitory activity was evaluated in human retinal endothelial cells treated with VEGF and/or EPO. Endothelial cell proliferation, migration, and tube formation were assessed, in addition to phosphorylation of ERK1/2. For the studies of the oxygen-induced retinopathy model, C57BL/6 mice were exposed to 75% oxygen from postnatal day (P)7 to P12, and then returned to room air. Tetrac and tetrac-nanoparticle (tetrac-NP) were administered at P12 and P15 by either intraperitoneal or intravitreal injection. Retinal neovascularization was quantitated at P18. Tetrac significantly inhibited pro-angiogenic effects of VEGF and/or EPO on retinal endothelial cells, indicating that the angiogenic effects of both growth factors are dependent on integrin αvβ3. Retinal neovascularization in the OIR model was significantly inhibited by both tetrac and tetrac-NP. These results indicate that the integrin αvβ3 antagonist, tetrac, is an effective inhibitor of retinal angiogenesis. The ability of tetrac to inhibit the pro-angiogenic effect of both VEGF and EPO on retinal endothelial cells suggests that tetrac (and antagonism of integrin αvβ3) is a viable therapeutic strategy for proliferative diabetic retinopathy.
Keywords: Angiogenesis, Retinal endothelial cells, Integrin αvβ3, VEGF, Erythropoietin, Oxygen-induced retinopathy
1. Introduction
Retinal angiogenesis is a major cause of blindness in ischemic retinopathies including diabetic retinopathy and retinopathy of prematurity. Significant advances have been made in identifying growth factors involved in promoting retinal angiogenesis. Vascular endothelial growth factor (VEGF) has been identified as a major mediator (Adamis et al., 1994; Aiello, 2005; Aiello et al., 1994), leading to the development of anti-VEGF drugs for proliferative diabetic retinopathy (PDR) in patients (Adamis et al., 2006; Avery et al., 2006). Although VEGF plays a major role in PDR, additional growth factors have been identified as mediators of proliferative diabetic retinopathy, including erythropoietin. The importance of erythropoietin (EPO) is highlighted by the significant increase in intraocular levels of EPO in proliferative diabetic retinopathy (Katsura et al., 2005; Watanabe et al., 2005). A polymorphism in the EPO promoter is significantly associated with PDR (Tong et al., 2008). Furthermore, blockade of EPO inhibits retinal neovascularization in the mouse oxygen-induced retinopathy (OIR) model (Watanabe et al., 2005).
In addition to growth factors, other classes of molecules have been implicated in angiogenesis, including integrins, which serve as receptors for specific extracellular matrix proteins. Integrin αvβ3, in particular, is not generally expressed on quiescent microvessels but is dramatically increased in response to angiogenic growth factors (Brooks et al., 1994). Neovascular ocular tissue from patients with proliferative diabetic retinopathy showed a selective upregulation of integrin αvβ3 and αvβ5 (Friedlander et al., 1996; Robbins et al., 1994). Integrin αvβ3 is thought to be a critical regulator of angiogenesis, since pharmacologic inhibition of this integrin blocks neovascularization in multiple animal models (Stupack and Cheresh, 2004). Systemic administration of cyclic peptides mimicking the RGD binding motif of integrin αvβ3 inhibited retinal neovascularization in the mouse oxygen-induced retinopathy model (Luna et al., 1996), indicating that integrin αvβ3 is a promising therapeutic target for ocular angiogenesis.
Integrin αvβ3 is known to be important for the pro-angiogenic effects of growth factors including VEGF. It has been found to interact physically with VEGFR2, forming a complex with VEGFR2 upon stimulation with VEGF. Blockade of αvβ3 results in diminished VEGFR2 auto-phosphorylation and signaling (Somanath et al., 2009a). The synergistic connection between integrin αvβ3 and VEGF with regard to promotion of angiogenesis has been demonstrated both in vitro and in vivo by multiple studies (Somanath et al., 2009b). Although integrin αvβ3 has been demonstrated to be important for the pro-angiogenic effects of additional growth factors including FGF-2 (Somanath et al., 2009a), it is not known whether the angiogenic effects of erythropoietin are dependent on integrin αvβ3.
Tetra-iodothyroacetic acid (tetrac) has emerged as a small molecule integrin ligand that acts as an integrin αvβ3 antagonist. Integrin αvβ3 bears a cell surface receptor site for thyroid hormone for L-thyroxine (T4) that mediates pro-angiogenic activity of T4 (Bergh et al., 2005). Tetraiodothyroacetic acid is a deaminated analogue of T4 that blocks the pro-angiogenic actions of T4 by inhibiting T4 binding to the integrin. In addition, Tetrac blocks the pro-angiogenic actions of VEGF and FGF-2 via integrin αvβ3 (Mousa et al., 2008), indicating that Tetrac may be useful for blocking the angiogenic actions of multiple factors via integrin αvβ3. Indeed, Tetrac as well as a nanoparticulate formulation of Tetrac have been found to inhibit both tumor-related angiogenesis and tumor growth in several experimental models (Yalcin et al., 2010a; Yalcin et al., 2009; Yalcin et al., 2010b). In this study, we sought to determine the effects of tetrac on the angiogenic actions of both VEGF and erythropoietin in cultured human retinal endothelial cells. In addition, we investigated the effect of Tetrac and a nanoparticulate formulation on retinal angiogenesis in vivo, in the oxygen-induced retinopathy model.
2. Materials and methods
2.1. Reagents
Human recombinant VEGF was purchased from R&D Systems (Minneapolis, MN). Recombinant Erythropoietin (EPO) was from PROSPEC (Rehobot, Israel). Tetrac and tetrac-NP were synthesized as previously described (Glinskii et al., 2009; Yalcin et al., 2009; Yalcin et al., 2010b). Anti-phospho-p44/42 MAPK (Thr202/Tyr204), anti-p44/42 MAPK, anti-phospho-Akt, and anti-Akt antibodies were from Cell Signaling Technologies (Beverly, MA). Other chemicals and reagents were obtained from Sigma Chemical Co. (St. Louis, MO) unless otherwise indicated.
2.2.Retinal endothelial cell culture
Human retinal endothelial cells (HRECs; Cell Systems, Kirkland, WA) were cultured in EBM2 medium containing EGM2-MV (Clonetics) and used between passages 4 and 10. HRECs were cultured in fibronectin-coated dishes. Cells were grown in 5% CO2 at 37°C, and media were changed every 2 to 3 days. For these studies, cells were cultured in 6-well or 24-well plates. All studies were performed using HREC passage 6–9.
2.3. Animal studies
Oxygen-induced retinopathy was elicited in mice as previously described (Smith et al., 1994). In brief, at postnatal day (P) 7, C57BL/6J mice were exposed to 75% O2, along with their nursing mothers for 5 days, and then returned to room air. On P12, the mice were returned to room air and administered tetrac at P12 and P15 by intraperitoneal injection (1 mg/kg or 10 mg/kg of tetrac or tetrac-NP) and tetrac or tetrac-NP by intravitreal injection (right eye: 1 µl of vehicle; left eye: 1 µl of tetrac or tetrac-NP), as previously described (Duh et al., 2002). The mice were euthanized at P18, and retinal flatmounts prepared. Retinal flat mounts were stained with Griffonia Simplicfonia (GS) lectin for quantitation of avascular retinal area and retinal neovascularization as described (Connor et al., 2009). Mice were used in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University School of Medicine, and mouse studies adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.4.Western blot analysis
HRECs were cultured in 6-well plates. Confluent cells were serum-starved overnight. HRECs were then pretreated with tetrac at the indicated concentrations or vehicle for 15 min and incubated with 25 ng/ml VEGF or 25 IU EPO for another 15 min. Total cell lysates were prepared and analysed by immunoblotting as described (Xu et al., 2006a). Briefly, cells were washed with phosphate-buffered saline (PBS) and lysed with Laemmli sample buffer (BioRad Laboratories, Hercules, Calif). The protein samples from total cell lysates were separated by SDS-PAGE and transferred to Hybond ECL nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ). After incubating with primary and secondary antibodies, proteins were detected by using the Supersignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, Ill).
2.5. Cell proliferation assay
Endothelial cell proliferation was assessed as previously described (Xu et al., 2006b) by measuring the incorporation of [3H] thymidine (Perkin Elmer, Boston MA) into DNA. HRECs were seeded into a 24-well plate. After 24 h, media were replaced by 10% calf serum in DMEM, treated with 25 ng/ml VEGF or 25 IU/ml EPO, and cells incubated for another 24 h. The cells were pulsed with 1 µCi/mL [3H] thymidine during the final 6 h. Cells were then washed twice with PBS and twice with cold 5% trichloroacetic acid (TCA) to remove the unincorporated [3H] thymidine. Cells were then washed twice with ethanol/ether (3:1 vol) and solubilized in 200 µL of 0.1 N NaOH, followed by neutralization in 0.1 N HCl. Aliquots of samples were added to 8 mL scintillation fluid, and radioactivity was determined with a liquid scintillation counter.
2.6. Modified Boyden Chamber Migration Assay
Endothelial cell migration assays were performed as previously described (Maiti et al., 2008). Modified Boyden chambers containing polycarbonate membranes (Transwell, 8 µm pore size; Corning Inc.) coated with 10 µg/ml collagen were used. Serum-starved HRECs were trypsinized and resuspended in EBM2 containing 0.1% bovine serum albumin. After preincubation with tetrac for 15 min, the cells were seeded onto the upper chamber at 1 × 105 cell per insert. The lower chamber contained EBM2 with 0.1% bovine serum albumin and 25 ng/ml VEGF or 25 IU/ml EPO. After incubation at 37°C for 4 h, the stationary cells on the top of the membrane were removed by a cotton swab, and the migrated cells on the bottom of the membrane were stained with DAPI. Ten photographs for each well were randomly taken under microscope (Zeiss Axiovert 200) at 20x objective, and cells were counted using the Image J program (Abràmoff et al., 2004).
2.7. Tube Formation Assay
Endothelial cell tube formation by HRECs was performed as previously described (Im et al., 2005). A collagen gel mixture consisting of 80% (v/v) bovine type I collagen (Advanced Biomatrix), 0.02 N NaOH, 20 mM HEPES (GIBCO-BRL), 2 mg/ml NaHCO3, 0.5 µg/ml fibronectin, 0.5 µg/ml laminin, and 10.5 mg/ml RPMI powder (GIBCO-BRL) was prepared. For the lower gel layer, 400 µl of the gel mixture per well was added to each well of the 24-well plates and incubated at 37°C for 2 h. After polymerization, 1 × 105 HRECs were seeded in each well and incubated with EGM2-MV and for 24 h at 37°C. The medium was removed and 150 µl of the gel mixture was added to each well. To polymerize the upper gel layer, the plates were incubated at 37°C for 2 h. Finally, 500 µl of medium supplemented with EBM2 medium with or without 25 ng/ml VEGF or 25 IU/ml EPO was added in the presence or absence of tetrac. After 48 h, three different fields per well were randomly chosen and photographed. Images were processed using Adobe Photoshop (Adobe Systems, San Jose, CA), and the data were imported as a TIFF file into NIH Image. After calibration with a stage micrometer, the total length of all tubes within a field was measured.
2.8. Cell Viability Assay
96-well plates were coated with fibronectin. 100 µl of HREC suspension (7,000 cells) were seeded in each well in the presence or absence of tetrac and incubated overnight at 37°C in a humidified, 5% CO2 atmosphere. The viability was determined after 48 hrs using the CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay (Promega) according to the manufacturer’s protocol. Each tetrac concentration group (from 10−7 to 10−5 M) included 10 replicate wells.
2.9 Statistical Analysis
Each experimental condition was assayed at least in triplicate. Results were reported as mean ± SD. An unpaired Student’s t test was used to determine statistical significance. P < 0.05 was considered statistically significant.
3. Results
3.1. Tetrac inhibits the mitogenic effects of VEGF and EPO on retinal endothelial cells
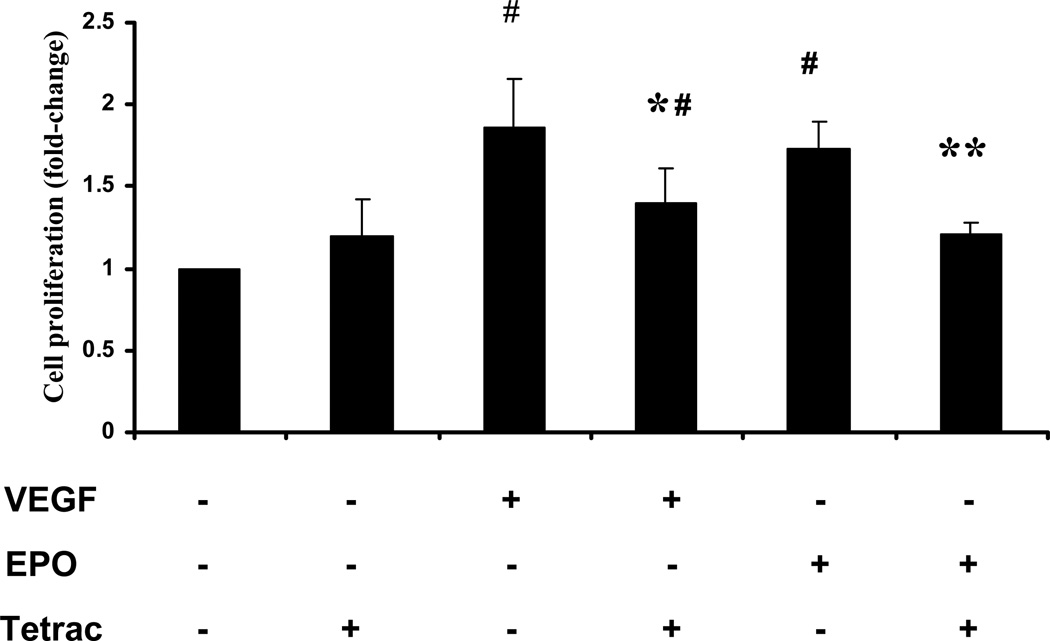
VEGF and EPO are known to have multiple pro-angiogenic effects on endothelial cells, including stimulation of endothelial cell proliferation. To investigate whether tetrac decreases proliferation of human retinal endothelial cells (HREC) by VEGF or EPO, we utilized the [3H] thymidine incorporation assay. HRECs were treated with VEGF or EPO in the presence or absence of tetrac, and [3H] thymidine incorporation measured 24 h after growth factor addition. 25 ng/ml VEGF induced a 1.9 fold increase in retinal endothelial cell proliferation (p<0.001, Figure 1). Tetrac (10−5 M) significantly suppressed VEGF-induced proliferation by about 51% (p<0.05). 25 IU/ml EPO induced a 1.7 fold increase in endothelial cell proliferation (p<0.001, Figure 1). Tetrac (10−5 M) significantly suppressed EPO-induced proliferation by about 71% (p<0.05). Tetrac did not significantly affect basal endothelial cell proliferation (Figure 1). In addition, treatment of HRECs with tetrac (at doses from 10−7 to 10−5 M) for 48 hours had no significant effect on cell viability (data not shown).
Fig. 1.
Effect of tetrac on induction of endothelial cell proliferation by VEGF and EPO. After incubation at 37°C for 18 h with 25 ng/ml VEGF or 25 IU/ml EPO in the presence or absence of 10−5 M tetrac, [3H] thymidine was added, and HRECs were incubated for another 6 h. [3H] thymidine incorporation in HRECs was then measured by scintillation counter. Values represent the mean ± SEM (n=3). #Denotes P < 0.01 vs. control group, *denotes P= 0.05 vs. VEGF treated group. **denotes P =0.05 vs. EPO treated group.
3.2.Tetrac inhibits VEGF- and erythropoietin-induction of ERK 1/2 phosphorylation
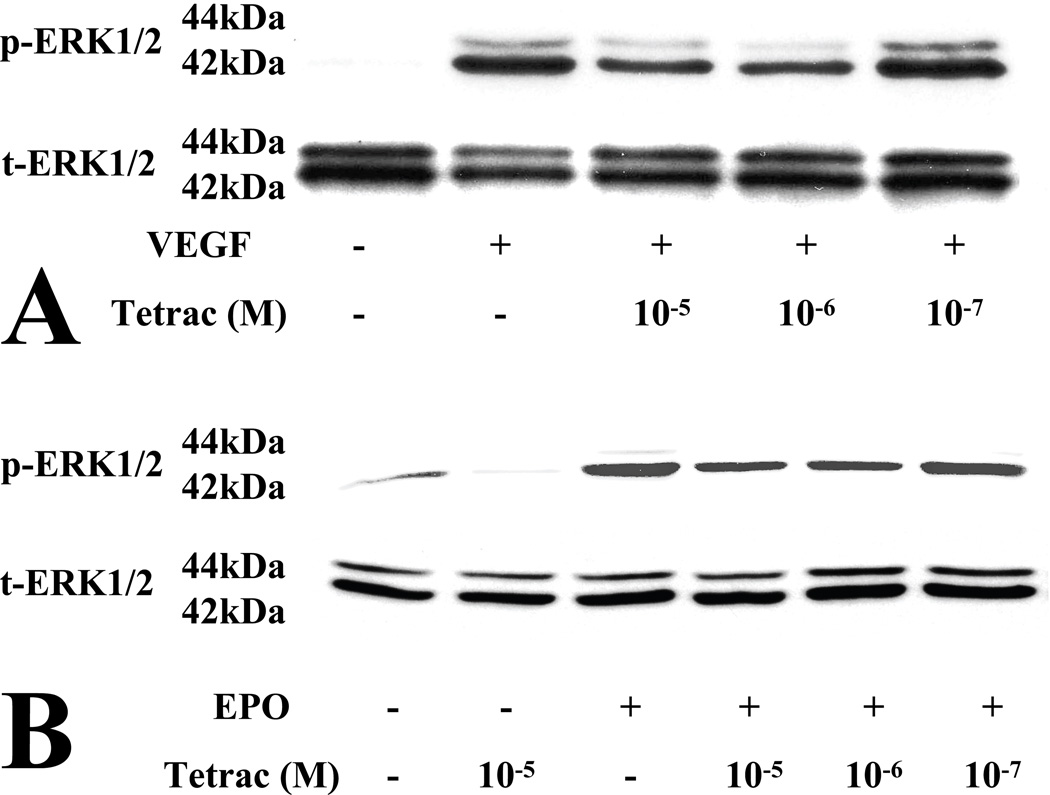
ERK 1/2 activation is known to be important for endothelial cell proliferation. Both VEGF (Zachary, 2003) and EPO (Watanabe et al., 2005) activate ERK in endothelial cells. Furthermore, inhibition of integrin αvβ3 is known to inhibit VEGFR2 signaling (Somanath et al., 2009a). We therefore investigated whether tetrac blocks phosphorylation of ERK1/2 by VEGF and EPO in HRECs. Both VEGF and EPO activated the phosphorylation of ERK in HREC, as expected (Figure 2). Pretreatment of HREC with tetrac significantly inhibited both VEGF- and EPO-induction of ERK in a dose-dependent fashion (Figure 2). In addition to growth factor-induction of ERK, tetrac also significantly inhibited basal phosphorylation of ERK (Figure 2). In the immunoblots displayed in Figure 2, analysis by Image J software was performed to quantitate phosphorylated ERK1/2, normalized to total ERK1/2. Tetrac (10−5 M) suppressed basal phosphorylation of ERK1/2 by 78% (B). Tetrac suppressed VEGF-induced phosphorylation of ERK1/2 by a maximum of 58% (10−6 M; A) and EPO-induced phosphorylation of ERK1/2 by a maximum of 59% (10−6 M; B).
Fig. 2.
Inhibition of growth factor-induced phosphorylation of ERK1/2 by tetrac HRECs were pretreated with vehicle or tetrac at the indicated concentrations (10−5, 10−6 and 10−7 M) for 15 minutes and then treated with 25 ng/ml VEGF (A) or 25 IU/ml EPO (B) for 15 min. Activation of ERK1/2 was examined by western blot, using antibodies to phospho-ERK and total-ERK. Immunoblots are representative of at least three independent experiments.
3.3.Tetrac Inhibits VEGF Stimulation of Retinal Endothelial Cell Migration
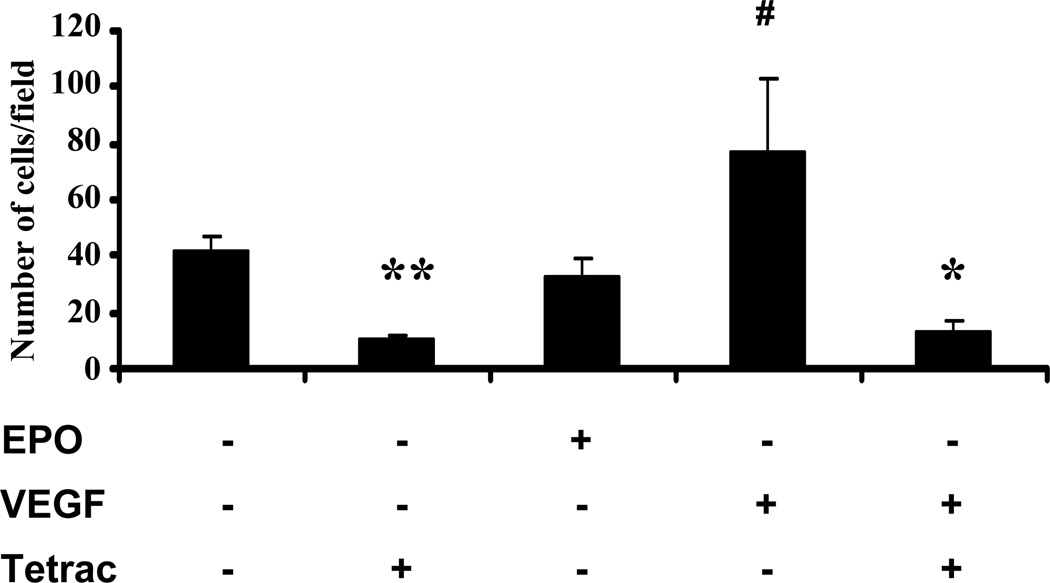
In addition to proliferation, we also investigated whether tetrac inhibits growth factor-induced human retinal endothelial cell migration. For this purpose, we utilized the modified Boyden chamber assay, using VEGF and EPO as chemotactic agents. As shown in Fig. 3, VEGF (25 ng/ml) increased migration of HREC by 1.8 fold compared with control (p< 0.001). Addition of tetrac inhibited basal endothelial cell migration (p<0.01) and completely abrogated VEGF-induction of endothelial cell migration (p< 0.001). EPO treatment did not increase migration of HRECs (Figure 3). Correlating with its effect on basal ERK activation, tetrac alone also significantly inhibited basal endothelial cell migration (Figure 3).
Fig. 3.
Effects of tetrac on growth factor-induction of endothelial cell migration. HREC migration was assessed using the modified Boyden chamber assay as described in Methods. HRECs were treated with VEGF or EPO in presence or absence of 10−5 M tetrac. After incubation at 37°C for 4 h, the number of migrated cells were counted. Values represent the mean ± SEM (n=3). #Denotes significant increase (P < 0.001) vs. control group, *denotes significant decrease (P < 0.001) vs. VEGF treated group. **denotes significant decrease (P < 0.01) vs. control group.
3.4.Tetrac Blocks VEGF Induction of Retinal Endothelial Tube Formation
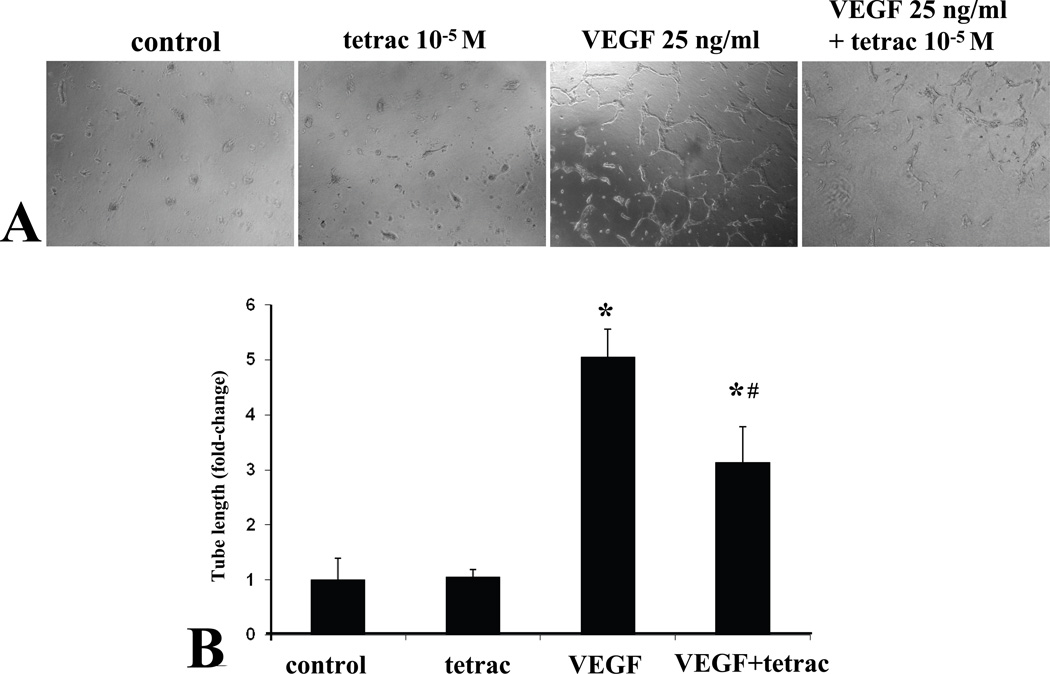
The endothelial tube formation assay is an informative endpoint, since it incorporates multiple endothelial cell activities including proliferation, migration, and organization. We employed a method that has been used successfully for bovine retinal endothelial cells, particularly for VEGF-induced angiogenesis (Im et al., 2005; Ojima et al., 2006). Consistent with previous reports for bovine retinal endothelial cells, VEGF treatment significantly increased human retinal endothelial tube formation (Fig. 4A and B). Concomitant treatment with tetrac significantly inhibited VEGF induction of HREC tube formation (Fig. 4A and B). Tetrac alone did not affect basal HREC tube formation as compared with control.
Fig. 4.
Inhibition of VEGF-induced retinal endothelial tube formation by tetrac. (A) Human retinal endothelial cells were seeded within a collagen sandwich gel, and then medium containing tetrac or vehiclein the presence or absence of VEGF was added. After 48 h, three randomly selected fields were photographed. Representative images are shown. (B) The total length of all tubes within a field was measured. Values are expressed as means ± SE (n = 3). *Denotes P < 0.01 as compared with the control, #Denotes P = 0.02 as compared with the VEGF.
3.5.Intraperitoneal Administration of Tetrac Inhibits Retinal Neovascularization in Oxygen-induced Retinopathy
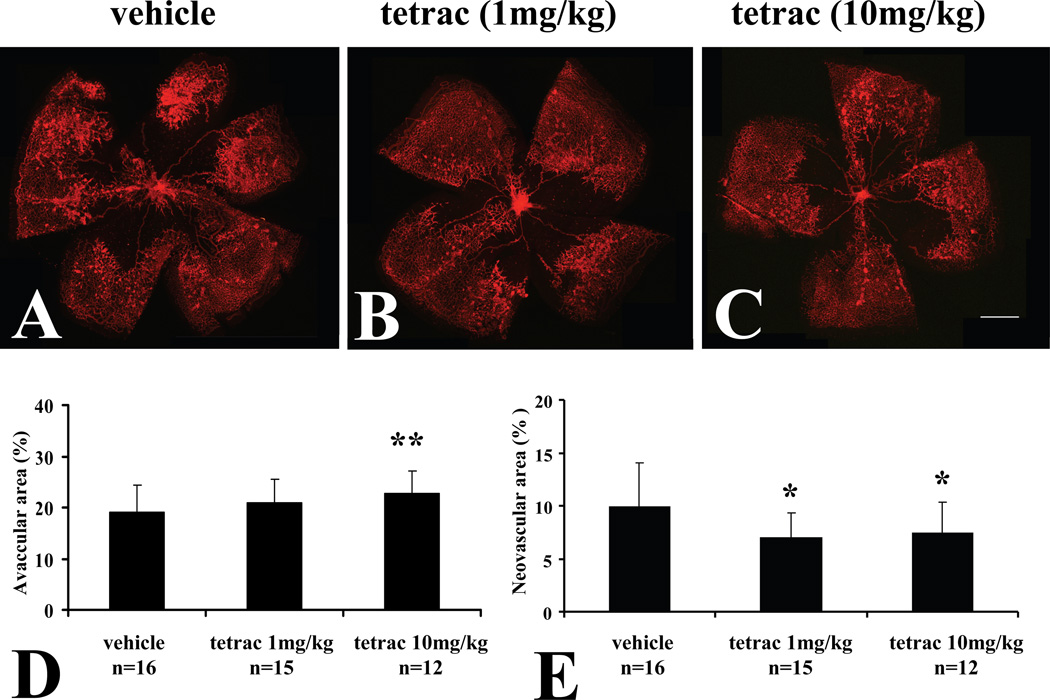
Since tetrac inhibited pro-angiogenic effects of both VEGF and EPO on cultured retinal endothelial cells, we were interested in investigating its effects on retinal neovascularization in vivo. For this purpose, we employed the mouse oxygen-induced retinopathy (OIR) model (Smith et al., 1994). In this model, C57BL/6 mice are exposed to 75% oxygen from postnatal day 7 (P7) to P12, which results in extensive retinal vaso-obliteration. On return of these mice to room air on P12, the inner retina becomes relatively hypoxic, leading to the formation of retinal neovascularization in 100% of animals by P17-18 (Smith et al., 1994). We first utilized systemic administration by the intraperitoneal route. Tetrac was injected into the peritoneal cavity of neonatal C57BL/6 mice at P12 and P15, and the retinal vasculature assessed at P18.
Analysis of the retinas revealed that intraperitoneal injection of tetrac (Figure 5) significantly reduced retinal neovascularization. Intraperitoneal administration of 1 mg/kg (Fig. 5B) and 10 mg/kg (Fig. 5C) of tetrac reduced retinal neovascularization by 29.5% (p=0.02) and 24.8% (p=0.03), respectively, compared to vehicle (Fig. 5E). Intraperitoneal administration of 1 mg/kg tetrac did not have a significant effect on avascular retinal area, whereas administration of 10 mg/kg tetrac showed a slight increase in avascular retinal area as compared with vehicle (p=0.03; Figure 5D).
Fig. 5.
Inhibition of retinopathy by intraperitoneal administration of tetrac in OIR. Representative flatmounts of OIR at P18 from mice injected with vehicle at P12 and P15 (A). Lectin-stained tufts show new capillary buds induced by the OIR model. Representative flatmounts of retinas of OIR from mice at P18 injected with 1mg/kg tetrac at P12 and P15 (B), and mice at P18 injected with 10mg/kg tetrac at P12 and P15 (C). Scale bars: 500 µm. (D) Quantitation of central vascular drop-out as percentage of total retinal area. (E) Quantitation of neovascular tufts as percentage of total retinal area shows a statistically significant decrease in both 1mg/ml and 10mg/kg tetrac-injected animals. Values are expressed as means ± SE *Denotes significant decrease (P<0.03) vs. vehicle injected group. ** Denotes significant increase (P=0.03) vs. vehicle injected group.
3.6.Intravitreal Administration of Tetrac and Tetrac-nanoparticle Inhibits Retinal Neovascularization in OIR
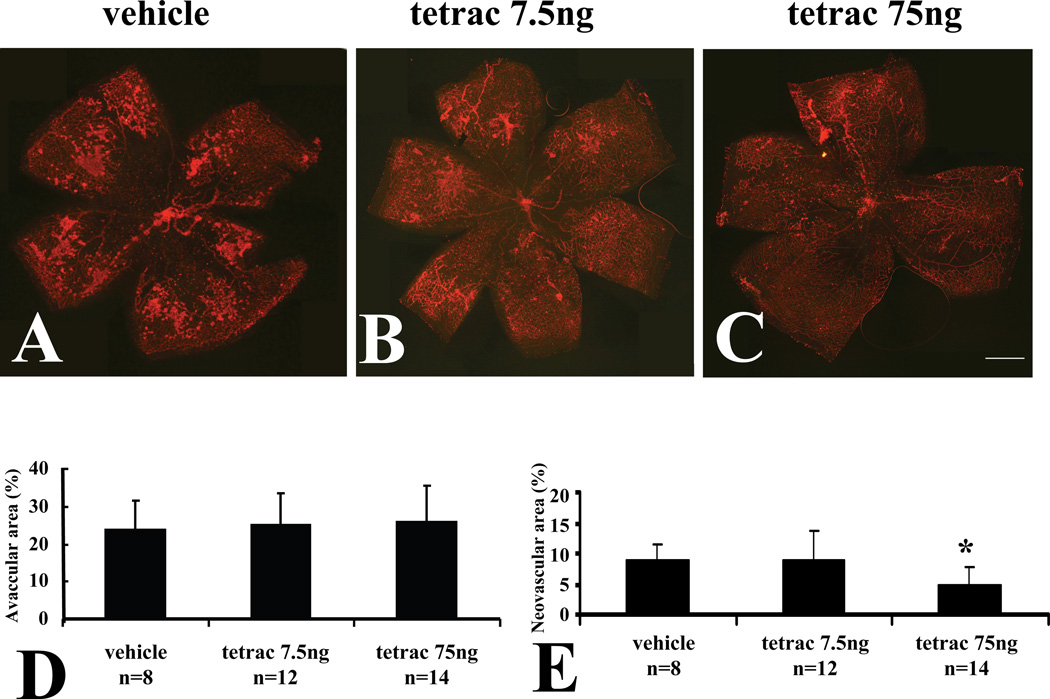
We also examined the effect of intravitreal administration of tetrac. Tetrac was injected by the intravitreal route in C57BL/6 mice at P12 and P15, and the retinal vasculature assessed at P18. Analysis of the retinas revealed that intravitreal injection of tetrac (Figure 6) significantly reduced retinal neovascularization in a dose-dependent fashion. Intravitreal administration of 7.5 ng tetrac (Figure 6B) had no significant effect on retinal neovascularization compared with vehicle, whereas intravitreal administration of 75 ng of tetrac (Figure 6C) reduced retinal neovascularization by 44.8% (p<0.01) in retinal neovascularization comparing with eyes of no treatment mice (Fig. 6E). Intravitreal administration of Tetrac had no significant effect on avascular retinal area at P18 (Fig. 6D).
Fig. 6.
Inhibition of retinopathy by intravitreal administration of tetrac in OIR. Representative flatmounts of OIR at P18 from mice injected with vehicle at P12 and P15 (A). Lectin-stained tufts show new capillary buds induced by the OIR model. Representative flatmounts of retinas of OIR from mice at P18 injected with 7.5ng tetrac at P12 and P15 (B), and mice at P18 injected with 75ng tetrac at P12 and P15 (C). Scale bars: 500 µm. (D) Quantitation of central vascular drop-out. (E) Quantitation of tufting shows a statistically significant decrease in 75 ng tetrac-injected animals. Values are expressed as means ± SE *Denotes P=0.01 vs. vehicle injected group.
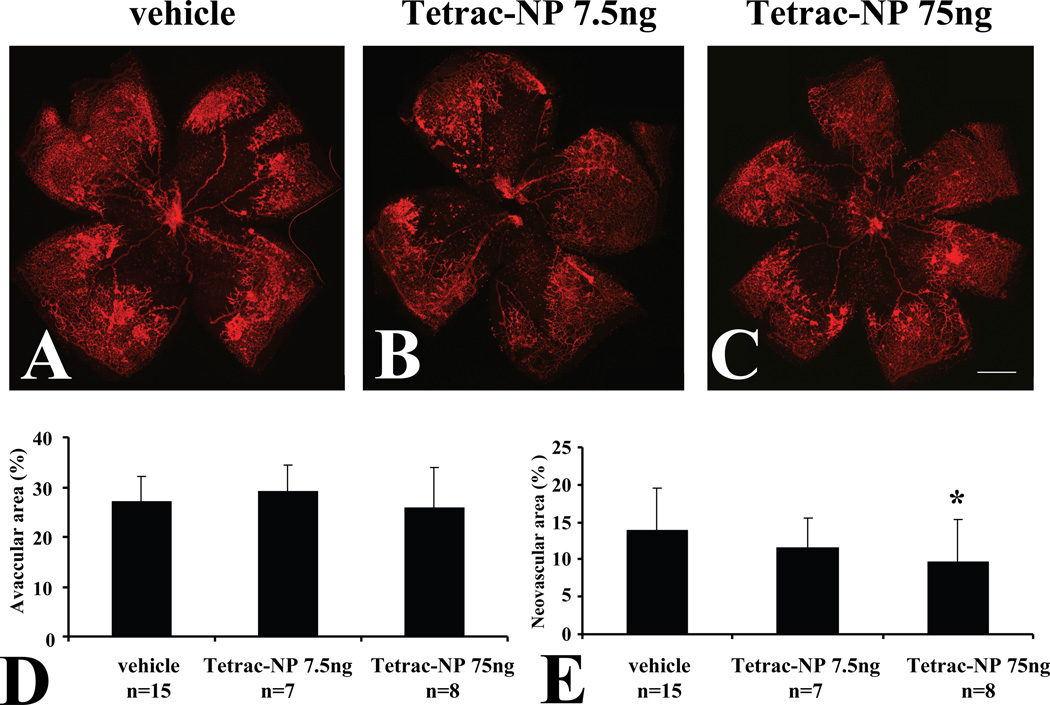
In addition to tetrac, we also evaluated the effect of intravitreal injection of a nanoparticulate form of tetrac, which has been shown to arrest both tumor-related angiogenesis and tumor-related growth in vivo (Yalcin et al., 2010a; Yalcin et al., 2010b). Intravitreal administration of 7.5 ng of tetrac (Figure 7B) had no statistically significant effect on retinal neovascularization compared with vehicle, whereas intravitreal administration of 75 ng of tetrac-NP (Figure 7C) significantly reduced retinal neovascularization by 30.9% (p=0.04), compared with vehicle (Fig. 7E). Intravitreal administration of Tetrac-NP had no significant effect on avascular retinal area at P18 (Fig. 7D).
Fig. 7.
Inhibition of retinopathy by intravitreal administration of tetrac-NP in OIR. Representative flatmounts of OIR at P18 from mice injected with vehicle at P12 and P15 (A). Lectin-stained tufts show new capillary buds induced by the OIR model. Representative flatmounts of retinas of OIR from mice at P18 injected with 7.5 ng tetrac-NP at P12 and P15 (B), and mice at P18 injected with 75 ng tetrac-NP at P12 and P15 (C). Scale bars: 500 µm. (D) Quantitation of central vascular drop-out. (E) Quantitation of tufting shows a statistically significant decrease in both 7.5 ng and 75 ng tetrac-NP-injected animals. Quantities of tetrac-NP are expressed in tetrac “equivalent.” Values are expressed as means ± SE *Denotes P<0.04 vs. vehicle injected group.
4. Discussion
Anti-angiogenic agents targeting VEGF are now being used in the treatment of proliferative diabetic retinopathy as well as other diseases involving retinal neovascularization (Jardeleza and Miller, 2009). Antagonists of integrin αvβ3 may be a viable therapeutic strategy as well, in part because they can inhibit the promotion of angiogenesis by VEGF and other growth factors including FGF-2 (Somanath et al., 2009a). Tetraiodothyroacetic acid (tetrac) has emerged as an antagonist of integrin αvβ3 (Bergh et al., 2005) that is being investigated as a therapeutic agent for tumors (Yalcin et al., 2010a; Yalcin et al., 2009; Yalcin et al., 2010b). The awareness of the antagonism by tetrac of the thyroid hormone receptor on integrin αvβ3, as well as tetrac’s associated anti-angiogenic properties, emerged from the discovery of the pro-angiogenic properties of T4 and T3 (Mousa et al., 2006). It has been determined that integrin αvβ3 contains a cell surface receptor site for thyroid hormone that is responsible for the pro-angiogenic effects of of T4 (Bergh et al., 2005). Tetrac is a deamination product of T4 that was shown initially to block the pro-angiogenic effect of thyroid hormone by blocking thyroid hormone binding to its cell surface receptor, integrin αvβ3 (Bergh et al., 2005). Tetrac has no thyromimetic activity at the hormone receptor site (Davis et al., 2004), but has pleiotropic effects on survival pathway gene expression in cancer cells (Glinskii et al., 2009; Yalcin et al., 2010b). Tetrac has also been shown to block the pro-angiogenic actions of VEGF and FGF-2 (Mousa et al., 2008) and platelet-derived growth factor (PDGF) (SA Mousa and PJ Davis: personal communication) on cultured human dermal microvascular endothelial cells, as well as the chick chorioallantoic membrane and mouse matrigel models of angiogenesis. This anti-angiogenic effect of tetrac on multiple pro-angiogenic molecules further increases its attractiveness as a therapeutic agent.
Because of its antagonistic effects on integrin αvβ3, as well as its anti-angiogenic effects in systemic disease, we investigated the potential of tetrac as an anti-angiogenic agent for retinal neovascularization. Given the major importance of VEGF in retinal neovascularization, we explored the effect of tetrac on VEGF-induction of angiogenesis in retinal microvascular endothelial cells. We found that tetrac significantly blocks multiple pro-angiogenic effects of VEGF, including retinal endothelial cell proliferation, ERK1/2 signaling, migration, and tube formation. Interestingly, in contrast to its inhibitory effect on VEGF activation of ERK1/2, tetrac did not have a similar inhibitory effect on VEGF activation of Akt in human retinal endothelial cells (data not shown). This indicates that tetrac may differentially regulate angiogenic pathways downstream of VEGF in retinal endothelial cells.
Although VEGF is a major stimulator of retinal neovascularization in PDR, other growth factors likely play an important role as well. Of particular note, erythropoietin has emerged as a likely major stimulator of PDR (Aiello, 2005). Vitreous levels of EPO are significantly higher in PDR compared to no diabetes (Katsura et al., 2005; Watanabe et al., 2005). Blockade of EPO inhibits retinal neovascularization in the mouse OIR model (Chen et al., 2008; Watanabe et al., 2005). Finally, a polymorphism in the EPO promoter is significantly associated with PDR in humans (Tong et al., 2008). For these reasons, a more complete anti-angiogenic effect in PDR will likely require the ability to block multiple growth factors. For this reason, we also examined the ability of tetrac to block the pro-angiogenic effects of EPO. We found that tetrac blocks both EPO-induction of retinal endothelial cell proliferation and ERK1/2 activation.
Of interest, although the pro-angiogenic effect of multiple growth factors is known to be dependent on integrin αvβ3, the interdependence of integrins and EPO have not previously been reported. Our results demonstrating the inhibition of EPO’s pro-angiogenic effects by tetrac indicate that EPO’s pro-angiogenic effect is also modulated by integrin αvβ3. This further highlights the potential therapeutic benefit of integrin αvβ3 antagonism for PDR.
In our assays with retinal microvascular endothelial cells, EPO stimulated both proliferation and ERK phosphorylation. However, in contrast to published studies (Anagnostou et al., 1990; Ribatti et al., 1999; Zhande and Karsan, 2007) with other endothelial cells, we did not find a stimulatory effect of EPO on retinal endothelial cell migration or tube formation or Akt induction, under the assay conditions tested. We were therefore not able to test the effect of tetrac on these other endpoints for EPO.
In light of tetrac’s ability to block the angiogenic stimulation of retinal endothelial cells by both VEGF and EPO, our results with tetrac are encouraging that tetrac might have potential for blocking retinal neovascularization in vivo. For this purpose, we investigated both tetrac as well as a nanoparticulate preparation of tetrac (tetrac-NP) that is being investigated for the treatment of thyroid tumors (Yalcin et al., 2010a; Yalcin et al., 2010b). We found that both tetrac and tetrac-NP significantly blocked retinal neovascularization in the oxygen-induced retinopathy model. This indicates that tetrac or tetrac-NP may be a suitable therapeutic agent for retinal neovascularization and proliferative diabetic retinopathy. Although the degree of inhibition of neovascularization was relatively modest (maximal inhibition of 45%), this study demonstrates an initial proof of concept regarding the therapeutic use of tetrac. It should be noted that the first studies of anti-VEGF molecules in the OIR model yielded a 47% and 37% reduction in neovascularization accomplished with Flt and Flk chimeric proteins, respectively (Aiello et al., 1995). Enhancements in either bioavailability or bioactivity of tetrac (for instance, by chemical modification) could be worthwhile toward the goal of clinical translation.
In summary, our data indicate that the integrin αvβ3 antagonist, tetrac, significantly blocks both VEGF- and EPO-induction of angiogenesis in cultured retinal microvascular endothelial cells. In addition, tetrac (and a nanoparticulate formulation of tetrac) significantly blocks retinal neovascularization in vivo in the oxygen-induced retinopathy model. This study implicates the therapeutic benefits of antagonizing the L-thyroxine receptor site of integrin alpha-v-beta3 and identifies tetraiodothyroacetic acid as a new pharmacologic agent for integrin alpha-v-beta3 antagonism in retinal neovascular disorders. Integrin αvβ3 is known to be important for the pro-angiogenic effects of several growth factors, including VEGF and FGF-2. Our results indicating the importance of this integrin for erythropoietin further highlights the attractiveness of therapeutic targeting of integrin αvβ3. Taken together, our data suggest that tetrac and tetrac-NP have potential as a new therapeutic strategy for proliferative diabetic retinopathy, and further support the use of integrin αvβ3 antagonists for this condition.
Highlights.
The integrin αvβ3 antagonist, tetra-iodothyroacetic acid (tetrac), inhibited the pro-angiogenic effects of VEGF and erythropoietin on retinal endothelial cells, in assays including proliferation, migration, and tube formation; this indicates that erythropoietin’s pro-angiogenic effects are modulated by integrin αvβ3.
Tetra-iodothyroacetic acid (tetrac) administered by both the intravitreal and intraperitoneal route inhibited retinal neovascularization in the oxygen-induced retinopathy model.
Tetrac may be a viable therapeutic strategy for proliferative diabetic retinopathy.
Acknowledgments
Supported by NIH EY18138, CLF-MTAP (Charitable Leadership Foundation-Medical Technology Acceleration Program), and an RPB Career Development Award to EJD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abràmoff MD, Magalhães PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Adamis AP, Altaweel M, Bressler NM, Cunningham ET, Jr, Davis MD, Goldbaum M, Gonzales C, Guyer DR, Barrett K, Patel M. Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology. 2006;113:23–28. doi: 10.1016/j.ophtha.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353:839–841. doi: 10.1056/NEJMe058142. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci U S A. 1990;87:5978–5982. doi: 10.1073/pnas.87.15.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695. doi: 10.1016/j.ophtha.2006.05.064. e1691-1615. [DOI] [PubMed] [Google Scholar]
- Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S, Davis PJ. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology. 2005;146:2864–2871. doi: 10.1210/en.2005-0102. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LE. Erythropoietin siRNA suppresses retinal neovascularization in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FB, Mousa SA, O'Connor L, Mohamed S, Lin HY, Cao HJ, Davis PJ. Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ Res. 2004;94:1500–1506. doi: 10.1161/01.RES.0000130784.90237.4a. [DOI] [PubMed] [Google Scholar]
- Duh EJ, Yang HS, Suzuma I, Miyagi M, Youngman E, Mori K, Katai M, Yan L, Suzuma K, West K, Davarya S, Tong P, Gehlbach P, Pearlman J, Crabb JW, Aiello LP, Campochiaro PA, Zack DJ. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci. 2002;43:821–829. [PubMed] [Google Scholar]
- Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci U S A. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinskii AB, Glinsky GV, Lin HY, Tang HY, Sun M, Davis FB, Luidens MK, Mousa SA, Hercbergs AH, Davis PJ. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac) Cell Cycle. 2009;8:3554–3562. doi: 10.4161/cc.8.21.9963. [DOI] [PubMed] [Google Scholar]
- Im E, Venkatakrishnan A, Kazlauskas A. Cathepsin B regulates the intrinsic angiogenic threshold of endothelial cells. Mol Biol Cell. 2005;16:3488–3500. doi: 10.1091/mbc.E04-11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardeleza MS, Miller JW. Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin Ophthalmol. 2009;24:87–92. doi: 10.1080/08820530902800330. [DOI] [PubMed] [Google Scholar]
- Katsura Y, Okano T, Matsuno K, Osako M, Kure M, Watanabe T, Iwaki Y, Noritake M, Kosano H, Nishigori H, Matsuoka T. Erythropoietin is highly elevated in vitreous fluid of patients with proliferative diabetic retinopathy. Diabetes Care. 2005;28:2252–2254. doi: 10.2337/diacare.28.9.2252. [DOI] [PubMed] [Google Scholar]
- Luna J, Tobe T, Mousa SA, Reilly TM, Campochiaro PA. Antagonists of integrin alpha v beta 3 inhibit retinal neovascularization in a murine model. Lab Invest. 1996;75:563–573. [PubMed] [Google Scholar]
- Maiti D, Xu Z, Duh EJ. Vascular endothelial growth factor induces MEF2C and MEF2-dependent activity in endothelial cells. Invest Ophthalmol Vis Sci. 2008;49:3640–3648. doi: 10.1167/iovs.08-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa SA, Bergh JJ, Dier E, Rebbaa A, O'Connor LJ, Yalcin M, Aljada A, Dyskin E, Davis FB, Lin HY, Davis PJ. Tetraiodothyroacetic acid, a small molecule integrin ligand, blocks angiogenesis induced by vascular endothelial growth factor and basic fibroblast growth factor. Angiogenesis. 2008;11:183–190. doi: 10.1007/s10456-007-9088-7. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Davis FB, Mohamed S, Davis PJ, Feng X. Pro-angiogenesis action of thyroid hormone and analogs in a three-dimensional in vitro microvascular endothelial sprouting model. Int Angiol. 2006;25:407–413. [PubMed] [Google Scholar]
- Ojima T, Takagi H, Suzuma K, Oh H, Suzuma I, Ohashi H, Watanabe D, Suganami E, Murakami T, Kurimoto M, Honda Y, Yoshimura N. EphrinA1 inhibits vascular endothelial growth factor-induced intracellular signaling and suppresses retinal neovascularization and blood-retinal barrier breakdown. Am J Pathol. 2006;168:331–339. doi: 10.2353/ajpath.2006.050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell'Era P, Nico B, Roncali L, Dammacco F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- Robbins SG, Brem RB, Wilson DJ, O'Rourke LM, Robertson JE, Westra I, Planck SR, Rosenbaum JT. Immunolocalization of integrins in proliferative retinal membranes. Invest Ophthalmol Vis Sci. 1994;35:3475–3485. [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Somanath PR, Ciocea A, Byzova TV. Integrin and growth factor receptor alliance in angiogenesis. Cell Biochem Biophys. 2009a;53:53–64. doi: 10.1007/s12013-008-9040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009b;12:177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol. 2004;64:207–238. doi: 10.1016/S0070-2153(04)64009-9. [DOI] [PubMed] [Google Scholar]
- Tong Z, Yang Z, Patel S, Chen H, Gibbs D, Yang X, Hau VS, Kaminoh Y, Harmon J, Pearson E, Buehler J, Chen Y, Yu B, Tinkham NH, Zabriskie NA, Zeng J, Luo L, Sun JK, Prakash M, Hamam RN, Tonna S, Constantine R, Ronquillo CC, Sadda S, Avery RL, Brand JM, London N, Anduze AL, King GL, Bernstein PS, Watkins S, Jorde LB, Li DY, Aiello LP, Pollak MR, Zhang K. Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proc Natl Acad Sci U S A. 2008;105:6998–7003. doi: 10.1073/pnas.0800454105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, Suzuma I, Ohashi H, Ojima T, Murakami T, Kobayashi T, Masuda S, Nagao M, Yoshimura N, Takagi H. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- Xu Z, Maiti D, Kisiel W, Duh EJ. Tissue factor pathway inhibitor-2 is upregulated by vascular endothelial growth factor and suppresses growth factor-induced proliferation of endothelial cells. Arterioscler Thromb Vasc Biol. 2006a;26:2819–2825. doi: 10.1161/01.ATV.0000248731.55781.87. [DOI] [PubMed] [Google Scholar]
- Xu Z, Yu Y, Duh EJ. Vascular endothelial growth factor upregulates expression of ADAMTS1 in endothelial cells through protein kinase C signaling. Invest Ophthalmol Vis Sci. 2006b;47:4059–4066. doi: 10.1167/iovs.05-1528. [DOI] [PubMed] [Google Scholar]
- Yalcin M, Bharali DJ, Dyskin E, Dier E, Lansing L, Mousa SS, Davis FB, Davis PJ, Mousa SA. Tetraiodothyroacetic acid and tetraiodothyroacetic acid nanoparticle effectively inhibit the growth of human follicular thyroid cell carcinoma. Thyroid. 2010a;20:281–286. doi: 10.1089/thy.2009.0249. [DOI] [PubMed] [Google Scholar]
- Yalcin M, Bharali DJ, Lansing L, Dyskin E, Mousa SS, Hercbergs A, Davis FB, Davis PJ, Mousa SA. Tetraidothyroacetic acid (tetrac) and tetrac nanoparticles inhibit growth of human renal cell carcinoma xenografts. Anticancer Res. 2009;29:3825–3831. [PubMed] [Google Scholar]
- Yalcin M, Dyskin E, Lansing L, Bharali DJ, Mousa SS, Bridoux A, Hercbergs AH, Lin HY, Davis FB, Glinsky GV, Glinskii A, Ma J, Davis PJ, Mousa SA. Tetraiodothyroacetic acid (tetrac) and nanoparticulate tetrac arrest growth of medullary carcinoma of the thyroid. J Clin Endocrinol Metab. 2010b;95:1972–1980. doi: 10.1210/jc.2009-1926. [DOI] [PubMed] [Google Scholar]
- Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- Zhande R, Karsan A. Erythropoietin promotes survival of primary human endothelial cells through PI3K-dependent, NF-kappaB-independent upregulation of Bcl-xL. Am J Physiol Heart Circ Physiol. 2007;292:H2467–H2474. doi: 10.1152/ajpheart.00649.2006. [DOI] [PubMed] [Google Scholar]