Abstract
Human adipose tissue stromal/stem cells (ASCs) are known to induce proliferation of resting T cells under ambient (21%) O2 conditions; however, ASCs exist physiologically under lower oxygen (5% O2) conditions in adipose tissue. The effects of low oxygen levels on ASC immunomodulation of T cells are unknown. In this study, we show that ASCs stimulated proliferation of naive CD4+ T cells and the percentage of CD25+ T cells was significantly increased under both low and ambient O2. Forkhead box P3 (FoxP3) and transforming growth factor beta (TGF-β) mRNA expression were significantly increased when ASCs were cocultured with CD4+ T cells under low compared with ambient O2 conditions. The low O2-induced regulatory T cells (iTregs) exhibited functionality when added to mixed lymphocyte reactions as demonstrated by inhibition of peripheral blood mononuclear cell proliferation, and by >300-fold increase in FoxP3 mRNA, and >2-fold increase in TGF-β mRNA expression. These results demonstrate that under physiologically relevant low O2 conditions, direct contact of human ASCs with naive CD4+ T cells induced functional iTregs.
Introduction
Due to their inherent multipotency, relative ease of isolation from the stromal vascular fraction (SVF) of adipose tissue, and the ability to enhance vascularization, adipose tissue stromal/stem cells (ASCs) represent a favorable cell-based therapy tool for tissue engineering, regenerative medicine, and reconstructive surgery [1,2]. In a murine xenograft model using polymerized poly (ethylene) glycol-diacrylate for breast reconstruction, ASCs were reported to regenerate functional, highly vascularized adipose tissue following transplantation [3,4]. In addition, ASCs possess important immunoregulatory effects, including immunosuppression of a number of immune cells under varying conditions [5,6]. Under ambient (21%) O2 conditions in vitro, ASCs inhibited proliferation of alloactivated peripheral blood mononuclear cells (PBMCs) and CD4+ T cells, and stimulated proliferation of resting CD4+ T cells [7,8]. T-cell proliferation continued after extended culture in the absence of ASCs [6,7,9].
Whereas they represent a small fraction of all CD4+ T cells (∼10%), naive T cells have the potential to differentiate into multiple functional phenotypes, including induced regulatory T cells (iTregs), T helper type I (Th1), Th2, or Th17 cells [10]. Chen et al. demonstrated that naive, peripheral CD4+CD45RA+ T cells were converted into anergic/suppressor iTregs, CD25+ and CD45RB−/low, through costimulation of T-cell receptors (TCRs) and treatment with transforming growth factor β (TGF-β) [11]. These iTregs were not only refractory to further TCR stimulation, but they also inhibited normal T-cell proliferation in vitro when placed in mixed lymphocyte reactions (MLRs) [11].
ASC activation or suppression of one or more specific T-cell subsets may significantly affect the observed overall growth inhibition or stimulation of T cells [5–7,9,12–17]. Several studies have demonstrated that ASCs inhibit cells of both the innate and adaptive immune response through secretion of soluble factors such as prostaglandin E2, TGF-β1, hepatocyte growth factor, and production of factors that include inducible nitric oxide synthase and indoleamine 2,3-dioxygenase [7,9,12,13,20–22]. Another potential mechanism of immune interference involves ASC-mediated induction of iTregs from naive CD4+ T cells by direct contact. Crop et al. demonstrated that ASCs had the potential to activate resting immune cells when directly cocultured with PBMCs [7]. The activated population was CD4+ CD25high CD127− FoxP3+ and possessed suppressive capacity [7]. Moreover, ASCs were demonstrated to recruit iTregs to both the lymphoid organs and adipose grafts in vivo [7,15,23]. In breast, colorectal, pancreatic, and other cancers, Treg activation suppresses the host immune response to tumor-specific antigens through downregulation of CD8+ cytotoxic T cells [21–24]. Treg cell-mediated immune suppression is one of the more crucial tumor immune evasion mechanisms and the main obstacle to successful tumor immunotherapy [13,24–29]. Taken together, these data suggest that, when placed in direct contact with naive CD4+ T cells, ASCs may induce and recruit iTregs that have the potential to suppress the cytotoxic T-cell response within adipose tissue grafts, thereby contributing to breast cancer recurrence [18,19].
Kronsteiner et al. [15] demonstrated that the stimulation method and the cellular environment may alter CD4+ T-cell cytokine secretions, create an inflammatory milieu, and effectively alter ASC effects on specific cells of the immune system. Low O2 content was demonstrated to significantly alter the ASC transcriptome and the secretion of pro- and anti-inflammatory cytokines that are involved in T-cell activation and suppression [8,30–33,42,45]. However, ASC immunomodulatory effects on specific subpopulations of CD4+ T cells under low O2 conditions are unknown.
Although most studies of ASC immune suppression have been conducted under ambient (21%) O2 conditions [7,9,15,16,34–38], physiological O2 levels are ∼5% in intact adipose tissue and in fat grafts used for breast and other tissue reconstruction [39,40]. We hypothesized that under physiological low oxygen, ASCs would stimulate induced Tregs (iTregs) from naive CD4+ T cells derived from breast cancer patients. The objective of the present study was therefore to investigate the ASC immunomodulatory behavior on naive CD4+ T cells under physiological conditions similar to those present in adipose tissue or in newly transplanted allogeneic fat grafts. In a newly transplanted ASC-supplemented fat graft, ASCs may contribute to the suppression of the cell-mediated immune response to tumor cells by inducing a Treg phenotype from naive CD4+ T cells.
Materials and Methods
Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Norcross, GA) unless otherwise specified.
Isolation, collection, and culture of human ASC
The procedures for isolation, collection, and culture of ASCs were previously described [41]. ASCs were obtained from the subcutaneous abdominal adipose tissue region of five healthy female, Caucasian patients between ages 28–61 years with a mean±standard deviation (SD) of 42±12.9 years. The patients displayed a mean body mass index (kg/m2) (±SD) of 26.4±5.7. All tissues were acquired from elective procedures in local plastic surgery offices as approved by the Pennington Biomedical Research Center Institution Review Board (No. 23040), with patients' informed consent. Briefly, liposuction tissues were transported to Pennington Biomedical in a saline solution within 2 h postsurgery. The tissue was washed with phosphate-buffered saline and digested with 0.1% collagenase type I (Worthington Biochemicals, Brunswich NJ), 1% bovine serum albumin, and 2 mM CaCl2 for 60 min at 37°C with intermittent shaking. The floating adipocytes were separated from the SVF by centrifugation (300 g) for 5 min at room temperature. The supernatant containing mature adipocytes was aspirated and discarded and the remaining pellet was identified as the SVF. The SVF cells were suspended and plated immediately in T175 flasks in the stromal medium [DMEM/F-12 Ham's, 10% fetal bovine serum (FBS; Hyclone, Logan, UT, www.hyclone.com), 100 U penicillin/100 g streptomycin/0.25 g fungizone] at a density of 0.156 mL of tissue digest/cm2 of surface area for expansion and culture. This initial passage of the primary cell culture was referred to as passage 0 (P0). ASCs were used between passages 2 and 3 for experiments (table in Supplementary Fig. S1a; Supplementary Data are available online at www.liebertpub.com/scd).
Low O2 cell culture
In vitro low O2 experiments were performed with a Single Set point Oxygen & CO2 controller inserted in a standard incubator (Biospherix C-Chamber Hypoxia Chamber; Bio-Spherix, Lacona, NY, www.biospherix.com) that continuously infused a calibrated gas mixture (95% N2, 5% CO2). Experiments were performed at oxygen concentrations of 21% (ambient O2) or 5% (low O2).
ASC exposure to inflammatory conditions under low O2
To observe ASC behavior under the influence of an inflammatory milieu, cells were kept in the stromal medium supplemented with 400 U/mL recombinant human interferon-gamma (IFN-γ) (R&D Systems, Minneapolis, MN) under low O2 (5% O2) or ambient O2 (21% O2) culture conditions using a Single Set point O2 and CO2 controller as described under the Low O2 Cell Culture section. After 5 days of culture, untreated or IFN-γ-treated cells were subjected to flow cytometry analysis, used for direct or indirect coculture experiments, or the cytokine secretion profile was determined by applying conditioned supernatants onto the Human Proteome Profiler Array Kit, panel A (R&D Systems).
Western immunoblot
ASCs were cultured 3 days under 5% O2 or 21% O2. The total protein was quantified using a Biorad protein assay (Life Science Research, Hercules, CA). Fifty micrograms of protein was loaded onto a 15% denaturing acrylamine gel, separated by electrophoresis, transferred to nitrocellulose membrane, and probed sequentially with primary murine monoclonal antibodies corresponding to amino acids 432–528 of human hypoxia-inducible factor-1 alpha (HIF-1α; Abcam HIF1A67) and human GAPDH (ab8245). Images were scanned using a LI-COR Odyssey scanner (LI-COR Biosciences, Lincoln, NE).
Flow cytometry for ASC expression of immune-stimulatory markers on ASCs
ASCs were treated with 400 U/mL IFN-γ under low O2 or ambient O2 culture, as described under the ASC Exposure to Inflammatory Conditions Under Low O2 section. Adherent cells were removed from culture flasks by incubation with 0.25% trypsin-EDTA at 37°C for 5 min, washed two times with the stromal medium, and resuspended in a cell staining buffer (Biolegend, San Diego, CA) at a final concentration of 1×106 cells/mL. Cell suspensions were incubated with antibodies against CD86-phycoerythrin (PE), CD80-fluorescein isothiocyanate (FITC), CD40-PE, human leukocyte antigen A,B,C-related (HLA-A,B,C)-allophycocyanin (APC), or human leukocyte antigen D-related (HLA-DR)-FITC (BD Biosciences, San Jose, CA) at room temperature for 30 min, protected from light. After two washes with a cell staining buffer (Biolegend), flow cytometric analysis was performed using a BD Biosciences fluorescence-activated cell sorter (FACS) Beckman-Coulter Galios flow cytometer with two lasers and eight detectors running the Galios acquisition software (BD Biosciences).
Isolation of PBMCs
PBMCs (n=60) were obtained through the Louisiana Cancer Research Consortium (LCRC) Biospecimen Core. Peripheral blood samples were collected from patients with breast cancer (Supplementary Fig. S2). PBMCs were isolated from the collected heparinized peripheral blood by density gradient centrifugation using Ficoll Isopaque (d¼1.077; Amersham Biosciences; Upsala, Sweden) and frozen at −150°C until use. The demographic characteristics of the patients are also presented in Supplementary Table S1.
Isolation of naive CD4+ T cells from PBMCs by QuadroMACs
Naive CD4+ T cells were isolated from PBMCs (Miltenyi Biotech, Auburn, CA) by negative selection using quadroMACS (Miltenyi Biotech). PBMCs were thawed and plated in a complete medium [RPMI 1640, supplemented with 10% (v/v) FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, and 1% (v/v) nonessential amino acids (all media supplements obtained from Gibco, Gaithersburg, MD)]. To check for purity, both fractions (negatively selected naive CD4+ T cells and the flow-through fraction) were stained with CD4-PE and CD45RA-FITC (Biolegend). Flow cytometric analysis was performed on a FACS Beckman-Coulter Galios flow cytometer.
Direct coculture of ASCs with naive CD4+ T cells under low O2
For direct coculture, ASCs were seeded in the stromal medium in 6-well plates at concentrations ranging from 1,000 cells/well to 100,000 cells/well. Following 24 h of attachment, ASCs were treated with 400 U/mL human recombinant IFN-γ (Sigma-Aldrich) for 5 days, as described above under the ASC Exposure to Inflammatory Conditions Under Low O2 section. ASCs were treated with 50 μg/mL mitomycin c (Sigma-Aldrich) for 1 h to inhibit proliferation at mitosis. Isolated naive CD4+ T cells were then plated at 100,000 cells per well with the ASC monolayers, yielding ratios of 1:100 (1,000 ASC:100,000 T cells), 1:10 (10,000 ASC:100,000 T cells), and 1:1 (100,000 ASC:100,000 T cells). ASC-naive T-cell cocultures were cultured under low O2 and ambient O2 culture conditions for 3 days. Naive T-cell proliferation was assessed using the bromodeoxyuridine (BrdU) assay.
Flow cytometry for regulatory T-cell immunophenotype
Naive CD4+ T cells were cocultured with IFN-γ-treated ASCs under low O2 or ambient O2 culture as described under the Direct Coculture of ASCs with naive CD4+ T Cells Under Low O2 section. T cells were then collected and cell suspensions were incubated with antibodies against CD4-FITC, CD127-PE, and CD25-PE for 30 min. Cells were fixed and permeabilized using the cell fixation/permeabilization buffer (Biolegend) and resuspended with antibodies against Forkhead box P3 (FoxP3)-Alexa Fluor 647 at 4°C. Flow cytometric analysis was performed using a Beckman-Coulter Galios flow cytometer (BD Biosciences).
Functional analysis of T cells cultured previously with ASCs
Naive T cells were cocultured with IFN-γ-treated ASCs for 3 days under low O2 or ambient O2 culture, as described under the Direct Coculture of ASCs with naive CD4+ T Cells Under Low O2 section. T cells were collected and added into mixed lymphocyte reactions (MLRs) at a ratio of 1:10 with 2 μM PKH-26-labeled PBMCs (Sigma-Aldrich) for 7 days. PBMC proliferation was assessed by the BrdU assay. PBMC suspensions from functional assays were incubated with antibodies against CD4-FITC, CD127-PE, and CD25-PE for 30 min, and CXCR3-APC-cyanin 7 (Cy7) for 30 min. Flow cytometric analysis was performed using a BD FACSAria.
Quantitative real time polymerase chain reaction (qRT-PCR) for TGF-β and FoxP3 gene expression
Total RNA was purified from human naive CD4+ T cells that were cocultured with ASCs under low O2 and ambient O2 conditions for 3 days using the Qiagen AllPrep DNA/RNA/Protein Mini Kit (Qiagen, Inc., Valencia, CA; www.qiagen.com) according to the manufacturer's specifications. One-step reverse transcriptase-polymerase chain reactions were performed with ∼500 ng of total RNA using the iScript One-Step RT-PCR Kit for probes (Bio-Rad Laboratories, Hercules, CA). The complete reaction mix was incubated with the oligonucleotide primer and probe sets specific for human TGF-β (forward: 5′-TCGCCTACCAGCTCATGCATAACA-3′; reverse: 5′-TGAAGCTCTTCCAGGTGTCAACGA-3′; probe: 5′-/56-FAM/TCAGGGACATTGACGTGATCATGGG/3BHQ_1/3′) and FoxP3 (forward: 5′-GGT TCA CAC GCA TGT TTG CCT TCT-3′; reverse: 5′-GCA CAA AGC ACT TGT GCA GAC TCA-3′; probe: 5′-/56-FAM/AGA AAC CAT/ZEN/CCT GCC ACC TGG AAG AA/3BHQ_1/3′) in a real-time thermal detection system (Bio-Rad Laboratories). All results were normalized relative to GAPDH expression control.
Statistical analyses
Statistical analysis of data was performed using Graphpad Prism v5.0 software; the level of P value was set at 0.05. The statistical analyses performed were as follows: two-way analysis of variances (two-way ANOVAs), followed by Bonferroni's post-tests were used for CyQUANT and BrdU proliferation experiments, differentiation, and array experiments. Linear regression analyses were performed on T-cell proliferation following direct coculture, and the determination coefficients (R2 values) were given to evaluate the relationship between the ASC:naive T-cell coculture ratio and T-cell proliferation. The coefficient of determination was such that 0≤R2≤1, and denoted the strength of the association between the ASC:naive T-cell coculture ratio and proliferation, where 1 represents the strongest correlation. Results were reported as a positive or negative correlation. A positive correlation reflected an increase in growth as the number of ASCs plated increased. A negative correlation reflected a decrease in growth as the number of ASCs plated increased.
Results
Effect of low O2 on ASC and naive CD4+ T-cell secretomes
ASCs isolated from five healthy female, Caucasian donors (Supplementary Fig. S1a) were cultured under ambient O2 (21%) or low O2 (5%) conditions. Naive CD4+ T cells were isolated from the peripheral blood of five donors who were diagnosed with breast cancer (Supplementary Table S1) and cultured 1, 3, and 5 days in low O2 or ambient O2 culture. Low O2 culture did not affect viability or proliferation of ASCs or naive T cells (data not shown, Supplementary Fig. S1b, c). The percent purity for naive T-cell isolation using the MACs separation procedure averaged 92.5% (Supplementary Fig. S1d, e). To demonstrate that low O2 exposure stimulates hypoxia-induced pathways in ASCs, ASCs from five different donors were exposed to low O2 for 3 days. The protein was extracted and probed for HIF-1α by western immunoblot (Supplementary Fig. S1f).
We previously demonstrated that exposure to low physiological O2 levels for 3 days altered ASC secretion of extracellular matrix remodeling proteins and type I cytokines that are involved in tissue fibrosis [41]. To determine the effects of low O2 on the secretome of naive CD4+ T cells alone, the conditioned medium from naive CD4+ T cells cultured in ambient O2 or low O2 was used to probe a human Proteome Profiler array that contained 36 different cytokines, chemokines, and acute-phase proteins important in T-cell growth, differentiation, gene expression, migration, immunity, and inflammation. All 36 proteins present on the array were detected in the secretomes of naive CD4+ T cells cultured in both ambient O2 and low O2 (Supplementary Figs. S2 and S3). Thirteen proteins were not significantly altered when comparing culture in ambient O2 to culture in low O2, including interleukin (IL)-4 and IL-5, which are involved in Th2 differentiation, and IL-12p70, which is involved in Th1 differentiation [24]. Seven proteins were significantly decreased under low O2 culture, including the cytokines IL-6 (P<0.05) and IL-23 (P<0.001) that stimulate Th17 differentiation, while inhibiting Treg cell differentiation/expansion [24,43]. Other protein secretions that were significantly decreased under low O2 culture included soluble intercellular adhesion molecule-1 (P<0.001), IL-8 (P<0.001), monocyte chemoattractant protein-1 (MCP-1, P<0.001), and macrophage inhibitory protein-1α and β (MIP-1-α, P<0.01; MIP-1β, P<0.01). Sixteen proteins were significantly increased under low O2 culture, including IL-10 (P<0.001) that is involved in Treg differentiation [44] (Supplementary Figs. S2 and S3). These data suggested that culture of naive T cells in low O2 may favor a Treg secretory profile.
Low O2 treatment reduced expression of HLA-DR on IFN-γ-stimulated ASCs
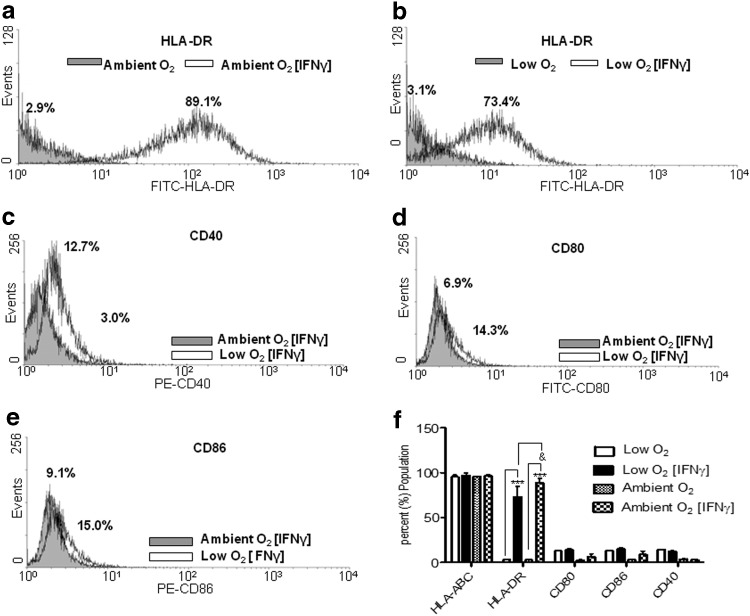
Following culture of ASCs in ambient or low O2 for 5 days concurrent with IFN-γ exposure, flow cytometry was performed to measure surface expression of mixed lymphocyte reaction class II receptor HLA-DR, the MHC class I receptor HLA-A,B,C, and costimulatory molecules CD40, CD80, and CD86. As expected, significant HLA-A,B,C surface expression was detected on ASCs cultured under both ambient O2 (untreated: 96.1%±0.1%; treated: 96.8±3.0; Fig. 1f) and low O2 (untreated: 95.5%±4.1%; treated: 97.1%±5.6%; Fig. 1f). Two-way ANOVAs reflected no significant difference in HLA-A,B,C expression on IFN-γ-treated ASCs under ambient O2 or low O2. HLA-DR expression was significantly increased under both ambient O2 (untreated: 3.1%±1.6%; treated: 89.1%±10.1%; P<0.001; Fig. 1a) and low O2 (untreated: 3.1%±0.8%; treated: 73.4%±25%; P<0.001; Fig. 1b) following IFN-γ treatment. Analyses of mean fluorescent intensities of ambient O2 and low O2 demonstrated that a higher percentage of IFN-γ-stimulated ASCs expressed HLA-DR under ambient O2 culture (Fig. 1f). IFN-γ treatment did not significantly alter surface expression of the costimulatory molecules CD40, CD80, and CD86 on ASCs cultured in ambient O2 or low O2 (Fig. 1c–f). These data indicated that low O2 culture did not significantly affect IFN-γ-induced expression of costimulatory molecules CD40, CD80, and CD86 on ASCs; however, low O2 culture did reduce IFN-γ-induced expression of HLA-DR.
FIG. 1.
Expression of mixed lymphocyte reaction class I/II and costimulatory molecules on adipose tissue stromal/stem cells (ASCs) following IFN-γ treatment. Human leukocyte antigen D-related (HLA-DR) expression on the surface of ASCs following interferon-gamma (IFN-γ) treatment under (a) ambient (21% O2) conditions and (b) low (5% O2) culture conditions for 5 days. (c, d, and e) Expression of costimulatory molecules CD40, CD80, and CD86, respectively, on ASCs under ambient and low O2 culture conditions. (f) Quantification of flow analyses following ASC culture under low or ambient O2 culture conditions. Three independent sets of experiments were performed. Data are reported as mean (μ)±standard error (SE). ***P<0.0001 comparing HLA-DR expression in an unstimulated ASC to IFN-γ-stimulated ASC, &P<0.05 comparing HLA-DR expression in ASCs under low O2 to ambient O2.
ASC stimulated naive CD4+ T-cell proliferation
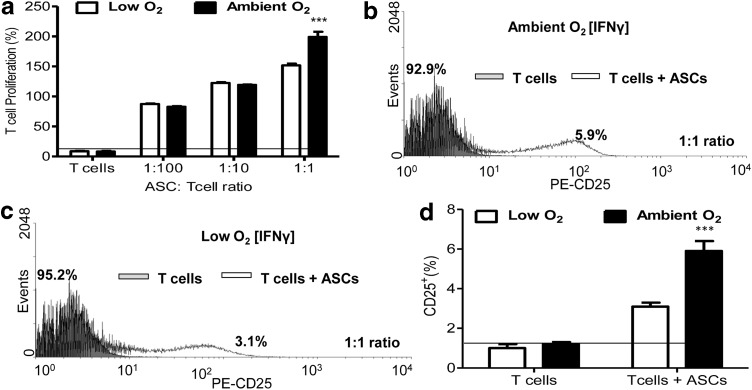
Increasing numbers of IFN-γ-stimulated ASCs were cocultured with naive CD4+ T cells (Fig. 2a, ASCs: T cells; 1:100, 1:10, and 1:1) for 3 days under ambient O2 or low O2. ASCs significantly induced T-cell proliferation at all ratios (Fig. 2a). This stimulation of proliferation was cell number dependent, as T-cell proliferation increased as the ASC number increased (Fig. 2a). The highest T-cell proliferation was observed following a 1:1 ratio of coculture, and a higher percentage of T cells proliferated following coculture with ASCs under ambient O2 at this ratio [mean (μ)±SD: ambient O2, 199.1%±8.7%; low O2, 151.7%±2.9%; P<0.0001, Fig. 2a. T cells were collected and flow cytometry was performed for expression of T-cell early activation marker CD25. The percentage of CD25− T cells was designated in the gray peaks and labeled. The percentage of CD25+ T cells was designated in the clear peaks (Fig. 2) and labeled accordingly. Flow cytometric analyses revealed that the percentage of CD25+ T cells was significantly increased (P<0.001) when naive CD4+ T cells were cocultured with ASCs at a 1:1 ratio under both low O2 and ambient O2, and CD25 expression was higher in cells under ambient O2 conditions (Fig. 2b–d). In contrast to direct coculture, indirect coculture of ASCs with naive CD4+ T cells in a Boyden chamber showed no significant difference in T-cell proliferation under low O2 or ambient O2 culture (data not shown), demonstrating that ASCs stimulated proliferation and activation of naive T cells under both ambient O2 and low O2, and that these effects were cell number and cell contact dependent.
FIG. 2.
Naive T-cell proliferation and activation following direct coculture with ASCs under low O2 and ambient O2 culture conditions. Naive CD4+ T cells were isolated from peripheral blood of breast cancer patients. ASCs were treated with IFN-γ for 5 days, and then treated with 50 μg/mL mitomycin c to inhibit growth. Naive CD4+ T cells were cocultured with IFN-γ-treated ASCs at cell concentrations of 1:100 (1,000 ASCs:100,000 T cells), 1:10 (10,000 ASCs:100,000 T cells), and 1:1 (100,000 ASCs:100,000 T cells) for 3 days. T-cell proliferation was quantified through bromodeoxyuridine (BrdU) incorporation. CD4+ T-cell proliferation (%)=(ETcell+ASC/ETcell)×100, where ETcell+ASC=proliferation absorbance measurement for naive T-cell+ ASC group, and ETcell=proliferation absorbance measurement for T-cell group. (a) Percent (%) T-cell proliferation following coculture at varying ratios. Percent (%) CD25+ T cells were evaluated following 1:1 coculture with ASCs by flow cytometry under (b) ambient and (c) low O2 conditions. (d) Quantification of flow cytometric analyses in panels b and c for CD25 expression of T cells following coculture of ASCs with naive T cells. Three independent sets of experiments were performed for each treatment. Data are reported as mean (μ)±SE. Significance: ***P<0.001.
ASCs induced CD4+ CD25high FoxP3+ iTreg phenotype from naive CD4+ T cells
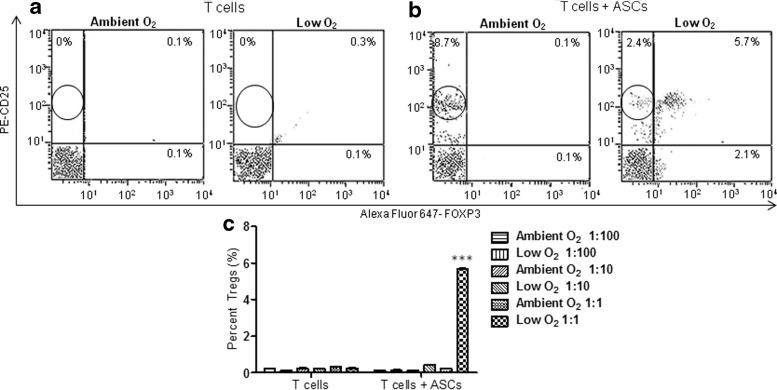
Naive CD4+ T cells were cocultured with IFN-γ-stimulated ASCs at ratios of 1:100, 1:10, and 1:1, under ambient O2 and low O2 culture. Flow cytometric analyses were performed for detection of CD4, CD25, and FoxP3, markers of iTregs. Consistent with the CD25 results in Fig. 2, the surface expression of CD25high on T cells was significantly increased when naive T cells were directly cocultured with ASCs at a 1:1 ratio under both ambient O2 (8.7%±0.03%) and low O2 (2.4%±0.07%) conditions (Fig. 3a, b). Remarkably, the percentage of cells coexpressing CD25 and FoxP3 significantly increased following coculture with ASCs at a 1:1 ratio under low O2, but not under ambient O2 (ambient O2: T alone, 0.3%±0.03%; T+ASC, 0.21%±0.03%; low O2: T alone, 0.3%±0.03%; T+ASC, 5.7%±0.07%; P<0.0001, Fig. 3a–c).
FIG. 3.
Expression of CD25 and Forkhead box P3 (FoxP3) from naive CD4+ T cells following coculture with ASCs under ambient and low O2 culture conditions. IFN-γ-treated ASCs were cocultured with naive CD4+ T cells for 3 days under low or ambient O2 culture conditions at ratios of 1:100 (1,000 ASCs:100,000 T cells), 1:10 (10,000 ASCs:100,000 T cells), and 1:1 (100,000 ASCs:100,000 T cells). T cells were collected and stained for fluorescein isothiocyanate (FITC)-CD4, phycoerythrin (PE)-CD25, and Alexa Fluor 647-FoxP3 (AF-FoxP3) and flow cytometry was used to calculate percent Treg cells present in the gated CD4+ population. Representative flow cytometric analyses of CD25- and FoxP3-positive (a) T cells alone, and (b) T cells following coculture with ASCs at a 1:1 ratio under low and ambient O2 culture conditions. (c) Representative quantification of percentage of Tregs following coculture of ASCs with T cells. Three independent sets of experiments were performed for each treatment. Data are reported as mean (μ)±SE. ***P<0.001.
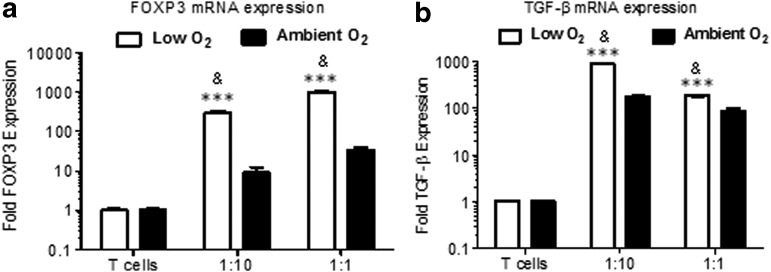
Coexpression of TGF-β and FoxP3 has been associated with the Treg phenotype [46]. To confirm that the proliferating population induced by ASCs was iTregs, mRNA expression of FoxP3 and TGF-β in naive T cells was measured by qRT-PCR following coculture at ASC/T-cell ratios of 1:1 and 1:10. FoxP3 mRNA in T cells was 200-fold higher in cocultures under low O2 compared with ambient O2 at the 1:10 cell ratio (2ΔΔCt: ambient O2, 9.0±3.2; low O2, 302.3±32.4; P<0.001), and 300-fold higher at the 1:1 cell ratio (2ΔΔCt: ambient O2, 33.6±5.1; low O2, 982.3±99; P<0.001; Fig. 4a). Similarly, TGF-β mRNA expression was more than sevenfold higher in cocultures under low O2 compared with ambient O2 in the 1:10 cell ratio (2ΔΔCt: ambient O2, 177.3±7.5; low O2, 883.2±9.8; P<0.001), and twofold higher in the 1:1 cell ratio (2ΔΔCt: ambient O2, 85.5±13.1; low O2, 186.5±7.5; P<0.001; Fig. 4b). Taken together, these data demonstrated that following direct contact for a brief period of time, ASCs induced a Treg phenotype from naive CD4+ T cells. Taken together, these data demonstrated that following direct contact for a brief period of time, ASCs induced a Treg phenotype from naive CD4+ T cells. These data also suggested that extended culture may have induced higher detectable percentages of iTregs by flow cytometric analyses. In addition, extended culture may have induced higher detectable percentages of iTregs by flow cytometric analyses.
FIG. 4.
FoxP3 and transforming growth factor beta (TGF-β) gene expression in T cells following coculture with ASCs. IFN-γ-treated ASCs were cocultured with naive CD4+ T cells for 3 days under low or ambient O2 culture conditions at ratios of 1:10 (10,000 ASCs:100,000 T cells) and 1:1 (100,000 ASCs:100,000 T cells). T cells were collected, RNA was isolated, and one-step quantitative real-time polymerase chain reaction (qRT-PCR) was performed following coculture of ASCs with T cells using forward and reverse primers and probes for human (a) FoxP3 and (b) TGF-β. Three independent sets of experiments were performed for each treatment. Data are reported as mean (μ)±SE. ***P<0.0001 comparing low O2 to ambient O2 culture for each group. &P<0.001 comparing T cells alone under low O2 culture to T cells+ASCs under low O2 culture, and T cells alone under ambient O2 culture to T cells+ASCs under ambient O2 culture.
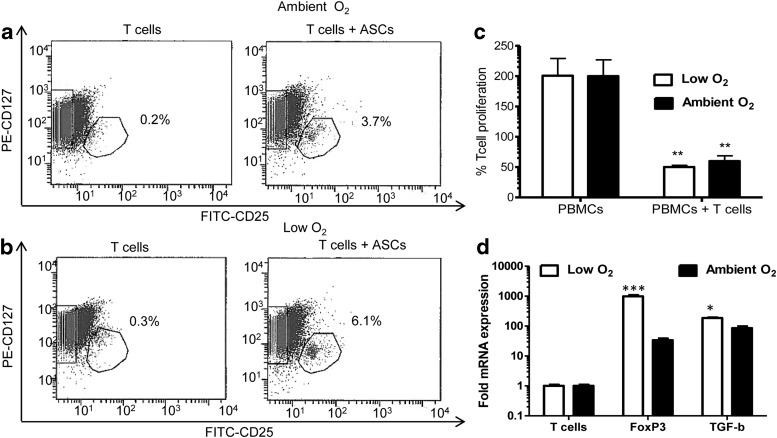
ASCs induced CD127−/low CD4+CD25+/highFoxP3+ functionally active iTregs
To determine whether iTregs induced by ASCs were functional, and to assess the impact of low O2, naive T cells were cocultured with IFN-γ-treated ASCs under low O2 or ambient O2 culture at a 1:1 ratio. T cells were then collected, sorted for the CD127− CD25+ fraction (Fig. 5a, b), and added to MLRs with PBMCs at a 1:10 ratio for 7 days under low O2 or ambient O2 conditions. CD25+ CD127− T cells that were cocultured with ASCs under low O2 and ambient O2 significantly inhibited PBMC proliferation by 40% and 30%, respectively (low O2: PBMCs alone, 200%±28.6%; PBMCs+Tregs, 50.1%±2.9%; ambient O2: PBMCs alone, 200.1%±26.8%; PBMCs+Tregs, 59.9%±8.7%; P<0.01, Fig. 5c). FoxP3 mRNA in previously cocultured T cells was 300-fold higher following cocultures under low O2 compared with ambient O2 (2ΔΔCt: ambient O2, 31.6±3.1; low O2, 990.3±98; P<0.001; Fig. 5d). TGF-β mRNA expression was more than twofold higher (2ΔΔCt: ambient O2, 84.5±12.1; low O2, 180.5±6.5; P<0.05; Fig. 5d).
FIG. 5.
Expression of human adipose tissue-specific markers on induced regulatory T cells (iTregs) following coculture with ASCs. IFN-γ-treated ASCs were cocultured with naive CD4+ T cells for 3 days under low or ambient O2 culture conditions at ASC:T-cell ratios of 1:10 (10,000 ASCs:100,000 T cells) and 1:1 (100,000 ASCs:100,000 T cells). T cells were collected, incubated with antibodies for FITC-CD25 and PE-CD127, and subjected to flow cytometry to sort for CD127−/low CD25+ iTregs under (a) ambient and (b) low O2 culture conditions. Isolated iTregs were cocultured for 7 days with allogeneic PBMCs isolated from human breast cancer patients at a 10:1 ratio (100,000 PBMCs:10,000 iTregs) under low and ambient culture conditions. (c) T-cell proliferation was quantified by BrdU analysis in parallel experiments. % T-cell proliferation=(ETcell+ASC/ETcell)×100 where ETcell+ASC=proliferation absorbance measurement for the naive T-cell+ASC group, and ETcell=proliferation absorbance measurement for the T-cell group. (d) mRNA was isolated and one-step qRT-PCR was performed following ASC-T-cell coculture using forward and reverse primers and probes for human FoxP3 and TGF-β. Three independent sets of experiments were performed for each treatment. Data are reported as mean (μ)±SE. *P<0.05, **P<0.01, ***P<0.0001.
Discussion
In the present study, we demonstrated that ASCs stimulated proliferation of naive CD4+ T cells from breast cancer patients under both low O2 and ambient O2. The low O2 iTregs exhibited functionality when added to MLRs as demonstrated by 40% inhibition of PBMC proliferation, and by >300-fold increase in FoxP3 mRNA and >2-fold increase in TGF-β mRNA.
A low O2 concentration (5%) is representative of O2 levels in tissues in vivo during T-cell activation, clonal expansion, and differentiation [48–50]. Atkuri et al. [48] demonstrated that primary T cells cultured at ambient O2 (21%) significantly altered the intracellular redox state (stimulated production of an abnormally elevated amount of oxidized intracellular glutathione), whereas T cells cultured at an O2 level of 5% maintained the balance of intracellular glutathione and its oxidized form that more closely resembled the in vivo status. Toussaint et al. [50] demonstrated that the observed increased T-cell proliferative response in many studies following extended culture under ambient O2 conditions may be a skewed T-cell response. Moreover, based on the proliferation index and T-cell yield, Duggan et al. [49] demonstrated that CD4/CD28 antibody-mediated T-cell proliferation was higher at ambient O2 than at low O2. This was partly due to intracellular nitric oxide (NO) levels, which were skewed higher at ambient O2 [49]. Because high levels of intracellular NO and sustained CD69 tend to downregulate T-cell responses in vivo, the lower proliferative T-cell responses in vitro under low O2 may be considered as a reflection of in vivo regulatory mechanisms.
Crop et al. demonstrated that ASCs stimulated PBMC proliferation under ambient oxygen conditions, independent of cell contact [7]. The present study demonstrated that when placed in direct contact with naive CD4+ T cells from breast cancer patients under ambient O2 culture conditions, ASCs induced iTregs in vitro. Both soluble mediators from the ASCs, as well as direct contact with ASCs may contribute to iTreg induction. Of note, there were several differences in the experimental design in the present study compared with previous studies. The present study selected for the naive CD4+ T-cell subpopulation from breast cancer patients before coculture analyses. We cannot conclude whether the gene expression changes identified would also occur in CD4+ T cells from healthy donors. It is possible that naive CD4+ T cells from healthy patients may not respond to ASC cytokine/chemokine/growth factor secretions in the same manner, and further investigation on T-cell activation in breast cancer is warranted. Multiple studies cocultured MSCs with PBMCs for 1 week before measuring effects on iTreg induction [7,11,12]. Because of the setup for the hypoxia c-chamber and to prevent ischemia/reperfusion variable interference, the present study measured proliferation following only 3 days of coculture. The present study supports and further extends the observations of Kronsteiner et al. [15], who reported that ASC immunomodulatory effects on different immune cells may vary significantly depending on the culture conditions utilized [51–52].
These data are consistent with previously published reports that, under ambient O2 conditions, ASCs cocultured with peripheral blood leukocytes from breast cancer patients induced expression of CD4+ CD25+ FoxP3+ T cells [38,53–55]. In addition, recent studies have reported a relationship between leukocyte infiltration, an increased ratio of iTreg to CD4+ T cells in primary tumors, and tumor recurrence in breast cancer patients [38,47,56]. In a retrospective study on triple negative breast cancer (stage I to III), Kim et al. [56] found that an increased number of FoxP3+ Treg cells were significantly correlated with tumors that exhibited lymph node metastases. There were also significant correlations between the increased ratio of FoxP3+ Treg to CD4+ T cells with lymph node metastasis and expression of the estrogen receptor (ER), p53, and Ki-67 positivity. A lower ratio of FoxP3+ Treg to CD4+ T cells was significantly associated with triple-negative breast cancer. Disease-free survival of analyzed patients was also significantly associated with the number of FoxP3+ Tregs [56].
Taken together, data from these previous studies and the present study suggest that ASC usage to supplement subcutaneous adipose tissue transplanted grafts could possibly contribute to breast cancer recurrence by inducing an iTreg cell phenotype from circulating naive CD4+ T cells and subsequent downregulation of cytotoxic T-lymphocyte activity. Further studies are required to explore changes in gene expression in healthy donors compared with breast cancer patients.
Supplementary Material
Acknowledgments
This work has been supported, in part, by the Biospecimen Core Laboratory of the LCRC and the National Institute of General Medical Sciences of the National Institutes of Health under award number P20GM103518. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
All authors declare that they have no competing interests except for J.M.G. who is the cofounder of LaCell, LLC, a company focusing on the development of ASCs for research and clinical applications.
References
- 1.Cherubino M. and Marra KG. (2009). Adipose-derived stem cells for soft tissue regeneration. Regen Med 9:109–117 [DOI] [PubMed] [Google Scholar]
- 2.Gimble JM, Katz AJ. and Bunnell BA. (2007). Adipose-derived stem cells for regenerative medicine. Circ Res 2007, 100:1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moioli EK, Chen M, Yang R, Shah B, Wu J. and Mao JJ. (2010). Hybrid adipogenic implants from adipose stem cells for soft tissue reconstruction in vivo. Tissue Eng Part A 16:3299–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimuro K. (2010). Hybrid adipogenic implants from adipose stem cells for soft tissue reconstruction in vivo. Tissue Eng Part A 16:3299–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abumaree M, Al JM, Pace RA. and Kalionis B. (2011). Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev 2:375–392 [DOI] [PubMed] [Google Scholar]
- 6.Singer NG. and Caplan AI. (2011). Mesenchymal stem cells mechanisms of inflammation. Annu Rev Pathol 6:457–478 [DOI] [PubMed] [Google Scholar]
- 7.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Weimar W. and Hoogduijn MJ. (2010). Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev 19:1843–1853 [DOI] [PubMed] [Google Scholar]
- 8.Wang JA, He A, Hu X, Jiang Y, Sun Y, Jiang J, Gui C, Wang Y. and Chen H. (2009). Anoxic preconditioning: a way to enhance the cardioprotection of mesenchymal stem cells. Int J Cardiol 133:410–412 [DOI] [PubMed] [Google Scholar]
- 9.Delarosa O, Sanchez-Correa B, Morgado S, Ramirez C, Del RB, Menta R, Lombardo E, Tarazona R. and Casado JG. (2011). Human adipose-derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer-mediated lysis. Stem Cells Dev 21:1333–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy KM. (2011). Janeway's Immunobiology, 8th edition. Garland Science, New York, NY [Google Scholar]
- 11.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G. and Wahl SM. (2003). Conversion of peripheral CD4+. J Exp Med 198:1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghannam S, Pene J, Torcy-Moquet G, Jorgensen C. and Yssel H. (2010). Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a t regulatory cell phenotype. Immunology 185:302–312 [DOI] [PubMed] [Google Scholar]
- 13.Hegyi B, Kudlik G, Monostori E. and Uher F. (2012). Activated T-cells and pro-inflammatory cytokines differentially regulate prostaglandin E2 secretion by mesenchymal stem cells. Biochem Biophys Res Commun 419:215–220 [DOI] [PubMed] [Google Scholar]
- 14.Hoogduijn MJ, Crop MJ, Peeters AM, Van Osch GJ, Balk AH, Ijzermans JN, Weimar W. and Baan CC. (2007). Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev 16:597–604 [DOI] [PubMed] [Google Scholar]
- 15.Kronsteiner B, Wolbank S, Peterbauer A, Hackl C, Redl H, van Griensven M. and Gabriel C. (2011). Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev 20:2115–2126 [DOI] [PubMed] [Google Scholar]
- 16.Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S, Farinazzo A, Constantin G, Bedogni G, Bedogni A. and Bonetti B. (2012). Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A 18:1264–1272 [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Yuan J, Zhou B, Lee AJ, Lee AJ, Ghawji M, Jr., and Yoo TJ. (2011). The therapeutic efficacy of human adipose tissue-derived mesenchymal stem cells on experimental autoimmune hearing loss in mice. Immunology 133:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muehlberg FL, Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM, et al. (2009). Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis 30:589–597 [DOI] [PubMed] [Google Scholar]
- 19.Uccelli A, Morretta L. and Pistoia V. (2008). Mesenchymal stem cells in health and disease. Nat Rev 9:726–736 [DOI] [PubMed] [Google Scholar]
- 20.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J. and Arnalich-Montiel F. (2012). Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 12:574–591 [DOI] [PubMed] [Google Scholar]
- 21.Singer NG. and Caplan AI. (2011). Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 6:457–478 [DOI] [PubMed] [Google Scholar]
- 22.Yanez R, Oviedo A, Aldea M, Bueren JA. and Lamana ML. (2010). Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res 316:3109–3123 [DOI] [PubMed] [Google Scholar]
- 23.Kuo YR, Chen CC, Goto S, Lee IT, Huang CW, Tsai CC, Wang CT. and Chen CL. (2011). Modulation of immune response and T-cell regulation by donor adipose-derived stem cells in a rodent hind-limb allotransplant model. Plast Reconstr Surg 128:661e–672e [DOI] [PubMed] [Google Scholar]
- 24.Bilate AM. and Lafaille JJ. (2012). Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol 30:733–758 [DOI] [PubMed] [Google Scholar]
- 25.Engelhardt BG, Jagasia SM, Crowe JE, Jr., Griffith ML, Savani BN, Kassim AA, Lu P, Weitkamp JH, Moore DJ, et al. (2012). Predicting posttransplantation diabetes mellitus by regulatory T-cell phenotype: implications for metabolic intervention to modulate alloreactivity. Blood 119:2417–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichihara F, Kono K, Takahashi A, Kawaida H, Sagai H. and Fuji H. (2003). Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 9:4404–4408 [PubMed] [Google Scholar]
- 27.Lanca T. and Silva-Santos B. (2012). The split nature of tumor-infiltrating leukocytes implications for cancer surveillance and immunotherapy. Oncoimmunology 1:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L, Xu W, Wen Z. and Xiong S. (2011). In situ prior proliferation of CD4+ CCR6+ regulatory T cells facilitated by TGF-β secreting DCs is crucial for their enrichment and suppression in tumor immunity. PLoS One 6:e20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou W. (2006). Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6:295–307 [DOI] [PubMed] [Google Scholar]
- 30.Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, Poitou C, Basdevant A, Stich V, et al. (2008). Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 9:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS. and Sung JH. (2009). Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 17:540–547 [DOI] [PubMed] [Google Scholar]
- 32.Leroux L, Descamps B, Tojais NF, Seguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, et al. (2010). Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther 18:1545–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosova I, Dao M, Capoccia B, Link D. and Nolta JA. (2008). Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26:2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi EW, Shin IS, Park SY, Park JH, Kim JS, Yoon EJ, Kang SK, Ra JC. and Hong SH. (2012). Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum 64:243–253 [DOI] [PubMed] [Google Scholar]
- 35.Koenen TB, Stienstra R, van Tits LJ, Joosten LA, van Velzen JF, Hijmans A, Pol JA, van der Vliet JA, Netea MG, et al. (2011). The inflammasome and caspase-1 activation: a new mechanism underlying increased inflammatory activity in human visceral adipose tissue. Endocrinology 152:3769–3778 [DOI] [PubMed] [Google Scholar]
- 36.O'Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin II, Jobe BA, Roberts CT, Jr., Slifka MK. and Marks DL. (2009). Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 33:978–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Rourke RW, White AE, Metcalf MD, Olivas AS, Mitra P, Larison WG, Cheang EC, Varlamov O, Corless CL, Roberts CT., Jr. and Marks DL. (2011). Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia 54:1480–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR. and Ghaderi A. (2011). Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-â1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol 2:116–122 [DOI] [PubMed] [Google Scholar]
- 39.Keith B. and Simon MC. (2007). Hypoxia-inducible factors, stem cells, and cancer. Cell 129:465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tremolada C, Palmieri G. and Ricordi C. (2010). Adipocyte transplantation and stem cells: plastic surgery meets regenerative medicine. Cell Transplant 19:1217–1223 [DOI] [PubMed] [Google Scholar]
- 41.Frazier TP, Gimble JM, Kheterpal I. and Rowan BG. (2013). Impact of low oxygen on the secretome of human adipose-derived stromal/stem cell primary cultures. Biochimie 20:1–11 [DOI] [PubMed] [Google Scholar]
- 42.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Pescatori MSAP, van IJcken WFJ, Dahlke MH, Eggenhofer E, Weimar W. and Hoogduijn MJ. (2010). Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin Exp Immunol 3:474–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL. and Kuchroo VK. (2006). Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238 [DOI] [PubMed] [Google Scholar]
- 44.O'Garra A, Vieira PL, Vieira P. and Goldfeld AE. (2004). IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest 114:1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Alwayn IP, Weimar W. and Hoogduijn MJ. (2009). Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation 87:896–906 [DOI] [PubMed] [Google Scholar]
- 46.Dons EM, Raimondi G, Cooper DK. and Thomson A. (2012). Induced regulatory T cells: mechanisms of conversion and suppressive potential. Hum Immunol 73:328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cipolletta D, Kolodin D, Benoist C. and Mathis D. (2011). Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol 23:431–437 [DOI] [PubMed] [Google Scholar]
- 48.Atkuri KR, Herzenberg LA, Niemi AK, Cowan T. and Herzenberg LA. (2007). Importance of culturing primary lymphocytes at physiological oxygen levels. Proc Natl Acad Sci U S A 104:4547–4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duggan O, Hyland P, Annett K, Freeburn R, Barnett C, Pawelec G. and Barnett Y. (2004). Effects of a reduced oxygen tension culture system on human T cell clones as a function of in vitro age. Exp Gerontol 39:525–530 [DOI] [PubMed] [Google Scholar]
- 50.Toussaint O, Weemaels G, Debacq-Chainiaux F, Scharffetter-Kochanek K. and Wlaschek M. (2011). Artefactual effects of oxygen on cell culture models of cellular senescence and stem cell biology. J Cell Physiol 226:315–321 [DOI] [PubMed] [Google Scholar]
- 51.Wion D, Christen T, Barbier EL. and Coles JA. (2009). PO(2) matters in stem cell culture. Cell Stem Cell 5:242–243 [DOI] [PubMed] [Google Scholar]
- 52.Csete M. (2005). Oxygen in the cultivation of stem cells. Ann N Y Acad Sci 1049:1–8 [DOI] [PubMed] [Google Scholar]
- 53.Sakaguchi S, Sakaguchi N, Asano M, Itoh M. and Toda M. (1995). Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155:1151–1164 [PubMed] [Google Scholar]
- 54.Shevach EM. (2000). Regulatory T cells in autoimmmunity. Annu Rev Immunol 18:423–449 [DOI] [PubMed] [Google Scholar]
- 55.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC. and O'Garra A. (2004). IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol 172:5986–5993 [DOI] [PubMed] [Google Scholar]
- 56.Kim ST, Jeong H, Woo OH, Seo JH, Kim A, Lee ES, Shin SW, Kim YH, Kim JS. and Park KH. (2012). Tumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. Am J Clin Oncol 36:224–231 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.