Summary
There has been a great level of enthusiasm to down-regulate overactive N-methyl-d-aspartate (NMDA) receptors to protect neurons from excitotoxicity. NMDA receptors play pivotal roles in basic brain development and functions as well as in neurological disorders and diseases. However, mechanistic understanding of antagonism in NMDA receptors is limited due to complete lack of antagonist-bound structures for the l-glutamate-binding GluN2 subunits. Here we report the crystal structures of GluN1/GluN2A NMDA receptor ligand-binding domain (LBD) heterodimers in complex with GluN1- and GluN2-targeting antagonists. The crystal structures reveal that the antagonists, D-(−)-2-Amino-5-phosphonopentanoic acid (d-AP5) and 1-(Phenanthrene-2-carbonyl)piperazine-2,3-dicarboxylic acid (PPDA), have discrete binding modes and mechanisms for opening of the bilobed architecture of GluN2A LBD compared to the agonist-bound form. The current study shows distinct ways by which the conformations of NMDA receptor LBDs may be controlled and coupled to receptor inhibition and provides possible strategies to develop therapeutic compounds with higher subtype-specificity.
Keywords: NMDA receptors, Ligand-binding domain, Antagonists
Introduction
Situated in the midst of the synaptic transmission paradigm, NMDA receptors have long been studied from the perspectives of both basic neuroscience and clinical science. These receptors belong to the large family of ionotropic glutamate receptors (iGluRs), which bind the neurotransmitter l-glutamate and meditate the majority of the fast excitatory neurotransmission in the mammalian brain (Kandel et al., 1995; Traynelis et al., 2010). Pharmacological identification of those receptors have been led by a key finding that NMDA induces current formation in neurons (Watkins and Evans, 1981) followed by the important discovery that the NMDA-induced current can be specifically inhibited by antagonists such as D-α-aminoadipate and D-(−)-2-Amino-5-phosphonopentanoic acid (d-AP5) (Evans et al., 1982). Thus, the discovery of d-AP5 played a critical role in confirming the presence of iGluRs in neurons.
NMDA receptors are obligatory hetero-tetramers composed of eight splicing variants of GluN1 (1–4 a or b) and GluN2 (A-D) and/or GluN3 (A-B) of which GluN1 and GluN3 bind glycine and GluN2 binds l-glutamate and NMDA (Benveniste and Mayer, 1991; Clements and Westbrook, 1991). Consistent with the hetero-tetrameric assembly, activation of GluN1/GluN2 NMDA receptors requires concurrent binding of glycine and l-glutamate (Johnson and Ascher, 1987). Combinations of different GluN2 subunits (A-D) with the splicing variants of GluN1 subunits result in formation of ion channels with distinct spatiotemporal expression patterns (Monyer et al., 1994) and pharmacological and electrophysiological properties (Vicini et al., 1998). Subtype-specific targeting of NMDA receptors has been an important challenge especially from the clinical point of view as dysfunctional NMDA receptors are implicated in various neurological disorders and injuries including depression, schizophrenia, Alzheimer’s and Parkinson’s disease, and stroke (Paoletti, 2011). Decades of efforts have led to identification of allosteric compounds with subtype-selectivity including ifenprodil (GluN2B) (Gallagher et al., 1996), TCN201 (GluN2A) (Bettini et al., 2010), QNZ46 (GluN2D) (Acker et al., 2013; Mosley et al., 2010), and CIQ (GluN2C/D) (Mullasseril et al., 2010). Of these, ifenprodil and its analogues have been used most extensively and successfully in neuroscience research, while others have only been used with minimal success to date. For example, it is difficult to specifically inhibit GluN2A in neurons with TCN201 because its effect is robustly altered at different glycine concentrations (Hansen et al., 2012). A phenanthrene-based competitive antagonist with moderate selectivity for GluN2C/D over GluN2A/B, such as 1-(Phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid (PPDA), was identified more than a decade after d-AP5 (Feng et al., 2004). However, in general, competitive antagonists with high degree of subtype-specificity have been slow to develop due to complete lack of structural information that shows binding modes of antagonists at the GluN2 subunits.
Structural studies on iGluRs in the past fifteen years have led to understanding of subunit assembly, ligand recognition, and regulatory mechanism of gating at the molecular level (Mayer, 2011). Thus far, the most advanced subfamily members with respect to structural studies are AMPA and kainate receptors with the intact tetrameric structures obtained by x-ray crystallography and electron microscopy, respectively (Schauder et al., 2013; Sobolevsky et al., 2009). Studies on NMDA receptors have been limited to the extracellular domains, including an amino terminal domain (ATD) (Farina et al., 2011; Karakas et al., 2009, 2011) and a ligand-binding domain (LBD) of GluN1 (Furukawa and Gouaux, 2003; Furukawa et al., 2005; Inanobe et al., 2005; Yao et al., 2013), GluN2 subunits (Furukawa et al., 2005; Vance et al., 2011), and GluN3 subunits (Yao et al., 2008). Furthermore, the structural information of the antagonist-bound form of NMDA receptors is restricted to that of GluN1 LBD (Furukawa and Gouaux, 2003; Inanobe et al., 2005). Thus, despite the historical importance, the molecular mechanism underlying inhibition of NMDA receptors by d-AP5 at GluN2 LBD has remained enigmatic. Moreover, it is not known how phenanthrene-based compounds such as PPDA (Feng et al., 2004) with a drastically distinct chemical structure from d-AP5 can function as an antagonist with some degree of subtype-specificity (Figure 1). The limited understanding of this neuropharmacological problem is largely due to technical difficulties associated with crystallization of the antagonist-bound GluN2 subunit. In this study, we overcome the technical challenge and report crystal structures of heterodimeric GluN1/GluN2A LBD in complex with four combinations of ligands: glycine/l-glutamate, glycine/d-AP5, glycine/(−)-PPDA and 5,7-dichlorokynurenic acid (DCKA)/l-glutamate. The high resolution crystal structures along with site-directed mutagenesis and electrophysiology pinpoint the elements necessary for binding of ligands with distinct chemical nature. Comparison of the four crystal structures shows the pattern of global protein conformational alterations in the context of the GluN1/GluN2A heteromer, thereby showing the plausible mechanism for antagonism.
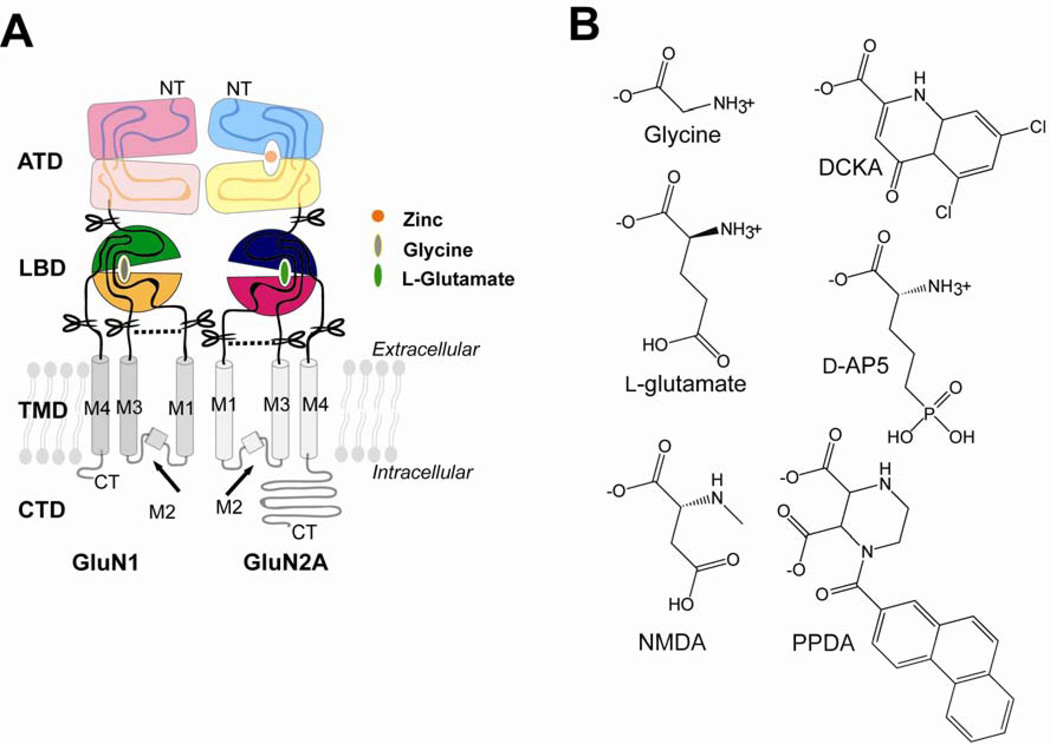
Figure 1. Domain organization of NMDA receptor subunits and ligands.
(A) NMDA receptor subunits are modular proteins composed of an amino terminal domain (ATD), a ligand-binding domain (LBD), a transmembrane domain (TMD), and a cytosolic domain (CTD). Their modular domains are oriented such that the N-terminus (NT) and the C-terminus (CT) are located at the extracellular and cytoplasmic regions, respectively. In this study, LBDs from GluN1 and GluN2A are isolated (scissors) by tethering two peptide fragments between TMD M1 and M3 by a Gly-Thr dipeptide linker (dashed line). (B) Ligands for GluN1 and GluN2 subunits. Glycine and 5,7-dichlorokynurenic acid (DCKA) are an agonist and an antagonist, respectively, for GluN1. l-glutamate and N-methyl-d-aspartate (NMDA) are agonists for GluN2 LBD. D-(−)-2-Amino-5-phosphonopentanoic acid (d-AP5) and 1-(Phenanthrene-2-carbonyl-)piperazine-2,3-dicarboxylic acid (PPDA) with distinct chemical structures both act as competitive antagonists at the GluN2 LBD.
Results
Structural study on antagonist-NMDA receptor complex
To capture the structural representation of competitive antagonism in NMDA receptors, we sought to crystallize the GluN1/GluN2A LBD heterodimer in complex with antagonists for GluN1, DCKA, and GluN2, d-AP5 and PPDA (Figure 1). Based on the previous work, it is well established that iGluRs are arranged as a dimer of two dimers at the extracellular domains (Mayer, 2011) and that the NMDA receptor LBDs form GluN1–GluN2 heterodimers (Furukawa et al., 2005). To date, there are several antagonist-bound structures in the context of GluN1 LBD (Furukawa and Gouaux, 2003; Inanobe et al., 2005), but not in the context of the physiologically relevant GluN1/GluN2A LBD heterodimer. Furthermore, no antagonist-bound structure of LBDs for any GluN2 subunits is presently available due to difficulties in crystallizing the antagonist-receptor complex. Thus, instead of co-crystallizing the receptor-ligand complexes, we have sought to take an alternative approach involving soaking of crystals of GluN1/GluN2A LBDs in complex with glycine and l-glutamate (GluN1/GluN2A LBD-gly/glu) (Furukawa et al., 2005) against the crystallization buffer containing DCKA and l-glutamate (DCKA/glu), glycine and d-AP5 (gly/d-AP5), and glycine and the racemic mixture of (+)- and (−)-PPDA enantiomers (gly/PPDA).
The GluN1/GluN2A LBD-gly/glu crystals obtained in the previously published condition had a limited size (~15 µm×15 µm×100 µm) with radiation decay that disallowed collection of a complete data set (Furukawa et al., 2005). Soaking of those small crystals against antagonist containing solutions resulted in severe cracking and prevented diffraction analysis. Thus, we first screened for the new crystallization condition for GluN1/GluN2A LBD-gly/glu and found that the vapor diffusion method in the presence of PEG2000 MME at pH 7 and 17°C (see Experimental Procedures) produced substantially larger crystals (~50 µm×50 µm×500 µm) than the one previously reported. Importantly, these new crystals withstood soaking against the antagonist containing solutions and provided complete x-ray diffraction data sets at high resolution for all of the above states (Table S1). Extensive soaking of the GluN1/GluN2A LBD-gly/glu crystals against a buffer containing d-AP5 (1 mM), PPDA (0.1 mM), or DCKA (1 mM) as described in “Experimental Procedures” resulted in large alterations in the cell unit dimensions but with no change in the space group (Table S1). Structures for the antagonist-bound GluN1/GluN2A LBDs were solved by molecular replacement (MR) using the following structural coordinates as search probes: the two split fragments of the glycine-bound GluN1 LBD (PDB code: 1PB7) (Furukawa and Gouaux, 2003) and the l-glutamate-bound GluN2A LBD (Furukawa et al., 2005) (PDB code: 2A5S) containing the upper lobe and the lower lobe of the respective clamshell-like structures. With the improved crystals and datasets, we were able to acquire clear electron density for 565 residues out of 575 possible residues for GluN1/GluN2A LBD-gly/glu, 22 more residues than the previous GluN1/GluN2A LBD-gly/glu structure (Furukawa et al., 2005). The newly resolved regions of the structure included the motifs, Loop1 and Loop2 in GluN1, the hinge loop of the bilobed structure in GluN2A, the N-terminal ends in GluN1 LBD and GluN2A LBD, and the C-terminal end of GluN1 LBD. For GluN1/GluN2A LBD-gly/d-AP5, GluN1/GluN2A LBD-gly/PPDA, and GluN1/GluN2A LBD-DCKA/glu structures, electron density for 545, 556, and 554 residues out of 575 possible residues were observed, respectively.
Overall architecture and antagonist binding site
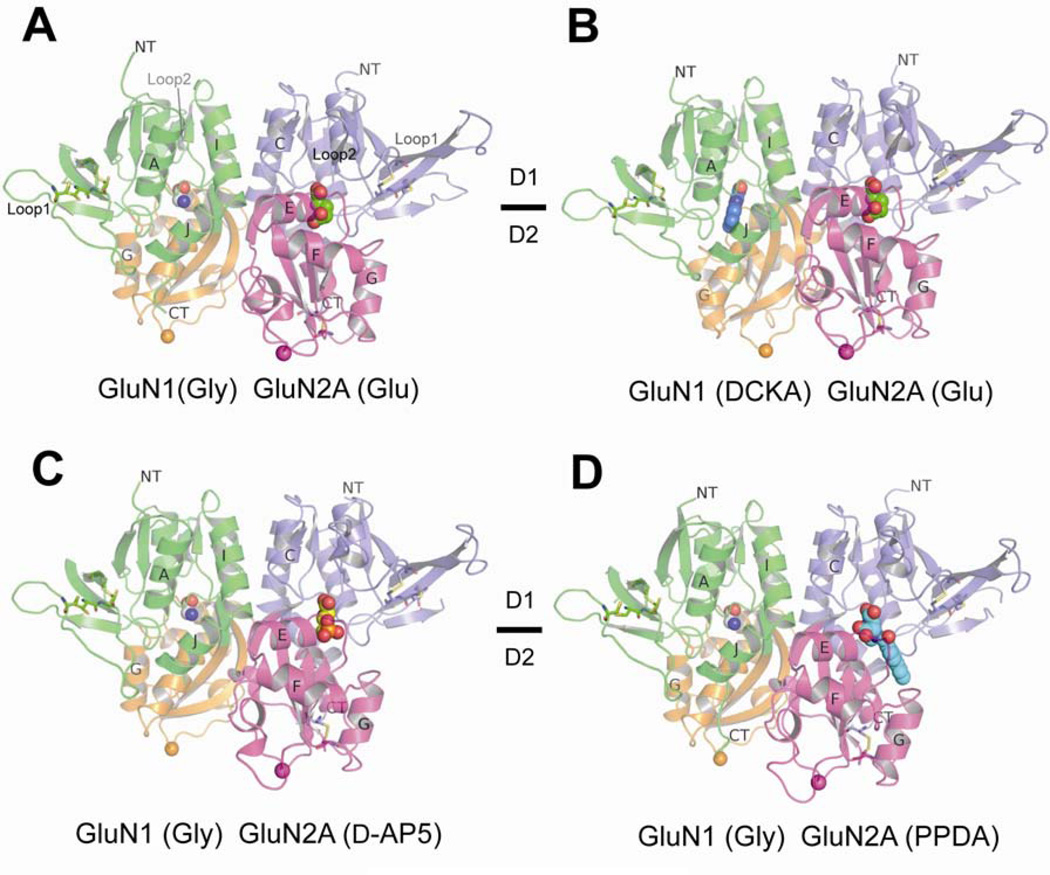
The high resolution crystallography (1.85 – 2.1 Å) conducted in this study led to unambiguous identification of antagonist binding sites as well as detailed understanding of the ligand-binding modes and the pattern of overall protein conformational alterations. Both GluN1 and GluN2A LBDs have bilobed clamshell-like structures composed of upper (D1) and lower (D2) domains (Figure 2). All of the NMDA receptor subunits contain three pairs of disulfide bonds, one located in the lower lobe and the other two located in the Loop 1 motif, which is specific to NMDA receptors (Figure 2) (Furukawa and Gouaux, 2003; Furukawa et al., 2005). The ligand binding sites are located at the inter D1-D2 cleft in both GluN1 and GluN2A but with strict specificity for glycine and DCKA in GluN1 and l-glutamate, d-AP5, and PPDA in GluN2A (Figure 2A). Even though the crystals for GluN1/GluN2A LBD in complex with glycine and l-glutamate were grown in the different condition from the previous one (Furukawa et al., 2005), the modes of glycine and l-glutamate binding as well as the overall structure is close to identical with root-mean-square deviation (rmsd) of 0.44 Å over 543 Cα positions (Figure S1). In all of the four crystal structures with different ligands bound, the GluN1 and GluN2A LBDs are assembled as heterodimers in the back-to-back arrangement where the GluN1 and GluN2A ligand-binding sites are facing away from each other (Figure 2). However, the overall conformations of the GluN1/GluN2A LBD structures in different ligand combinations are highly distinct from one another as described in the later section.
Figure 2. Structures of GluN1/GluN2A LBDs in complex with various ligands.
The four crystal structures of GluN1/GluN2A LBDs in complex with glycine (Gly; gray sphere) and l-glutamate (Glu; green sphere) (panel A), DCKA (blue sphere) and l-glutamate (panel B), glycine and d-AP5 (yellow sphere; panel C), and glycine and PPDA (cyan sphere; panel D). The GluN1 and GluN2A LBDs exist as a heterodimer in the asymmetric unit of the orthorhombic crystals obtained in this study (Table S1). The structures are oriented so that the N-terminal ends (NT) from both GluN1 and GluN2A face top of this figure. GluN1 and GluN2A LBDs are shaped like bilobed clamshells composed of upper (D1) and lower (D2) domains colored green and orange, respectively, in GluN1 and blue and magenta, respectively, in GluN2A. All of the ligands studied here bind at the D1-D2 interface. Sticks represent three disulfide bonds conserved among the NMDA receptor family members. The spheres at the bottom of the structures represent the Cα of the glycine residue in the Gly-Thr dipeptide linker, where loops leading to the M1 TMD and from the M3 TMD would be located in the full-length receptors.
Antagonist binding in GluN1/GluN2A LBD
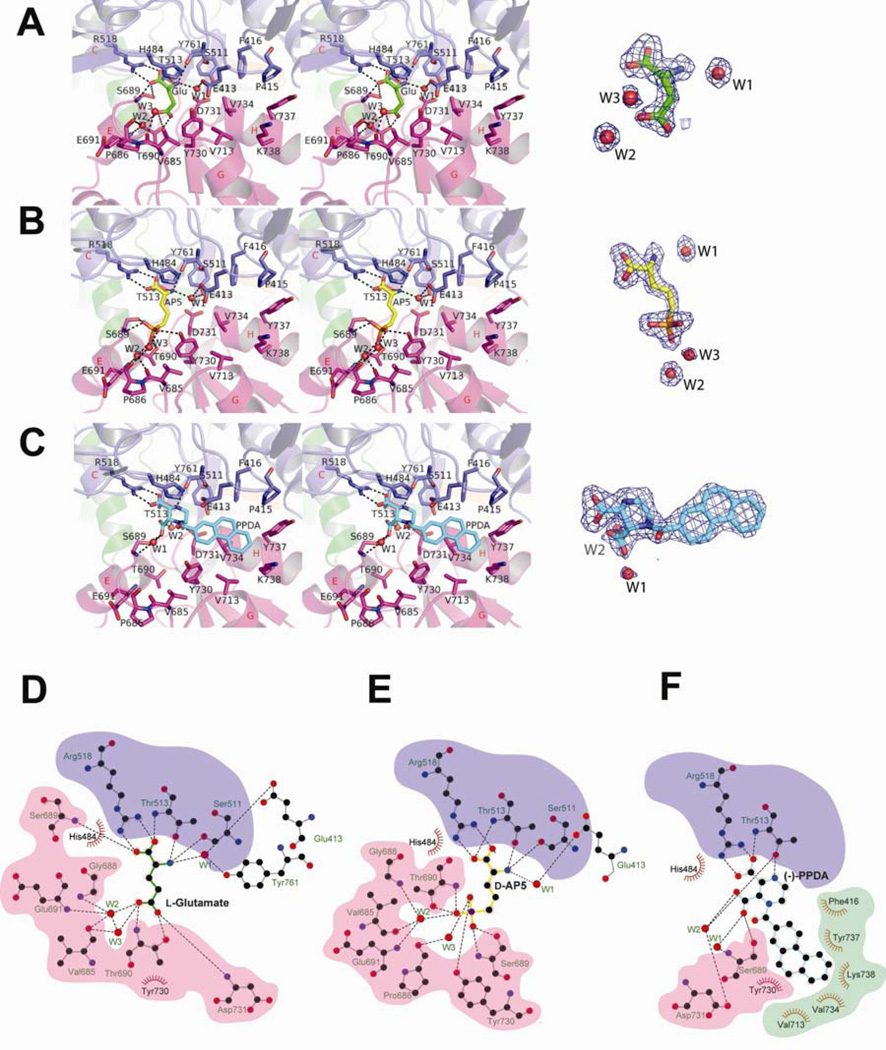
The current crystallographic study provides high quality electron density, which shows the exact positions of ligands as well as amino acid residues and water molecules involved in compound recognition. In GluN2A, binding of both D-AP5 and PPDA occur at the inter-D1-D2 cleft in the bilobed clashell-like structure of GluN2A but through different chemistry. In general, D-AP5 binds to GluN2A mostly via polar interactions involving similar residues to those participating in l-glutamate binding (Figure 3A, 3B, 3D, and 3E). Residues from D1 including Ser511, Thr513, and Arg518 form polar interactions with the α-carboxylate and α-amino groups of d-AP5 whereas His484 “caps” the entire amino group through van der Waals interaction. Binding residues in D2 are similar between the l-glutamate-bound and the d-AP5-bound structures, however, the placement of the bulky phosphono group in d-AP5 pushes and rearranges the orientation of the D2 residues by forming direct and water-mediated polar interactions with them. The phosphono group is “locked” by seven polar interactions mediated directly by Ser689, Thr690, and Tyr730 and indirectly by Val685, Pro686, Gly688, and Glu691 via water (W2 and W3; Figure 3B and 3E).
Figure 3. Antagonist binding in GluN2A LBD.
(A–C) Stereoview of the ligand binding sites located at the D1-D2 interface. Binding of l-glutamate (green sticks; panel A), the GluN2 antagonists involve residues from both D1 and D2 for both D-AP5 (cyan sticks; panel B), and PPDA (yellow sticks; panel C). Dashed lines represent polar interactions. The current crystallographic study shows unambiguous electron density of l-glutamate (A), d-AP5 (B) and PPDA (C) as well as critical water molecules for binding (red spheres; W1-3). The electron density (blue mesh) represents the Fo-Fc omit map contoured at 3σ. (D–F) Schematic representation of l-glutamate (D), d-AP5 (E), and (−)-PPDA (F) binding sites. The crystal structures around the ligand binding sites were analyzed by LIGPLOT v.4.5.3 (Wallace et al., 1995). Dotted lines represent polar interactions whereas red arcs with spokes represent hydrophobic interactions. Red spheres marked by “W” represent water molecules at the binding sites. Residues involved in binding of l-glutamate and/or d-AP5 have blue (from D1) and magenta (from D2) backgrounds. Residues uniquely involved in binding of (−)-PPDA have an emerald green background. Orientations of the D2 residues are different between the l-glutamate-bound, the d-AP5-bound, and the (−)-PPDA-bound GluN2A LBDs due to a large domain movement of D2 relative to D1.
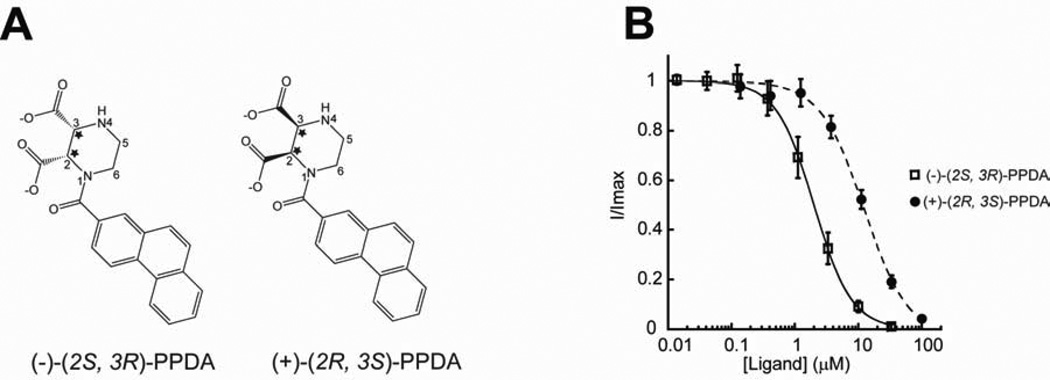
The crystallographic study on GluN1/GluN2A LBD-PPDA provides important insights into stereoselectivity and ligand recognition. First, (−)-(2S, 3R)-PPDA ((−)-PPDA) has a preferential binding over (+)- (2R, 3S)-PPDA ((+)-PPDA). The crystal structure clearly shows exclusive binding of the (−)-PPDA enantiomer even though the crystals were soaked against a buffer containing a racemic mixture of (+)- and (−)-PPDA (Figure 4A). In the previous study, only the (−)-PPDA enantiomer has been tested and shown to inhibit NMDA receptors (Feng et al., 2004). Thus, to understand whether or not (−)-PPDA is the only inhibitory component in the racemic mixture, we have synthesized individual enantiomers and tested them for their ability to inhibit the GluN1/GluN2A and GluN1/GluN2D NMDA receptor currents using two electrode voltage clamp (TEVC) on Xenopus oocytes injected with cRNAs encoding rat NMDA receptor subunits. Measurement of dose-response inhibition by (+)-PPDA and (−)-PPDA shows that both enantiomers can inhibit GluN1/GluN2A and GluN1/GluN2D NMDA receptors, however, with 6–7-fold weaker potency for (+)-PPDA than (−)-PPDA (Figure 4B). Both enantiomers have 5–6-fold higher potency toward GluN1/GluN2D than GluN1/GluN2A indicating that (+) and (−) enantiomers have a similar degree of specificity toward GluN2D over GluN2A (Figure 4B, Table S2). The second important observation is that binding of (−)-PPDA involves distinct residues and chemistry from d-AP5 except for the conserved polar interactions between the amino group moiety (the nitrogen at the 4-position and the carboxylate group at the 3-position of piperazine ring) and Thr513 and Arg518 (Figure 3C, 3F, and 4A). The majority of the binding is mediated by hydrophobic interactions involving the phenanthrene rings of (−)-PPDA, which are oriented toward the hydrophobic core of the GluN2A LBD around Helix H by the piperazine ring stabilized in the chair configuration (Figure 3C). Consequently, the phenanthrene rings are surrounded by clusters of hydrophobic residues including Phe416, Val713, Val734, and Tyr737 and the methylene group of Lys738 whose ε-NH3+ is salt bridged to Glu714 and, thus, is capable of forming hydrophobic interaction (Dyson et al., 2006) (Figure 3C and 3F; residues with green background).
Figure 4. GluN1/GluN2A NMDA receptors selectively bind (−)-PPDA over (+)-PPDA.
(A) Enantiomers of PPDA showing two chiral centers (stars) at the 2 and 3 positions of the piperazine ring. PPDA used in this crystallographic study is the enantiomeric mixture available commercially (TOCRIS). However, the electron density indicates the exclusive presence of (−)-(2S, 3R)-PPDA as shown in Figure 3C. (B) Inhibition of glycine/l-glutamate ion channel current by (+)-(2S, 3R)-PPDA (filled circle) or (−)-(2S, 3R)-PPDA (square) in the GluN1-1a/GluN2A NMDA receptors. The currents are formed by coapplication of 100 µM of glycine and 5 µM of l-glutamate and inhibited by various concentrations of PPDA. All of the recordings are done using the TEVC method. Error bars represent s.d.
The crystal structure of GluN1/GluN2A LBD in complex with DCKA/l-glutamate shows the antagonist-bound GluN1 LBD in the context of GluN1–GluN2A heterodimer for the first time. The GluN1 LBD portion of the GluN1/GluN2A LBD-DCKA/glu structure is highly similar to the monomeric GluN1 LBD-DCKA structure (Furukawa and Gouaux, 2003) with rmsd of 0.91 Å over 274 Cα positions and 0.55 Å over 266 Cα positions for protomer A and B, respectively, even though their crystallization conditions are highly distinct from one another (Figure S2). Furthermore, the pattern of DCKA binding at the ligand-binding site, including the water-mediated polar interaction, is identical between those two structures (Figure S2). Thus, the overall protein conformation of the GluN1 LBD-DCKA and the chemistry for ligand recognition of DCKA appear to follow the strict rule regardless of crystallization conditions or of the presence or absence of GluN2A LBD. The above observation could also imply that known cooperativity between the glycine binding site in GluN1 and the l-glutamate binding site in GluN2 (Mayer et al., 1989; Regalado et al., 2001) may not occur through the GluN1–GluN2A heterodimer interface in the present structures. Instead, the possible GluN1–GluN2 interaction sites that mediate the glycine-l-glutamate binding cooperativity may involve the interfaces between the two LBD heterodimers or plausible interfaces between LBD and either ATD or TMD in the context of the heterotetrameric subunit assembly. Thus, understanding the structure-based mechanism of glycine-l-glutamate binding cooperativity would likely require a structure of the intact heterotetrameric NMDA receptor.
Mutational analysis of GluN2A antagonist binding site
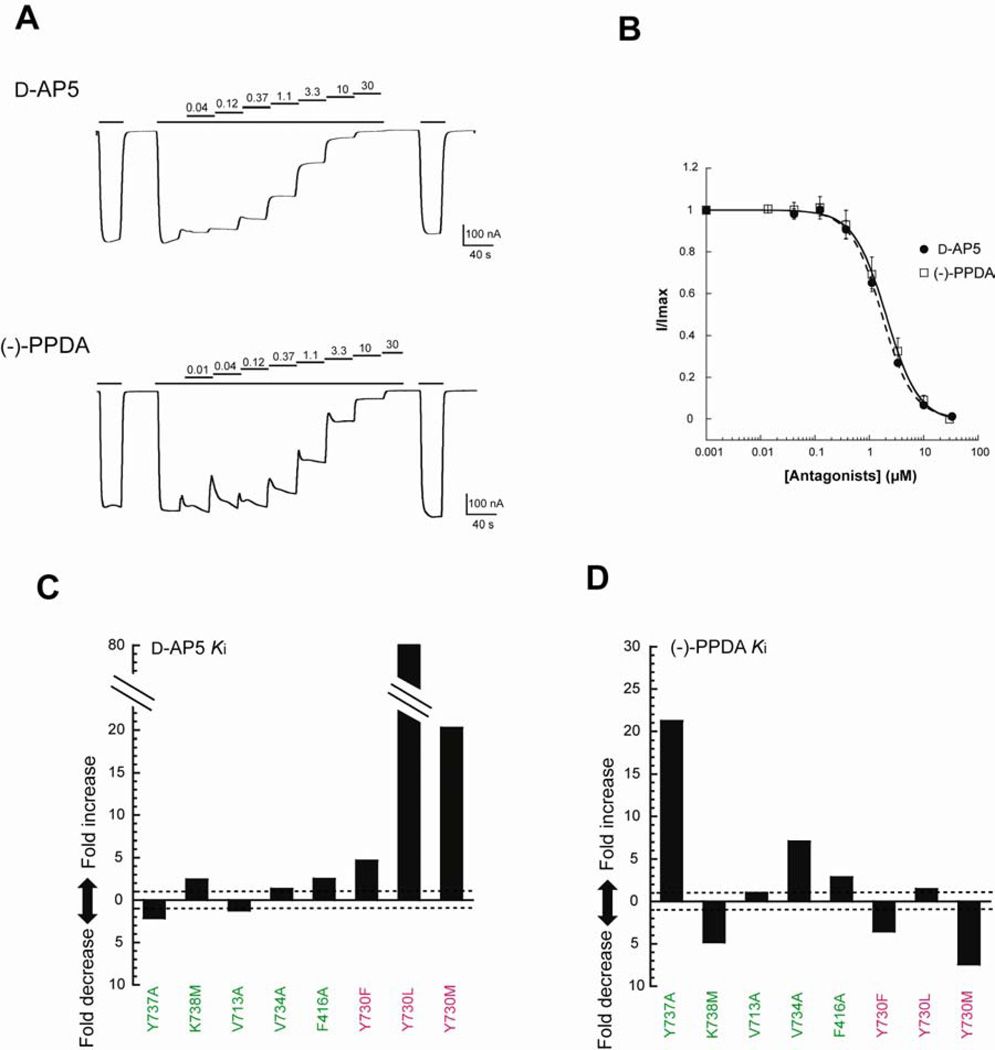
Inspection of the ligand-binding site clearly shows distinct binding modes between d-AP5 and (−)-PPDA involving different structural elements in the GluN2A ligand-binding site. To validate the physiological relevance of the structural observation and to further characterize the chemical nature of the ligand binding site, we carried out mutational analysis of residues involved in antagonist binding by measuring current inhibition by TEVC. Mutagenesis on Phe416, Val713, Tyr730, Val734, Tyr737, and Lys738 affected sensitivity to l-glutamate, whereas that on Ser689 or Thr690 completely abolished a response to l-glutamate. Thus, normalized potency of d-AP5 and (−)-PPDA were calculated by determining EC50 values of l-glutamate and IC50 values for d-AP5 and (−)-PPDA at fixed l-glutamate concentrations, and by converting EC50 and IC50 into Ki values using Cheng-Prusoff equation (Cheng and Prusoff, 1973) for each of the tested mutants (Figure 5, Table S3).
Figure 5. Mutagenesis of the ligand-binding site.
Residues surrounding the antagonist binding site are mutated and tested for inhibition of ion channel activities to validate physiological relevance of the crystal structures. (A) Typical dose-response inhibition pattern of the wild type GluN1/GluN2A NMDA receptor current assessed by TEVC. In this recording, currents formed by application of 100 µM of glycine and 5 µM l-glutamate are inhibited by various concentrations of d-AP5 (top panel) or (−)-PPDA (bottom panel). Note that there is no significant rundown of currents before and after the dose-response experiment. (B) All of the data points can be fit to a one-binding-site model of Hill equation. (C–D) Fold increase of Ki values for d-AP5 (C) and (−)-PPDA (D). Ki values were calculated by the Cheng-Prusoff equation using EC50 values for l-glutamate and IC50 values for the antagonists for every mutant (Table S3).
The mutational analysis indeed verifies the involvement of distinct residues in binding of d-AP5 and (−)-PPDA and thus, validates the physiological relevance of the crystal structures obtained in this study. In general, mutation of residues surrounding the phenanthrene rings of (−)-PPDA (Figure 3F; residues in emerald green background) affects potency of (−)-PPDA with little or no effect on potency of d-AP5. Among those mutations, GluN2A Val734Ala, Tyr737Ala, and Lys738Met, have significant effects on the (−)-PPDA potency but with only minor effects on the d-AP5 potency (Figure 5C and 5D). An intriguing observation is that while Val734Ala and Tyr737Ala both decreases the (−)-PPDA sensitivity by reduction of van der Waals interaction with the phenanthrene ring, Lys738Met increases the (−)-PPDA sensitivity by strengthening the interaction likely through aromatic-sulfur interaction (Zauhar et al., 2000). Among GluN2s, GluN2A is the only subunit with lysine in the 738 position whereas the other three subunits (GluN2B-D) contain methionine at this position. Consistently, GluN1/GluN2A NMDA receptor is the subtype that is least sensitive to (−)-PPDA (Feng et al., 2004). Thus, we propose that the preferential binding of (−)-PPDA toward GluN2B/C/D over GluN2A containing NMDA receptors derives from different modes of interaction with the phenanthrene ring at the 738 position. Mutations on other residues surrounding the phenanthrene ring, Phe416Ala and Val713Ala, have minor effects on sensitivity to both (−)-PPDA and d-AP5, consistent with the structural observation that those residues are further away from the phenanthrene ring compared to Val734, Tyr737, or Lys738, and therefore, not at the ideal position to form a strong van der Waals interaction. Tyr730 participates in binding of d-AP5 through polar interaction with the phosphono group and, to a minor extent, of (−)-PPDA through van der Waals interaction with the piperazine ring. The Tyr730Phe mutation results in five-fold decrease in sensitivity of d-AP5 by reduction in number of polar interactions whereas it results in increase in (−)-PPDA sensitivity perhaps by strengthening of hydrophobic interactions due to absence of hydroxyl group (Figure 5C and 5D).
Binding specificity in NMDA receptors and non-NMDA receptors
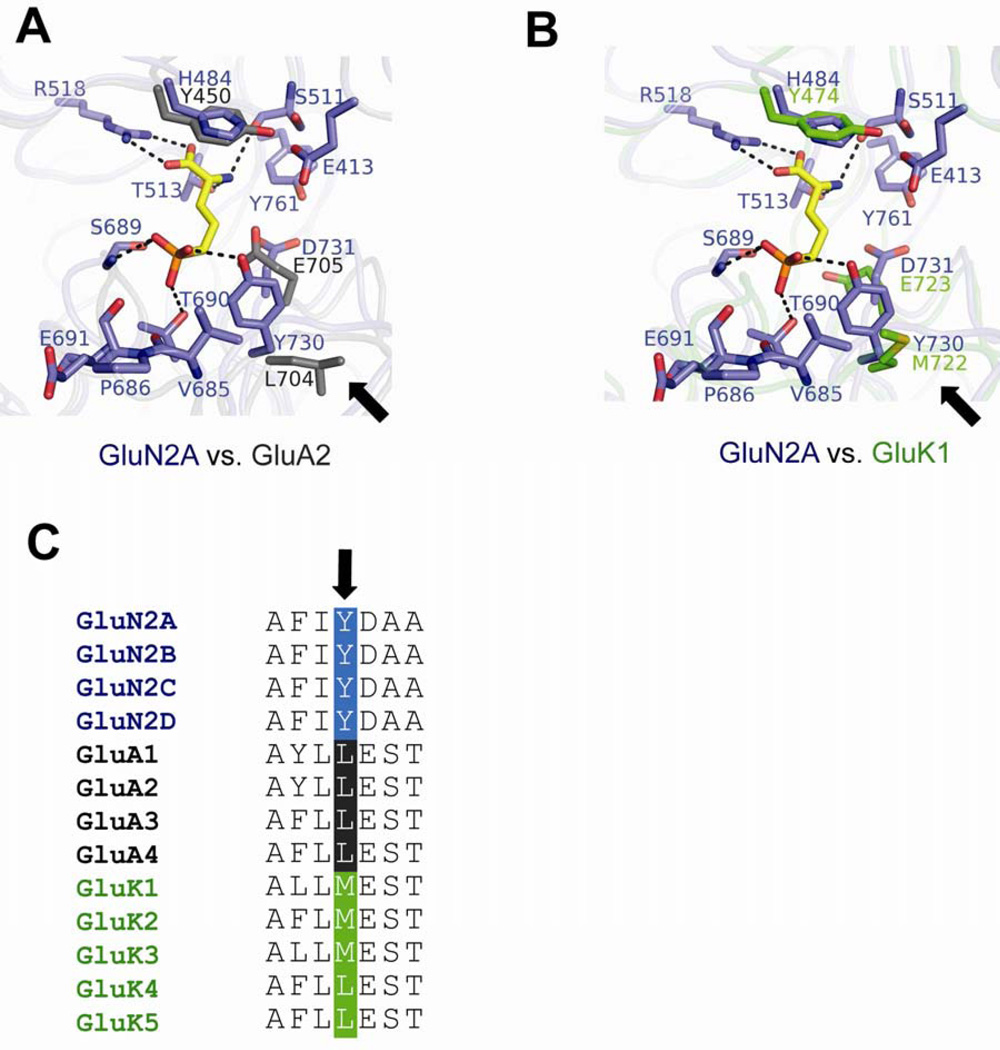
Specific inhibition of NMDA-induced currents by antagonists was crucial in confirming the existence of the iGluRs (Watkins and Evans, 1981). d-AP5 was one of the first antagonists discovered to specifically inhibit NMDA-induced current while (−)-PPDA was later reported to inhibit NMDA receptors with subtype specificity toward GluN2C/GluN2D containing NMDA receptors (Feng et al., 2004). Structural comparison between GluN1/GluN2A LBD and non-NMDA receptor LBDs show elements that may play a role in distinguishing NMDA receptors and non-NMDA receptors such as AMPA and kainate receptors. The residues from D1 in direct contact with or in vicinity of d-AP5 are mostly conserved in non-NMDA receptors except that the equivalent residue to GluN2A His484 is tyrosine in AMPA and kainate receptors (Figure 6A and 6B). As described above, direct and indirect polar interactions in D2 are mediated by side chains of Ser689, Thr690, and Tyr730, and nitrogen and oxygen atoms from the main chain. While GluN2A Ser689 and Thr690 are conserved in all of the l-glutamate binding subunits in iGluRs, Tyr730 is unique to the GluN2 subunits in NMDA receptor family (Figure 6C). The equivalent residues of GluN2A Tyr730 are GluA2 Leu704 and GluK1 Met722, which cannot form direct polar interaction or “cap” the binding site as GluN2A Tyr730 does. Consistent with the structural and primary sequence analyses, mutating GluN2A Tyr730 to leucine or methionine dramatically reduces d-AP5 sensitivity (~20-fold and ~80-fold increase in Ki values in Tyr730Leu and Tyr730Met, respectively) while it causes little or no change in sensitivity to (−)-PPDA. However, mutating GluA2 Leu704 and GluK1 Met722 to tyrosine does not confer sensitivity to d-AP5 in those non-NMDA receptors, indicating that specific binding of d-AP5 to NMDA receptors is not determined solely by the tyrosine residue in the binding pocket (data not shown). Overall, Tyr730 is a critical but not the only factor that facilitates specific inhibition of NMDA receptor by d-AP5.
Figure 6. Comparison of GluN2A LBD and non-NMDAR LBDs.
(A–B) Superposition of GluA2 (panel A) and GluK1 (panel B) LBDs onto the GluN2A LBD-D-AP5 structure. The LBD structures of GluA2 (PDB code: 1FTL) and GluK1 (PDB code: 2F34) are split into D1 and D2 domains and superposed onto the respective domains of GluN2A LBD-d-AP5 structure by the program LSQKAB (Kabsch, 1976). The GluN2A residues involved in binding of d-AP5 are shown in blue sticks whereas d-AP5 is shown in yellow sticks. Side chains of non-NMDA receptor residues that are involved in ligand-binding and are different from GluN2 subunits are shown in gray (GluA2) and green (GluK1). A notable difference is found at the position of GluN2A Tyr730 (arrows) where the equivalent residues in GluA2 and GluK1 are Leu704 (gray sticks) and Met722 (green sticks), respectively. (C) Sequence alignment of the glutamate binding iGluR subunits around GluN2A Tyr730 (arrow). Tyrosine is conserved in the NMDA subfamily (blue) whereas the equivalent residue in the AMPA (black) and kainate (green) subfamilies is either leucine or methionine.
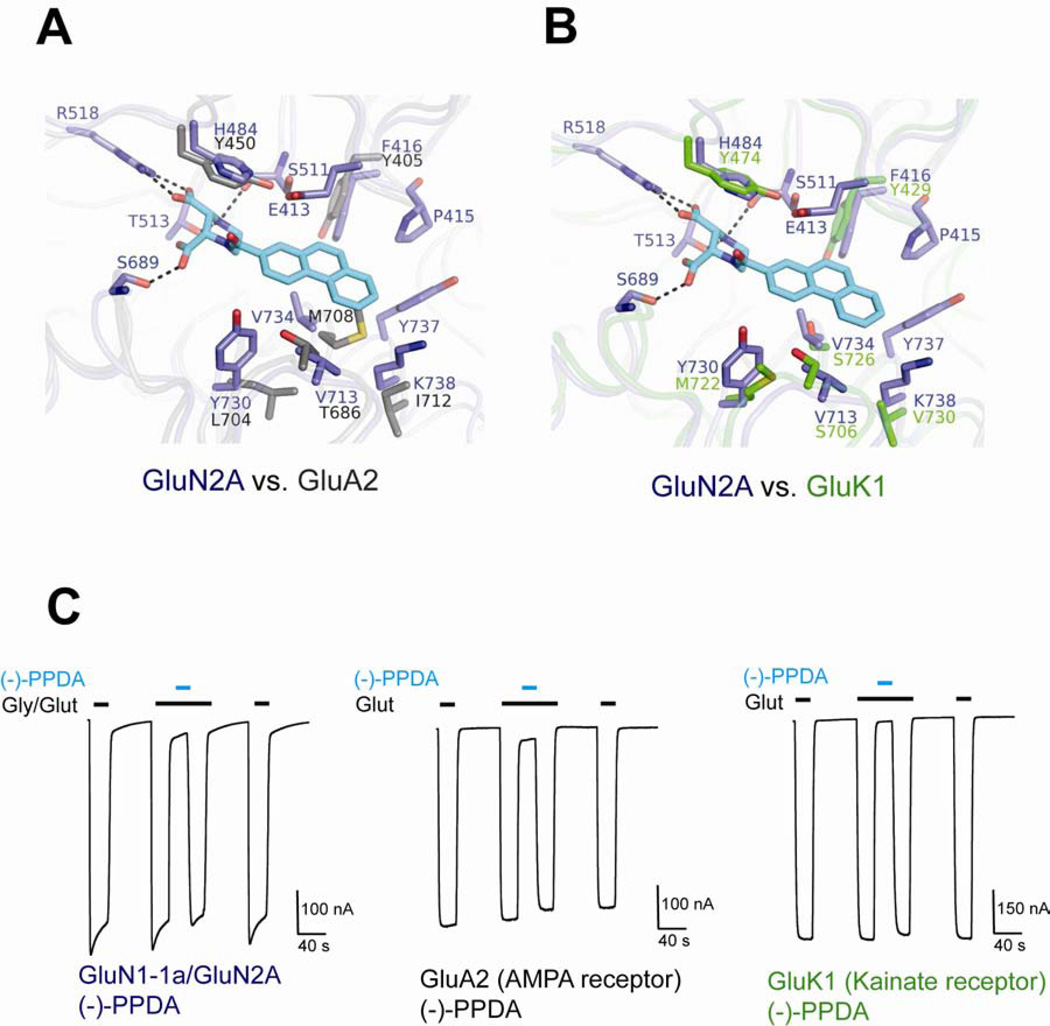
In contrast to d-AP5, the structural comparison between GluN1/GluN2A LBD and GluA2 or GluK1 LBD shows no apparent molecular features that may interfere with binding of (−)-PPDA in non-NMDA receptors, but instead, points out a reasonable possibility of (−)-PPDA binding to occur (Figure 7A and 7B). To validate this structural observation, we assessed the inhibition pattern of (−)-PPDA on l-glutamate induced currents produced by non-desensitizing mutants of GluA2 Leu483Tyr flip (Stern-Bach et al., 1998) and GluK1 Tyr506Cys Leu768Cys (Weston et al., 2006) using TEVC. The application of 100 µM (−)-PPDA in the presence of agonists completely inhibited not only GluN1/GluN2A NMDA receptors, but also GluA2 AMPA receptors, and GluK1 kainate receptors (Figure 7C). Indeed, both GluA2 and GluK1 respond to (−)-PPDA with Ki values of 7.85 µM and 1.17 µM, respectively, which are comparable to Ki of 0.82 µM in GluN1/GluN2A receptors (Table S3). These results are consistent with the recent report that some piperazine-2,3-dicarboxylate derivatives can act on both kainate and NMDA receptors (Irvine et al., 2012). The current study clearly shows that (−)-PPDA is a general antagonist that acts on all of the l-glutamate-binding iGluR subunits. A recent study has shown that the Tyr506Cys/Leu768Cys mutations in GluK1 receptors desynchronize receptor activation (Dawe et al., 2013). However, the overall interpretation that (−)-PPDA acts on GluK1 receptors with comparable potency to the GluN1/GluN2A NMDA receptors should remain intact.
Figure 7. (−)-PPDA binding on GluN2A LBD and non-NMDAR LBDs.
(A–B) Superposition of GluA2 (panel A) and GluK1 LBDs (panel B) onto the GluN2A LBD-(−)-PPDA structure. The structural superposition is done in the same manner as in Figure 6. Side chains of non-NMDA receptor residues that are involved in ligand-binding and are different from GluN2 subunits are shown in gray (GluA2) and green (GluK1). In both GluA2 and GluK1 receptors, there appear to be no residue that may prevent (−)-PPDA from binding. (C) (−)-PPDA can robustly inhibit currents produced by GluN1-1a/GluN2A NMDA receptors, GluA2 AMPA receptors (Leu483Tyr, flip), and GluK1 kainate receptors (Tyr506Cys Leu768Cys). Here NMDA receptor and non-NMDA receptor currents are induced by 100 µM glycine/30 µM glutamate and 30 µM glutamate, respectively, and inhibited by application of 100 µM (−)-PPDA in the presence of agonists. All of the recordings were done using TEVC on Xenopus oocytes injected with cRNAs encoding GluN1/GluN2A NMDA receptor, GluA2 AMPA receptor mutant, and GluK1 kainate receptor mutant. The Ki values for (−)-PPDA inhibition are 7.85 µM and 1.17 µM for GluA2 (flip, Leu483Tyr) and GluK1 (Tyr506Cys Leu768Cys), respectively.
Antagonist-mediated conformational change in GluN1/GluN2A LBD
Extensive studies on non-NMDA receptor LBDs and the crystal structure of the intact GluA2 receptors demonstrated that LBDs form two-fold symmetric homodimers that further assemble into tetramers corroborating with the idea that the basic functional unit in iGluRs is a dimer (Mayer, 2011; Sobolevsky et al., 2009). In the GluA2 and GluK1 LBDs, conformational alteration of the bilobed structures were observed in a way that agonist-bound and antagonist-bound structures were stabilized in closed and open conformations, respectively (Mayer et al., 2006; Sun et al., 2002). Furthermore, in GluA2, apo state is stabilized in the open cleft conformation similar to the antagonist-bound structure (Armstrong and Gouaux, 2000). This ligand-dependent conformational variation results in changes in the distances between the linkers adjacent to the transmembrane ion channel domains (defined as Ile633 in GluA2 and Ile653 in GluK1) in the dimer pairs of GluK1 and GluA2 LBDs where agonist binding separates the linker distances. The linker movement resulting from the conformational alteration of the LBDs has been suggested to be coupled, at least in part, to a gating event in the ion channel (Mayer et al., 2006; Sun et al., 2002).
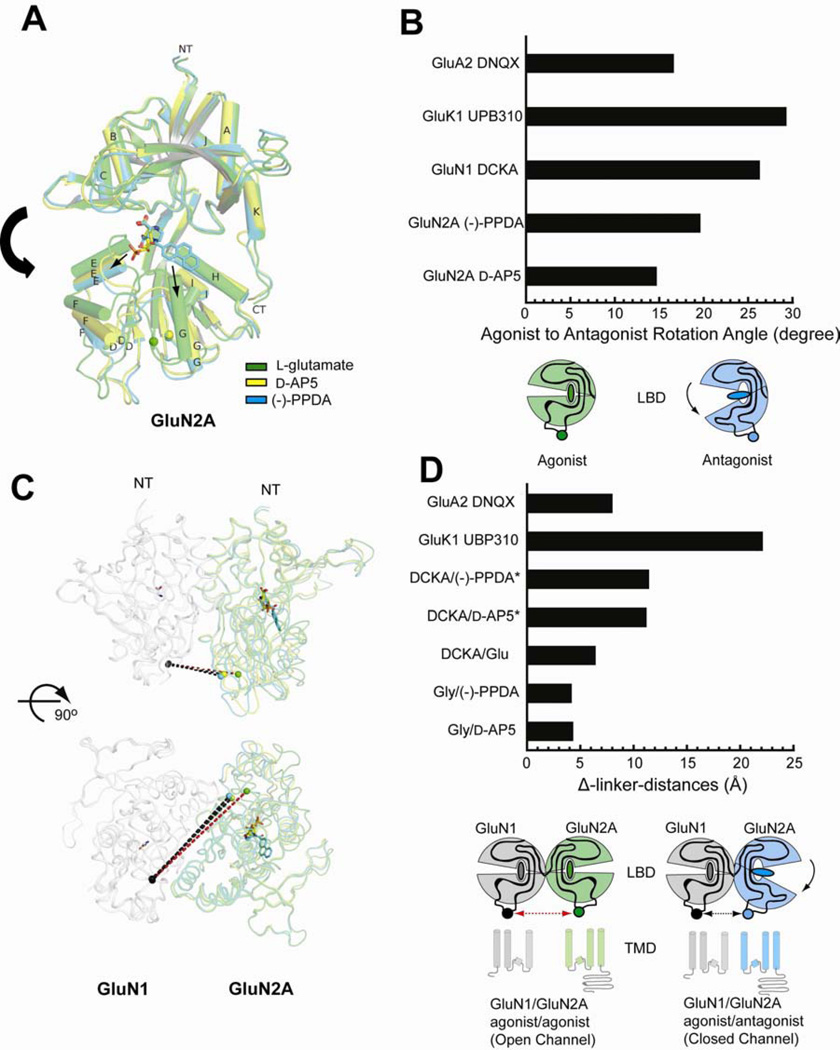
The current study structurally demonstrates the pattern of conformational alterations in NMDA receptors, for the first time, in the context of the GluN1/GluN2A heterodimer. In NMDA receptors, GluN1 LBD has been shown to be stabilized in open-cleft conformations in the presence of antagonists, DCKA (Furukawa and Gouaux, 2003) and cycloleucine (Inanobe et al., 2005), or in the apo-state (Yao et al., 2013). The structure of GluN1/GluN2A LBD in complex with the GluN1 antagonist, DCKA, and l-glutamate shows that the GluN1 LBD is in the open-cleft conformation similar to the one observed in protomer A in the previous study on GluN1 LBD-DCKA (Furukawa and Gouaux, 2003) (Figure S3). Compared to the glycine/l-glutamate-bound GluN1/GluN2A LBD structure, the DCKA/l-glutamate-bound structure has ~26° opening in the GluN1 LBD cleft while little or no change is observed in the pattern of the GluN1–GluN2A heterodimeric arrangement or in the conformation of GluN2A LBD (rmsd = 0.51 Å over 277 Cα positions within GluN2A LBD). (Figure S3).
The structures of GluN1/GluN2A LBD in complex with glycine/d-AP5 or with glycine/(−)-PPDA show opening of the GluN2A LBD structure, however, to different extents. Binding of d-AP5 and (−)-PPDA results in opening of the bilobed structure of the GluN2A LBD by 14.4° and 19.4°, respectively, compared to the l-glutamate-bound form while it causes little or no change in the GluN1–GluN2A subunit arrangement and the GluN1 conformation (rmsd = 0.48 Å for d-AP5 and 0.62 Å for (−)-PPDA over 282 Cα positions; Figure 8A and 8B). Opening of the GluN2A LBD cleft is mediated through movement of D2 (lower lobe) while D1 is locked by inter-subunit interactions in the GluN1/GluN2A LBD heterodimers. Stabilization of the open cleft conformation of GluN2A LBD by d-AP5 and (−)-PPDA occurs through distinct mechanisms involving discrete elements of the ligand binding site. In the d-AP5-bound form, movement of Helix E caused by the placement of the phosphono group appears to be the driving force for the opening of the bilobed structure whereas in the (−)-PPDA-bound form, movement of Helix G and H by the placement of the bulky phenanthrene group may be the cause of the cleft opening (Figure 8A; arrows). Consistently, there is no direct contact between d-AP5 and Helix G and H whereas there is only one contact between (−)-PPDA and Helix E. The cleft opening by both d-AP5 and (−)-PPDA is mediated through rigid-body movement of D2 (rmsd = 0.39 Å over 101 Cα positions in D2 between d-AP5-bound and (−)-PPDA-bound structures) despite different modes of ligand-receptor interactions causing such conformational change.
Figure 8. Binding of d-AP5 and (−)-PPDA induce different degree of domain movement.
(A) Superposition of GluN1/GluN2A LBD structures in complex with l-glutamate (green), d-AP5 (yellow), and (−)-PPDA (cyan). The Cαs of D1 in GluN2A in d-AP5-bound or (−)-PPDA-bound forms and l-glutamate-bound form are superposed to observe the differences in the extent of domain opening caused by the GluN2-antagonist binding. d-AP5 and (−)-PPDA induce opening of the clamshell-like architecture through mobilization of Helix E and Helix G, respectively. Spheres at the bottom of the structures represent Cα of GluN2A Val662 next to the M3 transmembrane region. GluN1 LBD is omitted from this figure for clarity. (B) Analysis of domain opening of antagonist-bound LBD structures relative to the l-glutamate-bound LBD structures in GluA2 (PDB code: 1FTJ for l-glutamate vs. 1FTL for DNQX), GluK1 (PDB code: 2F36 for l-glutamate vs. 2F34 for UBP310) GluN1, and GluN2A. The rotation angles are measured using the program Dyndom (Hayward and Lee, 2002). Below the bar graph is the schematic presentation of domain opening resulting from binding of an antagonist. (C) The same superposition as “panel A” but showing GluN1 LBD in complex with glycine (light gray) viewed from the side (top panel) and from the bottom or the membrane plane (bottom panel). Spheres in GluN1 represent Cα of Ile664. Dashed lines show distances between GluN1 Ile664 and GluN2A Val662, which are plausible measures of the TMD movement. The linker distance for GluN1/GluN2A LBD-gly/glu is shown in red whereas that for GluN1/GluN2A LBD-gly/d-AP5 or GluN1/GluN2A LBD-gly/PPDA is in black. (D) Differences in the GluN1 Ile664 - GluN2A Val662 distances between antagonist-bound (black dash in panel C) and the agonist-bound (glycine and l-glutamate; red dash in panel C) structures (Δ-linker-distances). The inter-linker distances in the DCKA/PPDA and DCKA/d-AP5 forms (asterisks) are calculated using hypothetical models built by superposing the D1 of the DCKA/glu-bound GluN1/GluN2A LBD onto that of GluN1/GluN2A LBD structures bound to gly/d-AP5 or gly/(−)-PPDA. In GluA2, distances between the Cαs of Ile663 in the GluA2 LBD homodimers in the l-glutamate-bound (PDB code: 1FTJ) and DNQX-bound (PDB code: 1FTL) forms are measured. In GluK1, distances between the Cαs of Ile653 in the GluK1 LBD homodimers in l-glutamate-bound (PDB code: 2F36) and UBP310-bound (PDB code: 2F34) forms are measured. Below the bar graph is the schematic presentation to show that the antagonist-induced domain opening of LBD may result in a decrease in the inter-linker distance (arrows between two spheres), thereby causing the transmembrane ion channel to close.
The GluN1/GluN2A LBD structures in the various ligand combinations reveal the movement of the linkers leading to the transmembrane ion channel. Binding of antagonists results in shortening of the linker distances (defined as Ile664 in GluN1 and Val662 in GluN2A) in the GluN1–GluN2A heterodimer pairs. The differences in the linker distances in comparison with the glycine/l-glutamate-bound form (Δ-linker-distance) are 6.4 Å, 4.3 Å, and 4.3 Å for the DCKA/l-glutamate, glycine/d-AP5, and glycine/(−)-PPDA forms, respectively, (Figure 8C and 8D). The ~5° difference in the “openness” of the bilobed structures between GluN2A-d-AP5 and GluN2A-(−)-PPDA does not alter the location of GluN2A Val662, thus, the GluN1–GluN2A Δ-linker-distance of the glycine/d-AP5 is similar to that of the glycine/(−)-PPDA forms. Overall, Δ-linker-distances are minor compared to those observed in the homodimeric non-NMDA receptor LBDs (Figure 8D) because the NMDA receptor antagonists only bind to either GluN1 or GluN2A in the heterodimeric GluN1/GluN2A LBDs whereas non-NMDA receptor antagonists bind to both subunits in the homodimeric non-NMDA receptor LBDs. The above observation implies that even a small perturbation of the LBD-TMD linker may be sufficient to cause inhibition in NMDA receptors. Superposition of the d-AP5- or (−)-PPDA-bound GluN2A LBD structure onto the GluN1/GluN2A LBD-DCKA/l-glutamate structure shows the plausible state where LBDs from both subunits are occupied with antagonists. In those cases, Δ-linker-distances are calculated to be 12 Å and 13 Å, which are comparable to the distance observed in the non-NMDA receptors (Figure 8D). Despite extensive efforts we have been unable to obtain a structure of GluN1/GluN2A LBD in complex with DCKA and d-AP5 or (−)-PPDA.
How could the conformational alterations in LBDs change positioning of linkers in the context of the tetrameric arrangement in NMDA receptors? To speculate on this question, we have constructed the heterotetrameric model of NMDA receptors by superposing the GluN1/GluN2A LBD structures onto the intact tetrameric GluA2 AMPA receptor (Sobolevsky et al., 2009) based on the assumptions that the tetrameric subunit arrangement is similar between NMDA receptors and AMPA receptors within LBD and that the GluN1 and GluN2A subunits are arranged in the 1-2-1-2 format as suggested recently (Riou et al., 2012; Sobolevsky et al., 2009) (Figure S4). The heterotetrameric models bound to different ligands show that the areas of tetragon defined by the four linkers (spheres in Figure S4) as vertices in the antagonist-bound forms are consistently smaller compared to the equivalently defined area in the glycine/l-glutamate-bound form. Even though this observation is highly speculative, the models suggest that changes in the linker positioning in the tetrameric subunit arrangement may be correlated to opening and closing of the ion channels.
Discussion
In this study, we show structural representations of competitive antagonism of NMDA receptors in the context of the heterodimeric GluN1/GluN2A LBDs. This has been achieved by obtaining the crystal structures of GluN1/GluN2A LBDs bound to competitive GluN2 antagonists, d-AP5 and (−)-PPDA, and a competitive GluN1 antagonist, DCKA. Overall, these antagonist-bound GluN1/GluN2A LBD structures along with the agonist-bound GluN1/GluN2A LBD structure provide important insights into the pharmacology of antagonist binding as well as the pattern of ligand-induced conformational movements that may be coupled to regulation of the ion channel activity.
Obtaining the crystal structures of the GluN2 subunit in complex with antagonists required a rather unexpected technical tweak involving soaking of the GluN1/GluN2A LBD crystals in complex with glycine and l-glutamate against the crystallization buffers containing antagonists. The GluN1/GluN2A LBD crystals grown in the novel condition accommodated large conformational changes caused by binding of antagonists with substantial changes in cell unit dimensions (Table S1). The experimental outcome is valid for the following reasons: 1) a series of structure-based mutagenesis at the antagonist binding site coupled to the electrophysiological experiments strongly shows the physiological relevance of the ligand-binding modes (Figure 5); and 2) the DCKA-bound GluN1/GluN2A LBD heterodimer structure in the current study has a very similar open conformation in the GluN1 to the one previously observed in the GluN1 LBD-DCKA structure derived from crystals grown in a different condition (Furukawa and Gouaux, 2003) (Figure S2 and S3). Taken together, the soaking method implemented here can now serve as an excellent crystallographic tool to visualize binding modes of NMDA receptor compounds and support structure-based drug design to move forward.
The crystal structures and structure-based mutagenesis provide structural elements in the binding pocket, which are crucial for specific binding of compounds to NMDA receptors (vs. non-NMDA receptors) and NMDA receptor subtypes (GluN2A-D). On the one hand, the difference between NMDA receptors and non-NMDA receptors is marked by GluN2A Tyr730, which is uniquely present in all of the NMDA receptor GluN2 subunits and forms polar interaction with the phosphono group of d-AP5 (Figure 3B and Figure 6). Indeed, d-AP5 is highly specific to NMDA receptors with no inhibitory effect as high as 100 µM, which clearly explains why Watkins and colleagues were able to distinguish the NMDA-induced currents from others (Davies and Watkins, 1982). On the other hand, the structural elements that control subtype-specific binding of GluN2-targeting antagonists lie in the hydrophobic pocket that extends from the core of the l-glutamate binding site where the phenanthrene ring in (−)-PPDA is located (Figure 3F; emerald green background). Amongst all the residues in the hydrophobic pocket, Lys738 is unique to GluN2A and is methionine in all the other NMDA receptor subunits (GluN2B-D) at the equivalent position. Thus, taking all the above information together, we speculate that a compound harboring a phosphono group that mediates interaction with GluN2A Tyr730 and a substituted phenanthrene-like moiety that extends toward the hydrophobic pocket (Figure 3F; emerald green background) and promotes interactions with GluN2A K738 but disfavors interactions with methionine side chains present in GluN2B-D would specifically inhibit GluN2A containing NMDA receptors with no cross-effects on non-NMDA receptors. In this regard, NVP-AAM077 and SDZ220-040 (Figure S5), which contain a phosphono group similar to d-AP5 (light orange background in Figure S5) and aromatic rings that may extend toward the hydrophobic pocket (light green background in Figure 3F and S5) with nanomolar potency (Frizelle et al., 2006; Urwyler et al., 1996), would likely serve as reasonable starting compounds for development of a subtype-specific competitive inhibitor for NMDA receptors. NVP-AAM077 has been previously shown to have 5-fold specificity toward the GluN2A containing NMDA receptors over the GluN2B containing NMDA receptors (Frizelle et al., 2006). It would be important to obtain crystal structures of GluN1/GluN2A LBDs in complex with NVP-AAM077 and SDZ220-040 to visualize how the aromatic groups are oriented in the hydrophobic pocket (Figure 3C and 3F) and understand how to modify the design of the aromatic groups to promote interaction with GluN2A K738 and gain GluN2A-specificity.
Our crystallographic study shows that the binding of d-AP5 and (−)-PPDA both stabilize the open cleft conformation of the bilobed architecture of GluN2A LBD compared to the l-glutamate-bound form despite the substantial difference in binding modes. The current study is the first to show that the GluN2 LBD undergoes a similar pattern of ligand-induced conformational changes to glycine-binding GluN1 LBD and non-NMDA receptor LBDs (Armstrong and Gouaux, 2000; Furukawa and Gouaux, 2003; Mayer, 2005; Mayer et al., 2006). Of importance to note is the observation that the extent of opening in the GluN2A LBD conformation is significantly different between the d-AP5-bound form (14.4°) and the (−)-PPDA-bound form (19.4°), however, the potency of inhibition is similar to each other implying that the degree of domain opening may not be correlated to effectiveness of the antagonist activity.
The ligand-induced opening and closing of the LBD bilobes alters the distances between the linkers that are tethered to TMD1 and TMD3 of the ion channel in the GluN1–GluN2A heterodimer pair (Figure 8). In GluA2 AMPA receptor and GluK1 kainate receptor LBD homodimers, the differences in linker distances are as large as 8 to 20 Å between the agonist-bound state and the antagonist-bound state or the apo state (Mayer et al., 2006; Sun et al., 2002). This conformational movement has been proposed to be coupled, at least in part, to ligand-gating of the ion channels. The LBD crystal structures presented in this study are GluN1/GluN2A heterodimers containing glycine/d-AP5, glycine/(−)-PPDA, and DCKA/l-glutamate, which uniquely represent the functional state where the activity of NMDA receptors is inhibited by an antagonist bound to one subunit while the other subunit contains an agonist. In such structures, the Δ-linker-distance is small especially in the case of glycine/d-AP5 and glycine/(−)-PPDA (4.3 Å). This structural observation is somewhat consistent with the recent findings that constraining LBD-TMD linkers dramatically reduces open probability (Kazi et al., 2013; Talukder and Wollmuth, 2011). Precise correlation between the linker movement and the inhibition potency in NMDA receptors remains an open question.
Experimental Procedures
Expression and Purification of GluN1 and GluN2A LBDs
GluN2A LBD was expressed as a fusion protein to small ubiquitin-like modifier (SUMO). This construct contains a hexa-histidine tag at the N-terminus of SUMO followed by LBD in the pET22b vector. The SUMO-GluN2A LBD proteins were expressed in OrigamiB (DE3) cells (Novagen) as described previously (Vance et al., 2011). The cell lysate was subjected to a Nickel-NTA Chelating Sepharose chromatography, followed by digestion by ubiquitin ligase protease-1 to remove SUMO, Q-Sepharose ion exchange chromatography, and SP-Sepharose ion exchange chromatography (GE Healthcare). The new expression method using the SUMO-fusion approach in combination with the new purification scheme above improved the protein yield at least by 2.5-fold compared to the previous method (Furukawa et al., 2005). GluN1 LBD proteins were expressed and purified using the previous method (Furukawa and Gouaux, 2003).
Crystallography
The purified GluN1 and GluN2A LBD proteins were individually concentrated to 6 mg/ml, mixed so that a 1:1 weight ratio was achieved, and dialyzed against a buffer containing 10 mM HEPES-NaOH (pH7.0), 100 mM NaCl, 10 µM glycine, and 1 mM l-glutamate overnight. The GluN1/GluN2A LBD crystals were produced by vapor diffusion at 17°C in hanging drops containing 2:1 protein to reservoir solution composed of 18% polyethylene glycol monomethylether 2000 (PEG2000MME), 100 mM HEPES-NaOH (pH 7.0), and 75 mM NaCl. Antagonist-bound crystals were obtained by soaking the gly/glu crystals against the reservoir solution supplemented with glycine (10 µM) and either d-AP5 (1 mM), or PPDA (0.1 mM), or L-glutamate (1 mM) and DCKA (1 mM). The crystals were soaked against the buffer containing 0.1 mM of antagonists for 48 h and transferred to the buffer containing 1 mM of antagonists (0.1 mM for PPDA). After 48 h, the crystals were soaked against the same buffer containing 18% glycerol and flash frozen by liquid nitrogen. The x-ray diffraction data was collected at X25 beamline at the National Synchrotron Light Source at Brookhaven National Laboratory and at the ID23-B beamline at the Advanced Photon System at the Argonne National Laboratory and processed using HKL2000 (Otwinowski and Minor, 1997). All of the structures were determined by molecular replacement using four search probes: GluN1 LBD Domain1 (Thr396-Gln536/Ser765-Arg794), GluN1 LBD Domain2 (Gly537-Arg755), GluN2A LBD Domain 1 (Asn404-Glu530/Gly760-His801), and GluN2A LBD Domain 2 (Thr531-Thr759) made from the GluN1/GluN2A LBD coordinate (PDB code: 2A5T) (Furukawa et al., 2005) and using the program Phaser (McCoy et al., 2007). Structural refinement and model building were performed with PHENIX (Adams et al., 2002) and Coot (Emsley and Cowtan, 2004), respectively.
Electrophysiology
Recombinant GluN1/GluN2A NMDA receptors were expressed by co-injecting 0.01–0.05 ng of the wild-type or mutant rat GluN1 and GluN2A cRNAs at a 1:2 ratio (w/w) into defolliculated Xenopus laevis oocytes. For GluA2 Leu483Tyr flip and GluK1 Tyr506Cys/Leu768Cys, 1–5 ng of cRNAs were injected per oocyte. The two-electrode voltage-clamp recordings were performed using agarose-tipped microelectrodes (0.4–1.0 MΩ) filled with 3 M KCl at a holding potential of −60 mV. The bath solution contained 5 mM HEPES, 100 mM NaCl, 0.3 mM BaCl2 and 10 mM Tricine at pH 7.4 (adjusted with KOH). Currents were evoked by applications of 100 µM of glycine and various concentrations of l-glutamate. For dose-response curves, various concentrations of antagonists were applied at a given l-glutamate concentration. The data were acquired and analyzed by the program Pulse (HEKA). Dose-response curves were plotted and fitted to calculate EC50 values of l-glutamate and IC50 values of antagonists using the program Kaleida graph (Synergy Software). Ki values were calculated by Cheng-Prusoff equation: Ki = IC50/(1 + [l-glutamate]/EC50).
Synthesis of PPDA enantiomers
Reagents were obtained from Aldrich Chemical and used without further purification. Optima grade solvents were obtained from Fisher Scientific, degassed with argon, and purified on a solvent drying system as described (Pangborn et al., 1996) unless otherwise specified. Dichloromethane (HPLC grade) and tetrahydrofuran (99%+) were purchased from Aldrich and dried over 4 Å molecular sieves before used. Reactions were performed in flame-dried glassware under positive Argon pressure with magnetic stirring. Cold baths were generated as follows: 0°C, wet ice/water; −40°C, dry ice/CH3CN; −78°C, dry ice/acetone. Analytical thin-layer c hromatography (TLC) was carried out on commercial Aldrich aluminium-supported silica gel plates (thickness: 200 µm) with fluorescent indicator (F-254). Visualization was accomplished by UV light or stained with 5% phosphomolybdic acid in ethanol. Column chromatography was performed by the method of Still (Still et al., 1978) with 32–63 µm silica gel (Woelm). For more details, refer to Supplementary Experimental Procedures.
Supplementary Material
Acknowledgements
We thank the beamline staff at the 23-ID-B beamline at the Advanced Photon System in the Argonne National Laboratory and at the X25 beamline at the National Synchrotron Light Source in the Brookhaven National Laboratory for their excellent beamline supports. We also thank Mark Mayer for the plasmids containing wild type and mutant GluA2 and GluK1 receptors used for cRNA synthesis. Stephen Traynelis and members of Furukawa laboratory are thanked for critical comments on the manuscript. This work was supported by National Institute of Health (MH085926 to HF), Robertson Research Fund of Cold Spring Harbor Laboratory (to HF), and Japan Promotion of Science (to NT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers
Coordinates and structure factors for GluN1/GluN2A LBDs complexed to glycine/Lglutamate, DCKA/l-glutamate, glycine/d-AP5, and glycine/(−)-PPDA have been deposited in the Protein Data Bank under accession codes 4NF8, 4NF4, 4NF5, and 4NF6, respectively.
References
- Acker TM, Khatri A, Vance KM, Slabber C, Bacsa J, Snyder JP, Traynelis SF, Liotta DC. Structure-Activity Relationships and Pharmacophore Model of a Noncompetitive Pyrazoline Containing Class of GluN2C/GluN2D Selective Antagonists. J Med Chem. 2013;56:6434–6456. doi: 10.1021/jm400652r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Benveniste M, Mayer ML. Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophys J. 1991;59:560–573. doi: 10.1016/S0006-3495(91)82272-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, Negri M, Andreetta F, Senar-Sancho SA, Guiral L, et al. Identification and characterization of novel NMDA receptor antagonists selective for NR2A- over NR2B-containing receptors. J Pharmacol Exp Ther. 2010;335:636–644. doi: 10.1124/jpet.110.172544. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of anenzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Clements JD, Westbrook GL. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991;7:605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- Davies J, Watkins JC. Actions of D and L forms of 2-amino 5-phosphonovalerate and 2-amino 4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982;235:378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- Dawe GB, Musgaard M, Andrews ED, Daniels BA, Aurousseau MR, Biggin PC, Bowie D. Defining the structural relationship between kainate-receptor deactivation and desensitization. Nat Struct Mol Biol. 2013;20:1054–1061. doi: 10.1038/nsmb.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson HJ, Wright PE, Scheraga HA. The role of hydrophobic interactions in initiation and propagation of protein folding. Proc Natl Acad Sci U S A. 2006;103:13057–13061. doi: 10.1073/pnas.0605504103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Evans RH, Francis AA, Jones AW, Smith DA, Watkins JC. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. British journal of pharmacology. 1982;75:65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina AN, Blain KY, Maruo T, Kwiatkowski W, Choe S, Nakagawa T. Separation of domain contacts is required for heterotetrameric assembly of functional NMDA receptors. The Journal of neuroscience. 2011;31:3565–3579. doi: 10.1523/JNEUROSCI.6041-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene 2-carbonyl) piperazin-2,3-dicarboxylic acid. Br J Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizelle PA, Chen PE, Wyllie DJ. Equilibrium constants for (R)-[(S) 1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo 1,2,3,4-tetrahydroquinoxalin 5 -yl)-methyl]-phosphonic acid (NVP-AAM077) acting at recombinant NR1/NR2A and NR1/NR2B N-methyl-D-aspartate receptors: Implications for studies of synaptic transmission. Mol Pharmacol. 2006;70:1022–1032. doi: 10.1124/mol.106.024042. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. Embo J. 2003;22:2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- Gallagher MJ, Huang H, Pritchett DB, Lynch DR. Interactions between ifenprodil and the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1996;271:9603–9611. doi: 10.1074/jbc.271.16.9603. [DOI] [PubMed] [Google Scholar]
- Hansen KB, Ogden KK, Traynelis SF. Subunit-selective allosteric inhibition of glycine binding to NMDA receptors. J Neurosci. 2012;32:6197–6208. doi: 10.1523/JNEUROSCI.5757-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S, Lee RA. Improvements in the analysis of domain motions inproteins from conformational change: DynDom version 1.50. J Mol Graph Model. 2002;21:181–183. doi: 10.1016/s1093-3263(02)00140-7. [DOI] [PubMed] [Google Scholar]
- Inanobe A, Furukawa H, Gouaux E. Mechanism of partial agonist action at the NR1 subunit of NMDA receptors. Neuron. 2005;47:71–84. doi: 10.1016/j.neuron.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Irvine MW, Costa BM, Dlaboga D, Culley GR, Hulse R, Scholefield CL, Atlason P, Fang G, Eaves R, Morley RM, et al. Piperazine-2,3-dicarboxylicacid derivatives as dual antagonists of NMDA and GluK1-containing kainate receptors. J Med Chem. 2012;55:327–341. doi: 10.1021/jm201230z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kabsch W. A solution for the best rotation to relate two sets of vectors. Acta Crystallogr A. 1976;32:922–923. [Google Scholar]
- Kandel ER, Schwartz JH, Jessel TM. Essentials of Neural Science and Behavior. East Norwalk: Appelton & Lange; 1995. [Google Scholar]
- Karakas E, Simorowski N, Furukawa H. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. Embo J. 2009;28:3910–3920. doi: 10.1038/emboj.2009.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E, Simorowski N, Furukawa H. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature. 2011;475:249–253. doi: 10.1038/nature10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazi R, Gan Q, Talukder I, Markowitz M, Salussolia CL, Wollmuth LP. Asynchronous movements prior to pore opening in NMDA receptors. J Neurosci. 2013;33:12052–12066. doi: 10.1523/JNEUROSCI.5780-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML. Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying kainate receptor selectivity. Neuron. 2005;45:539–552. doi: 10.1016/j.neuron.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Emerging models of glutamate receptor ion channel structure and function. Structure. 2011;19:1370–1380. doi: 10.1016/j.str.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Ghosal A, Dolman NP, Jane DE. Crystal structures of the kainate receptor GluR5 ligand binding core dimer with novel GluR5-selectiveantagonists. J Neurosci. 2006;26:2852–2861. doi: 10.1523/JNEUROSCI.0123-06.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Vyklicky L, Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989;338:425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. Journal of Applied Crystallography. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mosley CA, Acker TM, Hansen KB, Mullasseril P, Andersen KT, Le P, Vellano KM, Brauner-Osborne H, Liotta DC, Traynelis SF. Quinazolin 4-one derivatives: A novel class of noncompetitive NR2C/D subunit-selective N-methyl-D-aspartate receptor antagonists. J Med Chem. 2010;53:5476–5490. doi: 10.1021/jm100027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan H, Kurtkaya NL, Santangelo R, Orr AG, Le P, Vellano KM, et al. A subunit selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat Commun. 2010;1:90. doi: 10.1038/ncomms1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pangborn AB, Giardello MA, Grubbs RH, Rosen RK, Timmers F. Safe and Convenient Procedure for Solvent Purification. J Organometallics. 1996;15:1518–1520. [Google Scholar]
- Paoletti P. Molecular basis of NMDA receptor functional diversity. The European journal of neuroscience. 2011;33:1351–1365. doi: 10.1111/j.1460-9568.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- Regalado MP, Villarroel A, Lerma J. Intersubunit cooperativity in the NMDA receptor. Neuron. 2001;32:1085–1096. doi: 10.1016/s0896-6273(01)00539-6. [DOI] [PubMed] [Google Scholar]
- Riou M, Stroebel D, Edwardson JM, Paoletti P. An alternating GluN1-2-1-2 subunit arrangement in mature NMDA receptors. PLoS One. 2012;7:e35134. doi: 10.1371/journal.pone.0035134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder DM, Kuybeda O, Zhang J, Klymko K, Bartesaghi A, Borgnia MJ, Mayer ML, Subramaniam S. Glutamate receptor desensitization is mediated by changes in quaternary structure of the ligand binding domain. Proc Natl Acad Sci U S A. 2013;110:5921–5926. doi: 10.1073/pnas.1217549110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- Still WC, Khan M, Mitra A. Rapid chromatographic technique for preparative separations with moderate resolution. J Org Chem. 1978:2923–2925. [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Talukder I, Wollmuth LP. Local constraints in either the GluN1 or GluN2 subunit equally impair NMDA receptor pore opening. J Gen Physiol. 2011;138:179–194. doi: 10.1085/jgp.201110623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwyler S, Campbell E, Fricker G, Jenner P, Lemaire M, McAllister KH, Neijt HC, Park CK, Perkins M, Rudin M, et al. Biphenyl-derivatives of 2-amino 7-phosphono-heptanoic acid, a novel class of potent competitive N-methyl-D-aspartate receptor antagonists--II Pharmacological characterization in vivo. Neuropharmacology. 1996;35:655–669. doi: 10.1016/0028-3908(96)84637-5. [DOI] [PubMed] [Google Scholar]
- Vance KM, Simorowski N, Traynelis SF, Furukawa H. Ligand-specific deactivation time course of GluN1/GluN2D NMDA receptors. Nat Commun. 2011;2:294. doi: 10.1038/ncomms1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995;8:127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
- Watkins JC, Evans RH. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Weston MC, Schuck P, Ghosal A, Rosenmund C, Mayer ML. Conformational restriction blocks glutamate receptor desensitization. Nat Struct Mol Bio. 2006;l13:1120–1127. doi: 10.1038/nsmb1178. [DOI] [PubMed] [Google Scholar]
- Yao Y, Belcher J, Berger AJ, Mayer ML, Lau AY. Conformational Analysis of NMDA Receptor GluN1, GluN2, and GluN3 Ligand-Binding Domains Reveals Subtype-Specific Characteristics. Structure. 2013 doi: 10.1016/j.str.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Harrison CB, Freddolino PL, Schulten K, Mayer ML. Molecular mechanism of ligand recognition by NR3 subtype glutamate receptors. Embo J. 2008;27:2158–2170. doi: 10.1038/emboj.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauhar RJ, Colbert CL, Morgan RS, Welsh WJ. Evidence for a strong sulfur-aromatic interaction derived from crystallographic data. Biopolymers. 2000;53:233–248. doi: 10.1002/(SICI)1097-0282(200003)53:3<233::AID-BIP3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.