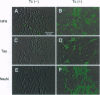
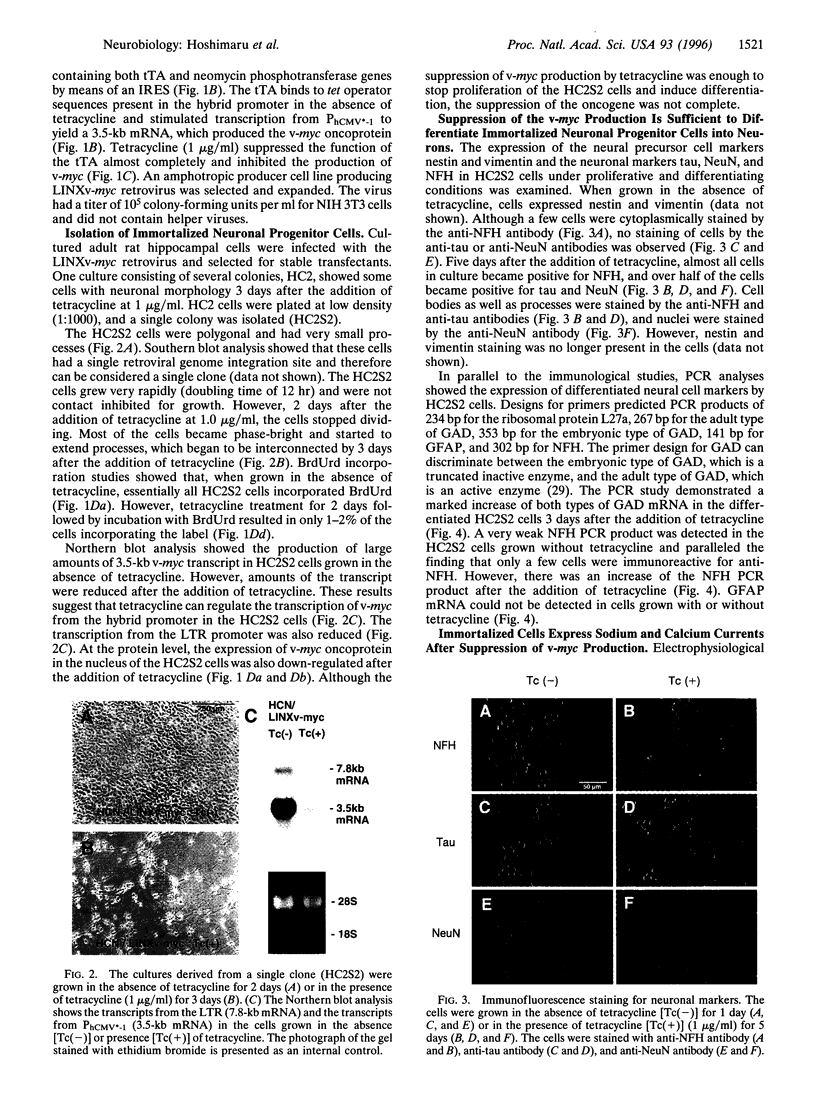
Abstract
A regulatable retroviral vector in which the v-myc oncogene is driven by a tetracycline-controlled transactivator and a human cytomegalovirus minimal promoter fused to a tet operator sequence was used for conditional immortalization of adult rat neuronal progenitor cells. A single clone, HC2S2, was isolated and characterized. Two days after the addition of tetracycline, the HC2S2 cells stopped proliferating, began to extend neurites, and expressed the neuronal markers tau, NeuN, neurofilament 200 kDa, and glutamic acid decarboxylase in accordance with the reduced production of the v-myc oncoprotein. Differentiated HC2S2 cells expressed large sodium and calcium currents and could fire regenerative action potentials. These results suggest that the suppression of the v-myc oncogene may be sufficient to make proliferating cells exit from cell cycles and induce terminal differentiation. The HC2S2 cells will be valuable for studying the differentiation process of neurons.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barres B. A., Chun L. L., Corey D. P. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron. 1989 Apr;2(4):1375–1388. doi: 10.1016/0896-6273(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Bartlett P. F., Reid H. H., Bailey K. A., Bernard O. Immortalization of mouse neural precursor cells by the c-myc oncogene. Proc Natl Acad Sci U S A. 1988 May;85(9):3255–3259. doi: 10.1073/pnas.85.9.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond R. W., Wyborski R. J., Gottlieb D. I. Developmentally regulated expression of an exon containing a stop codon in the gene for glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8771–8775. doi: 10.1073/pnas.87.22.8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen K. C., Robinson P. A., Wion D., Anderton B. H. Partial sequence of the rat heavy neurofilament polypeptide (NF-H). Identification of putative phosphorylation sites. FEBS Lett. 1988 Dec 5;241(1-2):213–218. doi: 10.1016/0014-5793(88)81064-0. [DOI] [PubMed] [Google Scholar]
- Cepko C. L. Immortalization of neural cells via retrovirus-mediated oncogene transduction. Annu Rev Neurosci. 1989;12:47–65. doi: 10.1146/annurev.ne.12.030189.000403. [DOI] [PubMed] [Google Scholar]
- Cepko C. Immortalization of neural cells via oncogene transduction. Trends Neurosci. 1988 Jan;11(1):6–8. doi: 10.1016/0166-2236(88)90039-2. [DOI] [PubMed] [Google Scholar]
- Efrat S., Fusco-DeMane D., Lemberg H., al Emran O., Wang X. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3576–3580. doi: 10.1073/pnas.92.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eves E. M., Tucker M. S., Roback J. D., Downen M., Rosner M. R., Wainer B. H. Immortal rat hippocampal cell lines exhibit neuronal and glial lineages and neurotrophin gene expression. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4373–4377. doi: 10.1073/pnas.89.10.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein D. L., Weinmaster G. A., Milner R. J. Isolation of cDNA clones encoding rat glial fibrillary acidic protein: expression in astrocytes and in Schwann cells. J Neurosci Res. 1992 May;32(1):1–14. doi: 10.1002/jnr.490320102. [DOI] [PubMed] [Google Scholar]
- Frederiksen K., Jat P. S., Valtz N., Levy D., McKay R. Immortalization of precursor cells from the mammalian CNS. Neuron. 1988 Aug;1(6):439–448. doi: 10.1016/0896-6273(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Furth P. A., St Onge L., Böger H., Gruss P., Gossen M., Kistner A., Bujard H., Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F. H., Ray J., Fisher L. J. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- Gossen M., Bonin A. L., Bujard H. Control of gene activity in higher eukaryotic cells by prokaryotic regulatory elements. Trends Biochem Sci. 1993 Dec;18(12):471–475. doi: 10.1016/0968-0004(93)90009-c. [DOI] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U., McKay R. D. The use of cell lines in neurobiology. Trends Neurosci. 1990 Apr;13(4):132–137. doi: 10.1016/0166-2236(90)90004-t. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Bossone S. A., Patel A. J. myc function and regulation. Annu Rev Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- Mehler M. F., Rozental R., Dougherty M., Spray D. C., Kessler J. A. Cytokine regulation of neuronal differentiation of hippocampal progenitor cells. Nature. 1993 Mar 4;362(6415):62–65. doi: 10.1038/362062a0. [DOI] [PubMed] [Google Scholar]
- Mullen R. J., Buck C. R., Smith A. M. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992 Sep;116(1):201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Palmer T. D., Miller A. D., Reeder R. H., McStay B. Efficient expression of a protein coding gene under the control of an RNA polymerase I promoter. Nucleic Acids Res. 1993 Jul 25;21(15):3451–3457. doi: 10.1093/nar/21.15.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J., Gage F. H. Spinal cord neuroblasts proliferate in response to basic fibroblast growth factor. J Neurosci. 1994 Jun;14(6):3548–3564. doi: 10.1523/JNEUROSCI.14-06-03548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfranz P. J., Cunningham M. G., McKay R. D. Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell. 1991 Aug 23;66(4):713–729. doi: 10.1016/0092-8674(91)90116-g. [DOI] [PubMed] [Google Scholar]
- Rubin L. L., Philpott K. L., Brooks S. F. Apoptosis: the cell cycle and cell death. Curr Biol. 1993 Jun 1;3(6):391–394. doi: 10.1016/0960-9822(93)90211-6. [DOI] [PubMed] [Google Scholar]
- Ryder E. F., Snyder E. Y., Cepko C. L. Establishment and characterization of multipotent neural cell lines using retrovirus vector-mediated oncogene transfer. J Neurobiol. 1990 Mar;21(2):356–375. doi: 10.1002/neu.480210209. [DOI] [PubMed] [Google Scholar]
- Snyder E. Y. Grafting immortalized neurons to the CNS. Curr Opin Neurobiol. 1994 Oct;4(5):742–751. doi: 10.1016/0959-4388(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Stockschlaeder M. A., Storb R., Osborne W. R., Miller A. D. L-histidinol provides effective selection of retrovirus-vector-transduced keratinocytes without impairing their proliferative potential. Hum Gene Ther. 1991 Spring;2(1):33–39. doi: 10.1089/hum.1991.2.1-33. [DOI] [PubMed] [Google Scholar]
- White L. A., Eaton M. J., Castro M. C., Klose K. J., Globus M. Y., Shaw G., Whittemore S. R. Distinct regulatory pathways control neurofilament expression and neurotransmitter synthesis in immortalized serotonergic neurons. J Neurosci. 1994 Nov;14(11 Pt 1):6744–6753. doi: 10.1523/JNEUROSCI.14-11-06744.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore S. R., White L. A. Target regulation of neuronal differentiation in a temperature-sensitive cell line derived from medullary raphe. Brain Res. 1993 Jun 25;615(1):27–40. doi: 10.1016/0006-8993(93)91111-5. [DOI] [PubMed] [Google Scholar]
- Wool I. G., Chan Y. L., Paz V., Olvera J. The primary structure of rat ribosomal proteins: the amino acid sequences of L27a and L28 and corrections in the sequences of S4 and S12. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):69–73. doi: 10.1016/0167-4781(90)90143-p. [DOI] [PubMed] [Google Scholar]
- Wyborski R. J., Bond R. W., Gottlieb D. I. Characterization of a cDNA coding for rat glutamic acid decarboxylase. Brain Res Mol Brain Res. 1990 Aug;8(3):193–198. doi: 10.1016/0169-328x(90)90016-7. [DOI] [PubMed] [Google Scholar]