Abstract
Cell polarization is an evolutionarily conserved process that facilitates asymmetric distribution of organelles and proteins, is an evolutionarily conserved property that is modified dynamically during physiological processes such as cell division, migration, and morphogenesis. The plasticity with which cells change their behavior and phenotype in response to cell intrinsic and extrinsic cues is an essential feature of normal physiology. In disease states such as cancer, cells lose their ability to behave normally in response to physiological cues. A molecular understanding of mechanisms that alter the behavior of cancer cells is limited. Cell polarity proteins are an recognized class of molecules that can receive and interpret both intrinsic and extrinsic signals to modulate cell behavior. In this review, we discuss how cell polarity proteins regulate a diverse array of biological processes and how they can contribute to alterations in the behavior of cancer cells.
Keywords: morphogenesis, 3D, oncogenes, tumor suppressor
INTRODUCTION
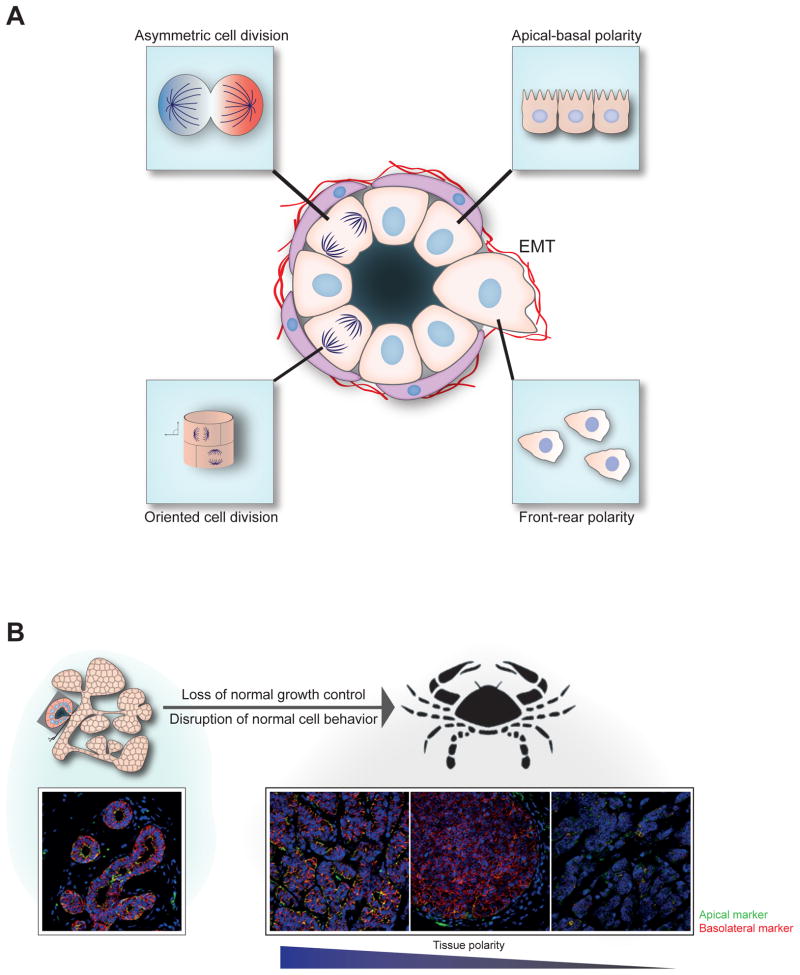
Cells in all organs possess an asymmetric distribution of proteins along their membranes and within their intracellular spaces. The ability to develop and maintain spatial asymmetry is an evolutionarily conserved property observed from yeast to human. Among the multiple cell types in humans, columnar epithelia display a highly sophisticated type of membrane asymmetry referred to as apical-basal polarity. The apical membrane, in contact with the luminal space, displays surface modifications such as microvilli and cilia and is involved in functions including secretion, absorption, and excretion. The basolateral membrane scaffolds the epithelial tissue to the underlying connective tissue and has cell junctions, including tight junctions (TJs), adherens junctions (AJs) gap junctions, desmosomes, and hemidesmosomes. These junctional complexes regulate communication between cells and with the extracellular matrix (ECM) and also limit the paracellular permeability of fluid and ions between the lumen and the interstitium (Handler 1989). The ability of columnar epithelial cells to maintain normal apical-basal polarity is essential for their normal physiological functions and tissue integrity. In addition to apical-basal polarity, epithelial cells engage in different types of cell polarization events during homeostasis and during periodic tissue-remodeling events, such as those observed in colon or breast epithelia. Dividing epithelial cells regulate the plane of cell division to retain or release the daughter cell from the basement membrane, a process controlled by spindle orientationa and referred to as spindle polarity. Cell migration associated with tissue remodeling and morphogenesis involves the alignment of intracellular organelles and proteins to control the direction of cell migration, a process referred to as front-rear polarity. In addition, epithelia align with their neighbors and engage in long-range patterning to maintain tissue order, a process referred to as planar cell polarity (PCP) (Zallen 2007). During both development and normal homeostasis, epithelial cells interact with each other and with the surrounding environment, including the ECM, to maintain tissue organization and function (Figure 1a).
Figure 1.
Cell polarity in normal epithelial organs and cancer. (a) Different types of polarity that are likely to regulate morphogenesis of epithelial structures. Planar cell polarity, used by epithelial cells to coordinate polarity between cells; apical-basal polarity, used by epithelial cells to asymmetrically distribute proteins along the apical-basal axis; front-rear polarity, used by migrating cells to maintain directionality; and mitotic spindle polarity, used for controlling position of daughter cells. (b, upper) Cancer is a consequence of both loss of normal growth control and disruption of normal cell behaviors indicated above. (b, lower) The immunostaining images of normal human breast and cancer with an apical marker (ZO-1) and a basolateral marker (E-cadherin). Normal tissue shows the presence of normal apical-basal polarity and overall organization of ducts with a layer of epithelial cells (tissue polarity); tumor tissue exhibits progressive loss of apical-basal and tissue polarity. Abbreviations: EMT, epithelial to mesenchymal transition
Cancer: Back to the Future
Cancerous growth is associated with an increase in cell number and loss of normal tissue organization. The mechanisms that initiate a cancerous growth may be a result of either genetic and epigenetic events in target cells or to changes in the microenvironment and tissue organization. The cause-and-effect relationship between genetic/epigentic changes and disruption of tissue structure is not clear, because normal tissue exists in an equilibrium of proliferation, morphogenesis, and differentiation. Changes to this equilibrium can result in unregulated proliferation, which in turn can activate adaptive stress pathways that induce changes to the genome of the cells. Thus, disruption of tissue integrity likely can create a permissive environment that allows cells to accumulate genetic aberrations and gain the ability to divide uncontrollably. The dynamic relationship between tissue order and uncontrolled proliferative state, in which one begets the other, makes it challenging to distinguish clearly the cause from the effect, and the effect can seem like the cause.
The mechanisms that initiate cancerous growth haven been a topic of debate for more than 100 years. Between 1894 and 1895, Hugo Ribbert, a histopathologist, reported that tumors result when the balance between the cell and surrounding tissue is disturbed (Triolo 1964). He proposed that epithelial cells resume proliferation upon loss of a restrictive influence from their surrounding connective tissue and referred to this idea as the tissue-tension hypothesis (Triolo 1964). Several alternative/competing hypotheses for the origin of cancer were proposed during the 1890s and the early part of twentieth century. The most prominent among those, which continues to significantly influence cancer research today, is the cell autonomy hypothesis proposed by Hauser, Adami, and Ewing between 1895 and 1907 (Triolo 1964). Although this view dominated cancer research for several decades, there is a resurgence of interest in the domineering role of microenvironment on cancer cell biology, which is fueled by compelling evidence using animal models and cell culture studies (Bissell & Radisky 2001).
Cancer as a Disease of Dysregulated Morphogenesis
Normal tissue homeostasis is maintained by a dynamic equilibrium between processes such as cell proliferation, cell death, tissue morphogenesis, and differentiation. Loss of this equilibrium will initiate disease states including cancer. Studies on cultured epithelial cells grown in a 3D matrix have provided surprising new insights into the relationship between cell proliferation and higher-order tissue organization. In contrast to cells grown in monolayer cultures, contact inhibition cannot explain the proliferation control observed during 3D morphogenesis of organoid structures. For example, tumor-derived epithelial cell lines that retain expression of the cell-cell adhesion protein, E-cadherin, and have cobblestone morphology in monolayer cultures undergo confluency-induced proliferation arrest in monolayer cultures. Furthermore, reexpression of E-cadherin in E-cadherin--negative cell lines is sufficient to confer an epithelial phenotype and regain the ability to undergo contact inhibition (McNeill et al 1990), which suggests that E-cadherin--mediated cell-cell junctions are necessary and sufficient to mediate contact inhibition in monolayer cultures. However, cancer-derived cell lines, whether or not they express E-cadherin, fail to form proliferation-arrested organoids when grown in 3D culture (Debnath & Brugge 2005, Kenny et al. 2007, Petersen et al. 1992) demonstrating that E-cadherin-mediated adhesion is not sufficient for confer proliferation-arrest in 3D organoids. Although it can promote cell proliferation, overexpression of cyclin D1, a known oncogene in breast cancer, is not sufficient to overcome contact inhibition of human mammary epithelial cells in monolayer cultures. However, the same cells, when grown in a 3D matrix, fail to undergo proliferation arrest (Debnath et al. 2003b). Transgenic mouse models overexpressing cyclin D1 in the mammary epithelium are poor at inducing tumorigenesis, which suggests that signals that drive proliferation by deregulating proliferation control, although they may be necessary for the cancer process, are certainly not sufficient to initiate cancer (Wang et al. 1994). Furthermore, genes differentially expressed in cells undergoing proliferation arrest in monolayer represent pathways that are not shared by the genes differentially expressing during prolfieration arrest in 3D culture demonstrating that the mechanism by which cells undergo prolfieration arrest during morphogenesis is likely to be distinct from those used during contact-inhibition (Yu et al 2012).
Dysregulation of cell polarity is an important step during transformation of 3D organized epithelial structures. In contrast to overexpression of cyclin D1, activation of ErbB2, a receptor tyrosine kinase that is amplified and overexpressed in 30% of breast cancers, was sufficient to reinitiate proliferation and disrupt 3D epithelial organization in proliferation-arrested 3D acini (Muthuswamy et al. 2001). ErbB2 requires an interaction with the Par cell polarity complex to transform 3D organized epithelial structures. Inhibition of the ErbB2-Par pathway failed to transform normal acini-like structures into multiacinar structures; however, it was not required for ErbB2 to induce cell proliferation (Aranda et al. 2006), which demonstrates that transformation of 3D organized epithelial structures requires a cooperative interaction between the loss of cell polarity and induction of cell proliferation.
Thus, carcinoma should be viewed as a consequence of a combined effect of dysregulated morphogenesis and uncontrolled proliferation and not simply as a consequence of an increase in cell number.
Cell Polarity and Control of Cell Behavior
Whereas the importance of cell proliferation in cancer is well recognized, the role cell polarity plays is only beginning to be appreciated. If cell proliferation and death are analogous to acceleration and brakes in a car, and metabolism is analogous to fuel, cell polarity can be compared with the steering wheel, which controls direction and maintains spatial relationships in traffic. Improper steering can result in significant damage even to a nonspeeding car, and conversely, proper steering can prevent damage to a speeding car. similarly, we posit that loss of control over cell polarity can disrupt normal cell behavior and lead to initiation and progression of cancer. In this review, we will discuss how cell polarity can integrate extrinsic and intrinsic regulators of cell behavior during establishment and maintenance of normal tissue structure (Figure 1b).
CELL POLARITY PROTEINS AND THEIR ALTERATIONS IN CANCER
The general steps of cell polarity, including its establishment and maintenance, are highly conserved through evolution from bacteria to humans. Over the past few decades, genes responsible for the regulation of cell polarity have been identified using genetic screening in Caenorhabditis elegans and Drosophila melanogaster. Please see Tepass (2012) in this volume for a comprehensive summary of polarity studies in Drosophila. Here, we focus on studies that relate to mammalian epithelial cells and highlight those polarity proteins that are altered in cancer cells.
The proteins that regulate cell polarity can be grouped, for the sake of simplicity, into protein complexes according to their known localization: the subapically localized Par and Crumbs complexes, the basolaterally localized Scribble complex, and the planar polarizing Vang and Frizzled complexes.
PAR Complex
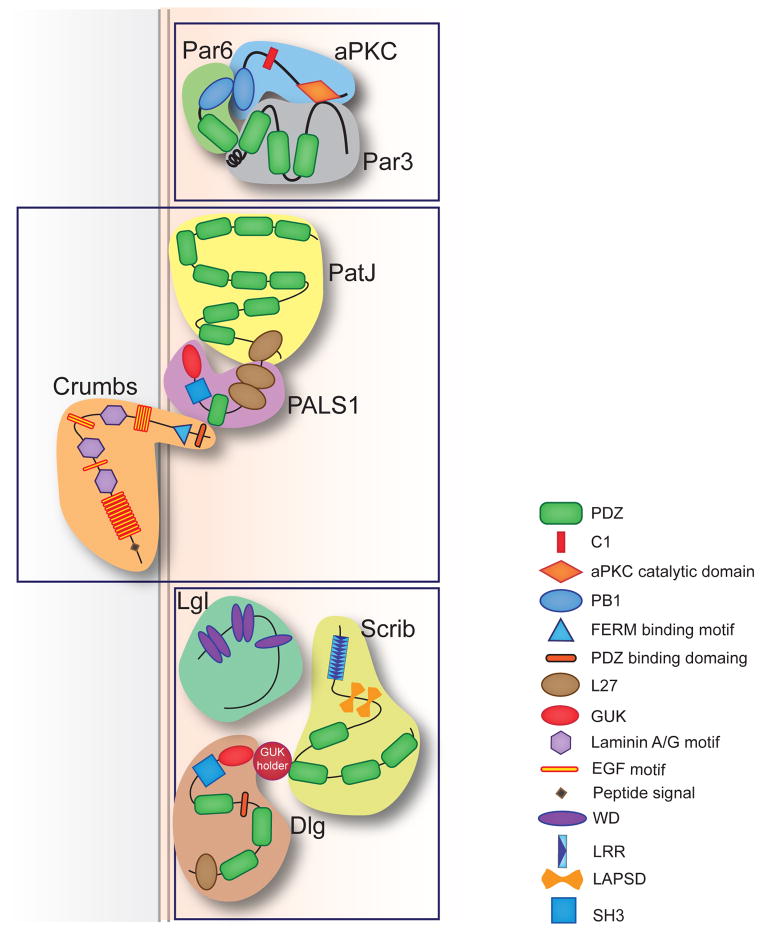
The par (partitioning defective) genes were identified as proteins required for asymmetric division during early embryogenesis in C. elegans; mutations in par genes lead to defects in cell cleavage (Kemphues et al. 1988). The products of par genes have diverse biological function: PAR-1 [MARK2 (microtubule-affinity-regulating kinase)] and Par4 (also known as LKB1 in human, official gene name STK11) are serine/threonine protein kinases, Par3 (PARD3) and Par6 (isoforms, PARD6A, PARD6B, and PARD6G) are scaffold proteins containing (post synaptic density 95; discs large; Zonula occludens-1 (PDZ) domain, Par2 (human homolog unknown) is a RING finger protein that may function as an E3-ubiquitin ligase (Hao et al. 2006, Moore & Boyd 2004), and Par5 is a 14-3-3 protein. In mammalian epithelia, PARD3, PARD6, and atypical protein kinase C (aPKC) form a Par complex that localizes to apical TJs and regulates apical junction formation (Hirose et al. 2002). Par6 binds to aPKC through its Phox Bem 1 (PB1) domains and functions both as an inhibitor of aPKC kinase activity and as a targeting subunit to recruit aPKC substrates, including PARD3 (Hirano et al. 2005). Recruitment of CDC42-GTP to the PARD6/aPKC complex activates aPKC kinase activity (Joberty et al. 2000) towards its subtrates PARD3 (Izumi et al. 1998, Nagai-Tamai et al. 2002). Phosphorylated PARD3 is excluded from the apical domain and localizes to AJs, in which it functions independently from PARD6 and aPKC (Morais-de-Sa et al. 2010). Phosphorylation of Par3 can also negatively regulate aPKC to prevent its ectopic activation (Lin et al. 2000) (Figure 2a).
Figure 2.
Cell polarity proteins in mammalian epithelia. Shown are the protein diagrams of the (a) subapically localized Par complex, (b) Crumbs complex, and (c) basolaterally localized Scribble complex and the protein interactions within the complexes, as reported previously. (a) In the Par complex, Par6 binds to aPKC through its N-terminal PB1 domain, and its PDZ domain binds to Par3. Par3 also interacts with aPKC through its C-terminal tail. (b) In the Crumbs complex, the cytoplasmic tail of the transmembrane protein Crumbs contains a PDZ-binding domain that interacts directly with the PDZ domain of PALS1. The interactions between PALS1 and PATJ involve the binding of the L27 domain of each protein. (c) Scribble associates indirectly with Dlg via a GUK holder protein. The interaction between Lgl and the rest of the complex is not yet clear. (d) The core PCP molecules distribute asymmetrically along the proximal-distal axis. Vang-like protein binds to Prickle (Pk) at the proximal end of the cell. The transmembrane protein Frizzled (Fzd) binds to Dgo and Dsh at the distal end of the cell. CELSR1 localizes to both ends to recruit VANGL (Vang) or Fzd to the membrane. Abbreviations: Par, partitioning defective; aPKC, atypical protein kinase C; Dlg, discs large; Lgl, lethal giant larvae; PATJ, Pals1 associated tight junction; PB1, Phox Bem 1; PDZ, Post synaptic density 95/Discs large/Zonula occludens-1; PCP, planar cell polarity; GUK, guanylate kinase.
An emerging body of evidence implicates Par-complex proteins in cancer biology. PARD6B is amplified and overexpressed in breast cancer (Nolan et al. 2008).. PARD6 interacts with the oncogenic receptor tyrosine HER2/ErbB2 in human mammary epithelial cells (Aranda et al. 2006), and associates with TGFβRI and regulates epithelial to mesenchymal transition (EMT) (Viloria-Petit et al. 2009); the PARD6/PARD3 complex interacts with discoidin domain receptor 1 to regulate collective migration of cancer cells (Hidalgo-Carcedo et al. 2011). Zen et al. (2009) recently showed that PARD3 was deleted frequently in esophageal cancer cells. Overexpression of aPKC is observed in multiple cancers, including hepatocellular carcinoma, pancreatic adenocarcinoma, and breast cancer (Huang & Muthuswamy 2010), and it correlates with poor clinical prognosis in ovarian cancer (Eder et al. 2005).
Crumbs Complex
Another apical module, the Crumbs complex, comprises a transmembrane protein, Crumbs (isoforms CRB1-3); Pals1 (MPP5); and Pals1-associated TJ protein (PATJ, official gene name INADL) (Figure 2b). During polarization, the Crumbs complex specifies apical identity by recruiting actin cytoskeleton regulators Moesin and β H-spectrin (Medina et al 2002). In Drosophila, embryos with crumbs mutation fail to assemble or stabilize a zonula adherens junction from a spot AJ, which leads to breakdown of the epithelial structure and extensive cell death (Tepass et al. 1990). Downregulation of CRB3 was required for transformation of immortal baby mouse kidney cells (Karp et al. 2008), which suggests that CRB3 may function as a tumor suppressor.
Scribble Complex
The Scribble complex is composed of three proteins, Scribble (SCRIB), Discs Large (isoforms, DLG1–5), and Lethal(2) giant larvae (Lgl) (isoforms, LLGL1 and 2). Scribble associates indirectly with Dlg via a linker protein called guanylate kinase (GUK) Holder (Mathew et al. 2002) and binds directly to LLGL2 via SCRIB’s LRR (leucine-rich repeat) domain. The Scribble complex localizes to the lateral domain and is required for restricting the apical domain by antognizing the apical Par complex (Figure 2) (St Johnston & Sanson 2011). In Drosophila, mutations of Scrib cause a broad defect in the epithelial organization and lead to embryo lethality (Bilder & Perrimon 2000). In addition to complete loss, mislocalization of SCRIB inactives protein function (Audebert et al 2004). Consistently, SCRIB, DLG and LLGL are lost of mislocalized in multiple cancers, including lung, prostate, breast, and colon and downregulation of Scribble results in transformation of mammary epithelial and prostate epithelial cells (Huang & Muthuswamy 2010). Aberrant splicing of LLGL1 and expression of the truncated protein is associated with poor differentiation and large tumor size of hepatocellular carcinoma (Lu et al. 2009).
Planar Cell Polarity Complex
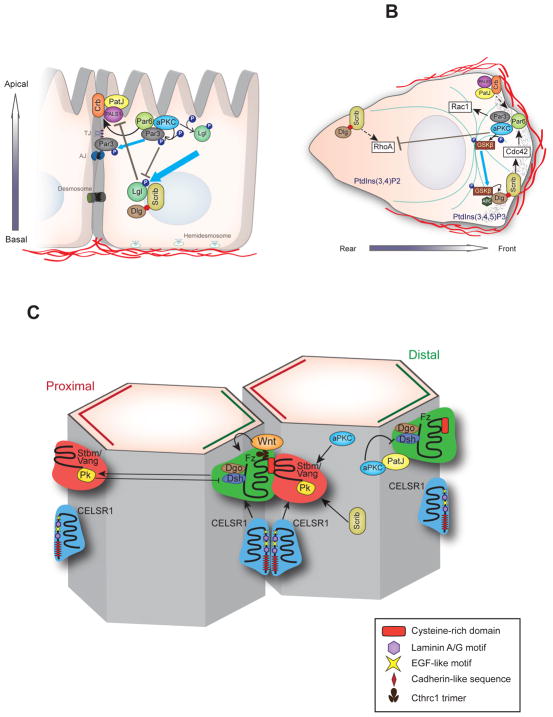
Two classes of molecules, the core planar cell polarity (PCP) proteins and other modulators of PCP, regulate planar cell polarization. The core PCP regulators include Vang-like protein (isoforms, VANGL1 and 2); a four-pass transmembrane protein that binds to its cytoplasmic partner Prickle (isoforms PRICKLE1-3) at the proximal end of the cell. A complex containing seven-pass transmembrane protein, Frizzled (isoforms, FZD 1–10) and its ligand, the noncanonical Wnts (WNT5A, 4, and 11); and an Fzd cytoplasmic binding protein, Dishevelled (DSH) form at the distal end of the cell. Normal proximal – distal orientation of these complexes is required for the distal orientation of hair in the Drosophila wing.. However, other core PCP regulators such as Flamingo/Starry night (isoforms CELSR1, 2, and 3), a seven-pass transmembrane atypical cadherin, localizes to both ends (Strutt & Strutt 2009) of the proximal – distal axis. In mammals, CELSR1 interacts with VANGL2 and FZD6 to recruit them to the cell membrane (Devenport et al. 2011). WNT5a interacts with receptor tyrosine-like orphan receptor 2 (ROR2), which activates a noncanonical Wnt pathway and synergistically regulates convergent extension movements during planar polarization (Oishi et al. 2003). VANGL2 can be phosphorylated and activated by this WNT5a/ROR2 interaction in a WNT5a concentration--dependent manner to regulate chondrocyte polarity along the proximal-distal axis during limb bud elongation (Gao et al. 2011) (Figure 2d).
Other modulators of PCP include the large protocadherins Fat (Ft, four isoforms, FAT1–4), which have 34 cadherin repeats that interact with Dachsous (Ds, two isforms, DCHS1 and 2) and a cadherin molecule in the adjacent cell to regulate planar polarization (Ishikawa et al. 2008, McNeill 2010). In Drosophila, Ft interacts with a transcriptional corepressor, Atrophin (human homolog ATN1) (Fanto et al. 2003), and represses expression of four jointed (FJX1), a Golgi complex localized kinase that phosphorylates Ds (Ishikawa et al. 2008) to reduce its affinity for Ft (Brittle et al. 2010) in order to form an autoregulatory loop. The relationship between Ft/Ds and the core PCP proteins is unknown.
PCP proteins are altered widely in cancer. Loss of VANGL2 promotes migration and metalloproteinase-dependent invasion in cancer cells (Cantrell & Jessen 2010). Downregulation of PRICKLE1 is associated with high β-catenin activity and increased cell proliferation in hepatocellular carcinoma cells, which suggests that PRICKLE1 is a putative tumor suppressor (Chan et al. 2006). WNT5a and ROR2 are implicated in multiple cancers, including melanoma, prostate, and breast (O’Connell et al. 2010, Ren et al. 2011, Yamamoto et al. 2010). Among the FATs, FAT1 is overexpressed and mislocalized in melanoma cells and breast cancers (Kwaepila et al. 2006, Sadeqzadeh et al. 2011) and FAT4 is deleted or silenced in breast cancers (Qi et al. 2009).
Cross Talk Between Polarity Complexes
During the establishment and maintenance of cell polarity, polarity complexes interact with each other. PARD6 interacts with CRUMBS during establishment of TJs (Lemmers et al. 2004) and regulates exclusion of PARD3 from the subapical domain during photoreceptor remodeling (Walther & Pichaud 2010). SCRIB, LLGL and DLG restrict the activity of the Crumbs complex to the apical domain (Bilder & Perrimon 2000). Thus, the basolateral Scribble complex and the apical Par and Crumbs complexes antagonize each other during establishment of cell polarity (Figure 3a).
Figure 3.
The central role of polarity protein complexes is retooled and rewired during cell polarization under different cellular contexts. The figure illustrates the changes in localization of polarity proteins and regulation of downstream targets during the transition between (a) apical-basal polarity and (b) front-rear polarity. Abbreviations: aPKC, atypical protein kinase C; Dlg, discs large; Lgl, lethal giant larvae; ECM, extracellular matrix; PATJ, Pals1 associated tight junction; GSK, glycogen synthase kinase, PItdIns, phosphatidylinositol; P, phosphorylation.
During establishment of apical-basal polarity, all PCP proteins are located apically, which suggests that apical-basal and PCP pathways are intertwined. For example, in Drosophila eyes, the TJ protein dPatj binds the cytoplasmic tail of Fz1 and recruits aPKC, which in turn phosphorylates and inhibits Fz1 (Courbard et al. 2009). During PCP signaling, aPKC/dPatj is downregulated, whereas the PARD3 (Bazooka) levels are elevated (Djiane et al. 2005). In Xenopus oocytes, aPKC binds to a maternal PCP core protein, VANGL2, to promote asymmetric distribution of post-Golgi complex-v-SNARE protein, VAMP1, and acetylated microtubules required for embryonic patterning (Cha et al. 2011). Basolateral polarity protein SCRIB can bind to PCP component VANGL2 through its PDZ3 domain and cooperate with VANGL2 in PCP establishment (Courbard et al. 2009).
The above studies demonstrate the complex network of interactions that occur during cell polarization and highlight the need for understanding how polarity proteins induce cell polarization as well as how they engage in both cooperative and antagonistic interactions between each other to establish and maintain cell polarization. A better understanding of the mechanisms that regulate polarization also is necessary to elucidate how cells modulate behavioral plasticity during tissue morphogenesis.
CELL POLARITY PROTEINS AS INTEGRATORS OF INTRINSIC AND EXTRINSIC SIGNALS
Establishment and maintenance of cell and planar polarity is regulated by both cell-intrinsic and cell-extrinsic factors. We highlight how some of the cell-intrinsic factors, such as protein trafficking and microtubule and actin dynamics, and the cell extrinsic factors, such as cell-cell and cell-matrix interaction, regulate the polarization process and how polarity proteins in turn affect these processes.
Intrinsic Factor: Protein Trafficking
Protein trafficking is highly regulated in polarized epithelial cells. Epithelial cells use both exocytic (transport of newly synthesized proteins) and endocytic (recycling of membrane proteins) mechanisms during establishment and maintenance of cell polarity (Nelson & Yeaman 2001). Proteins targeted to the plasma membrane are first transported from the endoplasmic reticulum to the Golgi apparatus by the coatmer protein complex II (COPII), a coat protein complex that initiates budding from the endoplasmic reticulum. Proteins are then transported through the Golgi compartments by COPI, a protein complex that initiates budding of vesicles from the Golgi. Proteins use both intrinsic sorting signals and the adaptor protein (AP) complex for transport between the trans-Golgi network (TGN) and the plasma membrane. The AP complex recognizes the sorting signals in the cytoplasmic domain of membrane proteins and facilitates their assembly into vesicles that are sorted to apical or basolateral membranes. Apical sorting signals include glycosylphosphatidylinositol (GPI)-anchor, N- or O-linked glycosylation, a specialized transmembrane domain, and cytoplasmic determinants. Basolateral sorting signals include tyrosine-based cytoplasmic domains, dileucine and monoleucine motifs, pleomorphic sequences, and juxtamembrane sequences (Mellman & Nelson 2008). In general, the basolateral signals are dominant over apical signals. AP-1B and AP4 are involved in sorting proteins to the basolateral membrane; in particular, AP-1B is the only AP-1 complex that sorts basolateral proteins in epithelia (Mellman & Nelson 2008). There is limited evidence for interaction between cell polarity proteins and the members of the AP complex. However, mutation of Nak, a protein that interacts with the AP-1 and AP-2 complex, and mutation of the proteins that are part of the AP-2α subunit complex induce mislocalization of Dlg from the basolateral membrane in the Drosophila salivary gland (Peng et al. 2009).
Vesicles containing sorted proteins then dock and fuse with the plasma membrane through the involvement of SNARE proteins. Syntaxins (STX), members of the SNARE family, act as target membrane receptors and display distinct membrane domain specificity. STX1A and STX1B are present only in intracellular structures; STX2 is found in both the apical and basolateral surface, whereas STX3 and 4 are restricted to the apical or basolateral domains, respectively (Low et al. 1996). LLGL1 binds to STX4, but not to STX2 or 3, to target vesicles to the basolateral domain (Musch et al. 2002, Zhang et al. 2005). In addition, both SCRIB and DLG interact with STX4 in mammalian cells (Massimi et al. 2008). Mutations in Drosophila Avalance (homolog of human STX7 and STX12) result in basolateral localization of Crumbs by regulating endocytic trafficking (Lu & Bilder 2005). Although these initial evidences demonstrate the existence of a relationship between cell polarity proteins and the vesicle targeting machinery, they most importantly, highlight the gap in our knowledge of how cell polarity and vesicle trafficking machinery interact to generate intracellular asymmetry.
Another important class of vesicular trafficking regulators is the Rab GTPases. There are eleven Rabs in yeast and more than 60 in mammals. Rabs have a large number of effectors and have been implicated in all the steps in protein trafficking. Among them, RAB8 and RAB11 have a direct link to polarity establishment and lumen formation. RAB11 regulates recycling endosome trafficking and is required for junctional maintenance of E-cadherin and Crumbs as well as lumen formation (Desclozeaux et al. 2008, Roeth et al. 2009). RAB8 and RAB11 target PARD3 to the apical membrane in Madin-Darby canine kidney (MDCK) cells by interacting with the exocyst complex (Bryant et al. 2010). A significant body of evidence demonstrates a role for Rabs in cancer, in which they are thought to function as tumor suppressors, oncogenes, or regulators of cell migration and invasion (Stenmark 2009); however, little is known about the relationship between Rabs and cell polarity proteins.
Retromer is a protein complex involved in retrograde transport of transmembrane proteins from endocytic vesicles to the trans-Golgi network for recycling (Bonifacino & Hurley 2008). Recent studies demonstrate a role for retromers in regulation of cell polarity and possible cancer processes. Crumbs interacts with VPS35, a component of the retromer complex, and loss of function mutation of VPS35 leads to loss of Crumbs and phenocopies crb mutation in Drosophila follicular epithelia (Pocha et al. 2011). Interestingly, a comparative genomics study identified GOLPH3, a retromer-interacting protein, as overexpressed in cancers of the lung, ovary, prostate, breast, and skin, which highlights a potential role for retromer biology in cancer (Scott et al. 2009). GOLPH3 can activate mammalian target of rapamycin (mTOR), which in turn can promote cell growth. Overexpression of GOLPH3 can also promote retromer mediated endosome to golgi trafficking of transmembrane proteins by interacting VPS35 a member of the retromer complex. Consistent with this possibility, VPS35 is mutated frequently in colorectal and gastric cancers (An et al. 2011). However, there is little known about the relationship between retromer vesicle trafficking, cell polarity, and cancer initiation and progression.
Intrinsic Factors: Microtubules and Golgi Orientation
Microtubules are comprised of α- and β-tubulin polymers and several microtubule-associated proteins. The tubulin subunits are organized in a polarized manner, with a fast-growing plus end and a slow-growing minus end. Most of the microtubules in a cell are generated by the microtubule organizing center (MTOC), which is composed of two centrioles and a β-tubulin cloud. In many cell types, the minus ends are attached to the MTOC, and the plus ends radiate toward the cell periphery. In columnar epithelial cells, the microtubule ends are released from the centrosome after nucleation (Keating et al. 1997). The microtubules then form bundles along the apical-basal axis, with the plus ends oriented toward the basal side and the minus ends oriented toward the apical side of the cell. Proper microtubule organization is essential for the polarized structure, because it serves as tracks for transporting organelles, determining cell segregation axes during division, directing cell migration, and forming axoneme organelles such as cilia or flagella.
With the exception of apical-basal polarized epithelial cells, other cell types organize Golgi complexes around centrosomes. Microtubule-disrupting drugs such as colchicine and vinblastine induce fragmentation of stacks of Golgi cisternae into ministacks, which disperse throughout the cytoplasm. Removal of the drugs restores microtubules and restored organization to Golgi stacks (Moskalewski et al. 1975). Direct contact between Golgi complex and microtubule were obtained using cells expressing vesicular stomatitis virus glycoprotein (VSVG) as a marker for the Golgi complex where Golgi stacks were shown to associate with microtubules during reassembly (Rogalski & Singer 1984)..
We now know that Golgi complex elements interact directly with microtubule motors, including dynein and kinesin. Dynein is a minus end–directed, microtubule-binding motor protein complex composed of heavy, intermediate, and light chains and an AAA ATPase domain that hydrolyze ATP and generate energy for movement (Hirokawa 1998). Microinjection of antidynein antibody disrupts Golgi complex organization, which provides the initial demonstration of a role for microtubule motors in Golgi organization (Vaisberg et al. 1996, Lippincott-Schwartz et al. 1995). Either homozygous loss or downregulation of dynein heavy chain 1 results in fragmentation of Golgi stacks, which demonstrates that dynein motors mediate formation of Golgi stacks. The dynein complex interacts with dynactin, a protein complex that increases dynein processivity on microtubules (King & Schroer 2000, Schroer 2004). Actin related protein 1 (Arp1), a component of the dynactin complex, interacts with Golgi complex--associated βIII spectrin and facilitates an interaction between Golgi membranes and the dynein-dynactin microtubule motor complex. Kinesins (14 isoforms, KIF 1–14) are plus end--directed motors that are heteroteramers of two kinesin heavy chains and two light chains. Loss of KIFC3 induces Golgi fragmentation under cholesterol depletion conditions (Xu et al. 2002). Formation of Golgi stacks involves a dynein-dynactin complex to mediate minus end--directed transport of ministacks, which then assemble into the ribbonlike network proximal to the centrosome. Overexpression of dynamitin disrupts the dynactin protein complex and induces fragmentation of Golgi stacks (Burkhardt et al. 1997), which demonstrates a direct role for the dynactin complex in Golgi complex compaction.
In polarized epithelial cells, the Golgi complex is positioned in juxtaposition to the face of the nuclei proximal to the apical membrane (Barr & Egerer 2005, Lacy 1957) (Figure 3a). Golgi fragmentation does not significantly affect general vesicle transport but significantly impairs polarized secretion of VSVG particles to the apical membrane (Yadav et al. 2009). In migrating cells, the MTOC and the Golgi complex orient toward the direction of migration (Kupfer et al. 1987) (Figure 3b). This directional orientation facilitates the polarized, microtubule-dependent exocytosis toward the cell membranes at the leading edge of migrating cells (Schmoranzer et al. 2003). Golgi reassembly and stacking proteins and golgins play important roles during assembly of the Golgi complex (Ramirez & Lowe 2009). Golgins are coiled-coil proteins present in the cytoplasmic phase of the Golgi membrane, and loss of golgins GMAP210 and golgin-160 induces Golgi fragmentation and impairs directional cell migration as well as wound healing (Rios et al. 2004, Yadav et al. 2009). This evidence suggests a role for normal Golgi complex stacking in cell polarization and directional migration.
Polarity proteins are emerging as key regulators of microtubule dynamics and Golgi complex dynamics. MARK2, binds to and phosphorylates microtubule-associated protein, which leads to microtubule destabilization (Drewes et al. 1998). During gastrulation in Drosophila, aPKC maintains the symmetrical organization of AJs by promoting microtubule dissociation with the centrosome and by reaching microtubule/actin balance at AJs. aPKC might accomplish this by promoting release of AJ/Bazooka from dynein. Without aPKC, AJs form atypical planar-polarized puncta at gastrulation (Harris & Peifer 2007). In migrating cells, PARD3 interacts directly with dynein light intermediate chain (LIC2) and microtubule ends at cell-cell contact (Schmoranzer et al. 2009), whereas DLG1 and CDC42 control dynein association with microtubules by scaffold protein GKAP (Manneville et al. 2010). Both mechanisms converge to serve as a cortical factor for assisting proper positioning of the centrosome at the cell center. CRB3 associates with PARD3, PARD6, and aPKC to regulate ciliogenesis, which is mediated by microtubular motor KIF3/kinesin-II (Fan et al. 2004).
Drugs that dysregulate microtubule dynamics are used widely to treat cancer patients, and they may also induce Golgi fragmentation (Wehland et al. 1983) and dysregulation of vesicle trafficking in cancer cells (Sonee et al. 1998). Consistent with this notion, there is an emerging body of evidence on the role of Golgi complex in cancer, in particular the relationship between cell division and regulation of Golgi complex dynamics. Although mammalian cells in interphase maintain an intact Golgi stack structure, they are disassembled upon cell cycle entry and reassembled during cytokinesis (Roth 1999). Cancer-derived cell lines such as MCF-7 and primary colon cancers have fragmented Golgi organization (Kellokumpu et al. 2002). The relationship between cell polarity proteins and Golgi organization and orientation during cancer initiation and progression is an under-studied area of cancer cell biology.
Intrinsic Factor: Actin
Actin microfilaments are critical components of the cytoskeletal network. Actin filament (F-actin) turnover and dynamic regulation of actin cytoskeleton architecture are critical for cell morphology and behavior, particularly for cell adhesion and protrusion, processes that play very important roles in cancer.
In epithelial cells, both AJs and TJs associate with the actin cytoskeleton, and maturation and stabilization of the junctions involve reorganization of the actin cytoskeleton. PARD3 is required for TJ formation by binding and inhibiting LIMK2, which phosphorylates and inactivates the F-actin-severing protein, cofilin (Chen & Macara 2006). Cofilin-dependent depolymerization of actin is associated with TJ disassembly (Ivanov et al. 2004, Nagumo et al. 2008), which suggests that PARD3 regulates actin dynamics by inhibiting actin-severing proteins. Paradoxically, PARD3 inhibits the activation of Rac, a promoter of actin polymerization, by interacting with and inhibiting a Rac-GEF, TIAM1, at cell contacts (Chen & Macara 2005). Thus, PARD3 is likely to promote TJ biogenesis by maintaining a balance between positive and negative regulators of actin dynamics (Figure 3a). In addition, CDC42, PARD6, and aPKC are required for AJ stability via regulating WASP and Arp2/3-mediated endocytosis (Georgiou et al. 2008). In migrating cells, the segregation of CDC42 and RAC at the leading edge from RHOA in the trailing edge leads to a different cytoskeleton structure (Figure 3b). CDC42 and RAC1 promote actin nucleation mainly nucleation of highly branched to induce membrane protrusion, whereas RHOA activates ROCK1 to induce F-actin and induce actomyosin contraction and tail formation (Heasman & Ridley 2008, Iden & Collard 2008). When cells attach to ECM, focal adhesions provide the main sites for cell adhesion, and associate with actin stress fibers to control cell movement. Assembly of stress fibers and focal adhesions is regulated by RHOA-mediated downstream effectors such as mDia1/2 and ROCK1. PARD3 interacts with focal adhesion kinase (FAK), and depletion of PARD3 inhibits adhesion-induced activation of FAK, which suggests that PARD3 functions to link focal adhesion, actin reorganization, and cell migration (Itoh et al. 2010). Whereas the role played by actin dynamics in the migration and invasion of cancer cells is well known, the mechanisms by which cell polarity proteins regulate actin dynamics are only beginning to be understood. A better understanding of how cell polarity proteins regulate actin dynamics is likely to provide insights into behavior of tumor epithelial cells during processes such as metastasis.
Extrinsic Factor: Neighboring Cells
Evidence for cell-cell interactions regulating cell polarization can be observed during early development. In the 8-cell stage of mouse embryonic development, cell-cell interaction induces cell surface asymmetry, and such asymmetry is required for further development of the outer trophectoderm and inner cell mass in blastocysts (Johnson & Ziomek 1981, Ziomek & Johnson 1980). Inhibition of E-cadherin junctions using an anti--E-cadherin antibody blocks proper polarization and produces blastocysts without an inner cell mass (Shirayoshi et al. 1983). Blocking cell-cell contact in the MDCK monolayer by using an E-cadherin antibody disrupts apical-basal polarity demonstrating a critical role for cell-cell contact during establishment of apical-basal polarity (Imhof et al. 1983). MDCK cells in suspension culture form a polarized epithelium in which the apical membrane faces the growth medium and the basolateral membrane faces the central lumen (Wang et al. 1990), which suggests that adhesion between cells is sufficient to generate a free apical surface and initiate polarization. E-cadherin initiates polarity by orienting junction microtubules for targeting basolateral vesicles to the cell-cell contacts (Nejsum & Nelson 2007). However, studies in Drosophila cells during cellularization demonstrate that apical polarization of Par-3 can occur in the absence of Drosophila E-cadherin, which suggests the presence of E-cadherin--independent mechanisms of initiation of polarization (Harris & Peifer 2004, McGill et al. 2009).
TJs form a tight seal between adjacent cells and also demarcate the apical and basolateral membrane domains of a cell. This precise localization of TJ proteins, including ZO-1 and occludin, is regulated by the microtubule cytoskeleton, and vesicle trafficking machinery (Cereijido et al. 1998, Decaens & Cassio 2001, Subramanian et al. 2007). Crumbs and Par polarity protein complexes are essential for the apical TJ formation (Hurd et al. 2003, Shin et al. 2006). Although TJs may be dispensable for the initial establishment of membrane polarity, they are involved in maintaining cell polarity. Expression of mutant junctional adhesion molecule A (F11R), an integral membrane protein at TJs, leads to loss of apical-basal polarity and impairs cyst formation in MDCK cells (Rehder et al. 2006). In addition, TJs are likely to play important roles in regulating growth control. ZONAB, a TJ-associated transcription factor, is sequestered at sites of cell-cell contact in confluent monolayers, whereas, in subconfluent cultures that lack TJs, ZONAB translocates to the nucleus to induce expression of ERBB2 (Du et al. 2010) and also interacts with cyclin-dependent kinase 4 (CDK4) to promote cell proliferation (Sourisseau et al. 2006).
Cell polarity proteins are thought to play an active role in the establishment of cell-cell junctions; however, our understanding of the mechanisms by which this occurs is poor. Following the E-cadherin engagement, SCRIB is recruited to the junction site (Navarro et al. 2005), where it is thought to stabilize E-cadherin-catenin association and promotes cell adhesion (Qin et al. 2005, Yoshihara et al. 2011). Phosphorylation of SCRIB at Ser1601 excludes it from E-cadherin junctions and likely to interfere with its function at cell junctions. PARD3 associates directly with TJ proteins F11R, nectins, and afadin/AF-6 (RASSF5) at nascent junctions and promotes junction formation (Ebnet et al. 2001, Ooshio et al. 2007, Takekuni et al. 2003). PARD3 also binds to and inhibits regulators or TJ biogenesis such as Rac exchange factor, TIAM1, and cofilin kinase LIMK2 (Chen & Macara 2005, 2006; Mertens et al. 2005).
Loss of E-cadherin at cell-cell junctions is associated strongly with metastasis of multiple carcinomas, including that of the breast, colon, lung, prostate, and thyroid. Loss of cadherins or cell-cell adhesion molecules likely decreases cell-cell cohesion (Foty & Steinberg 1997). Although silencing of E-cadherin gene expression and aberrant cadherin endocytosis are observed in metastatic cancer cells, tumor epithelia likely have additional mechanisms that compromise E-cadherin--based cell-cell interactions, because a large number of metastatic tumor cells still express normal levels of E-cadherin at the cell surface. It is possible that these metastatic tumor epithelia have decreased cell-cell cohesion strength and hence have gained the ability to leave the primary site (Coman 1944). Neither the mechanisms that regulate cell-cell cohesion nor the role cell polarity proteins play in modulating E-cadherin function are well understood.
Intercellular communication by direct passing of molecules and ions between cells is mediated by gap junctions that are composed of homo- or heterohexamers of connexins. In polarized epithelial cells, connexin43 (CX43) and CX45 bind directly to the TJ molecule ZO-1 and colocalize with the TJ at the apical border, whereas CX32-containing gap junctions are scattered throughout the lateral membrane (Guerrier et al. 1995, Kausalya et al. 2001). Although the role played by connexins during establishment of apical-basal polarity is unknown, loss of CX43 impairs reorientation of MTOC and the Golgi complex during directional migration of mouse embryo fibroblasts in a CX43 tubulin-binding domain--dependent manner (Francis et al. 2011, Giepmans et al. 2001). It is still unclear whether cell polarity proteins can regulate gap junctions. A bidirectional signaling ligand, EphrinB1, interacts with CX43 and regulate its distribution and also inhibits TJ formation by interfering with the interaction between PARD6 and CDC42 (Davy et al. 2006, Lee et al. 2008). If EphrinB1 coordinates a relationship between TJ and gap junctions remains to be determined. In non-small cell lung cancer, decreases in CX43 protein levels correlate with loss of ZO-1 and E-cadherin (Jinn & Inase 2010) suggesting existence of mechanisms by which different cell-cell junction proteins are co-regulated. These observations also highlight the significant gap in our understanding of the relationship between cell polarity proteins and cell junctions during normal epithelial organ morphogenesis and cancer.
Extrinsic Factor: Basement Membrane
ECM proteins are assembled into an organized meshwork and associate with the surface of the cells that produced them. ECM not only provides structural support for tissues, it also guides cell development and patterning as well as regulates fate decisions (Muschler & Streuli 2010). Interactions between the epithelia and the ECM play critical roles during the establishment of cell and tissue polarity. Our understanding of the relationship between cell polarity pathways and interaction with ECM is poor. Here we highlight the key components of ECM and the cell surface molecules as well as the initial evidence that demonstrates a role for a dynamic relationship between cell polarity pathways and cell-ECM interactions during the establishment and maintenance of cell polarity.
The basement membrane (BM) underlying epithelia contains ECM proteins laminins, nidogens, perlecan, agrin (AGRN), and collagen IV (Hynes 2009). Laminin is a heterotrimer of five α, four β, and three γ chains and is required for assembly of BM. Nidogens (entactins) link laminin and collagen IV and play a vital role in BM stabilization. Agrins and perlecans provide collateral linkage between laminin, the cell surface, and the underlying cytoskeleton. Collagen IV is a heterotrimer of one of the six α chains. The most common collagen IV variant present in most BMs is a trimer of two α1 and one α2 subunit (Yurchenco 2011).
Assembly of BM is initiatied by binding of the C-terminal laminin globular domains to cell surface receptors such as integrins, and dystroglycan. The N-terminal laminin globular domain also interacts with cell surface integrins to align laminin into a triple helix parallel to the cell surface. The β and γ subunits of laminin interact with other BM proteins such as agrins and nidogens, which stabilizes the interactions with Collagen IV and with the cell surface..
BM receptors, integrins, are required for establishing polarity in different cell substratum: α2β1 and β4 are required in collagen, and β1-integrins (α1β1, α2β1, α3β1, α6β1, and α7β1) in laminins. α2β1 and β4 integrins induce cell polarization in a Rac-dependent manner. The α3β1 integrins are implicated in positioning of the mitotic spindle in epithelial cells within a cyst, which is essential for lumen formation (Myllymaki et al. 2011). Changes in ECM components and receptors play important roles during cell polarization and development. Heterozygous loss of laminin α1 results in defective polarization of columnar epiblast epithelia without affecting epiblast differentiation during early mouse development (Murray & Edgar 2000). Laminin-induced polarization of mammary epithelial cells requires an interaction with the extracellular domain of dystroglycan (Weir et al. 2006). Thus, interaction with ECM directs epithelial cell polarization.
Regulators of cell polarity play important roles in remodeling ECM to activate a positive feedback loop that reinforces establishment and maintenance of cell polarity. Most ECM is secreted by stromal cells; however, in MDCK cells, the developing epithelium also secrete laminin and assemble it on the cell surface. This process requires RAC activation, and expression of a dominant-negative RAC impairs laminin assembly and disrupts cyst morphogenesis, which can be rescued by exogenous laminin (O’Brien et al. 2001). MARK2 facilitates basolateral laminin assembly by promoting basolateral localization of dystroglycan, a laminin receptor (Masuda-Hirata et al. 2009). Loss of RASSF5 interferes with laminin deposition and α6β1 laminin receptor localization, which results in inhibition of cavitation during mouse embryoid body formation (Komura et al. 2008) demonstrating a relationship between cell polarity proteins and modulation of cell-matrix interactions.
Loss of the basement membrane is associated with the transition from in situ to invasive carcinoma (Albrechtsen et al. 1981). In addition to changes in the basement membrane, tumor tissues possess interstitial matrices, such as type I collagen and fibronectin, as well as changes in expression of matrix-remodeling enzymes, namely, matrix metalloproteinases and lsysyl oxidases (Egeblad & Werb 2002). Changes in ECM stiffness are regulated by lysyl oxidases and matrix metaolloproteases are thought to play important roles during both normal development and tumor progression (Paszek et al. 2005). While we are beginning to recognize the importance of ECM in cancer, very little is known about the relationship between the cell polarity machinery and ECM remodeling.
DYNAMICS OF CELL BEHAVIOR DURING MORPHOGENESIS
Tissue morphogenesis is a highly regulated process. There is a large knowledge gap in our understanding of the mechanisms that control organ size, diameter of the luminal space, and size and number of the cells that compose the ducts and acini. Epithelial cells cultured in a 3D ECM, unlike cells grown in monolayer cultures, recapitulate numerous features of glandular organs seen in vivo, including the formation of acini-like spheroids of defined size with a hollow lumen, apical-basal polarization of cells composing these acini, and the basal deposition of basement membrane components collagen IV and laminin-332 (Debnath & Brugge 2005). During 3D morphogenesis, cells use integrins and dystroglycans to contact the ECM, and they use cadherins and desmosomes to contact their neighbors (O’Brien et al. 2002a, b). As discussed above, these initial contacts are required for polarization of cells to create a basolateral surface and an apical surface with distinct membrane protein compositions. Although development of asymmetry can occur in monolayer cultures, formation of multicellular structures with a hollow central lumen is unique to 3D cultures. Cells surrounding the lumen point their apical poles toward the lumen. The ability to polarize is needed for lumen formation, and inhibition of polarization affects laminin assembly around 3D cysts (O’Brien et al. 2001). The mechanisms by which cells establish polarization and 3D organization are only beginning to be understood. Cell culture models, including MDCK and MCF-10A epithelial cells, have been the models of choice for understanding the dynamics of 3D morphogenesis (Debnath et al. 2003a, Elia & Lippincott-Schwartz 2001). Although almost all aspects of cell biology, including cell proliferation and cell death, are regulated dynamically during morphogenesis, in this article we discuss the dynamics of mitotic spindle position and EMT during 3D morphogenesis, two emerging, yet poorly understood, regulators of morphogenesis.
Spindle Orientation
Oriented cell division can influence the position of daughter cells in a 3D structure and can influence the size and shape of developing structures. Although epithelial cells in a monolayer divide parallel to the monolayer, morphogens such as hepatocyte growth factor can induce spindle repositioning, which forces a cell to change the plane of division with the possibility of developing branched structures (Yu et al. 2003). Cadherins provide the cortical cue for positioning of mitotic spindles, and this function of cadherin is thought to be independent of its role during establishment of cell polarity (den Elzen et al 2009). During development of the renal tubule, 95% of dividing epithelial cells orient their spindles parallel to the tubule axis, which mediates tubule elongation (Fischer et al. 2006). The autosomal recessive form of polycystic kidney disease is characterized by increase in renal tube diameter and cyst formation, which correlate with more than 70% of cells losing their ability to orient their division parallel to the axis of the tubule (Figure 1a).
Cell polarity proteins are emerging as important regulators of spindle orientation during morphogenesis. Development of lumen in the intestinal epithelial cell line Caco-2 requires spindle positioning perpendicular to the centroid of the cyst; disruption of CDC42 or PARD6b randomizes spindle orientation and results in formation of multiple lumens with normal apical-basal polarization (Durgan et al. 2011, Jaffe et al. 2008). TUBA, a CDC42 GEF, localizes to the apical cortex of dividing cells and regulates orientation of mitotic spindle and lumen formation. As with CDC42, loss of TUBA results in development of multilumens (Qin et al. 2010). In addition to functioning at the apical cortex, CDC42 and its GEF, Intersectin 2 (ITCN2), localize to centrosomes and regulate correct spindle orientation and lumen formation during morphogenesis (Rodriguez-Fraticelli et al. 2010). NUMA/LGN/Gα i localizes to cortex and is required for spindle orientation during cystogenesis. Depletion of LGN results in spindle orientiation defects and disruption of normal lumen formation (Du et al. 2002, Zheng et al. 2010). The NUMA/LGN/Gα i functions as a receptor for the dynein-dynactin complex (Gaetz & Kapoor 2004, Merdes et al. 2000). LGN localizes to both the apical and basal cortex but is excluded from the middle of the cell (proximal to the metaphase plate), which facilitates attachment of astral microtubules and orientation of the mitotic spindle in HeLa cells (Gaglio et al. 1995). PARD6G is required for spindle orientation during neurulation in zebrafish. Morphogenesis of the neural tube cavity, the neurocoel, requires PARD6G. Mutations in PARD6G and CDC42 result in mitotic spindle orientation defects during neural tube morphogenesis (Kieserman & Wallingford 2009, Munson et al. 2008). These observations demonstrate that positioning of the mitotic spindle in ductal structures is an ordered process, and they also highlight the need for better understanding of the molecular interactions that regulate this process. Furthermore, there is a great deal we do not know; for example, the role of mitotic spindle positioning during development of branched ducts and during disruption of normal ductal structure in cancer is not known..
Reversible Mesenchymal Transitions
Stimulation of MDCK cysts with hepatocyte growth factor induces formation of branched, tubule-like structures, a process that models renal tubulogenesis during embryonic development. The cells at the branching site adopt a mesenchymal state and switch from apical-basal polarity to front-rear polarity (Maeshima et al. 2000, Montesano et al. 1991, Yu et al. 2003, Zegers et al. 2003) (Figure 1a). The front-rear polarized cells have distinctive organization between the leading edge and the trailing edge, which differ in various properties including lipid composition and organelle orientation. The leading edge is enriched with phosphatidylinositol-3,4,5-triphosphate [PtdIns(3,4,5)P3]owing to uneven distribution of PI3K and PTEN in the cells (Funamoto et al. 2002). Polarity proteins act in concert with small GTPase to regulate cytoskeleton dynamics at the leading edge. PARD3, aPKC, and V12RAP1 colocalize during V12RAP1 induced polarization of T cells (Gerard et al 2007). Dlg and Scribble accumulate at the uropod; however, little is known about their interaction with RhoGTP in migrating cells (Iden & Collard 2008). Re-establishment of apical-basal polarity and regression of mesenchymal properties during establishment of a tubule structure at branch point requires a poorly understood sequence of events (Figure 3b).
In addition to branching morphogenesis, all cell ingression events are accomplished by EMT. For example, during gastrulation, invaginated mesoderm precursor cells undergo EMT to form mesoderm cells that dissociate, change shape, and occupy space between the epiblast and the visceral endoderm. The formation of definitive endoderm and morphogenesis of different organs require the cells to epithelialize, aggregate, and reestablish cell adhesions and apical-basal polarity (Ferrer-Vaquer et al. 2010). The role cell polarity proteins play during this process is poorly understood.
EMT is thought to play important roles during the development of metastasis in epithelial-derived cancers. Transcriptional repressors that induce EMT, ZEB1 and SNAI1/SNAI2, bind directly to the promoter elements of cell polarity proteins CRB3 and LLGL2 and repress their mRNA expression (Davalos et al. 2011). Apart from their direct regulation by EMT core signals, cell polarity proteins also regulate migration and invasion by modulating signaling pathways. For example, TGFβ binds and phosphorylates PARD6A at TJs, enabling recruitment of the E3 ubiquitin ligase SMURF1 to degrade RHOA. Importantly, in mammary gland tumors, blockade of Par6 phosphorylation suppresses metastasis to the lung (Viloria-Petit et al. 2009). The metastatic cells supposedly revert to an epithelial phenotype by undergoing a mesenchymal to epithelial transition at the secondary metastatic site. The plasticity with which epithelial cells transition between the mesenchymal and the epithelial states is critical for both normal development and cancer progression. The precise mechanisms that regulate these transitions need to be understood.
Cell Polarity Phenotypic Plasticity
Our recent results show that disruption of multiple polarity proteins in oncogene-naïve epithelial cells induces phenotypic plasticity, in which the cells acquire invasive behavior in response to a tumorlike microenvironment such as rigid ECM and inflammatory cytokines (Chatterjee et al 2012). However, the cells behave like epithelial cells under normal growth factor conditions in a bed of soft ECM. This plastic state may be similar to the previously described partial-EMT, or metastable or hybrid state (Thiery et al. 2009), which is thought to allow cells to transition between an epithelial and a mesenchymal phenotype. Tumor epithelia in basal type, an aggressive form of breast cancer, coexpress both epithelial and mesenchymal proteins (Sarrio et al. 2008), which suggests that the hybrid state is observed in human cancers.
The plastic differentiation state can provide a significant advantage for migrating/invading epithelial cells. Migrating cells need to reorganize their cytoskeleton, and vesicle trafficking and targeting machinery in order to transit from apical-basal polarity to front-rear polarity (Nelson 2009). During this transition, polarity proteins function as core signaling proteins that are retooled and rewired (Nelson 2009). Because many of the cell polarity proteins are signaling scaffolds, changes in expression or localization of a single polarity protein can significantly affect multiple downstream effectors and induce changes in cell behavior. In addition, transient changes in expression of one polarity protein can induce changes in localization and function of other cell polarity proteins, which together can affect cell behavior during morphogenesis, development, and cancer metastasis.
Summary and perspectives
Epithelial cells alter their behavior dynamically during development and normal homeostasis, however, we know little about the mechanisms that regulate the dynamic plasticity of cell behavior during normal physiology. It is likely that changes in this dynamic equilibrium will drive changes in cell biological properties associated with cancer. In this regard, cell polarity proteins are an underexplored class of molecules that integrate diverse signals to control the behavior of cells during division, migration, and morphogenesis. How changes in cell polarity proteins alter behavior of epithelial cells and the role that plays in cancer remains to be investigated.
There is also a significant need to develop ways to define and quantify polarity changes in cancer. The conventional method of monitoring the localization of membrane proteins such as E-cadherin, ZO-1 and mucins to monitor apical-basal polarity is misleading and inconclusive. In fact, there are multiple cancer-derived epithelial cell lines that possess normal membrane polarity, but lack the ability to undergo normal 3D morphogenesis to develop into proliferation-arrested, polarized duct-like structures, and retain the ability to form tumors in vivo. It is likely that in these cancer-derived cell lines, one or more of the different types of polarity is dysregulated, and hence demonstrates a need to create and use more comprehensive ways to define cell polarity changes in cancer. Exploring changes in the ability of cancer epithelial cells to establish and maintain intracellular organalle polarity will likely identify new ways of monitoring changes in cell polarity at the level of a single cell. In addition, to cell polarity, cancer tissues have loss of tissue polarity, however, it is not clear how changes in tissue organization can be defined and quantified. In this regard, developing methods to monitor changes in PCP will likely identify novel opportunites to monitor changes in tissue order.
In this review, we have attempted to summarize the different roles cell polarity proteins play during normal physiology and identify the initial evidence that supports a role for cell polarity proteins during the initiation and progression of cancer. We find that most of the evidence so far either demonstrates a correlation between dysregulation of cell polarity and cancer, or supports the notion that changes in polarity proteins can function as a cooperating event during the initiation and progression of cancer. Moving forward, it would be important to determine if dysregulation of cell polarity proteins is selected-for during cancer initiation and progression, and if dysregulation of polarity proteins alone can initiate cancer by directly activating or inactivativing pathways(s) implicated in cancer. Such an understanding can allow identification of therapeutic opportunities for restoring polarity and promoting differentiation of cancer epithelia.
Acknowledgments
We would like to thank Jennifer Haynes for critical reading of this manuscript. This work was supported by CA098830, BC075024, Era of Hope Scholar award from DOD Breast Cancer Research Program; Lee K Margaret Lau Chair for breast cancer research and Campbell Family Institute For Breast cancer research to SKM. Support from the Ontario Institute for Cancer Research through funding provided by the Government of Ontario to PCB. This was also funded in part by the Ontario Ministry of Health and Long Term Care. The views expressed do not necessarily reflect those of the OMOHLTC
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Albrechtsen R, Nielsen M, Wewer U, Engvall E, Ruoslahti E. Basement-membrane changes in breast-cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981;41:5076–81. [PubMed] [Google Scholar]
- An CH, Kim YR, Kim HS, Kim SS, Yoo NJ, Lee SH. Frameshift mutations of vacuolar protein sorting genes in gastric and colorectal cancers with microsatellite instability. Hum Pathol. 2011;43:40–47. doi: 10.1016/j.humpath.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8:1235–45. doi: 10.1038/ncb1485. [DOI] [PubMed] [Google Scholar]
- Barr FA, Egerer J. Golgi positioning: are we looking at the right MAP? J Cell Biol. 2005;168:993–98. doi: 10.1083/jcb.200501088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–80. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–36. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr Biol. 2010;20:803–10. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–45. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–84. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell VA, Jessen JR. The planar cell polarity protein Van Gogh-Like 2 regulates tumor cell migration and matrix metalloproteinase-dependent invasion. Cancer Lett. 2010;287:54–61. doi: 10.1016/j.canlet.2009.05.041. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Valdes J, Shoshani L, Contreras RG. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol. 1998;60:161–77. doi: 10.1146/annurev.physiol.60.1.161. [DOI] [PubMed] [Google Scholar]
- Cha SW, Tadjuidje E, Wylie C, Heasman J. The roles of maternal Vangl2 and aPKC in Xenopus oocyte and embryo patterning. Development. 2011;138:3989–4000. doi: 10.1242/dev.068866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DW, Chan CY, Yam JWP, Ching YP, Ng IOL. Prickle-1 negatively regulates Wnt/β-catenin pathway by promoting dishevelled ubiquitination/degradation in liver cancer. Gastroenterology. 2006;131:1218–27. doi: 10.1053/j.gastro.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7:262–69. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- Chen X, Macara IG. Par-3 mediates the inhibition of LIM kinase 2 to regulate cofilin phosphorylation and tight junction assembly. J Cell Biol. 2006;172:671–78. doi: 10.1083/jcb.200510061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman DR. Decreased mutual adhesiveness, a property of cells from squamous cell carcinomas. Cancer Res. 1944;4:625–29. [Google Scholar]
- Courbard JR, Djiane A, Wu J, Mlodzik M. The apical/basal-polarity determinant Scribble cooperates with the PCP core factor Stbm/Vang and functions as one of its effectors. Dev Biol. 2009;333:67–77. doi: 10.1016/j.ydbio.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, et al. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene. 2011;31:2062–74. doi: 10.1038/onc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:1763–76. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–88. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003a;30:256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Debnath J, Walker SJ, Brugge JS. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J Cell Biol. 2003b;163:315–26. doi: 10.1083/jcb.200304159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaens C, Cassio D. Spatiotemporal expression of catenins, ZO-1, and occludin during early polarization of hepatic WIF-B9 cells. Am J Physiol Cell Physiol. 2001;280:C527–39. doi: 10.1152/ajpcell.2001.280.3.C527. [DOI] [PubMed] [Google Scholar]
- Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, et al. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am J Physiol Cell Physiol. 2008;295:C545–56. doi: 10.1152/ajpcell.00097.2008. [DOI] [PubMed] [Google Scholar]
- Devenport D, Oristian D, Heller E, Fuchs E. Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nat Cell Biol. 2011;13:893–902. doi: 10.1038/ncb2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhom G. The cancer cell and the connective tissue. A historical retrospect. Der Pathol. 1994;15:271–78. doi: 10.1007/s002920050054. [DOI] [PubMed] [Google Scholar]
- Djiane A, Yogev S, Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005;121:621–31. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci. 1998;23:307–11. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- Du D, Xu F, Yu L, Zhang C, Lu X, et al. The tight junction protein, occludin, regulates the directional migration of epithelial cells. Dev Cell. 2010;18:52–63. doi: 10.1016/j.devcel.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Du QS, Taylor L, Compton DA, Macara IG. LGN blocks the ability of NuMA to bind and stabilize microtubules: a mechanism for mitotic spindle assembly regulation. Curr Biol. 2002;12:1928–33. doi: 10.1016/s0960-9822(02)01298-8. [DOI] [PubMed] [Google Scholar]
- Durgan J, Kaji N, Jin D, Hall A. Par6B and atypical PKC regulate mitotic spindle orientation during epithelial morphogenesis. J Biol Chem. 2011;286:12461–74. doi: 10.1074/jbc.M110.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer zu Brickwedde MK, et al. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) EMBO J. 2001;20:3738–48. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder AM, Sui X, Rosen DG, Nolden LK, Cheng KW, et al. Atypical PKCι contributes to poor prognosis through loss of apical-basal polarity and Cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:12519–24. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Elia N, Lippincott-Schwartz J. Culturing MDCK cells in three dimensions for analyzing intracellular dynamics. Curr Protoc Cell Biol. 2001;43(4):22.1–18. doi: 10.1002/0471143030.cb0422s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Hurd TW, Liu CJ, Straight SW, Weimbs T, et al. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol. 2004;14:1451–61. doi: 10.1016/j.cub.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, et al. The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development. 2003;130:763–74. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- Ferrer-Vaquer A, Viotti M, Hadjantonakis A-K. Transitions between epithelial and mesenchymal states and the morphogenesis of the early mouse embryo. Cell Adhes Migr. 2010;4:447–57. doi: 10.4161/cam.4.3.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- Folsch H, Pypaert M, Maday S, Pelletier L, Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol. 2003;163:351–62. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foty RA, Steinberg MS. Measurement of tumor cell cohesion and suppression of invasion by E-or P-cadherin. Cancer Res. 1997;57:5033–36. [PubMed] [Google Scholar]
- Francis R, Xu X, Park H, Wei CJ, Chang S, et al. Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PLoS ONE. 2011;6:e26379. doi: 10.1371/journal.pone.0026379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–23. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Gaetz J, Kapoor TM. Dynein/dynactin regulate metaphase spindle length by targeting depolymerizing activities to spindle poles. J Cell Biol. 2004;166:465–71. doi: 10.1083/jcb.200404015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T, Saredi A, Compton DA. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J Cell Biol. 1995;131:693–708. doi: 10.1083/jcb.131.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol. 2002;4:605–9. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–76. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol. 2008;18:1631–38. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Giepmans BNG, Verlaan I, Moolenaar WH. Connexin-43 interactions with ZO-1 and α- and β-tubulin. Cell Commun Adhes. 2001;8:219–23. doi: 10.3109/15419060109080727. [DOI] [PubMed] [Google Scholar]
- Gravotta D, Deora A, Perret E, Oyanadel C, Soza A, et al. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci USA. 2007;104:1564–69. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier A, Fonlupt P, Morand I, Rabilloud R, Audebet C, et al. Gap junctions and cell polarity: Connexin32 and connexin43 expressed in polarized thyroid epithelial cells assemble into separate gap junctions, which are located in distinct regions of the lateral plasma membrane domain. J Cell Sci. 1995;108:2609–17. doi: 10.1242/jcs.108.7.2609. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Handler JS. Overview of epithelial polarity. Annu Rev Physiol. 1989;51:729–40. doi: 10.1146/annurev.ph.51.030189.003501. [DOI] [PubMed] [Google Scholar]
- Hao Y, Boyd L, Seydoux G. Stabilization of cell polarity by the C. elegans RING protein PAR-2. Dev Cell. 2006;10:199–208. doi: 10.1016/j.devcel.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev Cell. 2007;12:727–38. doi: 10.1016/j.devcel.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJC, Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J Cell Biol. 2004;167:135–47. doi: 10.1083/jcb.200406024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, et al. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13:49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Yoshinaga S, Takeya R, Suzuki NN, Horiuchi M, et al. Structure of a cell polarity regulator, a complex between atypical PKC and Par6 PB1 domains. J Biol Chem. 2005;280:9653–61. doi: 10.1074/jbc.M409823200. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–26. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hirose T, Izumi Y, Nagashima Y, Tamai-Nagai Y, Kurihara H, et al. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J Cell Sci. 2002;115:2485–95. doi: 10.1242/jcs.115.12.2485. [DOI] [PubMed] [Google Scholar]
- Huang L, Muthuswamy SK. Polarity protein alterations in carcinoma: a focus on emerging roles for polarity regulators. Curr Opin Genet Dev. 2010;20:41–50. doi: 10.1016/j.gde.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol. 2003;5:137–42. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–19. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol. 2008;9:846–59. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- Imhof BA, Vollmers HP, Goodman SL, Birchmeier W. Cell-cell interaction and polarity of epithelial cells: specific perturbation using a monoclonal antibody. Cell. 1983;35:667–75. doi: 10.1016/0092-8674(83)90099-5. [DOI] [PubMed] [Google Scholar]
- Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–4. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Nakayama M, Nishimura T, Fujisue S, Nishioka T, et al. Identification of focal adhesion kinase (FAK) and phosphatidylinositol 3-kinase (PI3-kinase) as Par3 partners by proteomic analysis. Cytoskeleton. 2010;67:297–308. doi: 10.1002/cm.20444. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell. 2004;15:2639–51. doi: 10.1091/mbc.E04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, et al. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–33. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Betson M, Mulloy R, Foster R, Levay M, et al. p190A RhoGAP is a glycogen synthase kinase-3-β substrate required for polarized cell migration. J Biol Chem. 2008;283:20978–88. doi: 10.1074/jbc.M802588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinn Y, Inase N. Connexin 43, E-cadherin, β-catenin and ZO-1 expression, and aberrant methylation of the connexin43 gene in NSCLC. Anticancer Res. 2010;30:2271–78. [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–39. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Ziomek CA. Induction of polarity in mouse 8-cell blastomeres: specificity, geometry, and stability. J Cell Biol. 1981;91:303–8. doi: 10.1083/jcb.91.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp CM, Tan TT, Mathew R, Nelson D, Mukherjee C, et al. Role of the polarity determinant Crumbs in suppressing mammalian epithelial tumor progression. Cancer Res. 2008;68:4105–15. doi: 10.1158/0008-5472.CAN-07-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausalya PJ, Reichert M, Hunziker W. Connexin45 directly binds to ZO-1 and localizes to the tight junction region in epithelial MDCK cells. FEBS Lett. 2001;505:92–96. doi: 10.1016/s0014-5793(01)02786-7. [DOI] [PubMed] [Google Scholar]
- Keating TJ, Peloquin JG, Rodionov VI, Momcilovic D, Borisy GG. Microtubule release from the centrosome. Proc Natl Acad Sci USA. 1997;94:5078–83. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellokumpu S, Sormunen R, Kellokumpu I. Abnormal glycosylation and altered Golgi structure in colorectal cancer: dependence on intra-Golgi pH. FEBS Lett. 2002;516:217–24. doi: 10.1016/s0014-5793(02)02535-8. [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–20. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieserman EK, Wallingford JB. In vivo imaging reveals a role for Cdc42 in spindle positioning and planar orientation of cell divisions during vertebrate neural tube closure. J Cell Sci. 2009;122:2481–90. doi: 10.1242/jcs.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- Komura H, Ogita H, Ikeda W, Mizoguchi A, Miyoshi J, Takai Y. Establishment of cell polarity by afadin during the formation of embryoid bodies. Genes Cells. 2008;13:79–90. doi: 10.1111/j.1365-2443.2007.01150.x. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Kronebusch PJ, Rose JK, Singer SJ. A critical role for the polarization of membrane recycling in cell motility. Cell Motil Cytoskelet. 1987;8:182–89. doi: 10.1002/cm.970080210. [DOI] [PubMed] [Google Scholar]
- Kwaepila N, Burns G, Leong ASY. Immunohistological localisation of human FAT1 (hFAT) protein in 326 breast cancers. Does this adhesion molecule have a role in pathogenesis? Pathology. 2006;38:125–31. doi: 10.1080/00313020600559975. [DOI] [PubMed] [Google Scholar]
- Lacy D. The Golgi apparatus in neurons and epithelial cells of the common limpet Patella vulgata. J Biophys Biochem Cytol. 1957;3:779–96. doi: 10.1083/jcb.3.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Nishanian TG, Mood K, Bong YS, Daar IO. EphrinB1 controls cell-cell junctions through the Par polarity complex. Nat Cell Biol. 2008;10:979–86. doi: 10.1038/ncb1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Medina E, et al. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell. 2004;15:1324–33. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–47. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Cole NB, Marotta A, Conrad PA, Bloom GS. Kinesin is the motor for microtubule-mediated Golgi-to-ER membrane traffic. J Cell Biol. 1995;128:293–306. doi: 10.1083/jcb.128.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Chapin SJ, Weimbs T, Komuves LG, Bennett MK, Mostov KE. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1996;7:2007–18. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–39. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- Lu XF, Feng XJ, Man XB, Yang G, Tang L, et al. Aberrant splicing of Hugl-1 is associated with hepatocellular carcinoma progression. Clin Cancer Res. 2009;15:3287–96. doi: 10.1158/1078-0432.CCR-08-2078. [DOI] [PubMed] [Google Scholar]
- Maeshima A, Zhang YQ, Furukawa M, Naruse T, Kojima I. Hepatocyte growth factor induces branching tubulogenesis in MDCK cells by modulating the activin-follistatin system. Kidney Int. 2000;58:1511–22. doi: 10.1046/j.1523-1755.2000.00313.x. [DOI] [PubMed] [Google Scholar]
- Manneville JB, Jehanno M, Etienne-Manneville S. Dlg1 binds GKAP to control dynein association with microtubules, centrosome positioning, and cell polarity. J Cell Biol. 2010;191:585–98. doi: 10.1083/jcb.201002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimi P, Narayan N, Thomas M, Gammoh N, Strand S, et al. Regulation of the hDlg/hScrib/Hugl-1 tumour suppressor complex. Exp Cell Res. 2008;314:3306–17. doi: 10.1016/j.yexcr.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Masuda-Hirata M, Suzuki A, Amano Y, Yamashita K, Ide M, et al. Intracellular polarity protein PAR-1 regulates extracellular laminin assembly by regulating the dystroglycan complex. Genes Cells. 2009;14:835–50. doi: 10.1111/j.1365-2443.2009.01315.x. [DOI] [PubMed] [Google Scholar]
- Mathew D, Gramates LS, Packard M, Thomas U, Bilder D, et al. Recruitment of Scribble to the synaptic scaffolding complex requires GUK-holder, a novel DLG binding protein. Curr Biol. 2002;12:531–39. doi: 10.1016/s0960-9822(02)00758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MA, McKinley RF, Harris TJ. Independent cadherin-catenin and Bazooka clusters interact to assemble adherens junctions. J Cell Biol. 2009;185:787–96. doi: 10.1083/jcb.200812146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H. Planar cell polarity: Keeping hairs straight is not so simple. Cold Spring Harb Perspect Biol. 2010;2:a003376. doi: 10.1101/cshperspect.a003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–45. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A, Heald R, Samejima K, Earnshaw WC, Cleveland DW. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J Cell Biol. 2000;149:851–61. doi: 10.1083/jcb.149.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens AEE, Rygiel TP, Olivo C, van der Kammen R, Collard JG. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol. 2005;170:1029–37. doi: 10.1083/jcb.200502129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Schaller G, Orci L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell. 1991;66:697–711. doi: 10.1016/0092-8674(91)90115-f. [DOI] [PubMed] [Google Scholar]
- Moore R, Boyd L. Analysis of RING finger genes required for embryogenesis in C. elegans. Genesis. 2004;38:1–12. doi: 10.1002/gene.10243. [DOI] [PubMed] [Google Scholar]
- Morais-de-Sa E, Mirouse V, St Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141:509–23. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalewski S, Thyberg J, Lohmander S, Friberg U. Influence of colchicine and vinblastine on the Golgi complex and matrix deposition in chondrocyte aggregates: an ultrastructural study. Exp Cell Res. 1975;95:440–54. doi: 10.1016/0014-4827(75)90569-8. [DOI] [PubMed] [Google Scholar]
- Munson C, Huisken J, Bit-Avragim N, Kuo T, Dong PD, et al. Regulation of neurocoel morphogenesis by Pard6γb. Dev Biol. 2008;324:41–54. doi: 10.1016/j.ydbio.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P, Edgar D. Regulation of programmed cell death by basement membranes in embryonic development. J Cell Biol. 2000;150:1215–21. doi: 10.1083/jcb.150.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch A, Cohen D, Yeaman C, Nelson WJ, Rodriguez-Boulan E, Brennwald PJ. Mammalian homolog of Drosophila tumor suppressor lethal (2) giant larvae interacts with basolateral exocytic machinery in Madin-Darby canine kidney cells. Mol Biol Cell. 2002;13:158–68. doi: 10.1091/mbc.01-10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschler J, Streuli CH. Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb Perspect Biol. 2010;2:a003202. doi: 10.1101/cshperspect.a003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–92. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllymaki SM, Teravainen TP, Manninen A. Two distinct integrin-mediated mechanisms contribute to apical lumen formation in epithelial cells. PLoS ONE. 2011;6:e19453. doi: 10.1371/journal.pone.0019453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S. Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells. 2002;7:1161–71. doi: 10.1046/j.1365-2443.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- Nagumo Y, Han J, Bellila A, Isoda H, Tanaka T. Cofilin mediates tight-junction opening by redistributing actin and tight-junction proteins. Biochem Biophys Res Commun. 2008;377:921–25. doi: 10.1016/j.bbrc.2008.10.071. [DOI] [PubMed] [Google Scholar]
- Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, et al. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24:4330–39. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- Nejsum LN, Nelson WJ. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J Cell Biol. 2007;178:323–35. doi: 10.1083/jcb.200705094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb Perspect Biol. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Yeaman C. Protein trafficking in the exocytic pathway of polarized epithelial cells. Trends Cell Biol. 2001;11:483–86. doi: 10.1016/s0962-8924(01)02145-6. [DOI] [PubMed] [Google Scholar]
- Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, et al. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res. 2008;68:8201–09. doi: 10.1158/0008-5472.CAN-07-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–38. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Zegers MM, Mostov KE. Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002a;3:531–37. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Zegers MMP, Mostov KE. Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002b;3:531–37. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- O’Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, et al. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene. 2010;29:34–44. doi: 10.1038/onc.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–54. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Ooshio T, Fujita N, Yamada A, Sato T, Kitagawa Y, et al. Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. J Cell Sci. 2007;120:2352–65. doi: 10.1242/jcs.03470. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Peng YH, Yang WK, Lin WH, Lai TT, Chien CT. Nak regulates Dlg basal localization in Drosophila salivary gland cells. Biochem Biophys Res Commun. 2009;382:108–13. doi: 10.1016/j.bbrc.2009.02.139. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–68. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocha SM, Wassmer T, Niehage C, Hoflack B, Knust E. Retromer controls epithelial cell polarity by trafficking the apical determinant Crumbs. Curr Biol. 2011;21:1111–17. doi: 10.1016/j.cub.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Qi C, Zhu YT, Hu LP, Zhu YJ. Identification of Fat4 as a candidate tumor suppressor gene in breast cancers. Int J Cancer. 2009;124:793–98. doi: 10.1002/ijc.23775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol. 2005;171:1061–71. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Meisen WH, Hao Y, Macara IG. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol. 2010;189:661–69. doi: 10.1083/jcb.201002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez IB, Lowe M. Golgins and GRASPs: holding the Golgi together. Semin Cell Dev Biol. 2009;20:770–79. doi: 10.1016/j.semcdb.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Rehder D, Iden S, Nasdala I, Wegener J, Brickwedde MKMZ, et al. Junctional adhesion molecule-A participates in the formation of apico-basal polarity through different domains. Exp Cell Res. 2006;312:3389–403. doi: 10.1016/j.yexcr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ren DY, Minami Y, Nishita M. Critical role of Wnt5a-Ror2 signaling in motility and invasiveness of carcinoma cells following Snail-mediated epithelial-mesenchymal transition. Genes Cells. 2011;16:304–15. doi: 10.1111/j.1365-2443.2011.01487.x. [DOI] [PubMed] [Google Scholar]
- Rios RM, Sanchis A, Tassin AM, Fedriani C, Bornens M. GMAP-210 recruits gamma-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell. 2004;118:323–35. doi: 10.1016/j.cell.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli AE, Vergarajauregui S, Eastburn DJ, Datta A, Alonso MA, et al. The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J Cell Biol. 2010;189:725–38. doi: 10.1083/jcb.201002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeth JF, Sawyer JK, Wilner DA, Peifer M. Rab11 helps maintain apical crumbs and adherens junctions in the Drosophila embryonic ectoderm. PLoS ONE. 2009;4:e7634. doi: 10.1371/journal.pone.0007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski AA, Singer SJ. Associations of elements of the Golgi apparatus with microtubules. J Cell Biol. 1984;99:1092–100. doi: 10.1083/jcb.99.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MG. Inheriting the Golgi. Cell. 1999;99:559–62. doi: 10.1016/s0092-8674(00)81544-5. [DOI] [PubMed] [Google Scholar]
- Sadeqzadeh E, de Bock CE, Zhang XD, Shipman KL, Scott NM, et al. Dual processing of FAT1 cadherin protein by human melanoma cells generates distinct protein products. J Biol Chem. 2011;286:28181–91. doi: 10.1074/jbc.M111.234419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–97. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- Schmoranzer J, Fawcett JP, Segura M, Tan S, Vallee RB, et al. Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr Biol. 2009;19:1065–74. doi: 10.1016/j.cub.2009.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoranzer J, Kreitzer G, Simon SM. Migrating fibroblasts perform polarized, microtubule-dependent exocytosis towards the leading edge. J Cell Sci. 2003;116:4513–19. doi: 10.1242/jcs.00748. [DOI] [PubMed] [Google Scholar]
- Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–79. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- Scott KL, Kabbarah O, Liang MC, Ivanova E, Anagnostou V, et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459:1085–90. doi: 10.1038/nature08109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–35. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Shirayoshi Y, Okada TS, Takeichi M. The calcium-dependent cell-cell adhesion system regulates inner cell mass formation and cell surface polarization in early mouse development. Cell. 1983;35:631–38. doi: 10.1016/0092-8674(83)90095-8. [DOI] [PubMed] [Google Scholar]
- Sonee M, Barrón E, Yarber FA, Hamm-Alvarez SF. Taxol inhibits endosomal-lysosomal membrane trafficking at two distinct steps in CV-1 cells. Am J Physiol Cell Physiol. 1998;275:C1630–C39. doi: 10.1152/ajpcell.1998.275.6.C1630. [DOI] [PubMed] [Google Scholar]
- Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, et al. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26:2387–98. doi: 10.1128/MCB.26.6.2387-2398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Bio. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Asymmetric localisation of planar polarity proteins: mechanisms and consequences. Semin Cell Dev Biol. 2009;20:957–63. doi: 10.1016/j.semcdb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Subramanian VS, Marchant JS, Ye D, Ma TY, Said HM. Tight junction targeting and intracellular trafficking of occludin in polarized epithelial cells. Am J Physiol Cell Physiol. 2007;293:C1717–26. doi: 10.1152/ajpcell.00309.2007. [DOI] [PubMed] [Google Scholar]
- Takekuni K, Ikeda W, Fujito T, Morimoto K, Takeuchi M, et al. Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem. 2003;278:5497–500. doi: 10.1074/jbc.C200707200. [DOI] [PubMed] [Google Scholar]
- Tang N, Marshall WF, McMahon M, Metzger RJ, Martin GR. Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. Science. 2011;333:342–45. doi: 10.1126/science.1204831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol. 2012;28 doi: 10.1146/annurev-cellbio-092910-154033. In press. [DOI] [PubMed] [Google Scholar]
- Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–99. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Vaisberg EA, Grissom PM, McIntosh JR. Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J Cell Biol. 1996;133:831–42. doi: 10.1083/jcb.133.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viloria-Petit AM, David L, Jia JY, Erdemir T, Bane AL, et al. A role for the TGFβ-Par6 polarity pathway in breast cancer progression. Proc Natl Acad Sci USA. 2009;106:14028–33. doi: 10.1073/pnas.0906796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther RF, Pichaud F. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr Biol. 2010;20:1065–74. doi: 10.1016/j.cub.2010.04.049. [DOI] [PubMed] [Google Scholar]
- Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990;95(Pt 1):137–51. doi: 10.1242/jcs.95.1.137. [DOI] [PubMed] [Google Scholar]
- Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–71. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- Wehland J, Henkart M, Klausner R, Sandoval IV. Role of microtubules in the distribution of the Golgi apparatus: effect of taxol and microinjected anti-α-tubulin antibodies. Proc Natl Acad Sci USA. 1983;80:4286–90. doi: 10.1073/pnas.80.14.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir ML, Oppizzi ML, Henry MD, Onishi A, Campbell KP, et al. Dystroglycan loss disrupts polarity and β-casein induction in mammary epithelial cells by perturbing laminin anchoring. J Cell Sci. 2006;119:4047–58. doi: 10.1242/jcs.03103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Takeda S, Nakata T, Noda Y, Tanaka Y, Hirokawa N. Role of KIFC3 motor protein in Golgi positioning and integration. J Cell Biol. 2002;158:293–303. doi: 10.1083/jcb.200202058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Puri S, Linstedt AD. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell. 2009;20:1728–36. doi: 10.1091/mbc.E08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, et al. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036–46. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- Yoshihara K, Ikenouchi J, Izumi Y, Akashi M, Tsukita S, Furuse M. Phosphorylation state regulates the localization of Scribble at adherens junctions and its association with E-cadherin-catenin complexes. Exp Cell Res. 2011;317:413–22. doi: 10.1016/j.yexcr.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Yu W, O’Brien LE, Wang F, Bourne H, Mostov KE, Zegers MM. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol Biol Cell. 2003;14:748–63. doi: 10.1091/mbc.E02-06-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3:a004911. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–63. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Zegers MMP, Yu W, O’Brien LE, Wang F, Bourne H, Mostov KE. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol Biol Cell. 2003;14:748–63. doi: 10.1091/mbc.E02-06-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K, Yasui K, Gen Y, Dohi O, Wakabayashi N, et al. Defective expression of polarity protein PAR-3 gene (PARD3) in esophageal squamous cell carcinoma. Oncogene. 2009;28:2910–18. doi: 10.1038/onc.2009.148. [DOI] [PubMed] [Google Scholar]
- Zhang H, Macara IG. The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev Cell. 2008;14:216–26. doi: 10.1016/j.devcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang P, Gangar A, Zhang J, Brennwald P, et al. Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J Cell Biol. 2005;170:273–83. doi: 10.1083/jcb.200502055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Zhu H, Wan Q, Liu J, Xiao Z, et al. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J Cell Biol. 2010;189:275–88. doi: 10.1083/jcb.200910021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziomek CA, Johnson MH. Cell-surface interaction induces polarization of mouse 8-cell blastomeres at compaction. Cell. 1980;21:935–42. doi: 10.1016/0092-8674(80)90457-2. [DOI] [PubMed] [Google Scholar]