Abstract
Adult Philadelphia chromosome–positive (Ph+) or BCR-ABL–positive (BCR-ABL+) acute lymphoblastic leukemia (all) is an acute leukemia previously associated with a high relapse rate, short disease-free survival, and poor overall survival. In adults, allogeneic hematopoietic cell transplant in first remission remains the only proven curative strategy for transplant-eligible patients. The introduction of tyrosine kinase inhibitors (tkis) in the treatment of patients with Ph+ or BCR-ABL+ all has significantly improved the depth and duration of complete remission, allowing more patients to proceed to transplantation. Although tkis are now considered a standard of care in this setting, few randomized trials have examined the optimal use of tkis in patients with Ph+ all. Questions of major importance remain, including the best way to administer these medications, the choice of tki to administer, and the schedule and the duration to use. We present the results of a systematic review of the literature with consensus recommendations based on the available evidence.
Keywords: Acute lymphoblastic leukemia, all, hematology, Philadelphia chromosome–positive, Ph+, tyrosine kinase inhibitors
1. INTRODUCTION
Acute lymphoblastic leukemia (all) is the most common cancer in children. Approximately one third of cases occur in adults1,2. Treatment has produced remarkable improvements in outcome for children with all, but the outlook for adults remains poor3. Treatment with chemotherapy alone or with chemotherapy and allogeneic transplantation in adults with all results in 5-year overall survival (os) between 45% and 53% for those less than 60 years of age4 and less than 10% for those 60 years of age and older3. Until recently, the outcome of adults with Philadelphia chromosome–positive (Ph+) all has been even worse, particularly if treatment includes only chemotherapy and not allogeneic transplantation.
The Philadelphia chromosome is the most common chromosomal abnormality in adults with all, and the prevalence of Ph+ or BCR-ABL–positive (BCR-ABL+) all increases with age, from 12.7% in adolescents to 44% in patients 35–44 years of age5. Patients with Ph+ all are older and, at presentation, have higher white blood cell counts and higher blast counts than do patients without the translocation6. Although these patients often respond to remission-induction therapy, rates of complete remission (cr) are lower than they are for all without the Philadelphia chromosome (83% vs. 93%)3, and 5-year os is much poorer because of a high relapse rate. Allogeneic transplantation has been the treatment of choice for eligible adult patients with Ph+ all who have a matched sibling or unrelated donor.
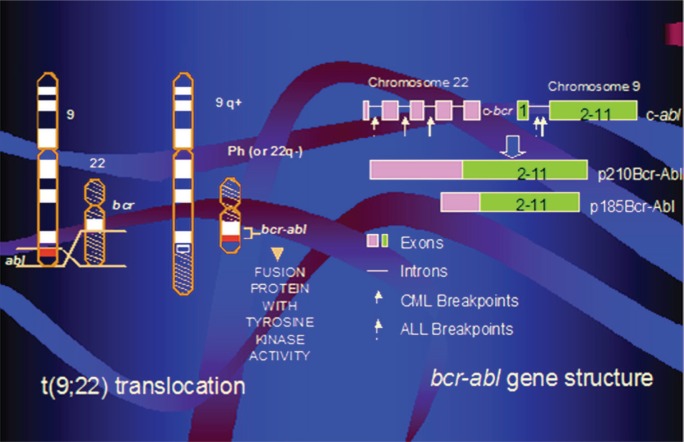
The Philadelphia chromosome arises from a translocation of the ABL gene on chromosome 9 to the BCR gene on chromosome 22, yielding a chimeric BCR-ABL fusion gene7. Depending on the translocation breakpoint, either a p190 Bcr-Abl or a p210 Bcr-Abl protein results, each being a constitutively active tyrosine kinase central to the pathogenesis of all. The aberrant tyrosine kinase alters signalling pathways that control cell proliferation, survival, and self-renewal, leading to leukemogenesis (Figure 1).
FIGURE 1.
Philadelphia chromosome BCR-ABL gene. cml = chronic myeloid leukemia; all = acute lymphoblastic leukemia.
The development of tyrosine kinase inhibitors (tkis), which inhibit the constitutively activated aberrant tyrosine kinases, has revolutionized therapy and outcomes for patients with chronic myeloid leukemia (cml), a disease also characterized by the presence of the Ph+ chromosome8. Although clinically different, Ph+ all shares the constitutive expression of aberrant Bcr-Abl tyrosine kinases central to the pathogenesis of cml. Many reports describing the use of tkis in the treatment of patients with Ph+ all have now been published.
For the present evidence-based guideline, a panel of physicians with expertise in the treatment of acute leukemia (YAM, SC, SL, BL, MM, LS, RT) and in methodology analysis (NS, VP) systematically reviewed the literature examining the role of tkis in the treatment of adults with Ph+ all. The goal of the guideline is to provide evidence-based recommendations for the use of tkis in five key areas. We suggest that a guideline is required because of the absence of adequately powered randomized controlled trials addressing the use of tkis in adults with Ph+ all. Furthermore, although reports of observational studies in patients with Ph+ all are encouraging, they are not nearly as definitive as the corresponding initial observational studies in patients with cml. For those reasons, physicians can benefit from guidance in this area.
Our purpose in creating the present document was to systematically review and synthesize the evidence and then to formulate recommendations about the use of tkis in the management of adults with Ph+ all.
1.1. Target Population
These guidelines are intended for adults with Ph+ or BCR-ABL+ all.
1.2. Target Users
These guidelines are for use by physicians responsible for the care of patients with Ph+ or BCR-ABL+ all.
2. METHODS
2.1. Development of the Guideline
A panel was convened to develop an evidence-based practice guideline for the use of tkis in patients with Ph+ or BCR-ABL+ all. The panel members included hematologists responsible for the care of such patients. Also included on the panel were methodology experts. The panel was asked to identify important clinical questions about the use of tkis in patients with Ph+ or BCR-ABL+ all. The methodology experts refined key questions, which were used to identify search terms (Table i).
TABLE I.
Search strategy
| Ovid medline search |
| (((exp leukemia, lymphoid/ OR precursor cell lymphoblastic leukemia-lymphoma/ OR precursor b-cell lymphoblastic leukemia-lymphoma/ OR precursor t-cell lymphoblastic leukemia-lymphoma/) AND (exp philadelphia chromosome/ OR philadelphia.mp)) OR (Philadelphia-positive acute lymphocytic leukemia OR Ph+ ALL).mp) AND (dasatinib OR nilotinib OR imatinib OR tyrosine kinase inhibitor OR TKI).mp) limited to English language and publication date ≥1998 |
| Ovid embase search |
| ((Philadelphia-positive acute lymphocytic leukemia.mp OR Ph+ ALL.mp OR (Acute Lymphocytic Leukemia/ AND (exp philadelphia chromosome/ OR philadelphia.mp))) AND (dasatinib OR nilotinib OR imatinib OR tyrosine kinase inhibitor OR TKI).mp) limited to English language and publication date ≥1998 |
| Cochrane Library search |
| ((Philadelphia):ti,ab,kw AND (leukemia):ti,ab,kw) OR (PH ALL):ti,ab,kw) AND ((tyrosine kinase inhibitor):ti,ab,kw OR (tki):ti,ab,kw OR (dasatinib):ti,ab,kw OR (nilotinib):ti,ab,kw OR (imatinib):ti,ab,kw) limited to English language and publication date ≥1998 |
| Grey literature search |
Web sites of these international associations were searched for conference and trial abstracts, limited to English language and publication date ≥2004:
|
2.2. Formulation of Recommendations
The panel generated recommendations after group discussion of the evidence. The panel considered both the level of evidence and the quality of the studies. Two moderators with guideline expertise (NS, VP) provided initial guidance to the panel to make recommendations based on a formal grading scheme (described next).
2.2.1. Levels of Evidence and Grading of Recommendations
The levels of evidence and grading of recommendations were adapted from the Canadian Task Force on Preventive Health Care9 and are illustrated in Table ii. For each recommendation, the level of evidence is explicitly stated.
TABLE II.
Levels of evidence and recommendation grades according to the Canadian Task Force on Preventive Health Carea
| Levels of evidence | |
| i | Evidence from one or more randomized controlled trials |
| ii-1 | Evidence from controlled trials without randomization |
| ii-2 | Evidence from cohort or case–control analytic studies, preferably from more than one centre or research group |
| ii-3 | Evidence from comparisons between times or places with or without the intervention (dramatic results in uncontrolled experiments could be included here) |
| iii | Opinions of respected authorities, based on clinical experience; descriptive studies or reports of expert committees |
| Recommendation grades | |
| a | There is good evidence to recommend the action. |
| b | There is fair evidence to recommend the action. |
| c | The existing evidence is conflicting and does not allow for a recommendation for or against use of the action; however, other factors might influence decision-making. |
| d | There is fair evidence to recommend against the action. |
| e | There is good evidence to recommend against action. |
| i | There is insufficient evidence (in quantity or quality, or both) to make a recommendation; however, other factors might influence decision-making. |
Available at http://www.canadiantaskforce.ca.
2.2.2. Consensus
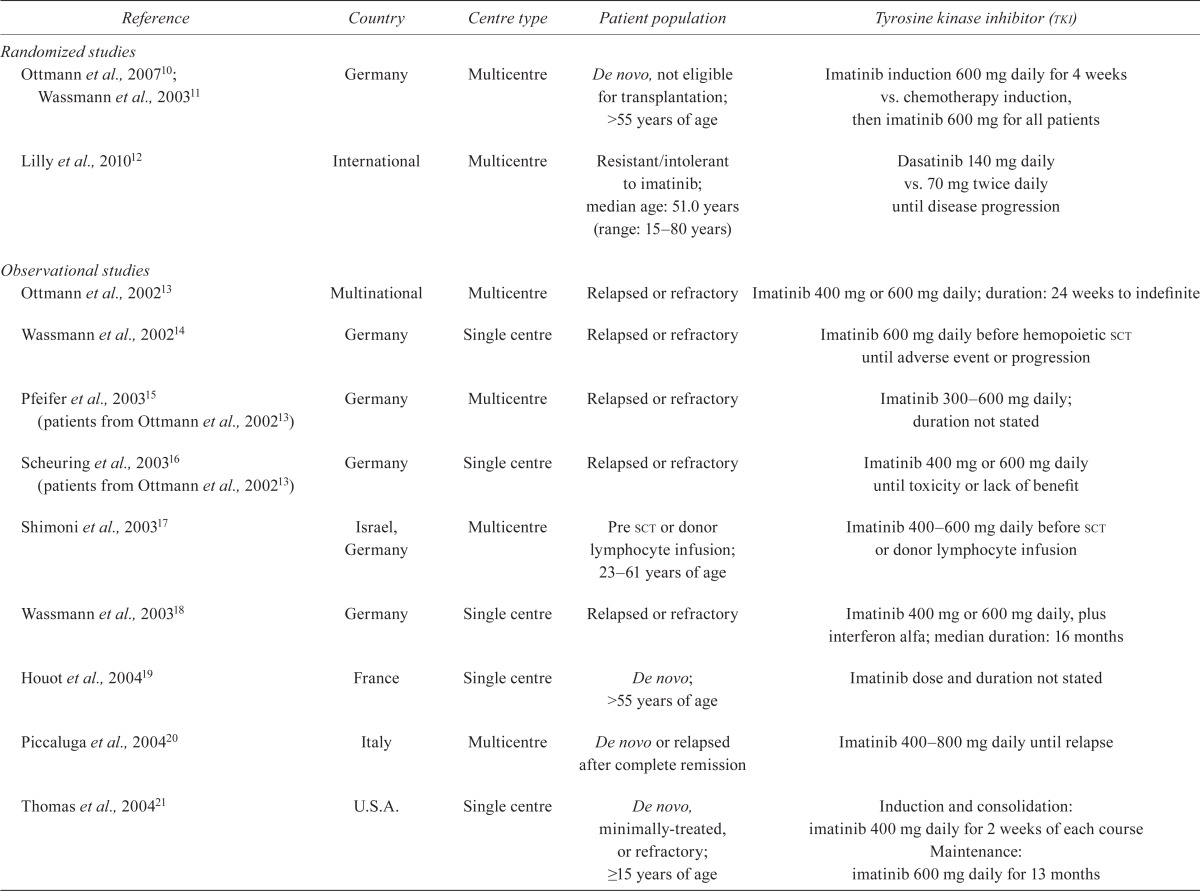
Areas of disagreement were resolved through consensus with all panel members. The guideline is organized by clinical question. The relevant background, a summary of the evidence, the consensus process, and the recommendation statement (accompanied by level of evidence and grade of recommendation) follow each question. All evidence is summarized in the evidence tables (Tables iii–v).
TABLE III.
Study characteristics
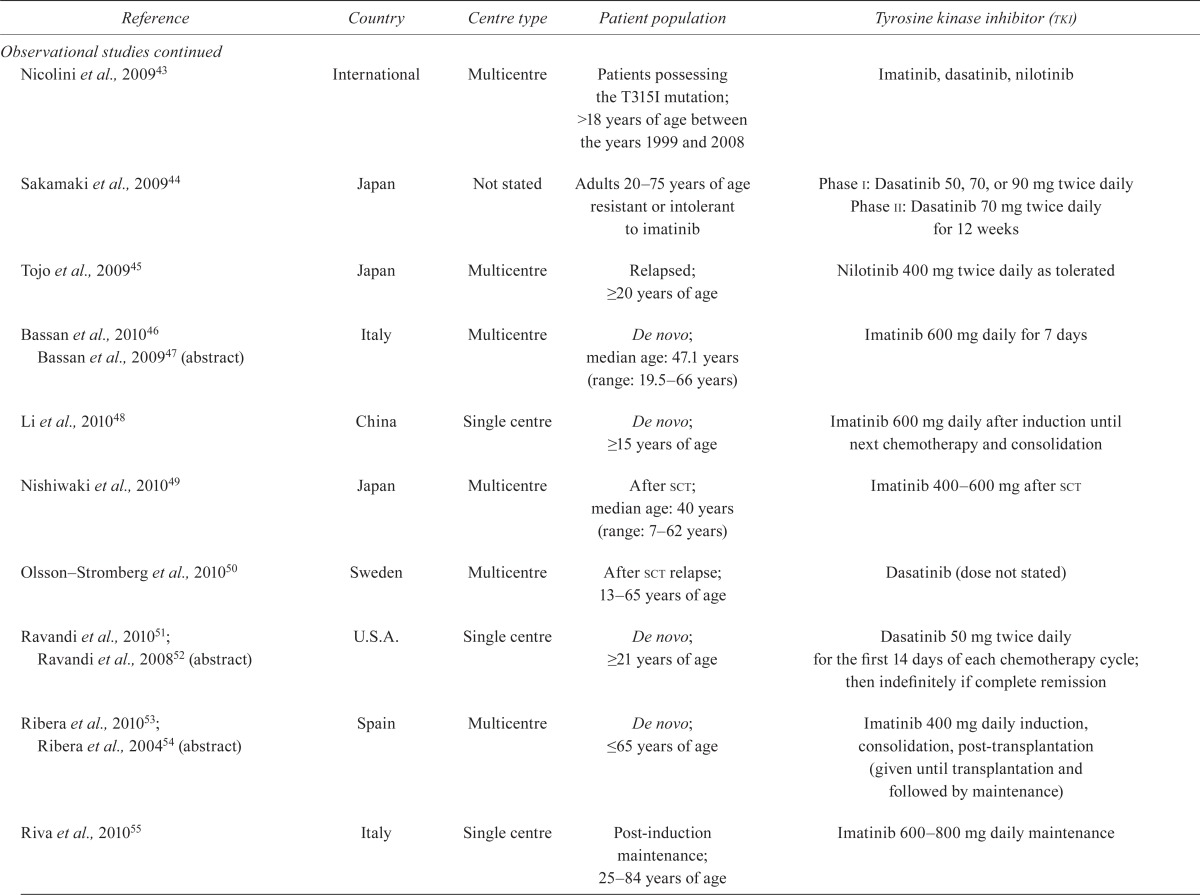

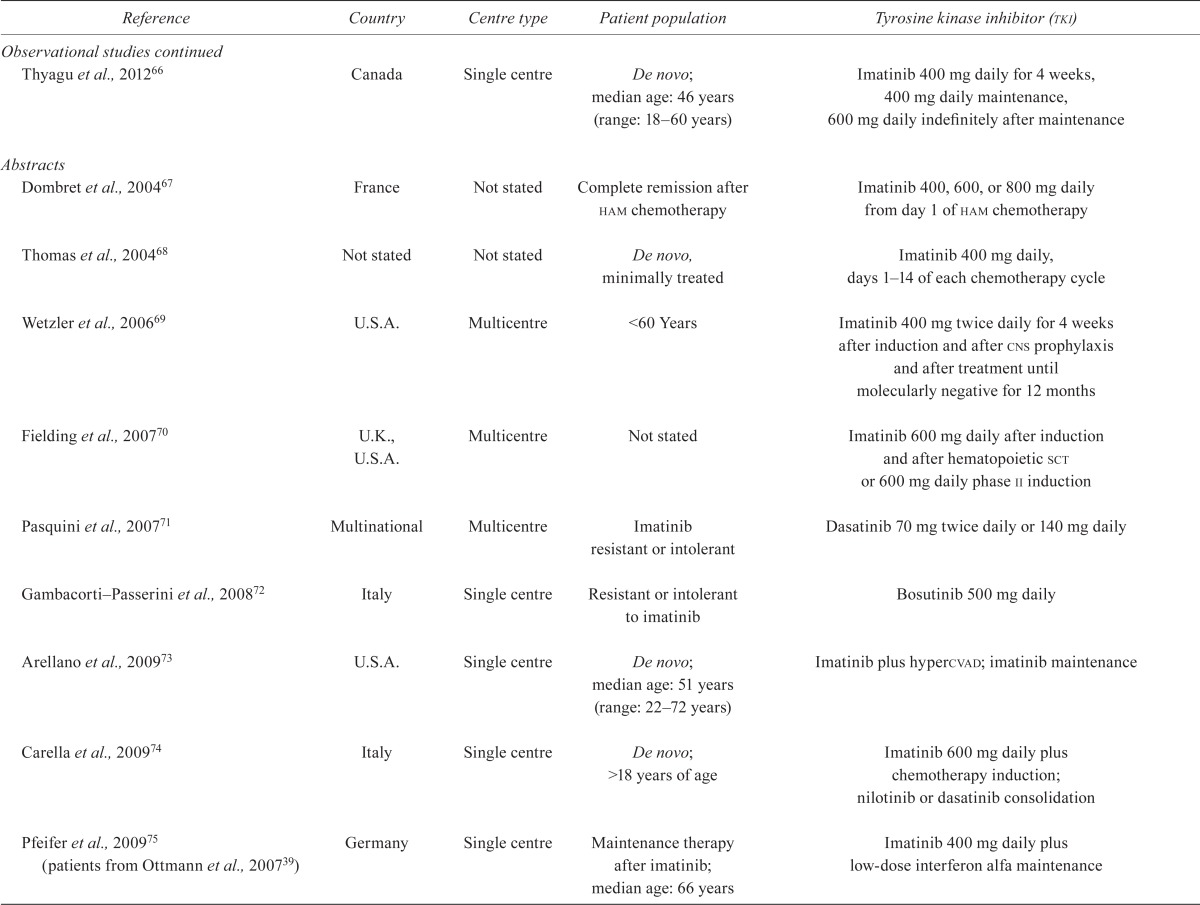
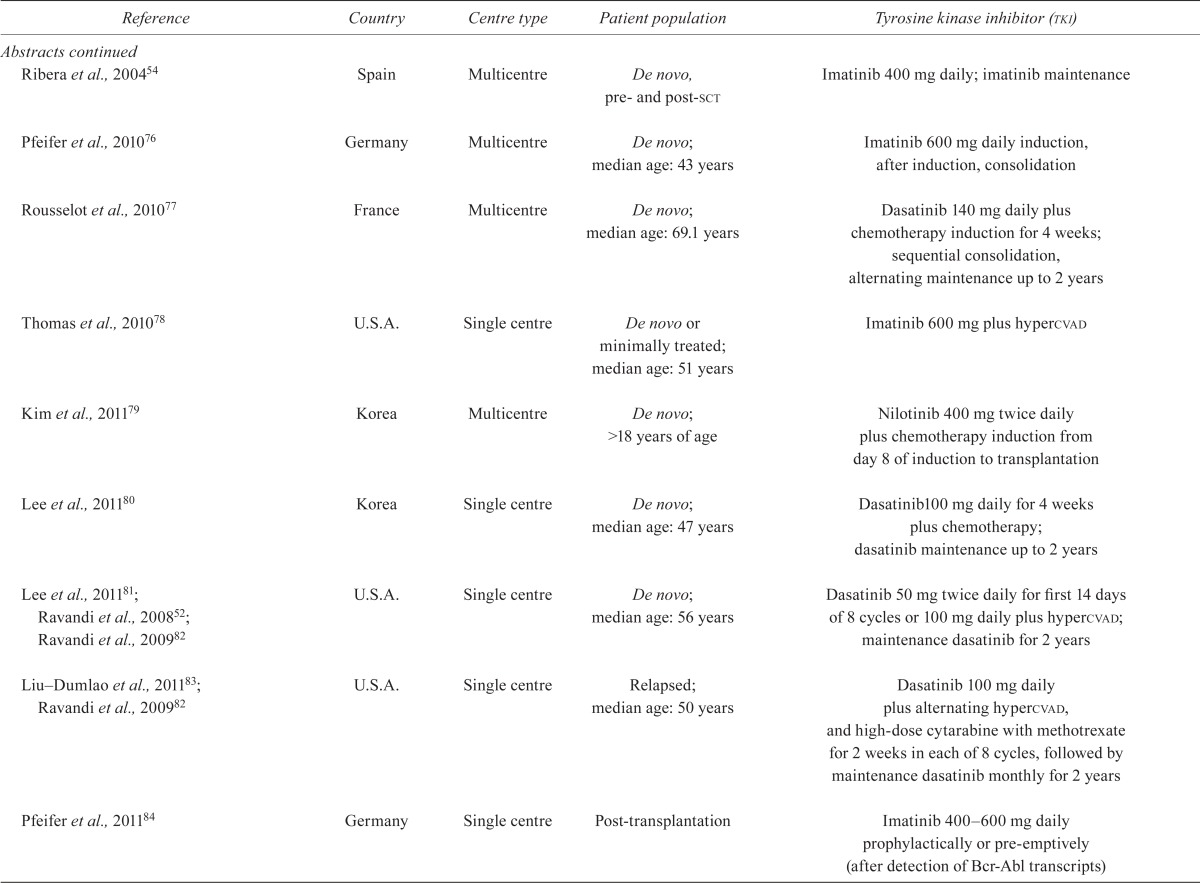
| Reference | Country | Centre type | Patient population | Tyrosine kinase inhibitor (tki) |
|---|---|---|---|---|
| Randomized studies | ||||
| Ottmann et al., 200710; Wassmann et al., 200311 | Germany | Multicentre | De novo, not eligible for transplantation; >55 years of age | Imatinib induction 600 mg daily for 4 weeks vs. chemotherapy induction, then imatinib 600 mg for all patients |
| Lilly et al., 201012 | International | Multicentre | Resistant/intolerant to imatinib; median age: 51.0 years (range: 15–80 years) | Dasatinib 140 mg daily vs. 70 mg twice daily until disease progression |
| Observational studies | ||||
| Ottmann et al., 200213 | Multinational | Multicentre | Relapsed or refractory | Imatinib 400 mg or 600 mg daily; duration: 24 weeks to indefinite |
| Wassmann et al., 200214 | Germany | Single centre | Relapsed or refractory | Imatinib 600 mg daily before hemopoietic sct until adverse event or progression |
| Pfeifer et al., 200315 (patients from Ottmann et al., 200213) | Germany | Multicentre | Relapsed or refractory | Imatinib 300–600mg daily; duration not stated |
| Scheuring et al., 200316 (patients from Ottmann et al., 200213) | Germany | Single centre | Relapsed or refractory | Imatinib 400 mg or 600 mg daily until toxicity or lack of benefit |
| Shimoni et al., 200317 | Israel, Germany | Multicentre | Pre sct or donor lymphocyte infusion; 23–61 years of age | Imatinib 400–600 mg daily before sct or donor lymphocyte infusion |
| Wassmann et al., 200318 | Germany | Single centre | Relapsed or refractory | Imatinib 400 mg or 600 mg daily, plus interferon alfa; median duration: 16 months |
| Houot et al., 200419 | France | Single centre | De novo; >55 years of age | Imatinib dose and duration not stated |
| Piccaluga et al., 200420 | Italy | Multicentre | De novo or relapsed after complete remission | Imatinib 400–800 mg daily until relapse |
| Thomas et al., 200421 | U.S.A. | Single centre | De novo, minimally-treated, or refractory; ≥15 years of age | Induction and consolidation: imatinib 400 mg daily for 2 weeks of each course Maintenance: imatinib 600 mg daily for 13 months |
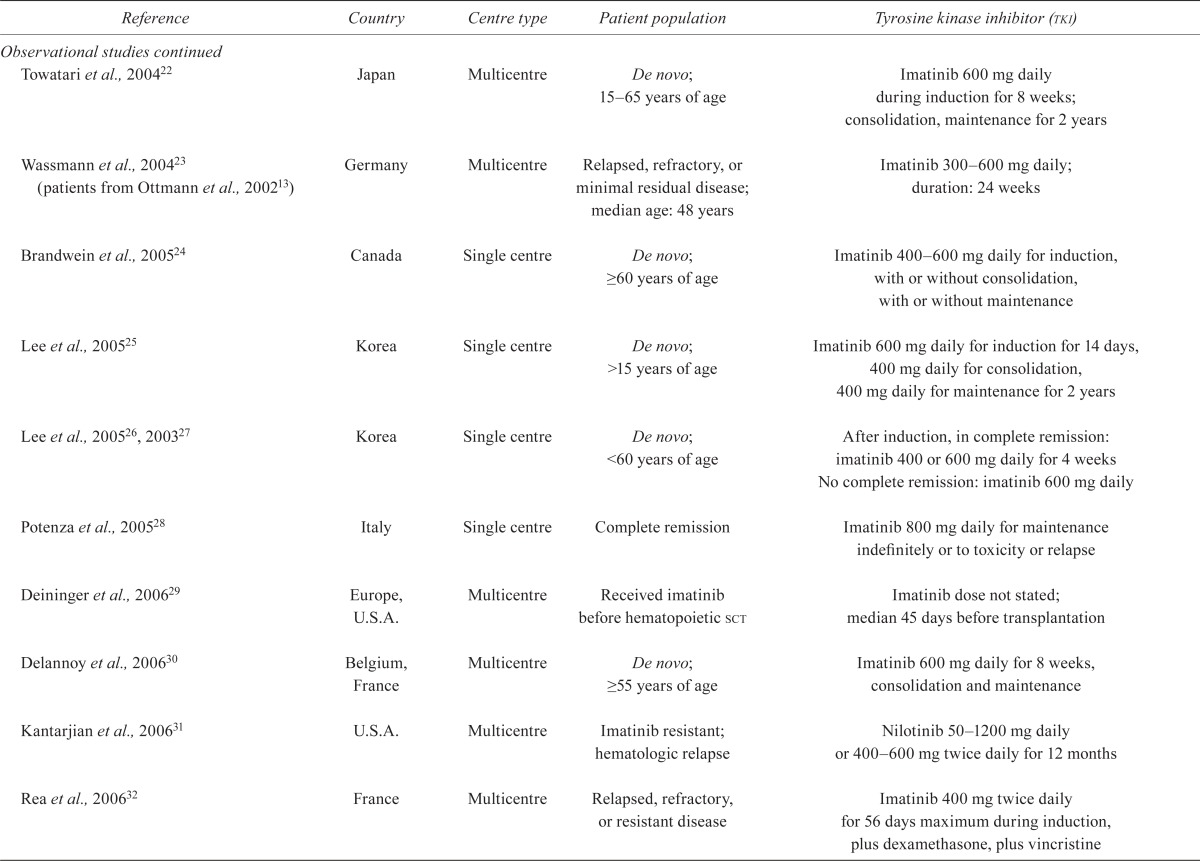
| Towatari et al., 200422 | Japan | Multicentre | De novo; 15–65 years of age | Imatinib 600 mg daily during induction for 8 weeks; consolidation, maintenance for 2 years |
| Wassmann et al., 200423 (patients from Ottmann et al., 200213) | Germany | Multicentre | Relapsed, refractory, or minimal residual disease; median age: 48 years | Imatinib 300–600mg daily; duration: 24 weeks |
| Brandwein et al., 200524 | Canada | Single centre | De novo; ≥60 years of age | Imatinib 400–600 mg daily for induction, with or without consolidation, with or without maintenance |
| Lee et al., 200525 | Korea | Single centre | De novo; >15 years of age | Imatinib 600 mg daily for induction for 14 days, 400 mg daily for consolidation, 400 mg daily for maintenance for 2 years |
| Lee et al., 200526, 200327 | Korea | Single centre | De novo; <60 years of age | After induction, in complete remission: imatinib 400 or 600 mg daily for 4 weeks No complete remission: imatinib 600 mg daily |
| Potenza et al., 200528 | Italy | Single centre | Complete remission | Imatinib 800 mg daily for maintenance indefinitely or to toxicity or relapse |
| Deininger et al., 200629 | Europe, U.S.A. | Multicentre | Received imatinib before hematopoietic sct | Imatinib dose not stated; median 45 days before transplantation |
| Delannoy et al., 200630 | Belgium, France | Multicentre | De novo; ≥55 years of age | Imatinib 600 mg daily for 8 weeks, consolidation and maintenance |
| Kantarjian et al., 200631 | U.S.A. | Multicentre | Imatinib resistant; hematologic relapse | Nilotinib 50–1200 mg daily or 400–600 mg twice daily for 12 months |
| Rea et al., 200632 | France | Multicentre | Relapsed, refractory, or resistant disease | Imatinib 400 mg twice daily for 56 days maximum during induction, plus dexamethasone, plus vincristine |
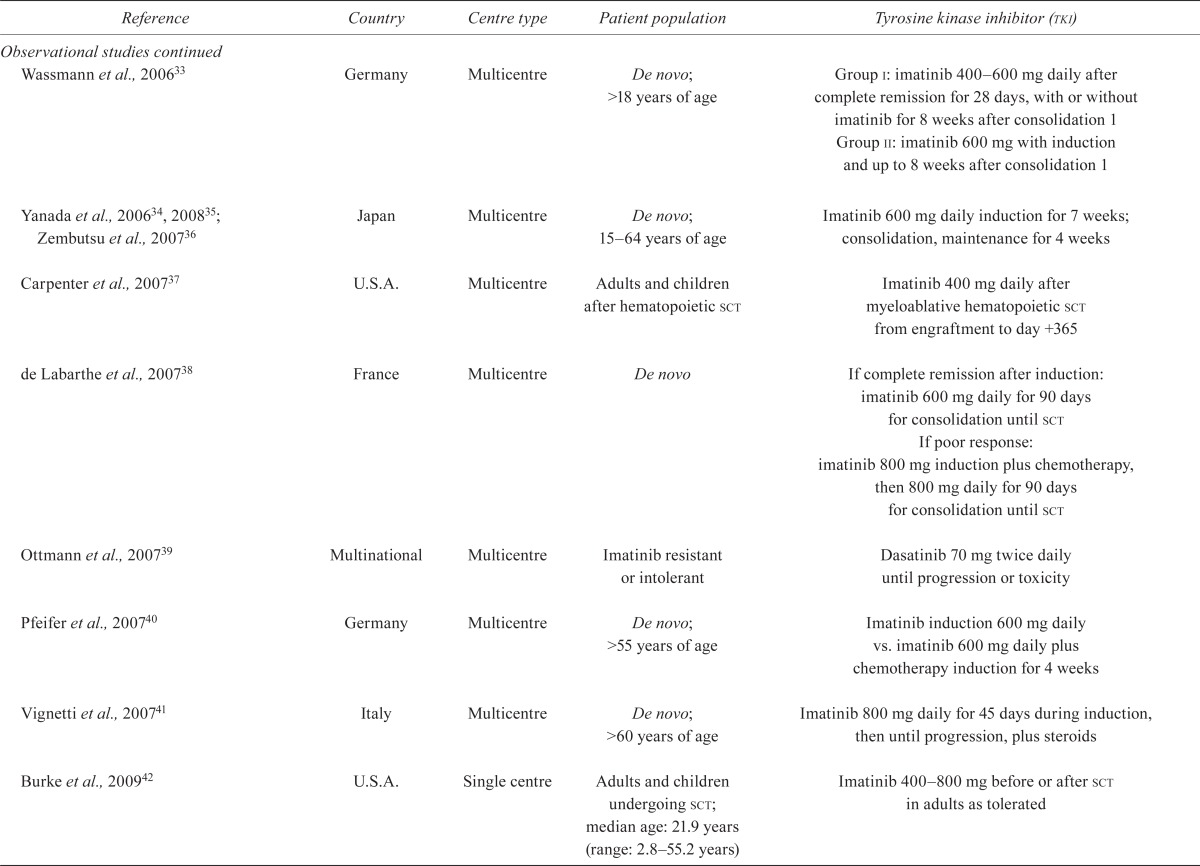
| Wassmann et al., 200633 | Germany | Multicentre | De novo; >18 years of age | Group i: imatinib 400–600 mg daily after complete remission for 28 days, with or without imatinib for 8 weeks after consolidation 1 Group ii: imatinib 600 mg with induction and up to 8 weeks after consolidation 1 |
| Yanada et al., 200634, 200835; Zembutsu et al., 200736 | Japan | Multicentre | De novo; 15–64 years of age | Imatinib 600 mg daily induction for 7 weeks; consolidation, maintenance for 4 weeks |
| Carpenter et al., 200737 | U.S.A. | Multicentre | Adults and children after hematopoietic sct | Imatinib 400 mg daily after myeloablative hematopoietic sct from engraftment to day +365 |
| de Labarthe et al., 200738 | France | Multicentre | De novo | If complete remission after induction: imatinib 600 mg daily for 90 days for consolidation until sct If poor response: imatinib 800 mg induction plus chemotherapy, then 800 mg daily for 90 days for consolidation until sct |
| Ottmann et al., 200739 | Multinational | Multicentre | Imatinib resistant or intolerant | Dasatinib 70 mg twice daily until progression or toxicity |
| Pfeifer et al., 200740 | Germany | Multicentre | De novo; >55 years of age | Imatinib induction 600 mg daily vs. imatinib 600 mg daily plus chemotherapy induction for 4 weeks |
| Vignetti et al., 200741 | Italy | Multicentre | De novo; >60 years of age | Imatinib 800 mg daily for 45 days during induction, then until progression, plus steroids |
| Burke et al., 200942 | U.S.A. | Single centre | Adults and children undergoing sct; median age: 21.9 years (range: 2.8–55.2 years) | Imatinib 400–800 mg before or after sct in adults as tolerated |
| Nicolini et al., 200943 | International | Multicentre | Patients possessing the T315I mutation; >18 years of age between the years 1999 and 2008 | Imatinib, dasatinib, nilotinib |
| Sakamaki et al., 200944 | Japan | Not stated | Adults 20–75 years of age resistant or intolerant to imatinib | Phase i: Dasatinib 50, 70, or 90 mg twice daily Phase ii: Dasatinib 70 mg twice daily for 12 weeks |
| Tojo et al., 200945 | Japan | Multicentre | Relapsed; ≥20 years of age | Nilotinib 400 mg twice daily as tolerated |
| Bassan et al., 201046 Bassan et al., 200947 (abstract) | Italy | Multicentre | De novo; median age: 47.1 years (range: 19.5–66 years) | Imatinib 600 mg daily for 7 days |
| Li et al., 201048 | China | Single centre | De novo; ≥15 years of age | Imatinib 600 mg daily after induction until next chemotherapy and consolidation |
| Nishiwaki et al., 201049 | Japan | Multicentre | After sct; median age: 40 years (range: 7–62 years) | Imatinib 400–600 mg after sct |
| Olsson–Stromberg et al., 201050 | Sweden | Multicentre | After sct relapse; 13–65 years of age | Dasatinib (dose not stated) |
| Ravandi et al., 201051; Ravandi et al., 200852 (abstract) | U.S.A. | Singlecentre | De novo; ≥21 years of age | Dasatinib 50 mg twice daily for the first 14 days of each chemotherapy cycle; then indefinitely if complete remission |
| Ribera et al., 201053; Ribera et al., 200454 (abstract) | Spain | Multicentre | De novo; ≤65 years of age | Imatinib 400 mg daily induction, consolidation, post-transplantation (given until transplantation and followed by maintenance) |
| Riva et al., 201055 | Italy | Single centre | Post-induction maintenance; 25–84 years of age | Imatinib 600–800 mg daily maintenance |
| Chen et al., 201156 | China | Single centre | After sct; 26 adults, 3 children | Imatinib 400 mg daily after sct for at least 1 month |
| Foà et al., 201157; Foà et al., 200758 (abstract) | Italy | Multicentre | De novo; median age: 53.6 years (range: 23.8–76.5 years) | Dasatinib 70 mg twice daily for 84 days during induction |
| Mizuta et al., 201159; Yanada et al., 200634, 200835; Zembutsu et al., 200736 | Japan | Multicentre | De novo; median age: 38 years (range: 15–64 years) | Imatinib 600 mg daily induction, consolidation, maintenance |
| Wang et al., 201160 | China | Single centre | De novo; median age: 30 years (range: 15–50 years) | Imatinib plus chemotherapy vs. chemotherapy alone (dose not stated) |
| Bose et al., 201261 | U.S.A. | Multicentre | Hematologic relapse (cml, aml); ≥18 years of age | Flavopiridol 30mg/m2 each week and imatinib 400 mg daily escalating to 60mg/m2 and 1000 mg respectively for 3 weeks every 4 weeks |
| Caocci et al., 201262 | Italy | Single centre | Post sct; median age: 41 years (range: 18–56 years) | Dasatinib 50–100 mg daily for post-sct maintenance |
| Chen et al., 201263 | China | Single centre | After sct; median age: 28.5 years (range: 3–51 years) | Imatinib 400 mg daily for 3–12 months after sct until complete molecular remission was sustained for at least 3 months (260 mg twice daily, >17 years of age) |
| Lee et al., 201264 | Korea | Single centre | De novo; median age: 34 years (range: 15–59 years) | Imatinib 100 mg daily for 4 weeks’ induction, consolidation |
| Pfeifer et al., 201265 (patients from Ottmann et al., 200739; Ottmann et al., 200213; Wassmann et al., 200423) | Germany | Multicentre | Hematologic relapse and de novo; 17–79 years of age | Imatinib 400 mg or 600 mg daily for 4 weeks |
| Thyagu et al., 201266 | Canada | Single centre | De novo; median age: 46 years (range: 18–60 years) | Imatinib 400 mg daily for 4 weeks, 400 mg daily maintenance, 600 mg daily indefinitely after maintenance |
| Abstracts | ||||
| Dombret et al., 200467 | France | Not stated | Complete remission after ham chemotherapy | Imatinib 400, 600, or 800 mg daily from day 1 of ham chemotherapy |
| Thomas et al., 200468 | Not stated | Not stated | De novo, minimally treated | Imatinib 400 mg daily, days 1–14 of each chemotherapy cycle |
| Wetzler et al., 200669 | U.S.A. | Multicentre | <60 Years | Imatinib 400 mg twice daily for 4 weeks after induction and after cns prophylaxis and after treatment until molecularly negative for 12 months |
| Fielding et al., 200770 | U.K., U.S.A. | Multicentre | Not stated | Imatinib 600 mg daily after induction and after hematopoietic sct or 600 mg daily phase ii induction |
| Pasquini et al., 200771 | Multinational | Multicentre | Imatinib resistant or intolerant | Dasatinib 70 mg twice daily or 140 mg daily |
| Gambacorti–Passerini et al., 200872 | Italy | Single centre | Resistant or intolerant to imatinib | Bosutinib 500 mg daily |
| Arellano et al., 200973 | U.S.A. | Single centre | De novo; median age: 51 years (range: 22–72 years) | Imatinib plus hypercvad; imatinib maintenance |
| Carella et al., 200974 | Italy | Single centre | De novo; >18 years of age | Imatinib 600 mg daily plus chemotherapy induction; nilotinib or dasatinib consolidation |
| Pfeifer et al., 200975 (patients from Ottmann et al., 200739) | Germany | Single centre | Maintenance therapy after imatinib; median age: 66 years | Imatinib 400 mg daily plus low-dose interferon alfa maintenance |
| Ribera et al., 200454 | Spain | Multicentre | De novo, pre- and post-sct | Imatinib 400 mg daily; imatinib maintenance |
| Pfeifer et al., 201076 | Germany | Multicentre | De novo; median age: 43 years | Imatinib 600 mg daily induction, after induction, consolidation |
| Rousselot et al., 201077 | France | Multicentre | De novo; median age: 69.1 years | Dasatinib 140 mg daily plus chemotherapy induction for 4 weeks; sequential consolidation, alternating maintenance up to 2 years |
| Thomas et al., 201078 | U.S.A. | Single centre | De novo or minimally treated; median age: 51 years | Imatinib 600 mg plus hypercvad |
| Kim et al., 201179 | Korea | Multicentre | De novo; >18 years of age | Nilotinib 400 mg twice daily plus chemotherapy induction from day 8 of induction to transplantation |
| Lee et al., 201180 | Korea | Single centre | De novo; median age: 47 years | Dasatinib100 mg daily for 4 weeks plus chemotherapy; dasatinib maintenance up to 2 years |
| Lee et al., 201181; Ravandi et al., 200852; Ravandi et al., 200982 | U.S.A. | Single centre | De novo; median age: 56 years | Dasatinib 50 mg twice daily for first 14 days of 8 cycles or 100 mg daily plus hypercvad; maintenance dasatinib for 2 years |
| Liu–Dumlao et al., 201183; Ravandi et al., 200982 | U.S.A. | Single centre | Relapsed; median age: 50 years | Dasatinib 100 mg daily plus alternating hypercvad, and high-dose cytarabine with methotrexate for 2 weeks in each of 8 cycles, followed by maintenance dasatinib monthly for 2 years |
| Pfeifer et al., 201184 | Germany | Single centre | Post-transplantation | Imatinib 400–600mg daily prophylactically or pre-emptively (after detection of Bcr-Abl transcripts) |
| Brummendorf et al., 201285 | Multinational | Multicentre | Relapsed after tki | Bosutinib 500 mg daily |
| Cortes et al., 201286 | U.S.A. | Multicentre | Resistant | Ponatinib 45 mg daily |
sct= stem-cell transplantation; cml= chronic myelogenous leukemia; aml= acute myeloid leukemia; ham= cytarabine, mitoxantrone; cns= central nervous system; hypercvad= hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone.
TABLE V.
Outcomes
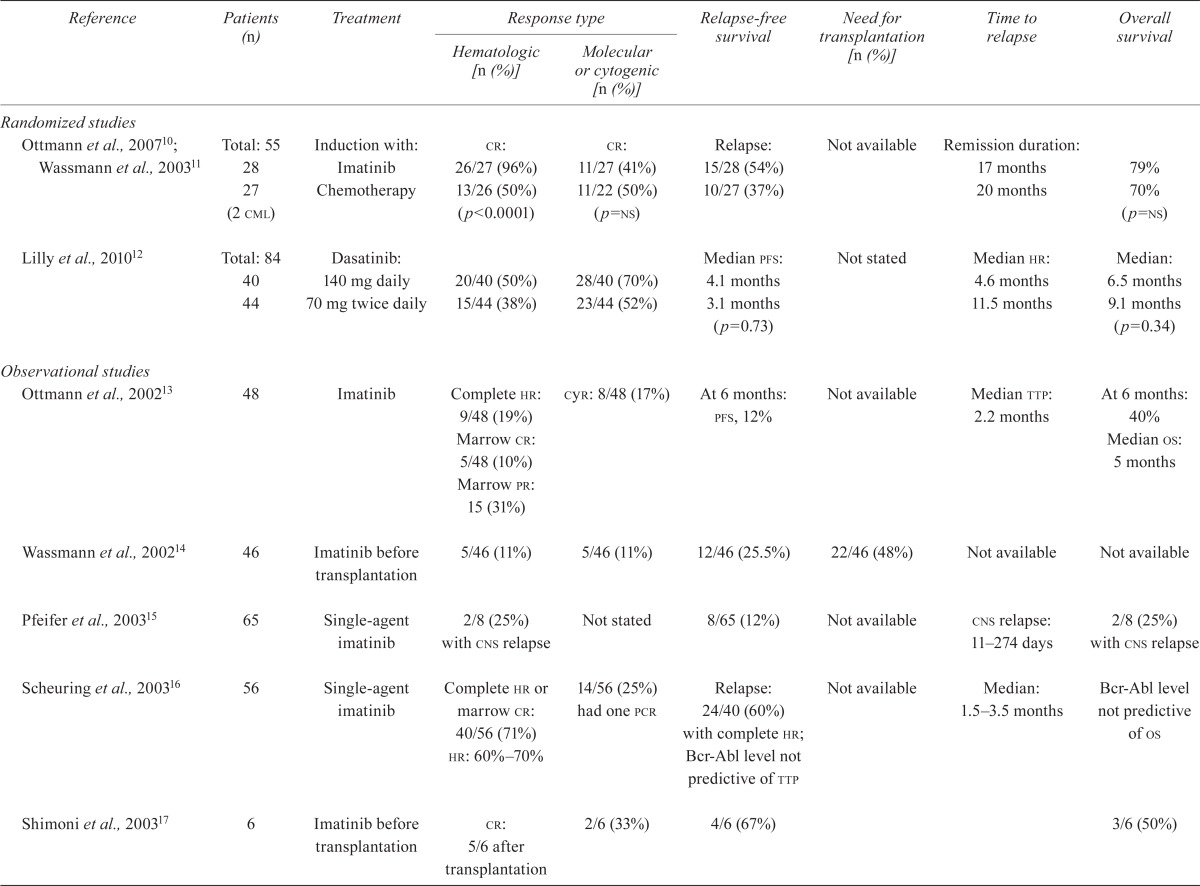
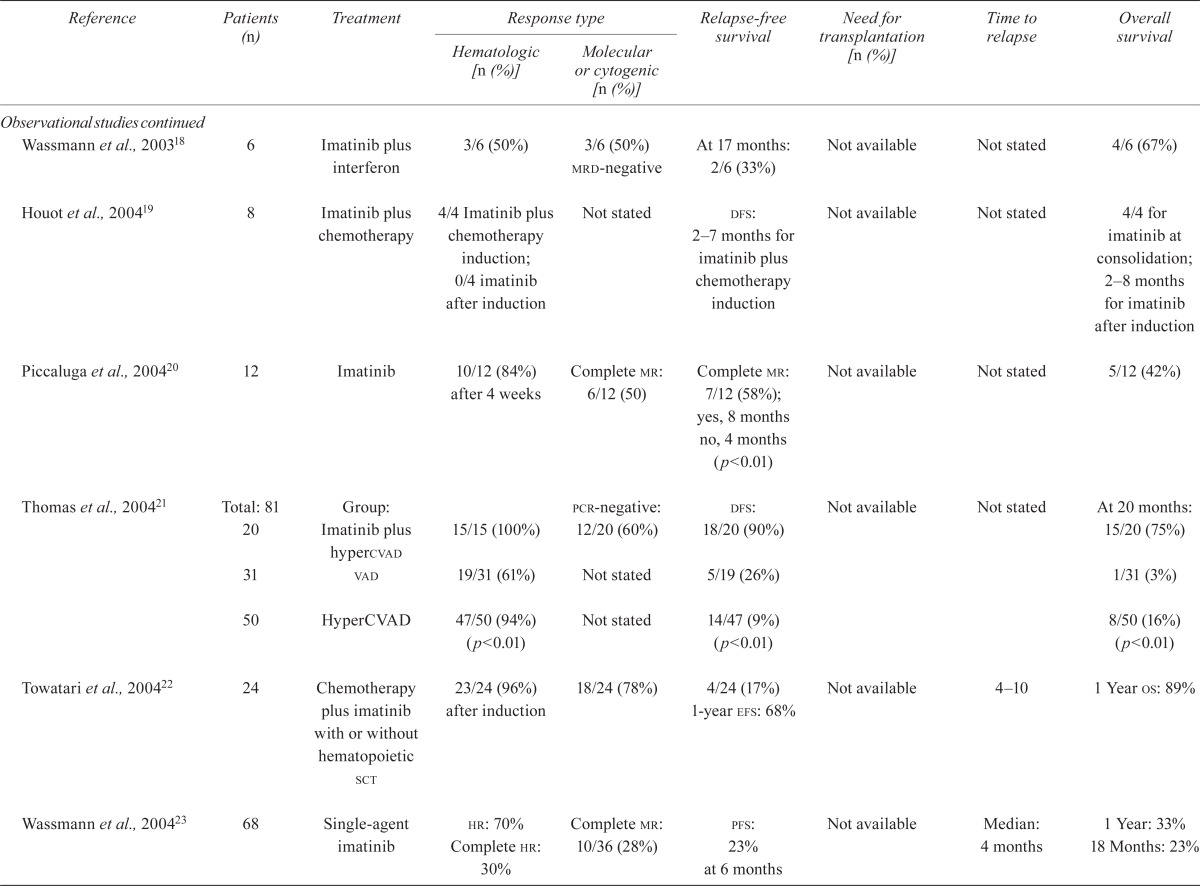
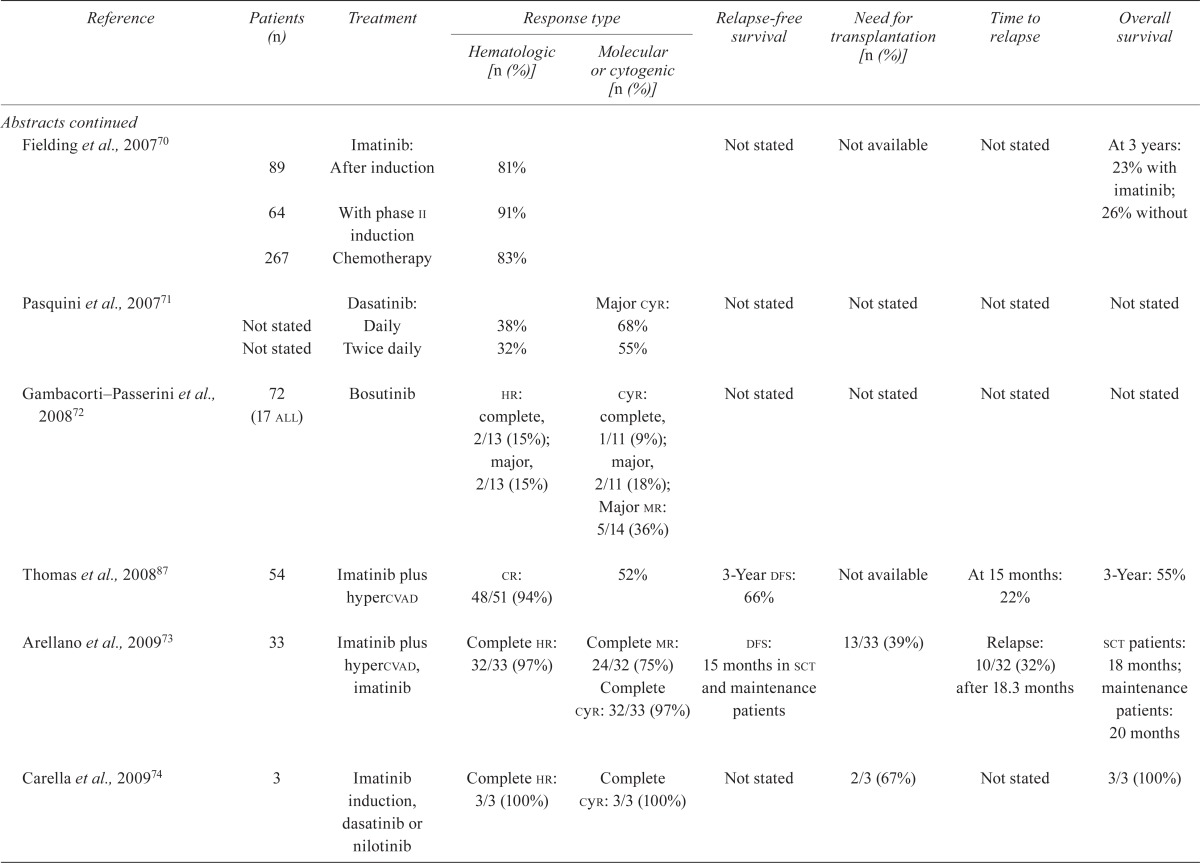
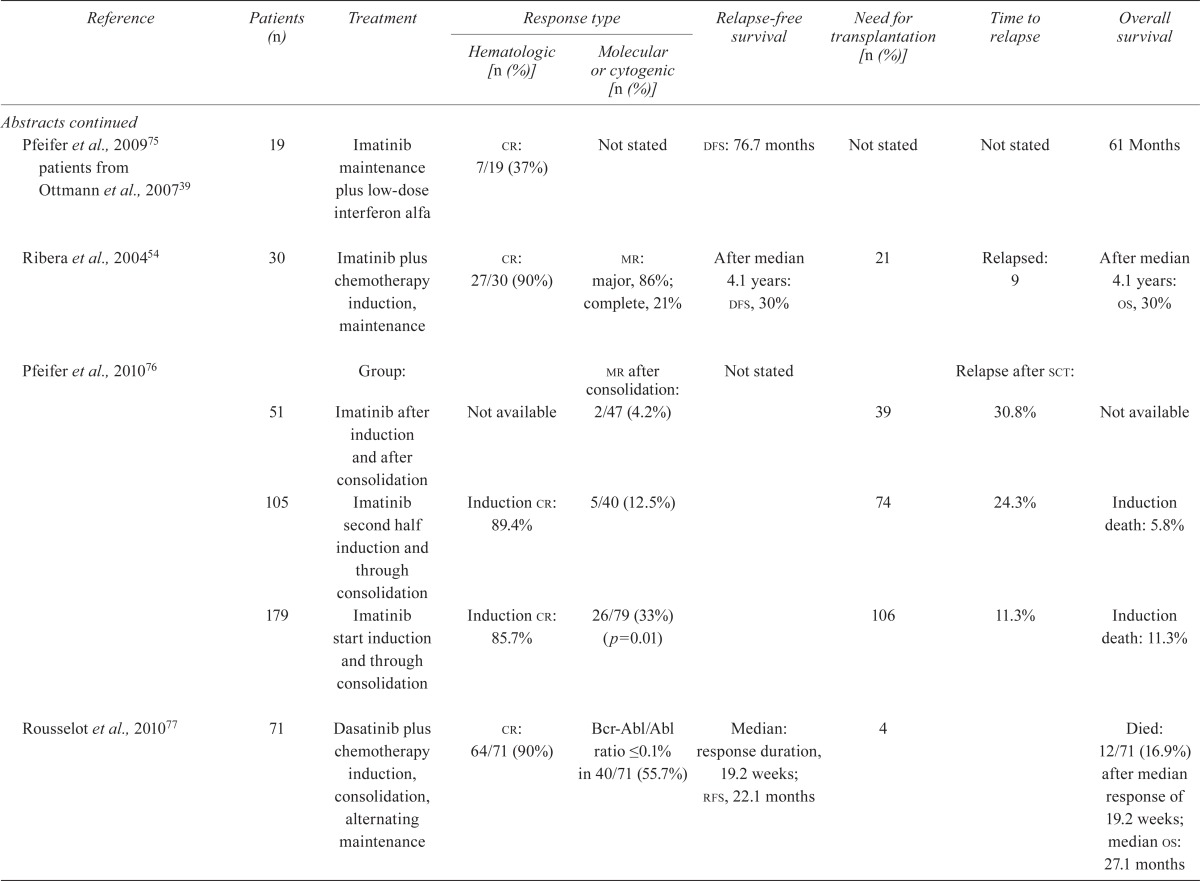
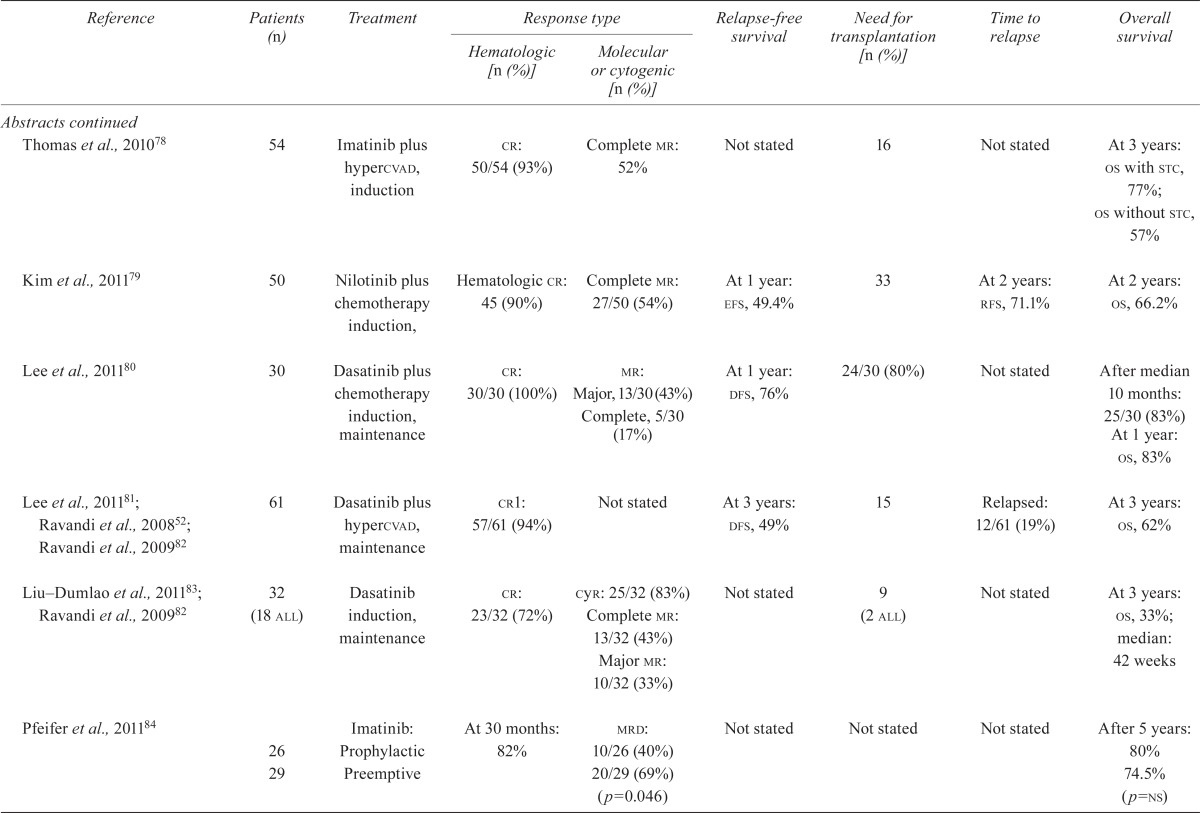
| Reference | Patients (n) | Treatment |
Response type
|
Relapse-free survival | Need for transplantation [n (%)] | Time to relapse | Overall survival | |
|---|---|---|---|---|---|---|---|---|
| Hematologic [n (%)] | Molecular or cytogenic [n (%)] | |||||||
| Randomized studies | ||||||||
| Ottmann et al., 200710; Wassmann et al., 200311 | Total: 55 28 27 (2 cml) |
Induction with: Imatinib Chemotherapy |
cr: 26/27 (96%) 13/26 (50%) (p<0.0001) |
cr: 11/27 (41%) 11/22 (50%) (p=ns) |
Relapse: 15/28 (54%) 10/27 (37%) |
Not available | Remission duration: 17 months 20 months |
79% 70% (p=ns) |
| Lilly et al., 201012 | Total: 84 40 44 |
Dasatinib: 140 mg daily 70 mg twice daily |
20/40 (50%) 15/44 (38%) |
28/40 (70%) 23/44 (52%) |
Median pfs: 4.1 months 3.1 months (p=0.73) |
Not stated | Median hr: 4.6 months 11.5 months |
Median: 6.5 months 9.1 months (p=0.34) |
| Observational studies | ||||||||
| Ottmann et al., 200213 | 48 | Imatinib | Complete hr: 9/48 (19%) Marrow cr: 5/48 (10%) Marrow pr: 15 (31%) |
cyr: 8/48 (17%) | At 6 months: pfs, 12% | Not available | Median ttp: 2.2 months | At 6 months: 40% Median os: 5 months |
| Wassmann et al., 200214 | 46 | Imatinib before transplantation | 5/46 (11%) | 5/46 (11%) | 12/46 (25.5%) | 22/46 (48%) | Not available | Not available |
| Pfeifer et al., 200315 | 65 | Single-agent imatinib | 2/8 (25%) with cns relapse | Not stated | 8/65 (12%) | Not available | cns relapse: 11–274 days | 2/8 (25%) with cns relapse |
| Scheuring et al., 200316 | 56 | Single-agent imatinib | Complete hr or marrow cr: 40/56 (71%) hr: 60%–70% |
14/56 (25%) had one pcr | Relapse: 24/40 (60%) with complete hr; Bcr-Abl level not predictive of ttp | Not available | Median: 1.5–3.5 months | Bcr-Abl level not predictive of os |
| Shimoni et al., 200317 | 6 | Imatinib before transplantation | cr: 5/6 after transplantation | 2/6 (33%) | 4/6 (67%) | 3/6 (50%) | ||
| Wassmann et al., 200318 | 6 | Imatinib plus interferon | 3/6 (50%) | 3/6 (50%) mrd-negative | At 17 months: 2/6 (33%) | Not available | Not stated | 4/6 (67%) |
| Houot et al., 200419 | 8 | Imatinib plus chemotherapy | 4/4 Imatinib plus chemotherapy induction; 0/4 imatinib after induction | Not stated | dfs: 2–7 months for imatinib plus chemotherapy induction | Not available | Not stated | 4/4 for imatinib at consolidation; 2–8 months for imatinib after induction |
| Piccaluga et al., 200420 | 12 | Imatinib | 10/12 (84%) after 4 weeks | Complete mr: 6/12 (50) | Complete mr: 7/12 (58%); yes, 8 months no, 4 months (p<0.01) |
Not available | Not stated | 5/12 (42%) |
| Thomas et al., 200421 | Total: 81 20 |
Group: Imatinib plus hypercvad | 15/15 (100%) | pcr-negative: 12/20 (60%) | dfs: 18/20 (90%) | Not available | Not stated | At 20 months: 15/20 (75%) |
| 31 | vad | 19/31 (61%) | Not stated | 5/19 (26%) | 1/31 (3%) | |||
| 50 | HyperCVAD | 47/50 (94%) (p<0.01) | Not stated | 14/47 (9%) (p<0.01) | 8/50 (16%) (p<0.01) | |||
| Towatari et al., 200422 | 24 | Chemotherapy plus imatinib with or without hematopoietic sct | 23/24 (96%) after induction | 18/24 (78%) | 4/24 (17%) 1-year efs: 68% | Not available | 4–10 | 1 Year os: 89% |
| Wassmann et al., 200423 | 68 | Single-agent imatinib |
hr: 70% Complete hr: 30% |
Complete mr: 10/36 (28%) | pfs: 23% at 6 months | Not available | Median: 4 months | 1 Year: 33% 18 Months: 23% |
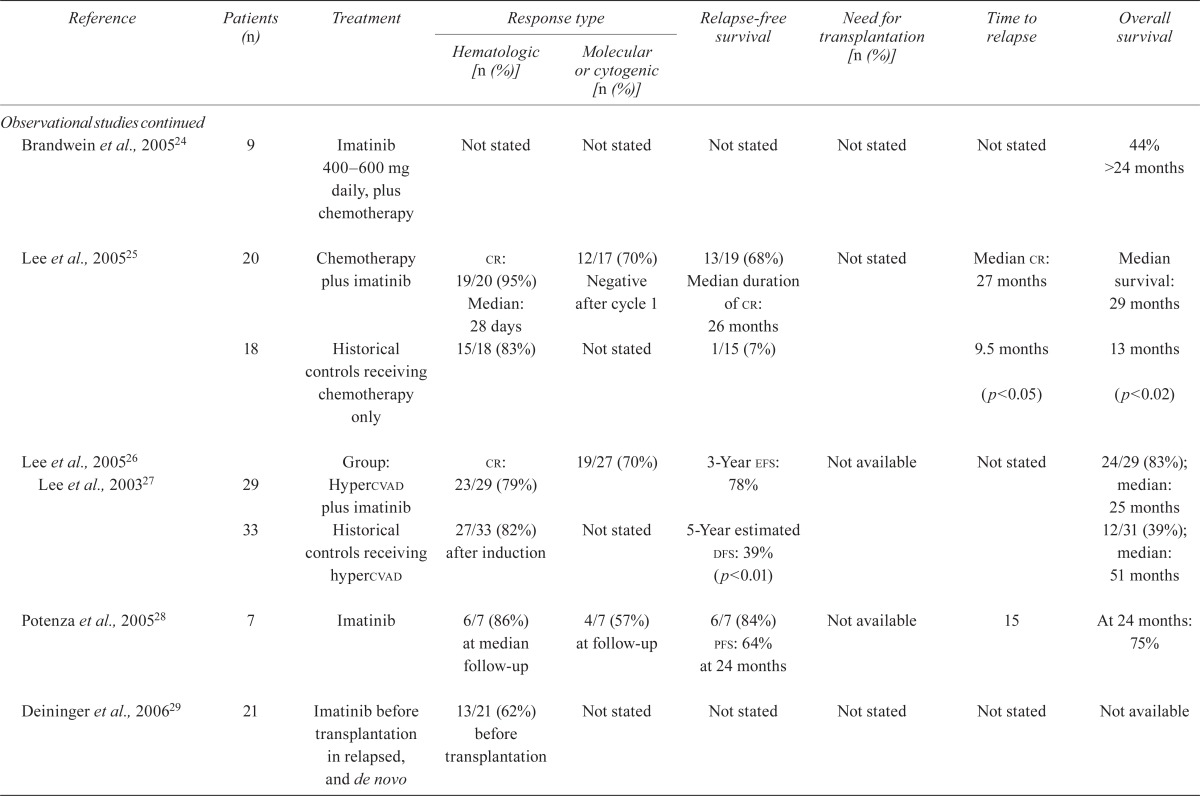
| Brandwein et al., 200524 | 9 | Imatinib 400–600mg daily, plus chemotherapy | Not stated | Not stated | Not stated | Not stated | Not stated | 44% >24 months |
| Lee et al., 200525 | 20 | Chemotherapy plus imatinib |
cr: 19/20 (95%) Median: 28 days |
12/17 (70%) Negative after cycle 1 |
13/19 (68%) Median duration of cr: 26 months |
Not stated | Median cr: 27 months | Median survival: 29 months |
| 18 | Historical controls receiving chemotherapy only | 15/18 (83%) | Not stated | 1/15 (7%) | 9.5 months (p<0.05) | 13 months (p<0.02) | ||
| Lee et al., 200526 Lee et al., 200327 | 29 | Group: Hypercvad plus imatinib | cr: 23/29 (79%) | 19/27 (70%) | 3-Year efs: 78% | Not available | Not stated | 24/29 (83%); median: 25 months |
| 33 | Historical controls receiving hypercvad | 27/33 (82%) after induction | Not stated | 5-Year estimated dfs: 39% (p<0.01) | 12/31 (39%); median: 51 months | |||
| Potenza et al., 200528 | 7 | Imatinib | 6/7 (86%) at median follow-up | 4/7 (57%) at follow-up | 6/7 (84%) pfs: 64% at 24 months |
Not available | 15 | At 24 months: 75% |
| Deininger et al., 200629 | 21 | Imatinib before transplantation in relapsed, and de novo | 13/21 (62%) before transplantation | Not stated | Not stated | Not stated | Not stated | Not available |
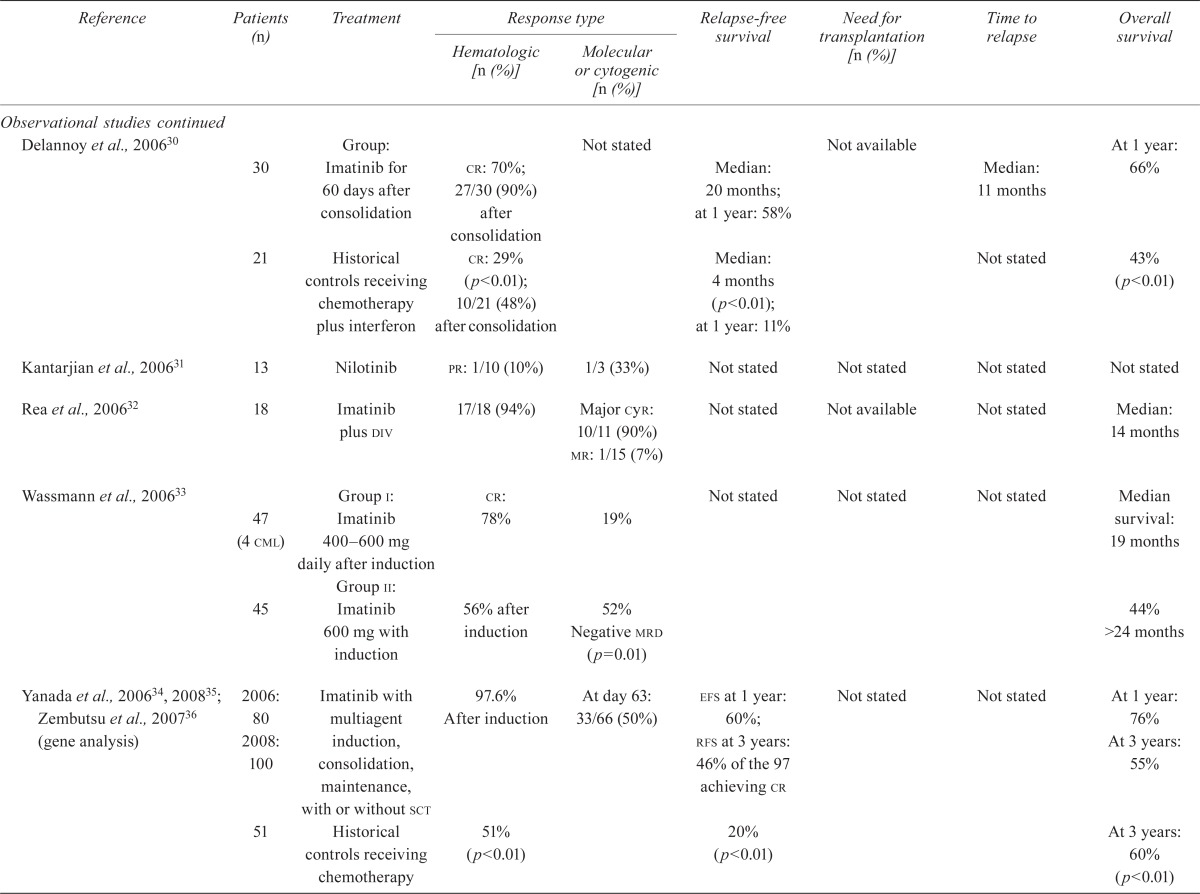
| Delannoy et al., 200630 | 30 | Group: Imatinib for 60 days after consolidation | cr: 70%; 27/30 (90%) after consolidation | Not stated | Median: 20 months; at 1 year: 58% | Not available | Median: 11 months | At 1 year: 66% |
| 21 | Historical controls receiving chemotherapy plus interferon |
cr: 29% (p<0.01); 10/21 (48%) after consolidation |
Median: 4 months (p<0.01); at 1 year: 11% | Not stated | 43% (p<0.01) | |||
| Kantarjian et al., 200631 | 13 | Nilotinib | pr: 1/10 (10%) | 1/3 (33%) | Not stated | Not stated | Not stated | Not stated |
| Rea et al., 200632 | 18 | Imatinib plus div | 17/18 (94%) | Major cyr: 10/11 (90%) mr: 1/15 (7%) |
Not stated | Not available | Not stated | Median: 14 months |
| Wassmann et al., 200633 | 47 (4 cml) | Group i: Imatinib 400–600mg daily after induction | cr: 78% | 19% | Not stated | Not stated | Not stated | Median survival: 19 months |
| 45 | Group ii: Imatinib 600 mg with induction | 56% after induction | 52% Negative mrd (p=0.01) | 44% >24 months | ||||
| Yanada et al., 200634, 200835; Zembutsu et al., 200736 (gene analysis) | 2006: 80 2008: 100 |
Imatinib with multiagent induction, consolidation, maintenance, with or without sct | 97.6% After induction | At day 63: 33/66 (50%) |
efs at 1 year: 60%; rfs at 3 years: 46% of the 97 achieving cr |
Not stated | Not stated | At 1 year: 76% At 3 years: 55% |
| 51 | Historical controls receiving chemotherapy | 51% (p<0.01) | 20% (p<0.01) | At 3 years: 60% (p<0.01) | ||||
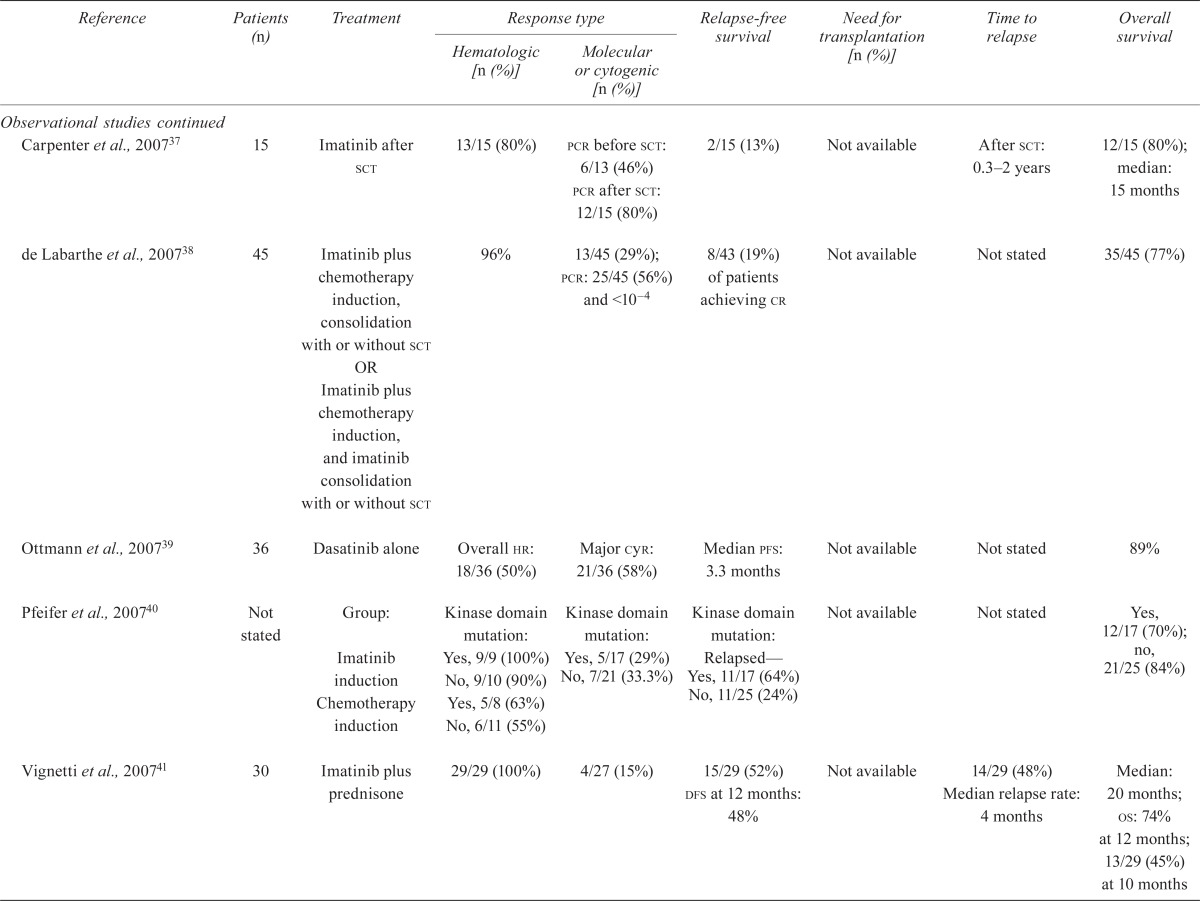
| Carpenter et al., 200737 | 15 | Imatinib after sct | 13/15 (80%) |
pcr before sct: 6/13 (46%) pcr after sct: 12/15 (80%) |
2/15 (13%) | Not available | After sct: 0.3–2 years | 12/15 (80%); median: 15 months |
| de Labarthe et al., 200738 | 45 | Imatinib plus chemotherapy induction, consolidation with or without sct OR Imatinib plus chemotherapy induction, and imatinib consolidation with or without sct | 96% | 13/45 (29%); pcr: 25/45 (56%) and <10–4 |
8/43 (19%) of patients achieving cr | Not available | Not stated | 35/45 (77%) |
| Ottmann et al., 200739 | 36 | Dasatinib alone | Overall hr: 18/36 (50%) | Major cyr: 21/36 (58%) | Median pfs: 3.3 months | Not available | Not stated | 89% |
| Pfeifer et al., 200740 | Not stated | Group: Imatinib induction Chemotherapy induction |
Kinase domain mutation: Yes, 9/9 (100%) No, 9/10 (90%) Yes, 5/8 (63%) No, 6/11 (55%) |
Kinase domain mutation: Yes, 5/17 (29%) No, 7/21 (33.3%) |
Kinase domain mutation: Relapsed— Yes, 11/17 (64%) No, 11/25 (24%) |
Not available | Not stated | Yes, 12/17 (70%); no, 21/25 (84%) |
| Vignetti et al., 200741 | 30 | Imatinib plus prednisone | 29/29 (100%) | 4/27 (15%) | 15/29 (52%) dfs at 12 months: 48% |
Not available | 14/29 (48%) Median relapse rate: 4 months |
Median: 20 months; os: 74% at 12 months; 13/29 (45%) at 10 months |
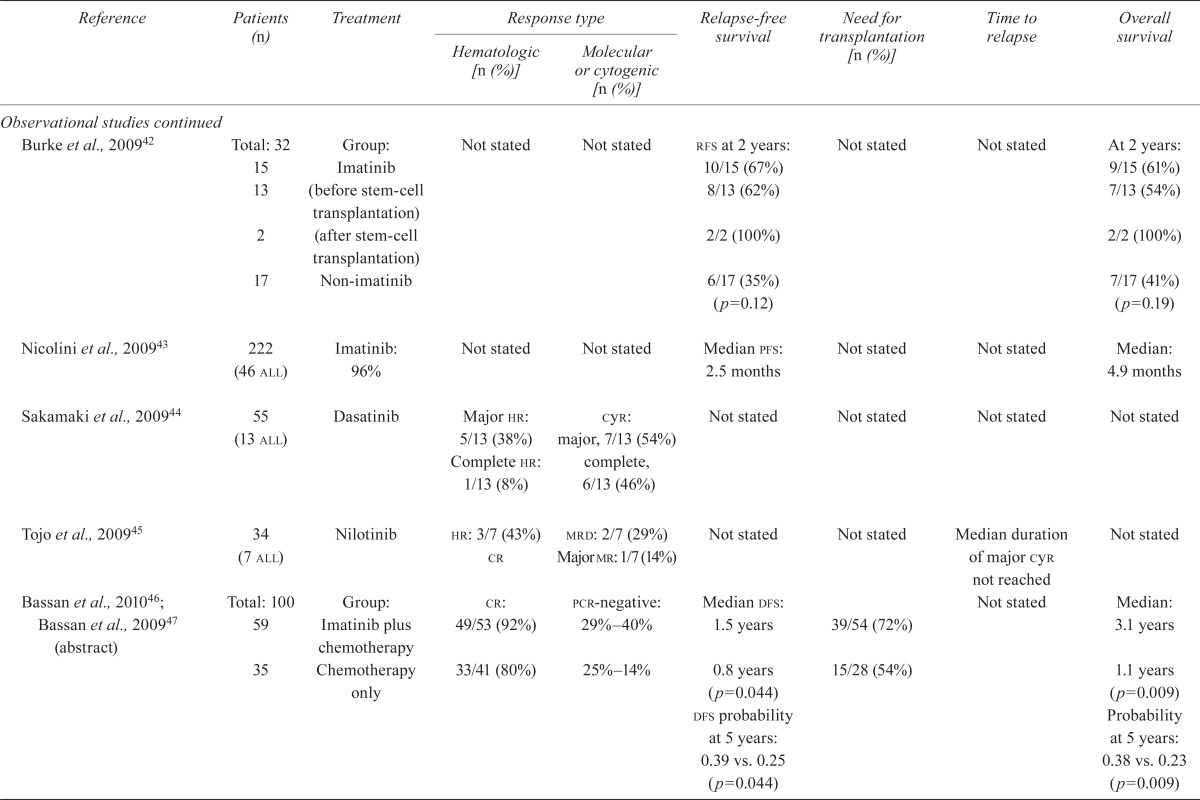
| Burke et al., 200942 | Total: 32 | Group: | Not stated | Not stated | rfs at 2 years: | Not stated | Not stated | At 2 years: |
| 15 | Imatinib | 10/15 (67%) | 9/15 (61%) | |||||
| 13 | (before stem-cell transplantation) | 8/13 (62%) | 7/13 (54%) | |||||
| 2 | (after stem-cell transplantation) | 2/2 (100%) | 2/2 (100%) | |||||
| 17 | Non-imatinib | 6/17 (35%) (p=0.12) | 7/17 (41%) (p=0.19) | |||||
| Nicolini et al., 200943 | 222 (46 all) | Imatinib: 96% | Not stated | Not stated | Median pfs: 2.5 months | Not stated | Not stated | Median: 4.9 months |
| Sakamaki et al., 200944 | 55 (13 all) | Dasatinib | Major hr: 5/13 (38%) Complete hr: 1/13 (8%) |
cyr: major, 7/13 (54%) complete, 6/13 (46%) |
Not stated | Not stated | Not stated | Not stated |
| Tojo et al., 200945 | 34 (7 all) | Nilotinib |
hr: 3/7 (43%) cr |
mrd: 2/7 (29%) Major mr: 1/7 (14%) |
Not stated | Not stated | Median duration of major cyr not reached | Not stated |
| Bassan et al., 201046; Bassan et al., 200947 (abstract) | Total: 100 59 |
Group: Imatinib plus chemotherapy | cr: 49/53 (92%) | pcr-negative: 29%–40% | Median dfs: 1.5 years | 39/54 (72%) | Not stated | Median: 3.1 years |
| 35 | Chemotherapy only | 33/41 (80%) | 25%–14% | 0.8 years (p=0.044) dfs probability at 5 years: 0.39 vs. 0.25 (p=0.044) |
15/28 (54%) | 1.1 years (p=0.009) Probability at 5 years: 0.38 vs. 0.23 (p=0.009) |
||
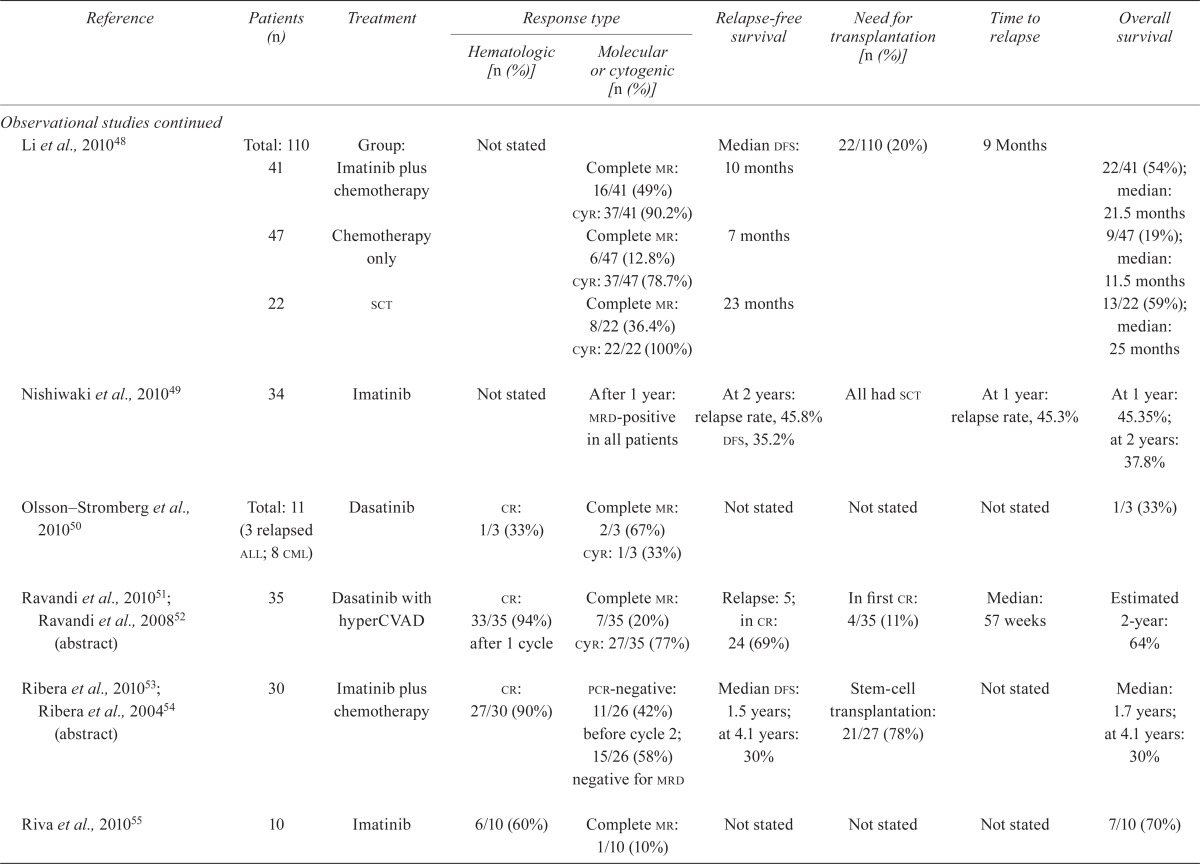
| Li et al., 201048 | Total: 110 41 |
Group: Imatinib plus chemotherapy | Not stated | Complete mr: 16/41 (49%) cyr: 37/41 (90.2%) |
Median dfs: 10 months | 22/110 (20%) | 9 Months | 22/41 (54%); median: 21.5 months |
| 47 | Chemotherapy only | Complete mr: 6/47 (12.8%) cyr: 37/47 (78.7%) |
7 months | 9/47 (19%); median: 11.5 months |
||||
| 22 | sct | Complete mr: 8/22 (36.4%) cyr: 22/22 (100%) |
23 months | 13/22 (59%); median: 25 months |
||||
| Nishiwaki et al., 201049 | 34 | Imatinib | Not stated | After 1 year: mrd-positive in all patients | At 2 years: relapse rate, 45.8% dfs, 35.2% |
All had sct | At 1 year: relapse rate, 45.3% | At 1 year: 45.35%; at 2 years: 37.8% |
| Olsson–Stromberg et al., 201050 | Total: 11 (3 relapsed all; 8 cml) |
Dasatinib | cr: 1/3 (33%) | Complete mr: 2/3 (67%) cyr: 1/3 (33%) |
Not stated | Not stated | Not stated | 1/3 (33%) |
| Ravandi et al., 201051; Ravandi et al., 200852 (abstract) | 35 | Dasatinib with hyperCVAD | cr: 33/35 (94%) after 1 cycle | Complete mr: 7/35 (20%) cyr: 27/35 (77%) |
Relapse: 5; in cr: 24 (69%) | In first cr: 4/35 (11%) | Median: 57 weeks | Estimated 2-year: 64% |
| Ribera et al., 201053; Ribera et al., 200454 (abstract) | 30 | Imatinib plus chemotherapy | cr: 27/30 (90%) | pcr-negative: 11/26 (42%) before cycle 2; 15/26 (58%) negative for mrd | Median dfs: 1.5 years; at 4.1 years: 30% | Stem-cell transplantation: 21/27 (78%) | Not stated | Median: 1.7 years; at 4.1 years: 30% |
| Riva et al., 201055 | 10 | Imatinib | 6/10 (60%) | Complete mr: 1/10 (10%) | Not stated | Not stated | Not stated | 7/10 (70%) |
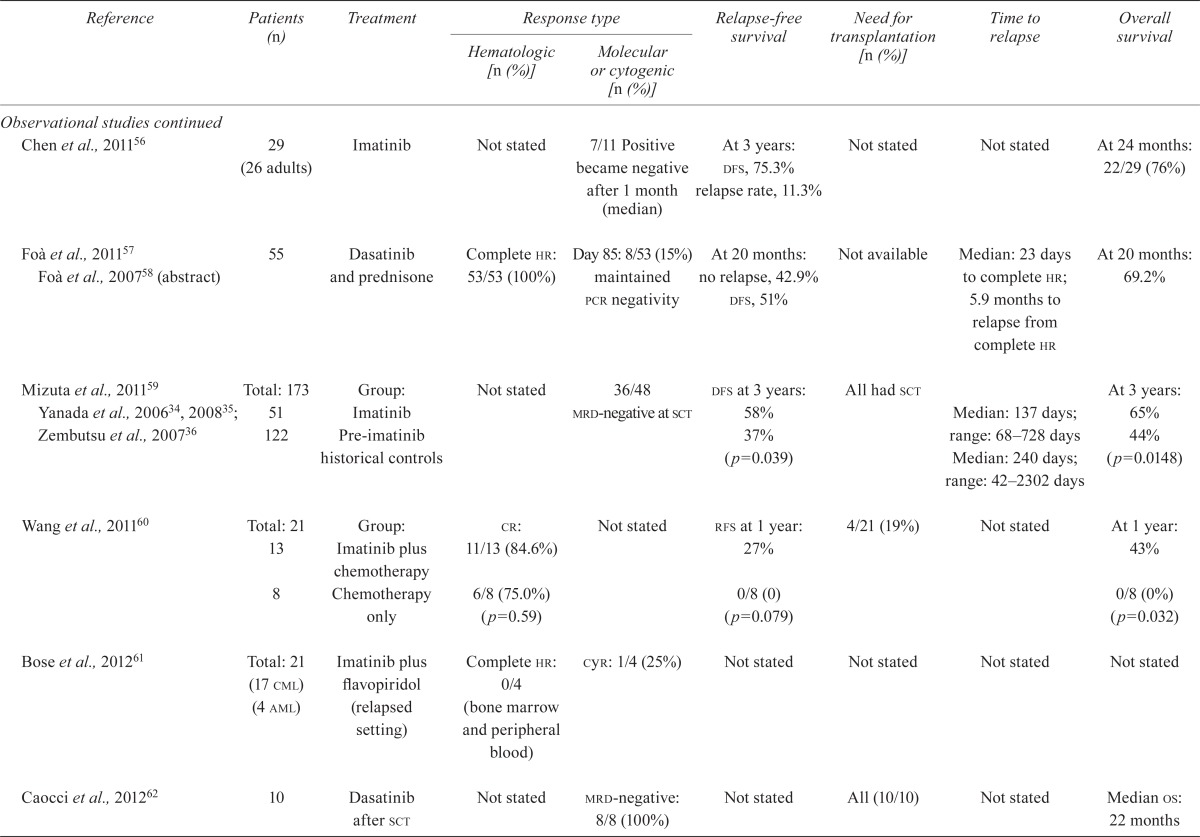
| Chen et al., 201156 | 29 (26 adults) | Imatinib | Not stated | 7/11 Positive became negative after 1 month (median) | At 3 years: dfs, 75.3% relapse rate, 11.3% |
Not stated | Not stated | At 24 months: 22/29 (76%) |
| Foà et al., 201157 Foà et al., 200758 (abstract) | 55 | Dasatinib and prednisone | Complete hr: 53/53 (100%) | Day 85: 8/53 (15%) maintained pcr negativity | At 20 months: no relapse, 42.9% dfs, 51% | Not available | Median: 23 days to complete hr; 5.9 months to relapse from complete hr | At 20 months: 69.2% |
| Mizuta et al., 201159 Yanada et al., 200634, 200835; Zembutsu et al., 200736 | Total: 173 | Group: Imatinib | Not stated | 36/48 | dfs at 3 years: 58% | All had sct | Median: 137 days; range: 68–728 days | At 3 years: 65% |
| 51 122 |
Pre-imatinib historical controls | mrd-negative at sct | 37% (p=0.039) | Median: 240 days; range: 42–2302 days | 44% (p=0.0148) | |||
| Wang et al., 201160 | Total: 21 13 |
Group: Imatinib plus chemotherapy | cr: 11/13 (84.6%) | Not stated | rfs at 1 year: 27% | 4/21 (19%) | Not stated | At 1 year: 43% |
| 8 | Chemotherapy only | 6/8 (75.0%) (p=0.59) | 0/8 (0) (p=0.079) | 0/8 (0%) (p=0.032) | ||||
| Bose et al., 201261 | Total: 21 (17 cml) (4 aml) |
Imatinib plus flavopiridol (relapsed setting) | Complete hr: 0/4 (bone marrow and peripheral blood) | cyr: 1/4 (25%) | Not stated | Not stated | Not stated | Not stated |
| Caocci et al., 201262 | 10 | Dasatinib after sct | Not stated | mrd-negative: 8/8 (100%) | Not stated | All (10/10) | Not stated | Median os: 22 months |
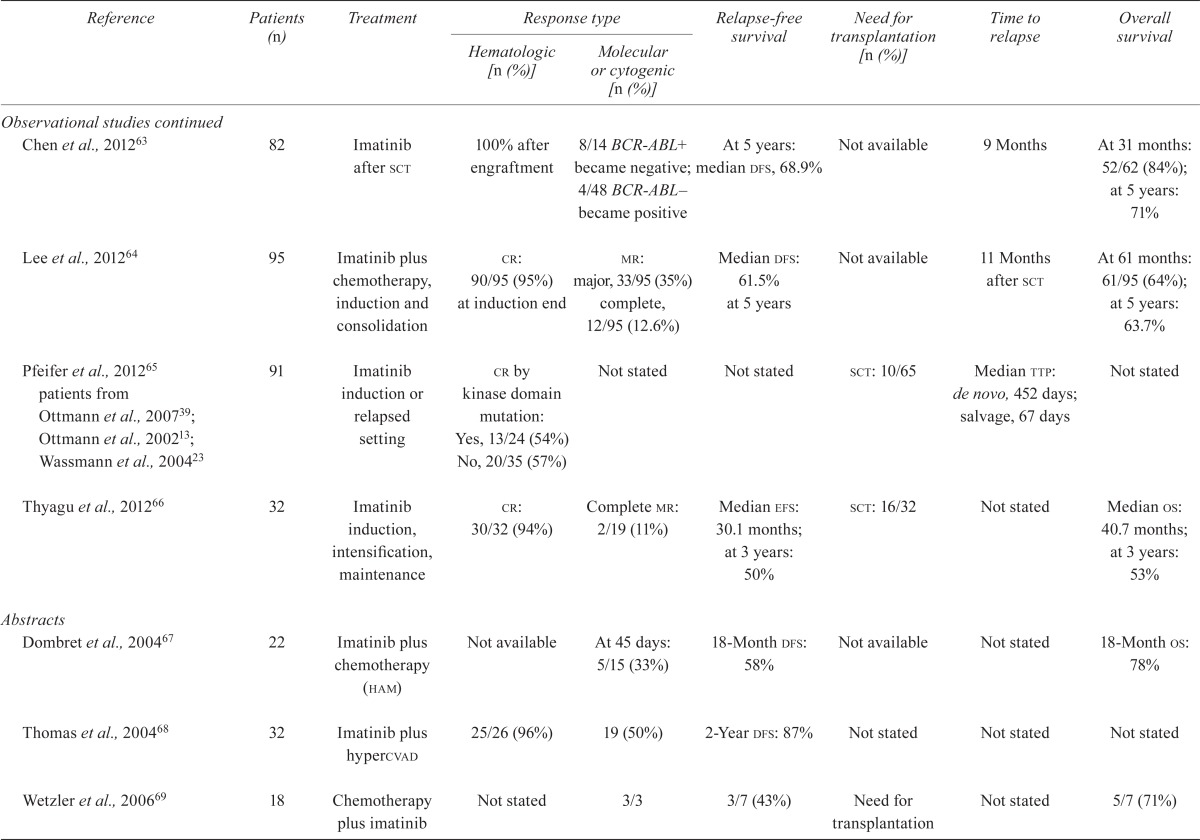
| Chen et al., 201263 | 82 | Imatinib after sct | 100% after engraftment | 8/14 BCR-ABL+ became negative; 4/48 BCR-ABL– became positive |
At 5 years: median dfs, 68.9% | Not available | 9 Months | At 31 months: 52/62 (84%); at 5 years: 71% |
| Lee et al., 201264 | 95 | Imatinib plus chemotherapy, induction and consolidation | cr: 90/95 (95%) at induction end |
mr: major, 33/95 (35%) complete, 12/95 (12.6%) |
Median dfs: 61.5% at 5 years | Not available | 11 Months after sct | At 61 months: 61/95 (64%); at 5 years: 63.7% |
| Pfeifer et al., 201265 patients from Ottmann et al., 200739; Ottmann et al., 200213; Wassmann et al., 200423 | 91 | Imatinib induction or relapsed setting |
cr by kinase domain mutation: Yes, 13/24 (54%) No, 20/35 (57%) |
Not stated | Not stated | sct: 10/65 | Median ttp: de novo, 452 days; salvage, 67 days | Not stated |
| Thyagu et al., 201266 | 32 | Imatinib induction, intensification,maintenance | cr: 30/32 (94%) | Complete mr: 2/19 (11%) | Median efs: 30.1 months; at 3 years: 50% | sct: 16/32 | Not stated | Median os: 40.7 months; at 3 years: 53% |
| Abstracts | ||||||||
| Dombret et al., 200467 | 22 | Imatinib plus chemotherapy (ham) | Not available | At 45 days: 5/15 (33%) | 18-Month dfs: 58% | Not available | Not stated | 18-Month os: 78% |
| Thomas et al., 200468 | 32 | Imatinib plus hypercvad | 25/26 (96%) | 19 (50%) | 2-Year dfs: 87% | Not stated | Not stated | Not stated |
| Wetzler et al., 200669 | 18 | Chemotherapy plus imatinib | Not stated | 3/3 | 3/7 (43%) | Need for transplantation | Not stated | 5/7 (71%) |
| Fielding et al., 200770 | 89 | Imatinib: After induction | 81% | Not stated | Not available | Not stated | At 3 years: 23% with imatinib; | |
| 64 | With phase ii induction | 91% | 26% without | |||||
| 267 | Chemotherapy | 83% | ||||||
| Pasquini et al., 200771 | Not stated | Dasatinib: Daily | 38% | Major cyr: 68% | Not stated | Not stated | Not stated | Not stated |
| Not stated | Twice daily | 32% | 55% | |||||
| Gambacorti–Passerini et al., 200872 | 72 (17 all) | Bosutinib |
hr: complete, 2/13 (15%); major, 2/13 (15%) |
cyr: complete, 1/11 (9%); major, 2/11 (18%); Major mr: 5/14 (36%) |
Not stated | Not stated | Not stated | Not stated |
| Thomas et al., 200887 | 54 | Imatinib plus hypercvad | cr: 48/51 (94%) | 52% | 3-Year dfs: 66% | Not available | At 15 months: 22% | 3-Year: 55% |
| Arellano et al., 200973 | 33 | Imatinib plus hypercvad, imatinib | Complete hr: 32/33 (97%) | Complete mr: 24/32 (75%) Complete cyr: 32/33 (97%) |
dfs: 15 months in sct and maintenance patients | 13/33 (39%) | Relapse: 10/32 (32%) after 18.3 months | sct patients: 18 months; maintenance patients: 20 months |
| Carella et al., 200974 | 3 | Imatinib induction, dasatinib or nilotinib | Complete hr: 3/3 (100%) | Complete cyr: 3/3 (100%) | Not stated | 2/3 (67%) | Not stated | 3/3 (100%) |
| Pfeifer et al., 200975 patients from Ottmann et al., 200739 | 19 | Imatinib maintenance plus low-dose interferon alfa | cr: 7/19 (37%) | Not stated | dfs: 76.7 months | Not stated | Not stated | 61 Months |
| Ribera et al., 200454 | 30 | Imatinib plus chemotherapy induction, maintenance | cr: 27/30 (90%) |
mr: major, 86%; complete, 21% |
After median 4.1 years: dfs, 30% | 21 | Relapsed: 9 | After median 4.1 years: os, 30% |
| Pfeifer et al., 201076 | Group: | mr after consolidation: | Not stated | Relapse after sct: | ||||
| 51 | Imatinib after induction and after consolidation | Not available | 2/47 (4.2%) | 39 | 30.8% | Not available | ||
| 105 | Imatinib second half induction and through consolidation | Induction cr: 89.4% | 5/40 (12.5%) | 74 | 24.3% | Induction death: 5.8% | ||
| 179 | Imatinib start induction and through consolidation | Induction cr: 85.7% | 26/79 (33%) (p=0.01) | 106 | 11.3% | Induction death: 11.3% | ||
| Rousselot et al., 201077 | 71 | Dasatinib plus chemotherapy induction, consolidation, alternating maintenance | cr: 64/71 (90%) | Bcr-Abl/Abl ratio ≤0.1% in 40/71 (55.7%) | Median: response duration, 19.2 weeks; rfs, 22.1 months | 4 | Died: 12/71 (16.9%) after median response of 19.2 weeks; median os: 27.1 months | |
| Thomas et al., 201078 | 54 | Imatinib plus hypercvad, induction | cr: 50/54 (93%) | Complete mr: 52% | Not stated | 16 | Not stated | At 3 years: os with stc, 77%; os without stc, 57% |
| Kim et al., 201179 | 50 | Nilotinib plus chemotherapy induction, | Hematologic cr: 45 (90%) | Complete mr: 27/50 (54%) | At 1 year: efs, 49.4% | 33 | At 2 years: rfs, 71.1% | At 2 years: os, 66.2% |
| Lee et al., 201180 | 30 | Dasatinib plus chemotherapy induction, maintenance | cr: 30/30 (100%) |
mr: Major, 13/30 (43%) Complete, 5/30 (17%) |
At 1 year: dfs, 76% | 24/30 (80%) | Not stated | After median 10 months: 25/30 (83%) At 1 year: os, 83% |
| Lee et al., 201181; Ravandi et al., 200852; Ravandi et al., 200982 | 61 | Dasatinib plus hypercvad, maintenance | cr1: 57/61 (94%) | Not stated | At 3 years: dfs, 49% | 15 | Relapsed: 12/61 (19%) | At 3 years: os, 62% |
| Liu–Dumlao et al., 201183; Ravandi et al., 200982 | 32 (18 all) | Dasatinib induction, maintenance | cr: 23/32 (72%) |
cyr: 25/32 (83%) Complete mr: 13/32 (43%) Major mr: 10/32 (33%) |
Not stated | 9 (2 all) | Not stated | At 3 years: os, 33%; median: 42 weeks |
| Pfeifer et al., 201184 | 26 | Imatinib: Prophylactic | At 30 months: | mrd: 10/26 (40%) | Not stated | Not stated | Not stated | After 5 years: 80% |
| 29 | Preemptive | 82% | 20/29 (69%) (p=0.046) | 74.5% (p=ns) | ||||
| Brummendorf et al., 201285 | 570 (164 cml or all) | Bosutinib |
hr: complete, 14%; major, 28% (≥ 65 years); complete, 25%; major, 30% (≤65 years) |
cyr: complete, 19%; major, 23% (≥65 years); complete, 22%; major, 32% (≤65 years) |
Not stated | Not stated | Not stated | Not stated |
| Cortes et al., 201286 | 81 (60 cml; 5 all) | Ponatinib | Major hr: 17/46 (37%) |
cyr: major, 14/41 (34%) complete, 11/41 (27%) |
Not stated | Not stated | Not stated | Not stated |
pfs = progression-free survival; hr = hematologic response; cr = complete remission; cml = chronic myeloid leukemia; ns = nonsignificant; hypercvad = hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; cyr = cytogenic response; pcr = polymerase chain reaction; dfs = disease-free survival; mrd = minimal residual disease; mr = molecular response; sct = stem-cell transplantation; all = acute lymphoblastic leukemia; os = overall survival; ttp = time to progression; rfs = relapse-free survival; efs = event-free survival; pr = partial response; div = dexamethasone, imatinib, vincristine; cns = central nervous system; ham = cytarabine, mitoxantrone.
TABLE IV.
Quality of the studies
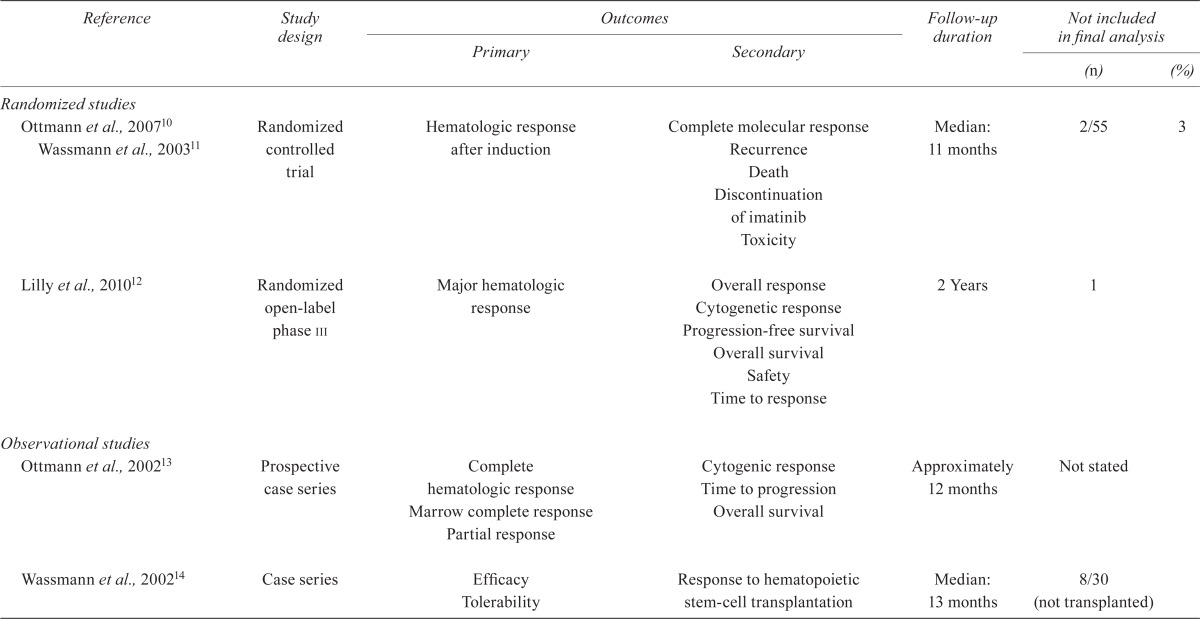
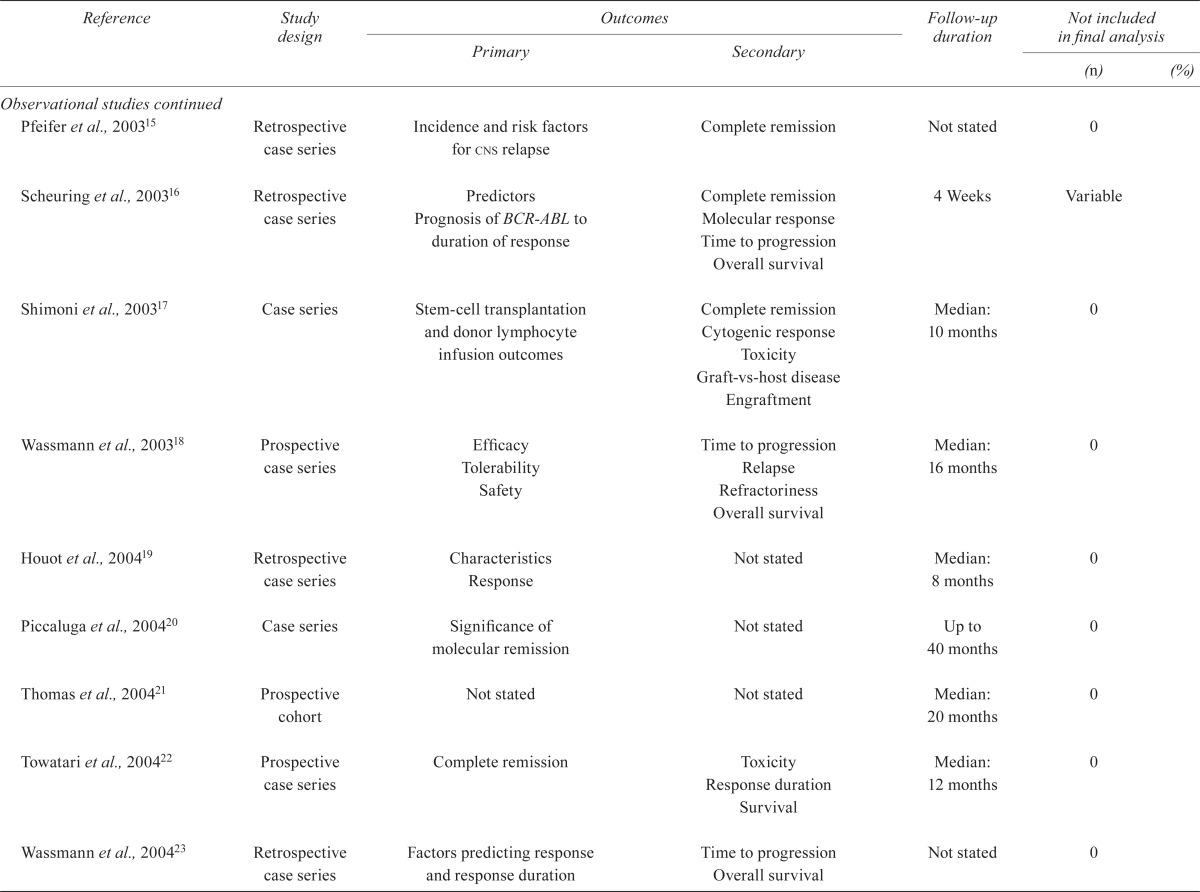
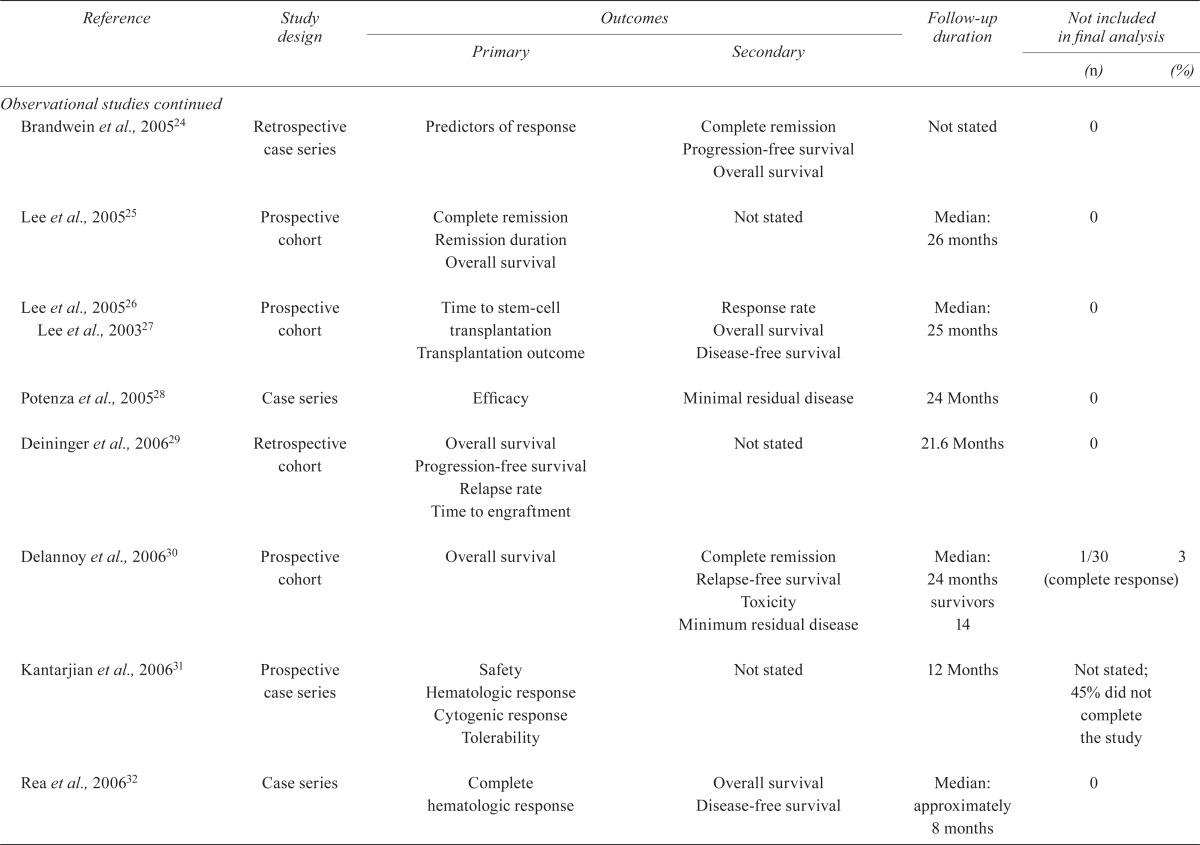
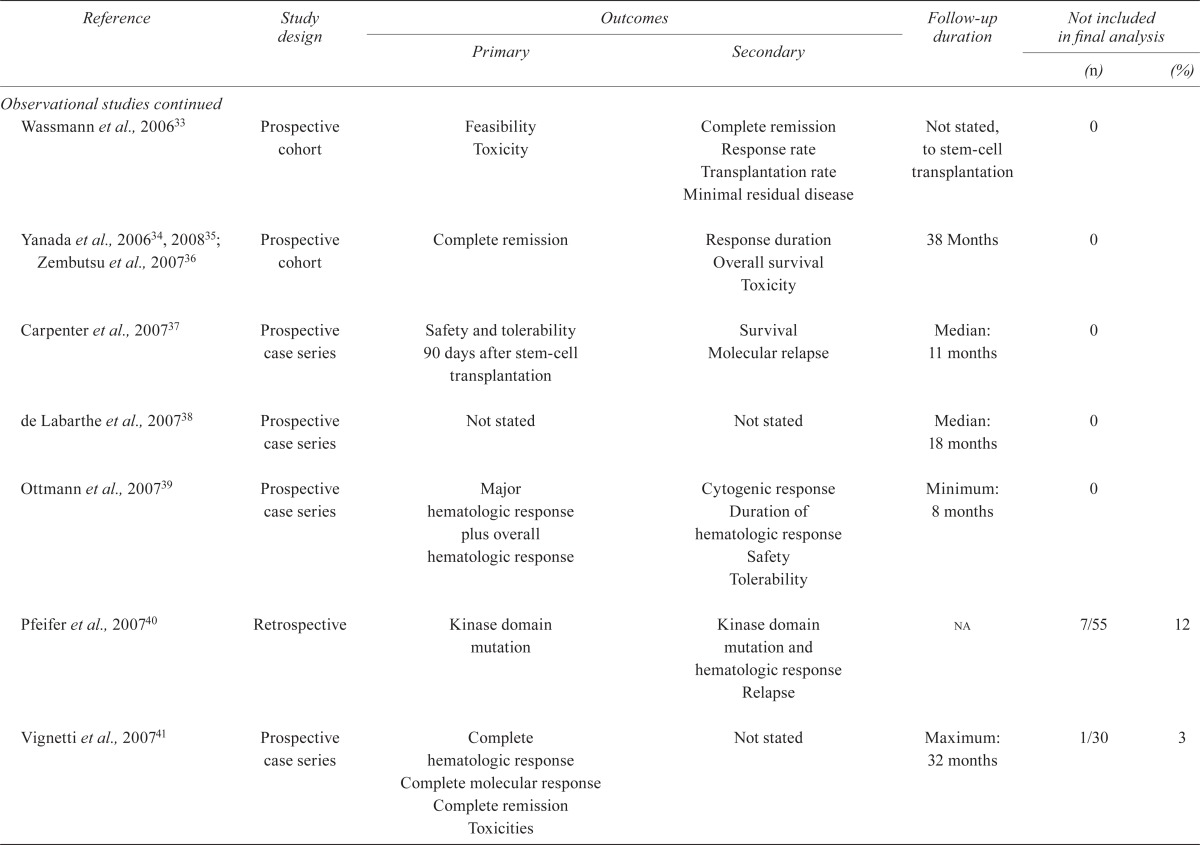
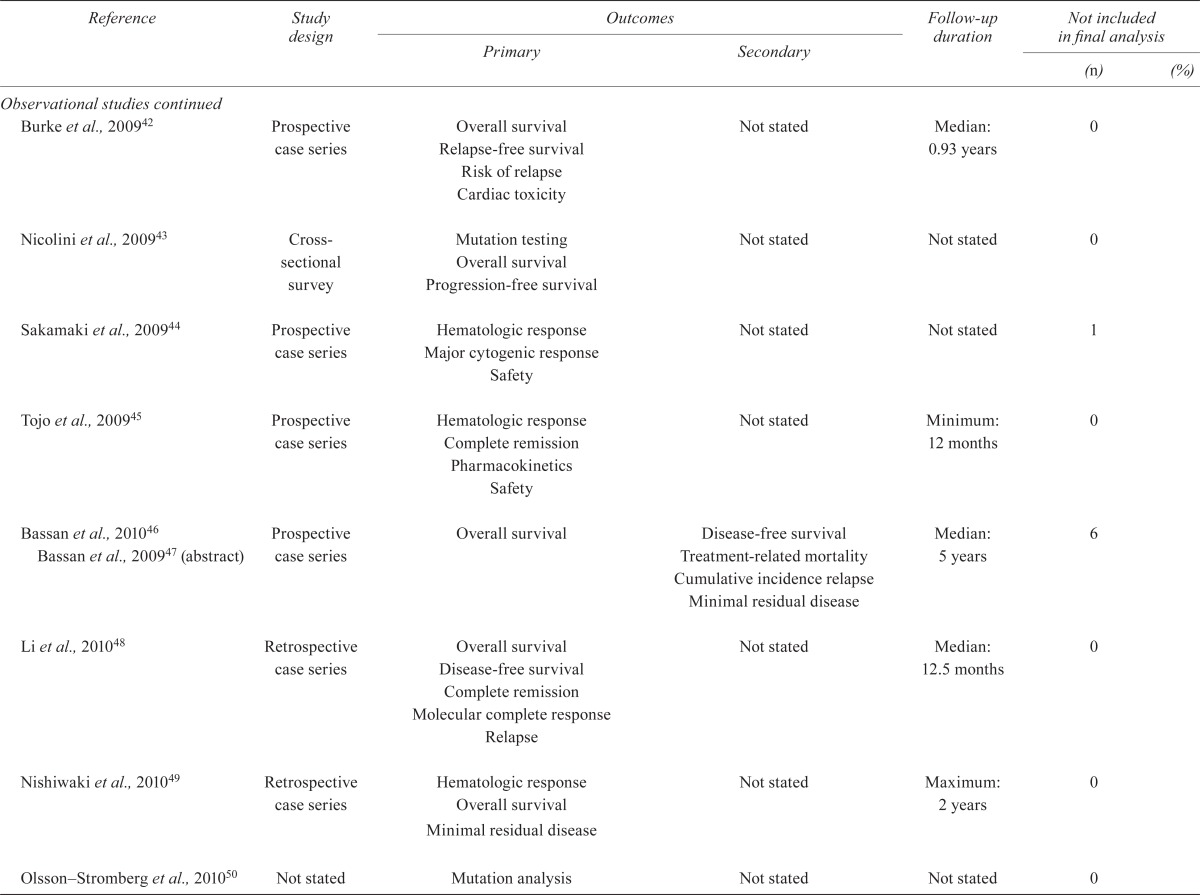
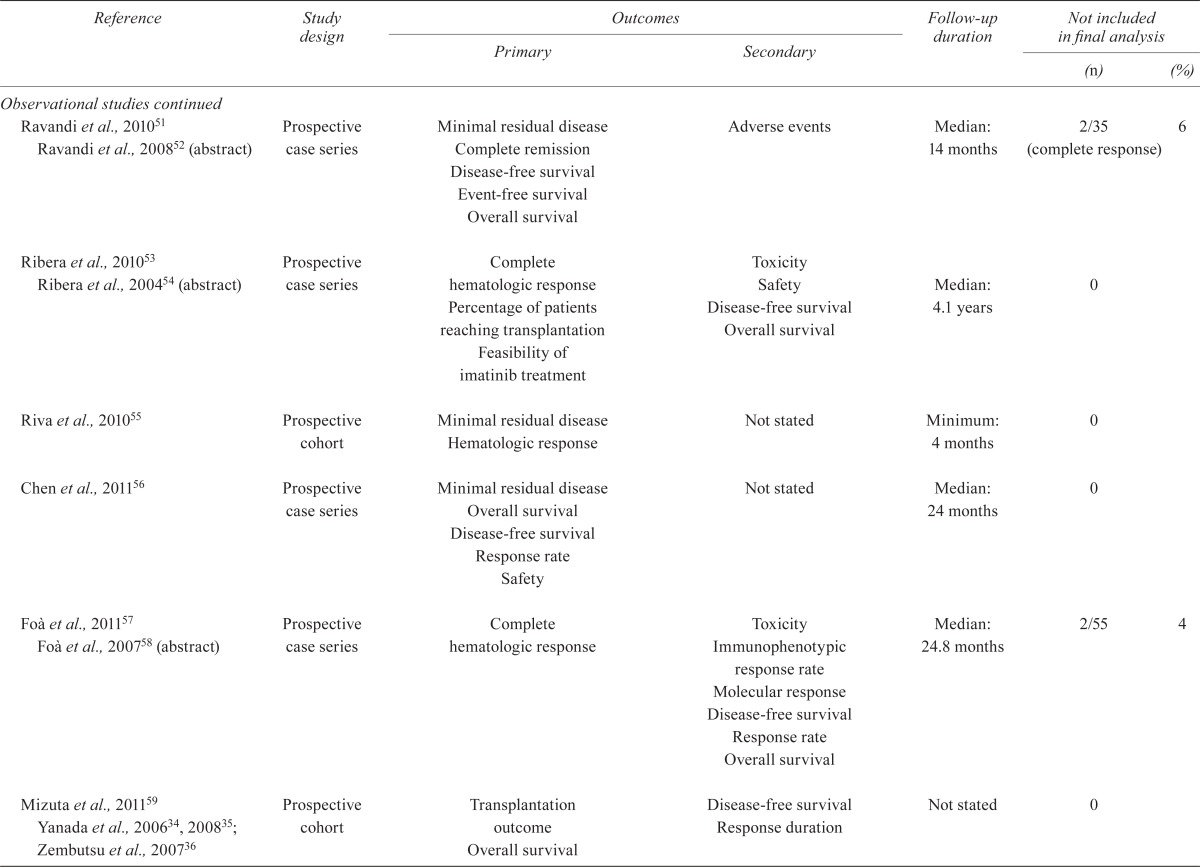
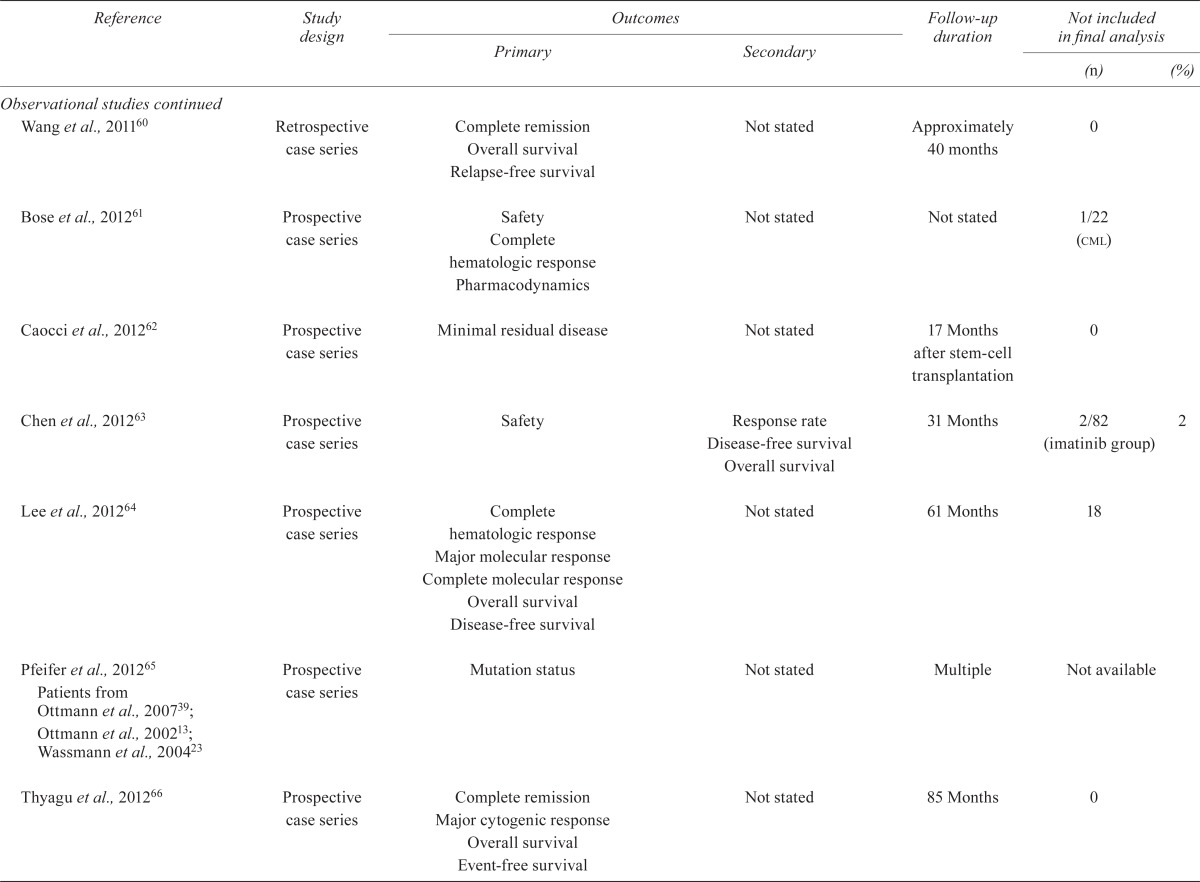
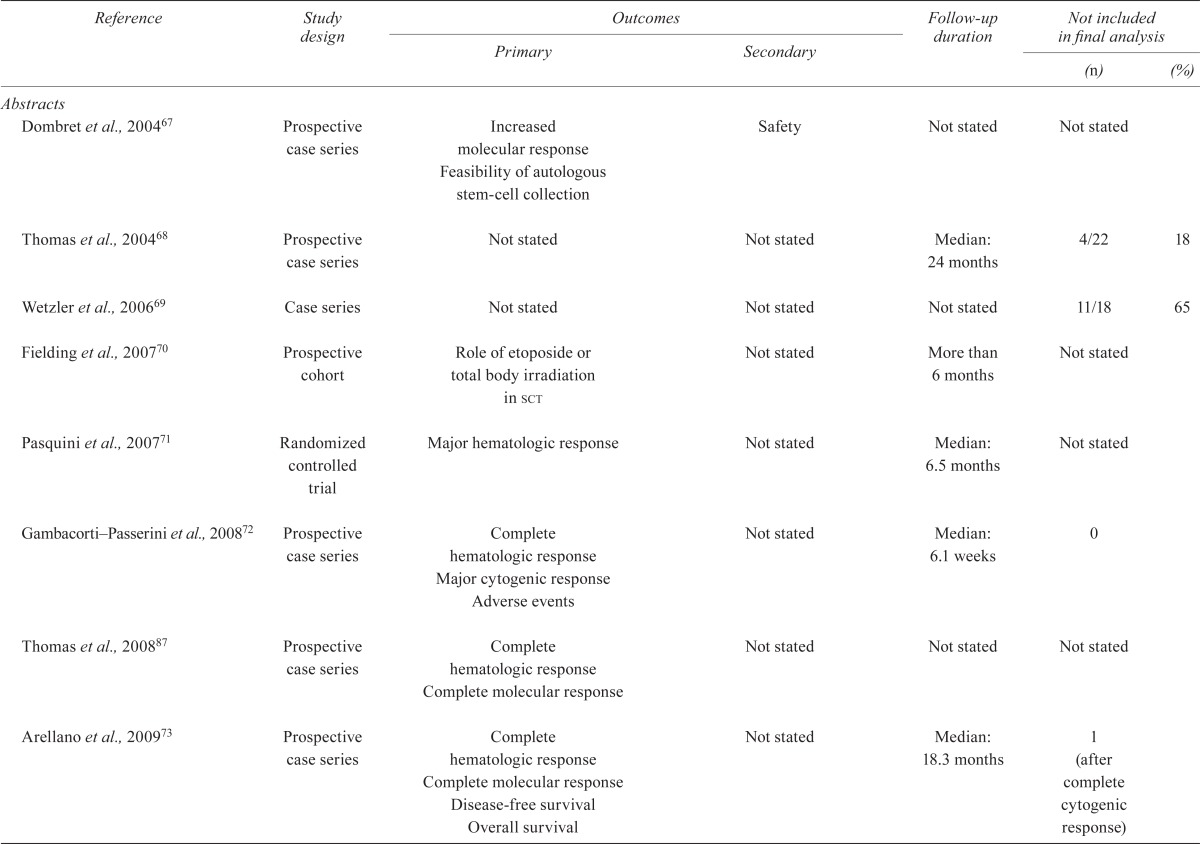
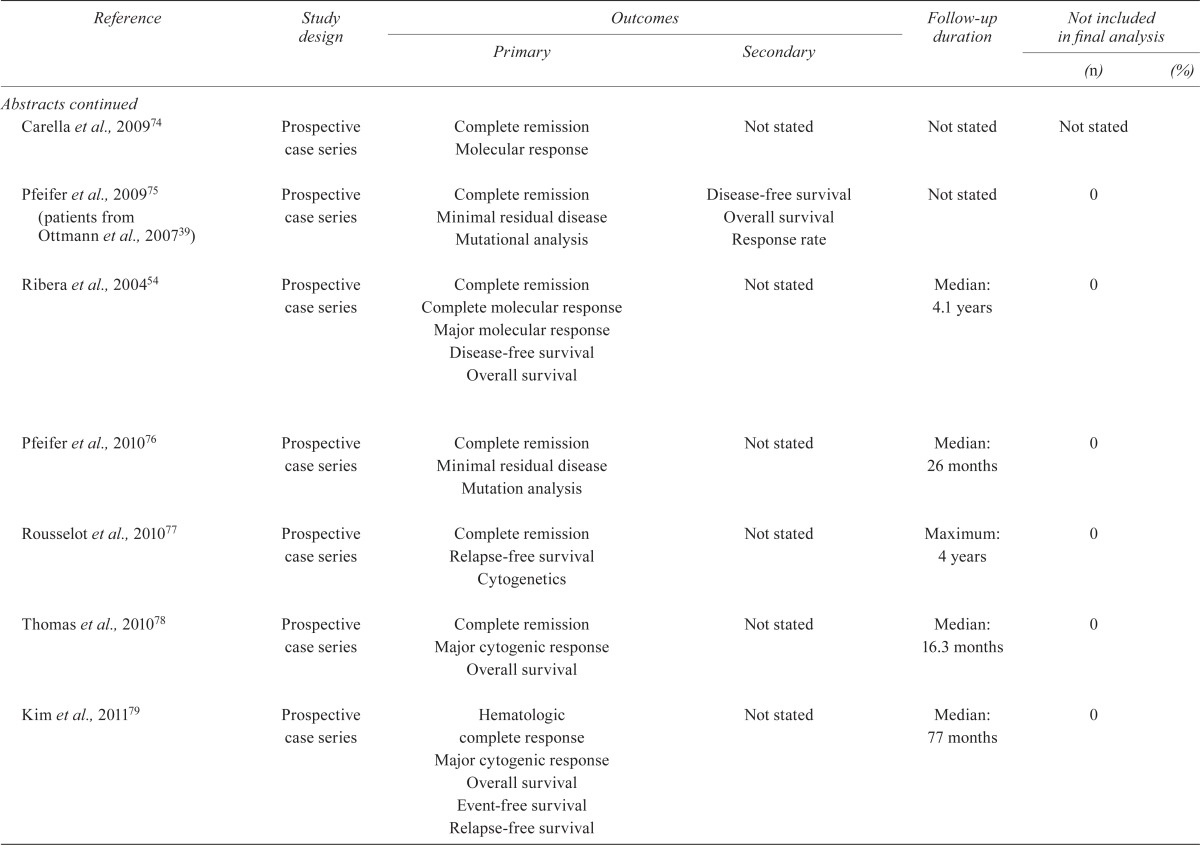
| Reference | Study design |
Outcomes
|
Follow-up duration |
Not included in final analysis
|
||
|---|---|---|---|---|---|---|
| Primary | Secondary | (n) | (%) | |||
| Randomized studies | ||||||
| Ottmann et al., 200710 Wassmann et al., 200311 | Randomized controlled trial | Hematologic response after induction | Complete molecular response Recurrence Death Discontinuation of imatinib Toxicity |
Median: 11 months | 2/55 | 3 |
| Lilly et al., 201012 | Randomized open-label phase iii | Major hematologic response | Overall response Cytogenetic response Progression-free survival Overall survival Safety Time to response |
2 Years | 1 | |
| Observational studies | ||||||
| Ottmann et al., 200213 | Prospective case series | Complete hematologic response Marrow complete response Partial response |
Cytogenic response Time to progression Overall survival |
Approximately 12 months | Not stated | |
| Wassmann et al., 200214 | Case series | Efficacy Tolerability | Response to hematopoietic stem-cell transplantation | Median: 13 months | 8/30 (not transplanted) | |
| Pfeifer et al., 200315 | Retrospective case series | Incidence and risk factors for cns relapse | Complete remission | Not stated | 0 | |
| Scheuring et al., 200316 | Retrospective case series | Predictors Prognosis of BCR-ABL to duration of response | Complete remission Molecular response Time to progression Overall survival |
4 Weeks | Variable | |
| Shimoni et al., 200317 | Case series | Stem-cell transplantation and donor lymphocyte infusion outcomes | Complete remission Cytogenic response Toxicity Graft-vs-host disease Engraftment |
Median: 10 months | 0 | |
| Wassmann et al., 200318 | Prospective case series | Efficacy Tolerability Safety |
Time to progression Relapse Refractoriness Overall survival |
Median: 16 months | 0 | |
| Houot et al., 200419 | Retrospective case series | Characteristics Response |
Not stated | Median: 8 months | 0 | |
| Piccaluga et al., 200420 | Case series | Significance of molecular remission | Not stated | Up to 40 months | 0 | |
| Thomas et al., 200421 | Prospective cohort | Not stated | Not stated | Median: 20 months | 0 | |
| Towatari et al., 200422 | Prospective case series | Complete remission | Toxicity Response duration Survival |
Median: 12 months | 0 | |
| Wassmann et al., 200423 | Retrospective case series | Factors predicting response and response duration | Time to progression Overall survival |
Not stated | 0 | |
| Brandwein et al., 200524 | Retrospective case series | Predictors of response | Complete remission Progression-free survival Overall survival |
Not stated | 0 | |
| Lee et al., 200525 | Prospective cohort | Complete remission Remission duration Overall survival |
Not stated | Median: 26 months | 0 | |
| Lee et al., 200526 Lee et al., 200327 | Prospective cohort | Time to stem-cell transplantation Transplantation outcome |
Response rate Overall survival Disease-free survival |
Median: 25 months | 0 | |
| Potenza et al., 200528 | Case series | Efficacy | Minimal residual disease | 24 Months | 0 | |
| Deininger et al., 200629 | Retrospective cohort | Overall survival Progression-free survival Relapse rate Time to engraftment |
Not stated | 21.6 Months | 0 | |
| Delannoy et al., 200630 | Prospective cohort | Overall survival | Complete remission Relapse-free survival Toxicity Minimum residual disease |
Median: 24 months survivors 14 | 1/30 (complete response) | 3 |
| Kantarjian et al., 200631 | Prospective case series | Safety Hematologic response Cytogenic response Tolerability |
Not stated | 12 Months | Not stated; 45% did not complete the study | |
| Rea et al., 200632 | Case series | Complete hematologic response | Overall survival Disease-free survival |
Median: approximately 8 months | 0 | |
| Wassmann et al., 200633 | Prospective cohort | Feasibility Toxicity |
Complete remission Response rate Transplantation rate Minimal residual disease |
Not stated, to stem-cell transplantation | 0 | |
| Yanada et al., 200634, 200835; Zembutsu et al., 200736 | Prospective cohort | Complete remission | Response duration Overall survival Toxicity |
38 Months | 0 | |
| Carpenter et al., 200737 | Prospective case series | Safety and tolerability 90 days after stem-cell transplantation | Survival Molecular relapse |
Median: 11 months | 0 | |
| de Labarthe et al., 200738 | Prospective case series | Not stated | Not stated | Median: 18 months | 0 | |
| Ottmann et al., 200739 | Prospective case series | Major hematologic response plus overall hematologic response | Cytogenic response Duration of hematologic response Safety Tolerability |
Minimum: 8 months | 0 | |
| Pfeifer et al., 200740 | Retrospective | Kinase domain mutation | Kinase domain mutation and hematologic response Relapse |
na | 7/55 | 12 |
| Vignetti et al., 200741 | Prospective case series | Complete hematologic response Complete molecular response Complete remission Toxicities |
Not stated | Maximum: 32 months | 1/30 | 3 |
| Burke et al., 200942 | Prospective case series | Overall survival Relapse-free survival Risk of relapse Cardiac toxicity |
Not stated | Median: 0.93 years | 0 | |
| Nicolini et al., 200943 | Cross-sectional survey | Mutation testing Overall survival Progression-free survival |
Not stated | Not stated | 0 | |
| Sakamaki et al., 200944 | Prospective case series | Hematologic response Major cytogenic response Safety |
Not stated | Not stated | 1 | |
| Tojo et al., 200945 | Prospective case series | Hematologic response Complete remission Pharmacokinetics Safety |
Not stated | Minimum: 12 months | 0 | |
| Bassan et al., 201046 Bassan et al., 200947 (abstract) | Prospective case series | Overall survival | Disease-free survival Treatment-related mortality Cumulative incidence relapse Minimal residual disease |
Median: 5 years | 6 | |
| Li et al., 201048 | Retrospective case series | Overall survival Disease-free survival Complete remission Molecular complete response Relapse |
Not stated | Median: 12.5 months | 0 | |
| Nishiwaki et al., 201049 | Retrospective case series | Hematologic response Overall survival Minimal residual disease |
Not stated | Maximum: 2 years | 0 | |
| 2Olsson–Stromberg et al., 201050 | Not stated | Mutation analysis | Not stated | Not stated | 0 | |
| Ravandi et al., 201051 Ravandi et al., 200852 (abstract) | Prospective case series | Minimal residual disease Complete remission Disease-free survival Event-free survival Overall survival |
Adverse events | Median: 14 months | 2/35 (complete response) | 6 |
| Ribera et al., 201053 Ribera et al., 200454 (abstract) | Prospective case series | Complete hematologic response Percentage of patients reaching transplantation Feasibility of imatinib treatment |
Toxicity Safety Disease-free survival Overall survival |
Median: 4.1 years | 0 | |
| Riva et al., 201055 | Prospective cohort | Minimal residual disease Hematologic response |
Not stated | Minimum: 4 months | 0 | |
| Chen et al., 201156 | Prospective case series | Minimal residual disease Overall survival Disease-free survival Response rate Safety |
Not stated | Median: 24 months | 0 | |
| Foà et al., 201157 Foà et al., 200758 (abstract) | Prospective case series | Complete hematologic response | Toxicity Immunophenotypic response rate Molecular response Disease-free survival Response rate Overall survival |
Median: 24.8 months | 2/55 | 4 |
| Mizuta et al., 201159 Yanada et al., 200634, 200835; Zembutsu et al., 200736 | Prospective cohort | Transplantation outcome Overall survival |
Disease-free survival Response duration |
Not stated | 0 | |
| Wang et al., 201160 | Retrospective case series | Complete remission Overall survival Relapse-free survival |
Not stated | Approximately 40 months | 0 | |
| Bose et al., 201261 | Prospective case series | Safety Complete hematologic response Pharmacodynamics |
Not stated | Not stated | 1/22 (cml) | |
| Caocci et al., 201262 | Prospective case series | Minimal residual disease | Not stated | 17 Months after stem-cell transplantation | 0 | |
| Chen et al., 201263 | Prospective case series | Safety | Response rate Disease-free survival Overall survival |
31 Months | 2/82 (imatinib group) | 2 |
| Lee et al., 201264 | Prospective case series | Complete hematologic response Major molecular response Complete molecular response Overall survival Disease-free survival |
Not stated | 61 Months | 18 | |
| Pfeifer et al., 201265 Patients from Ottmann et al., 200739; Ottmann et al., 200213; Wassmann et al., 200423 | Prospective case series | Mutation status | Not stated | Multiple | Not available | |
| Thyagu et al., 201266 | Prospective case series | Complete remission Major cytogenic response Overall survival Event-free survival |
Not stated | 85 Months | 0 | |
| Abstracts | ||||||
| Dombret et al., 200467 | Prospective case series | Increased molecular response Feasibility of autologous stem-cell collection |
Safety | Not stated | Not stated | |
| Thomas et al., 200468 | Prospective case series | Not stated | Not stated | Median: 24 months | 4/22 | 18 |
| Wetzler et al., 200669 | Case series | Not stated | Not stated | Not stated | 11/18 | 65 |
| Fielding et al., 200770 | Prospective cohort | Role of etoposide or total body irradiation in sct | Not stated | More than 6 months | Not stated | |
| Pasquini et al., 200771 | Randomized controlled trial | Major hematologic response | Not stated | Median: 6.5 months | Not stated | |
| Gambacorti–Passerini et al., 200872 | Prospective case series | Complete hematologic response Major cytogenic response Adverse events |
Not stated | Median: 6.1 weeks | 0 | |
| Thomas et al., 200887 | Prospective case series | Complete hematologic response Complete molecular response |
Not stated | Not stated | Not stated | |
| Arellano et al., 200973 | Prospective case series | Complete hematologic response Complete molecular response Disease-free survival Overall survival |
Not stated | Median: 18.3 months | 1 (after complete cytogenic response) | |
| Carella et al., 200974 | Prospective case series | Complete remission Molecular response |
Not stated | Not stated | Not stated | |
| Pfeifer et al., 200975 (patients from Ottmann et al., 200739) | Prospective case series | Complete remission Minimal residual disease Mutational analysis |
Disease-free survival Overall survival Response rate |
Not stated | 0 | |
| Ribera et al., 200454 | Prospective case series | Complete remission Complete molecular response Major molecular response Disease-free survival Overall survival |
Not stated | Median: 4.1 years | 0 | |
| Pfeifer et al., 201076 | Prospective case series | Complete remission Minimal residual disease Mutation analysis |
Not stated | Median: 26 months | 0 | |
| Rousselot et al., 201077 | Prospective case series | Complete remission Relapse-free survival Cytogenetics |
Not stated | Maximum: 4 years | 0 | |
| Thomas et al., 201078 | Prospective case series | Complete remission Major cytogenic response Overall survival |
Not stated | Median: 16.3 months | 0 | |
| Kim et al., 201179 | Prospective case series | Hematologic complete response Major cytogenic response Overall survival Event-free survival Relapse-free survival |
Not stated | Median: 77 months | 0 | |
| Lee et al., 201180 | Prospective case series | Complete remission Minimal residual disease Major molecular response |
Not stated | Median: 1.4 months | 0 | |
| Lee et al., 201181 Ravandi et al., 200852; Ravandi et al., 200982 | Prospective case series | Complete remission Disease-free survival Overall survival |
Not stated | Median: 10 months | 6/36 | |
| Liu–Dumlao et al., 201183 Ravandi et al., 200982 | Prospective case series | Complete remission Cytogenic response Molecular response |
Not stated | Median: 26.1 months | Not stated | |
| Pfeifer et al., 201184 | Prospective case series | Minimal residual disease Remission duration Tolerability |
Not stated | Median: 139 weeks | 0 | |
| Brummendorf et al., 201285 | Prospective case series | Major hematologic response Complete hematologic response |
Not stated | Median: 30 months | Not stated | |
| Major cytogenic response Complete cytogenic response |
Not stated | Median: 31 months | 0 | |||
| Cortes et al., 201286 | Prospective case series | Adverse events Major hematologic response Molecular response Cytogenic response Dose-limiting toxicities |
Not stated | Median: 56 weeks | 0 | |
2.3. Identification and Selection of Studies
The medline, embase, and Cochrane Library databases and abstracts from key conferences were systematically searched to December 2012. The full search strategy is presented in Table i.
The panel selected studies that met these inclusion criteria:
Randomized controlled trial, cohort or case series, systematic review, or guideline
Published in English
Original reports and abstracts
- Studies were excluded if they
- did not report clinical outcome,
- were letters or reviews, or
- included 5 or fewer patients with Ph+ or BCR-ABL+ all.
We also excluded studies in which outcomes for patients with cml could not be differentiated from outcomes for patients with all, unless the patients with cml accounted for fewer than 5% of cohort members.
2.4. Data Extraction
Three reviewers assessed the citations for inclusion (LS, SL, SC). Data were abstracted by the panel members, verified by an author with methodology expertise (NS), and entered into predefined data abstraction forms. The content of the data abstraction forms was used to generate tables describing trial design, quality, and outcomes (Tables iii–v). The tables were used as a basis for discussion by the panel and to generate evidence-based conclusions.
3. RESULTS
3.1. Literature Search Results
The search retrieved 277 papers and thirty-six conference abstracts; eighty articles and the thirty-six conference abstracts were examined further for inclusion. Studies were excluded if patients with all could not be differentiated from patients with cml88–92; if they summarized results from previous studies of tkis8,93; or if treatment did not include tkis94. Several studies reported data from a cohort of patients that were already reported and thus are counted as one report. The evidence tables were therefore generated using sixty-eight studies described in forty-seven papers and twenty-one abstracts (Table iii).
3.2. Recommendations
3.2.1. Question 1
For which patients with Ph+ or BCR-ABL+ all (de novo patients, patients in whom prior therapy has failed, and patients with relapsed disease) should tkis be considered?
Evidence:
Of the sixty-eight included studies examining the efficacy of tkis in patients with Ph+ or BCR-ABL+ all (Tables iii–v), four examined the addition of tkis after transplantation and were not included in the discussion related to Question 1. Three studies included de novo and relapsed patients in their analysis and did not stratify results accordingly. Those studies were also not included in the discussion related to Question 1, leaving sixty-one studies for consideration.
De Novo Population:
One randomized study of 56 newly diagnosed older patients with Ph+ all compared imatinib with chemotherapy during remission induction10,11. It demonstrated a clear benefit for patients randomized to imatinib during induction compared with patients randomized to chemotherapy alone. Patients who received imatinib during induction had a higher rate of hematologic response, which was the primary outcome of the study. The overall cr rate was statistically higher in the imatinib induction group (96.3% vs. 50%, p < 0.0001). Patients in both arms subsequently received imatinib with consolidation chemotherapy, and the 18-month disease-free survival (dfs) and os were similar in both groups [29.5% and 57.2% for the imatinib group vs. 34.6% and 41% for the chemotherapy group, p = nonsignificant (ns)].
Thirty-five observational studies had examined the efficacy of induction with tkis, with or without chemotherapy, in patients with Ph+ all. Three studies included a mix of de novo and relapsed patients and did not stratify the results by exposure to prior therapies29,43,65; those studies were therefore excluded during the discussion of this question. Of the remaining thirty-five studies, twenty-nine administered imatinib, five administered dasatinib, and one administered nilotinib. In thirty prospective studies examining 1677 patients, cr rates ranged from 56% to 100%, with cr rates exceeding 80% in twenty-eight of thirty-one studies. Median dfs ranged from 10 months to 20 months, and median os ranged from 20 months to 40 months. In addition, 5-year os ranged from 40% to 60%.
A prospective study comparing imatinib plus chemotherapy with chemotherapy alone showed improvement for both the cr rate (92% vs. 80%) and the median os (3.1 years vs. 1.1 years, p = 0.009) in the imatinib-plus-chemotherapy group46. In addition, one abstract reported a comparison of imatinib after induction, imatinib with the second phase of induction, and chemotherapy alone70. The abstract reported comparable rates of cr and os at 3 years in patients treated with or without imatinib (os: 23% vs. 26%). Among the reviewed studies that addressed this question, the latter study was important, but a notable outlier. Finally, four studies described improved relapse-free survival (rfs) or os for treated patients compared with historical controls21,25,26,30,35,59.
Five retrospective studies in 186 patients examined the efficacy of tki induction, showing similar results. One study comparing imatinib plus chemotherapy with imatinib alone showed statistically comparable cr rates (85% vs. 75%, p = ns), but improved os with the addition of imatinib to chemotherapy (43% vs. 0% for chemotherapy alone at 1 year, p = 0.032)60. In addition, a second retrospective study comparing imatinib plus chemotherapy with chemotherapy alone observed improved median os in the imatinib-plus-chemotherapy group (21.5 months vs. 11.5 months)48.
Relapsed Population:
In the relapsed population, no studies comparing tkis with chemotherapy were located. One randomized study examined the efficacy of a tki in relapsed patients with Ph+ all. That study randomized 85 patients who had relapsed on imatinib or who were resistant to imatinib to receive dasatinib 140 mg once daily or dasatinib 70 mg twice daily12. Compared with patients randomized to 70 mg dasatinib twice daily, those randomized to 140 mg dasatinib once daily had a higher cr rate (50% vs. 38%), but a similar median os (6.5 months vs. 9.1 months, p = 0.34).
Twenty-four observational studies (twenty prospective, four retrospective) have examined the efficacy of a tki for the treatment of relapsed patients with Ph+ all. Of those studies, fourteen gave imatinib13–16,23,29,32,37,42,49,55,56,61,63,75, five gave dasatinib44,50,71,83,88, two gave nilotinib50,75, two gave bosutinib72,85, and one gave ponatinib86. The tkis were given with or without chemotherapy. In twenty prospective studies examining 1164 patients, cr rates varied widely (from 0% to 90%), and os rates ranged from 30% to 70% at 1 year (median os: 5–60 months). In the four retrospective studies examining 223 patients, the cr rate ranged from 20% to 70%, and the os rate ranged from 20% to 45% at 1 year.
Consensus:
The panel agreed that the reported duration of follow-up was insufficient to make a definitive statement about the effect of tkis on long-term os. Some panel members felt that the observation that tkis improve early outcome in Ph+ and BCR-ABL+ all, allowing more patients to undergo allogeneic transplantation (which remains the definitive curative therapy for this condition), was sufficient to imply that an os benefit is likely. Other panel members reserved judgement until a longer-term os benefit is actually reported.
Recommendation 1(A):
Use a tki in all newly diagnosed patients with Ph+ or BCR-ABL+ all to increase complete response (hematologic, cytogenetic, and molecular), prolong time to relapse, improve transplant eligibility, and improve leukemia- free survival. (Level of evidence: i to ii-2; Grade of recommendation: A)
Clinical Considerations:
This recommendation applies to both transplant-eligible and non-transplant-eligible patients, including elderly patients.
The evidence about the effects of tkis on os is conflicting. The panel agreed that there was definitive evidence that, compared with standard therapy without a tki or with no therapy respectively, the addition of a tki to standard therapy or the use of a tki alone for Ph+ and BCR-ABL+ all improves hematologic, cytogenetic, and molecular response; lengthens time to progression (ttp); and improves progression-free survival (pfs).
The panel also agreed that improved pfs was a particularly relevant clinical outcome because clinical progression in this disease is rapid without a tki and a late survival benefit can be realized only if patients survive beyond the first 12–24 months after diagnosis. Again, delaying the ttp would allow for an increased number of patients to proceed to allogeneic transplantation, particularly those in search of an unrelated donor.
Recommendation 1(B):
Use a tki in patients with relapsed or refractory Ph+ and BCR-ABL+ all. (Level of evidence: ii-2; Grade of recommendation: A)
Clinical Considerations:
Most of the evidence supports the use of imatinib at a dose of 400 mg orally twice daily for patients with de novo, relapsed, or refractory Ph+ or BCR-ABL+ all.
For patients with Ph+ or BCR-ABL+ all who progress or relapse while receiving imatinib, it is reasonable to administer a second- or subsequent-generation tki with or without further additional chemotherapy. The comparative clinical data from the published clinical literature are insufficient to make a recommendation about the best second- or subsequent-generation tki to use in this setting. However, the observation of clinically significant dasatinib levels in the central nervous system90 is noted by the panel as a significant potential advantage of dasatinib and suggests that dasatinib would be an appropriate agent to use in this circumstance. The panel also noted the reported efficacy of ponatinib in patients with cml who have the T315I mutation and would recommend consideration of the use of this agent if there is evidence of the T315I mutation in Ph+ or BCR-ABL+ all.
Although there are no data suggesting that the use of a tki alone or in addition to chemotherapy is curative in either the first-line or the relapsed and refractory settings, the use of a tki alone or in combination with chemotherapy might prolong clinical response enough to allow the patient to proceed to an allogeneic transplant, which can be curative.
Even in non-transplant-eligible patients, the hematologic response to tki, although often short-lived, might improve quality of life.
3.2.2. Question 2
When in the clinical course of a patient with Ph+ or BCR-ABL+ all should a tki be administered?
Question 2(A):
Should a tki be administered during induction, consolidation, and pre-transplantation?
Evidence:
De Novo Population: In the only randomized controlled trial in de novo patients10,11, 55 individuals not eligible for transplantation were given imatinib during induction and were compared with patients given only chemotherapy during induction. Patients in both arms subsequently received imatinib. This study demonstrated a clear benefit for patients randomized to imatinib during induction compared with those randomized to chemotherapy alone during induction. Patients who received imatinib during induction had a higher rate of hematologic response, which was the primary outcome of the study. The overall cr rate was statistically higher in the imatinib induction group (96.3% vs. 50%, p < 0.0001). Patients in both arms subsequently received imatinib with consolidation chemotherapy, and the 18-month dfs and os were similar in both groups (29.5% and 57.2% respectively for the imatinib group vs. 34.6% and 41% for the chemotherapy group, p = ns).
Thirty-eight observational studies in 1863 de novo patients and 334 patients who were a mixture of de novo and relapsed (from three studies) gave a tki during induction. In twelve of the thirty-eight studies, a tki was given only during induction. Twenty studies gave a tki during consolidation or for maintenance (or both) in addition to during induction. Three studies gave imatinib before allogeneic transplantation17,29,42.
In some studies, imatinib was started during induction, as soon as Ph+ or BCR-ABL+ status was determined26,34–36. In other studies, imatinib was started after remission was achieved, and the drug administered either continuously25.27,30 or intermittently21. In one study, imatinib was administered in two different ways in the same study (either after cr or during induction and after cr)33. The rate of hematologic response was higher in the group receiving imatinib after induction (78%) compared with the group receiving imatinib during induction (56%), although no difference in rfs or os was observed. In a second study, imatinib was given as consolidation after induction with chemotherapy, or with a second phase of induction70. The cr rate was higher in patients given imatinib in the second induction phase than in those given imatinib as consolidation (91% vs. 81%). A third study compared patients given imatinib after induction and consolidation with patients given imatinib in the second half of induction and through consolidation or at the beginning of induction and through consolidation76. Molecular response was greatest in the group given imatinib from the beginning of induction (33% vs. 12.5% vs. 4.2% respectively, p = 0.01).
In most studies, better outcomes were observed when imatinib was started earlier and administered for a longer period, although that was not the case in one study33.
Relapsed Population:
The only randomized trial in relapsed patients examined the efficacy of two different doses of dasatinib after failure of imatinib or imatinib intolerance12. Dasatinib was administered orally at either 140 mg once daily or 70 mg twice daily. Treatment continued until disease progression, unacceptable toxicity, or withdrawal at the request of the patient or investigator. Compared with patients given 70 mg dasatinib twice daily, those given 140 mg dasatinib once daily experienced a higher cr rate (50% vs. 38%), but a statistically similar median os (6.5 months vs. 9.1 months, p = 0.34).
Eighteen observational studies examined the efficacy of a tki given during induction, maintenance, consolidation, or pre-transplantation for the treatment of relapsed patients with Ph+ or BCR-ABL+ all. As discussed earlier, three of the studies included both de novo and relapsed patients who had been given a tki. In five of the eighteen, a tki was given during induction for only a limited duration23,31,32,44,71; ten studies gave a tki indefinitely until progression or toxicity11,14–16,45,61,72,85,86,88; and one study gave imatinib from 24 weeks onwards (that is, indefinitely)13. In addition, one study gave imatinib only as maintenance55. Finally, one study gave imatinib during induction and maintenance83.
Consensus:
The panel suggests introducing a tki as soon as the diagnosis of Ph+ or BCR-ABL+ all is confirmed.
Recommendation 2(A):
Use a tki during induction, consolidation, and pre-transplantation. The panel found most evidence supports the use of imatinib at an oral dose of 400 mg twice daily. The data about the use of other tkis (dasatinib, nilotinib, bosutinib, ponatinib) in these settings was felt to be less substantive. (Level of evidence: ii; Grade of recommendation: B)
Clinical Considerations:
In the clinical reports, tkis have been administered during various phases of treatment and in both an intermittent and a continuous manner. The panel recommends that a tki be started as soon as the Ph+ or BCR-ABL+ status is established and that it be continued without a break and indefinitely until progression, intolerance, or allogeneic transplantation.
Question 2(B):
Should a tki be administered in the post-transplantation period?
Evidence:
Ten studies in 376 patients gave a tki in the post-transplantation period: eight studies administered imatinib, and two studies administered dasatinib37,42,49,50,53,56,62,63,70,84. Seven studies gave a tki only in the post-transplantation period. In those studies, the cr rate ranged from 33% to 100%, and the dfs rate ranged from 35% to 100%. One study gave imatinib plus chemotherapy as induction, consolidation, and post-transplantation53. Imatinib was started after transplantation in 13 of 21 patients (62%). After a median follow-up of 4.1 years, the median dfs and os were 1.5 years and 1.7 years respectively. In addition, a second study gave imatinib pre-transplantation (n = 13) or post-transplantation (n = 2)42. The rfs rate was 62% in patients given imatinib pre-transplantation and 100% in patients given imatinib post-transplantation; however, the number of patients in this report—and especially in the post-transplantation group—was particularly small.
Consensus:
There are compelling theoretical reasons to consider the use of tkis after transplantation as either consolidation or maintenance therapy. The frequency of relapse, the rapidity of relapse, the favourable toxicity profile of the tkis, and the simplicity of therapy (an oral medication administered once or twice daily) all favour consideration of a consolidation or maintenance strategy after transplantation. Of course, for the subset of patients with Ph+ or BCR-ABL+ all who are cured with allogeneic transplantation, administration of a tki after transplantation exposes them to the risk of adverse effects with no discernible benefit. Poor hematologic tolerance was also noted in one study when the tki was introduced early after transplantation53. However, clinicians can neither accurately predict who will relapse after allogeneic transplantation nor reliably distinguish the group of patients at higher risk of relapse from those who are cured. Although the presence of minimal residual disease (mrd) before transplantation is perceived as a risk for relapse and the persistence of mrd after transplantation is more predictive of disease progression, neither observation allows clinicians to reliably predict who will relapse.
Given the limited number of reports about the use of tkis after transplantation, the panel could not make an evidence-based recommendation. The panel acknowledges that some centres use a post-transplantation tki-based consolidation or maintenance strategy. No evidence-based recommendation can be made about the dose or duration of tki administration in the post-transplant setting.
Recommendation 2(B):
The evidence is insufficient to recommend for or against the routine use of a tki as consolidation or maintenance therapy after allogeneic transplantation. (Level of evidence: iii; Grade of recommendation: C)
Clinical Considerations:
Although the panel could not make any evidence-based recommendations about the routine use of a tki after allogeneic transplantation, the panel was unanimous in recommending that a tki be started as soon as there is any evidence (molecular or cytogenetic) of Ph+ or BCR-ABL+ disease.
3.2.3. Question 3
Is there a difference in efficacy between the various tkis in patients with Ph+ or BCR-ABL+ all?
Evidence:
No randomized controlled trials have compared the efficacy of the various tkis in patients with Ph+ or BCR-ABL+ all. The panel therefore examined the response rates for the various tkis, recognizing that this approach involves comparison of different reports, with accompanying limitations. The panel also studied reports in which patients with Ph+ or BCR-ABL+ all had progressed while taking one tki and were evaluated for subsequent response to a second tki.
Imatinib:
As discussed earlier, one randomized study of 56 newly diagnosed older patients with Ph+ all compared imatinib with chemotherapy during remission induction10,11. That study demonstrated a clear benefit for patients randomized to imatinib compared with patients randomized to chemotherapy alone during induction, with cr rates of 96% in the imatinib group and 50% in the chemotherapy group.
Forty-three observational studies used imatinib: twenty-nine in de novo patients, fourteen in relapsed patients, and three in mixed de novo and relapsed patients. In the de novo setting, cr rates ranged from 56% to 100%, with rates exceeding 80% in most studies. Median dfs ranged from 10 months to 20 months, and os ranged from a median of 20 to 40 months. In addition, the 5-year os rate ranged from 40% to 60%. In the relapsed setting, cr rates varied widely (0%–90%), and os rates ranged from 30% to 70% at 1 year, with median os ranging from 5 months to 60 months.
Dasatinib:
Eleven studies examined the efficacy of dasatinib: five in de novo patients51,58,77,80,81 and six in relapsed patients12,44,50,71,83,88. In the de novo population, cr rates ranged from 90% to 100%, and the os rate was approximately 65% at 2 years (median os: 27 months). In the relapsed setting, one randomized study compared two different oral doses of dasatinib (140 mg once daily vs. 70 mg twice daily)12. Compared with patients given 70 mg dasatinib twice daily, those given 140 mg dasatinib once daily experienced a higher cr rate (50% vs. 38%), but a statistically similar median os (6.5 months vs. 9.1 months, p = 0.34). In the remaining observational studies, cr rates ranged from 8% to 72%, and os rates ranged from 33% to 80% at 1 year (median os: approximately 10 months).
Nilotinib:
Three studies in a total of 70 patients examined the efficacy of nilotinib: one in de novo patients and two in relapsed patients31,45,79. In the de novo study, induction with nilotinib plus chemotherapy was given to 50 patients with Ph+ all. Results demonstrated a cr rate of 90%, with an os rate of 66% at 2 years79. In one study in the relapsed setting, a partial response of 10% was found31. In the second study in the relapsed setting, a cr rate of 43% was achieved45.
Bosutinib:
Two studies in 1019 relapsed patients, including some patients with Ph+ all, examined the efficacy of bosutinib72,85. In both studies, bosutinib was given at an oral dose of 500 mg once daily. Results showed cr rates ranging from 14% to 25% and cytogenetic response rates ranging from 9% to 22%.
Ponatinib:
One study in 81 relapsed patients with Ph+ all examined the efficacy of ponatinib86. Oral ponatinib was escalated from 45 mg once daily. A major hematologic response was reported in 17 of 46 patients (37%) and a cytogenetic response was reported in 11 of 41 patients (27%).
Consensus:
Although there are no comparative studies of the various tkis for patients with Ph+ or BCR-ABL+ all, imatinib has been extensively studied. A tki should be initiated as soon as the diagnosis of Ph+ or BCR-ABL+ all is established by standard cytogenetic, fluorescence in situ hybridization, or molecular studies. A tki can be used with any chemotherapeutic regimen, or it can be used with steroids alone or as a single agent. Imatinib in combination with prednisone is a reasonable option for induction and consolidation in elderly patients in whom allogeneic transplantation or aggressive chemotherapy is not an option.
Recommendation 3(A):
Use imatinib either alone or in combination with steroids or chemotherapy as soon as Ph+ or BCR-ABL+ is determined in patients with acute lymphoblastic leukemia. (Level of evidence: ii; Grade of recommendation: B)
Clinical Considerations:
Imatinib is typically administered orally at a dose of either 600 mg once daily or 400 mg twice daily when used with steroids or chemotherapy. It can be used orally at a dose of 400 mg twice daily when used alone.
Dasatinib12,44,50,51,58,71,77,80,81,83,88, nilotinib31,45,79, bosutinib31,45,79, and ponatinib86 have been studied in patients who are resistant or intolerant to imatinib. Those agents have produced hematologic and cytogenetic responses in that group of patients. In patients who are resistant to imatinib, the duration of response to subsequent tkis has been short.
Dasatinib at an oral dose of 50 mg or 70 mg twice daily can be used for patients who are intolerant to imatinib. Most of the published data describe the use of an oral dose of 70 mg twice daily, but the panel would also consider 50 mg twice daily because the latter dose might be associated with a lower incidence of pleural effusion.
Nilotinib at an oral dose of 400 mg twice daily, bosutinib at an oral dose of 500 mg daily, and ponatinib at an oral dose of up to 60 mg daily can also be used for patients who are either intolerant to imatinib or another tki, or who progress while receiving imatinib or another tki.
There are theoretical advantages to the initial use of a second- or subsequent-generation tki, including higher potency (dasatinib, nilotinib, bosutinib, ponatinib), broader spectrum of activity (dasatinib, ponatinib), and central nervous system penetration (dasatinib); however, the panel felt that the data were currently insufficient to recommend the up-front use of a second- or subsequent-generation tki outside the setting of a clinical trial.
Because most patients with Ph+ or BCR-ABL+ all will have been treated with imatinib alone or in combination with steroids or chemotherapy at presentation, it is reasonable to consider a second- or subsequent-generation tki either alone or in combination with steroids or chemotherapy for patients with refractory or relapsed disease. Most of the published clinical experience has used dasatinib at an oral dose of 70 mg twice daily. However, the panel is aware of studies in patients with cml which suggest that a lower oral dose of 100 mg once daily is better tolerated and equally effective in that disease. No definitive recommendation about the dose of dasatinib to be used in patients with refractory or relapsed Ph+ or BCR-ABL+ all can be made at this time. However, the panel felt that most published data favoured 70 mg orally twice daily.
Although the panel is aware of three studies using nilotinib31,45,79, three studies using bosutinib50,58,75, and one study using ponatinib86 in patients with Ph+ or BCR-ABL+ all, very few data are available, and the duration of follow-up with the use of those agents is less than that with imatinib and dasatinib in the relapsed and refractory setting.
3.2.4. Question 4
How long should therapy with a tki continue in a patient with Ph+ or BCR-ABL+ all?
Evidence:
No randomized studies have examined the optimal duration of treatment with tkis during induction, consolidation, or maintenance.
Induction:
Thirty-nine studies examined induction treatment with tkis in de novo patients. In nine of those studies, a tki was given only at induction for a range of 1–12 weeks and for a limited number of courses; however, a number of studies gave a tki indefinitely or until progression. Two studies gave a tki indefinitely after induction28,51,66, three studies gave a tki until progression or until molecular negativity was attained20,41,69, and one study gave a tki until transplantation38.
In twenty studies, a tki was given for consolidation or maintenance (or both) as well as for induction. In those studies, the duration of induction was typically 4 weeks, but ranged from 2 weeks to 8 weeks. In one study, a tki was given after induction until the next chemotherapy course48. Three studies gave imatinib before transplantation17,29,42. In one study giving a tki before transplantation, imatinib was given for a median of 45 days29.
Relapsed Setting:
In the relapsed setting, a tki was typically given indefinitely as tolerated12–14,16,39,45. However, several studies used other schedules: dasatinib for 12 weeks44; imatinib for 3 weeks every 4 weeks61; nilotinib for 12 months31; imatinib for a maximum of 56 days32; and imatinib for 24 months23. Finally, one study gave dasatinib for 2 weeks of every 8-week cycle, followed by monthly maintenance with dasatinib for 2 years83.
Maintenance Setting:
Nineteen studies gave a tki during maintenance treatment. Duration of maintenance ranged from 4 weeks to 2 years, with one study giving maintenance indefinitely66. Several studies gave a tki as maintenance after transplantation37,42,49,50,53,56,62,63,70,84. Individual studies also gave imatinib for at least 1 month after transplantation56, until a complete molecular remission was sustained for 3 months63, starting at engraftment and continuing for at least 1 year37, and indefinitely as tolerated42.
Consensus:
No study has examined the impact on disease control of tkis used upon achievement of cr, but the panel recommended continuing therapy until disease progression or transplantation, provided that the tki is well tolerated.
Recommendation 4(A):
Continue a tki beyond completion of chemotherapy until transplantation or disease progression. (Level of evidence: iii; Grade of recommendation: B)
Clinical Considerations:
The panel found no clinical evidence to support intermittent drug administration or drug holidays. Similarly, there was neither clinical evidence to support the use of more than one tki at the same time, nor evidence to support the use of a strategy of alternating tkis in an individual patient. Such strategies could be the subject of future investigation.
3.2.5. Question 5
Which measure of disease response should be used to follow patients with Ph+ or BCR-ABL+ all?
Evidence:
Studies examining the efficacy of tkis used a variety of outcome measures, including hematologic response (hr) such as cr, complete hr, and overall response rate; molecular response (mr) such as mrd, cytogenetic response (cyr), complete cyr, major cyr, complete mr, and major mr; and measures of duration of response such as pfs, dfs, rfs, event-free survival, and os.
Data on hr were reported in fifty-eight of sixty-eight studies; mr data were available for fifty-six studies; duration of response was available in forty-six studies; and os results were available in fifty-seven studies.
Consensus:
Hematologic cr is a poor predictor of os in patients with Ph+ or BCR-ABL+ all in almost all contexts except after allogeneic transplantation. However, failure to achieve a hematologic response is highly predictive of poor os. In contrast, molecular monitoring by quantification of the Bcr-Abl transcript level in bone marrow or peripheral blood in assays capable of detecting disease reduction to molecular transcript levels as low as 10−5 is much more predictive of rfs (and sometimes os).
Although widely practiced, the value of monitoring is not well established. The best frequency for molecular assessment and whether the source of cells for testing should be blood or marrow is not known. Although marrow assessment is thought to be more sensitive, the panel felt that use of a quantitative assay to measure the reverse transcriptase–polymerase chain reaction level of Bcr-Abl transcripts in peripheral blood once per month would be a reasonable strategy. The panel felt that the initial 2 years of therapy represent the period during which the risk of relapse is greatest, but acknowledged that no data are available to inform a recommendation about the duration of monitoring. In the absence of clinical data, the panel recommended long-term monitoring for patients in whom the resulting information would lead to a medical intervention and a change in treatment strategy.
Recommendation 5(A):
To aid in prognostic evaluation, use quantitative assessment of the Bcr-Abl transcript level to follow molecular status after the patient achieves a hematologic remission. (Level of evidence: iii; Grade of recommendation: B)
Clinical Considerations:
The panel felt that using a quantitative assay to measure the reverse transcriptase– polymerase chain reaction level of Bcr-Abl transcripts in peripheral blood once per month would be a reasonable and practical monitoring strategy.
Achievement of a complete hr is a poor predictor of os in patients with Ph+ or BCR-ABL+ all after conventional chemotherapy without a tki because almost all patients relapse after first responding. However, failure to achieve a hr is highly predictive of poor os. In contrast, molecular monitoring by quantification of the Bcr-Abl transcript level in bone marrow or peripheral blood in assays capable of detecting disease reduction to 10−5 is predictive of rfs and sometimes os in most series where that outcome was examined. Conversely, reappearance of Bcr-Abl transcripts when they were previously absent usually predicts relapse.
Thus, although widely practiced, the value of monitoring has not been critically or explicitly evaluated in large trials. The value of such testing in predicting relapse cannot therefore be calculated with confidence. This issue is further complicated by the variety of combination chemotherapy regimens and schedules that have used with tkis, making widely applicable recommendations about the frequency and timing of molecular monitoring difficult. Nevertheless, common practice from larger series includes molecular monitoring at the end of various treatment blocks (induction, consolidation, intensification, and so on) and frequently after definitive treatment is completed (that is, after transplantation or maintenance, as appropriate). The best frequency for molecular assessment and the best source of cells for testing (blood or marrow) is therefore not entirely known. Furthermore, relapses in Ph+ all can occur rapidly, and the true value of molecular monitoring is to allow for earlier interventions that would make a difference in the ultimate outcome. Thus, the utility and impact of molecular monitoring has not been formally demonstrated.
Information about the importance of monitoring for BCR-ABL mutations in patients with Ph+ or BCR-ABL+ all is insufficient.
4. AUTHORSHIP
In these evidence-based guidelines, YA, SC, SL, BL, MM, LS, and RT systematically reviewed the data and created recommendations. SC wrote the article, and all authors reviewed the manuscript. NS and VP were responsible for the methodology analyses. After the initial version of the manuscript was written, SC asked Anna Christofides, a medical writer, to update the literature search; based on the updated search, SC and AC revised relevant aspects of the descriptive text. The conclusions and recommendations were not changed.
5. ACKNOWLEDGMENTS
The authors acknowledge medical writing support from Anna Christofides msc rd.
6. CONFLICT OF INTEREST DISCLOSURES
No authors reported financial conflicts of interest connected with this publication. This work was supported by unrestricted grants from Novartis and Bristol–Myers Squibb.
7. REFERENCES
- 1.United States, National Institutes of Health, National Cancer Institute (nci) Childhood Acute Lymphoblastic Leukemia Treatment (PDQ) [Web page] Bethesda, MD: NCI; n.d. [Available at: http://www.cancer.gov/cancertopics/pdq/treatment/childALL/HealthProfessional; cited January 28, 2013] [Google Scholar]
- 2.Be the Match. Acute lymphoblastic leukemia (all) [Web page] Minneapolis, MN: U.S. National Marrow Donor Program; n.d. [Available at: http://bethematch.org/For-Patients-and-Families/Learning-about-your-disease/Acute-Lymphoblastic-Leukemia-(ALL)/; cited February 7, 2013] [Google Scholar]
- 3.Rowe JM, Buck G, Burnett AK, et al. on behalf of the mrc/ncriAdult Leukemia Working Party Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international all trial: mrc ukall xii/ecog E2993. Blood. 2005;106:3760–7. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 4.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (mrc ukall xii/ecog E2993) Blood. 2008;111:1827–33. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 5.Burmeister T, Schwartz S, Bartram CR, Gokbuget N, Hoelzer D, Thiel E, on behalf of the gmall study group Patients’ age and BCR-ABL frequency in adult B-precursor all: a retrospective analysis from the gmall study group. Blood. 2008;112:918–19. doi: 10.1182/blood-2008-04-149286. [DOI] [PubMed] [Google Scholar]
- 6.Bloomfield CD, Peterson LC, Yunis JJ, Brunning RD. The Philadelphia chromosome (Ph1) in adults presenting with acute leukaemia: a comparison of Ph1+ and Ph1– patients. Br J Haematol. 1977;36:347–58. doi: 10.1111/j.1365-2141.1977.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 7.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–48. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 8.Brave M, Goodman V, Kaminskas E, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome–positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res. 2008;14:352–9. doi: 10.1158/1078-0432.CCR-07-4175. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Vist GE, et al. on behalf of the gradeWorking Group grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottmann OG, Wassmann B, Pfeifer H, et al. on behalf of the gmall Study Group Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ all) Cancer. 2007;109:2068–76. doi: 10.1002/cncr.22631. [DOI] [PubMed] [Google Scholar]
- 11.Wassmann B, Gokbuget N, Scheuring UJ, et al. A randomized multicenter open label phase ii study to determine the safety and efficacy of induction therapy with imatinib (Glivec, formerly STI571) in comparison with standard induction chemotherapy in elderly (>55 years) patients with Philadelphia chromosome–positive (Ph+/BCR-ABL+) acute lymphoblastic leukemia (all) (csti571ade 10) Ann Hematol. 2003;82:716–20. doi: 10.1007/s00277-003-0728-8. [DOI] [PubMed] [Google Scholar]
- 12.Lilly MB, Ottmann OG, Shah NP, et al. Dasatinib 140 mg once daily versus 70 mg twice daily in patients with Ph-positive acute lymphoblastic leukemia who failed imatinib: results from a phase 3 study. Am J Hematol. 2010;85:164–70. doi: 10.1002/ajh.21615. [DOI] [PubMed] [Google Scholar]
- 13.Ottmann OG, Druker BJ, Sawyers CL, et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome–positive acute lymphoid leukemias. Blood. 2002;100:1965–71. doi: 10.1182/blood-2001-12-0181. [DOI] [PubMed] [Google Scholar]
- 14.Wassmann B, Pfeifer H, Scheuring U, et al. Therapy with imatinib mesylate (Glivec) preceding allogeneic stem cell transplantation (sct) in relapsed or refractory Philadelphia-positive acute lymphoblastic leukemia (Ph+ all) Leukemia. 2002;16:2358–65. doi: 10.1038/sj.leu.2402770. [DOI] [PubMed] [Google Scholar]
- 15.Pfeifer H, Wassmann B, Hofmann WK, et al. Risk and prognosis of central nervous system leukemia in patients with Philadelphia chromosome–positive acute leukemias treated with imatinib mesylate. Clin Cancer Res. 2003;9:4674–81. [PubMed] [Google Scholar]
- 16.Scheuring UJ, Pfeifer H, Wassmann B, et al. Early minimal residual disease (mrd) analysis during treatment of Philadelphia chromosome/BCR-ABL–positive acute lymphoblastic leukemia with the Abl-tyrosine kinase inhibitor imatinib (STI571) Blood. 2003;101:85–90. doi: 10.1182/blood-2002-02-0360. [DOI] [PubMed] [Google Scholar]
- 17.Shimoni A, Kroger N, Zander AR, et al. Imatinib mesylate (STI571) in preparation for allogeneic hematopoietic stem cell transplantation and donor lymphocyte infusions in patients with Philadelphia-positive acute leukemias. Leukemia. 2003;17:290–7. doi: 10.1038/sj.leu.2402808. [DOI] [PubMed] [Google Scholar]
- 18.Wassmann B, Scheuring U, Pfeifer H, et al. Efficacy and safety of imatinib mesylate (Glivec) in combination with interferon-alpha (ifn-alpha) in Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+all) Leukemia. 2003;17:1919–24. doi: 10.1038/sj.leu.2403093. [DOI] [PubMed] [Google Scholar]
- 19.Houot R, Tavernier E, Le QH, Lhéritier V, Thiebaut A, Thomas X. Philadelphia chromosome–positive acute lymphoblastic leukemia in the elderly: prognostic factors and treatment outcome. Hematology. 2004;9:369–76. doi: 10.1080/10245330400001983. [DOI] [PubMed] [Google Scholar]
- 20.Piccaluga PP, Malagola M, Amabile M, et al. The achievement of molecular complete remission during treatment with imatinib mesylate correlates with relapse-free survival in BCR/ABL-positive acute lymphoid leukemia patients. Haematologica. 2004;89:1269–71. [PubMed] [Google Scholar]
- 21.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome–positive acute lymphocytic leukemia with hyper-cvad and imatinib mesylate. Blood. 2004;103:4396–407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 22.Towatari M, Yanada M, Usui N, et al. on behalf of the Japan Adult Leukemia Study Group Combination of intensive chemotherapy and imatinib can rapidly induce high-quality complete remission for a majority of patients with newly diagnosed BCR-ABL–positive acute lymphoblastic leukemia. Blood. 2004;104:3507–12. doi: 10.1182/blood-2004-04-1389. [DOI] [PubMed] [Google Scholar]
- 23.Wassmann B, Pfeifer H, Scheuring UJ, et al. Early prediction of response in patients with relapsed or refractory Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ all) treated with imatinib. Blood. 2004;103:1495–8. doi: 10.1182/blood-2003-01-0154. [DOI] [PubMed] [Google Scholar]
- 24.Brandwein JM, Gupta V, Wells RA, et al. Treatment of elderly patients with acute lymphoblastic leukemia—evidence for a benefit of imatinib in BCR-ABL positive patients. Leuk Res. 2005;29:1381–6. doi: 10.1016/j.leukres.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Lee KH, Lee JH, Choi SJ, et al. Clinical effect of imatinib added to intensive combination chemotherapy for newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia. Leukemia. 2005;19:1509–16. doi: 10.1038/sj.leu.2403886. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Kim YJ, Min CK, et al. The effect of first-line imatinib interim therapy on the outcome of allogeneic stem cell transplantation in adults with newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia. Blood. 2005;105:3449–57. doi: 10.1182/blood-2004-09-3785. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Kim DW, Kim YJ, et al. Minimal residual disease-based role of imatinib as a first-line interim therapy prior to allogeneic stem cell transplantation in Philadelphia chromosome–positive acute lymphoblastic leukemia. Blood. 2003;102:3068–70. doi: 10.1182/blood-2003-04-1180. [DOI] [PubMed] [Google Scholar]
- 28.Potenza L, Luppi M, Riva G, Marasca R, Martinelli S, Torelli G. Efficacy of imatinib mesylate as maintenance therapy in adults with acute lymphoblastic leukemia in first complete remission. Haematologica. 2005;90:1275–7. [PubMed] [Google Scholar]
- 29.Deininger M, Schleuning M, Greinix H, et al. on behalf of the European Blood and Marrow Transplantation Group The effect of prior exposure to imatinib on transplant-related mortality. Haematologica. 2006;91:452–9. [PubMed] [Google Scholar]
- 30.Delannoy A, Delabesse E, Lheritier V, et al. Imatinib and methylprednisolone alternated with chemotherapy improve the outcome of elderly patients with Philadelphia-positive acute lymphoblastic leukemia: results of the graall afr09 study. Leukemia. 2006;20:1526–32. doi: 10.1038/sj.leu.2404320. [DOI] [PubMed] [Google Scholar]
- 31.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinibresistant cml and Philadelphia chromosome–positive all. N Engl J Med. 2006;354:2542–51. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 32.Rea D, Legros L, Raffoux E, et al. on behalf of the Intergroupe Français des Leucémies Myéloïdes Chronique and the Group for Research in Adult Acute Lymphoblastic Leukemia High-dose imatinib mesylate combined with vincristine and dexamethasone (div regimen) as induction therapy in patients with resistant Philadelphia-positive acute lymphoblastic leukemia and lymphoid blast crisis of chronic myeloid leukemia. Leukemia. 2006;20:400–3. doi: 10.1038/sj.leu.2404115. [DOI] [PubMed] [Google Scholar]
- 33.Wassmann B, Pfeifer H, Goekbuget N, et al. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ all) Blood. 2006;108:1469–77. doi: 10.1182/blood-2005-11-4386. [DOI] [PubMed] [Google Scholar]
- 34.Yanada M, Naoe T. Imatinib combined chemotherapy for Philadelphia chromosome–positive acute lymphoblastic leukemia: major challenges in current practice. Leuk Lymphoma. 2006;47:1747–53. doi: 10.1080/10428190600634085. [DOI] [PubMed] [Google Scholar]
- 35.Yanada M, Sugiura I, Takeuchi J, et al. on behalf of the Japan Adult Leukemia Study Group Prospective monitoring of Bcr-Abl1 transcript levels in patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia undergoing imatinib-combined chemotherapy. Br J Haematol. 2008;143:503–10. doi: 10.1111/j.1365-2141.2008.07377.x. [DOI] [PubMed] [Google Scholar]
- 36.Zembutsu H, Yanada M, Hishida A, et al. Prediction of risk of disease recurrence by genome-wide cdna microarray analysis in patients with Philadelphia chromosome–positive acute lymphoblastic leukemia treated with imatinib-combined chemotherapy. Int J Oncol. 2007;31:313–22. [PubMed] [Google Scholar]
- 37.Carpenter PA, Snyder DS, Flowers ME, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome–positive leukemia. Blood. 2007;109:2791–3. doi: 10.1182/blood-2006-04-019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Labarthe A, Rousselot P, Huguet–Rigal F, et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome–positive acute lymphoblastic leukemia: results of the graaph-2003 study. Blood. 2007;109:1408–13. doi: 10.1182/blood-2006-03-011908. [DOI] [PubMed] [Google Scholar]
- 39.Ottmann OG, Larson RA, Kantarjian HM, et al. Nilotinib in patients (pts) with relapsed/refractory Philadelphia chromosome– positive acute lymphoblastic leukemia (Ph+ all) who are resistant or intolerant to imatinib [abstract 2815] Blood. 2007;110 doi: 10.1182/blood-2007-02-073528. [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/110/11/2815?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Nilotinib+in+Patients+%28pts%29+with+Relapsed%2FRefractory+Philadelphia+Chromosome-Positive+Acute+Lymphobl&searchid=1&FIRSTINDEX=0&volume=110&issue=11&resourcetype=HWCIT; cited February 6, 2014] [DOI] [Google Scholar]
- 40.Pfeifer H, Wassmann B, Pavlova A, et al. Kinase domain mutations of BCR-ABL frequently precede imatinib-based therapy and give rise to relapse in patients with de novo Philadelphia-positive acute lymphoblastic leukemia (Ph+ all) Blood. 2007;110:727–34. doi: 10.1182/blood-2006-11-052373. [DOI] [PubMed] [Google Scholar]
- 41.Vignetti M, Fazi P, Cimino G, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome–positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell’Adulto (gimema) LAL0201-B protocol. Blood. 2007;109:3676–8. doi: 10.1182/blood-2006-10-052746. [DOI] [PubMed] [Google Scholar]
- 42.Burke MJ, Trotz B, Luo X, et al. Allo-hematopoietic cell transplantation for Ph chromosome–positive all: impact of imatinib on relapse and survival. Bone Marrow Transplant. 2009;43:107–13. doi: 10.1038/bmt.2008.296. [DOI] [PubMed] [Google Scholar]
- 43.Nicolini FE, Mauro MJ, Martinelli G, et al. Epidemiologic study on survival of chronic myeloid leukemia and Ph(+) acute lymphoblastic leukemia patients with BCR-ABL T315I mutation. Blood. 2009;114:5271–8. doi: 10.1182/blood-2009-04-219410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakamaki H, Ishizawa K, Taniwaki M, et al. Phase 1/2 clinical study of dasatinib in Japanese patients with chronic myeloid leukemia or Philadelphia chromosome–positive acute lymphoblastic leukemia. Int J Hematol. 2009;89:332–41. doi: 10.1007/s12185-009-0260-2. [DOI] [PubMed] [Google Scholar]
- 45.Tojo A, Usuki K, Urabe A, et al. A phase i/ii study of nilotinib in Japanese patients with imatinib-resistant or -intolerant Ph+ cml or relapsed/refractory Ph+ all. Int J Hematol. 2009;89:679–88. doi: 10.1007/s12185-009-0327-0. [DOI] [PubMed] [Google Scholar]
- 46.Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28:3644–52. doi: 10.1200/JCO.2010.28.1287. [DOI] [PubMed] [Google Scholar]
- 47.Bassan R, Rossi G, Pogliani EM, et al. Short chemotherapyphased imatinib (im) pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ all) [abstract 2029] Blood. 2009;114 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/114/22/2029?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Bassan+Rossi+Pogliani+%C2%93Short+Chemotherapy-Phased+Imatinib%C2%94&searchid=1&FIRSTINDEX=0&volume=114&issue=22&resourcetype=HWCIT; cited March 15, 2014] [Google Scholar]
- 48.Li Y, Zou D, Zhao Y, Mi Y, Wang J, Qiu L. Clinical characteristics and outcomes of adults with Philadelphia chromosome positive and/or BCR-ABL positive acute lymphoblastic leukemia: a single center study from China. Leuk Lymphoma. 2010;51:488–96. doi: 10.3109/10428190903370361. [DOI] [PubMed] [Google Scholar]
- 49.Nishiwaki S, Miyamura K, Kato C, et al. Impact of posttransplant imatinib administration on Philadelphia chromosome– positive acute lymphoblastic leukaemia. Anticancer Res. 2010;30:2415–18. [PubMed] [Google Scholar]
- 50.Olsson–Stromberg U, Hermansson M, Lundan T, et al. Molecular monitoring and mutation analysis of patients with advanced phase cml and Ph+ all receiving dasatinib. Eur J Haematol. 2010;85:399–404. doi: 10.1111/j.1600-0609.2010.01506.x. [DOI] [PubMed] [Google Scholar]
- 51.Ravandi F, O’Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-cvad for the frontline treatment of patients with Philadelphia chromosome– positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–7. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravandi F, Thomas D, Kantarjian H, et al. Phase ii study of combination of hypercvad with dasatinib in frontline therapy of patients with Philadelphia chromosome (Ph) positive acute lymphoblastic leukemia (all) [abstract 2921] Blood. 2008;112 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/112/11/2921?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Ravandi+Thomas+Kantarjian+Dasatinib+%22Frontline+Therapy%22&searchid=1&FIRSTINDEX=0&volume=112&issue=11&resourcetype=HWCIT; cited March 15, 2014] [Google Scholar]
- 53.Ribera JM, Oriol A, González M, et al. on behalf of the Programa Español de Tratamiento en Hematología and the Grupo Español de Trasplante Hemopoyético Groups Concurrent intensive chemotherapy and imatinib before and after stem cell transplantation in newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Final results of the cstibes02 trial. Haematologica. 2010;95:87–95. doi: 10.3324/haematol.2009.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribera JM, Oriol A, Gonzalez M, et al. Treatment of Philadelphia chromosome (Ph)-positive acute lymphoblastic leukemia (all) with concurrent chemotherapy and imatinib mesylate [abstract 4483] Blood. 2004;104 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/104/11/4483?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Ribera+Oriol+Gonzalez+%C2%93Acute+Lymphoblastic+Leukemia%C2%94+%C2%93Imatinib+Mesylate%C2%94&searchid=1&FIRSTINDEX=0&volume=104&issue=11&resourcetype=HWCIT; cited March 15, 2014] [Google Scholar]
- 55.Riva G, Luppi M, Barozzi P, et al. Emergence of BCR-ABL–specific cytotoxic T cells in the bone marrow of patients with Ph+ acute lymphoblastic leukemia during long-term imatinib mesylate treatment. Blood. 2010;115:1512–18. doi: 10.1182/blood-2009-06-230391. [DOI] [PubMed] [Google Scholar]
- 56.Chen H, Liu KY, Xu LP, et al. Administration of imatinib in the first 90 days after allogeneic hematopoietic cell transplantation in patients with Philadelphia chromosome–positive acute lymphoblastic leukemia. Chin Med J (Engl) 2011;124:246–52. [PubMed] [Google Scholar]
- 57.Foà R, Vitale A, Vignetti M, et al. on behalf of the gimema Acute Leukemia Working Party Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118:6521–8. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- 58.Foà R, Vignetti M, Vitale A, et al. Dasatinib as front-line monotherapy for the induction treatment of adult and elderly Ph+ acute lymphoblastic leukemia (all) patients: interim analysis of the gimema Prospective Study LAL1205 [abstract 7] Blood. 2007;110 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/110/11/7?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Dasatinib+as+Front-Line+Monotherapy+for+the+Induction+Treatment+of+Adult+and+Elderly+Ph%2B+Acute+Lymph&searchid=1&FIRSTINDEX=0&volume=110&issue=11&resourcetype=HWCIT ; cited February 6, 2014] [Google Scholar]
- 59.Mizuta S, Matsuo K, Yagasaki F, et al. Pre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL–positive acute lymphoblastic leukemia. Leukemia. 2011;25:41–7. doi: 10.1038/leu.2010.228. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Gu M, Mi Y, Qiu L, Bian S, Wang J. Clinical characteristics and outcomes of mixed phenotype acute leukemia with Philadelphia chromosome positive and/or BCR-ABL positive in adult. Int J Hematol. 2011;94:552–5. doi: 10.1007/s12185-011-0953-1. [DOI] [PubMed] [Google Scholar]
- 61.Bose P, Perkins EB, Honeycut C, et al. Phase i trial of the combination of flavopiridol and imatinib mesylate in patients with Bcr-Abl(+) hematological malignancies. Cancer Chemother Pharmacol. 2012;69:1657–67. doi: 10.1007/s00280-012-1839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caocci G, Vacca A, Ledda A, et al. Prophylactic and pre-emptive therapy with dasatinib after hematopoietic stem cell transplantation for Philadelphia chromosome–positive acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2012;18:652–4. doi: 10.1016/j.bbmt.2011.12.587. [DOI] [PubMed] [Google Scholar]
- 63.Chen H, Liu KY, Xu LP, et al. Administration of imatinib after allogeneic hematopoietic stem cell transplantation may improve disease-free survival for patients with Philadelphia chromosome–positive acute lymphoblastic leukemia. J Hematol Oncol. 2012;5:29. doi: 10.1186/1756-8722-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee S, Kim DW, Cho BS, et al. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2012;26:2367–74. doi: 10.1038/leu.2012.164. [DOI] [PubMed] [Google Scholar]
- 65.Pfeifer H, Lange T, Wystub S, et al. Prevalence and dynamics of BCR-ABL kinase domain mutations during imatinib treatment differ in patients with newly diagnosed and recurrent BCR-ABL positive acute lymphoblastic leukemia. Leukemia. 2012;26:1475–81. doi: 10.1038/leu.2012.5. [DOI] [PubMed] [Google Scholar]
- 66.Thyagu S, Minden MD, Gupta V, et al. Treatment of Philadelphia chromosome–positive acute lymphoblastic leukaemia with imatinib combined with a paediatric-based protocol. Br J Haematol. 2012;158:506–14. doi: 10.1111/j.1365-2141.2012.09182.x. [DOI] [PubMed] [Google Scholar]
- 67.Dombret H, Witz F, De Botton S, et al. Imatinib combined with intensive ham chemotherapy as consolidation of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph1-all). Preliminary results of the AFR03 phase i/ii study [abstract 2741] Blood. 2004;104 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/104/11/2741?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Dombret+Witz+%C2%93De+Botton%C2%94+%C2%93HAM+Chemotherapy%C2%94&searchid=1&FIRSTINDEX=0&volume=104&issue=11&resourcetype=HWCIT; cited March 15, 2014] [Google Scholar]
- 68.Thomas DA, Faderl S, Cortes J, et al. Update of the hypercvad and imatinib mesylate regimen in Philadelphia (Ph) positive acute lymphocytic leukemia (all) [abstract 2738] Blood. 2004;104 doi: 10.1182/blood-2003-08-2958. [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/104/11/2738?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Thomas+Faderl+Cortes+%C2%93Hyper-CVAD+and+Imatinib+Mesylate%C2%94&searchid=1&FIRSTINDEX=0&volume=104&issue=11&resourcetype=HWCIT; cited March 15, 2014] [DOI] [PubMed] [Google Scholar]
- 69.Wetzler M, Stock W, Owzar K, et al. Sequential imatinib and chemotherapy yield reverse-transcriptase polymerase chain reaction (rt-pcr)–negative peripheral stem cell collections in Philadelphia (Ph) chromosome positive acute lymphoblastic leukemia (all)—preliminary results of calgb 10001 [abstract 6549] J Clin Oncol. 2006;24 [Available online at: http://meeting.ascopubs.org/cgi/content/abstract/24/18_suppl/6549; cited February 6, 2014] [Google Scholar]
- 70.Fielding AK, Richards SM, Lazarus HM, et al. Does imatinib change the outcome in Philadelphia chromosome positive acute lymphoblastic leukaemia in adults? Data from the ukallxii/ecog2993 Study [abstract 8] Blood. 2007;110 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/110/11/8?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Does+Imatinib+Change+the+Outcome+in+Philapdelphia+Chromosome+Positive+Acute+Lymphoblastic+Leukaemia+&searchid=1&FIRSTINDEX=0&volume=110&issue=11&resourcetype=HWCIT; cited February 4, 2014] [Google Scholar]
- 71.Pasquini R, Ottmann OG, Goh YT, et al. Dasatinib 140 mg qd compared to 70 mg bid in advanced-phase cml or Ph(+) all resistant or intolerant to imatinib: one-year results of CA180-035 [abstract 7025] J Clin Oncol. 2007;25 [Available online at: http://meeting.ascopubs.org/cgi/content/abstract/25/18_suppl/7025; cited February 6, 2014] [Google Scholar]
- 72.Gambacorti–Passerini C, Kantarjian HM, Baccarani M, et al. Activity and tolerance of bosutinib in patients with ap and bp cml and Ph+ all [abstract 7049] J Clin Oncol. 2008;26 [Available online at: http://meeting.ascopubs.org/cgi/content/abstract/26/15_suppl/7049; cited February 26, 2014] [Google Scholar]
- 73.Arellano M, Muringampurath–John D, Shenoy PJ, et al. Imatinib mesylate and hyper-cvad (im-hcvad) for Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ all) [abstract 4103] Blood. 2009;114 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/114/22/4103?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=+Arellano+%C2%93Muringampurath-John%C2%94+Shenoy+%C2%93Imatinib+Mesylate+and+Hyper-CVAD%C2%94&searchid=1&FIRSTINDEX=0&volume=114&issue=22&resourcetype=HWCIT; cited March 15, 2014] [Google Scholar]
- 74.Carella AM, Catania G, Pica G, et al. Adult Philadelphia-positive acute lymphoblastic leukemia (Ph + all) treated at diagnosis with imatinib followed by 2nd line tki (imatinib or dasatinib) and stem cell transplantation [abstract 4109] Blood. 2009;114 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/114/22/4109?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Carella+Catania+Pica+%C2%93Treated+at+Diagnosis+with+Imatinib%C2%94+&searchid=1&FIRSTINDEX=0&volume=114&issue=22&resourcetype=HWCIT; cited March 15, 2014] [Google Scholar]
- 75.Pfeifer H, Wassmann B, Kabisch A, et al. Imatinib and interferon- alpha maintenance therapy for patients with Philadelphia-positive acute lymphoblastic leukemia (Ph+ all) ineligible for allogeneic stem cell transplantation (sct) [abstract 2041] Blood. 2009;114:2042. [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/114/22/2042?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Imatinib+and+Interferon-Alpha+Maintenance+Therapy+for+Patients+with+Philadelphia-Positive+Acute+Lymp&searchid=1&FIRSTINDEX=0&volume=114&issue=22&resourcetype=HWCIT; cited February 5, 2014] [Google Scholar]
- 76.Pfeifer H, Goekbuget N, Volp C, et al. Long-term outcome of 335 adult patients receiving different schedules of imatinib and chemotherapy as front-line treatment for Philadelphia-positive acute lymphoblastic leukemia (Ph+ all) [abstract 173] Blood. 2010;116 doi: 10.1182/blood-2005-11-4386. [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/116/21/173?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Long-Term+Outcome+of+335+Adult+Patients+Receiving+Different+Schedules+of+Imatinib+and+Chemotherapy+a&searchid=1&FIRSTINDEX=0&volume=116&issue=21&resourcetype=HWCIT; cited February 6, 2014] [DOI] [PubMed] [Google Scholar]
- 77.Rousselot P, Cayuela JM, Hayette S, et al. Dasatinib (Sprycel) and low intensity chemotherapy for first-line treatment in elderly patients with de novo Philadelphia positive all (ewall-ph-01): kinetic of response, resistance and prognostic significance [abstract 172] Blood. 2010;116 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/116/21/172?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Dasatinib+%28Sprycel%28R%29%29+and+Low+Intensity+Chemotherapy+for+First-Line+Treatment+In+Elderly+Patients+w&searchid=1&FIRSTINDEX=0&volume=116&issue=21&resourcetype=HWCIT; cited February 6, 2014] [Google Scholar]
- 78.Thomas DA, O’Brien SM, Faderl S, et al. Long-term outcome after hyper-cvad and imatinib (im) for de novo or minimally treated Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph-all) [abstract 6506] J Clin Oncol. 2010;28 [Available online at: http://meetinglibrary.asco.org/content/52502-74; cited March 15, 2014] [Google Scholar]
- 79.Kim DY, Joo YD, Lee JH, et al. Nilotinib combined with multi-agent chemotherapy for adult patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia: interim results of Korean Adult ALL Working Party phase 2 study [abstract 1517] Blood. 2011;118 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/118/21/1517?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=+Kim+Joo+Lee+%C2%93Nilotinib+Combined+with+Multi-Agent+Chemotherapy%C2%94&searchid=1&FIRSTINDEX=0&volume=118&issue=21&resourcetype=HWCIT; cited February 6, 2014] [Google Scholar]
- 80.Lee S, Kim DW, Kim YJ, et al. First-line dasatinib plus conventional chemotherapy in adults with newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ all): interim analysis of the Korean Prospective Phase ii Study [abstract 1516] ASH Annual Meeting Abstracts. 2011;118 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/118/21/1516?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Dasatinib+Plus+Conventional+Chemotherapy+in+Adults+with+Newly+Diagnosed+Philadelphia+Chromosome-Posi&searchid=1&FIRSTINDEX=0&volume=118&issue=21&resourcetype=HWCIT; cited February 6, 2014] [Google Scholar]
- 81.Lee HJ, Kantarjian HM, Thomas DA, et al. Long-term followup of combined hypercvad (hcvad) regimen with dasatinib (Db) in the front line therapy of patients (pts) with Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (all) [abstract 1512] Blood. 2011;118 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/118/21/1512?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Long-Term+Follow-up+of+Combined+Hypercvad+%28hCVAD%29+Regimen+with+Dasatinib+%28Db%29+in+the+Front+Line+Thei&searchid=1&FIRSTINDEX=0&volume=118&issue=21&resourcetype=HWCIT; cited February 6, 2014] [Google Scholar]
- 82.Ravandi F, Kantarjian HM, Thomas DA, et al. Phase ii study of combination of the hypercvad regimen with dasatinib in the front line therapy of patients with Philadelphia chromosome (Ph) positive acute lymphoblastic leukemia (all) [abstract 837] Blood. 2009;114 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/118/21/1517?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=+Kim+Joo+Lee+%C2%93Nilotinib+Combined+with+Multi-Agent+Chemotherapy%C2%94&searchid=1&FIRSTINDEX=0&volume=118&issue=21&resourcetype=HWCIT; cited March 15, 2014] [Google Scholar]
- 83.Liu–Dumlao T, O’Brien S, Cortes JE, et al. Combination of the hypercvad regimen with dasatinib in patients with relapsed Philadelphia chromosome (Ph) positive acute lymphoblastic leukemia (all) or lymphoid blast phase of chronic myeloid leukemia (cml-lb) [abstract 2578] ASH Annual Meeting Abstracts. 2011;118 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/118/21/2578?sid=32cdd5f4-c93e-4710-8076-dba1267b5cbf; cited February 5, 2014] [Google Scholar]
- 84.Pfeifer H, Wassmann B, Bethge WA, et al. Updated long-term results of a randomized comparison of prophylactic and pre-emptive imatinib following allogeneic stem cell transplantation for Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ all) [abstract 247] ASH Annual Meeting Abstracts. 2011;118 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/118/21/247?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Updated+Long-Term+Results+of+a+Randomized+Comparison+of+Prophylactic+and+Pre-Emptive+Imatinib+Follow&searchid=1&FIRSTINDEX=0&volume=118&issue=21&resourcetype=HWCIT; cited February 6, 2014] [Google Scholar]
- 85.Brummendorf TH, Gambacorti–Passerini C, Schafhausen P, et al. Efficacy and safety of bosutinib (bos) for Philadelphia chromosome–positive (Ph+) leukemia in older versus younger patients (pts) [abstract 6511] J Clin Oncol. 2012;30 [Available online at: http://meetinglibrary.asco.org/content/100244-114; cited February 6, 2014] [Google Scholar]
- 86.Cortes J, Kim D, Pinilla–Ibarz J. pace: a pivotal phase ii trial of ponatinib in patients with cml and Ph+ all resistant or intolerant to dasatinib or nilotinib, or with the T315I mutation [presentation] J Clin Oncol. 2012;30 [Available online at: http://meetinglibrary.asco.org/content/69655?media=vm; cited February 6, 2014] [Google Scholar]
- 87.Thomas DA, Kantarjian HM, Cortes J, et al. Outcome after frontline therapy with the hyper-cvad and imatinib mesylate regimen for adults with de novo or minimally treated Philadelphia chromosome (Ph) positive acute lymphoblastic leukemia (all) [abstract 2931] Blood. 2008;112 [Available online at: http://abstracts.hematologylibrary.org/cgi/content/abstract/112/11/2931?maxtoshow=&hits=10&RESULTFORMAT=&fulltext=Thomas+Kantarjian+Cortes+%C2%93Frontline+Therapy+with+the+Hyper-CVAD+and+Imatinib+Mesylate%C2%94&searchid=1&FIRSTINDEX=0&volume=112&issue=11&resourcetype=HWCIT; cited March 15, 2014] [Google Scholar]
- 88.Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110:2309–15. doi: 10.1182/blood-2007-02-073528. [DOI] [PubMed] [Google Scholar]
- 89.Soverini S, Colarossi S, Gnani A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the gimema Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12:7374–9. doi: 10.1158/1078-0432.CCR-06-1516. [DOI] [PubMed] [Google Scholar]
- 90.Porkka K, Koskenvesa P, Lundan T, et al. Dasatinib crosses the blood–brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome–positive leukemia. Blood. 2008;112:1005–12. doi: 10.1182/blood-2008-02-140665. [DOI] [PubMed] [Google Scholar]
- 91.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–42. doi: 10.1056/NEJM200104053441402. [Erratum in: N Engl J Med 2001;345:232] [DOI] [PubMed] [Google Scholar]
- 92.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinibresistant Philadelphia chromosome–positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 93.Capdeville R, Krahnke T, Hatfield A, Ford JM, Van Hoomissen I, Gathmann I. Report of an international expanded access program of imatinib in adults with Philadelphia chromosome positive leukemias. Ann Oncol. 2008;19:1320–6. doi: 10.1093/annonc/mdn050. [DOI] [PubMed] [Google Scholar]
- 94.Radich J, Gehly G, Lee A, et al. Detection of Bcr-Abl transcripts in Philadelphia chromosome–positive acute lymphoblastic leukemia after marrow transplantation. Blood. 1997;89:2602–9. [PubMed] [Google Scholar]