Abstract
Vitamin E is the major lipid-soluble component in the cell antioxidant defence system and is exclusively obtained from the diet. It has numerous important roles within the body because of its antioxidant activity. Oxidation has been linked to numerous possible conditions and diseases, including cancer, ageing, arthritis and cataracts; vitamin E has been shown to be effective against these. Platelet hyperaggregation, which can lead to atherosclerosis, may also be prevented by vitamin E; additionally, it also helps to reduce the production of prostaglandins such as thromboxane, which cause platelet clumping. The current literature review discusses the functions and roles of vitamin E in human health and some diseases as well as the consequences of vitamin E deficiency. The main focus of the review is on the tocopherol class of the vitamers.
Keywords: Vitamin E, Health, Tocopherols, Antioxidants
Vitamin E is the collective term given to a group of fat-soluble compounds first discovered in 1922 by Evans and Bishop; these compounds have distinct antioxidant activities essential for health.1 Vitamin E is present in fat-containing foods2 and, as the fat-soluble property of the vitamin allows it to be stored within the fatty tissues of animals and humans, it does not have to be consumed every day. The vitamin E group (i.e. chroman-6-ols), collectively termed tocochromanols (divided into tocopherols and tocotrienols), includes all of the tocol and tocotrienol derivatives which qualitatively exhibit the biological activity of d-alpha-tocopherol.
There are eight naturally occurring forms of vitamin E; namely, the alpha, beta, gamma and delta classes of tocopherol and tocotrienol, which are synthesised by plants from homogentisic acid. Alpha-and gamma-tocopherols are the two major forms of the vitamin, with the relative proportions of these depending on the source. The richest dietary sources of vitamin E are edible vegetable oils as they contain all the different homologues in varying proportions [Table 1]. Among the tocopherols, the alpha- and gamma-tocopherols are found in the serum and the red blood cells, with alpha-tocopherol present in the highest concentration.3 Beta- and delta-tocopherols are found in the plasma in minute concentrations only. The preferential distribution of alpha-tocopherol in humans over the other forms of tocopherol stems from the faster metabolism of the other forms and from the alpha-tocopherol transfer protein (alpha-TTP). It is due to the binding affinity of alpha-tocopherol with alpha-TTP that most of the absorbed beta-, gamma-and delta-tocopherols are secreted into the bile and excreted in the faeces, while alpha-tocopherol is largely excreted in the urine. The alpha-tocopherol form also accumulates in the non-hepatic tissues, particularly at sites where free radical production is greatest, such as in the membranes of the mitochondria and endoplasmic reticulum in the heart and lungs.
Table 1:
Vitamin E content in vegetable oils
| Oil | Alpha-tocopherol | G-tocopherol | D-tocopherol | A-tocotrienol |
|---|---|---|---|---|
| in mg of tocopherol per 100 g | ||||
| Coconut | 0.5 | 0 | 0.6 | 0.5 |
| Maize (corn) | 11.2 | 60.2 | 1.8 | 0 |
| Palm | 25.6 | 31.6 | 7.0 | 14.3 |
| Olive | 5.1 | Trace amounts | 0 | 0 |
| Peanut | 13.0 | 21.4 | 2.1 | 0 |
| Soybean | 10.1 | 59.3 | 26.4 | 0 |
| Wheatgerm | 133.0 | 26.0 | 27.1 | 2.6 |
| Sunflower | 48.7 | 5.1 | 0.8 | 0 |
Source: Slover HT. Tocopherols in foods and fats.4
This review mainly focuses on the current developments in vitamin E research in the context of their importance to human health and disease prevention. The data obtained from a survey of clinical trials or systematic reviews have been included here due to the difficulty of proving the efficacy of vitamin E supplementation, and in order to describe the evidence-based results.
Chemistry of Vitamin E
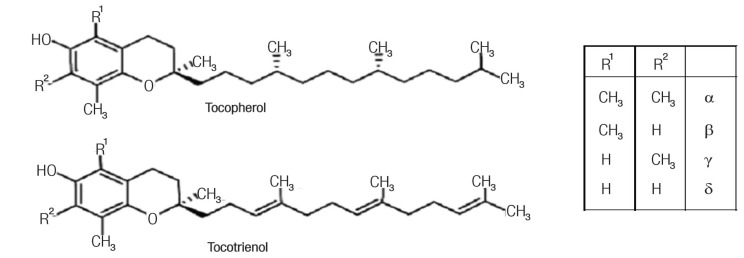
The term ‘tocopherol’ signifies the methyl-substituted derivatives of tocol and is not synonymous with the term ‘vitamin E’. Natural tocochromanols comprise two homologous series: tocopherols with a saturated side chain and tocotrienols with an unsaturated side chain. Tocopherols and tocotrienols have the same basic chemical structure, which is characterised by a long isoprenoid side chain attached at the 2 position of a 6-chromanol ring, as shown in Figure 1.
Figure 1:
The structures of a tocopherol and tocotrienol.
Adapted from: Colombo ML. An update on vitamin E, tocopherol and tocotrienol: Perspectives.10
Tocotrienols differ from tocopherols in that they possess a farnesyl rather than a saturated isoprenoid C16 side chain. Natural tocopherols occur in the RRR-configuration while the synthetic form contains eight different stereoisomers and is called all-rac-alpha-tocopherol. Tocotrienols possess only the chiral stereocenter at C-2 and naturally occurring tocotrienols exclusively possess the 2R,3’E,7’E configuration.5 The receptors and enzymes in the body are highly stereoselective and interact exclusively with one of the enantiomers of a chiral molecule in a process called chiral recognition. As a result, only one enantiomer has the desired effect on the body, while the others may have either no effect or an adverse effect.6 Vitamin E isoforms are not interconvertible inside the human body.7
Sources and Recommended Intakes
Vitamin E is found in various foods and oils. Nuts, seeds and vegetable oils contain high amounts of alpha-tocopherol, and significant amounts are also available in green leafy vegetables and fortified cereals. Some of the richest sources of vitamin E, along with their tocopherol content and percent daily values, are shown in Tables 1 and 2.
Table 2:
Selected dietary sources of vitamin E (alpha-tocopherol)
| Food and recommended intake | Alpha-tocopherol content in mg per serving | Percent daily value |
|---|---|---|
| Wheat germ oil, 1 tablespoon | 20.3 | 100 |
| Sunflower seeds, dry roasted, 1 ounce | 7.4 | 37 |
| Almonds, dry roasted, 1 ounce | 6.8 | 34 |
| Sunflower oil, 1 tablespoon | 5.6 | 28 |
| Safflower oil, 1 tablespoon | 4.6 | 25 |
| Hazelnuts, dry roasted, 1 ounce | 4.3 | 22 |
| Peanut butter, 2 tablespoons | 2.9 | 15 |
| Peanuts, dry roasted, 1 ounce | 2.2 | 11 |
| Corn oil, 1 tablespoon | 1.9 | 10 |
| Spinach, boiled, ½ cup | 1.9 | 10 |
| Broccoli, chopped, boiled, ½ cup | 1.2 | 6 |
| Soybean oil, 1 tablespoon | 1.1 | 6 |
| Kiwifruit, 1 medium | 1.1 | 6 |
| Mango, sliced, ½ cup | 0.7 | 4 |
| Tomato, raw, 1 medium | 0.7 | 4 |
| Spinach, raw, 1 cup | 0.6 | 3 |
Adapted from: United States Department of Agriculture (USDA), Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 25.8
No specific recommendations regarding the intake of vitamin E have been made officially, and the optimal supplementation dosage of mixed tocopherols is still undetermined. When obtained from food sources alone, vitamin E has no documented evidence of toxicity. However, evidence of pro-oxidant damage has been found to be associated with supplements, but usually only at very high doses (for example at >1,000 mg/day).9 The recommended dietary allowances (RDAs) for vitamin E (alpha-tocopherol) are shown in Table 3.
Table 3:
Recommended dietary allowances for vitamin E (alpha-tocopherol)
| Age | RDA in mg (IU) | |
|---|---|---|
| Males | Females | |
| 0–6 months* | 4 (6) | 4 (6) |
| 7–12 months* | 5 (7.5) | 5 (7.5) |
| 1–3 years | 6 (9) | 6 (9) |
| 4–8 years | 7 (10.4) | 7 (10.4) |
| 9–13 years | 11 (16.4) | 11 (16.4) |
| >14 years | 15 (22.4) | 15 (22.4) |
| In pregnancy | 15 (22.4) | |
| If lactating | 19 (28.4) | |
RDA = recommended dietary allowances; IU = international units.
Adequate intake.
Source: Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Vitamin C, Vitamin E, Selenium, and Carotenoids.63
Interactions with Dietary Factors
Vitamin E is heavily dependent on vitamin C, vitamin B3, selenium and glutathione. A diet high in vitamin E cannot have an optimal effect unless it is also rich in foods that provide these other nutrients. It was found that a cooperative interaction between vitamin C and vitamin E is quite probable, while one between vitamin C and beta-carotene is improbable and one may exist between vitamin E and beta-carotene.10 Interactions were also found between thiols, tocopherols and other compounds which enhance the effectiveness of the cellular antioxidant defence systems.11
In 2007, reports from the Women’s Health Study (WHS) demonstrated that vitamin E supplements decrease the risk of mortality from thromboembolism and that alpha-tocopherol decreases the tendency for clotting in normal healthy women.12 In addition, vitamin E supplements in humans were also seen to increase the under-carboxylation of prothrombin,13 suggesting that vitamin E decreases the vitamin K status in humans.
Functions of Vitamin E
Prevention of Oxidative Stress
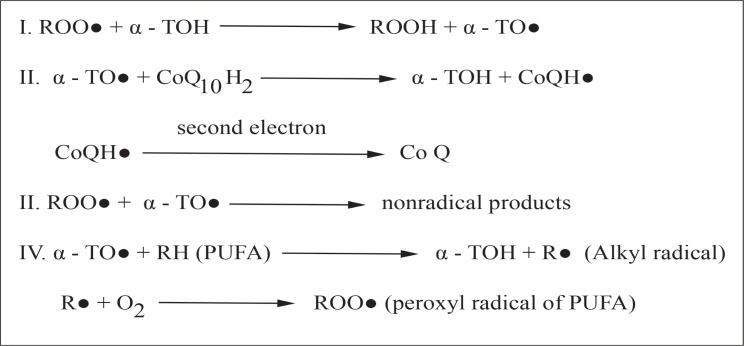
Vitamin E is a potent chain-breaking antioxidant that inhibits the production of reactive oxygen species molecules when fat undergoes oxidation and during the propagation of free radical reactions.14 It is primarily located in the cell and organelle membranes where it can exert its maximum protective effect, even when its concentration ratio may be only one molecule for every 2,000 phospholipid molecules. It acts as the first line of defence against lipid peroxidation, protecting the cell membranes from free radical attack [Figure 2]. Studies have shown that a mixture of tocopherols has a stronger inhibitory effect on lipid peroxidation induced in human erythrocytes compared to alpha-tocopherol alone.15 Due to its peroxyl radical-scavenging activity, it also protects the polyunsaturated fatty acids present in membrane phospholipids and in plasma lipoproteins.16 The tocopheroxyl radicals formed can either: (1) oxidise other lipids; (2) undergo further oxidation producing tocopheryl quinones; (3) form non-reactive tocopherol dimers by reacting with another tocopheroxyl radical, or (4) be reduced by other antioxidants to tocopherol.
Figure 2:
The mechanism of vitamin E (alpha-tocopherol)-mediated low-density lipoprotein lipid peroxidation.
Adapted from: Rathore GS, Suthar M, Pareek A, Gupta RN. Nutritional antioxidants: A battle for better health.17
It has been found that alpha-tocopherol mainly inhibits the production of new free radicals, while gamma-tocopherol traps and neutralises the existing free radicals. Oxidation has been linked to numerous possible conditions/diseases including: cancer, ageing, arthritis and cataracts. Thus, vitamin E might help prevent or delay the chronic diseases associated with reactive oxygen species molecules.
Protection of the Cell Membranes
Vitamin E increases the orderliness of the membrane lipid packaging, thus allowing for a tighter packing of the membrane and, in turn, greater stability to the cell. In 2011, Howard et al. showed that vitamin E is necessary for maintaining proper skeletal muscle homeostasis and that the supplementation of cultured myocytes with alpha-tocopherol promotes plasma membrane repair.18 This occurs because the membrane phospholipids are prominent targets of oxidants and vitamin E efficiently prevents lipid peroxidation. Conversely, in the absence of alpha-tocopherol supplementation, the exposure of the cultured cells to an oxidant challenge strikingly inhibits the repair. Comparative measurements reveal that in order to promote the repair, an antioxidant must associate with the membranes, as alpha-tocopherol does, or be capable of alpha-tocopherol regeneration. Thus, vitamin E promotes membrane repair by preventing the formation of oxidised phospholipids that theoretically might interfere with the membrane fusion events.
Regulation of Platelet Aggregation and Protein Kinase C Activation
An increase in the concentration of alpha-tocopherol in the endothelial cells has been found to inhibit platelet aggregation and to release prostacyclin from the endothelium. This effect was thought to occur because of the downregulation of the intracellular cell adhesion molecule (ICAM-1) and the vascular cell adhesion molecule (VCAM-1), thereby decreasing the adhesion of blood cell components to the endothelium. Also, due to their upregulation by vitamin E in the arachidonic acid cascade, the increase in the expression of cytosolic phospholipase A2,19 and cyclooxygenase-1,20 increases the release of prostacyclin, which is a potent vasodilator and inhibitor of platelet aggregation in humans.21 A few other studies suggest that tocopherols appear to inhibit platelet aggregation through the inhibition of protein kinase C (PKC)22 and the increased action of nitric oxide synthase.23
The natural RRR-configuration form of alpha-tocopherol has been shown to be twice as potent as the other all-racemic (synthetic) alpha-tocopherols in inhibiting PKC activity.24 This occurs because of the attenuating effect of alpha-tocopherol on the generation of membrane-derived diacylglycerol (a lipid which facilitates PKC translocation and thus increases its activity); additionally, alpha-tocopherol increases the activity of protein phosphatase type 2A, which inhibits PKC autophosphorylation and, consequently, its activity. Mixed tocopherols are more effective than alpha-tocopherol in inhibiting platelet aggregation. Adenosine diphosphate-induced platelet aggregation decreased significantly in healthy people who were given gamma-tocopherol-enriched vitamin E (100 mg of gamma-tocopherol, 40 mg of delta-tocopherol and 20 mg of alpha-tocopherol per day), but not in those receiving pure alpha-tocopherol alone (100 mg per day) or in the controls.25
Vitamin E in Disease Prevention
Vitamin E has been found to be very effective in the prevention and reversal of various disease complications due to its function as an antioxidant, its role in anti-inflammatory processes, its inhibition of platelet aggregation and its immune-enhancing activity.
Cardiovascular Diseases
Cardiovascular complications basically arise because of the oxidation of low-density lipoproteins present in the body and the consequent inflammation.26 Gamma-tocopherol is found to improve cardiovascular functions by increasing the activity of nitric oxide synthase, which produces vessel-relaxing nitric oxide.27 It does this by trapping the reactive nitrogen species (peroxynitriten) molecules and thus enhancing the endothelial function. Researchers have found that the supplementation of 100 mg per day of gamma-tocopherol in humans leads to a reduction in several risk factors for arterial clotting, such as platelet aggregation and cholesterol.28 In another study, mixed tocopherols were found to have a stronger inhibitory effect on lipid peroxidation and the inhibition of human platelet aggregation than individual tocopherols alone,25 suggesting a synergistic platelet-inhibitory effect. Apart from tocopherols, tocotrienols were also found to inhibit cholesterol biosynthesis by suppressing 3-hydroxy-3-methylglutaryl-CoA (HMGCoA) reductase, resulting in less cholesterol being manufactured by the liver cells.29 Contradictory to this, most of the recent large interventional clinical trials have not shown cardiovascular benefits from vitamin E supplementation and report that the use of vitamin E was associated with a significantly increased risk of a haemorrhagic stroke in the participants.30 Thus, it was suggested that understanding the potential uses of vitamin E in preventing coronary heart disease might require longer studies with younger participants.
Cancer
Vitamin E also possesses anti-cancer properties. This is possibly because of the various functions of vitamin E which include: the stimulation of the wild-type p53 tumor suppressor gene; the downregulation of mutant p53 proteins; the activation of heat shock proteins, and an anti-angiogenic effect mediated by the blockage of transforming growth factor alpha.31 Alpha-, gamma-and delta-tocopherols have emerged as vitamin E molecules with functions clearly distinct from each other in anti-cancer activity as well. Alpha-tocopherol was found to inhibit the production of PKC and collagenase,32 which facilitate cancer cell growth. In this context gamma-tocopherol was found to be more effective than alpha-tocopherol in its growth inhibitory effect on human prostate cancer cell lines, whereas delta-tocopherol has shown growth inhibitory activity against mouse mammary cancer cell lines.33
Gamma-tocopherol inhibits the growth of cancer cells in cultures through a number of mechanisms. It traps free radicals, including the reactive nitrogen species molecules that cause mutations in the deoxyribonucleic acid strands and malignant transformations in the cells.34 It also downregulates the control molecules known as cyclins, stopping the cancerous cell cycle in the middle and thus preventing their proliferation.35 Gamma-tocopherol has also been found to be superior to alpha-tocopherol in: inducing apoptosis; triggering a number of cell-death-inducing pathways;36 stimulating peroxisome proliferator-activated receptor gamma activity, especially in colon cancer cells,37 and in reducing the formation of new blood vessels in tumours, thus depriving them of the nutrients they need to thrive.38 In this context, tocotrienols were also found to have antiproliferative and apoptotic activities on normal and cancerous cells in humans, which could be due to the induction of apoptosis by a mitochondria-mediated pathway, or due to the suppression of cyclin D which would therefore arrest the cell cycle.39 They also inhibit vascularisation and suppress 3-hydroxy-3-methyl coenzyme A (HMG-CoA) reductase activity, thus preventing malignant proliferation.
Jiang et al. showed that, of the various forms of vitamin E, gamma-tocopherol, particularly in combination with delta-tocopherol, induced apoptosis in androgen-sensitive prostate cancer cells within three days of treatment, while alpha-tocopherol alone did not have the same effect.40 The gamma and delta E fractions appear to induce apoptosis by interrupting the synthesis of sphingolipid in the membranes of human prostate cancer cells.40 The fractions do this by inducing the release of cytochrome c, the activation of caspase-9 and caspase-3, the cleavage of polyadenosine diphosphate (ADP)-ribose polymerase (PARP) and the involvement of caspase-independent pathways. Recently, Chen reported that, of the tocopherols tested, the gamma form was the most potent anti-cancer form of the vitamin. They also found a novel anti-cancer mechanism of vitamin E: gamma-tocopherol, they created a new agent which was found to be 20-fold more effective. They did so by removing a string of chemical groups dangling from the head group of gamma-tocopherol which enhanced its anti-cancer effect. Chen et al. said that gamma-tocopherol was more effective than other tocopherols because of its chemical structure which is more effective in attaching and thus shutting off the Akt enzyme.38 These findings suggest that an agent based on the chemical structure of one form of vitamin E could help to prevent and treat numerous types of cancer—particularly those associated with a mutation in the PTEN gene, a fairly common cancer-related genetic defect that keeps protein kinase B (Akt) active. Chen studied both alpha and gamma forms of the vitamin E molecule; both inhibited the Akt enzyme in very targeted ways, but the gamma structure emerged as the more powerful form of the vitamin. In effect, the vitamin halted Akt activation by attracting Akt and the PHLPP1 protein to the same region of a cell where the vitamin was absorbed in the fat-rich cell membrane. The PHLPP1 tumour suppressor protein then launched a chemical reaction that inactivated Akt, rendering it unable to keep cancer cells alive.
Apart from these findings, the role of vitamin E in cancer prevention remains controversial. The reports from the Cancer Institute of New Jersey show that gamma- and delta-tocopherols can prevent colon, lung, breast and prostate cancers, while alpha-tocopherol had no such effect. In addition, human trials and surveys aiming to study the association between vitamin E intake and cancer have found that vitamin E is not beneficial in most cases. Both the Heart Outcomes Prevention Evaluation—The Ongoing Outcomes (HOPE-TOO) trial and the WHS study evaluated whether vitamin E supplements might protect people from cancer and found no significant reduction in the risk of developing cancer in individuals taking daily doses of 400 IU or 600 IU of vitamin E.41,42
Cataracts
Cataracts are one of the commonest causes of significant vision loss in older people. They basically occur due to the accumulation of proteins damaged by free radicals. Several observational studies have revealed a potential relationship between vitamin E supplements and the risk of cataract formation. Leske et al. found that lens clarity was superior in participants who took vitamin E supplements and those with higher blood levels of the vitamin.43 In another study, a long-term supplementation of vitamin E was associated with the slower progression of age-related lens opacification.44 However, in the randomised Age-Related Eye Disease Study (AREDS), vitamin E had no apparent effect on cataract development/progression over an average of 6.3 years.45 Overall, the available evidence is insufficient to conclude that vitamin E supplements, taken alone or in combination with other antioxidants, can reduce the risk of cataract formation.
Alzheimer’s Disease
Alzheimer’s disease (AD) occurs as a result of protein oxidation and lipid peroxidation via a free radical mechanism, where the beta amyloid protein induces cytotoxicity through a mechanism involving oxidative stress and hydrogen peroxide, leading to neuronal cell death and, finally, AD. Vitamin E can block the production of hydrogen peroxide and the resulting cytotoxicity. It reduces beta amyloid-induced cell death in rat hippocampal cell cultures46 and PC12 cells47 and attenuates the excitatory amino acid-induced toxicity in neuroblastoma cells.48 The Alzheimer’s Disease Cooperative Study in 1997 showed that vitamin E may slow disease progression in patients with moderately severe AD. High doses of vitamin E delayed the loss of the patient’s ability to carry out daily activities and their consequent placement in residential care for several months.49 In another study, it was found that subjects with AD had reduced concentrations of plasma antioxidant micronutrients, suggesting that inadequate antioxidant activity is a factor in this disease. High plasma levels of vitamin E are associated with a reduced risk of AD in older patients and this neuroprotective effect is related to the combination of different forms of vitamin E rather than to alpha-tocopherol alone.50 A study published in 2009 examined the effects of taking 2,000 IU of vitamin E with and without an AD drug on 847 people. It concluded that vitamin E plus a cholinesterase inhibitor may be more beneficial than taking either agent alone.51
At the biomarker level, Mangialasche et al. demonstrated that plasma levels of tocopherols and tocotrienols together with automated magnetic resonance imaging (MRI) measures can help to differentiate patients with AD and mild cognitive impairment (MCI) from the control subjects, and prospectively predict the MCI conversion into AD.49 This therefore suggests the potential role of nutritional biomarkers detected in plasma-tocopherols and tocotrienols as indirect indicators of AD pathology.52 However, researchers have recommended that patients should not take vitamin E to treat AD without the supervision of a physician, as in high doses it can interact negatively with other medications, including those prescribed to lower cholesterol.
Human Immunodeficiency Virus and Acquired Immunodeficiency Syndrome
Vitamin E is an important anti-inflammatory agent that is often found to be deficient in human immunodeficiency virus (HIV)-positive individuals; however, it is not known whether vitamin E supplementation is beneficial either at every or any stage of HIV infection. At a dose of 400 IU, vitamin E was shown to restore delayed skin hypersensitivity reactions and interleukin-2 production, and at high doses it was shown to stimulate T helper cell (CD4 T-cell) proliferation.53 In 1997, Tang et al. studied the association between serum vitamin A and E levels with HIV-1 disease progression. In this study, it was found that men with serum vitamin E levels above 23.5 μm/L had a significantly reduced risk of disease progression. A strong correlation was noted in this cohort between the intake of supplements containing vitamin E at the point of entry into the study and high blood levels of vitamin E.54
A study on murine acquired immunodeficiency syndrome (AIDS) using a 15-fold increase in dietary vitamin E showed the normalisation of immune parameters that are altered in HIV/AIDS.55 Apart from this, an increase in dietary vitamin E has also been shown to protect against the side-effects of azidothymidine, such as bone marrow toxicity.56 Related studies on bone marrow cultures from stage IV AIDS patients using d-alpha-tocopherol supplementation revealed similar results.57 Nevertheless, it has also been reported that higher vitamin E levels pre-infection were found to be associated with increased mortality. Thus, further research is needed to elucidate the role vitamin E plays in the pathogenesis of HIV-1.58
Immunity
It has now been proven that vitamin E stimulates the body’s defences, enhances humoral and cell immune responses and increases phagocytic functions. It has a pronounced effect in infectious diseases where immune phagocytosis is involved, but is less effective in the case of cell-mediated immune defences. Its supplementation significantly enhances both cell-mediated and humoral immune functions in humans, especially in the elderly. A daily intake of 200 mg of vitamin E improved the antibody response to various vaccines in healthy subjects who showed no adverse side-effects to vitamin E supplementation.59 Vitamin E also enhanced resistance to viral diseases in elderly subjects, where higher plasma vitamin E levels correlated with a reduced number of infections over a three-year period.60 A recent study by Kutty et al. showed that a daily supplementation of vitamin E can enhance the immune response to a specific antigen.61
Besides the above mentioned diseases, vitamin E has also been found to play a beneficial role in other diseases, such as photodermatitis, menstrual pain/dysmenorrhoea, pre-eclampsia and tardive dyskinesia, when taken along with vitamin C.62
Vitamin E Deficiency
Vitamin E deficiency is quite rare in humans. It happens almost exclusively in people with an inherited or acquired condition that impairs their ability to absorb the vitamin (for instance, cystic fibrosis, short bowel syndrome or bile duct obstruction) and in those who cannot absorb dietary fat or have rare disorders of fat metabolism. Recent reports have shown that the alpha-TTP regulates the secretion of alpha-tocopherol from the liver cells and that missense mutations of some arginine residues at the surface of the alpha-TTP can cause severe vitamin E deficiency in humans.63 The wild-type alpha-TTP was found to bind phosphatidylinositol phosphates (PIPs), whereas the arginine mutants did not—where the PIPs in the target membrane promoted the inter-membrane transfer of alpha-tocopherol by alpha-TTP. The resulting symptoms of vitamin E deficiency include muscle weakness, vision problems, immune system changes, numbness, difficulty in walking and tremors as well as a poor sense of balance. Apart from these symptoms, deficiency can also lead to neuromuscular problems such as spinocerebellar ataxia and myopathies,24 dysarthria, an absence of deep tendon reflexes, the loss of both vibratory sensations and positive Babinski reflexes.24 Vitamin E deficiency can also cause anaemia due to the oxidative damage to the red blood cells,24 retinopathy64–67 and the impairment of the immune response.59–61 If untreated, vitamin E deficiency may result in blindness, heart disease, permanent nerve damage and impaired thinking. Some reports also suggest that vitamin E deficiency can even result in male infertility.24
Conclusion
Vitamin E was first used as a supplement in Canada by the physicians Shute and Shute; based on the positive results it achieved, they began using it regularly in their practices. Since then, well-designed experimental and clinical studies have progressed steadily and increased our knowledge of vitamin E. The antioxidative properties of vitamin E have been found to play a vital role in the battle against various diseases such as atherosclerosis, oxidative stress, cancer, cataract and AD, among others.
This review focussed on the important functions of vitamin E in some diseases; in addition to these, this vitamin has been found to be effective against asthma, allergies and diabetes, among others. Discussion of the dietary sources, RDA and the interaction of vitamin E supplements with other dietary factors, has demonstrated the need for and significance of vitamin E in the human context. Thus, raising public awareness of the role of dietary antioxidants in maintaining better health would benefit a number of lives.
Apart from the enormous benefits reported, there has always been debate about the exact function of vitamin E and its role in various diseases. There are many conflicting reports of positive and negative results on the same biological activities in the literature. The primary hindrance in determining the roles of vitamin E in human health is the lack of validated biomarkers for vitamin E intake and status, which would help to relate intakes to possible clinical outcomes. In conclusion, although the data surrounding vitamin E is contradictory, the current literature appears to support the view that the benefits outweigh the side-effects.
References
- 1.Niki E, Traber MG. A history of vitamin E. Ann Nutr Metab. 2012;61:207–12. doi: 10.1159/000343106. [DOI] [PubMed] [Google Scholar]
- 2.Zingg JM. Vitamin E: An overview of major research directions. Mol Aspects Med. 2007;28:400–422. doi: 10.1016/j.mam.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Chow CK. Distribution of tocopherols in human plasma and red blood cells. Am J Clin Nutr. 1975;28:756–60. doi: 10.1093/ajcn/28.7.756. [DOI] [PubMed] [Google Scholar]
- 4.Drotleff AM, Ternes W. Determination of RS,E/Z-tocotrienols by HPLC. J Chomatogr A. 2001;909:215–23. doi: 10.1016/s0021-9673(00)01110-9. [DOI] [PubMed] [Google Scholar]
- 5.Zingg JM. Molecular and cellular activities of vitamin E analogues. Mini Rev Med Chem. 2007;7:543–58. doi: 10.2174/138955707780619608. [DOI] [PubMed] [Google Scholar]
- 6.Ball GFM. Vitamins in Foods: Analysis, bioavailability, and stability. Boca Raton, Florida: CRC Press; 2006. pp. 119–36. [Google Scholar]
- 7.Brown KM, Morrice PC, Duthie GG. Erythrocyte vitamin E and plasma ascorbate concentrations in relation to erythrocyte peroxidation in smokers and non-smokers: Dose response to vitamin E supplementation. Am J Clin Nutr. 1997;65:496–502. doi: 10.1093/ajcn/65.2.496. [DOI] [PubMed] [Google Scholar]
- 8.Niki E, Noguchi N, Tsuchihashi H, Gotoh N. Interaction among vitamin C, vitamin E, and beta-carotene. Am J Clin Nutr. 1995;62:1322S–26S. doi: 10.1093/ajcn/62.6.1322S. [DOI] [PubMed] [Google Scholar]
- 9.Di Mascio P, Murphy ME, Sies H. Antioxidant defense systems: The role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991;53:194S–200S. [PubMed] [Google Scholar]
- 10.Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: Report from the Women’s Health Study. Circulation. 2007;116:1497–503. doi: 10.1161/CIRCULATIONAHA.107.716407. [DOI] [PubMed] [Google Scholar]
- 11.Booth SL, Golly I, Sacheck JM, Roubenoff R, Dallal GE, Hamada K, et al. Effect of vitamin E supplementation on vitamin K status in adults with normal coagulation status. Am J Clin Nutr. 2004;80:143–8. doi: 10.1093/ajcn/80.1.143. [DOI] [PubMed] [Google Scholar]
- 12.Burton GW, Ingold KU. Autoxidation of biological molecules: 1. The antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J Am Chem Soc. 1981;103:6472–7. [Google Scholar]
- 13.Liu M, Wallin R, Wallmon A, Saldeen T. Mixed tocopherols have a stronger inhibitory effect on lipid peroxidation than alpha-tocopherol alone. J Cardiovasc Pharmacol. 2002;39:714–21. doi: 10.1097/00005344-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Burton GW, Joyce A, Ingold KU. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983;221:281–90. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- 15.Howard AC, Anna K, McNeil AK, McNeil PL. Promotion of plasma membrane repair by vitamin E. Nat Commun. 2011;20:597. doi: 10.1038/ncomms1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran K, Wong JT, Lee E, Chan AC, Choy PC. Vitamin E potentiates arachidonate release and phospholipase A2 activity in rat heart myoblastic cells. Biochem J. 1996;319:385–91. doi: 10.1042/bj3190385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan AC, Wagner M, Kennedy C, Mroske C, Proulx P, Laneuville O, et al. Vitamin E up-regulates phospholipase A2, arachidonic acid release and cyclooxygenasin endothelial cells. Aktuel Ernährungsmed. 1998;23:1–8. [Google Scholar]
- 18.Szczeklik A, Gryglewski RJ, Domagala B, Dworski R, Basista M. Dietary supplementation with vitamin E in hyperlipoproteinemias: Effects on plasma lipid peroxides, antioxidant activity, prostacyclin generation and platelet aggregability. Thromb Haemost. 1985;54:425–30. [PubMed] [Google Scholar]
- 19.Freedman JE, Farhat JH, Loscalzo J, Keaney JF., Jr Alpha-tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism. Circulation. 1996;94:2434–40. doi: 10.1161/01.cir.94.10.2434. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Saldeen T, Romeo F, Mehta JL. Different isoforms of tocopherols enhance nitric oxide synthase phosphorylation and inhibit human platelet aggregation and lipid peroxidation: Implications in therapy with vitamin E. J Cardiovasc Pharmacol Ther. 2001;6:155–61. doi: 10.1177/107424840100600207. [DOI] [PubMed] [Google Scholar]
- 21.Brigelius-Flohé R, Traber MG. Vitamin E: Function and metabolism. FASEB J. 1999;13:1145–55. [PubMed] [Google Scholar]
- 22.Liu M, Wallmon A, Olsson-Mortlock C, Wallin R, Saldeen T. Mixed tocopherols inhibit platelet aggregation in humans: Potential mechanisms. Am J Clin Nutr. 2003;77:700–6. doi: 10.1093/ajcn/77.3.700. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: Modifications of low-density lipoprotein that increase its artherogenicity. N Engl J Med. 1989;320:915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 24.Li D, Saldeen T, Romeo F, Mehta JL. Relative effects of alpha-and gamma-tocopherol on low-density lipoprotein oxidation and superoxide dismutase and nitric oxide synthase activity and protein expression in rats. J Cardiovasc Pharmacol Ther. 1999;4:219–26. doi: 10.1177/107424849900400403. [DOI] [PubMed] [Google Scholar]
- 25.Singh I, Turner AH, Sinclair AJ, Li D, Hawley JA. Effects of gamma-tocopherol supplementation on thrombotic risk factors. Asia Pac J Clin Nutr. 2007;16:422–8. [PubMed] [Google Scholar]
- 26.McAnally JA, Gupta J, Sodhani S, Bravo L, Mo H. Tocotrienols potentiate lovastatin-mediated growth suppression in vitro and in vivo. Exp Biol Med (Maywood) 2007;232:523–31. [PubMed] [Google Scholar]
- 27.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shklar G, Oh SK. Experimental basis for cancer prevention by vitamin E. Cancer Invest. 2000;18:214–22. doi: 10.3109/07357900009031826. [DOI] [PubMed] [Google Scholar]
- 29.Ricciarelli R, Maroni P, Ozer N, Zingg JM, Azzi A. Age-dependent increase of collagenase expression can be reduced by alpha-tocopherol via protein kinase C inhibition. Free Radic Biol Med. 1999;27:729–37. doi: 10.1016/s0891-5849(99)00007-6. [DOI] [PubMed] [Google Scholar]
- 30.McIntyre BS, Briski KP, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med. 2000;224:292–301. doi: 10.1046/j.1525-1373.2000.22434.x. [DOI] [PubMed] [Google Scholar]
- 31.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. Gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: Physiological implications. Proc Natl Acad Sci U S A. 1997;94:3217–22. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002;16:1952–4. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi S, Takeshita K, Seeni A, Sugiura S, Tang M, Sato SY, et al. Suppression of prostate cancer in a transgenic rat model via gamma-tocopherol activation of caspase signaling. Prostate. 2009;69:644–51. doi: 10.1002/pros.20915. [DOI] [PubMed] [Google Scholar]
- 34.Stone WL, Krishnan K, Campbell SE, Qui M, Whaley SG, Yang H. Tocopherols and the treatment of colon cancer. Ann N Y Acad Sci. 2004;1031:223–33. doi: 10.1196/annals.1331.022. [DOI] [PubMed] [Google Scholar]
- 35.Wells SR, Jennings MH, Rome C, Hadjivassiliou V, Papas KA, Alexander JS. Alpha-, gamma- and delta-tocopherols reduce inflammatory angiogenesis in human microvascular endothelial cells. J Nutr Biochem. 2010;21:589–97. doi: 10.1016/j.jnutbio.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Wada S. Chemoprevention of tocotrienols: The mechanism of antiproliferative effects. Forum Nutr. 2009;61:204–16. doi: 10.1159/000212752. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. Gamma-tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A. 2004;101:17825–30. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CS. Study Shows How Vitamin E Can Help Prevent Cancer. From: www.researchnews.osu.edu/archive/silenceakt.htm Accessed: Dec 2013.
- 39.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 40.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women’s Health Study: A randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 41.Jacques PF, Taylor A, Moeller S, Hankinson SE, Rogers G, Tung W, et al. Long-term nutrient intake and 5-year change in nuclear lens opacities. Arch Ophthalmol. 2005;123:517–26. doi: 10.1001/archopht.123.4.517. [DOI] [PubMed] [Google Scholar]
- 42.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Opthalmol. 2001;119:1439–52. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman Y, Mattson MP. Secreted forms of beta-amyloid precursor protein protect hippocampal neurons against amyloid beta-peptide-induced oxidative injury. Exp Neurol. 1994;128:1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- 44.Behl C, Davis J, Cole GM, Schubert D. Vitamin E protects nerve cells from amyloid beta protein toxicity. Biochem Biophys Res Commun. 1992;186:944–50. doi: 10.1016/0006-291x(92)90837-b. [DOI] [PubMed] [Google Scholar]
- 45.Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. FASEB J. 1990;4:1624–33. [PubMed] [Google Scholar]
- 46.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336:1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 47.Mangialasche F, Kivipelto M, Mecocci P, Rizzuto D, Palmer K, Winblad B, et al. High plasma levels of vitamin E forms and reduced Alzheimer’s disease risk in advanced age. J Alzheimer’s Dis. 2010;20:1029–37. doi: 10.3233/JAD-2010-091450. [DOI] [PubMed] [Google Scholar]
- 48.Pavlik VN, Doody RS, Rountree SD, Darby EJ. Vitamin E use Is associated with improved survival in an Alzheimer’s disease cohort. Dement Geriatr Cogn Disord. 2009;28:536–40. doi: 10.1159/000255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mangialasche F, Westman E, Kivipelto M, Muehlboeck JS, Cecchetti R, Baglioni M, et al. Classification and prediction of clinical diagnosis of Alzheimer’s disease based on MRI and plasma measures of α-/γ-tocotrienols and γ-tocopherol. J Intern Med. 2013;273:602–21. doi: 10.1111/joim.12037. [DOI] [PubMed] [Google Scholar]
- 50.Meydani M. Vitamin E. Lancet. 1995;345:170–5. doi: 10.1016/s0140-6736(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 51.Tang AM, Graham NM, Semba RD, Saah AJ. Association between serum vitamin A and E levels and HIV-1 disease progression. AIDS. 1997;11:613–20. doi: 10.1097/00002030-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Huang DS, Watson RR. Vitamin E supplementation modulates cytokine production by thymocytes during murine AIDS. Immunol Res. 1993;12:358–66. doi: 10.1007/BF02935509. [DOI] [PubMed] [Google Scholar]
- 53.Ganser A, Greher J, Volkers B, Staszewski A, Hoelzer D. Azidothymidine in the treatment of ATDS. N Engl J Med. 1988;318:250–1. doi: 10.1056/NEJM198801283180412. [DOI] [PubMed] [Google Scholar]
- 54.Geissler RG, Ganser A, Ottmann OG, Gute P, Morawetz A, Guba P, et al. In vitro improvement of bone marrow-derived hematopoietic colony formation in HIV-positive patients by alpha-D-tocopherol and erythropoietin. Eur J Haematol. 1994;53:201–6. doi: 10.1111/j.1600-0609.1994.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 55.Graham SM, Baeten JM, Richardson BA, Bankson DD, Lavreys L, Ndinya-Achola JO, et al. Higher pre-infection vitamin E levels are associated with higher mortality in HIV-1-infected Kenyan women: A prospective study. BMC Infect Dis. 2007;7:63. doi: 10.1186/1471-2334-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects: A randomized controlled trial. JAMA. 1997;277:1380–6. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- 57.Chavance M, Herbeth B, Fournier C, Janot C, Vernhes G. Vitamin status, immunity and infections in an elderly population. Eur J Clin Nutr. 1989;43:827–35. [PubMed] [Google Scholar]
- 58.Radhakrishnan AK, Mahalingam D, Selvaduray KR, Nesaretnam K. Supplementation with natural forms of vitamin E augments antigen-specific TH1-type immune response to tetanus toxoid. Biomed Res Int. 2013;2013:782067. doi: 10.1155/2013/782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.University of Maryland Medical Center Vitamin E. From: www.umm.edu/health/medical/altmed/supplement/vitamine#ixzz2c7gfq93R Accessed: Dec 2013.
- 60.Kono N, Ohto U, Hiramatsu T, Urabe M, Uchida Y, Satow Y, et al. Impaired a-TTP-PIPs interaction underlies familial vitamin E deficiency. Science. 2013;340:1106–10. doi: 10.1126/science.1233508. [DOI] [PubMed] [Google Scholar]
- 61.Office of Dietary Supplements, National Institutes of Health Dietary Supplement Fact Sheet: Vitamin E. From: www.ods.od.nih.gov/factsheets/vitamine.asp Accessed: Aug 2010.
- 62.Food and Nutrition Board, Institute of Medicine . Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 63.Kowdley KV, Mason JB, Meydani SN, Cornwall S, Grand RJ. “Vitamin E deficiency and impaired cellular immunity related to intestinal fat malabsorption”. Gastroenterology. 1992;102(6):2139–42. doi: 10.1016/0016-5085(92)90344-x. [DOI] [PubMed] [Google Scholar]
- 64.Slover HT. Tocopherols in foods and fats. Lipids. 1971;6:291–6. [PubMed] [Google Scholar]
- 65.United States Department of Agriculture (USDA), Agricultural Research Service USDA National Nutrient Database for Standard Reference, Release 25. From: www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/SR25/nutrlist/sr25a323.pdf Accessed: Dec 2013.
- 66.Colombo ML. An update on vitamin E, tocopherol and tocotrienol: Perspectives. Molecules. 2010;15:2103–13. doi: 10.3390/molecules15042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rathore GS, Suthar M, Pareek A, Gupta RN. Nutritional antioxidants: A battle for better health. J Nat Pharmaceuticals. 2011;2:2–14. [Google Scholar]